Abstract
The lipidic cubic mesophase has been used to crystallize important membrane proteins for high-resolution structure determination. To date, however, no integral membrane enzymes have yielded to this method, the in meso. For a crystal structure to be meaningful the target protein must be functional. Using the in meso method with a membrane enzyme requires that the protein is active in the mesophase that grows crystals. Because the cubic phase is sticky and viscous and is bicontinuous topologically, quantitatively assessing enzyme activity in meso is a challenge. Here, we describe a procedure for characterizing the catalytic properties of the integral membrane enzyme, diacylglycerol kinase, reconstituted into the bilayer of the lipidic cubic phase. The kinase activity of this elusive crystallographic target was monitored spectrophotometrically using a coupled assay in a high-throughput, 96-well plate format. In meso, the enzyme exhibits classic Michaelis–Menten kinetics and works with a range of lipid substrates. The fact that the enzyme and its lipid substrate and product remain confined to the porous mesophase while its water-soluble substrate and product are free to partition into the aqueous bathing solution suggests a general and convenient approach for characterizing membrane enzymes that function with lipids in a membrane-like environment. The distinctive rheology of the cubic phase means that a procedural step to physically separate substrate from product is not needed. Because of its open, bicontinuous nature, the cubic phase offers the added benefit that the protein is accessible for assay from both sides of the membrane.
Keywords: coupled enzyme assay, DgkA, glycerophospholipid, phosphatidylglycerol phosphate synthase, PgsA
The lipidic cubic phase has been used to grow crystals of membrane proteins for high-resolution structure determination. This approach has had spectacular successes of late with the publication of structures for G-protein coupled receptors (1–7). The method has also been used with a variety of other membrane protein and peptide types that include photo-sensitive proteins like the assorted bacterial rhodopsins, a light harvesting complex and the photosynthetic reaction centers, a transporter, a channel, and an adhesin (8–15). Conspicuous by their absence on this list are the integral membrane enzyme class of proteins.
We have an interest in the structure-function relationships that pertain to membrane enzymes involved in glycerolipid metabolism. Our immediate focus is on the diacylglycerol kinase, DgkA, in Escherichia coli responsible for the ATP-dependent conversion of diacylglycerol to phosphatidic acid for use in membrane-derived oligosaccharide synthesis (16). A solution NMR structure of this small, hydrophobic trimer was published recently (17), and a high-resolution crystal structure is needed now to complement and extend the solution work. Efforts at crystallizing DgkA by conventional means have produced crystals but none of structure quality (18). The lipidic cubic mesophase (in meso) method is now being examined as an alternative approach that may yield the requisite crystal structure given the success it has had with other small, hydrophobic integral membrane proteins and peptides.
In support of such in meso crystallization trials it is important to demonstrate that the enzyme is functionally active in the cubic mesophase, in and from which crystals must grow. The cubic phase can be viewed as consisting of a single, highly curved, continuous lipid bilayer that permeates 3D space the midplane of which sits on a periodic minimal surface. The lipid membrane is bathed on either side by the aqueous component of the mesophase, which takes the form of continuous, branching networks of channels. Assaying for enzymatic activity in such a “bicontinuous” medium is not straightforward and is the focus of this work.
To demonstrate and to characterize the activity of an integral membrane enzyme in meso presents two major challenges. To begin with, the cubic phase is extremely viscous and sticky, having the rheology of a thick toothpaste. Accordingly, handling the mesophase is not trivial. However, these viscosity and stickiness features have been used to advantage in setting up and performing in meso assays, as described.
The second challenge arises because the mesophase is a porous, liquid crystalline medium in which the enzyme must be assayed. It can be viewed as a molecular sponge where the fabric of the sponge is a lipid bilayer and the holes in the sponge are filled with aqueous medium. Thus, like the common household sponge, water-soluble materials can diffuse into and out of the holes or aqueous channels that permeate the mesophase and that are continuous with the bulk medium. The water-soluble DgkA substrate, ATP, and product, ADP, must, respectively, diffuse into and out of the mesophase in the course of the assay to support kinase activity and for detection. The latter is done in the current application using a coupled enzyme system (19–21) that includes pyruvate kinase (PK), lactate dehydrogenase (LD), phosphoenolpyruvate (PEP), and nicotinamide adenine dinucleotide (NADH). Thus, ADP production as a result of DgkA activity leads to a decrease in NADH concentration in the bathing aqueous solution that can be monitored conveniently by following the change in dinucleotide absorption at 340 nm. In contrast, the DgkA enzyme as well as its lipid substrate and product are confined to the lipid bilayer of the mesophase and are free to move within the plane of that continuous membrane.
DgkA is a promiscuous enzyme. It can use a variety of lipid and surfactant alcohols as substrates for γ-phosphoryl group transfer from ATP (22–25). It has been shown to work with monoolein (22), which is also the lipid most commonly used to create the hosting mesophase for crystallogenesis. In the current investigation, therefore, monoolein serves double duty, as substrate and hosting mesophase lipid.
