Abstract
DNA topoisomerases of kinetoplastids represent a family of DNA processing enzymes that essentially solve the topological problems not only in nuclear DNA but also in kinetoplast DNA. We have, for the first time, identified a Leishmania donovani homologue of bacterial and eukaryotic IA type of topoisomerase III protein and termed as LdTopIIIβ. Complementation study of wild-type and mutant LdTopIIIβ with slow-growing topoisomerase III mutant yeast S. cerevisiae revealed the functional conservation of the leishmanial counterpart of topoisomerase IIIβ protein, the 327 tyrosine being the active site amino acid. A C-terminal deletion construct of LdTopIIIβ could not suppress the slow-growth phenotype of mutant yeast, indicating the requirement of C-terminal region for the enzyme function in vivo.LdTopIIIβ localized inside the nucleus and kinetoplast of the parasite. Taken together, our study indicates functional conservation and possible role of LdTopIIIβ in parasite DNA processing.
1. Introduction
DNA topoisomerases are ubiquitous enzymes found in all prokaryotic and eukaryotic cells and in some viruses. They are involved in all aspects of DNA metabolism such as replication, transcription, recombination, and chromosome segregation [1, 2]. These reactions are based on sequential breakage and rejoining of the DNA phosphodiester backbone [2–4]. Type I DNA topoisomerases catalyze the cleavage of one strand of DNA, whereas type II DNA topoisomerases catalyze the cleavage of a double-stranded DNA, requiring ATP as a cofactor [4].
Type I DNA topoisomerases are further classified in two subfamilies, IA and IB, based on differences in amino acid sequence and reaction mechanisms [5]. The type IA enzymes link covalently to cleaved DNA through the 5′-phosphate. They are represented by bacterial topoisomerase I and III and the eukaryotic topoisomerase III enzymes. Type IB topoisomerases, exemplified by eukaryotic topoisomerase I, in contrast, become attached to 3′-phosphate end of the cleaved strand of the DNA [4]. Type IA topoisomerases are highly conserved from bacteria to humans.
While the function of topoisomerase II and I are quite well established, the role of topoisomerase III in DNA metabolism is still being defined. Genes encoding topoisomerase III enzymes are highly conserved in evolution from bacteria to human, and the phenotypic consequences of loss of topoisomerase III function are generally quite severe. It has been shown to possess a weak, ATP-independent relaxation activity towards negatively supercoiled DNA only and strict dependence on magnesium [6].
The E. coli chromosome encodes two type IA topoisomerase, DNA topoisomerase I [7] and topoisomerase III [8, 9]. Loss of topoisomerase III in E. coli results in an increase in deletions arising from recombination events between direct repeats [10, 11]. Yeast cells express a single type IA topoisomerase, topoisomerase III encoded by the Top3 gene. In S. pombe, top3 is essential for viability and plays a role in chromosome segregation [12]. It has been shown that top3-ts mutant S. pombe cells are sensitive to the DNA damaging agents UV and MMS (methyl methanesulfonate) at the restrictive temperature revealing that topoisomerase III is involved in DNA damage survival [13]. In S. cerevisiae, top3Δ mutants are viable, but very slow-growing and have defects in S phase responses to DNA damage and in both mitotic and meiotic recombination [14, 15]. In vertebrates, there are two isoforms of topoisomerase III enzymes termed α and β [16–19]. Deletion of mouse topoisomerase IIIα gene led to embryonic lethality [20]. Deletion of mouse topoisomerase IIIβ gene displayed shortened lifespan and infertility [21, 22].
DNA topoisomerases of kinetoplastids represent a family of DNA processing enzymes that essentially solve the topological problems not only in the nuclear DNA but also in the kinetoplastid DNA. The IB type of bi-subunit topoisomerase I and topoisomerase II of the parasites which maintain vital cellular processes, are also proven target for clinically useful antitumor drugs [23]. Apart from this IB type of topoisomerase I, three type IA topoisomerases are there in the parasite genome, termed as topoisomerase IA, and two topoisomerase III. Topoisomerase IA of T. brucei has been reported and shown to be mitochondrial and essential for late theta structure resolution [24]. Very recently, a Topoisomerase IIIα from T. brucei has been shown to play a critical role in antigenic switching [25]. In the present study, for the first time, we have identified functionally active DNA topoisomerase IIIβ from kinetoplastid parasite L. donovani, which localized both inside the nucleus and kinetoplast of the parasite and rescued the topoisomerase III mutant yeast from slow-growth phenotype.
2. Materials and Methods
2.1. Parasite Culture and Maintenance
L. donovani strain AG 83 promastigotes were grown at 22°C in M199 liquid media supplemented 10% heat inactivated fetal calf serum. Transfected cells were maintained under the same conditions with 100 μg/mL G418.
2.2. Strains, Media, and Growth Conditions
The Escherichia coli strains used were DH5α and BL21 (DE3) pLysS. If required, ampicillin and chloramphenicol were used at 100 and 34 μg/mL final concentrations, respectively. The yeast strains used in the studies were W5909-3B (MAT alpha trp1-1 his3-11, 15 leu2-3, 112 ura3-1 RAD5 LYS2 MET15 ADE2) and W2633-4C (a/alpha top3:: TRP1/+) (kindly gifted by Dr. R Rothstein). The yeast cells were grown at 25°C on YEPD medium containing 1% peptone, 2% yeast extract, 2% dextrose and 1.5% agar or synthetic minimal media as required.
