Abstract
Pulmonary morbidity and mortality resulting from exposure to fine particulate matter (PM) increases with age. The present studies analyzed potential mechanisms underlying increased susceptibility of the elderly to PM using diesel exhaust (DE) as a model. Mice (2 m and 18 m) were exposed to DE (0, 300, and 1000 μg/m3) for 3 h once (single) or 3 h/day for 3 days (repeated). Bronchoalveolar lavage fluid (BAL), serum and lung tissue were collected 0 and 24 h later. Exposure to DE resulted in structural alterations in the lungs of older but not younger mice, including patchy thickening of the alveolar septa and inflammatory cell localization in alveolar spaces. These effects were most pronounced 24 h after a single exposure to the higher dose of DE. Significant increases in BAL nitrogen oxides were also noted in older mice, as well as expression of lipocalin 24p3, an oxidative stress marker in the lung with no effects in younger mice. Following DE inhalation, expression of Tumor Necrosis Factor alpha (TNFα) was upregulated in lungs of both younger and older mice; however, this was attenuated in older animals. Whereas exposure to DE resulted in increases in lung Interleukin-6 (IL-6) expression in both older and younger mice, IL-8 increased only in older animals. In younger mice, constitutive expression of manganese superoxide dismutase (MnSOD) decreased after DE exposure, while in older mice, constitutive MnSOD was not detectable and DE had no effect on expression of this antioxidant. Taken together, these results suggest that altered generation of inflammatory mediators and MnSOD may contribute to increased susceptibility of older mice to inhaled DE.
Keywords: Diesel exhaust, Aging, Lipocalin, Nitrogen oxides, Antioxidants
Introduction
Epidemiologic studies have demonstrated strong associations between increases in hourly or daily levels of air pollution and pulmonary morbidity and mortality (Dockery et al., 1992; Samet et al., 2000; USEPA, 2004). Of particular concern as a potential causal agent is ambient fine particulate matter (PM2.5; particles smaller than 2.5 μm in diameter). A significant source of fine PM in the atmosphere is DE which consists of a mixture of compounds in the gas and particle phases (Schlesinger, 2007). DE particles typically contain a carbon core that adsorbs many organic compounds including polycyclic aromatic and nitroaromatic hydrocarbons, heterocyclics, aldehydes, quinones and pyrenes (Schuetzle et al., 1981). These compounds stimulate both macrophages and bronchial epithelial cells to generate reactive oxygen species and proinflammatory cytokines (Hiura et al., 1999; Li et al., 2002). Exposure of humans and experimental animals to DE has been reported to cause airway inflammation, decrease lung function and aggravate asthma and chronic bronchitis (Salvi et al., 1999; Sydbom et al., 2001). Increased morbidity and mortality in individuals with existing cardiopulmonary disease have also been described (www.epa.gov/particles/health.html, 2008).
One of the most sensitive populations to the adverse health effects of inhaled PM is the elderly, typically individuals over 65 years of age. Aging is associated with deterioration of both innate and adaptive immunity (Kumar and Burns, 2008; Suvas, 2008). Most notable changes include age-related deficits in macrophage and neutrophil activity which are thought to underlie increased susceptibility of the elderly to respiratory infections (Liu and Kimura, 2007; Aprahamian et al., 2008; Murciano et al., 2008). Alterations in lung permeability and mechanics have also been described in elderly humans and rodents (Tankersley et al., 2003; Pride, 2005). These changes may also contribute to the increased sensitivity of older individuals to the adverse effects of inhaled pollutants.
As oxidative stress appears to be a common mechanism underlying the biological effects of PM and other air pollutants, a particularly important contributory factor may be age-related alterations in lung antioxidants and intracellular oxidative stress (Kelly et al., 2003). These include reduced levels of superoxide dismutase (SOD), vitamin C and dysregulation of vitamin B12 and folic acid metabolism in the lung (Ischiropoulos et al., 1990; van der Loo et al., 2003; Wolters et al., 2004). Aging is also associated with aberrant generation of reactive oxygen and nitrogen species by macrophages resulting in oxidative/nitrosative stress (Tasat et al., 2003). In the present studies, we compared the effects of inhaled DE in older and younger mice. We speculated that age-related decreases in antioxidant defense and alterations in production of inflammatory mediators contribute to the increased susceptibility of older animals to DE.
Materials and methods
Exposure system
A Yanmar, Model YDG 5500EE, 5.5 Kw, 406 cc, one-cylinder, two cycle, diesel-powered electrical generator was used as a source of diesel emissions. The generator was operated using premium low sulfur (<500 ppm; Petro, Inc.) diesel fuel and 40-weight motor oil (Proline HD40). A fraction of the total DE emissions was directed into the delivery system, where it was aged and diluted with HEPA-filtered air and then isokinetically drawn into the exposure chamber. The DE mass concentration in the delivery system and in the exposure chamber was controlled by adjusting the fraction of total DE directed into the system and the amount of dilution air. The flow of DE through the exposure chamber was maintained at 5 l/min by a pump downstream of the chamber. The engine was operated for 15–20 min before beginning animal exposure sessions to ensure that the aerosol mass concentrations were stable.
Exposure characterization
Particle mass concentrations in the chamber were measured in real-time by a SidePak personal aerosol mass monitor (TSI, Inc., Shoreview, MN), which was calibrated against gravimetric (filter) measurements of the DE. Particle number and volume distributions from 13 nm to 20 μm were determined using a Scanning Mobility Particle Sizer (SMPS) connected to a butanol-based condensation particle counter (CPC 3025A, TSI, Inc.) and an Aerodynamic Particle Sizer (APS) (TSI, Inc.). Data from the SMPS and the APS were combined using Data Merge Software Module (TSI, Inc.). Total particle number for each mass concentration was measured using stand-alone CPC 3025A which detects particles between 3 nm and 3 μm. Levels of co-pollutant gases (e.g., NO/NO2 and CO/CO2) were assessed using an IAQ RAE gas monitor with multi-gas sensors (RAE Systems). Particle number distribution measurements were made inside the delivery system (exhaust pipe from which diesel is drawn into the chamber) and inside the animal exposure chamber for both DE mass concentrations (300 and 1000 μg/m3). Characterization of DE particle size distribution and measurements of co-pollutant gases were performed prior to animal exposures while mass concentration measurements were made both prior to and during the animal exposures.
Animals and exposures
Young (2 m; average weight, 24 g; Harlan, Indianapolis, IN) and old (18 m; average weight, 41 g; National Institute of Aging, Bethesda, MD) CB6F1 male mice were used. Animals were housed in microisolator cages and maintained on sterile food (Picolab Rodent Diet 20, Fisher Scientific, Pittsburgh, PA) and pyrogen-free water ad libitum. Mice were randomly assigned to treatment groups. All animals received humane care in compliance with the institution's guidelines, as outlined in the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health. Animals were exposed to ultra-pure air (Messer Gas, Allentown, PA), 300 or 1000 μg/m3 DE (as PM2.5) in a 17-l whole body plexiglass divided enclosure (16″l×8″w×8″h) for 3 h one time (single) or 3 h per day on 3 consecutive days (repeated).
Sample collection
Animals were euthanized immediately (0 h) or 24 h after exposure. The 0-h time point was selected for evaluation of inflammatory alterations that occur immediately after exposure, while the 24-h time point assessed the persistence of early inflammatory changes and the appearance of later pro-inflammatory and early repair markers. Animals were killed by Nembutal (200 mg/kg, i.p.) and blood was collected by cardiac puncture. Serum samples were centrifuged (1000×g, 10 min, 4 °C), aliquoted and stored at −80 °C. The thoracic cavity was then opened and the trachea and lungs were exposed. The largest lobe was clamped at the bronchus and reserved for histology, immunohistochemistry and RNA analysis (see below). The remaining lobes were lavaged three times through the trachea with 1 ml sterile, pyrogen-free, ice-cold phosphate buffered saline (PBS). The lavage fluid was transferred to tubes containing 5 mM diethylene triamine penta acetic acid (DTPA), centrifuged (300×g, 8 min, 4 °C) and supernatants were analyzed for protein content. Cell pellets were resuspended in 50 μl of PBS. Samples (10 μl) were stained with trypan blue for measurement of cell number and viability using a hemocytometer. Differential cell counts were determined microscopically on cytospin preparations of Giemsa stained cells. For all treatment groups, greater than 98% of the cells were identified as alveolar macrophages and 1–2% as lymphocytes.
For RNA analysis, the largest lobe was removed and stored at −80 °C in tubes containing 200 μl RNALater (Sigma-Aldrich Corp., St. Louis, MO). For histological analysis and immunohistochemistry, the lobe was instilled with 3% cold paraformaldehyde in PBS, removed and placed on ice for 4 h. Samples were then transferred to 50% ethanol.
Measurement of BAL protein and LDH (lactate dehydrogenase) activity
Total protein content was quantified in cell-free BAL using a BCA Protein Assay kit (Pierce Biotechnologies, Inc., Rockford, IL) with bovine serum albumin (BSA) as the standard. Samples (10 μl) were analyzed in triplicate at 540 nm. LDH activity was determined using the CytoTox 96 non-radioactive cytotoxicity assay kit from Promega Corporation (Madison, WI). BAL samples (50 μl) were analyzed in triplicate at 490 nm. Data were normalized to the volume of BAL collected. Samples from five to six animals per treatment group were assayed.
Histology and immunohistochemistry
Histological sections (4 μm) were stained with hematoxylin and eosin. Specimens were analyzed by light microscopy using ProgRes Capture Pro v2.5 software. The extent of inflammatory changes including macrophage and neutrophil localization, alterations in alveolar epithelial barriers and edema were assessed blindly by a board certified veterinary pathologist (S.R.). For immunohistochemistry, tissue sections were deparaffinized, blocked in 100% serum (room temperature, 3–4h) and incubated overnight at 4 °C with rabbit IgG or anti-MnSOD antibody (1:400; Stressgen Biotechnologies, Inc., San Diego, CA.), followed by 30-min incubation with biotinylated secondary antibody (Vector Labs, Burlingame, CA). Binding was visualized using a Peroxidase Substrate Kit DAB (Vector Labs). For both histology and immunohistochemistry, three mice per treatment group were analyzed.
Measurement of serum TNFα
TNFα levels in serum were quantified using a Quantikine immunoassay kit from R&D Systems (Minneapolis, MN). Serum samples were diluted 2-fold and analyzed at 450 and 540 nm in duplicate in 96-well plates. A standard curve was generated using a four parametric logistic (4-PL SoftMax Pro v4 software, R&D Systems) curve fit. Samples from two to four animals per treatment group were assayed.
Measurements of nitrogen oxides (NOx)
NOx levels in BAL samples were analyzed using an Ionics/Sievers Nitric Oxide Analyzer 280 (NOA 280) as previously described (Atochina et al., 2004). Samples were treated with an excess of vanadium chloride in 1 M HCl (0.4 g/50 ml) at 95 °C to reduce nitrate, nitrite and S-nitrosothiols (SNO) and to release nitric oxide. Sodium nitrate (Sigma Aldrich, St. Louis, MO) was used as a standard. Samples from three to six animals per treatment group were assayed in duplicate. Data were normalized to the volume of BAL collected.
Real-time quantitative polymerase chain reaction (RQ-PCR)
Total mRNA was extracted from tissue samples using an RNeasy Mini kit (Qiagen, Valencia, CA). RNA was reverse-transcribed using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. Standard curves were generated using serial dilutions from pooled cDNA samples. Real-time PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) on a 7900HT thermocycler according to the manufacturer's protocol. All PCR primers were generated using Primer Express 3.0 software (Applied Biosystems). Samples from two to three animals per treatment group were analyzed and presented relative to GAPDH mRNA expression. Forward and reverse primer sequences were as follows: 24p3, AGG AAC GTT TCA CCC GCT TT, TGT TGT CGT CCT TGA GGC C; TNFα, AGG GAT GAG AAG TTC CCA AAT G, TGT GAG GGT CTG GCG CAT A; IL-6, CCA CGG CCT TCC CTA CTT C, GTT GGG AGT GGT ATC CTC TGT GA; IL-8: CAG CTG CCT TAA CCC CAT CA, CTT GAG AAG TCC ATG GCG AAA; and GAPDH, TGA AGC AGG CAT CTG AGG G, CGA AGG TGG AAG AGT GGG AG.
Statistical analysis
Data were analyzed in consultation with Rutgers University Department of Statistics using two-way ANOVA or unpaired t-test (unequal variances). A p-value of <0.05 was considered statistically significant. Changes in gene expression >2-fold were considered biologically significant.
Results
Aerosol characterization
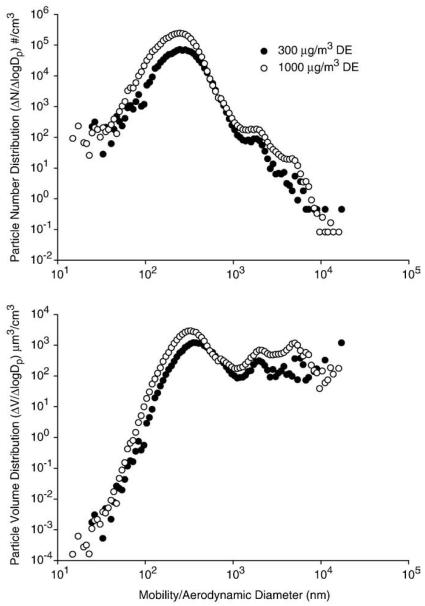
Two different DE mass concentrations (300 and 1000 μg/m3) were used in these studies. Data obtained by the SMPS and APS particle sizers inside the animal chamber revealed that there were no significant differences in the particle number or volume distributions between these two mass concentrations (Table 1 and Fig. 1). The number and mass median diameters were approximately 240 and 450 nm, respectively. The mass size distribution showed a distinct size mode peaking at ~350 nm accounting for most of the particle mass, with an extended tail indicating the presence of larger particles presumably formed through coagulation (Fig. 1). For both particle mass concentrations (300 and 1000 μg/m3), the coefficient of variation over the 3-h exposure period was ≤15%, demonstrating that the aerosol concentration was stable. According to the particle volume distribution data, the median mass diameter was 487 and 411 nm, and the mean mass diameter was approximately 2200 and 1676 nm, for the 300 and the 1000 μg/m3 mass concentrations, respectively (Fig. 1).
TABLE 1.
Characteristics of DE.
| Particle size distribution parameters | DE mass concentration |
|
|---|---|---|
| 300 μg/m3 | 1000 μg/m3 | |
| Number median diameter (nm) | 255±10 | 231±3 |
| Number mean diameter (nm) | 273±10 | 242±2 |
| Number mode diameter (nm) | 246±27 | 246±9 |
| Geometric mean diameter (nm) | 252±9 | 225±2 |
| Geometric standard deviation | 1.48±0.02 | 1.46±0.01 |
| Total particle number concentration per cm−3 | 3.6±0.4×104 | >1×105 |
|
| ||
| Co-pollutant gases, ppm | ||
|
| ||
| COx (CO + CO2) | 1200±116.8 | 2516.6±786.0 |
| NOx (NO + NO2) | 4.32±1.34 | 14.47±2.05 |
Particle size distribution characteristics and co-pollutant gas concentrations inside the exposure chamber were measured at the 300 and 1000 μg/m3 mass concentrations as described in Materials and methods. Each value is the average±SD (n=3).
Fig. 1.
Particle number (upper panel) and particle volume (lower panel) distribution inside the exposure chamber. Measurements (n = 3) were made at 300 and 1000 μg/m3 using an SMPS and a butanol-based CPC and APS. One representative sample at each mass concentration is shown.
Particle number, NOx and COx concentrations were greater for the 1000 μg/m3 exposures, relative to the 300 μg/m3 exposures (Table 1). Thus, the particle number concentration was approximately 3-fold greater at the 1000 μg/m3 mass concentration. As the PM mass concentration increased from 300 to 1000 μg/m3, the concentration of NOx (NO + NO2) increased by a factor of 3.3, from 4.32 to 14.47 ppm (Table 1). Increases in COx (CO + CO2) concentrations were also noted as the PM mass concentration increased from 300 to 1000 μg/m3. This is likely due to the fact that a larger fraction of the primary DE was directed into the delivery system to achieve the desired 1000 μg/m3 mass concentration when compared to the 300 μg/m3 concentration and not to contaminants in the dilution air. In the dilution air, CO levels were <1 ppm, CO2 levels were approximately 350 ppm, and NO/NO2 levels were <0.02 ppm (data not shown).
Effects of DE on lung histology
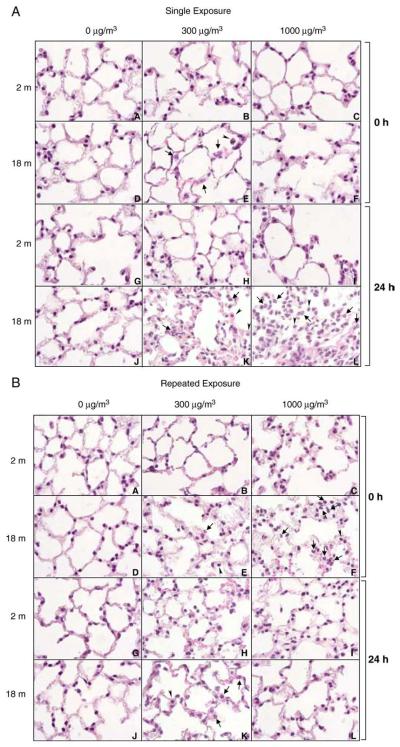
A single exposure of younger mice to DE had no major effects on lung histology (Fig. 2A). In contrast, in older animals, an increase in neutrophils was noted in the lung immediately after low dose (300 μg/m3) exposure, which became more discernable after 24 h. At the higher DE concentration (1000 μg/m3), inflammatory changes were even more pronounced and included greater numbers of neutrophils in the lung and focal inflammatory infiltrates consisting predominantly of plasma cells and macrophages (Fig. 2A). Structural changes were also noted in older but not younger animals, immediately after repeated exposure to DE. At the lower dose, these consisted of patchy thickening of alveolar septa, cytomegaly in alveolar septal walls and an increase in the number of macrophages in alveolar spaces (Fig. 2B). By 24-h post-exposure, focal thickening of alveolar septal walls and increased numbers of neutrophils and erythrocytes were also evident in alveolar walls and capillaries. These effects were more prominent at the higher dose of DE immediately after repeated exposure and, as observed after a single DE exposure, included increased numbers of neutrophils in the lung and focal infiltrates consisting of plasma cells and macrophages.
Fig. 2.
Effects of DE exposure on lung histology. Lung sections (4 μm), prepared from younger (2 m) and older (18 m) mice immediately (0 h) and 24 h after a single (upper panel) or repeated (lower panel) exposure to air (0 μg/m3) or DE (300 or 1000 μg/m3), were stained with H&E. One representative section from each treatment group is shown (magnification,×100). Arrow, neutrophils; arrowhead, alveolar macrophages.
Effects of DE on markers of lung injury and oxidative stress
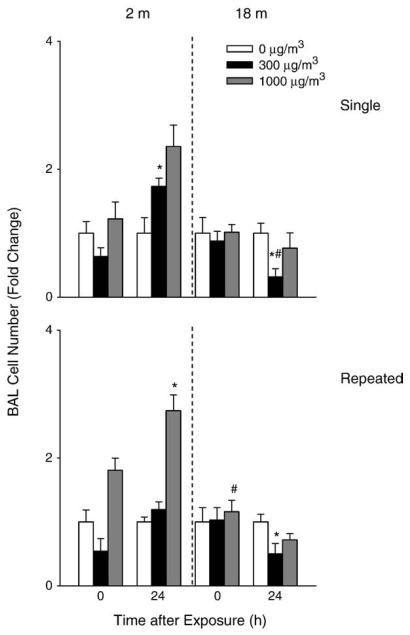
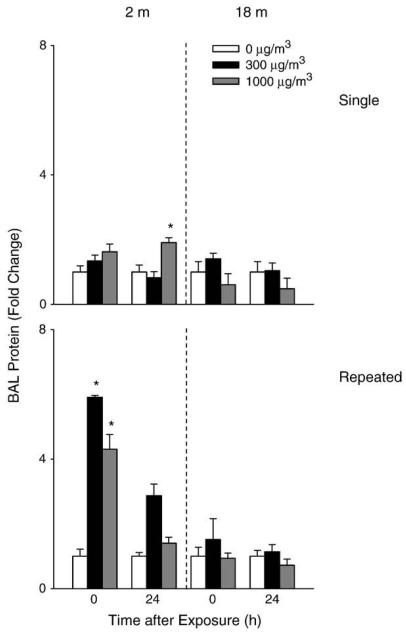
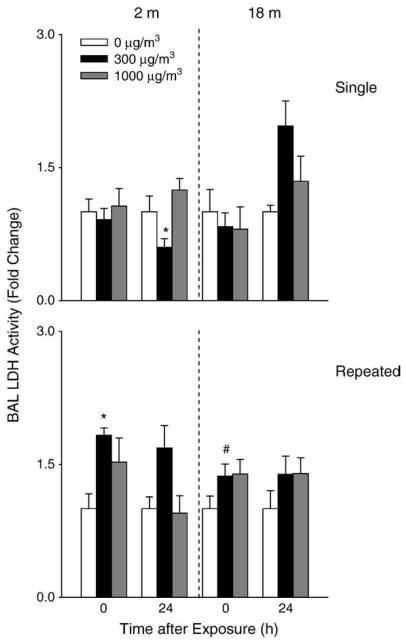
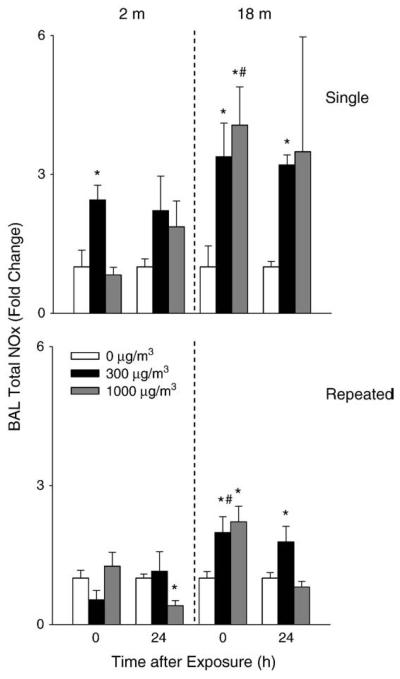
A 2-fold increase in BAL cell number was observed in younger mice 24 h after repeated exposure to high dose DE (Fig. 3). Repeated exposure of younger mice to DE also resulted in a significant increase in BAL protein levels (Fig. 4) as well as LDH activity (Fig. 5). In contrast, in older animals, a small but significant decrease in cell number was observed 24 h after exposure to DE, while no significant changes were noted in BAL protein or LDH activity. In older animals, exposure to DE resulted in an increase in NOx levels in BAL (Fig. 6). Although increases in BAL NOx were also noted in younger animals, this was significant only after a single exposure to the lower DE concentration.
Fig. 3.
Effects of DE on BAL cell number. BAL fluid was collected immediately (0 h) or 24 h after a single (upper panel) or repeated (lower panel) exposure of younger (2 m) and older (18 m) mice to air (0 μg/m3) or DE (300 or 1000 μg/m3). Viable cells in BAL were enumerated by trypan blue dye exclusion. Each bar is the mean±SE. Data are presented as fold change relative to control (0 μg/m3 DE). *Significantly different (p<0.05) from air-exposed mice; #Significantly different (p<0.05) from younger mice.
Fig. 4.
Effects of DE on BAL protein content. BAL fluid was collected immediately (0 h) or 24 hafter a single (upper panel) or repeated (lower panel) exposure of younger (2m) and older (18m) mice to air (0 μg/m3) or DE (300 or 1000 μg/m3) and analyzed in triplicate for total protein content using a BCA Protein Assay kit (Pierce Biotechnologies Inc). Data are presented as fold change relative to control (0 μg/m3 DE). Each bar is the mean±SE. *Significantly different (p<0.05) from air-exposed mice.
Fig. 5.
Effects of DE on BAL LDH activity. BAL fluid was collected immediately (0 h) or 24 h after a single (upper panel) or repeated (lower panel) exposure of younger (2 m) and older (18 m) mice to air (0 μg/m3) or DE (300 or 1000 μg/m3) and assayed in triplicate for LDH activity. Each bar is the mean±SE. Data are presented as fold change relative to control (0 μg/m3 DE). *Significantly different (p<0.05) from air-exposed mice; #Significantly different (p<0.05) from younger mice.
Fig. 6.
Effects of DE on BAL nitrogen oxides. BAL fluid was collected immediately (0 h) or 24 h after a single (upper panel) or repeated (lower panel) exposure of younger (2 m) and older (18 m) mice to air (0 μg/m3) or DE (300 or 1000 μg/m3) and assayed in duplicate for levels of nitrogen oxides. Each bar is the average±SE. Data are presented as fold change relative to control (0 μg/m3 DE). *Significantly different (p<0.05) from air-exposed mice; #Significantly different (p<0.05) from younger mice.
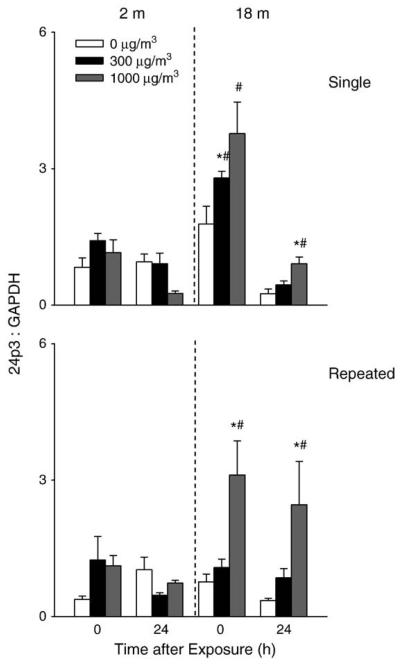
Acute phase proteins such as lipocalin 24p3 are rapidly produced in response to oxidative stress and tissue injury (Roudkenar et al., 2007). 24p3 mRNA expression was upregulated in the lung after both single and repeated exposure of older, but not younger mice to DE (Fig. 7). These effects were dose related and persisted for 24 h.
Fig. 7.
Effects of DE on lipocalin 24p3 mRNA expression. Lung tissue was collected immediately (0 h) or 24 h after a single (upper panel) or repeated (lower panel) exposure of younger (2 m) and older (18 m) mice to air (0 μg/m3) or DE (300 or 1000 μg/m3). RNA was extracted from the tissue and analyzed in triplicate by real-time PCR for lipocalin 24p3 expression. Data are presented relative to GAPDH mRNA expression. Each bar is the mean±SE. *Significantly different (p<0.05) from air-exposed mice; #Significantly different (p<0.05) from younger mice.
Effects of DE on production of inflammatory mediators
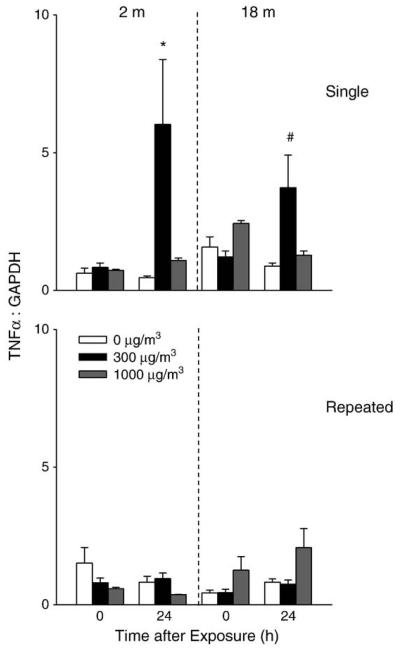
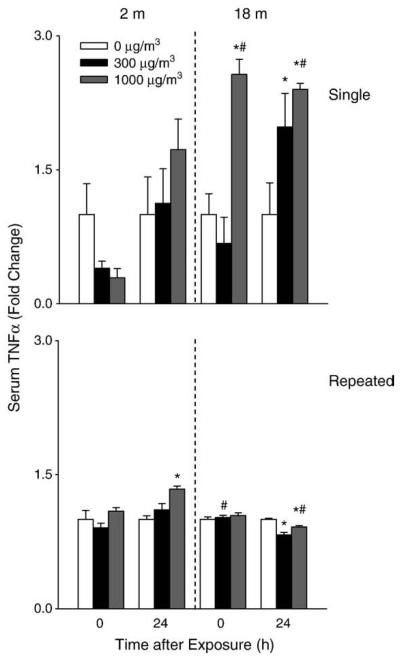
TNFα is a pro-inflammatory cytokine implicated in tissue injury and repair (Lehmann et al., 2005; Gosselin and Rivest, 2007). Exposure of younger mice to a single low dose of DE resulted in a 5-fold increase in lung TNFα which was noted after 24 h (Fig. 8). DE exposure also resulted in increased lung TNFα expression in older mice; however, this response was significantly attenuated relative to younger animals. In contrast, an increase in serum TNFα was observed following a single DE exposure in older mice, which persisted for 24 h, while no effects were noted in younger animals (Fig. 9). In general repeated exposure of the mice to DE had no major effect on TNFα mRNA expression in the lung or on TNFα levels in serum.
Fig. 8.
Effects of DE on TNFα expression. Lung tissue was collected immediately (0 h) or 24 h after a single (upper panel) or repeated (lower panel) exposure of younger (2 m) and older (18 m) mice to air (0 μg/m3) or DE (300 or 1000 μg/m3). RNA was extracted from the tissue and analyzed in triplicate by real-time PCR for TNFα expression. Data are presented relative to GAPDH mRNA expression. Each bar is the mean±SE. *Significantly different (p<0.05) from air-exposed mice; #Significantly different (p<0.05) from younger mice.
Fig. 9.
Effects of DE on serum TNFα. Serum was collected immediately (0 h) or 24 h after a single (upper panel) or repeated (lower panel) exposure of younger (2 m) and older (18 m) mice to air (0 μg/m3) or DE (300 or 1000 μg/m3). TNFα levels were quantified by ELISA. Each bar is the mean±SE. Data are presented as fold change relative to control (0 μg/m3 DE). *Significantly different (p<0.05) from air-exposed mice; #Significantly different (p<0.05) from younger mice.
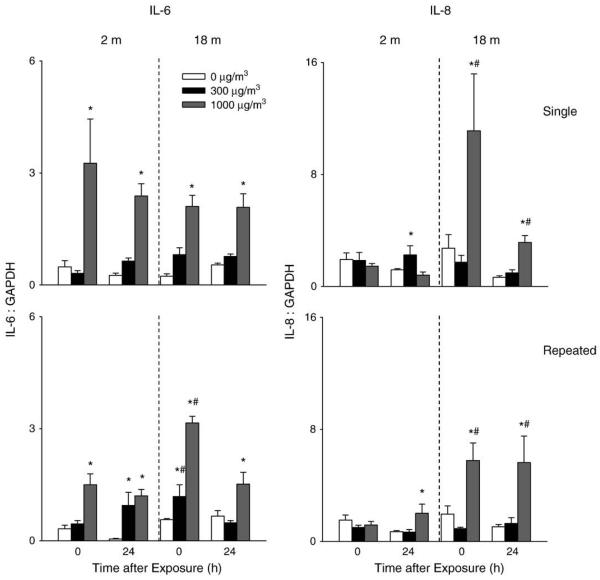
Significant increases in IL-6 and IL-8 mRNA expression were also observed in the lungs of older animals after exposure to DE. The effects of DE were dose related and persisted for 24 h (Fig. 10). Although IL-6 was also upregulated in younger mice, only a small increase in IL-8 mRNA expression was detected in these mice following a single exposure to low dose DE. Generally similar results were observed after repeated exposure to DE.
Fig. 10.
Effects of DE on IL-6 and IL-8 mRNA expression. Lung tissue was collected immediately (0 h) or 24 h after a single (upper panel) or repeated (lower panel) exposure of younger (2 m) and older (18 m) mice to air (0 μg/m3) or DE (300 or 1000 μg/m3). RNA was extracted from the tissue and analyzed in triplicate by real-time PCR for IL-6 and IL-8 expression. Data are presented relative to GAPDH mRNA expression. Each bar is the mean±SE. *Significantly different (p<0.05) from air-exposed mice; #Significantly different (p<0.05) from younger mice.
Effects of DE on MnSOD expression
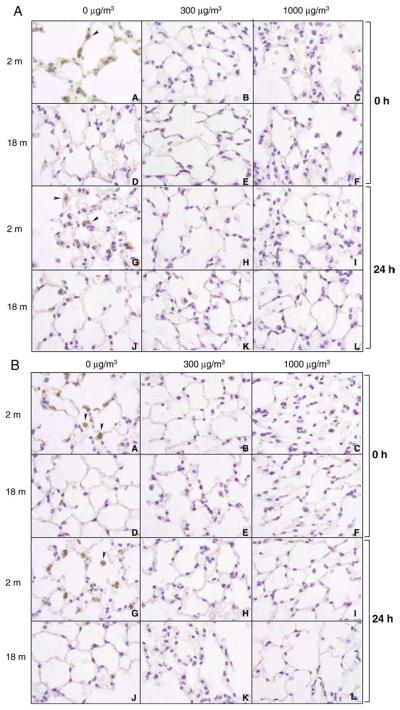
Immunohistochemical analysis of lung sections revealed significant staining for MnSOD in younger but not older mice (Fig. 11A). This was most prominent in alveolar macrophages. Exposure to DE (single or repeated) resulted in a persistent decrease in MnSOD expression in younger animals, while levels remained undetectable in older animals (Figs. 11A and B).
Fig. 11.
Effects of DE exposure on MnSOD expression. Lung sections (4 μm), prepared from younger (2 m) and older (18 m) mice immediately (0 h) and 24 h after a single (upper panel) or repeated (lower panel) exposure to air (0 μg/m3) or DE (300 or 1000 μg/m3), were stained with antibody to MnSOD. One representative section from each treatment group is shown. Magnification, ×100. Arrowhead, alveolar macrophage.
Discussion
The mechanisms underlying increases in the susceptibility of the elderly to the adverse effects of inhaled air pollutants are unknown. Innate immune system functioning declines with advancing age and this may contribute to xenobiotic-induced hyperresponsiveness. Altered oxidant/antioxidant balance may also be a key factor. To evaluate these possibilities, we compared inflammatory mediator and antioxidant expression in lungs of younger and older mice following exposure to inhaled DE.
Exposure of older but not younger mice to DE was associated with rapid and progressive morphological and structural alterations in the lung, which included neutrophil and macrophage accumulation in the tissue, focal cellular infiltrates of plasma cells and macrophages in the interstitum and patchy thickening of alveolar septa. These findings are consistent with previous reports of morphological and structural changes in lungs of older rodents and humans exposed to PM (Tankersley et al., 2003; Sunil et al., 2007a; Tsagareli et al., 2008) and confirm that aging is associated with increased sensitivity to PM-induced lung injury.
An accumulation of protein and inflammatory cells in BAL fluid and an increase in LDH activity have been used as markers of injury to the lower lung (Bhalla, 1999). Despite pronounced structural and histologic evidence of lung pathology in older mice after exposure to DE, no significant changes were noted in BAL protein or LDH activity. This suggests that these measures are not sensitive indicators of lung injury in the elderly. Alternatively, DE-induced injury may not be prominent in the lower lung of older mice. Interestingly, in older mice, BAL cell number was reduced 24 h following single or repeated exposure to DE. This is most likely due to increased adherence of inflammatory cells to alveolar epithelium, which is consistent with our histologic findings. In younger animals, increases in BAL cell number and protein were detected after single or repeated exposure to DE, whereas BAL LDH activity increased only after repeated exposure. However, no major structural alterations were noted in the lung of younger mice. These findings further indicate the limitations in the use of BAL protein and cell number as general markers of acute lung injury.
Nitric oxide possesses complex redox chemistry and is capable of forming a variety of cytotoxic reactive nitrogen species which are thought to contribute to lung pathology and disease (Dweik et al., 2001; Ricciardolo et al., 2006). At higher DE concentrations, biological effects may also be induced by the interaction between particles and co-pollutant gases, which are present at higher levels. The present studies demonstrate significantly greater levels of NOx in BAL from older animals following exposure to DE (single or repeated) relative to younger mice, providing additional evidence that elderly mice are more susceptible to adverse pulmonary effect of inhaled DE. NOx has been implicated in the pathogenesis of acute respiratory distress syndrome and bronchopulmonary dysplasia in animal models of lung injury (Linke et al., 2001; Haczku et al., 2002), and they may play a similar role in the increased responsiveness of elderly mice to inhaled DE.
Lipocalin 24p3 (Lcn2, NGAL) was originally identified as a liver-derived acute phase protein produced in response to hepatic injury (Liu and Nilsen-Hamilton, 1995). More recent studies have shown that 24p3 is upregulated in a number of tissues including the lung and kidney following injury, as well as in diseases such as colorectal and pancreatic cancer and inflammatory bowel disease (Nielsen et al., 1996; Missiaglia et al., 2004; Mishra et al., 2006; Roudkenar et al., 2007; Sunil et al., 2007b). It has also been suggested that 24p3 is important in the response of cells and tissues to oxidative stress (Roudkenar et al., 2007). We found that exposure of older, but not younger mice to DE, was associated with a rapid induction in 24p3 mRNA expression in the lung. cDNA microarray studies revealed a similar upregulation of 24p3 after exposure of mice to the combination of DE and lipopolysaccharide (Yanagisawa et al., 2004). Our findings that in older mice 24p3 is upregulated after exposure to DE alone suggest that this may be a highly sensitive biomarker of early oxidative stress in the elderly.
TNFα is a macrophage-derived cytokine implicated in the pathogenesis of lung injury induced by a number of air pollutants (Kelley, 1990; Schins and Borm, 1999; Fakhrzadeh et al., 2004; Gowdy et al., 2008). Recent studies suggest that TNFα plays a dual role in the pathogenic response, initially promoting inflammation and cytotoxicity and subsequently initiating tissue repair (Lehmann et al., 2005; Gosselin and Rivest, 2007). This latter activity is thought to be due to TNFα-induced upregulation of antioxidants, stimulation of epithelial cell proliferation and extracellular matrix turnover (Sasaki et al., 2000; Ryter et al., 2002; Chiu et al., 2003). In accord with previous studies (Saber et al., 2006; Gowdy et al., 2008), we observed a significant increase in expression of TNFα in the lung 24 h after a single exposure of younger mice to DE. Interestingly, this response was most notable at the lower DE dose. It may be that higher doses of DE cause suppression of inflammatory responses in the lung of younger mice (Yin et al., 2002). Although TNFα expression was also increased in lungs of older animals 24 h following a single exposure to DE, this response was attenuated relative to younger mice. A similar attenuation of TNFα production in lung or BAL fluid has previously been described in older animals after induction of endotoxin shock and after exposure to silica or terpene oxidation products (Corsini et al., 2004; Ito et al., 2007; Sunil et al., 2007a). Age-related decreases in production of TNFα have also been described in isolated monocytes and macrophages (Lloberas and Celada, 2002; Boehmer et al., 2004). After a single DE exposure, serum TNFα levels were increased significantly, but only in older mice. The origin of TNFα in serum is unknown and may reflect its rapid release in the lung following DE exposure. Alternatively, TNFα may be released by extra-pulmonary tissues responding to DE-induced oxidative stress and this remains to be determined.
IL-6 and IL-8 are known to be important in regulating inflammatory cell trafficking into injured tissues (Murtaugh et al., 1996; McClintock et al., 2008). Previous studies have reported DE-induced increases in IL-6 and IL-8 in cultured bronchial epithelial cells (Steerenberg et al., 1998). Similarly, we noted significant increases in IL-6 and IL-8 mRNA expression in lungs of younger and older animals after exposure to DE (single or repeated). Whereas the kinetics of IL-6 mRNA expression appeared to be independent of age, IL-8 expression increased in older animals immediately after DE exposure. These findings are consistent with reports of increased production of IL-8 and IL-8-like cytokines (e.g., MIP-2) with advancing age in rodents and humans (Himi et al., 1997; Pulsatelli et al., 2000). IL-8 is a potent chemoattractant for neutrophils (Matsuzaki et al., 2006). Our findings of increased expression of IL-8 in older mice following exposure to DE are consistent with our histologic data of increased accumulation of neutrophils in the lungs and with increased levels of NOx in BAL of these animals.
Antioxidants play a critical role in host defense by scavenging and detoxifying oxidants (Heffner and Repine, 1991; Lang et al., 2002). With increasing age, constitutive levels of tissue antioxidants and the capacity to respond to oxidative stress decline (Lykkesfeldt and Ames, 1999; Squier, 2001; Servais et al., 2005). Thus, while expression of antioxidant enzymes, such as SOD, rapidly decreases in younger animals as it is utilized following exposure to PM, in older mice this response is reduced or absent (Sagai et al., 1993; Sunil et al., 2007a). We found that MnSOD was constitutively expressed in lungs of younger animals, predominantly in alveolar macrophages. Moreover, exposure of younger animals to DE resulted in a rapid reduction in MnSOD expression. In contrast, constitutive expression of MnSOD was not evident in lungs of older mice and DE exposure did not alter its expression. It has been suggested that oxidative stress is a key mechanism underlying the adverse health effects of ambient PM (Li et al., 2004). A lack of constitutive MnSOD in the lung of older mice may contribute to their increased susceptibility to lung injury induced by inhaled DE.
In summary, the present studies demonstrate that single or repeated inhalation of DE results in significant structural and inflammatory changes in the lungs of older but not younger mice. However, no consistent differences were noted between the two exposure protocols, suggesting that the impact of diesel is rapid. Alternatively, more prolonged exposures may be required for differences in the effects of single and repeated DE exposures to be observed. It should be noted that the exposure doses used in our studies are relevant to human exposure in occupational and environmental settings (USEPA, 2002). Occupational exposures to DE can exceed 1000 μg/m3, and levels of PM in the world's largest cities have been shown to exceed 300 μg/m3 (WHO, 1992; USEPA, 2002). The data presented in our studies showing altered production of inflammatory mediators and reduced MnSOD in lungs of older mice suggest a potential mechanism for the increased susceptibility of the elderly to inhaled PM. However, at present we cannot exclude the possibility that some of these biological effects are due to different levels of NOx and COx in the DE. Identification of key inflammatory mediators involved in the response of older mice to DE may help in the design of new and effective approaches to mitigating pulmonary pathology in the geriatric population.
Acknowledgments
The authors acknowledge the help of Dr. Jianzhong Song, Ph.D. (Visiting Scholar, Department of Environmental Science, Rutgers University). This work was supported by grants from the Health Effects Institute, the American Lung Association of New Jersey, the USEPA and NIH Grants ES004738, ES013520, ES005022, GM034310, CA132624 and AR055073.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- Aprahamian T, Takemura Y, Goukassian D, Walsh K. Ageing is associated with diminished apoptotic cell clearance in vivo. Clin. Exp. Immunol. 2008;152:448–455. doi: 10.1111/j.1365-2249.2008.03658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atochina EN, Beck JM, Preston AM, Haczku A, Tomer Y, Scanlon ST, Fusaro T, Casey J, Hawgood S, Gow AJ, Beers MF. Enhanced lung injury and delayed clearance of Pneumocystis carinii in surfactant protein A-deficient mice: attenuation of cytokine responses and reactive oxygen-nitrogen species. Infect. Immun. 2004;72:6002–6011. doi: 10.1128/IAI.72.10.6002-6011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla DK. Ozone-induced lung inflammation and mucosal barrier disruption: toxicology, mechanisms, and implications. J. Toxicol. Environ. Health B Crit. Rev. 1999;2:31–86. doi: 10.1080/109374099281232. [DOI] [PubMed] [Google Scholar]
- Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J. Leukoc. Biol. 2004;75:342–349. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- Chiu H, Gardner CR, Dambach DM, Durham SK, Brittingham JA, Laskin JD, Laskin DL. Role of tumor necrosis factor receptor 1 (p55) in hepatocyte proliferation during acetaminophen-induced toxicity in mice. Toxicol. Appl. Pharmacol. 2003;193:218–227. doi: 10.1016/j.taap.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Corsini E, Giani A, Peano S, Marinovich M, Galli CL. Resistance to silica-induced lung fibrosis in senescent rats: role of alveolar macrophages and tumor necrosis factor-alpha (TNF) Mech. Ageing Dev. 2004;125:145–146. doi: 10.1016/j.mad.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Dockery DW, Schwartz J, Spengler JD. Air pollution and daily mortality: associations with particulates and acid aerosols. Environ. Res. 1992;59:362–373. doi: 10.1016/s0013-9351(05)80042-8. [DOI] [PubMed] [Google Scholar]
- Dweik RA, Comhair SA, Gaston B, Thunnissen FB, Farver C, Thomassen MJ, Kavuru M, Hammel J, Abu-Soud HM, Erzurum SC. NO chemical events in the human airway during the immediate and late antigen-induced asthmatic response. Proc. