Abstract
The xenobiotic receptors, constitutive androstane receptor (CAR), and pregnane X receptor (PXR) regulate and alter the metabolism of xenobiotic substrates. Among the 19 functional UDP-glucuronosyltransferases (UGTs) in humans, UGT2B7 is involved in the metabolism of many structurally diverse xenobiotics and plays an important role in the clearance and detoxification of many therapeutic drugs. To examine whether this gene is regulated by CAR and PXR in vivo, transgenic mice expressing the entire UGT2B7 gene (TgUGT2B7) were created. Gene expression profiles revealed that UGT2B7 is differentially expressed in liver, kidney, adipocytes, brain, and estrogen-sensitive tissues, such as ovary and uterus. Liver UGT2B7 expression levels were decreased when TgUGT2B7 mice were treated with the CAR ligand 1,4-b-s-[2-(3,5,-dichloropyridyloxy)] (TCPOBOP) but not the PXR ligand pregnenolone 16α-carbonitrile. Although TCPOBOP decreased the levels of UGT2B7 mRNA in TgUGT2B7 mice, it had no affect on Tg(UGT2B7)Car(−/−) mice, adding support for a CAR-dependent mechanism contributing toward UGT2B7 gene suppression. Expression of promoter constructs in HepG2 cells showed the CAR-dependent inhibition was linked to hepatocyte nuclear factor-4α (HNF4α)-mediated transactivation of the UGT2B7 promoter. The inhibitory effect of CAR on UGT2B7 gene expression was validated in chromatin immunoprecipitation assays in which TCPOBOP treatment blocked HNF4α binding to the UGT2B7 promoter. These results suggest that HNF4α plays an important role in the constitutive expression of hepatic UGT2B7, and CAR acts as a negative regulator by interfering with HNF4α binding activity.
Introduction
Located in the cellular endoplasmic reticulum, the family of UDP-glucuronosyltransferases (UGTs) plays a vital role in the metabolism and detoxification of numerous endogenous and exogenous compounds. There are 19 functional UGTs in humans, 9 are encoded by the UGT1 locus on chromosome 2 and the other by UGT2 genes on chromosome 4 (Mackenzie et al., 2005). The expression of these genes in human tissues is highly organized, with each tissue comprising its own complement of the UGTs (Tukey and Strassburg, 2000; Gregory et al., 2004). Among the human UGTs, UGT2B7 is expressed in many tissues and conveys broad substrate specificity. Some estimates indicate that UGT2B7 is responsible for the metabolism of 35% of all clinical drugs (Williams et al., 2004). In addition, UGT2B7 participates in the metabolism of bile acids, fatty acids, and steroids (Ritter et al., 1992).
Because UGT2B7 plays a key role in drug metabolism and is abundant in human liver (Izukawa et al., 2009) and intestine, efforts are underway to investigate the mechanisms leading to UGT2B7 gene control. In human liver, there is large interindividual variability in the expression of UGT2B7 (Izukawa et al., 2009), part of which has been linked to hepatocyte nuclear factor-1α (HNF1α) expression (Ormrod et al., 1999; Toide et al., 2002). In human Caco-2 cells, exposure to farnesoid X receptor (FXR) ligands, such as lithocholic acid, suppressed constitutive expression of UGT2B7 (Lu et al., 2005). Retinoic acids, which are also metabolized by UGT2B7 (Samokyszyn et al., 2000) but play a key role in nuclear receptor function by activating the retinoid X receptor (RXR), have also been shown to suppress UGT2B7 expression in Caco-2 cells (Lu et al., 2008). These results indicate that the family of xenobiotic nuclear receptors (XenRs), including FXR and possibly others that are expressed in liver and intestine such as the constitutive androstane receptor (CAR) and pregnane X receptor (PXR), may also be implicated in the control of the UGT2B7 gene.
The placement of human genes into mice that are expressed as transgenes serves as a powerful tool to examine the influence of hormones, steroids, and nuclear receptors toward influencing transcriptional control and function of the gene products. The generation of transgenic UGT1 (TgUGT1) mice expressing the human UGT1 locus has confirmed that the 9-UGT1A genes are expressed in a coordinated fashion (Chen et al., 2005) that resembles their expression pattern as mapped in human tissues (Strassburg et al., 1997a,b; Tukey and Strassburg, 2000). The treatment of TgUGT1 mice with ligands that activate the XenRs is a powerful tool to examine the role of these receptors in control and expression of the UGT1A genes, because the genes are regulated both through induction and tissue specificity (Chen et al., 2005; Verreault et al., 2006; Senekeo-Effenberger et al., 2007; Yueh and Tukey, 2007). The functional role of the human UGT1A1 gene in homeostatic control of serum bilirubin has been demonstrated in humanized UGT1 mice, which expresses the UGT1A genes in a complete Ugt1-null background (Fujiwara et al., 2010). We undertook a similar approach to examine the regulation of the human UGT2B7 gene.
The UGT2B7 gene spans 16 kb on chromosome 4 (Monaghan et al., 1994). We generated UGT2B7 transgenic mice (TgUGT2B7) with a bacterial artificial chromosome encoding the human UGT2B7 gene. Tissue-specific expression demonstrated by transcriptional levels revealed that the pattern of expression in TgUGT2B7 mice is comparable with what has been found for UGT2B7 expression in human tissues (Turgeon et al., 2001). Here, we describe experiments that suggest functional inhibitory cross-talk between HNF4α in liver of mice exposed to TCPOBOP, confirming a role for HNF4α and CAR toward the regulation of UGT2B7.
Materials and Methods
Animals.