Here, we show that the lipidic cubic phase is a suitable and a convenient system in which to reconstitute and to assay an integral membrane enzyme. Specifically, DgkA has been shown to reconstitute into the bilayer of the cubic phase prepared with monoolein in a functionally active form. The kinetic properties of the enzyme in meso have been characterized, and the kinase has been shown to work with a range of lipid substrates. The system and approach described here have general applicability as demonstrated in measurements made with the phosphatidylglycerol phosphate synthesizing enzyme, PgsA.
Results
Reconstitution.
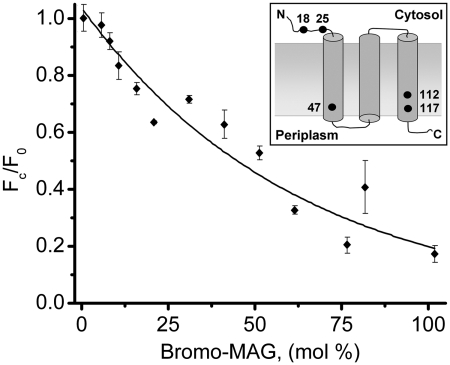
DgkA is a small, hydrophobic integral membrane protein (Fig. 1, Inset). The expectation therefore is that upon mixing with the lipid, monoolein, under conditions that produce the cubic phase the protein will reconstitute into the bilayer of the mesophase. This was demonstrated convincingly by fluorescence quenching. Thus, the intrinsic tryptophan fluorescence of DgkA was monitored as monoolein was replaced, mole for mole, by the quenching lipid, bromo-MAG. Bromo-MAG is a monoacylglycerol with a bromine atom on carbon atoms 9 and 10 of its stearoyl chain. Its phase behavior is very similar to that of monoolein, and the two are entirely miscible. The quenching curve (Fig. 1) is analogous to that observed with other integral membrane proteins known to reconstitute in meso (13, 15, 26) and is convincing evidence that DgkA similarly reconstitutes into the bilayer of the cubic phase.
Fig. 1.
Fluorescence quenching of DgkA reconstituted in the lipidic cubic mesophase. Quenching of intrinsic tryptophan fluorescence was effected by replacing monoolein, mole for mole, with bromo-MAG. Intrinsic fluorescence (Fc) was normalized to the unquenched fluorescence value (Fo) recorded in the absence of bromo-MAG. The inset shows a model of DgkA in the membrane with tryptophans identified by dots and sequence position.
Transport in the Mesophase.
It was proposed to monitor DgkA activity using a coupled enzyme system. Thus, the reconstituted enzyme must have access to both of its substrates, monoolein and ATP, during the course of the assay. Monoolein is immediately available in the bilayer of the cubic phase as hosting lipid. In contrast, ATP is provided in the bathing solution. The nucleotide must therefore diffuse into the aqueous channels and be available to the enzyme, which is distributed throughout the mesophase. At the same time, the product, ADP, must diffuse from its site of production through the aqueous channels of the cubic phase out and into the bathing solution. There it is available for reaction with the PK and LD enzyme couple that leads to a measurable drop in A340 reflecting consumption of NADH.
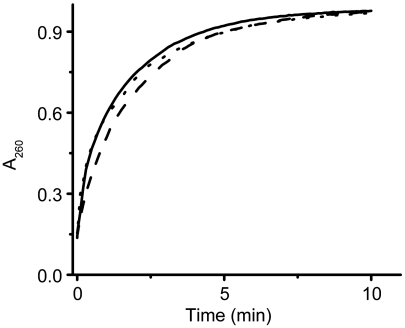
Before proceeding with the assay it was important to demonstrate that the cubic phase is porous and can support the diffusion of small, soluble organics under assay conditions. This was done by preparing the cubic phase with a buffer containing ADP. The mesophase, whose aqueous channels were thus preloaded with nucleotide, was placed in the well of a 96-well plate and covered with buffer solution mimicking assay conditions. Release of ADP from the cubic phase was conveniently monitored by following the rise in absorbance of the bathing solution at 260 nm. ADP floods out of the bolus with approximately 98% of the nucleotide released within 10 min (Fig. 2). This is expected behavior and clearly demonstrates that transport of the type needed for a successful assay of DgkA reconstituted in meso should be possible.
Fig. 2.
ADP release from the cubic phase. The mesophase was prepared by homogenizing monoolein with 8.8 mM ADP in 10 mM HEPES pH 7.5. Five microliters of the cubic phase was placed in a 96-well plate and the bolus was overlain with 0.2 mL 10 mM HEPES pH 7.5. A260 of the bathing solution was monitored as a function of time in a microplate reader at 30 °C with shaking. Release profiles are shown for triplicate samples. To facilitate absorbance measurements the polystyrene base of the microplate, which strongly absorbs at 260 nm, was replaced with the more transparent Crystal Clear Sealing Film (Hampton).
Kinase Activity. Monoolein as Substrate, LPA as Product.