2.3. Cloning of Topoisomerase IIIβ Gene from Leishmania donovani
LdTopIIIβ gene was PCR amplified from the genomic DNA of L. donovani parasites using the sense primer 5′-GGAAATTCCATATGGGCCGCAATGTGTTGATG-3′ and antisense primer 5′-CGGGATCCTCACCTGCGATCCTCGCGGTTGCC-3′ and was cloned in bacterial expression vector pET16b in Nde1 and BamH1 restriction sites, termed as LdTopIIIβ-pET16b.
2.4. Structural Analysis and Homology Modeling
Multiple sequence alignment of LdTopIIIβ sequences from various species was carried out using CLUSTAL W (http://expasy.org/tools). Three-dimensional models of LdTopIIIβ based on the crystal structure of E. coli topoisomerase III were generated using Swiss Prot (http://expasy.org/sprot). The generated files were opened in RasMol (http://www.rasmol.org/). The protein sequences were represented in ribbon format and the active site residues were represented in ball and stick format over the ribbon structure.
2.5. Construction of Expression Vectors and Transfection in Leishmania
LdTOPIIIβ genes was PCR amplified using LdTopIIIβ-pET16b as templates and was subcloned using the sense primer 5′-CGGGATCCATGGGCCGCA ATGTGTTGATG-3′ and antisense primer 5′-GATATCCCTGCGATCCTCGCGGTTGCC-3′ in BamH1 and EcoRV sites of Leishmania transfection vector pXG-B2863 (a kind gift from Dr. S. M. Beverley), to produce C-terminal-GFP-tagged full-length LdTopIIIβ protein and termed as LdTopIIIβ-GFP. The constructs and empty vector pXG-B2863 were transfected into L. donovani promastigotes separately by electroporation as described earlier [26]. Briefly, late log-phase promastigotes were harvested and washed twice in OPTI-MEM (GIBCO). Cells were finally suspended at a density of 1 × 108/mL and 0.4 mL was taken into a 0.2 mm ice-chilled electroporation cuvette. Thirty microgram of plasmid DNA was taken in 100 μL of electroporation buffer and added to the cells. After 10 min on ice, the cells were electroporated with a single pulse by Bio-Rad Gene Pulsar apparatus using 450 V and 550 μF capacitance. The cells were incubated on ice for further 5 min and then added to10 mL of drug-free growth medium. After 24 h of survival 10 μg/mL G418 was added and kept at 22°C. The transfected cells were monitored visually by microscope and drug concentration was increased gradually. Finally the transfected cells were routinely maintained in medium containing 100 μg/mL G418.
2.6. Fluorescence Microscopy
Localization of C-terminal GFP tagged chimeric LdTopIIIβ-GFP protein was visualized by fluorescence microscopy (Olympus IX81). Cell nucleus and kinetoplast were stained with DAPI. Differential visualization of the fluophores was achieved using a 488 nm excitation filters and 523 nm emission filter for GFP and 258 nm excitation and 361 nm emission filter for DAPI.
2.7. Construction of Mutants
The full-length LdTopIIIβ was subcloned in XbaI and BamH1 sites into the yeast shuttle vector pVT100U, a kind gift from Dr. Rolf Sternglanz [27] and termed as LdTopIIIβ-pVT using the sense primer 5′-GCTCTAGAATGGGCCGCAATGTGTTGATG-3′ and antisense primer 5′-CGGGATCCTCACCTGCGATCCTCGCGGTT-3′. For construction of C-terminal deletion construct of LdTopIIIβ, regions corresponding to amino acids 1-608 was PCR amplified using the primers 5′-GCTCTAGAATGGGCCGCAATGTGTTGATG-3′ (sense) and 5′-CGGGATCCGGCGGCGGAGATGGCGGAGAA-3′ (antisense) and was cloned in Xba1 and BamH1 sites of pVT100U vector.
2.8. Site-Directed Mutagenesis
Single mutations were introduced in LdTOPIIIβat position Tyr 327 (Y327). Mutagenesis was performed by using the QuikChangeXL site-directed kit (Stratagene, La Jolla, CA) according to the manufacturer's protocol. To carry out the desired mutations, LdTopIIIβ-pVT was used as templates for all mutagenesis experiments. For each mutation, the wild-type nucleotide was replaced using a specific pair of mutagenic primers. The following sense primer, along with the antisense counterparts (with codons in boldface and substitutions underlined), were used; for Y327 of LdTopIIIβ, sense primer was 5′-CCGCGGCTATATTTCGTTCCCTCGTACCCGAATCC-3′ and antisense primer was 5′-GGATTCGGTACGAGGGAACGAAATATAGCCGCGG-3′.
2.9. Complementation Assay
The top3 mutant yeast strain W2633-4C (a/alpha top3:: TRP1/+) (a kind gift from Dr. R Rothstein) was used for transformation with recombinant topo III proteins from L. donovani by the lithium acetate and polyethylene glycol method [28]. The transformants were cultured on solid synthetic minimal medium at 30°C for 2 days. Colonies were picked and cultured in tubes with 2 mL of synthetic minimal media at 30°C overnight.