The TgUGT2B7 mice were generated at the University of California San Diego Superfund Research Program Mouse Genetics Core Facility (San Diego, CA). A bacterial artificial chromosome (BAC) encoding the UGT2B7 gene (GenBank accession number RP13-644M16) was purified, microinjected into the pronucleus of CB6F1 mouse eggs, and transplanted into the oviduct of pseudopregnant C57BL/6N mice. For genotyping, DNA was isolated from tail clippings, and a 418-bp DNA fragment in exon 1 or a 292-bp DNA fragment in exon 6 was identified by PCR (exon 1: forward, 5′ GATTAAGAGATGGTCAGACC, reverse, 5′ CCACTTCTTCATGTCAAATATTTC; exon 6: forward, AATTCAACATGATCAACCAGTG, reverse, GTCTCACCTATCAGGTTTTCC). Founders containing the UGT2B7 gene were bred with Car-null mice (Dr. M. Negishi, National Institute of Environmental Health Sciences, Research Triangle Park, NC), and Tg(UGT2B7)Car(+/−) mice backcrossed to produce Tg(UGT2B7)Car(−/−) mice (genotyping for Car-null mice as described previously) (Ueda et al., 2002). All animals received food and water ad libitum and were housed in constant temperature rooms with a 12-h light/dark cycle. Mouse handling and experimental procedures were conducted in accordance with institutional guidelines.
UGT2B7 Promoter Activity.
A 4-kb UGT2B7 promoter element was cloned by PCR from the BAC DNA containing the UGT2B7 gene (GenBank accession number PR13-644M16) and subcloned into a pGL3 luciferase reporter plasmid. The primers for PCR cloning of the UGT2B7 promoter element were the following: −4 kb (forward KpnI, 5′-ATTTGGTACCCAGTTCTCAGTA; reverse BglII, 5′-atttagatcttcagtctgacac); −2.8 kb (forward KpnI, 5′-atttggtacctttgtgtgtcag; reverse BglII, 5′-aaagaagatcttctatgggta); −1.5 kb (forward KpnI, 5′-taaaggtaccaacagtttcata; reverse BglII, 5′-tgacagatcttgtttctgcag); and −0.4 kb (forward KpnI, 5′-attaggtaccatgtttagtcatt; reverse BglII, atttagatctggtgcaatgcaatg). Using the DNA fragment spanning from −1.0 kb to the translation start site (−1.0 kb, forward KpnI, 5′-atttggtacctaatgattaatgc, reverse XhoI, 5′-attactcgagacatcctggtgcaa), site-directed mutagenesis was carried out, altering two bases (underlined) on the HNF4α core sequences (HNF4α mutant; forward, 5′-tatgtactttgcattataagggtt; reverse, 5′-aacccttataatgcaaagtacata). For transient transfection experiments, HepG2 cells were seeded on 12-well plates 24 h before transfection. Cells were transfected with luciferase plasmids along with either pcDNA (Invitrogen, Carlsbad, CA), HNF4α-pcDNA, or VP-CAR expression vectors (Xie et al., 2003) using Lipofectamine 2000 (Invitrogen) based on the manufacturer's instructions. Cells were harvested with a lysis buffer (Promega, Madison, WI) 48 h after the transfection, and the supernatant was collected by a brief centrifugation. The promoter activities were measured by the expression of firefly luciferase and were normalized to the Renilla reniformis luciferase levels using a dual luciferase reporter assay kit (Promega).
Chromatin Immunoprecipitation.
Chromatin immunoprecipitation (CHIP) analysis was performed using the modified protocol based on the EZ-CHIP kit (Millipore Corp., Billerica, MA). HepG2 cells were transfected either with an HNF4α expression vector (HNF4α-pcDNA) or an HNF4α expression vector along with an activated CAR expression vector, VP-CAR (Xie et al., 2003). HepG2 cells were collected 48 h after the transfections and cross-linked in Dulbecco's modified Eagle's medium (Invitrogen) containing 1% formaldehyde. The procedures for cell lysis and sonication to shear DNA were followed according to the manufacturer's protocol (EZ-CHIP kit). One milliliter of cell extract in CHIP dilution buffer was precleared by incubation with 60 μl of Protein A Agarose/salmon sperm DNA (Millipore) overnight at 4°C. The cleared cellular extract was incubated with anti-HNF4α antibody (Santa Cruz Biotechnology, Santa Cruz, CA) for 2 h at 4°C. After precipitation with Protein A Agarose for 1h at 4°C, the antibody-chromatin complex was then transferred to a spin column (Qiagen, Valencia, CA) for three washes of 400 μl each with each of the following buffers: low-salt immune complex wash buffer, high-salt immune complex wash buffer, LiCl immune complex wash buffer, high-salt LiCl immune complex wash buffer (Okino et al., 2007), and Tris-EDTA buffer. The protein-DNA complexes were eluted in 200 μl of elution buffer and DNA was then reverse cross-linked and released from the complex as indicated in the EZ-CHIP instructions. After the DNA purification with spin columns, the purified DNA was further analyzed by real-time PCR with a pair of primers (HNF4α CHIP: forward, 5′-gtgtgaacagttcatttaccttc; reverse, 5′-ctggtgcaatgcaatgctgt) for the amplification and quantification of the UGT2B7 promoter region containing the HNF4α binding site.
Quantification of UGT2B7 Gene Transcripts by Real-Time PCR.
Total RNA was isolated from tissues using TRIzol (Invitrogen). One microgram of total RNA was used for the generation of cDNA with iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). After the cDNA synthesis, real-time PCRs were conducted to determine Ct values using the MX4000 Multiplex Quantitative PCR (Stratagene, La Jolla, CA). In brief, 1 μl of the cDNA template from the reverse transcription-polymerase chain reaction (RT-PCR) was used in 20 μl of reaction mixture containing 10 μl of 2× MESA GREEN qPCR MasterMix (Eurogentec, San Diego, CA) and 0.4 μM concentration of a pair of primers for the detection of the mRNA of UGT2B7 or internal control gene cyclophilin (qPCR UGT2B7: forward, 5′-gacttttggttcgaaatatttgaca; reverse, 5′-gaggaaactgaaaattccagg; qPCR cyclophilin: forward, 5′-cagacgccactgtcgcttt; reverse, 5′-tgtctttggaactttgtctgcaa). The thermal profile is as follows: 95°C for 10 min, 40 cycles of 95°C for 40 s, 58°C for 40 s, and 72°C for 60 s. After the amplification cycles were completed, the dissociation curve was generated at 95°C for 1 min followed by 41 dissociation cycles starting at 55°C for 30 s for each cycle and increasing by 1°C each cycle. Each sample was performed in triplicate and was quantified based on the formula ΔCt = Ct(UGT2B7) − Ct(cyclophilin).