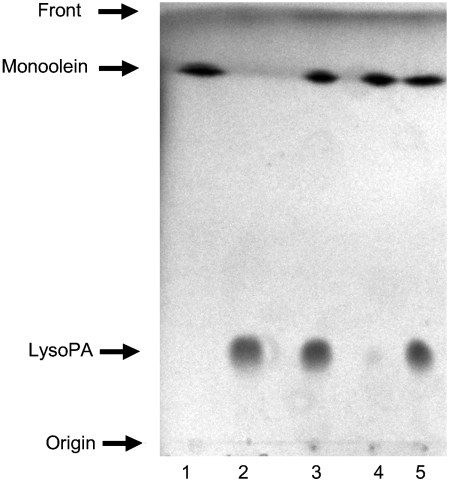
DgkA can use a variety of lipid substrates including monoacylglycerols. In the current study, monoolein creates the bilayer fabric of the cubic phase and the question of most immediate interest was, Can monoolein serve double duty—as a hosting, cubic phase-forming lipid and as a substrate for DgkA? The thin layer chromatographic (TLC) data (Fig. 3) clearly show that the enzyme is indeed active in meso and that lyso-phosphatidic acid (LPA) is a product of the DgkA reaction. Thus, monoolein can function both as a substrate and as a hosting lipid.
Fig. 3.
Thin layer chromatographic analysis of the lipid substrate for and product of DgkA action on monoolein in the cubic mesophase. Lane 1, monoolein standard. Lane 2, LPA standard. Lane 3, monoolein and LPA standards. Lane 4, reaction mix, no DgkA. Lane 5, reaction mix, with DgkA. The reaction was allowed to proceed overnight at 30 °C. The data in Lanes 4 and 5 clearly show DgkA-dependent conversion of monoolein to LPA (Lane 5). The control measurement in Lane 4 illustrates that conditions prevailing during assay support a low level of nonenzymatic phosporylation of monoolein by ATP, which is not surprising.
Kinase Activity Characterization.
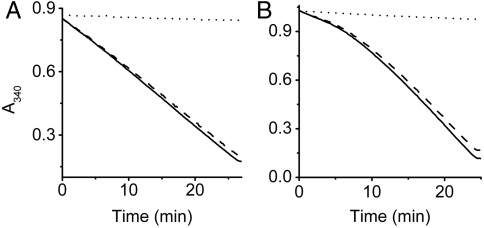
Before initiating kinetic characterization in meso control measurements of DgkA activity were carried out with the protein dispersed in a surfactant solution—the in surfo form of the protein. The substrate employed was dihexanoylglycerol (DHG), and measurements were made in the presence of cardiolipin, a DgkA activator (27). A typical progress curve recorded at 30 °C is shown in Fig. 4A. The corresponding specific activity is 120 U/mg protein, as expected (20). The in surfo assay was repeated with monoolein in place of DHG as substrate. With and without activating cardiolipin the specific activities were 37.4 ± 0.3 and 26.9 ± 0.3 U/mg, respectively. The cardiolipin and monoolein concentrations at which the assays were performed were 2.9 and 25.5 mol%, respectively.
Fig. 4.
Progress of the DgkA reaction in surfo and in meso monitored using a coupled enzyme assay. In A, the reaction was carried out in a micellar dispersion (in surfo) with DHG as substrate and cardiolipin as activator (Assay Mix 1, see SI Text). In B, the reaction was conducted with the enzyme reconstituted into the cubic mesophase formed by monoolein, which also serves as lipid substrate, and Assay Mix 2 (see SI Text). The progress curve recorded with enzyme (solid line) is corrected (dashed line) for background changes in A340 recorded in the absence of enzyme (dotted line). Initial and final A340 values reflect, respectively, the concentration of NADH in the reaction mix at the start of the reaction and when all NADH has been consumed.
Having shown that DgkA behaves in surfo as expected it was necessary to determine if it could be assayed conveniently in a 96-well format in meso with monoolein acting both as substrate and hosting lipid. Accordingly, the protein was reconstituted into the cubic phase, the mesophase was added to the well of a 96-well plate along with the assay solution (Assay Mix 2, see Methods), and the A340 of the bathing solution was monitored as a measure of kinase activity. The progress curve (Fig. 4B) begins with an initial lag followed by a region where the change in A340 becomes linear with time. The latter, linear region was used to calculate an operational specific activity of 16.5 U/mg under standard conditions (see SI Text). This result shows that the activity of the reconstituted kinase can indeed be monitored conveniently in a coupled assay system in meso.
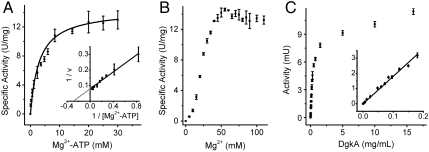
Operational Km and Vmax in Meso.
DgkA has two substrates, Mg2+-ATP and diacylglycerol. As noted, monoolein also functions as a substrate. But because monoolein serves as the hosting lipid in meso its concentration cannot be sensibly varied to quantify the catalytic characteristics of the enzyme, Km and Vmax, with respect to this substrate. Presumably, monoolein saturates the enzyme under assay conditions in meso. However, the concentration of Mg2+-ATP can be adjusted at will, and how this affected enzymatic activity in meso is illustrated in Fig. 5A. Classic Michaelis–Menten saturation behavior was observed with Km and Vmax values of 3.5 ± 0.3 mM Mg2+-ATP and 14.4 ± 0.4 U/mg, respectively. Corresponding values of 1.2 ± 0.5 mM Mg2+-ATP and 50 ± 7 U/mg, respectively, have been reported for DgkA in surfo with DHG as lipid substrate and cardiolipin as activator (20). These data support the view that DgkA reconstituted into the bilayer of the lipidic cubic phase functions as a classic, well behaved enzyme.