2.10. Expression of Recombinant LdTopIIIβ Using the Expressway Cell-Free E. coli Expression System (Invitrogen)
In vitro transcription and translation of LdTopIIIβ proteins were carried out according to the manufacturer's protocol. LdTopIIIβ-pET16b plasmids were used as DNA templates for synthesis of the protein. After the reaction is over, the crude bacterial lysate containing the newly synthesized protein was tested for activity.
2.11. DNA Relaxation Activity by LdTopIIIβ
The type IA DNA topoisomerases were assayed by decreased mobility of the relaxed isomers of supercoiled pBS (SK+) [pBluescript (SK+)] DNA in an agarose gel. Relaxation assay was carried out with the crude lysates containing the in vitro transcribed and translated LdTopIIIβ. Supercoiled pBS DNA (85%–95% were negatively supercoiled with the remaining being nicked circles) was used as substrate in the relaxation buffer (25 mM Tris-HCl, pH 7.5, 5% glycerol, 0.5 mM DTT, 2 mM MgCl2, 50 μg/mL BSA). The amount of supercoiled monomer DNA band fluorescence after EtBr (0.5 μg/mL) staining was visualized using Gel Doc 2000 under UV illumination (Bio-Rad Quality one Software).
3. Results
3.1. Type IA Topoisomerase Genes in Leishmania
A search of the Leishmania major genome database yielded three type IA topoisomerases. One is on chromosome 21, annotated as topoisomerase IA (LmjF21.0125) with an ORF of 2453 bp. Two other type IA topoisomerases are present on chromosome 28 and 36, respectively, both of which are annotated as topoisomerase III (LmjF28.1780 and LmjF36.3200, resp.).
3.2. Identification of Topoisomerase III Genes in Leishmania donovani
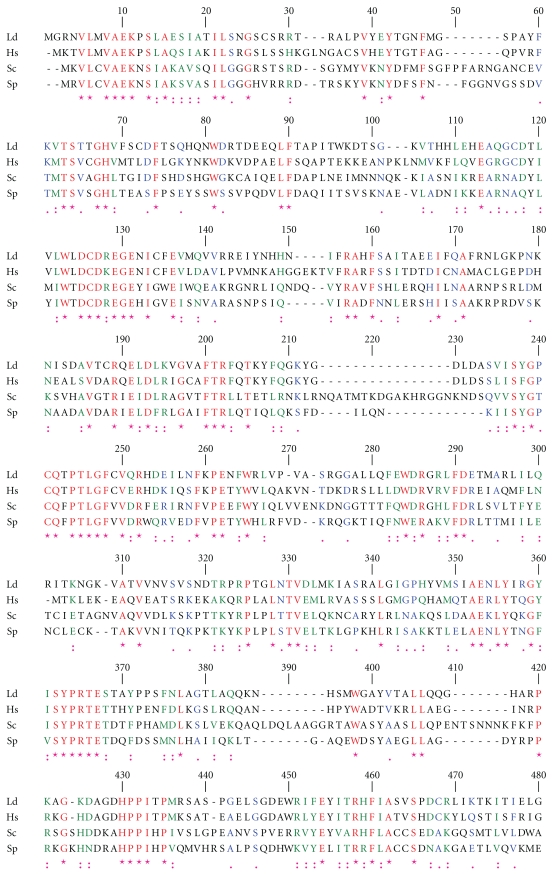
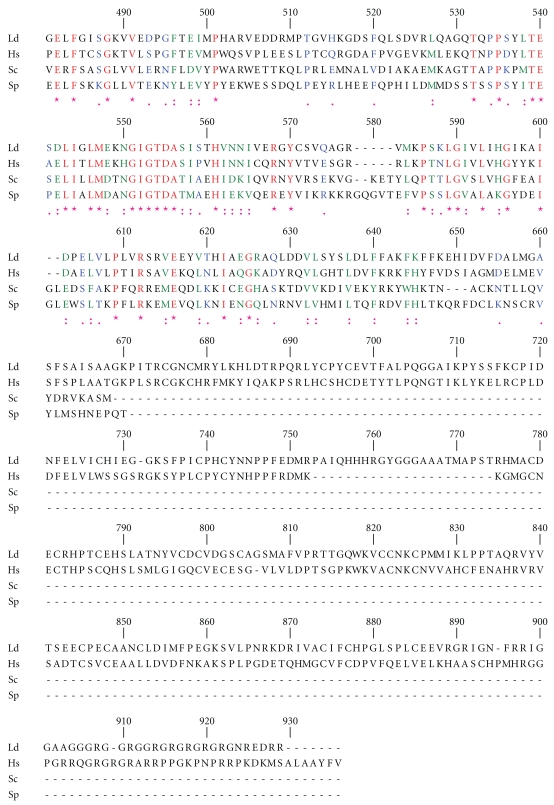
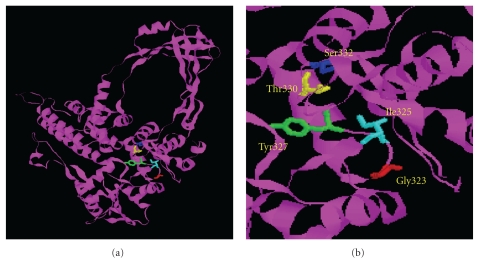
One of the two topoisomerase III genes present in L. major geneDB is 2601 bp (LmjF28.1780) and encodes a 95 kDa predicted protein. The other topoisomerase III ORF (LmjF36.3200) is 2844 bp, and encodes a 104 kDa predicted protein. Topoisomerase III gene with 2601 bp was PCR amplified from the genomic DNA of L. donovani, cloned and sequenced (GeneBank accession number GQ499197). Blast analysis of the sequence confirmed the topoisomerase III lineage of the protein and henceforth referred as LdTopIIIβ. The alignment of LdTopIIIβ with S. cerevisiae and S. pombe topoisomerase III and human topoisomerase III is shown in Figure 1. The active site tyrosine is located at the 327 position within a highly conserved GYISYPRTES sequence. The protein has 46.22% identity and 76.09% similarity with human topoisomerase IIIβ. It contains seven CXXC sequences instead of eight found in other topoisomerase IIIβ proteins. The intervening spacers are also highly conserved. Glycine (G) and arginine (R) rich clusters at the C-terminus end, which is another hallmark of topoisomerase IIIβ, are also present. It has a continuous stretch of 19 G and R residues in the C-terminus. Three-dimensional structure generated by Swiss Prot has been shown in Figure 2(a). Figure 2(b) shows the magnified view of the active site. The conserved amino acid residues are represented in ball and stick format and have been labeled. Homology comparisons of LdTopIIIβ with other IA type of topoisomerases have been provided in Table 1, which strongly indicates its topoisomerase III lineage.
