Abstract
The human brain has accumulated many useful building blocks over its evolutionary history, and the best knowledge of these has often derived from experiments performed in animal species that display finely honed abilities. In this article we review a model system at the forefront of investigation into the neural bases of information processing, plasticity, and learning: the barn owl auditory localization pathway. In addition to the broadly applicable principles gleaned from three decades of work in this system, there are good reasons to believe that continued exploration of the owl brain will be invaluable for further advances in understanding of how neuronal networks give rise to behavior.
Keywords: adaptive plasticity, auditory space map, barn owl (Tyto alba), orienting behavior, inferior colliculus, neuroethology, sound localization
Introduction
Barn owls (Tyto alba) are nocturnal hunters whose survival depends on sound localization (Payne 1971). Field observations in the 1960s by behaviorist Roger Payne led neuroethologist Masazaku Konishi to speculate that the owl brain might have a map of auditory space, which would accurately direct the birds’ talon strikes to prey rustling under groundcover. This was a contentious idea. At the time, the notion that the brain encoded incoming sensory events in the form of topographic maps was well established. In the visual or somatosensory cortex, for example, the spatial location of light or touch stimuli is represented by retinotopic or somatotopic maps, isomorphic representations of the corresponding epithelial surfaces, the retina or body surface. But the analogous representation in the auditory system would be a map of sound frequencies, not of sound source locations. If auditory space maps did exist they would have to be computed on the basis of binaural analysis—comparison of arrival times, intensity, and sound spectrum at each of the two ears. Had the brain evolved to perform this computational feat?
Indeed it had. In the late 1970s, Konishi and postdoctoral fellow Eric Knudsen reported conclusive evidence of an auditory space map in the owl’s inferior colliculus (Knudsen and Konishi 1978). The two major lines of investigation that emerged from this discovery have provided key insights into basic principles of neuroscience and continue to be active areas of research. One line of study focuses on elucidating the neural computations that give rise to the auditory map, and the other on how individual experience (i.e., learning) fine-tunes such computations. We outline some of the highlights of these topics.
Information Processing in the Auditory Localization Pathway
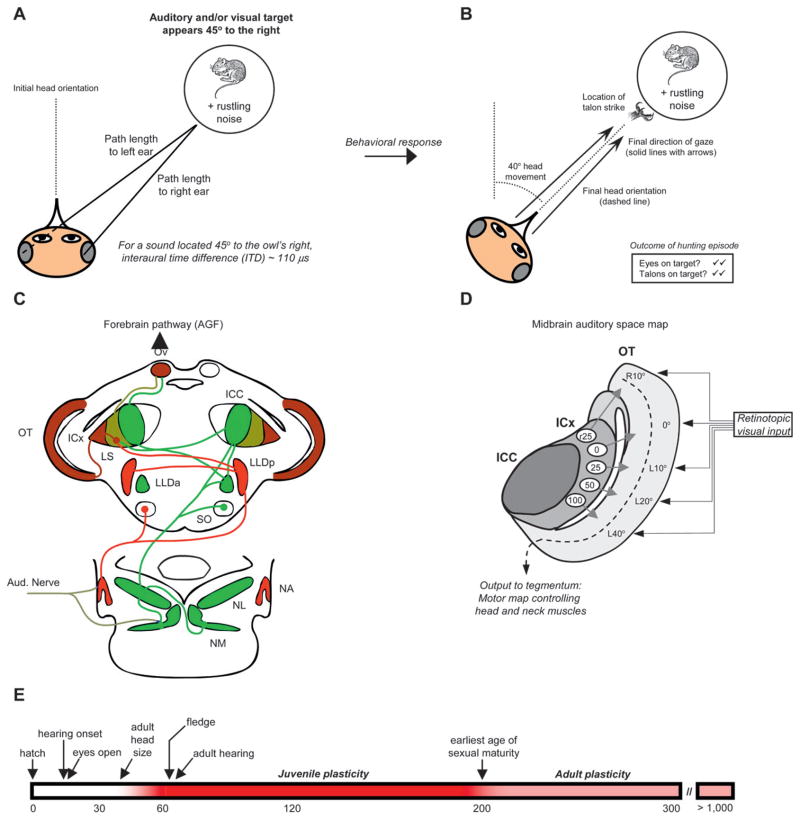
Shrews, most bats, cetaceans, and two species of birds use echolocation to navigate their environments. In contrast to this active process that uses biosonar emissions, owls and many mammals (including humans) rely on passive listening, involving a triangulation in which two copies of the sound are collected by each ear and compared by the brain to infer source direction (Konishi 2003). During periods of alert exploration, the computation may evoke an orienting response toward the “perceived” sound source, which, because of owls’ lack of a movable pinna and significant ocular movements (Steinbach 2004; Walls 1942), yields mainly two output variables: the azimuth and the elevation of a head turn (Figure 1).
Figure 1.
Auditory localization behavior and its neural basis in the barn owl. (A) Overhead view of an owl facing forward. A mouse that is both visible to the owl and whose movements produce an audible rustling noise (i.e., a bimodal target) appears 45° to the right. The different path lengths of sound to each of the two ears produce an interaural time difference (ITD), which the owl brain exploits to determine the azimuthal location of the sound source. For an average owl head size, the magnitude of this ITD is ~110 μs for sounds located at R45°. (B) The owl’s head movement in response to the bimodal stimulus. An accurate movement results in foveation of the target, extension of talons in the direction of gaze, and a successful hunt. (C) Auditory localization pathways. In the owl’s brainstem, two independent pathways process ITDs and interaural level differences (ILDs). These pathways originate in a bifurcation of the auditory nerve (AN) and converge in the lateral shell (LS) of the central nucleus of the inferior colliculus (ICC), which projects to the external nucleus of the inferior colliculus (ICx). The core of the ICC and LS project to the forebrain, via the auditory thalamus (nucleus ovoidalis, Ov), while the ICx projects to the optic tectum (OT). NA, cochlear nucleus angularis; NL, nucleus laminaris; NM, cochlear nucleus magnocellularis; LLDa, pars anterior of the dorsal nucleus of the lateral lemniscus; LLDp, pars posterior of the dorsal lateral lemniscus; SO, superior olive. For clarity, some connections between the thalamus and the brainstem (Wild 1987; Wild et al. 1993) are not shown. (D) Schematic diagram of a horizontal section through the right tectal lobe. Numbers in the ICx indicate the values of ITD to which each site is tuned. These change systematically from 25 μs right ear leading to progressively larger left ear leading values. The auditory space map is relayed to the OT where it merges with a visual space map derived from retinotopic input. The multimodal map in the OT is used to control orienting behavior. (E) Timeline of owl development. The owl’s capacity for plasticity during the juvenile period (dark red) differs in several ways from that of the adult period (light red). These distinctions are described in Figure 5 and the accompanying text.
The main cues used to compute source direction are left-right differences in timing, or interaural time differences (ITDs1), and left-right difference in sound level, or interaural level differences (ILDs1). Humans and other mammals with symmetric heads use both ITD and ILD cues for localization in the horizontal plane and monaural spectral cues for localization in the vertical plane (Heffner and Heffner 1992). Owls, too, use ITDs to determine the horizontal coordinate but uniquely use ILDs for the vertical component (Moiseff and Konishi 1983) because their craniofacial asymmetries create a systematic variation of ILD with source elevation (Moiseff 1989). Interestingly, while distinct morphological asymmetries have evolved in different species, from the position of the ear flaps in barn owls to the shape of the skull in Tengmalm’s owl (Aegolius funereus), they all appear to achieve the same functional goal (Volman and Konishi 1990).
Notwithstanding species-specific differences in the spatial pattern of ITD and ILD cues, the barn owl has proven to be an outstanding experimental model. In one sense it is indeed a “specialist” with a number of unique adaptations from inner ear to midbrain. In another sense, the computations performed in the barn owl brain likely represent building blocks of higher-order processes used across brain regions and across species. We describe these remarkable computations in the following two sections.
Detection of Binaural Cues
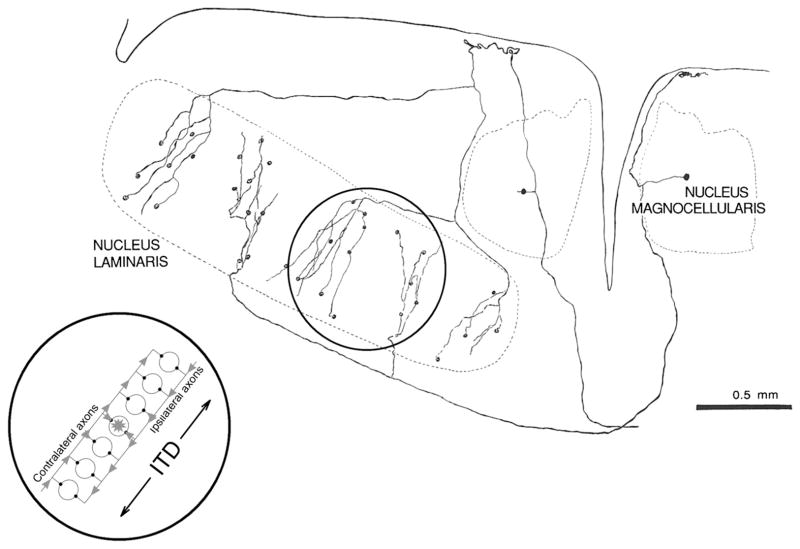
The brain’s measurement of ITD has been modeled as a coincidence detection system in which neurons respond when impulses arrive at the same time (Jeffress 1948). In the owl’s nucleus laminaris (NL; Figure 1C), impulses from each side are conveyed by myelinated axons of unusually low conduction velocity, with variable delays from one and the other side (Carr and Konishi 1990). These bilateral delay differences are within a physiologically relevant range of ITDs expected given the owl’s interaural distance and the speed of sound in air (Carr and Konishi 1990; Peña and Konishi 2001; Sullivan and Konishi 1986). NL neurons respond maximally when the conduction times from each side exactly cancel the difference in the arrival time of the sound to each ear (i.e., the ITD; Figure 2, inset) and are arranged topographically to form a map of the ITD. Although this neural scheme does not appear to be universal (Fitzpatrick et al. 2000; Harper and McAlpine 2004; Joris and Yin 2007; McAlpine et al. 2001; Schnupp and Carr 2009), experimental evidence strongly supports the idea that the model proposed by Jeffress (1948) applies to how ITD is encoded in the owl’s brain (Carr and Konishi 1990; Fischer et al. 2008; Peña and Konishi 2001; Wagner et al. 2007) and in that of other bird species (Köppl and Carr 2008) and reptiles (Carr et al. 2009).