Fig. 5.
Enzymatic activity of DgkA reconstituted into the cubic mesophase of monoolein and its dependence on substrate, activator and protein concentration. (A) A linear plot of DgkA saturation with respect to Mg2+-ATP concentration where the inset shows the Lineweaver–Burk representation for Km and Vmax determination (GraphPad Prism 5). (B) Specific activity of DgkA is shown as a function of total Mg2+ concentration. In this experiment, Mg2+ was added as magnesium acetate and ATP as the sodium salt. (C) Dependence of DgkA activity on enzyme loading in a fixed volume of the cubic phase.
Magnesium Optimization.
The kinase activity of DgkA in meso responds to Mg2+ concentration (Fig. 5B) in a way that is similar to its behavior in surfo with diolein as substrate (27). Based on these data our standard in meso assay mix (Assay Mix 2) includes magnesium acetate at 55 mM.
Enzyme Loading Effects.
The measured rate of kinase activity depended on the degree to which the mesophase was loaded with enzyme (Fig. 5C and Fig. S1). Rate increased linearly with loading initially as expected. The result indicates therefore that DgkA functions in meso as a well behaved catalyst. At higher concentrations, the rate dropped but became linear with loading again in the range of 4 to 16 mg protein/mL. This change in measured rate with concentration and with loading of the mesophase will be discussed.
Alternative Lipid Substrates.
As noted, DgkA can use a variety of lipid substrates. With a view to producing crystals of the enzyme by the in meso method there is the possibility that one or more of these may preferentially stabilize the protein and, in so doing, facilitate the growth of structure-grade crystals. Accordingly, in this study we examined different lipids that might act as a substrate for the enzyme under in meso conditions. The survey was limited to those lipids that form the cubic phase in a single lipid system.
The lipids included in this survey and their chemical structures and the recorded reaction rates under standard conditions are listed in Table S1. Several cis-monounsaturated monoacylglycerols (MAGs) were examined that differed in acyl chain length and position of the double bond along the chain. Activity remained relatively constant for MAGs with acyl chain lengths in the range from 16 to 18 carbons. However, when chain length was reduced to 14 carbons a significant drop in activity followed. Adding bromine to the chain, as in bromo-MAG, had a profound effect on activity, reducing it by three- to fourfold compared to the reference lipid, monoolein. It is of note that monoolein and bromo-MAG both have chains 18 carbon atoms long. Myverol is a food-grade lipid containing mono-, di-, and tri-acylglycerols, glycerol, and free fatty acids. The predominant component is monoolein (28). It was not unexpected therefore that the activity of DgkA in Myverol (17.6 ± 1.2 U/mg) is similar to that observed in monoolein.
The two other lipids included in this study resemble, but are not bone fide, MAGs. The first, PAL, is an amide lipid. It consists of a multiply branched 20 carbon phytanoyl chain linked to a glycerol analogue where one of the terminal ─CH2OH moieties is replaced by an amino group. It supported a very reasonable kinase activity in meso analogous to the rate observed with 7.7 MAG. The final lipid in the study was phytantriol, which is a 20 carbon, multiply branched phytane with hydroxyl groups attached to three sequential carbons at one end of the molecule. The enzyme shows very little activity with this cubic phase forming lipid.
Discussion
Reconstitution in Meso.
The most important outcome of this work is the demonstration that DgkA reconstitutes into the bilayer of the cubic phase and that it is enzymatically active therein. The fact that the protein contains five tryptophans distributed throughout its sequence (Fig. 1, Inset) made it relatively easy to show by fluorescence quenching that the protein resides in the bilayer following standard reconstitution procedures. Reconstituting the protein into pure bromo-MAG resulted in the quenching of approximately 80% of its intrinsic fluorescence (Fig. 1). The model that emerges from the recent solution NMR structure study places three of DgkA’s five tryptophans (Trp47, Trp112, Trp117) in the transmembrane region and thus susceptible to quenching. The fourth and fifth tryptophans (Trp18, Trp25) are expected to reside at the membrane–aqueous interface, although these are located in the N terminus of the protein (residues 1–25) that was deemed mobile and less well defined conformationaly in the NMR study. If indeed these telltale residues are at the interface then they are likely to succumb, at least partially, to quenching consistent with the data in Fig. 1.
Kinase Activity in Meso.
That DgkA was enzymatically active in meso was convincingly demonstrated directly by TLC (Fig. 3). Our next task was to determine if a high-throughput coupled assay system could be used with DgkA reconstituted into a sticky and viscous mesophase. The progress curve in Fig. 4B shows clearly this to be the case. The progress curve includes an initial lag phase followed by a region where rate is linear with time. A lag phase is expected. Enzymatic activity resides in protein distributed throughout a porous bolus that is accessed by one of its substrates, ATP, primarily by way of aqueous channels permeating the mesophase. Thus, ATP must diffuse from the bulk bathing solution into and throughout the aqueous channels to fuel the distributed kinase. At the same time, the water-soluble product, ADP, has to diffuse in these same permeating aqueous channels out of the bolus and into the bulk medium. There it can engage with the components of the coupled assay system that leads to a reduction in A340 reflecting kinase activity. Both of these processes take time, and together they account for the initial lag.