Figure 1.
Amino acid sequence alignment. Sequence of LdTopIIIβ (Ld) was aligned with the amino acid sequences of H. sapiens topoisomerase IIIβ (Hs), topoisomerase III from S. cerevisiae (Sc) and S. pombe (Sp) using CLUSTAL W. The amino acids are numbered on the top of the sequences. Active site motifs and other important conserved and identical residues are depicted in red. Green and blue indicate strongly similar and weakly similar amino acids, respectively.
Figure 2.
(a) Three-dimensional structure of LdTopIIIβ. A ribbon structure representation of LdTopIIIβ generated based on the crystal structure of E. coli topo isomerase III. Catalytically conserved residues are represented in ball and stick format over the ribbon structure. (b) Close up view of LdTopIIIβ in which the amino acid residues that are vital for enzyme action are labeled and represented in ball and stick format. The positions of the amino acids are also mentioned.
Table 1.
Amino acids homology comparisons between Leishmania donovani topoisomerase IIIβ (866 amino acids) and other members of typeIA topoisomerases.
| Type IA topoisomerases | Identity (%) | Similarity (%) | Size (amino acids) |
|---|---|---|---|
| LdTopIIIα | 28.54 | 59.66 | 947 |
| LdTopIA | 13.22 | 48.56 | 812 |
| Human TopIIIα | 27.14 | 55.47 | 1001 |
| Human TopIIIβ | 46.22 | 76.09 | 862 |
| S. cerevisiae TopIII | 22.96 | 47.33 | 656 |
| S. pombe TopIII | 22.56 | 49.32 | 622 |
3.3. Localization Study of LdTopIIIβ-GFP
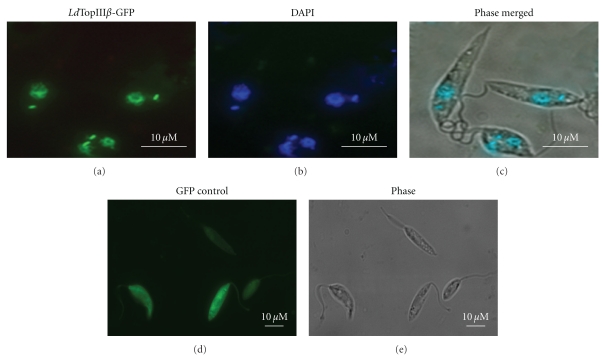
In silico search was carried out to determine possible localization of LdTopIIIβ protein. A 0.244 probability of mitochondrial transport was predicted by Mitoprot (http://expasy.org/tools) analysis and 73.9% cytoplasmic and 17.4% nuclear distribution was revealed by PSORT II analysis (http://expasy.org/tools). To determine the precise localization of the protein, full-length LdTopIIIβ (865 aa) was cloned in Leishmania expression vector as a C-terminal fusion protein with GFP, termed as LdTopIIIβ-GFP, and the construct was transfected in L. donovani parasites. Localization of LdTopIIIβ-GFP was viewed under fluorescence microscopy (Figure 3(a)). Nucleus and kinetoplast DNA was stained with DAPI (Figure 3(b)). Comparison of DAPI and GFP fluorescence and merged images (Figure 3(c)) revealed that LdTopIIIβ protein localized both inside the nucleus and kinetoplast of the parasites. Figures 3(d) and 3(e) show cytoplasmic distribution of control GFP protein in L. donovani parasites.
Figure 3.
Localization LdTopIIIβ. Wild-type construct of LdTopIIIβ was transfected in L. donovani parasites as C-terminally fused GFP proteins and viewed under fluorescence microscope (100x). Nucleus and kinetoplast DNA are visualized by DAPI staining.
3.4. LdTopIIIβ Suppresses the Yeast top3Δ Slow-Growth Phenotype
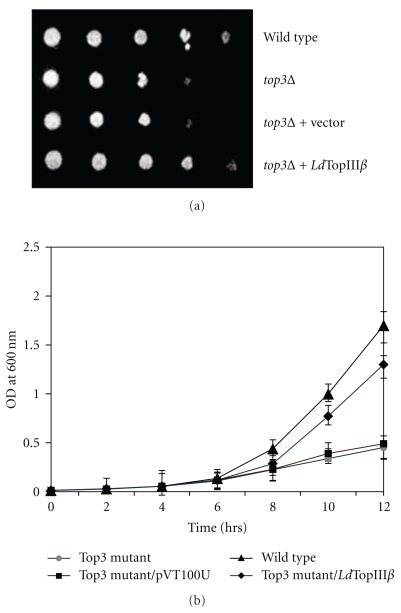
Mutation of the S. cerevisiae top3 gene is known to result in several phenotypes, including a growth rate which is only 50% that of wild-type [14]. In order to assess whether the LdTopIIIβ possesses functional similarity to the yeast topoisomerase III, we have used a functional complementation assay of LdTopIIIβ protein to rescue top3 mutant S. cerevisiae strain from slow-growing phenotype. We have cloned the LdTOPIIIβ gene in a shuttle vector pVT100U to generate LdTopIIIβ-pVT and transformed in top3Δ yeast S. cerevisiae and Ura+ colonies were selected. Yeast cells transformed with vector pVT100U served as control in the complementation assay. The LdTopIIIβ-pVT partially complemented the slow-growth of top3Δ yeast (Figure 4(a)). The improved growth rate was not observed in case of the vector control (Figure 4(a)). This observation suggests that LdTopIIIβ can be functionally expressed in yeast and shares functional similarity with S. cerevisiae top3 gene, which is consistent with earlier observations made with Drosophila and human topisomerase IIIβ proteins [19, 29]. To observe this complementation of LdTopIIIβ in liquid medium a yeast growth curve analysis was carried out (Figure 4(b)). Equal amounts of the wild-type, topoisomerase 3 mutant yeast cells, topoisomerase 3 mutant yeast cells containing empty vector (pVT100U) and topoisomerase 3 mutant yeast cells containing LdTopIIIβ (grown overnight at 30°C) were inoculated in fresh minimal medium and grown at 30°C. At every 2 hr interval up to 12 hrs, the growth was monitored and plotted.
Figure 4.
Functional complementation of LdTopIIIβ. (a) S. cerevisiae top3Δ strain was transformed with a vector pVT100U and the vector carrying wild-type LdTopIIIβ. Transformed cells were streaked on solid synthetic minimal media and incubated at 28°C. Ten-fold serial dilutions of exponentially growing wild-type strain, top3Δ strain, top3Δ strain harboring an empty vector, or top3Δ strain harboring plasmid encoded LdTopIIIβ, as indicated on the right, grown on the plate. (b) Growth rate of the above-described strains were measured in the liquid synthetic medium and OD600 was plotted against time. Results represent the means ± standard errors of three independent experiments.
3.5. Effects of Active Site Mutation of LdTopIIIβ on Complementation Ability
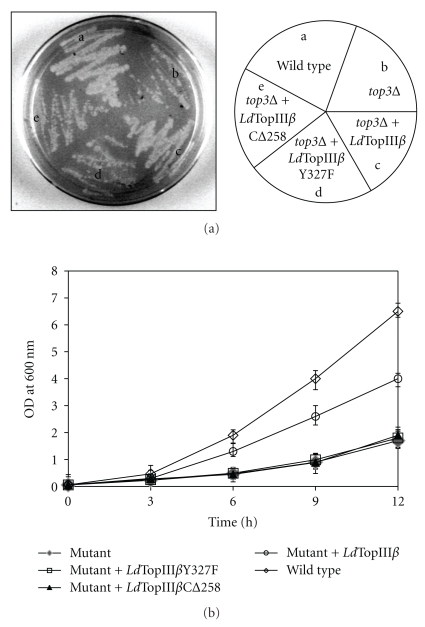
Tyrosine 327 of LdTopIIIβ was predicted to be the active site amino acid residue from sequence alignment analysis. In order to determine that LdTopIIIβ functionally complements the top3 mutant yeast and the growth recovery was not due to any compensatory mechanism induced by LdTopIIIβ we carried out site directed mutagenesis. We have mutated the active site residue of LdTopIIIβ to phenylalanine (Y327F) by site directed mutagenesis and transformed in top3 mutant yeast. Transformed cells were grown on plate, as well as in liquid minimal media. It was observed that the active site mutant construct could not suppress the slow-growth of top3 mutant S. cerevisiae (Figures 5(a) and 5(b)) confirming role of active site tyrosine 327 in functional conservation of LdTopIIIβ inside mutant yeast cells.
Figure 5.
Complementation assay with mutant LdTopIIIβ. (a) Topoisomerase III mutant yeast strain was transformed with the plasmid containing wild-type (c), active site mutant (d) and C-terminal deletion construct (e) of LdTopIIIβ, separately. Transformed cells were streaked on solid synthetic minimal media and incubated at 30°C. (b) Complementation assay as described above carried out in liquid synthetic medium and OD600 plotted against time. Results represent the means ± standard errors of three independent experiments.