Figure 2.
Overhead view of barn owl circuit for detection of interaural time difference (ITD). In the owl’s nucleus laminaris, phase-locked impulses are conveyed by cochlear nucleus magnocellularis axons from each side. The delays from one and the other side vary dorsoventrally in opposite directions. Coincidence-detector neurons respond maximally when impulses from the left and right sides arrive at the same time. Systematic variation in the neural delays produces a topographic representation of ITD that covers the range of ITD normally experienced by the animal (Carr and Konishi 1990). The process of detecting ITD has been modeled as a system implemented by coincidence-detector neurons and axonal delay lines (Jeffress 1948, inset).
NL neurons project to the central nucleus of the inferior colliculus (ICCc; Figure 1C). Both NL and ICCc neurons are tuned to ITD and narrowly tuned to frequency, leading to rate-ITD curves that look similar at first glance (Christianson and Peña 2007; Wagner et al. 1987, 2002, 2007). Recent studies showed, however, that ICCc cells respond to ITD more reliably (Christianson and Peña 2006). The ICCc receives input from the NL (Takahashi and Konishi 1988b) and from the anterior part of the dorsolateral lemniscal nucleus (LLDa; Figure 1C) (Adolphs 1993; Wild et al. 2001), which also receives input from the contralateral NL (Takahashi and Konishi 1988a). LLDa neurons show more reliable response to ITD than NL as well (Fischer et al. 2008). These results indicate that the output of similarly tuned NL neurons is summed to produce an averaged response with greater single-unit reliability.
The ILD is computed in a dedicated pathway where neurons, though unable to reliably adjust spike timing to sound phase, more efficiently signal sound-level changes (Köppl and Carr 2003; Sullivan and Konishi 1984). Neurons in the pars posterior of the dorsolateral lemniscus (LLDp; Figure 1) respond to sounds that are louder on the contralateral ear by means of reciprocal inhibition between LLDp nuclei (Manley et al. 1988; Mogdans and Knudsen 1994). The strength of inhibition varies dorsoventrally (Mogdans and Knudsen 1994; Takahashi et al. 1995), yielding systematic variation in ILD tuning along this axis and a precursor of the downstream topographic representation of elevation. In the owl’s midbrain neurons become sharply tuned to restricted ranges of ILD. Bilateral projections from LLDp to the lateral shell of the central nucleus of the inferior colliculus (ICCls1; Figure 1C) appear to be involved (Adolphs 1993), although the mechanism to generate nonmonotonic ILD tuning is poorly understood.
Integration of Binaural Cues
The ascending streams of binaural information converge in the external nucleus of the inferior colliculus (ICx1; Figure 1C), where they are used to compute a map of auditory space. Individual neurons in this map respond most strongly to sounds arising from one particular direction in the animal’s surroundings. For each ICx neuron, space specificity is largely due to combination selectivity to ITD and ILD and to frequency convergence (Mazer 1998; Takahashi and Konishi 1986). While the ITD and ILD tunings are robust determinants of the vertical and horizontal coordinates of spatial receptive fields (Moiseff and Konishi 1983; Olsen et al. 1989), convergence across frequency channels is required both to eliminate ambiguities inherent in the periodic nature of sound (Mazer 1998; Peña and Konishi 2000; Takahashi and Konishi 1986) and to account for frequency-dependent variation of spatial cues generated by direction-dependent filtering properties of the owl’s head and facial ruff (Hausmann et al. 2009; Keller et al. 1998; Olsen et al. 1989; Poganiatz et al. 2001; Spezio and Takahashi 2003). The end result is neuronal space specificity that is tuned to naturalistic sound properties (Nelson and Takahashi 2008; Reches and Gutfreund 2008; Wagner 1992; Witten et al. 2006) and matches behavioral acuity (Bala and Takahashi 2000; Bala et al. 2007; Saberi et al. 1998a,b).
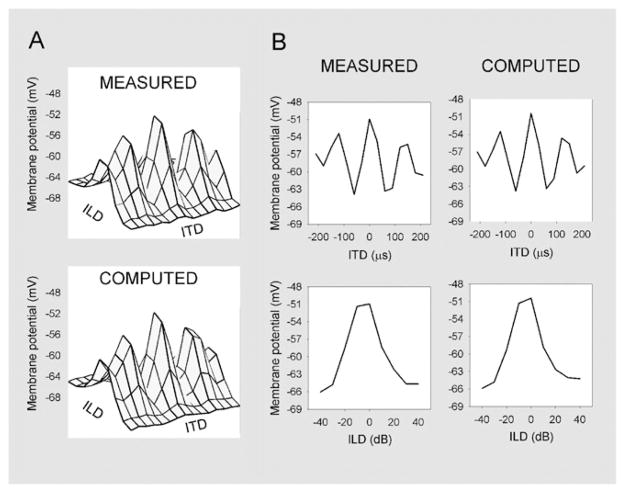
The biophysical mechanisms that underlie these computations have come under intense scrutiny. In vivo intracellular recordings showed that the spiking threshold of space-specific neurons is strategically located to narrow the spatial receptive fields, creating a spiking output that is significantly more sharply tuned to space than the subthreshold receptive field (Peña and Konishi 2000, 2002), and principal component analysis on these fields unmasked a multiplicative combination of inputs tuned to ITDs and ILDs (Peña and Konishi 2001, 2004) (Figure 3). Multiplication is a powerful operation that produces robust combination selectivity (Gabbiani et al. 2002; Koch 1999; Poggio 1990; Pouget and Sejnowski 1997; Salinas and Abbott 1996). A study of subjective lateral position based on lateralization-matching experiments in humans (Stern and Colburn 1978) and on physiological studies of auditory nerve activity (Kiang 1968) predicted that the estimate of lateral position is computed by the product of timing and intensity functions, which until then are processed in parallel. The similarity of the processing in the barn owl’s brain to this model is striking.
Figure 3.
Construction of auditory spatial receptive field by neuronal multiplication. Multiplicative combination of interaural time difference (ITD) and interaural level difference (ILD) inputs. (A) Experimental (measured) data matrix, consisting of the average membrane potential versus ITD and ILD recorded in neurons (top), and reconstruction of the matrix (computed) using a multiplication of ITD and ILD inputs (bottom). (B) ITD and ILD curves for measured post-synaptic potentials (left) and computed ITD and ILD inputs (right). The similarity between measured and computed curves indicates that the multiplicative model has remarkable power to predict the data. dB, decibel; mV, millivolts. Modified from Peña and Konishi (2001).
Understanding of the mechanism of spatial tuning in ICx has advanced considerably since Knudsen and Konishi (1978) discovered the map. However, further studies are necessary to understand its mechanism in terms of microcircuits, cellular properties, and synaptic effects.
Recent work has compiled the vast amount of data related to spatial tuning of ICx neurons into a model in which ITD- and ILD-dependent signals are multiplied within narrow frequency channels and frequency integration uses a linear-threshold mechanism (Fischer et al. 2009). This model embraces the diversity of spiking responses in ICCls (Fischer et al. 2007), addressing how a heterogeneous population can produce ICx responses; reconciles the observation of multiplicative responses to ITD and ILD with the presence of linear frequency integration in subthreshold ICx responses (Peña and Konishi 2000, 2001); and is consistent with experimental evidence that learning in the owl can elicit frequency-dependent connectivity changes in ICx (Gold and Knudsen 2000). Specific predictions of this model are yet to be tested.
Examples like these highlight the analytical power of mathematical models to represent how owls locate prey. However, they are snapshots of a conspicuous trend. The owl’s brain performs many algorithms that models have proposed. The running cross correlation, derived for coincidence detectors by Licklider (1959) and later used in several models of sound localization (Blauert and Cobben 1978; Jeffress and Robinson 1962; Saberi 1996; Sayers and Cherry 1957; Stern et al. 1988), accurately describes the NL neurons’ response to the ITD (Fischer et al. 2008). Noise reduction has been represented in sound localization models by time averaging (Sayers and Cherry, 1957); in the owl’s brain, an averaging-like process increases tuning reliability shortly after ITD detection (Christianson and Peña 2006; Fischer and Konishi 2008). Frequency convergence in the owl’s brain eliminates the ambiguity inherent in the periodic nature of sound (Mazer 1998; Peña and Konishi 2000; Saberi et al. 1998a,b; Singheiser et al. 2010; Takahashi and Konishi 1986), consistent with lateralization models for humans (Stern et al. 1988). As mentioned above, a multiplicative-like process, postulated by Stern and Colburn (1978), underlies the combination selectivity of space-specific neurons (Fischer et al. 2007, 2009; Peña and Konishi 2001).
The remarkable power of models to predict neural processing in the owl’s auditory system underscores the principles of neuroethology. In this specialized nocturnal hunter, developmental and evolutionary mechanisms have resulted in the convergence of optimal function and mathematical elegance.
Learning in the Auditory Localization Pathway
We have described the computations that interpret a particular set of binaural cues and translate them into a map of auditory space. But for any individual owl or human the cue values corresponding to particular locations change over time. This is especially evident during early and juvenile development as the head grows and the interaural distance lengthens. Even subtle postdevelopmental alterations in cranial, pinna, and torso morphology can significantly transform the spatial pattern of cues. Moreover, age-related changes in inner ear function can alter cue values at the input stage. To maintain an accurate representation of auditory space, the brain must adjust its interpretation of cue values on the basis of spatial information available from other modalities.
Barn owls have excellent night vision (Harmening et al. 2007) due to a dense concentration of rod-type photoreceptors in the area centralis, or fovea. They use vision for many essential behaviors—guiding flight through a forest or the rafters of a barn, landing on perches, and almost certainly for sizing up prey. Consider an owl flying above a field at night. It hears a noise, orients, and flies toward it. Upon close approach and with a bit of ambient moonlight or artificial lighting, the owl’s gaze falls on the auditory target. A field mouse or small vole is desirable, while a larger rat is unmanageable and dangerous. Should the visual assessment reveal suitable prey, the direction of gaze now instinctively guides the direction of talon strike (Figure 1B). Thus a successful hunt under most natural conditions relies on both audition and vision.
The integration of these modalities occurs in the optic tectum (OT1), which sits at the highest level of the midbrain auditory localization pathway (Figure 1D), where the auditory space map computed in the ICx aligns and integrates with a visual space map derived from retinotopic input (Knudsen 1982). Sensory stimuli of either modality can activate OT neurons, and because their output directly controls motor nuclei that innervate the head and neck muscles (Masino and Knudsen 1990, 1992, 1993), orienting movements can be elicited by targets that are visual only, auditory only, or bimodal in nature (Figures 1 and 4).
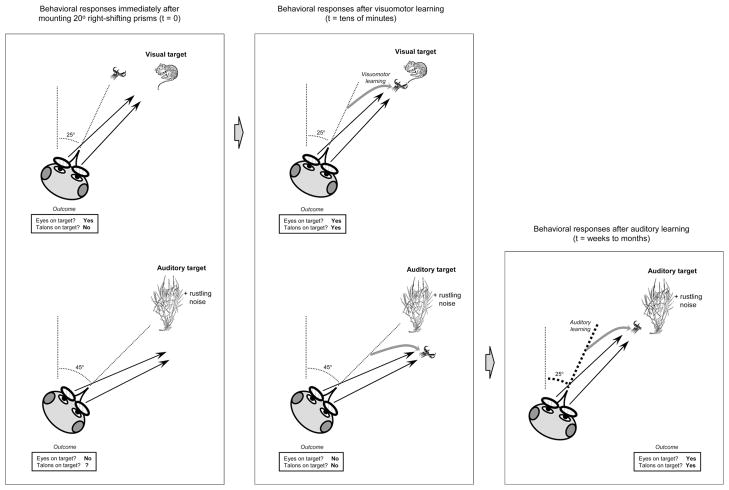
Figure 4.
Behavioral changes during prism adaptation. Head direction before and after orienting to target is indicated by thin dotted lines; final direction of gaze is indicated by solid lines with arrows, location of talon strike by talon icons. Visual targets are represented by mouse icons and auditory targets by mouse icons obscured under groundcover. Left panel: Behavioral responses to a visual (top) or auditory (bottom) target immediately after mounting of R20° prisms. The visual target elicits a head movement that accurately directs the owl’s optically displaced gaze. Because the talon strike normally follows head direction, the reach to target is miscalibrated and the mouse escapes. With the auditory target, the direction of the head movement is based solely on the owl’s interpretation of binaural cues (interaural time difference and interaural level difference); because these are unchanged by the prisms, the owl correctly orients its head toward the target—and the optically displaced gaze falls off target. If visual assessment is warranted, the owl may be reluctant to strike. If conditions are auditory only, the owl may strike and catch the mouse. Center panel: Behavioral responses after short-term prism experience. The owl makes a visuomotor adjustment (heavy grey arrow) so that the talon strike follows the optically displaced gaze. Once learned and reinforced by hundreds of daily interactions, this adaptive behavior becomes reflexive. As a result, reaching to auditory targets is now inaccurate. Right panel: Behavioral responses after long-term prism experience. Orienting and reaching to visual targets remains as illustrated in the center panel. After 8 weeks of prism rearing, however, the owl has learned to reinterpret the binaural cues that normally correspond with visual targets at R45° and now associates them with locations at R25°. This results in an adaptive orienting movement (heavy dotted line) to auditory targets. Now both the gaze and talon strike are accurate even for auditory-only targets. Behavioral integrity has been restored.
Bimodal targets provide the system with the richest information. Indeed, an important function of the OT is to associate particular values of binaural cues (ITD/ILD combinations) with the locations in the visual field that produce them. This is necessary because the values of ITD and ILD are shaped by epigenetic factors as described above; the owl genome by itself cannot know precisely how to interpret “ITD = 40 μs right ear leading.” By analyzing the success or failure of head turns to bimodal targets, or more generally daily bimodal experience (Witten and Knudsen 2005), the owl brain harnesses spatial information conveyed by the visual system to calibrate interpretation of these cues.
More than 20 years of elegant empirical studies support this model (Knudsen 2002). These experiments have used a variety of manipulations that have as a common goal the chronic alteration of cue-location relationships. Long-term effects on the brain are then assessed by electrophysiological recordings or behavioral measurements. Lid suture, ear plugging, and acoustic filtering (Gold and Knudsen 1999; Knudsen 1983, 1988; Mogdans and Knudsen 1992) are three distinct manipulations that lead to dramatic and predictable effects on auditory processing. These paradigms collectively demonstrate the robustness of the system as a learning machine. The best-studied manipulation, however, and the focus of the remainder of this review, involves the mounting of prismatic spectacles, or “prisms,” on the owl’s eyes (Knudsen and Brainard 1991).
Prisms are constructed from fresnel lenses, mounted in frames and secured to the owl via a surgically attached head bolt. They laterally displace the frontal visual field by a fixed amount (most commonly 23°) that immediately disrupts visually guided orienting behavior, resulting in missed prey strikes and landing accidents (Figure 4). The first learned response to prisms does not involve the auditory system but is a visuomotor adjustment that restores reaching accuracy to visual targets (Knudsen and Knudsen 1989b). A collateral effect of this rapid plasticity is that reaching to auditory-only targets becomes inaccurate (Figure 4, middle panel). With repeated practice over weeks to months, the owl resolves the dilemma by altering its auditory-driven orienting movements such that its optically displaced gaze falls on target (Knudsen and Knudsen 1985, 1989a; Figure 4, right panel). Visual assessment is once again possible, as is an accurate talon strike. If owls could move their eyes (more than ~2°, the maximum observed), this elaborate series of behavioral changes would not be necessary.
Learned changes in orienting behavior are primarily driven by rerouted auditory activity in the ICx (Brainard and Knudsen 1993; Figure 5A), a functional plasticity routinely quantified via electrophysiological recordings made from the OT as an adaptive shift in ITD tuning. While 57.5 μs is the maximum shift expected for 20° to 23° prisms, in practice the mean maximum shift displayed by juvenile owls is 42 μs—close may be good enough. Because it is far easier to measure adaptive shifts than changes in orienting behavior (owls are difficult to train and unresponsive to fluid rewards), the follow-up studies described below used electrophysiological recordings to monitor the progression of learning.
Figure 5.
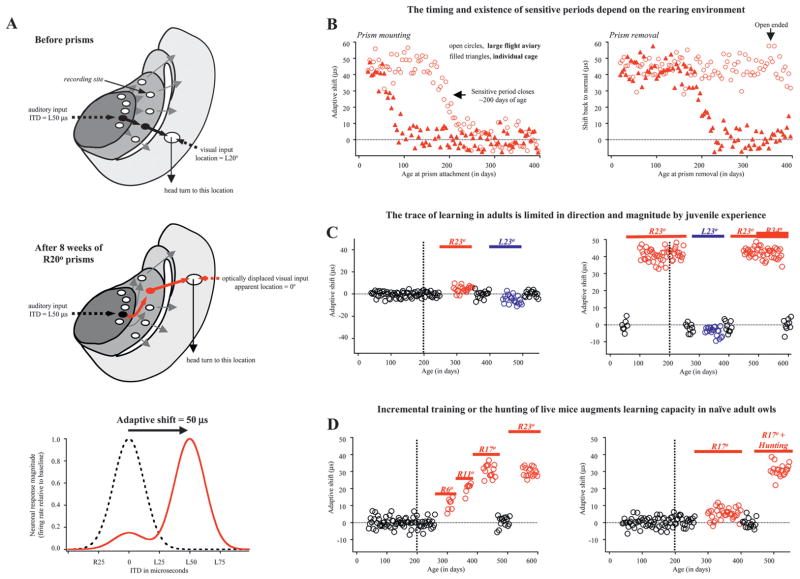
Effects of age and environment on the capacity for learning. Note: To facilitate presentation of results from a large number of studies, the graphs in B, C, and D were created with simulated data as different studies used slightly different methods for measuring adaptive shifts. The simulated data were generated in silico using the actual mean values and interindividual variances, after mapping onto a common reference frame (interaural time difference [ITD] shift in μs relative to the center of the nondisplaced visual spatial receptive field measured in degrees). (A) Neural basis of behavioral changes. Schematic diagram of a horizontal section through the right tectal lobe (see Figure 1D). The flow of neural activity in response to a sound producing an ITD of L50 μs is indicated by thick black arrows. Before prism mounting, this activity enters the optic tectum (OT) at a map location that drives a head turn to visual field location L20° (dotted black line). After 8 weeks of prism experience, activity in the ICx is rerouted (red curved arrow) and the newly calibrated response in the ICx is relayed to the OT along static connections (black arrows) that drive a head turn toward the optically displaced visual field location (dotted red line). Lower panel: ITD tuning curves recorded before (dotted black line) and after (solid red line) prism adaptation (the location of the recording site is indicated in the upper panel). Adaptive shifts are the basis of all analyses shown in the remaining panels. (B) Left panel: Magnitude of final adaptive shifts as a function of age at prism attachment. Shifts were measured after at least 8 weeks of experience with R23° prisms, sufficient time for owls to adapt. Each symbol represents data from a different owl. For birds raised in individual cages (filled triangles), the sensitive period for prism adaptation closes at approximately 60 days, whereas for those raised in large flight aviaries (open circles), the sensitive period remains open until approximately 200 d. Right panel: Magnitude of adaptive shifts back to normal as a function of age of prism removal. All of these owls had previously developed good adaptive shifts. Those in individual cages lost the ability to return to normal at approximately 200 d, whereas those in large flight aviaries retained the ability to return to normal throughout life. Collectively, these results show that (1) the housing environment can exert a powerful influence on learning capacity and (2) the capacity to relearn a normal mapping persists even after a lifetime of prism wearing. Thus, the normal mapping is privileged in ways the learned mapping is not. (C) Time courses of adaptive shifts for different directions and magnitudes of optical displacement. Each symbol represents data acquired at a different time from the same owl. Black symbols represent times when prisms are removed; red symbols, right-shifting prisms, and blue symbols, left-shifting prisms. All experiments shown in C were conducted using standard large aviary conditions (free flight, interaction with other owls, passive feeding on dead mice). Left panel: A naïve adult owl (>200 d at age of prism attachment) exhibits little adaptive adjustment to the sudden imposition of large optical displacements, R23° or L23° prisms. Right panel: An adult that had adapted to R23° prisms during the juvenile sensitive period can readapt to those prisms even after their removal for a long period. This enhanced capacity for learning does not extend to novel experience, however, because the same owl does not adapt to a different direction (L23°) or to larger displacement in the original direction (R34°). Thus the learning that results from juvenile experience is circumscribed by the parameters of that experience. (D) Two methods that augment learning in naïve adults. Left panel: Incremental training under standard large aviary conditions. A naïve adult adapts to a small optical displacement of R6° and subsequently makes adaptive shifts appropriate for R17° prisms. Consistent with characteristics of juvenile learning, the step-shifted adult reexpresses the normal mapping upon prism removal and reexpresses the learned mapping upon reexposure to a single large displacement. The trace induced by incremental training is also limited in direction and magnitude to the final step-shifted value. Right panel: A naïve adult exposed to R17° does not adapt under standard large aviary conditions, but does when it is required to hunt live mice.
Effects of Rearing Environment on the Capacity for Learning
In their natural environment owls are cavity dwellers. Whether the high canopy of a cactus palm or the recesses of a wooden barn, the cavities they seek are dark, cramped, and isolated from the outside world. This is where young owls spend their first 60 days of life before emerging to experience a rich auditory-visual environment, at which point their goal is to learn to associate an individualized pattern of binaural cues with the locations in the visual field that produce them. Their survival depends on it.
Is a rich environment necessary for hatchlings and fledglings to adapt to prisms? To answer this question, young owls were raised in either individual cages or large flight aviaries. The individual cages were nest-sized boxes with only small slits near the top to allow airflow, a housing environment that recreates the limited auditory-visual-motor experiences available in a natural nest (but sans social interactions). In contrast, the large flight aviaries offer fledglings the opportunity to fly, interact with other owls, and experience conditions similar to those encountered in the wild. Notably, for all of the experiments described in this section, hunting live prey was not part of the paradigm: the fledglings fed on dead mice that animal husbandry personnel scattered on the aviary floor once a day.
If prisms were mounted on young owls before the age of fledging (<60 d) the birds readily adapted regardless of housing environment (Brainard and Knudsen 1998). This result has been confirmed by both behavioral and physiological studies and indicates that the developing brain is quite open to changing its structure and function in order to represent the world as it is experienced. In contrast, if prisms were mounted after 60 days, the large flight aviary environment was required for successful adaptation; postfledged owls raised in individual cages did not adapt (Figure 5B), indicating the closure of a sensitive period during which the brain configures itself solely on the basis of the experienced pattern of neural activity and regardless of behavioral outcome.
Just how powerful is the rich experience available in large flight aviaries? Researchers began to experiment with different prism attachment ages (Brainard and Knudsen 1998; Knudsen and Knudsen 1986). They found that older juveniles—up to 200 days old (the approximate age of sexual maturity) at the time of prism mounting—readily adapted to prisms when housed in standard aviary conditions (i.e., with the capacity for free flight, interaction with other owls, passive feeding on dead prey). If prisms were mounted at older ages, owls did not adapt. The mechanism of this sensitive period closure remains to be determined.
An important related question is whether owls readapt to normal conditions when prisms are removed. Indeed they do. Most dramatically, prism-adapted owls living in large flight aviaries retain the ability to “snap back to normal” throughout life (Brainard and Knudsen 1998). Even owls that had been prism-reared since close to the age of eye opening (14–18 d) and had continuously worn prisms for years reexpressed a normal auditory space map and normal orienting behavior after prism removal in middle adulthood (Figure 5B). Although the time course of readaptation has not been thoroughly charted, anecdotal evidence suggests it is rapid, on the order of days to weeks at most. Thus, the normal mapping appears deeply hard-wired.
But the owl’s capacity to readapt to normal conditions can be limited by the housing environment. Although prism-adapted juveniles living in individual cages readily revert to normal following prism removal, they lose this ability at approximately 200 days (Brainard and Knudsen 1998; Figure 5B). Apparently, the hard-wired propensity of the normal mapping is not enough to overcome the absence of behavioral experience that is a hallmark of the impoverished cage environment. And again 200 days represents a crucial barrier. By analogy with other plastic circuits and systems, one could speculate that age-related consolidation of circuitry, perhaps involving the myelination of neural pathways (Cheng and Carr 2007) and potentially related to large changes in circulating levels of sex hormones, contributes in part to closure of the sensitive period.
The results described above answer many questions and raise new ones. First, how do the extent and nature of prism experience early in life circumscribe the individual’s lifelong capacity for learning? Second, is it possible to augment learning in naïve adults by using different training techniques? We consider these questions in the following sections.
Enhanced Capacity for Learning Exhibited by Prism-Adapted Owls
One clear enhancement demonstrated by prism-adapted adults is their ability to readapt to prisms after months to years of normal experience (Knudsen 1998; Figure 5C). This finding is evocative of learning in other systems, in which the development of skills early in life leaves a lasting “trace” in the brain that remains accessible even after long periods of disuse; for example, human toddlers transplanted from one language environment to another around the age of 18 months retain a superior ability as young adults to make phonetic distinctions in the first language, despite a complete lack of subsequent exposure or use (Kuhl 2004).
Does prism rearing boost learning capacity in general? To test this, prism-removed owls were challenged with optical displacements they had not experienced before (Knudsen 1998; Figure 5C). Owls that had adapted to R23° prisms as juveniles were readapted to normal and then fitted with L23° prisms, which demand shifts in the opposite direction. They did not adapt. When they were fitted with R34° prisms, which require shifts far greater than those they had expressed as juveniles, they adapted poorly, never shifting more than previously and often shifting less (juveniles <200 d do fully adapt to 34°). Finally, to verify that these owls had not simply lost their trace, they were refitted with R23° late in life—and adapted well. These results clearly indicate that the enhanced capacity for learning is limited in direction and magnitude by the specific characteristics of early life experience.
Teaching Old Owls New Tricks: The Effects of Active Hunting and Incremental Training
The conditions under which young and old animals effectively learn new skills can be strikingly different. For example, when naïve adult owls are required to hunt live mice for 1 hour per day they exhibit substantial adjustment to 23° prisms, as illustrated in Figure 5D (Bergan et al. 2005). This enhancement may result from the engagement of attention and/or increased sensory input provided by the hunting experience. Age-related dependence on the value or necessity of extrinsic context, especially heightened attention and arousal, has also been observed for plasticity occurring in the cortex (Kilgard and Merzenich 1998).
An alternative strategy to enhance learning is through incremental training (Linkenhoker and Knudsen 2002). Passively fed adult owls do not adapt to 17–23° prisms but do adapt to smaller optical displacements (~6°). Moreover, once fully adapted to the small displacement, these adults adapt to subsequent displacements of similar magnitude, and in this manner can be “step-shifted” to as much as 23° (Figure 5D). These findings reveal principles of widespread importance, as incremental training strategies have been successfully applied to other brain systems including skill learning (Clawson et al. 2001), language acquisition (Tallal et al. 1996), and operant conditioning (Perone et al. 1988).
Neural Mechanisms of Learning
As described above, the prism paradigm has been instrumental in disentangling the roles of age, housing, and training regimen on an individual’s capacity for learning. An equally important set of studies has investigated how, mechanistically, the brain accomplishes these feats.
Instructive Signal
After prism mounting, the optically displaced visual signal transduced by the central nervous system is stationary over time (Brainard and Knudsen 1993, 1995; DeBello and Knudsen 2004). That an unchanging signal from one network is used to adjust the response properties of another indicates that prism-induced adaptive plasticity is a form of instructed learning (Knudsen 1994). Instructed learning is a hallmark of many higher brain functions, such as the development of vocalizations in songbirds (Brainard and Doupe 2000) or the acquisition of expressive language in humans (Kuhl 2004).
Lesion reconstructions and latency analysis (Brainard and Knudsen 1993), pharmacological data (Feldman et al. 1996; Zheng and Knudsen 1999, 2001), and anatomical data (DeBello et al. 2001; Feldman and Knudsen 1997; McBride et al. 2008) indicate that the ICx is the major site of synaptic plasticity, at least for young juveniles (DeBello and Knudsen 2004). The visually based instructive signal acts here, but where does it come from? While an obvious candidate is the bimodal OT, early anatomical tracing studies failed to find a feedback connection from the OT to the ICx, and early physiological studies failed to find an overt trace of visually driven activity in the ICx—it appeared to be an auditory-only structure.
This view has been revised on the basis of more recent evidence. Improved tracing techniques revealed a sparse feedback connection from the OT to the ICx that exhibits many hallmarks of the instructive conduit (Hyde and Knudsen 2000; Luksch et al. 2000). This feedback connection, which is topographic in nature, originates primarily from intermediate and deep layers of the OT and from neurons whose dendritic architecture is positioned to sample both auditory and visual input, and it terminates in the region of the ICx where space-specific neurons are found. Moreover, restricted electrolytic lesions in the OT “freeze” the owl’s capacity for prism adaptation at—and only at—the corresponding map locations in the ICx (Hyde and Knudsen 2002). This is strong evidence that the OT is a source of instructive information, but leaves open the question of why no trace of visual activity had been observed in the ICx. One explanation is that the flow of information from the OT to the ICx is filtered, or gated, perhaps by attentional processes (top-down), or by specific features of auditory-visual stimuli (bottom-up), or both.
The first successful effort to tease open this gate used pharmacological manipulation to disinhibit the OT (Gutfreund et al. 2002). In baseline conditions, simple visual stimuli (LED flashes) were completely ineffective in driving ICx neurons. The gamma-aminobutyric acid (GABA) receptor antagonist bicuculline was then applied to a corresponding map location in the OT, and within minutes strong visually driven responses appeared. Moreover, for sounds and LED flashes presented simultaneously, the magnitude of the visually driven response in the ICx encoded the spatial mismatch between ITD value and LED location: instructive information had been delivered. This finding suggested that endogenous modulation of GABAergic inhibition is a proximate mechanism for opening or closing the gate. It also confirmed that the gate appears closed in lightly anesthetized owls exposed to artificial stimuli. The role, if any, of top-down attentional processes in opening the gate remains unknown.
In contrast, the nature of the stimulus itself has been found to play a role in facilitating access to the ICx (Bergan and Knudsen 2009). LED flashes were replaced with more dynamic visual stimuli such as a looming motion presented to the owl via a computer monitor. These stimuli modulated responses to simultaneously presented sounds even in the absence of pharmacological disinhibition. Because dynamic stimuli more closely represent qualities inherent to the natural visual scene, this finding suggests that specific features of visual stimuli can act as a salience filter and could indicate a bottom-up process that operates independent of attentional control.
Regardless of how the instructive signal arrives in the ICx, it must be decoded and translated into synaptic change. One candidate mechanism involves the cyclic adenosine mono-phosphate (cAMP) response element binding protein (CREB), an intracellular signaling molecule that integrates incoming synaptic activity and adjusts the neuron’s gene expression to strengthen or weaken recently activated synapses (Ahn et al. 1999; Barco et al. 2002; Kandel 2001; Marie et al. 2005; Pittenger et al. 2006). To test whether the instructive signal activates CREB, owls were provided a salient audiovisual experience (hunting live mice) and within 30 minutes sacrificed for histological analysis. CREB activation was analyzed using phosphorylation state-specific antibodies, confocal imaging, and immunofluorescent measurements at individual cell nuclei. In both normal and prism-adapted owls that experienced small auditory-visual mismatches, the distribution of cell-specific CREB activation values in the ICx exhibited a single peak. In contrast, the distribution in owls that experienced large auditory-visual mismatches (recently prism-mounted or -removed owls) exhibited two offset peaks: it appeared certain cells had received a synaptic “reward,” others a “punishment.” This bidirectional regulation could promote shifts in auditory tuning by strengthening adaptive responses while weakening normal ones (Figure 5A).
These results support a model in which CREB provides a readout of instructive information (Nichols and DeBello 2008). While the specific gene products operating downstream of CREB are not known, transcriptome-wide analysis of actively adapting owls found downregulation of ubiquitin (Swofford and DeBello 2007), a key component of protein degradation machinery that has been implicated in activity-dependent synaptic turnover (Zhao et al. 2003). These results point toward a role for precisely targeted synapse formation and elimination in driving functional plasticity of the auditory space map.
We now examine direct evidence documenting rewiring of the circuit.
Cellular and Synaptic Mechanisms
The core of the learning circuit consists of a series of topographically organized axonal projections that connect specific locations in ICCls to their cognate spatial map locations in the ICx (Figure 4A). Anatomical reconstruction of individually labeled axonal arbors revealed that the spatial density of ICCl axons terminating in the ICx peaks in the region appropriate to drive normal responses to ITD, at least in normal juvenile and adult owls (DeBello et al. 2001). In prism-adapted owls, however, the region of peak axonal density is broader and extends into territory corresponding to the direction of optical displacement. These newly sprouted axons represent the construction of a learned circuit. They are studded with boutons, morphological signatures of presynaptic terminals that connect directly to space-specific neurons (Rodriguez-Contreras et al. 2005) and exhibit a pharmacological profile consistent with the nascent appearance and subsequent strengthening of synapses through long-term potentiation (LTP) (Feldman and Knudsen 1998). These experience-induced synapses are very likely the principal source of learned responses.
The normally targeted axons and boutons persist anatomically, even after years of prism experience. Yet responses to normal values of ITD are appropriately weak in prism-adapted owls. Because the normal synapses are not withdrawn they might be diminished in strength (i.e., by long-term depression). If this occurs, the effect is small, as focal application of bicuculline unmasked strong responses in the ICx to normal values of ITD (Zheng and Knudsen 1999); the same ICx cell disinhibition had a comparatively weak effect in boosting responses to learned values of ITD. Thus, one mechanism that contributes to functional suppression of normal responses is an ITD-specific increase in local GABAergic inhibition. It is not known whether this reflects structural changes or synaptic weight changes, or both, in the inhibitory network.
The coexistent learned and normal circuits provide a macroanatomical framework that drives circuit function. One interesting question is whether the microanatomical connection patterns in each circuit (the wiring diagrams) are also modified during prism adaptation. Neuronal network simulations based on models of mammalian cortical and hippocampal neurons (Poirazi and Mel 2001) indicate that local rewiring could provide an enormous reservoir for the storage of learned skills and memories (prism adaptation is a form of procedural memory). A central prediction is that, after learning, functionally similar synapses tend to cluster together on short lengths of dendrites, where they are poised to drive nonlinear summation in the postsynaptic cell. Applied to the owl system, this theory predicts that learned synapses become more clustered than normal ones. A recent study using confocal microscopy for circuit reconstruction found evidence consistent with this prediction: learned synapses always occurred within a 20 μm window on the dendrites of space-specific ICx neurons, while synapses in the normal zone tended to be dispersed over greater distances (McBride et al. 2008). Follow-up studies using emerging tools for high-resolution, high-throughput reconstruction—for example, serial block face scanning electron microscopy (Denk and Horstmann 2004), array tomography (Micheva and Smith 2007), and automated transmission electron microscopy (Anderson et al. 2009)—are necessary to confirm, refute, or extend these observations.
The preservation of the normal circuit could explain the facility with which prism-adapted adult owls readapt to normal condition. By analogy, because these animals retain a good capacity to relearn the experiences of their youth, the learned circuit might persist after prism removal—and indeed it does for at least 2 months (Linkenhoker et al. 2005), the longest interval tested. The persistence of normal and learned circuits could represent the neural substrate, or trace of experience, that underlies the enhanced abilities of prism-adapted adults. The relative contributions of structural remodeling versus synaptic weight change to adult learning augmented by hunting or incremental training are not known.
The Role of the Forebrain Pathway in Stimulus Selection
We have focused in this review on the midbrain auditory localization pathway (Figure 1D), but a parallel pathway in the forebrain also mediates sound localization behavior. The pathways split at the level of the ICCc as the forebrain pathway proceeds through the auditory thalamus (nucleus ovoidalis) to field L, the functional equivalent of mammalian primary auditory cortex, and ultimately to the arcopallial gaze field (AGF), homologue of mammalian frontal eye fields. The AGF sends descending connections both to the midbrain (ICx and OT) and to the premotor nuclei that control head turns. Reversible inactivation of the forebrain pathway impairs memory for sound location (Knudsen and Knudsen 1996b) but does not disrupt rapid orienting movements (Knudsen and Knudsen 1996a; Knudsen et al. 1993). Reversible inactivation or electrolytic lesions of the midbrain pathway severely impair reflexive orienting without eliminating the capacity to localize sounds (Knudsen et al. 1993; Wagner 1993). This residual capacity is likely mediated by the direct connections between forebrain and premotor nuclei.
The adult owl brain is constantly bombarded with auditory and visual stimuli but restrains itself from orienting to most. In contrast, forebrain-lesioned animals often appear as automatons, reflexively orienting to unimportant noises or flashes. This raises the possibility that the descending connections from the AGF to the OT participate in stimulus selection. Like their counterparts in the midbrain, neurons in the AGF are tuned for the spatial location of auditory or visual stimuli. To probe the functional interactions between the AGF and the OT, researchers applied microstimulation to discrete sites in the AGF while simultaneously recording OT responses to auditory and visual stimuli (Winkowski and Knudsen 2006, 2007, 2008). They found that AGF microstimulation sharpened the tuning of OT neurons representing the same region of space (aligned sites), whereas responses at nonaligned sites were suppressed, resulting in an enhanced representation of stimuli at the location “preselected” by the forebrain. The AGF homologue in mammals directs spatial attention (Moore et al. 2003), so these findings support a model in which the forebrain directs covert shifts in attention that alter auditory processing in the midbrain. The role of this attention-like process in learning is not known.
Conclusions and Future Possibilities
Over the past three decades, the barn owl auditory localization pathway has emerged as a vital model system for neuroscience, in part because it drives a behavior on which survival depends: prey capture by sound localization. Investigations into the neural bases of this pathway are thus probing an architecture that has been honed for exceptional performance. While the underlying mechanisms include a few specialized features not found in other brain regions or species, they also include effective neural strategies for coincidence detection, enhancement of reliability, and combination selectivity (Figures 2 and 3). These computations are likely essential for the operation of many other circuits within and outside the owl auditory system. Natural selection often produces similar solutions to similar problems, regardless of whether the solutions arise once or multiple times independently over the course of brain evolution. Thus, the journey toward understanding these neural computations in owls, where the problem is well delineated by quantifiable stimulus parameters and behavioral output, may well provide a path toward revealing universal principles of information processing in the brain.
Because the spatial pattern of auditory cues experienced by an individual cannot be precisely known by that individual’s genome, the localization pathway must have coevolved with a powerful capacity for learning; the prism paradigm has elegantly proven this point (Figures 4 and 5). Moreover, analysis of the intrinsic and extrinsic factors that shape an owl’s capacity to adapt to prisms has illustrated principles of learning that operate across brain regions and across species. Along these lines we have highlighted the role of environment, age, and behavioral context, all of which have important implications for human society (Knudsen et al. 2006).
Another hallmark of the owl system is its amenability to integrative studies, which seek to enhance understanding of the connections across neural levels from system to circuit to neuron to synapse. Although the development of tools for molecular perturbation in owls has lagged, this technological barrier may soon crumble; when it does, the application of such techniques to circuits whose organization is already well understood should unlock new and powerful experimental designs. For example, the coexistence of normal and learned circuits provides a “before” and “after” snapshot of circuitry in the same tissue isolate, a unique tool for discovery of learning mechanisms. In the more distant future, the auditory localization pathway may be a prime candidate for connectome reconstruction and realistic computer simulations, steps toward a complete understanding of brain function from sensory stimulus to behavioral response.
Acknowledgments
The authors’ research is supported by grants from the National Institute of Deafness and Other Communication Disorders (DC007690 to JLP; DC005460 to WMD). We thank Jamie Mazer for contributing Figure 1C and two anonymous reviewers for helpful comments. Talon, mouse, and grass clipart in Figures 1 and 4 courtesy of FCIT (http://etc.usf.edu/clipart).
Footnotes
Abbreviations used in this article: ICCls, central nucleus of the inferior colliculus; ICx, external nucleus of the inferior colliculus; ILD, interaural level difference; ITD, interaural time difference; OT, optic tectum
Contributor Information
José L. Peña, Dominick P. Purpura Department of Neuroscience at the Albert Einstein College of Medicine in Bronx, New York.
William M. DeBello, Center for Neuroscience and the Department of Neurobiology, Physiology, and Behavior at the University of California, Davis.
References
- Adolphs R. Bilateral inhibition generates neuronal responses tuned to interaural level differences in the auditory brainstem of the barn owl. J Neurosci. 1993;13:3647–3668. doi: 10.1523/JNEUROSCI.13-09-03647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Ginty DD, Linden DJ. A late phase of cerebellar long-term depression requires activation of CaMKIV and CREB. Neuron. 1999;23:559–568. doi: 10.1016/s0896-6273(00)80808-9. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Jones BW, Yang JH, Shaw MV, Watt CB, Koshevoy P, Spaltenstein J, Jurrus E, Kannan UV, Whitaker RT, Mastronarde D, Tasdizen T, Marc RE. A computational framework for ultrastructural mapping of neural circuitry. PLoS Biol. 2009;7:e1000074. doi: 10.1371/journal.pbio.1000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala AD, Takahashi TT. Pupillary dilation response as an indicator of auditory discrimination in the barn owl. J Comp Physiol A. 2000;186:425–434. doi: 10.1007/s003590050442. [DOI] [PubMed] [Google Scholar]
- Bala AD, Spitzer MW, Takahashi TT. Auditory spatial acuity approximates the resolving power of space-specific neurons. PLoS One. 2007;2:e675. doi: 10.1371/journal.pone.0000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco A, Alarcon JM, Kandel ER. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell. 2002;108:689–703. doi: 10.1016/s0092-8674(02)00657-8. [DOI] [PubMed] [Google Scholar]
- Bergan JF, Knudsen EI. Visual modulation of auditory responses in the owl inferior colliculus. J Neurophysiol. 2009;101:2924–2933. doi: 10.1152/jn.91313.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergan JF, Ro P, Ro D, Knudsen EI. Hunting increases adaptive auditory map plasticity in adult barn owls. J Neurosci. 2005;25:9816–9820. doi: 10.1523/JNEUROSCI.2533-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauert J, Cobben W. Some consideration of binaural cross-correlation analysis. Acoustica. 1978;39:96–104. [Google Scholar]
- Brainard MS, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature. 2000;404:762–766. doi: 10.1038/35008083. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Knudsen EI. Experience-dependent plasticity in the inferior colliculus: A site for visual calibration of the neural representation of auditory space in the barn owl. J Neurosci. 1993;13:4589–4608. doi: 10.1523/JNEUROSCI.13-11-04589.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard MS, Knudsen EI. Dynamics of visually guided auditory plasticity in the optic tectum of the barn owl. J Neurophysiol. 1995;73:595–614. doi: 10.1152/jn.1995.73.2.595. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Knudsen EI. Sensitive periods for visual calibration of the auditory space map in the barn owl optic tectum. J Neurosci. 1998;18:3929–3942. doi: 10.1523/JNEUROSCI.18-10-03929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CE, Konishi M. A circuit for detection of interaural time differences in the brain stem of the barn owl. J Neurosci. 1990;10:3227–3246. doi: 10.1523/JNEUROSCI.10-10-03227.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CE, Soares D, Smolders J, Simon JZ. Detection of interaural time differences in the alligator. J Neurosci. 2009;29:7978–7990. doi: 10.1523/JNEUROSCI.6154-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SM, Carr CE. Functional delay of myelination of auditory delay lines in the nucleus laminaris of the barn owl. Dev Neurobiol. 2007;67:1957–1974. doi: 10.1002/dneu.20541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson GB, Peña JL. Noise reduction of coincidence detector output by the inferior colliculus of the barn owl. J Neurosci. 2006;26:5948–5954. doi: 10.1523/JNEUROSCI.0220-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson GB, Peña JL. Preservation of spectrotemporal tuning between the nucleus laminaris and the inferior colliculus of the barn owl. J Neurophysiol. 2007;97:3544–3553. doi: 10.1152/jn.01162.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clawson DM, Healy AF, Ericsson KA, Bourne LE., Jr Retention and transfer of Morse code reception skill by novices: Part-whole training. J Exp Psychol Appl. 2001;7:129–142. doi: 10.1037//1076-898x.7.2.129. [DOI] [PubMed] [Google Scholar]
- DeBello WM, Knudsen EI. Multiple sites of adaptive plasticity in the owl’s auditory localization pathway. J Neurosci. 2004;24:6853–6861. doi: 10.1523/JNEUROSCI.0480-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBello WM, Feldman DE, Knudsen EI. Adaptive axonal remodeling in the midbrain auditory space map. J Neurosci. 2001;21:3161–3174. doi: 10.1523/JNEUROSCI.21-09-03161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W, Horstmann H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. 2004;2:e329. doi: 10.1371/journal.pbio.0020329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE, Knudsen EI. An anatomical basis for visual calibration of the auditory space map in the barn owl’s midbrain. J Neurosci. 1997;17:6820–6837. doi: 10.1523/JNEUROSCI.17-17-06820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE, Knudsen EI. Pharmacological specialization of learned auditory responses in the inferior colliculus of the barn owl. J Neurosci. 1998;18:3073–3087. doi: 10.1523/JNEUROSCI.18-08-03073.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE, Brainard MS, Knudsen EI. Newly learned auditory responses mediated by NMDA receptors in the owl inferior colliculus. Science. 1996;271:525–528. doi: 10.1126/science.271.5248.525. [DOI] [PubMed] [Google Scholar]
- Fischer BJ, Konishi M. Variability reduction in interaural time difference tuning in the barn owl. J Neurophysiol. 2008;100:708–715. doi: 10.1152/jn.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BJ, Peña JL, Konishi M. Emergence of multiplicative auditory responses in the midbrain of the barn owl. J Neurophysiol. 2007;98:1181–1193. doi: 10.1152/jn.00370.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BJ, Christianson GB, Peña JL. Cross-correlation in the auditory coincidence detectors of owls. J Neurosci. 2008;28:8107–8115. doi: 10.1523/JNEUROSCI.1969-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BJ, Anderson CH, Peña JL. Multiplicative auditory spatial receptive fields created by a hierarchy of population codes. PLoS One. 2009;4:e8015. doi: 10.1371/journal.pone.0008015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick DC, Kuwada S, Batra R. Neural sensitivity to interaural time differences: Beyond the Jeffress model. J Neurosci. 2000;20:1605–1615. doi: 10.1523/JNEUROSCI.20-04-01605.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani F, Krapp HG, Koch C, Laurent G. Multiplicative computation in a visual neuron sensitive to looming. Nature. 2002;420:320–324. doi: 10.1038/nature01190. [DOI] [PubMed] [Google Scholar]
- Gold JI, Knudsen EI. Hearing impairment induces frequency-specific adjustments in auditory spatial tuning in the optic tectum of young owls. J Neurophysiol. 1999;82:2197–2209. doi: 10.1152/jn.1999.82.5.2197. [DOI] [PubMed] [Google Scholar]
- Gold JI, Knudsen EI. Abnormal auditory experience induces frequency-specific adjustments in unit tuning for binaural localization cues in the optic tectum of juvenile owls. J Neurosci. 2000;20:862–877. doi: 10.1523/JNEUROSCI.20-02-00862.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutfreund Y, Zheng W, Knudsen EI. Gated visual input to the central auditory system. Science. 2002;297:1556–1559. doi: 10.1126/science.1073712. [DOI] [PubMed] [Google Scholar]
- Harmening WM, Vobig MA, Walter P, Wagner H. Ocular aberrations in barn owl eyes. Vision Res. 2007;47:2934–2942. doi: 10.1016/j.visres.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Harper NS, McAlpine D. Optimal neural population coding of an auditory spatial cue. Nature. 2004;430:682–686. doi: 10.1038/nature02768. [DOI] [PubMed] [Google Scholar]
- Hausmann L, von Campenhausen M, Endler F, Singheiser M, Wagner H. Improvements of sound localization abilities by the facial ruff of the barn owl (Tyto alba) as demonstrated by virtual ruff removal. PLoS One. 2009;4:e7721. doi: 10.1371/journal.pone.0007721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner RS, Heffner EH. Evolution of sound localization in mammals. In: Webster DB, Fay RR, Popper AN, editors. The Evolutionary Biology of Hearing. New York: Springer; 1992. pp. 691–715. [Google Scholar]
- Hyde PS, Knudsen EI. Topographic projection from the optic tectum to the auditory space map in the inferior colliculus of the barn owl. J Comp Neurol. 2000;421:146–160. doi: 10.1002/(sici)1096-9861(20000529)421:2<146::aid-cne2>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Hyde PS, Knudsen EI. The optic tectum controls visually guided adaptive plasticity in the owl’s auditory space map. Nature. 2002;415:73–76. doi: 10.1038/415073a. [DOI] [PubMed] [Google Scholar]
- Jeffress LA. A place theory of sound localization. J Comp Physiol Psychol. 1948;41:35–39. doi: 10.1037/h0061495. [DOI] [PubMed] [Google Scholar]
- Jeffress LA, Robinson DA. Formulas for coefficient of interaural correlation for noise. J Acoust Soc Am. 1962;34:1658. [Google Scholar]
- Joris P, Yin TC. A matter of time: Internal delays in binaural processing. Trends Neurosci. 2007;30:70–78. doi: 10.1016/j.tins.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: A dialogue between genes and synapses. Biosci Rep. 2001;21:565–611. doi: 10.1023/a:1014775008533. [DOI] [PubMed] [Google Scholar]
- Keller CH, Hartung K, Takahashi TT. Head-related transfer functions of the barn owl: Measurement and neural responses. Hear Res. 1998;118:13–34. doi: 10.1016/s0378-5955(98)00014-8. [DOI] [PubMed] [Google Scholar]
- Kiang NY. A survey of recent developments in the study of auditory physiology. Ann Otol Rhinol Laryngol. 1968;77:656–675. doi: 10.1177/000348946807700406. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Auditory and visual maps of space in the optic tectum of the owl. J Neurosci. 1982;2:1177–1194. doi: 10.1523/JNEUROSCI.02-09-01177.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI. Early auditory experience aligns the auditory map of space in the optic tectum of the barn owl. Science. 1983;222:939–942. doi: 10.1126/science.6635667. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Early blindness results in a degraded auditory map of space in the optic tectum of the barn owl. Proc Natl Acad Sci U S A. 1988;85:6211–6214. doi: 10.1073/pnas.85.16.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI. Supervised learning in the brain. J Neurosci. 1994;14:3985–3997. doi: 10.1523/JNEUROSCI.14-07-03985.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI. Capacity for plasticity in the adult owl auditory system expanded by juvenile experience. Science. 1998;279:1531–1533. doi: 10.1126/science.279.5356.1531. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Instructed learning in the auditory localization pathway of the barn owl. Nature. 2002;417:322–328. doi: 10.1038/417322a. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Brainard MS. Visual instruction of the neural map of auditory space in the developing optic tectum. Science. 1991;253:85–87. doi: 10.1126/science.2063209. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Knudsen PF. Vision guides the adjustment of auditory localization in young barn owls. Science. 1985;230:545–548. doi: 10.1126/science.4048948. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Knudsen PF. The sensitive period for auditory localization in barn owls is limited by age, not by experience. J Neurosci. 1986;6:1918–1924. doi: 10.1523/JNEUROSCI.06-07-01918.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI, Knudsen PF. Vision calibrates sound localization in developing barn owls. J Neurosci. 1989a;9:3306–3313. doi: 10.1523/JNEUROSCI.09-09-03306.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI, Knudsen PF. Visuomotor adaptation to displacing prisms by adult and baby barn owls. J Neurosci. 1989b;9:3297–3305. doi: 10.1523/JNEUROSCI.09-09-03297.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI, Knudsen PF. Contribution of the forebrain archistriatal gaze fields to auditory orienting behavior in the barn owl. Exp Brain Res. 1996a;108:23–32. doi: 10.1007/BF00242901. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Knudsen PF. Disruption of auditory spatial working memory by inactivation of the forebrain archistriatum in barn owls. Nature. 1996b;383:428–431. doi: 10.1038/383428a0. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Konishi M. A neural map of auditory space in the owl. Science. 1978;200:795–797. doi: 10.1126/science.644324. [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Knudsen PF, Masino T. Parallel pathways mediating both sound localization and gaze control in the forebrain and midbrain of the barn owl. J Neurosci. 1993;13:2837–2852. doi: 10.1523/JNEUROSCI.13-07-02837.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI, Heckman JJ, Cameron JL, Shonkoff JP. Economic, neurobiological, and behavioral perspectives on building America’s future workforce. Proc Natl Acad Sci U S A. 2006;103:10155–10162. doi: 10.1073/pnas.0600888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. Biophysics of computation: Information processing in single neurons. New York: Oxford University Press; 1999. [Google Scholar]
- Konishi M. Coding of auditory space. Annu Rev Neurosci. 2003;26:31–55. doi: 10.1146/annurev.neuro.26.041002.131123. [DOI] [PubMed] [Google Scholar]
- Köppl C, Carr CE. Computational diversity in the cochlear nucleus angularis of the barn owl. J Neurophysiol. 2003;89:2313–2329. doi: 10.1152/jn.00635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köppl C, Carr CE. Maps of interaural time difference in the chicken’s brainstem nucleus laminaris. Biol Cybern. 2008;98:541–559. doi: 10.1007/s00422-008-0220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK. Early language acquisition: Cracking the speech code. Nat Rev Neurosci. 2004;5:831–843. doi: 10.1038/nrn1533. [DOI] [PubMed] [Google Scholar]
- Licklider JCR. Three auditory theories. In: Koch S, editor. Psychology: A Study of a Science. Vol. 1. New York: McGraw-Hill; 1959. pp. 41–144. [Google Scholar]
- Linkenhoker BA, Knudsen EI. Incremental training increases the plasticity of the auditory space map in adult barn owls. Nature. 2002;419:293–296. doi: 10.1038/nature01002. [DOI] [PubMed] [Google Scholar]
- Linkenhoker BA, von der Ohe CG, Knudsen EI. Anatomical traces of juvenile learning in the auditory system of adult barn owls. Nat Neurosci. 2005;8:93–98. doi: 10.1038/nn1367. [DOI] [PubMed] [Google Scholar]
- Luksch H, Gauger B, Wagner H. A candidate pathway for a visual instructional signal to the barn owl’s auditory system. J Neurosci. 2000;20:RC70. doi: 10.1523/JNEUROSCI.20-08-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GA, Köppl C, Konishi M. A neural map of interaural intensity differences in the brain stem of the barn owl. J Neurosci. 1988;8:2665–2676. doi: 10.1523/JNEUROSCI.08-08-02665.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie H, Morishita W, Yu X, Calakos N, Malenka RC. Generation of silent synapses by acute in vivo expression of CaMKIV and CREB. Neuron. 2005;45:741–752. doi: 10.1016/j.neuron.2005.01.039. [DOI] [PubMed] [Google Scholar]
- Masino T, Knudsen EI. Horizontal and vertical components of head movement are controlled by distinct neural circuits in the barn owl. Nature. 1990;345:434–437. doi: 10.1038/345434a0. [DOI] [PubMed] [Google Scholar]
- Masino T, Knudsen EI. Anatomical pathways from the optic tectum to the spinal cord subserving orienting movements in the barn owl. Exp Brain Res. 1992;92:194–208. doi: 10.1007/BF00227965. [DOI] [PubMed] [Google Scholar]
- Masino T, Knudsen EI. Orienting head movements resulting from electrical microstimulation of the brainstem tegmentum in the barn owl. J Neurosci. 1993;13:351–370. doi: 10.1523/JNEUROSCI.13-01-00351.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazer JA. How the owl resolves auditory coding ambiguity. Proc Natl Acad Sci U S A. 1998;95:10932–10937. doi: 10.1073/pnas.95.18.10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlpine D, Jiang D, Palmer AR. A neural code for low-frequency sound localization in mammals. Nat Neurosci. 2001;4:396–401. doi: 10.1038/86049. [DOI] [PubMed] [Google Scholar]
- McBride TJ, Rodriguez-Contreras A, Trinh A, Bailey R, DeBello WM. Learning drives differential clustering of axodendritic contacts in the barn owl auditory system. J Neurosci. 2008;28:6960–6973. doi: 10.1523/JNEUROSCI.1352-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva KD, Smith SJ. Array tomography: A new tool for imaging the molecular architecture and ultrastructure of neural circuits. Neuron. 2007;55:25–36. doi: 10.1016/j.neuron.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogdans J, Knudsen EI. Adaptive adjustment of unit tuning to sound localization cues in response to monaural occlusion in developing owl optic tectum. J Neurosci. 1992;12:3473–3484. doi: 10.1523/JNEUROSCI.12-09-03473.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogdans J, Knudsen EI. Representation of interaural level difference in the VLVp, the first site of binaural comparison in the barn owl’s auditory system. Hear Res. 1994;74:148–164. doi: 10.1016/0378-5955(94)90183-x. [DOI] [PubMed] [Google Scholar]
- Moiseff A. Bi-coordinate sound localization by the barn owl. J Comp Physiol A. 1989;164:637–644. doi: 10.1007/BF00614506. [DOI] [PubMed] [Google Scholar]
- Moiseff A, Konishi M. Binaural characteristics of units in the owl’s brainstem auditory pathway: Precursors of restricted spatial receptive fields. J Neurosci. 1983;3:2553–2562. doi: 10.1523/JNEUROSCI.03-12-02553.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Armstrong KM, Fallah M. Visuomotor origins of covert spatial attention. Neuron. 2003;40:671–683. doi: 10.1016/s0896-6273(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Nelson BS, Takahashi TT. Independence of echo-threshold and echo-delay in the barn owl. PLoS One. 2008;3:e3598. doi: 10.1371/journal.pone.0003598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols GS, DeBello WM. Bidirectional regulation of the cAMP response element binding protein encodes spatial map alignment in prism-adapting barn owls. J Neurosci. 2008;28:9898–9909. doi: 10.1523/JNEUROSCI.1385-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JF, Knudsen EI, Esterly SD. Neural maps of interaural time and intensity differences in the optic tectum of the barn owl. J Neurosci. 1989;9:2591–2605. doi: 10.1523/JNEUROSCI.09-07-02591.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RS. Acoustic location of prey by barn owls (Tyto alba) J Exp Biol. 1971;54:535–573. doi: 10.1242/jeb.54.3.535. [DOI] [PubMed] [Google Scholar]
- Peña JL, Konishi M. Cellular mechanisms for resolving phase ambiguity in the owl’s inferior colliculus. Proc Natl Acad Sci U S A. 2000;97:11787–11792. doi: 10.1073/pnas.97.22.11787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña JL, Konishi M. Auditory spatial receptive fields created by multiplication. Science. 2001;292:249–252. doi: 10.1126/science.1059201. [DOI] [PubMed] [Google Scholar]
- Peña JL, Konishi M. From postsynaptic potentials to spikes in the genesis of auditory spatial receptive fields. J Neurosci. 2002;22:5652–5658. doi: 10.1523/JNEUROSCI.22-13-05652.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña JL, Konishi M. Robustness of multiplicative processes in auditory spatial tuning. J Neurosci. 2004;24:8907–8910. doi: 10.1523/JNEUROSCI.2924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perone M, Galizio M, Baron A. The relevance of animal-based principles in the laboratory study of human operant conditioning. In: Cullen C, Davey G, editors. Human Operant Conditioning and Behavior Modification. New York: John Wiley and Sons; 1988. pp. 59–85. [Google Scholar]
- Pittenger C, Fasano S, Mazzocchi-Jones D, Dunnett SB, Kandel ER, Brambilla R. Impaired bidirectional synaptic plasticity and procedural memory formation in striatum-specific cAMP response element-binding protein-deficient mice. J Neurosci. 2006;26:2808–2813. doi: 10.1523/JNEUROSCI.5406-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poganiatz I, Nelken I, Wagner H. Sound-localization experiments with barn owls in virtual space: Influence of interaural time difference on head-turning behavior. J Assoc Res Otolaryngol. 2001;2:1–21. doi: 10.1007/s101620010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggio T. A theory of how the brain might work. Cold Spring Harbor Symp Quant Biol. 1990;55:899–910. doi: 10.1101/sqb.1990.055.01.084. [DOI] [PubMed] [Google Scholar]
- Poirazi P, Mel BW. Impact of active dendrites and structural plasticity on the memory capacity of neural tissue. Neuron. 2001;29:779–796. doi: 10.1016/s0896-6273(01)00252-5. [DOI] [PubMed] [Google Scholar]
- Pouget A, Sejnowski TJ. A new view of hemineglect based on the response properties of parietal neurones. Philos Trans R Soc Lond B Biol Sci. 1997;352:1449–1459. doi: 10.1098/rstb.1997.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reches A, Gutfreund Y. Stimulus-specific adaptations in the gaze control system of the barn owl. J Neurosci. 2008;28:1523–1533. doi: 10.1523/JNEUROSCI.3785-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Contreras A, Liu XB, DeBello WM. Axodendritic contacts onto calcium/calmodulin-dependent protein kinase type II-expressing neurons in the barn owl auditory space map. J Neurosci. 2005;25:5611–5622. doi: 10.1523/JNEUROSCI.3972-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saberi K. An auditory illusion predicted from a weighted cross-correlation model of binaural interaction. Psychol Rev. 1996;103:137–142. doi: 10.1037/0033-295x.103.1.137. [DOI] [PubMed] [Google Scholar]
- Saberi K, Farahbod H, Konishi M. How do owls localize interaurally phase-ambiguous signals? Proc Natl Acad Sci U S A. 1998a;95:6465–6468. doi: 10.1073/pnas.95.11.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saberi K, Takahashi Y, Konishi M, Albeck Y, Arthur BJ, Farahbod H. Effects of interaural decorrelation on neural and behavioral detection of spatial cues. Neuron. 1998b;21:789–798. doi: 10.1016/s0896-6273(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Salinas E, Abbott LF. A model of multiplicative neural responses in parietal cortex. Proc Natl Acad Sci U S A. 1996;93:11956–11961. doi: 10.1073/pnas.93.21.11956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers B, Cherry EC. Mechanism of binaural fusion in the hearing of speech. J Acoust Soc Am. 1957;29 [Google Scholar]
- Schnupp JW, Carr CE. On hearing with more than one ear: Lessons from evolution. Nat Neurosci. 2009;12:692–697. doi: 10.1038/nn.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singheiser M, Plachta DT, Brill S, Bremen P, van der Willigen RF, Wagner H. Target-approaching behavior of barn owls (Tyto alba): Influence of sound frequency. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010;196:227–240. doi: 10.1007/s00359-010-0508-6. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Takahashi TT. Frequency-specific interaural level difference tuning predicts spatial response patterns of space-specific neurons in the barn owl inferior colliculus. J Neurosci. 2003;23:4677–4688. doi: 10.1523/JNEUROSCI.23-11-04677.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach MJ. Owls’ eyes move. Br J Ophthalmol. 2004;88:1103. doi: 10.1136/bjo.2004.042291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RM, Colburn HS. Theory of binaural interaction based on auditory-nerve data. IV: A model for subjective lateral position. J Acoust Soc Am. 1978;64:127–140. doi: 10.1121/1.381978. [DOI] [PubMed] [Google Scholar]
- Stern RM, Zeiberg AS, Trahiotis C. Lateralization of complex binaural stimuli: A weighted-image model. J Acoust Soc Am. 1988;84:156–165. doi: 10.1121/1.396982. [DOI] [PubMed] [Google Scholar]
- Sullivan WE, Konishi M. Segregation of stimulus phase and intensity coding in the cochlear nucleus of the barn owl. J Neurosci. 1984;4:1787–1799. doi: 10.1523/JNEUROSCI.04-07-01787.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan WE, Konishi M. Neural map of interaural phase difference in the owl’s brainstem. Proc Natl Acad Sci U S A. 1986;83:8400–8404. doi: 10.1073/pnas.83.21.8400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford JA, DeBello WM. Transcriptome changes associated with instructed learning in the barn owl auditory localization pathway. Dev Neurobiol. 2007;67:1457–1477. doi: 10.1002/dneu.20458. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Konishi M. Selectivity for interaural time difference in the owl’s midbrain. J Neurosci. 1986;6:3413–3422. doi: 10.1523/JNEUROSCI.06-12-03413.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi TT, Konishi M. Projections of nucleus angularis and nucleus laminaris to the lateral lemniscal nuclear complex of the barn owl. J Comp Neurol. 1988a;274:212–238. doi: 10.1002/cne.902740207. [DOI] [PubMed] [Google Scholar]
- Takahashi TT, Konishi M. Projections of the cochlear nuclei and nucleus laminaris to the inferior colliculus of the barn owl. J Comp Neurol. 1988b;274:190–211. doi: 10.1002/cne.902740206. [DOI] [PubMed] [Google Scholar]
- Takahashi TT, Barberini CL, Keller CH. An anatomical substrate for the inhibitory gradient in the VLVp of the owl. J Comp Neurol. 1995;358:294–304. doi: 10.1002/cne.903580210. [DOI] [PubMed] [Google Scholar]
- Tallal P, Miller SL, Bedi G, Byma G, Wang X, Nagarajan SS, Schreiner C, Jenkins WM, Merzenich MM. Language comprehension in language-learning impaired children improved with acoustically modified speech. Science. 1996;271:81–84. doi: 10.1126/science.271.5245.81. [DOI] [PubMed] [Google Scholar]
- Volman SF, Konishi M. Comparative physiology of sound localization in four species of owls. Brain Behav Evol. 1990;36:196–215. doi: 10.1159/000115307. [DOI] [PubMed] [Google Scholar]
- Wagner H. On the ability of neurons in the barn owl’s inferior colliculus to sense brief appearances of interaural time difference. J Comp Physiol A. 1992;170:3–11. doi: 10.1007/BF00190396. [DOI] [PubMed] [Google Scholar]
- Wagner H. Sound-localization deficits induced by lesions in the barn owl’s auditory space map. J Neurosci. 1993;13:371–386. doi: 10.1523/JNEUROSCI.13-01-00371.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H, Takahashi T, Konishi M. Representation of interaural time difference in the central nucleus of the barn owl’s inferior colliculus. J Neurosci. 1987;7:3105–3116. doi: 10.1523/JNEUROSCI.07-10-03105.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner H, Mazer JA, von Campenhausen M. Response properties of neurons in the core of the central nucleus of the inferior colliculus of the barn owl. Eur J Neurosci. 2002;15:1343–1352. doi: 10.1046/j.1460-9568.2002.01970.x. [DOI] [PubMed] [Google Scholar]
- Wagner H, Asadollahi A, Bremen P, Endler F, Vonderschen K, von Campenhausen M. Distribution of interaural time difference in the barn owl’s inferior colliculus in the low- and high-frequency ranges. J Neurosci. 2007;27:4191–4200. doi: 10.1523/JNEUROSCI.5250-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls GL. The Vertebrate Eye and Its Adaptive Radiation. Bloomfield Hills MI: Cranbrook Institute of Science; 1942. [Google Scholar]
- Wild JM. Nuclei of the lateral lemniscus project directly to the thalamic auditory nuclei in the pigeon. Brain Res. 1987;408:303–307. doi: 10.1016/0006-8993(87)90393-3. [DOI] [PubMed] [Google Scholar]
- Wild JM, Karten HJ, Frost BJ. Connections of the auditory forebrain in the pigeon (Columba livia) J Comp Neurol. 1993;337:32–62. doi: 10.1002/cne.903370103. [DOI] [PubMed] [Google Scholar]
- Wild JM, Kubke MF, Carr CE. Tonotopic and somatotopic representation in the nucleus basalis of the barn owl, Tyto alba. Brain Behav Evol. 2001;57:39–62. doi: 10.1159/000047225. [DOI] [PubMed] [Google Scholar]
- Winkowski DE, Knudsen EI. Top-down gain control of the auditory space map by gaze control circuitry in the barn owl. Nature. 2006;439:336–339. doi: 10.1038/nature04411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkowski DE, Knudsen EI. Top-down control of multimodal sensitivity in the barn owl optic tectum. J Neurosci. 2007;27:13279–13291. doi: 10.1523/JNEUROSCI.3937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkowski DE, Knudsen EI. Distinct mechanisms for top-down control of neural gain and sensitivity in the owl optic tectum. Neuron. 2008;60:698–708. doi: 10.1016/j.neuron.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Knudsen EI. Why seeing is believing: Merging auditory and visual worlds. Neuron. 2005;48:489–496. doi: 10.1016/j.neuron.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Witten IB, Bergan JF, Knudsen EI. Dynamic shifts in the owl’s auditory space map predict moving sound location. Nat Neurosci. 2006;9:1439–1445. doi: 10.1038/nn1781. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Hegde AN, Martin KC. The ubiquitin proteasome system functions as an inhibitory constraint on synaptic strengthening. Curr Biol. 2003;13:887–898. doi: 10.1016/s0960-9822(03)00332-4. [DOI] [PubMed] [Google Scholar]
- Zheng W, Knudsen EI. Functional selection of adaptive auditory space map by GABAA-mediated inhibition. Science. 1999;284:962–965. doi: 10.1126/science.284.5416.962. [DOI] [PubMed] [Google Scholar]
- Zheng W, Knudsen EI. GABAergic inhibition antagonizes adaptive adjustment of the owl’s auditory space map during the initial phase of plasticity. J Neurosci. 2001;21:4356–4365. doi: 10.1523/JNEUROSCI.21-12-04356.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]