Following on from the lag is a region where A340 changes linearly with time, reflecting the establishment of a steady state. It is this linear region that was used to calculate an operational specific activity for the enzyme. The value recorded under standard conditions (see SI Text) was 14.4–16.5 U/mg. The specific activity recorded in surfo, with monoolein as substrate, was 26.9 U/mg. It is important to appreciate that the in surfo and in meso systems differ in many respects and similar specific activities are not necessarily expected.
To our knowledge, this is a unique demonstration of enzymatic activity and turnover for an integral membrane protein reconstituted in meso. Perhaps it is not surprising to find that this enzyme functions in the cubic mesophase in light of the fact that other membrane proteins, similarly disposed, have been shown to be biologically active. Thus, for example, the in meso form of the vitamin B12 transporter, BtuB, was shown to bind its substrate, cobalamin, with nanomolar affinity, as does the native protein in the outer membrane of E. coli (13). And OpcA, an adhesin from Neisseria, bound sialic acid in meso with an affinity similar to that recorded for the protein in surfo (15). Together, these and other data in the literature (29), support the view that the lipid bilayer of the cubic phase is a reasonable membrane mimetic. It also highlights the fact that the cubic phase is a generally useful system with which to study membrane proteins and that, despite its sticky and viscous nature, it is a tractable and versatile nanoporous biomaterial. In the current application, it works nicely in a multiwell format and is therefore well suited to economical, parallel, and high-throughput screening of the type that should benefit future crystallization and structure-function determination studies.
The Km and Vmax values for Mg2+-ATP reported here are operational in the sense that they reflect the particulars of the system under investigation. Thus, for example, the cubic phase is arranged in the assay well under standard conditions as a set of string-like boluses. The cubic phase that constitutes the bolus is an open and porous material into and out of which diffusion of water-soluble molecules and ions occurs. The aqueous channels permeating the mesophase are molecularly narrow with a diameter of 5–10 nm (30). Diffusion is therefore limited in rate but nonetheless significant as demonstrated in the release study (Fig. 2). It is clear, therefore, that the concentration of added ATP in the bathing solution is always going to be an upper limit to the nucleotide concentration experienced by the enzyme in meso. At time zero, those enzymes at the bolus surface will experience bulk concentration while DgkA molecules within the bolus have no ATP in their immediate vicinity. At steady state, a similar situation prevails at the surface of the bolus as at time zero. However, now enzymes in the bolus are operating under conditions where ATP concentration falls off from the surface toward the core of the bolus. Thus, the activity measured reflects the activity of enzymes operating over a range of ATP concentrations the maximum of which is that of the initial bulk bathing solution. Consequently, the operational Km value reported (3.5 mM) is an upper limit; the actual, intrinsic value is lower. For comparison, the corresponding values reported in the literature for DgkA in liposomes and in surfo are 0.2–1.8 mM (31) and 1.2 mM (20), respectively.
By the same reasoning, the activities reported for DgkA in meso must be considered operational values. Enzyme molecules at the surface of the bolus will produce ADP directly into the bulk bathing solution for immediate detection as a reduction in A340 by the coupled assay method. These then will exhibit and give rise to maximum measured activity. In contrast, enzymes within the bolus will operate at a lower concentration of ATP than prevails at the surface exhibiting lower activity. At the same time, the ADP produced must diffuse through and out of the bolus into the bathing solution for detection by the coupled assay system. Thus, the measured rate will reflect this movement of product from site of production and will be slower the closer is the enzyme to the cylindrical core of the bolus. The operational rate recorded for the bolus therefore is always going to be less than the true rate. The operational Vmax recorded in meso under standard conditions was 14.4 U/mg. For comparison, the Vmax value reported for DgkA in surfo with monoolein as substrate was 26.9 U/mg.
Despite the rationalization just provided it can be argued that the reduced Vmax in meso is attributable to unfolded and inactive enzyme. In the absence of a convenient, direct binding method to quantify active protein in meso we have approached the issue indirectly. A mutationally thermostabilized form of DgkA, CLLD (32), was shown to exhibit the same relative kinase activity in surfo and in meso as did wild type (Table S2). Given the extraordinary stability of CLLD, this finding corroborates the notion that the lower measured rates recorded in meso are a reflection of full enzyme activity in this membrane mimetic and not an indication of unfolded protein. Our ability to grow crystals of DgkA in meso (vide infra, Fig. S2) is consistent with this view.
Despite all of the above real but understandable complications with assaying DgkA in meso progress curves enter a phase where the measured rate is linear with time reflecting the establishment of a steady state under standard assay conditions. Further, when the rate in this linear region was monitored as a function of protein concentration in the mesophase and thus, in the assay solution, it too was linear with loading at lower protein concentrations (Fig. 5C). This is an important finding because it means that under standard assay conditions operational rates can be used as a reliable measure of the concentration of active protein and substrate.
At the higher protein loading the recorded kinase rate fell off (Fig. 5C). Reasons for this are likely to include a diffusion rate of water-soluble substrate into and product out of the mesophase that cannot cope with the elevated demands of the protein to access substrate and for product to diffuse away. It may also have to do with the changing characteristics of the mesophase that come about as the concentration of decyl maltoside (DM) detergent, brought along by the protein, rises. This will tend toward a lamellar and away from the cubic mesophase at higher surfactant levels (33, 34). As noted, the bulk lamellar phase is not compatible with the diffusion of water-soluble substrate and product of the type needed to support activity that can be monitored by coupled enzyme assay. For reference, a loading of 10 mg DgkA/mL corresponds to a DM concentration of approximately 0.1 M in meso, which is still well below the level needed to trigger a transition in meso (33, 34). Parenthetically, 10 mg/mL is within the concentration range used for in meso crystallization trials. Importantly, the protein loading study demonstrates that the enzyme is catalytically active under such conditions.
Toward in Meso Crystallogenesis and Structure-Function Studies.
The primary aim of this study was to determine if the integral membrane kinase, DgkA, was catalytically active (and assayable) upon incorporation into the lipidic cubic phase. Clearly, it is. The stage is therefore set to proceed with crystallization trials with a view to generating structure-grade crystals of functional DgkA by the in meso method for which diffraction quality crystals are already in hand (Fig. S2). The kinase has been shown to phosphorylate a variety of lipid substrates several of which should prove useful in future crystallographic studies aimed at deciphering the mode of action of the kinase and the origins of its substrate selectivity.
In this study, the lipidic cubic phase that incorporates an integral membrane kinase has been shown to be compatible with a microplate-based coupled assay of catalytic activity for convenient and high-throughput screening. The sticky and viscous nature of the cubic phase facilitated such measurements. The fact that the enzyme and its lipid substrate and product remain confined to the porous mesophase while the other water-soluble substrate and product are free to partition into the aqueous bathing solution suggests a general and convenient approach for characterizing integral membrane enzymes that function with lipids in a membrane-like environment. In support of this, the activity of a phosphatidylglycerol phosphate (PGP) synthesizing enzyme, PgsA, has been successfully assayed in meso (Figs. S3 and S4). Here, the lipid substrate, cytidine diphosphate-diacylglycerol (CDP-DAG), and product, PGP, remain in the host cubic phase; their water-soluble counterparts, glycerol 3-phosphate and cytidine monophosphate, respectively, move freely in and out of the PgsA laden bolus facilitating direct spectrophotometric assay. The distinctive rheology of the cubic phase means that a separate step to physically separate substrate from product is not always needed. Accordingly, if one of the water-soluble substrates or products can be measured spectrophotometrically the need for labeling, with radioisotopes for example, and for cumbersome lipid extraction (35) is dispensed with and activity can be monitored directly and continuously. Because of its open, bicontinuous nature, the cubic phase offers the advantage that the protein is accessible for assay from both sides of the membrane. By extension, the cubic mesophase system described should lend itself to the simultaneous reconstitution, functional characterization, and exploitation of integral membrane enzymes that catalyze sequential reactions in pathways of lipid metabolism.
Methods
Detailed procedures and additional results can be found in SI Text.
Molecular Biology, Protein Production, and Purification.
The dgkA gene was synthesised (Genscript) based on the sequence of dgkA in E. coli K12 and was cloned into pTrcHisB (Invitrogen) using NcoI and EcoRI. The amino acid sequence of the expressed protein is the same as that reported for pSD0005 (32) where the N-terminal methionine is replaced by a hexa-His tag-containing peptide (MGHHHHHHEL). The CLLD mutant gene was generated as for its wild-type counterpart. DgkA and CLLD production and purification were done following published procedures (36) with minor modifications (see SI Text).
Reconstitution in Meso.
DgkA was reconstituted into the bilayer of the cubic phase following a standard protocol (8). The diluted protein solution was homogenized with monoolein in a coupled syringe device (37) at room temperature (RT, 19–24 °C) using two volumes of protein solution for every three of monoolein. For spectroscopy, where transparent samples were needed, a slightly less hydrated phase was used; the corresponding volume ratio was 29∶71. Control samples that lacked enzyme were prepared with 1 % (w/v) DM in buffer in place of protein solution.
Kinase Activity Measurements.
A coupled assay system was used to monitor DgkA activity. Thus, the consumption of ATP in the phosphorylation of monoolein was coupled through the sequential action of PK and LD to the oxidation of NADH monitored by a change in A340 (19) in a 96-well plate format (20).
The kinase activity of DgkA reconstituted into the cubic phase was assayed under standard conditions as follows. The in meso assay mix (Assay Mix 2) contained 20 mM ATP, 0.1 mM EDTA, 0.1 mM EGTA, 55 mM magnesium acetate, 1 mM PEP, 0.2 mM DTT, 50 mM LiCl, 0.4 mM NADH, 20 U/mL of PK and LDH, and 75 mM PIPES pH 6.9. The protein loaded mesophase, prepared by homogenizing 3 volumes of monoolein with 2 volumes of DgkA solution at 66 μg/mL as above, was transferred to a 50-μL syringe (Hamilton) attached to a 50-step dispenser (Hamilton) and fitted with a 22-gauge needle. Five microliters of the mesophase was dispensed in 1-μL rod-shaped boluses along the sidewall of a well in a 96-well plate (Nunc) and out of the light path of the plate reader. Because the mesophase is sticky it remains in place on the sidewall during the assay. To start the reaction 0.2 mL Assay Mix 2 was added to each well, which was enough to cover the bolus. Immediately, the plate was placed in the microplate reader at 30 °C and shaken for 5 s. A340 readings were recorded every 15 s for 45 min with shaking for 2 s between each reading. DgkA-free blanks were run with each trial using mesophase prepared with buffer. Background rates, corresponding to nonenzymatic hydrolysis of ATP and to NADH photobleaching, were subtracted from those recorded in the presence of DgkA. Assays were done in triplicate. Average values in units (1 U = 1 μmol substrate converted to product per min) ± standard deviation are reported.
Spectrophotometry.
Absorbance and fluorescence measurements on the protein in surfo and in meso were carried out as previously described (13, 15, 26) with minor modifications (see SI Text).
Thin Layer Chromatography.
TLC was used to verify that LPA is a product of DgkA action on monoolein. Thus, 5 μL cubic phase with and without DgkA was incubated overnight in a 1.5-mL Eppendorf tube at 30 °C bathed in 0.2 mL of Assay Mix 2. Mesophase was harvested by centrifuging at 20,000 g for 10 min at 25 °C and used for lipid extraction (see SI Text). Three microliters of the extracted lipid and standard solutions in chloroform were spotted on silica gel TLC plates and developed in chloroform∶methanol∶acetone∶aceticacid∶water (10∶2∶4∶2∶1 by vol.). After staining with phosphomolybdic acid the plate was placed on a hot plate at 150 °C to develop.
Supplementary Material
Acknowledgments.
We thank M. Aherne and C. Doherty for technical assistance and A. Dukkipati and C. R. Sanders for helpful discussion. Supported by Science Foundation Ireland (07/IN.1/B1836), FP7 COST Action CM0902, and the National Institutes of Health (GM75915, P50GM073210, U54GM094599).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1101815108/-/DCSupplemental.
References
- 1.Rosenbaum DM, et al. GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science. 2007;318:1266–1273. doi: 10.1126/science.1150609. [DOI] [PubMed] [Google Scholar]
- 2.Cherezov V, et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaakola VP, et al. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 2008;322:1211–1217. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu B, et al. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010;330:1066–1071. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chien EY, et al. Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science. 2010;330:1091–1095. doi: 10.1126/science.1197410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasmussen SG, et al. Structure of a nanobody-stabilized active state of the beta(2) adrenoceptor. Nature. 2011;469:175–180. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenbaum DM, et al. Structure and function of an irreversible agonist-beta(2) adrenoceptor complex. Nature. 2011;469:236–240. doi: 10.1038/nature09665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caffrey M, Cherezov V. Crystallizing membrane proteins using lipidic mesophases. Nat Protoc. 2009;4:706–731. doi: 10.1038/nprot.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caffrey M. Crystallizing membrane proteins for structure determination: Use of lipidic mesophases. Annu Rev Biophys. 2009;38:29–51. doi: 10.1146/annurev.biophys.050708.133655. [DOI] [PubMed] [Google Scholar]
- 10.Landau EM, Rosenbusch JP. Lipidic cubic phases: A novel concept for the crystallization of membrane proteins. Proc Natl Acad Sci USA. 1996;93:14532–14535. doi: 10.1073/pnas.93.25.14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cherezov V, Clogston J, Papiz MZ, Caffrey M. Room to move: Crystallizing membrane proteins in swollen lipidic mesophases. J Mol Biol. 2006;357:1605–1618. doi: 10.1016/j.jmb.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 12.Katona G, Andreasson U, Landau EM, Andreasson LE, Neutze R. Lipidic cubic phase crystal structure of the photosynthetic reaction centre from Rhodobacter sphaeroides at 2.35 A resolution. J Mol Biol. 2003;331:681–692. doi: 10.1016/s0022-2836(03)00751-4. [DOI] [PubMed] [Google Scholar]
- 13.Cherezov V, et al. In meso structure of the cobalamin transporter, BtuB, at 1.95 A resolution. J Mol Biol. 2006;364:716–734. doi: 10.1016/j.jmb.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofer N, Aragao D, Caffrey M. Crystallizing transmembrane peptides in lipidic mesophases. Biophys J. 2010;99:L23–25. doi: 10.1016/j.bpj.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherezov V, et al. In meso crystal structure and docking simulations suggest an alternative proteoglycan binding site in the OpcA outer membrane adhesin. Proteins. 2008;71:24–34. doi: 10.1002/prot.21841. [DOI] [PubMed] [Google Scholar]
- 16.Raetz CR, Newman KF. Diglyceride kinase mutants of Escherichia coli: Inner membrane association of 1,2-diglyceride and its relation to synthesis of membrane-derived oligosaccharides. J Bacteriol. 1979;137:860–868. doi: 10.1128/jb.137.2.860-868.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Horn WD, et al. Solution nuclear magnetic resonance structure of membrane-integral diacylglycerol kinase. Science. 2009;324:1726–1729. doi: 10.1126/science.1171716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorch M, et al. How to prepare membrane proteins for solid-state NMR: A case study on the alpha-helical integral membrane protein diacylglycerol kinase from E. coli. Chembiochem. 2005;6:1693–1700. doi: 10.1002/cbic.200500054. [DOI] [PubMed] [Google Scholar]
- 19.Tanzer ML, Gilvarg C. Creatine and creatine kinase measurement. J Biol Chem. 1959;234:3201–3204. [PubMed] [Google Scholar]
- 20.Badola P, Sanders CR. Escherichia coli diacylglycerol kinase is an evolutionarily optimized membrane enzyme and catalyzes direct phosphoryl transfer. J Biol Chem. 1997;272:24176–24182. doi: 10.1074/jbc.272.39.24176. [DOI] [PubMed] [Google Scholar]
- 21.Czerski L, Sanders CR. Functionality of a membrane protein in bicelles. Anal Biochem. 2000;284:327–333. doi: 10.1006/abio.2000.4720. [DOI] [PubMed] [Google Scholar]
- 22.Bohnenberger E, Sandermann H., Jr Diglyceride kinase from Escherichia coli. Modulation of enzyme activity by glycosphingolipids. Biochim Biophys Acta. 1982;685:44–50. doi: 10.1016/0005-2736(82)90033-5. [DOI] [PubMed] [Google Scholar]
- 23.Schneider EG, Kennedy EP. Phosphorylation of ceramide by diglyceride kinase preparations from Escherichia coli. J Biol Chem. 1973;248:3739–3741. [PubMed] [Google Scholar]
- 24.Schneider EG, Kennedy EP. Partial purification and properties of diglyceride kinase from Escherichia coli. Biochim Biophys Acta. 1976;441:201–212. doi: 10.1016/0005-2760(76)90163-6. [DOI] [PubMed] [Google Scholar]
- 25.Walsh JP, Fahrner L, Bell RM. sn-1,2-diacylglycerol kinase of Escherichia coli. Diacylglycerol analogues define specificity and mechanism. J Biol Chem. 1990;265:4374–4381. [PubMed] [Google Scholar]
- 26.Liu W, Caffrey M. Gramicidin structure and disposition in highly curved membranes. J Struct Biol. 2005;150:23–40. doi: 10.1016/j.jsb.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Walsh JP, Bell RM. sn-1,2-Diacylglycerol kinase of Escherichia coli. Mixed micellar analysis of the phospholipid cofactor requirement and divalent cation dependence. J Biol Chem. 1986;261:6239–6247. [PubMed] [Google Scholar]
- 28.Clogston J, Rathman J, Tomasko D, Walker H, Caffrey M. Phase behavior of a monoacylglycerol: (myverol 18–99 K)/water system. Chem Phys Lipids. 2000;107:191–220. doi: 10.1016/s0009-3084(00)00182-1. [DOI] [PubMed] [Google Scholar]
- 29.Hochkoeppler A, et al. Photochemistry of a photosynthetic reaction center immobilized in lipidic cubic phases. Biotechnol Bioeng. 1995;46:93–98. doi: 10.1002/bit.260460202. [DOI] [PubMed] [Google Scholar]
- 30.Qiu H, Caffrey M. The phase diagram of the monoolein/water system: Metastability and equilibrium aspects. Biomaterials. 2000;21:223–234. doi: 10.1016/s0142-9612(99)00126-x. [DOI] [PubMed] [Google Scholar]
- 31.Pilot JD, East JM, Lee AG. Effects of bilayer thickness on the activity of diacylglycerol kinase of Escherichia coli. Biochemistry. 2001;40:8188–8195. doi: 10.1021/bi0103258. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Y, Bowie JU. Building a thermostable membrane protein. J Biol Chem. 2000;275:6975–6979. doi: 10.1074/jbc.275.10.6975. [DOI] [PubMed] [Google Scholar]
- 33.Misquitta Y, Caffrey M. Detergents destabilize the cubic phase of monoolein: Implications for membrane protein crystallization. Biophys J. 2003;85:3084–3096. doi: 10.1016/S0006-3495(03)74727-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ai X, Caffrey M. Membrane protein crystallization in lipidic mesophases: Detergent effects. Biophys J. 2000;79:394–405. doi: 10.1016/S0006-3495(00)76301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dowhan W. Phosphatidylglycerophosphate synthase from Escherichia coli. Methods Enzymol. 1992;209:313–321. doi: 10.1016/0076-6879(92)09039-6. [DOI] [PubMed] [Google Scholar]
- 36.Sanders CR, et al. Escherichia coli diacylglycerol kinase is an alpha-helical polytopic membrane protein and can spontaneously insert into preformed lipid vesicles. Biochemistry. 1996;35:8610–8618. doi: 10.1021/bi9604892. [DOI] [PubMed] [Google Scholar]
- 37.Cheng A, Hummel B, Qiu H, Caffrey M. A simple mechanical mixer for small viscous lipid-containing samples. Chem Phys Lipids. 1998;95:11–21. doi: 10.1016/s0009-3084(98)00060-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.