3.6. The C-Terminal Domain of LdTopIIIβ Is Essential for In Vivo Complementation
The Leishmania enzyme has a C-terminal segment of amino acids with no counterpart in yeast protein. The leishmanial protein contains Zn-binding motif at its C-terminus, which is absent in the topoisomerase III proteins of E. coli and yeast. The C-terminus residues of E. coli topoisomerase III have been previously shown to be involved in DNA binding [30]. To determine the role of the C-terminal stretch of LdTopIIIβ in functional complementation, we have made a C-terminal deletion construct (LdTopIIICΔ258) removing the 258 amino acids and transformed in topoisomerase III mutant yeast. The transformants were grown in plates and it was observed that the C-terminal deletion construct failed to rescue the mutant yeast from slow-growth (Figure 5(a)), suggesting essentiality of the C-terminal segment for functional complementation in vivo. To validate this observation in liquid medium we inoculated overnight grown cultures at 30°C in fresh minimal medium and monitored their growth at 3 hr intervals. The growth curve (Figure 5(b)) clearly indicates that LdTopIIICΔ258 could not functionally complement the slow-growing topoisomerase III mutant yeast. This indicates that the conserved C-terminal region between amino acid residues 608–866 contains important residues that are required for in vivo function of LdTopIIICΔ258. To get a better insight into the functional characteristics of the enzyme, we next sought to obtain recombinant LdTopIIIβ protein in vitro.
3.7. In Vitro Activity of Recombinant LdTopIII Protein
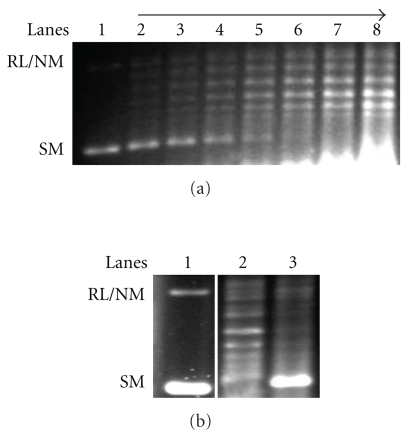
LdTopIIIβ was cloned in bacterial expression vector pET-16b and overexpressed in BL21 (DE3)-pLysS strain and induced with IPTG. But the overexpressed protein went to inclusion body and were found in the pellet as insoluble protein which could not be recovered in the soluble fraction in active state. However, to test the activity of the recombinant protein, we have used in vitro transcription-translation kit, which is specially designed for in vitro transcription and translation of target DNA to protein in a single reaction. The crude lysate containing the newly synthesized proteins were used for DNA relaxation assay. Figure 6(a) shows DNA relaxation by increasing amount of recombinant LdTopIIIβ (lanes 2–8). Lane 1 is the DNA control. The results clearly show that the recombinant protein containing lysates were able to relax the negatively supercoiled DNA. To test that the activity was not coming from the lysate itself, we have carried out DNA relaxation activity with the empty vector containing lysate which contained insignificant amount of activity, shown in Figure 6(b) (lane 3). Lane 2 shows DNA relaxation activity by recombinant LdTopIIIβ.
Figure 6.
DNA relaxation assay by recombinant LdTopIIIβ. (a) Negatively super coiled DNA was incubated with 1, 2, 3, 4, 5, 7 and 10 μL of recombinant LdTopIIIβ containing lysate for 30 min (lanes 2–8). Lane 1 is the DNA control. (b) DNA relaxation assay carried out with recombinant LdTopIIIβ (lane 2), and empty vector containing lysate (lane 3). Lane 1 is the DNA control.
4. Discussion
The type IA topoisomerases are among the most conserved proteins in nature, and their presence in all organisms is supported by extensive biochemical and genomic sequence data [2, 4]. This universal presence suggests that the type IA DNA topoisomerases play an indispensable role in one or more fundamental processes involving DNA, plausibly in the removal of double Holliday junctions [2]. Topoisomerases IIIα and IIIβ of kinetoplastid parasites seem to be orthologues of same kind of enzymes in other eukaryotes, notable for branching early within their respective groups. In the present study, for the first time we have identified functionally active DNA topoisomerase IIIβ from L. donovani. Blast sequence alignments suggested topoisomerase IIIβ from Leishmania has high homology with human and drosophila topoisomerase IIIβ. It shares many features, which are typical for other topoisomerase IIIβ proteins including the CXXC type of motifs and a long stretch of G and R residues at its C-terminus. GFP-fused LdTopIIIβ localized both inside the nucleus and the kinetoplast of L. donovani parasites indicating the involvement of LdTopIIIβ in DNA processing inside both the parasite organelle. Our results show for the first time the presence of an IA type of topoisomerase in the nucleus, as well as in the kinetoplast of Leishmania parasites. Previously, a IA type of topoisomerase from bacterial origin has been reported to be mitochondrial in T. brucei [24].
LdTopIIIβ could suppress the slow-growth phenotype of the mutant yeast indicating the functional conservation of topoisomerase III activity. The result is consistent with the earlier observations made with human and Drosophila topoisomerase IIIβ enzymes. The C-terminal deletion construct of LdTopIIIβ lacking its Zn binding domain was unable to rescue the topoisomerase III mutant yeast from slow-growing phenotype revealing that the C-terminal 258 amino acids were indispensable for functional complementation of LdTopIIIβ in vivo. Previous report reveals the requirement of the C-terminus region of bacterial topoisomerase III in substrate specificity [30]. It is possible that C-terminal end of the leishmanial topoisomerase IIIβ protein is essential for DNA binding which requires further investigations. Site directed mutagenesis study revealed that tyrosine at 327 position within the conserved amino acid stretch is the active site tyrosine of LdTopIIIβ and when this tyrosine is mutated to phenylalanine, the protein failed to complement the slow-growing mutant yeasts. The result indicates towards involvement of the functionally active LdTopIIIβ in rescue of the mutant yeast from slow-growth. Our attempts to purify recombinant LdTopIIIβ enzymes in active state from bacteria were unsuccessful as the proteins consistently went to inclusion body. But we were able to study, for the first time, the in vitro DNA relaxation activity the recombinant topoisomerase III protein from the kinetoplastid parasite Leishmania, when synthesized using cell free in vitro transcription-translation kit. Altogether, this is the first report of functionally active topoisomerase IIIβ protein from unicellular kinetoplastid parasite Leishmania.
The biological functions of eukaryotic topoisomerse III proteins are intriguing. Important nonoverlapping function of the two isozymes of topoisomerse III has been revealed by previous studies. The mouse-knockout experiments suggests, the α form is essential for embryonic development, whereas the β form is critical for life span [20, 21]. Genetic experiments in yeast have demonstrated that TOP3 plays a role in suppressing mitotic recombination and in resolving recombined homologous chromosomes during meiosis I [14, 31]. Preferential cleavage of plasmid-based R- and D-loops, has been reported by Drosophila topoisomerase IIIβ [32]. Furthermore, the combined action of either yeast or bacterial topoisomerse III and the DNA helicase RecQ can promote the formation of DNA catenanes [33]. The unwinding action of a RecQ type helicase appears to generate a DNA structure that can be recognized by a topoisomerase III. RecQ helicases are also conserved in kinetoplastide parasites. The only report of functionally significant topoisomerase IIIα from kinetoplastide parasite came very recently, which describes that topoisomerse IIIα from Trypanosoma brucei influences antigenic variation by monitoring expression-site-associated VSG switching [25]. Existence of functionally active topoisomerase III protein in Leishmania indicates towards its role in DNA metabolism in the parasites, which requires further studies and might emerge as a new therapeutic target that can be exploited against the deadly parasites.
Funding
This work was supported by Network Project (NWP038) of Council of Scientific and Industrial Research (CSIR), Government of India to H. K. Majumder. B. Banerjee was supported by Senior Research Fellowship from CSIR, Government of India.
Acknowledgments
The authors thank professor S. Roy, the Director of Indian Institute of Chemical Biology for his interest in this work. They are grateful to professor S. M. Beverley for kindly gifting the Leishmania transfection vector and Dr. R. Rothstein for topoisomerase III mutant yeast strains.
References
- 1.Hsiang YH, Hertzberg R, Hecht S, Liu LF. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. Journal of Biological Chemistry. 1985;260(27):14873–14878. [PubMed] [Google Scholar]
- 2.Wang JC. Cellular roles of DNA topoisomerases: a molecular perspective. Nature Reviews Molecular Cell Biology. 2002;3(6):430–440. doi: 10.1038/nrm831. [DOI] [PubMed] [Google Scholar]
- 3.Wang JC. DNA topoisomerases. Annual Review of Biochemistry. 1996;65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]
- 4.Champoux JJ. DNA topoisomerases: structure, function, and mechanism. Annual Review of Biochemistry. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- 5.Champoux JJ, Dulbecco R. An activity from mammalian cells that untwists superhelical DNA–a possible swivel for DNA replication (polyoma-ethidium bromide-mouse-embryo cells-dye binding assay) Proceedings of the National Academy of Sciences of the United States of America. 1972;69(1):143–146. doi: 10.1073/pnas.69.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim RA, Wang JC. Identification of the yeast TOP3 gene product as a single strand-specific DNA topoisomerase. Journal of Biological Chemistry. 1992;267(24):17178–17185. [PubMed] [Google Scholar]
- 7.Tse-Dinh YC, Wang JC. Complete nucleotide sequence of the topA gene encoding Escherichia coli DNA topoisomerase I. Journal of Molecular Biology. 1986;191(3):321–331. doi: 10.1016/0022-2836(86)90129-4. [DOI] [PubMed] [Google Scholar]
- 8.DiGate RJ, Marians KJ. Identification of a potent decatenating enzyme from Escherichia coli. Journal of Biological Chemistry. 1988;263(26):13366–13373. [PubMed] [Google Scholar]
- 9.DiGate RJ, Marians KJ. Molecular cloning and DNA sequence analysis of Escherichia coli topB, the gene encoding topoisomerase III. Journal of Biological Chemistry. 1989;264(30):17924–17930. [PubMed] [Google Scholar]
- 10.Pastorcic M. University of Chicago; 1982. Ph.D. thesis. [Google Scholar]
- 11.Srivenugopal KS, Lockshon D, Morris DR. Escherichia coli DNA topoisomerase III: purification and characterization of a new type I enzyme. Biochemistry. 1984;23(9):1899–1906. doi: 10.1021/bi00304a002. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin A, Wang SW, Toda T, Norbury C, Hickson ID. Topoisomerase III is essential for accurate nuclear division in Schizosaccharomyces pombe. Nucleic Acids Research. 1999;27(20):4050–4058. doi: 10.1093/nar/27.20.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh M, Choi IS, Park SD. Topoisomerase III is required for accurate DNA replication and chromosome segregation in Schizosaccharomyces pombe. Nucleic Acids Research. 2002;30(18):4022–4031. doi: 10.1093/nar/gkf531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallis JW, Chrebet G, Brodsky G, Rolfe M, Rothstein R. A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell. 1989;58(2):409–419. doi: 10.1016/0092-8674(89)90855-6. [DOI] [PubMed] [Google Scholar]
- 15.Chakraverty RK, Kearsey JM, Oakley TJ, et al. Topoisomerase III acts upstream of Rad53p in the S-phase DNA damage checkpoint. Molecular and Cellular Biology. 2001;21(21):7150–7162. doi: 10.1128/MCB.21.21.7150-7162.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanai R, Caron PR, Wang JC. Human TOP3: a single-copy gene encoding DNA topoisomerase III. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(8):3653–3657. doi: 10.1073/pnas.93.8.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seki T, Seki M, Onodera R, Katada T, Enomoto T. Cloning of cDNA encoding a novel mouse DNA topoisomerase III (topo IIIβ) possessing negatively supercoiled DNA relaxing activity, whose message is highly expressed in the testis. Journal of Biological Chemistry. 1998;273(44):28553–28556. doi: 10.1074/jbc.273.44.28553. [DOI] [PubMed] [Google Scholar]
- 18.Goulaouic H, Roulon T, Flamand O, Grondard L, Lavelle F, Riou JF. Purification and characterization of human DNA topoisomerase IIIα. Nucleic Acids Research. 1999;27(12):2443–2450. doi: 10.1093/nar/27.12.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson TM, Chen AD, Hsieh TS. Cloning and characterization of Drosophila topoisomerase IIIβ. Relaxation of hypernegatively supercoiled DNA. Journal of Biological Chemistry. 2000;275(3):1533–1540. doi: 10.1074/jbc.275.3.1533. [DOI] [PubMed] [Google Scholar]
- 20.Li W, Wang JC. Mammalian DNA topoisomerase IIIα is essential in early embryogenesis. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(3):1010–1013. doi: 10.1073/pnas.95.3.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwan KY, Wang JC. Mice lacking DNA topoisomerase IIIβ develop to maturity but show a reduced mean lifespan. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(10):5717–5721. doi: 10.1073/pnas.101132498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwan KY, Moens PB, Wang JC. Infertility and aneuploidy in mice lacking a type IA DNA topoisomerase IIIβ. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(5):2526–2531. doi: 10.1073/pnas.0437998100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das BB, Sengupta T, Ganguly A, Majumder HK. Topoisomerases of kinetoplastid parasites: why so fascinating? Molecular Microbiology. 2006;62(4):917–927. doi: 10.1111/j.1365-2958.2006.05428.x. [DOI] [PubMed] [Google Scholar]
- 24.Scocca JR, Shapiro TA. A mitochondrial topoisomerase IA essential for late theta structure resolution in African trypanosomes. Molecular Microbiology. 2008;67(4):820–829. doi: 10.1111/j.1365-2958.2007.06087.x. [DOI] [PubMed] [Google Scholar]
- 25.Kim H-S, Cross GAM. TOPO3α inlfluences antigenic variation by monitoring expression-site-associated VSG switching in Trypanosoma brucei. PLoS Pathogens. 2010;6(7) doi: 10.1371/journal.ppat.1000992. Article ID e1000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapler GM, Coburn CM, Beverley SM. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Molecular and Cellular Biology. 1990;10(3):1084–1094. doi: 10.1128/mcb.10.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vernet T, Dignard D, Thomas DY. A family of yeast expression vectors containing the phage f1 intergenic region. Gene. 1987;52(2-3):225–233. doi: 10.1016/0378-1119(87)90049-7. [DOI] [PubMed] [Google Scholar]
- 28.Chen D-C, Yang B-C, Kuo T-T. One-step transformation of yeast in stationary phase. Current Genetics. 1992;21(1):83–84. doi: 10.1007/BF00318659. [DOI] [PubMed] [Google Scholar]
- 29.Ng SW, Liu Y, Hasselblatt KT, Mok SC, Berkowitz RS. A new human topoisomerase III that interacts with SGS1 protein. Nucleic Acids Research. 1999;27(4):993–1000. doi: 10.1093/nar/27.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang HL, DiGate RJ. The carboxyl-terminal residues of Escherichia coli DNA topoisomerase III are involved in substrate binding. Journal of Biological Chemistry. 1994;269(12):9052–9059. [PubMed] [Google Scholar]
- 31.Gangloff S, De Massy B, Lane A, Rothstein R, Fabre F. The essential role of yeast topoisomerase III in meiosis depends on recombination. The EMBO Journal. 1999;18(6):1701–1711. doi: 10.1093/emboj/18.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson-Sali T, Hsieh TS. Preferential cleavage of plasmid-based R-loops and D-loops by Drosophila topoisomerase IIIβ. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(12):7974–7979. doi: 10.1073/pnas.122007999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harmon FG, DiGate RJ, Kowalczykowski SC. RecQ helicase and topoisomerase III comprise a novel DNA strand passage function: a conserved mechanism for control of DNA recombination. Molecular Cell. 1999;3(5):611–620. doi: 10.1016/s1097-2765(00)80354-8. [DOI] [PubMed] [Google Scholar]