In vivo studies with TgUGT2B7 and Car-null mice were conducted as follows. Age-matched groups of 8- to 10-week-old animals were used for all experiments. Wild-type, TgUGT2B7, Car(−/−), or Tg(UGT2B7)Car(−/−) (n = 3 or 4) mice were treated intraperitoneally every 24 h for 2 days with DMSO, PCN (10 mg/kg), dexamethasone (15 mg/kg), or TCPOBOP (4 mg/kg). All of the chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in 100 μl of DMSO for each injection. After 48 h, the liver tissues from each treatment group were pulverized in liquid nitrogen and used for preparation of microsomes and total RNA. Microsomal fractions for UGT2B7 catalytic assay were prepared as described previously (Yueh et al., 2003).
Glucuronidation Activity Assay.
UDP-glucuronyltransferase activities were determined using HDCA as substrate by thin-layer chromatography assay according to the method of Bansal and Gessner (1980) with modification. In brief, liver tissues were homogenized in a 5-fold volume of 1.15% ice-cold KCl, and microsomal fractions were prepared in buffer (50 mM Tris-HCl, pH 7.6, and 10 mM MgCl2) as described previously (Yueh et al., 2003). Each UGT assay was in a total volume of 100 μl of reaction mixture containing 50 mM Tris-HCl, pH 7.6, 10 mM MgCl2, 100 μM substrate, 500 μM uridine 5-diphosphoglucuronic acid, 0.04 μCi of UDP[14C]glucuronic acid, 8.5 mM saccharolactone, and 75 μg of microsomal protein. The reactions were performed at 37°C in a shaking water bath for 45 min. At the end of the reaction, 100 μl of ethanol was added, and the cell debris was pelleted by centrifugation. The supernatant was applied to thin-layer chromatography plates, and chromatography was performed in a mixture [35:35:10:20 (v/v)] of n-butanol/acetone/acetic acid/water. The resulting glucuronides were visualized with a PhosphorImager (Molecular Dynamics Storm 820; GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) and were removed and placed in scintillation fluid for quantification with a liquid scintillation counter (Beckman Instruments, Palo Alto, CA).
Reagents.
The BAC DNA containing UGT2B7 gene (PR13-644M16) was from Children's Hospital Oakland Research Institute. TCPOBOP, pregnenolone-16α-carbonitrile (PCN), dexamethasone, and DMSO were from Sigma-Aldrich. Restriction enzymes and T4 DNA ligase for subcloning were from New England Biolabs (Ipswich, MA). The Bradford assay for protein concentration analysis was from Bio-Rad Laboratories. Taq polymerase, the dual-luciferase reporter assay system and reporter plasmids, pGL3-basic vector, pGL3 promoter vector, and pRL-SV40 vector were from Promega. The expression vector for HNF4α (pcDNA-HNF4α) was a kind gift provided by Dr. Barbier at Laval University Hospital Research Center (Quebec, QC, Canada). The construct for the expression vector VP-CAR was described previously (Xie et al., 2003). Thin-layer chromatography plates for the catalytic assay were from Whatman (Clifton, NJ).
Results
Expression of UGT2B7 in Transgenic Mice.
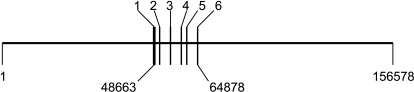
The organization of the UGT2B7 gene in the BAC DNA, consisting of a 5′-promoter region and six exons and introns, is shown in Fig. 1. The BAC clone was purified and microinjected into fertilized CB6F1 mouse eggs, and TgUGT2B7 transgenic mice were produced. The genotype analysis from tail DNA identified founders carrying sequences of exons 1 through 6. Three founders were used for breeding experiments to generate F1 progeny.
Fig. 1.
The gene arrangement of UGT2B7 in the BAC DNA. A 156-kb bacterial artificial chromosome encoding the UGT2B7 gene locus was used to generate the UGT2B7 transgenic mice. This is a representation of the UGT2B7 gene locus, ranging from 48 to 64 kb, in the BAC clone with six black lines denoting six exons.
To determine whether expression of the human gene in liver produced an intact mRNA, highly specific oligonucleotides were used to clone from reverse transcriptase product the full-length UGT2B7 RNA into pcDNA followed by expression in COS-1 cells. Cell lysates prepared from UGT2B7 pcDNA transfected COS-1 cells displayed catalytic activity toward hyodeoxycholic acid (Fig. 2A), a known substrate for UGT2B7. Enhanced levels of HDCA glucuronidation in liver microsomes from TgUGT2B7 mice compared with wild-type mice were also observed (Fig. 2B), confirming that expression of the UGT2B7 gene in transgenic mice produces a functional gene transcript.
Fig. 2.
Determination of UGT activity. A, RNA from TgUGT2B7 mouse liver was isolated, reverse-transcribed to cDNA, subcloned into a pcDNA3 expression vector, and heterologously expressed in COS-1 cells by transient transfections. After preparation of cell lysates, UGT activity was determined using HDCA as a substrate. UGT1A4-specific substrate amitriptyline was used as a negative control substrate. B, UGT activity was determined in liver microsomes prepared from TgUGT2B7 and wild-type mice (n = 4) using HDCA as substrate.
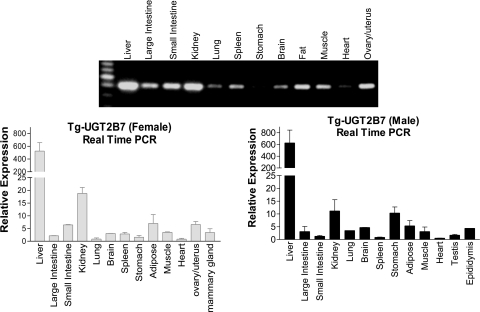
Examination of the constitutive expression pattern of the UGT2B7 gene was conducted by RT-PCR with UGT2B7-specific oligonucleotides to assess gene expression profiles. The oligonucleotides used in these experiments did not amplify gene transcripts from wild-type mouse liver RNA. Total RNA from different tissues was isolated from both male and female TgUGT2B7 mice. The intense UGT2B7 gene transcript was observed in liver and kidney tissues, with liver being the most prominent (Fig. 3). Lower levels of UGT2B7 gene expression products were shown in large and small intestines, adipose tissue, brain, muscle, ovary, and uterus. When we quantitated UGT2B7 gene expression using real-time PCR procedures, the expression levels matched the intensity of the banding patterns observed by RT-PCR. In experiments using human tissues, it has been demonstrated that the UGT2B7 is expressed abundantly in various tissues, including liver, kidney, small intestine, large intestine, mammary gland, and uterus (Turgeon et al., 2001; Izukawa et al., 2009; Ohno and Nakajin, 2009). Overall, the tissue expression profile of the UGT2B7 gene in transgenic mice corresponds well with that of humans, indicating that the TgUGT2B7 mice could be useful as an in vivo model to characterize UGT2B7 gene expression.
Fig. 3.
Tissue distribution of UGT2B7 transcript. Tissues from female and male TgUGT2B7 mice were used to prepare total RNA. The UGT2B7 gene expression levels in various tissues were examined by RT-PCR (female tissues) and real-time PCR using oligonucleotides specific for UGT2B7 gene products and normalized to cyclophilin RNA. The specificity of the PCR product was confirmed by direct sequence.
Regulation of Hepatic UGT2B7 Expression by PXR or CAR Ligands.
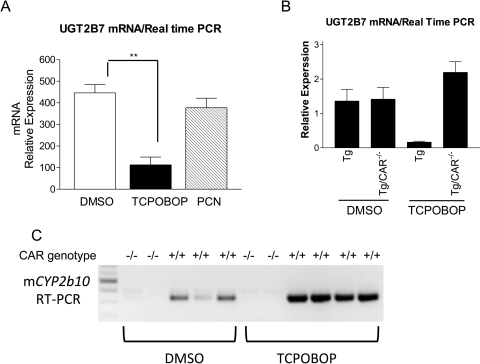
The effect of PXR and CAR activation of the UGT2B7 gene in TgUGT2B7 mice was evaluated after treatment with the PXR ligand PCN (10 mg/kg) or the CAR ligand TCPOBOP (4 mg/kg). After administration by the intraperitoneal route, quantitative RT-PCR analysis to quantitate UGT2B7 gene expression was conducted with RNA prepared from liver. PCN, a prototypical ligand of murine PXR, produced no effect on UGT2B7 gene expression. However, treatment with TCPOBOP, a potent ligand of the mouse CAR, inhibited hepatic UGT2B7 gene expression (Fig. 4A).
Fig. 4.
Inhibition of UGT2B7 expression by CAR ligand TCPOBOP and reversion of UGT2B7 inhibition in Car-null mice. A, age-matched TgUGT2B7 mice were treated with either DMSO, CAR ligand TCPOBOP, or PXR ligand PCN by intraperitoneal injection for 48 h. The liver tissues were used for preparation of total RNA. After the reverse transcription for cDNA synthesis, real-time PCR was conducted to determine the Ct value with cyclophilin as an internal control gene. B, TgUGT2B7, Tg(UGT2B7)Car(−/−), and wild-type mice were treated with DMSO or TCPOBOP by intraperitoneal injection for 48 h. RNA was isolated from the liver tissues, and the levels of UGT2B7 mRNA were measured by real-time PCR. C, the levels of mouse Cyp2b10 mRNA in liver tissues of treated mice were examined by RT-PCR (Cyp2b10 forward, 5′-aaagtcccgtggcaacttcc; reverse, 5′-catcccaaagtctctcatgg).
In efforts to determine whether CAR is tied to the regulation of the UGT2B7 gene, we crossed TgUGT2B7 mice with Car(−/−) mice to generate Tg(UGT2B7)Car(−/−) mice. Wild- type, TgUGT2B7, or Tg(UGT2B7)Car(−/−) mice were treated with either DMSO or TCPOBOP. RNA was prepared from liver tissues and the levels of UGT2B7 gene expression quantitated by quantitative RT-PCR. Compared with TgUGT2B7 mice, the interruption of the Car gene in DMSO-treated Tg(UGT2B7)Car(−/−) mice produced no change in UGT2B7 gene expression (Fig. 4B). TCPOBOP treatment of TgUGT2B7 mice resulted in more than an 80% reduction in gene expression. However, when Tg(UGT2B7)Car(−/−) mice were treated with TCPOBOP, UGT2B7 gene expression remained unchanged and was comparable with that of untreated mice. Cyp2b10 gene expression, a well known TCPOBOP-inducible CAR target gene, were substantially increased by treatment of TCPOBOP in TgUGT2B7 mice but not Tg(UGT2B7)Car(−/−) mice (Fig. 4C). Overall, these studies demonstrate that CAR functions as a negative regulator of the UGT2B7 gene in liver.
HNF4α Is Crucial for Constitutive UGT2B7 Expression in Liver.
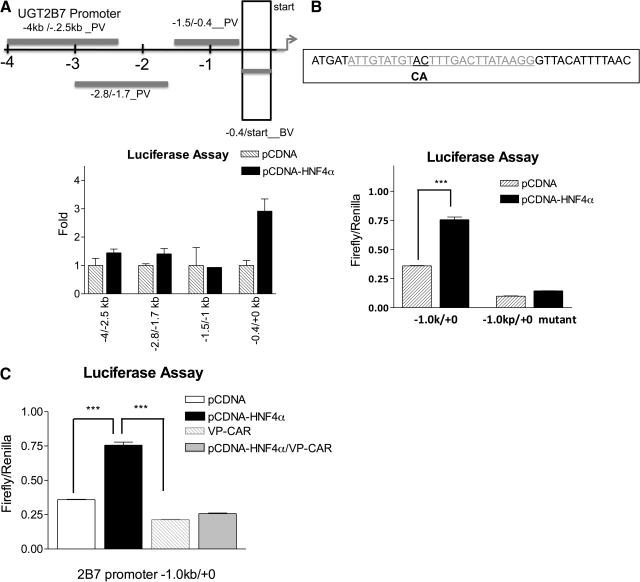
To study the molecular mechanisms that control constitutive expression of UGT2B7 in liver, 4 kb of the UGT2B7 promoter was cloned from the BAC DNA and subsequently subcloned into a luciferase reporter plasmid. HepG2 cells were transfected with the UGT2B7 promoter luciferase plasmids, and high promoter activity was observed in the 400-bp proximal promoter region (−367/+12) adjacent to the transcription start site (Fig. 5). Sequence analysis indicated that there is one consensus DR1 core sequence (TGTACT × TGACTT) for HNF4α binding within this region. When HepG2 cells were cotransfected with both a −0.4-kb UGT2B7 promoter-containing reporter plasmid (−0.4 kb/+ 0) and an HNF4α expression vector, the promoter activity was induced significantly, suggesting the presence of an HNF4α binding site in this region (Fig. 5A). A two-base mutation in the DR1 core sequence blocked HNF4α-mediated transactivation, confirming the involvement of HNF4α in constitutive UGT2B7 promoter activity (Fig. 5B).
Fig. 5.
Transactivation of UGT2B7 promoter by HNF4α and inhibition of HNF4α-mediated transactivation by CAR. A 4-kb portion of the UGT2B7 promoter was cloned, divided into four fragments, and subcloned into the luciferase reporter plasmids, pGL3 basic vector (BV) or promoter vector (PV). HepG2 cells were transiently transfected with UGT2B7 promoter-containing reporter plasmids, and luciferase activity was determined in the cytosolic fraction 48 h after transfections. A, UGT2B7 promoter activities were compared between cotransfection with a pcDNA plasmid or an HNF4α-containing expression vector, and values were normalized to R. reniformis luciferase activity by using a luciferase dual assay kit (Promega) and were shown as fold induction. B, two bases were mutated, from AC to CA, in DR1-like core sequence within the UGT2B7 promoter region (UGT2B7 promoter −1.0 kb/+0) by PCR-directed mutagenesis. The luciferase reporter plasmids containing either wild-type or mutated DR1 were transiently transfected into HepG2 cells. The promoter activities were normalized to R. reniformis luciferase activity and shown as firefly luciferase levels. C, HepG2 cells were transfected with the reporter plasmid containing the UGT2B7 promoter region (UGT2B7 promoter, −1.0 kb/+0) and cotransfected with HNF4α, VP-CAR, or HNF4α plus VP-CAR. Forty-eight hours after transfection, firefly luciferase activity was determined, and values were normalized to R. reniformis luciferase activity.
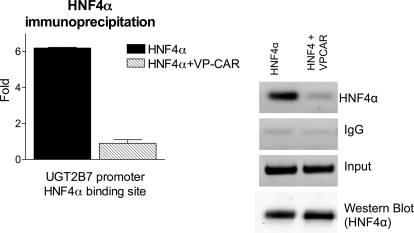
To explore the suppressive effect by CAR activation, HepG2 cells were transfected with an HNF4α expression vector with or without cotransfection of a CAR expression vector (VP-CAR). It is noteworthy that transfection with HNF4α alone increased promoter activity, and cotransfection of VP-CAR produced suppression of promoter activity (Fig. 5C). Similar results were observed when HNF4α transfected HepG2 cells were cotransfected with a CAR expression vector and treated with TCPOBOP for 48 h, indicating that CAR might interact with HNF4α and inhibit HNF4α-directed transactivation. To gain further insight into the possible interaction of CAR and HNF4α in regulating UGT2B7 transcription, HepG2 cells were transfected with an HNF4α expression vector with or without VP-CAR cotransfection followed by CHIP analysis. In CHIP studies using an HNF4α antibody, the precipitation of the DR1 element that contains the HNF4α binding site (−181/+11), quantitated by real-time PCR, was decreased in VP-CAR-cotransfected HepG2 cells (Fig. 6), indicating that the inhibition of HNF4α by CAR requires the inhibition in the binding of HNF4α to the direct repeat one site in the UGT2B7 promoter.
Fig. 6.
Chromatic immunoprecipitation analysis of HNF4α associated with the UGT2B7 5′ flanking region. HepG2 cells were either transfected with an HNF4α expression vector or cotransfected with an activated CAR expression vector (VP-CAR). Transfected HepG2 cells were collected 48 h after the transfections. Cells were fixed and sonicated for the preparation of sheared chromatin, and immunoprecipitations were performed using HNF4α antibody, or nonspecific IgG, as a negative control. After immunoprecipitation, associated DNA was amplified with a pair of primers targeting UGT2B7 gene region −181 to +11, quantitated by real-time PCR, and displayed by gel electrophoresis. Input and Western blot of HNF4α indicate equal amounts of lysates used before immunoprecipitation.
Discussion
Recent studies have indicated that the UGT2B7 gene plays an important role in drug metabolism and steroid homeostasis (Coffman et al., 1998; Barbier et al., 2000; Thibaudeau et al., 2006). The concern of species differences and lack of comprehensive knowledge regarding rodent UGT gene families prompted us to create a transgenic animal model containing a full-length human UGT2B7 gene. The present study delineates the use of this transgenic animal model to study the regulatory properties of the UGT2B7 gene. The expression pattern of UGT2B7 in various organs in TgUGT2B7 mice indicates that humoral and transcription factors meditating UGT2B7 gene expression resemble those patterns found in humans. The observation that liver tissue had the highest expression levels of UGT2B7 suggested that liver-specific factors were required for physiological transcriptional responses. It has been shown that HNF4α plays an important role in regulating hepatic expression of phase II enzymes and transporters in mice (Lu et al., 2010). We provide evidence that HNF4α is the contributing factor responsible for constitutive expression of hepatic UGT2B7. HNF4α regulates UGT2B7 gene expression by binding to a direct repeat motif of the AGGTCA sequence separated by one nucleotide (DR1) in the UGT2B7 5′-flanking promoter region. The HNF4α specificity and requirement for UGT2B7 gene activation was further confirmed by mutation of the DR1 core sequence, which eliminated the binding of HNF4α to the promoter and abolished promoter activity. Similar to our findings, mice lacking hepatic HNF4α had significantly lower gene expression of Ugt2b1 compared with wild-type mice (Lu et al., 2010), indicating that both hepatic expressions of human UGT2B7 and mouse Ugt2b1 are controlled by HNF4α.
It is well documented that XenRs, PXR, and CAR, act as xenobiotic sensors and mediate the induction of numerous xenobiotic-metabolizing enzymes. Induction of glucuronidation by xenobiotic receptors has been demonstrated using a number of clinical drugs and endogenous compounds. For example, CAR is a strong inducer of UGT1A1 (Huang et al., 2003; Xie et al., 2003), which proceeds through binding to a phenobarbital response element flanking the UGT1A1 gene promoter. We were surprised to observe that TCPOBOP treatment and activation of CAR in TgUGT2B7 mice led to a reduction in UGT2B7 gene expression. The specificity of CAR-mediated regulation is supported by findings that PXR-specific ligands, such as PCN and dexamethasone, had no effect on the repression of UGT2B7 transcription in transgenic mice. Combined with evidence that overexpression of CAR produced a decrease in promoter activity of HNF4α transactivation in HepG2 cells, UGT2B7 seems to be a candidate gene for CAR-associated transcriptional inhibition. In addition, the role for HNF4α in CAR-mediated inhibition of UGT2B7 expression was validated because CHIP assays revealed that CAR activation reduced HNF4α bound to the UGT2B7 chromatin. Activation of CAR inhibited HNF4α transactivation of UGT2B7 gene, which suggested that these two regulators are able to cross-talk in the regulation of UGT2B7 expression. Finally, the use of Car-null mice proved that the suppressive effect of TCPOBOP is linked to CAR, which acts as a transcriptional repressor in response to chemical activation by TCPOBOP and blocks HNF4α activation of UGT2B7 gene expression. By inhibiting HNF4α binding, CAR may prevent the changes in chromatin structure and consequent activation of UGT2B7 by HNF4α. In comparison with the antagonism between HNF4α and CAR for UGT2B7 gene regulation, a previous study showed that HNF4α inhibited PXR-mediated transactivation of CYP7A1 gene (Bhalla et al., 2004). The activated PXR did not affect the binding of HNF4α to CYP7A1. Instead, the association of HNF4α with cofactor peroxisome proliferator activating receptor coactivator 1 bound to the promoter was inhibited. HNF4α-dependent transactivation of UGT2B7 gene is mediated through the response element of the HNF4α binding site in the promoter region, and a two-base change in the response element drastically reduces the ability of HNF4α to bind DNA. When acting as a positive regulator, CAR binds to the regulatory region of the target genes. Without a functional binding site in the UGT2B7 promoter region, CAR is able to interact with HNF4α through a yet-to-be identified mechanism that possibly involves contact with other associated transcription factors and cofactors that are specifically associated with the UGT2B7 promoter region. For example, CAR could be inhibitory by competing for binding to common coactivators for HNF4α, such as peroxisome proliferator activating receptor coactivator 1. Thus, the UGT2B7-specific regulation of HNF4α and CAR may largely depend on the promoter context.
Regulation of the UGT2B7 gene at the transcription level is largely unstudied. Using human Caco-2 cells, UGT2B7 suppression by lithocholic acid has been linked to negative regulation by FXR (Lu et al., 2005). Likewise, retinoids (i.e., all trans-retinoic acid and 9-cis retinoic acid) were shown to inhibit UGT2B7 mRNA expression in this intestinal cell line. The fact that both lithocholic acid and retinoids are recognized as activators of CAR (Sakai et al., 2006; Chen et al., 2010) leads us to speculate that UGT2B7 down-regulation in these human intestinal cells might be partially caused by CAR activation. This down-regulation of CAR-dependent UGT2B7 gene expression might have implications in metabolism of therapeutic agents destined for glucuronidation by UGT2B7. Furthermore, CAR activation may lead to changes in the steady-state dynamics of steroids and bile acid homeostasis. A growing body of evidence shows the inhibitory effect of CAR on genes involved in hepatic glucose and lipid metabolism, bile acid biosynthesis (Ueda et al., 2002), such as phosphoenolpyruvate carboxykinase 1, glucose-6-phosphatase, and CYP7A1 activity (Miao et al., 2006). In combination, these studies implicate a diverse function of CAR as a negative regulator of genes associated with drug and xenobiotic, glucose, and lipid metabolism. Compared with DMSO-treated Tg(UGT2B7)Car(−/−) mice, TCPOBOP-treated Tg(UGT2B7)Car(−/−) mice exhibited higher UGT2B7 gene expression (∼150%); this finding is consistent with results from a number of other investigations (Bell and Michalopoulos, 2006; Tamasi et al., 2009) in which an induction of HNF4α by phenobarbital in the absence of CAR was observed. It is possible that TCPOBOP is able to influence HNF4α activity in the absence of CAR, with induced levels of HNF4α contributing to greater UGT2B7 gene expression.
Acknowledgments
We thank Autumn Bonner for assisting in formatting and assembling the manuscript.
This work was supported by the National Institutes of Health National Institute of Environmental Health Sciences [Grant P42-ES010337]; and the National Institutes of Health National Institute of General Medicine [Grant GM086713].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
doi:10.1124/mol.110.070649.
- UGT
- UDP-glucuronosyltransferase
- TCPOBOP
- 1,4-b-s-[2-(3,5,-dichloropyridyloxy)]
- CAR
- constitute androstane receptors
- PXR
- pregnane X receptor
- HNF
- hepatocyte nuclear factor
- HDCA
- hyodeoxycholic acid
- XenR
- xenobiotic receptor
- CHIP
- chromatin immunoprecipitation
- FXR
- farnesoid X receptor
- kb
- kilobase(s)
- BAC
- bacterial artificial chromosome
- bp
- base pair(s)
- PCR
- polymerase chain reaction
- qPCR
- quantitative polymerase chain reaction
- DMSO
- dimethyl sulfoxide
- RT-PCR
- reverse-transcription polymerase chain reaction
- PCN
- pregnenolone-16α-carbonitrile
- Tg
- transgenic.
Authorship Contributions
Participated in research design: Yueh, Mellon, and Tukey.
Conducted experiments: Yueh.
Contributed new reagents or analytic tools: Yueh and Mellon.
Performed data analysis: Yueh.
Wrote or contributed to the writing of the manuscript: Yeuh and Tukey.
References
- Bansal SK, Gessner T. (1980) A unified method for the assay of uridine diphosphoglucuronyltransferase activities toward various aglycones using uridine diphospho[U-14C]glucuronic acid. Anal Biochem 109:321–329 [DOI] [PubMed] [Google Scholar]
- Barbier O, Girard C, Breton R, Bélanger A, Hum DW. (2000) N-glycosylation and residue 96 are involved in the functional properties of UDP-glucuronosyltransferase enzymes. Biochemistry 39:11540–11552 [DOI] [PubMed] [Google Scholar]
- Bell AW, Michalopoulos GK. (2006) Phenobarbital regulates nuclear expression of HNF-4alpha in mouse and rat hepatocytes independent of CAR and PXR. Hepatology 44:186–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla S, Ozalp C, Fang S, Xiang L, Kemper JK. (2004) Ligand-activated pregnane X receptor interferes with HNF-4 signaling by targeting a common coactivator PGC-1alpha. Functional implications in hepatic cholesterol and glucose metabolism. J Biol Chem 279:45139–45147 [DOI] [PubMed] [Google Scholar]
- Chen S, Beaton D, Nguyen N, Senekeo-Effenberger K, Brace-Sinnokrak E, Argikar U, Remmel RP, Trottier J, Barbier O, Ritter JK, et al. (2005) Tissue-specific, inducible, and hormonal control of the human UDP-glucuronosyltransferase-1 (UGT1) locus. J Biol Chem 280:37547–37557 [DOI] [PubMed] [Google Scholar]
- Chen S, Wang K, Wan YJ. (2010) Retinoids activate RXR/CAR-mediated pathway and induce CYP3A. Biochem Pharmacol 79:270–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman BL, King CD, Rios GR, Tephly TR. (1998) The glucuronidation of opioids, other xenobiotics, and androgens by human UGT2B7Y(268) and UGT2B7H(268). Drug Metab Dispos 26:73–77 [PubMed] [Google Scholar]
- Fujiwara R, Nguyen N, Chen S, Tukey RH. (2010) Developmental hyperbilirubinemia and CNS toxicity in mice humanized with the UDP glucuronosyltransferase 1 (UGT1) locus. Proc Natl Acad Sci USA 107:5024–5029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory PA, Lewinsky RH, Gardner-Stephen DA, Mackenzie PI. (2004) Regulation of UDP glucuronosyltransferases in the gastrointestinal tract. Toxicol Appl Pharmacol 199:354–363 [DOI] [PubMed] [Google Scholar]
- Huang W, Zhang J, Chua SS, Qatanani M, Han Y, Granata R, Moore DD. (2003) Induction of bilirubin clearance by the constitutive androstane receptor (CAR). Proc Natl Acad Sci USA 100:4156–4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izukawa T, Nakajima M, Fujiwara R, Yamanaka H, Fukami T, Takamiya M, Aoki Y, Ikushiro S, Sakaki T, Yokoi T. (2009) Quantitative analysis of UDP-glucuronosyltransferase (UGT) 1A and UGT2B expression levels in human livers. Drug Metab Dispos 37:1759–1768 [DOI] [PubMed] [Google Scholar]
- Lu H, Gonzalez FJ, Klaassen C. (2010) Alterations in hepatic mRNA expression of phase II enzymes and xenobiotic transporters after targeted disruption of hepatocyte nuclear factor 4 alpha. Toxicol Sci 118:380–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Bratton S, Heydel JM, Radominska-Pandya A. (2008) Effect of retinoids on UDP-glucuronosyltransferase 2B7 mRNA expression in Caco-2 cells. Drug Metab Pharmacokinet 23:364–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Heydel JM, Li X, Bratton S, Lindblom T, Radominska-Pandya A. (2005) Lithocholic acid decreases expression of UGT2B7 in Caco-2 cells: a potential role for a negative farnesoid X receptor response element. Drug Metab Dispos 33:937–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie PI, Bock KW, Burchell B, Guillemette C, Ikushiro S, Iyanagi T, Miners JO, Owens IS, Nebert DW. (2005) Nomenclature update for the mammalian UDP glycosyltransferase (UGT) gene superfamily. Pharmacogenet Genomics 15:677–685 [DOI] [PubMed] [Google Scholar]
- Miao J, Fang S, Bae Y, Kemper JK. (2006) Functional inhibitory cross-talk between constitutive androstane receptor and hepatic nuclear factor-4 in hepatic lipid/glucose metabolism is mediated by competition for binding to the DR1 motif and to the common coactivators, GRIP-1 and PGC-1alpha. J Biol Chem 281:14537–14546 [DOI] [PubMed] [Google Scholar]
- Monaghan G, Clarke DJ, Povey S, See CG, Boxer M, Burchell B. (1994) Isolation of a human YAC contig encompassing a cluster of UGT2 genes and its regional localization to chromosome 4q13. Genomics 23:496–499 [DOI] [PubMed] [Google Scholar]
- Ohno S, Nakajin S. (2009) Determination of mRNA expression of human UDP-glucuronosyltransferases and application for localization in various human tissues by real-time reverse transcriptase-polymerase chain reaction. Drug Metab Dispos 37:32–40 [DOI] [PubMed] [Google Scholar]
- Okino ST, Quattrochi LC, Pookot D, Iwahashi M, Dahiya R. (2007) A dioxin-responsive enhancer 3′ of the human CYP1A2 gene. Mol Pharmacol 72:1457–1465 [DOI] [PubMed] [Google Scholar]
- Ormrod D, Holm K, Goa K, Spencer C. (1999) Epirubicin: a review of its efficacy as adjuvant therapy and in the treatment of metastatic disease in breast cancer. Drugs Aging 15:389–416 [DOI] [PubMed] [Google Scholar]
- Ritter JK, Chen F, Sheen YY, Lubet RA, Owens IS. (1992) Two human liver cDNAs encode UDP-glucuronosyltransferases with 2 log differences in activity toward parallel substrates including hyodeoxycholic acid and certain estrogen derivatives. Biochemistry 31:3409–3414 [DOI] [PubMed] [Google Scholar]
- Sakai H, Iwata H, Kim EY, Tsydenova O, Miyazaki N, Petrov EA, Batoev VB, Tanabe S. (2006) Constitutive androstane receptor (CAR) as a potential sensing biomarker of persistent organic pollutants (POPs) in aquatic mammal: molecular characterization, expression level, and ligand profiling in Baikal seal (Pusa sibirica). Toxicol Sci 94:57–70 [DOI] [PubMed] [Google Scholar]
- Samokyszyn VM, Gall WE, Zawada G, Freyaldenhoven MA, Chen G, Mackenzie PI, Tephly TR, Radominska-Pandya A. (2000) 4-Hydroxyretinoic acid, a novel substrate for human liver microsomal UDP-glucuronosyltransferase(s) and recombinant UGT2B7. J Biol Chem 275:6908–6914 [DOI] [PubMed] [Google Scholar]
- Senekeo-Effenberger K, Chen S, Brace-Sinnokrak E, Bonzo JA, Yueh MF, Argikar U, Kaeding J, Trottier J, Remmel RP, Ritter JK, et al. (2007) Expression of the human UGT1 locus in transgenic mice by 4-chloro-6-(2,3-xylidino)-2-pyrimidinylthioacetic acid (WY-14643) and implications on drug metabolism through peroxisome proliferator-activated receptor alpha activation. Drug Metab Dispos 35:419–427 [DOI] [PubMed] [Google Scholar]
- Strassburg CP, Manns MP, Tukey RH. (1997a) Differential down regulation of the UDP-glucuronosyltransferase 1A locus is an early event in human liver and biliary cancer. Cancer Res 57:2979–2985 [PubMed] [Google Scholar]
- Strassburg CP, Oldhafer K, Manns MP, Tukey RH. (1997b) Differential expression of the UGT1A locus in human liver, biliary, and gastric tissue: identification of UGT1A7 and UGT1A10 transcripts in extrahepatic tissue. Mol Pharmacol 52:212–220 [DOI] [PubMed] [Google Scholar]
- Tamasi V, Juvan P, Beer M, Rozman D, Meyer UA. (2009) Transcriptional activation of PPARalpha by phenobarbital in the absence of CAR and PXR. Mol Pharm 6:1573–1581 [DOI] [PubMed] [Google Scholar]
- Thibaudeau J, Lépine J, Tojcic J, Duguay Y, Pelletier G, Plante M, Brisson J, Têtu B, Jacob S, Perusse L, et al. (2006) Characterization of common UGT1A8, UGT1A9, and UGT2B7 variants with different capacities to inactivate mutagenic 4-hydroxylated metabolites of estradiol and estrone. Cancer Res 66:125–133 [DOI] [PubMed] [Google Scholar]
- Toide K, Takahashi Y, Yamazaki H, Terauchi Y, Fujii T, Parkinson A, Kamataki T. (2002) Hepatocyte nuclear factor-1alpha is a causal factor responsible for interindividual differences in the expression of UDP-glucuronosyltransferase 2B7 mRNA in human livers. Drug Metab Dispos 30:613–615 [DOI] [PubMed] [Google Scholar]
- Tukey RH, Strassburg CP. (2000) Human UDP-glucuronosyltransferases: metabolism, expression, and disease. Annu Rev Pharmacol Toxicol 40:581–616 [DOI] [PubMed] [Google Scholar]
- Turgeon D, Carrier JS, Lévesque E, Hum DW, Bélanger A. (2001) Relative enzymatic activity, protein stability, and tissue distribution of human steroid-metabolizing UGT2B subfamily members. Endocrinology 142:778–787 [DOI] [PubMed] [Google Scholar]
- Ueda A, Hamadeh HK, Webb HK, Yamamoto Y, Sueyoshi T, Afshari CA, Lehmann JM, Negishi M. (2002) Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol Pharmacol 61:1–6 [DOI] [PubMed] [Google Scholar]
- Verreault M, Senekeo-Effenberger K, Trottier J, Bonzo JA, Bélanger J, Kaeding J, Staels B, Caron P, Tukey RH, Barbier O. (2006) The liver X-receptor alpha controls hepatic expression of the human bile acid-glucuronidating UGT1A3 enzyme in human cells and transgenic mice. Hepatology 44:368–378 [DOI] [PubMed] [Google Scholar]
- Williams JA, Hyland R, Jones BC, Smith DA, Hurst S, Goosen TC, Peterkin V, Koup JR, Ball SE. (2004) Drug-drug interactions for UDP-glucuronosyltransferase substrates: a pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab Dispos 32:1201–1208 [DOI] [PubMed] [Google Scholar]
- Xie W, Yeuh MF, Radominska-Pandya A, Saini SP, Negishi Y, Bottroff BS, Cabrera GY, Tukey RH, Evans RM. (2003) Control of steroid, heme, and carcinogen metabolism by nuclear pregnane X receptor and constitutive androstane receptor. Proc Natl Acad Sci USA 100:4150–4155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueh MF, Huang YH, Hiller A, Chen S, Nguyen N, Tukey RH. (2003) Involvement of the xenobiotic response element (XRE) in Ah receptor-mediated induction of human UDP-glucuronosyltransferase 1A1. J Biol Chem 278:15001–15006 [DOI] [PubMed] [Google Scholar]
- Yueh MF, Tukey RH. (2007) Nrf2-Keap1 signaling pathway regulates human UGT1A1 expression in vitro and in transgenic UGT1 mice. J Biol Chem 282:8749–8758 [DOI] [PubMed] [Google Scholar]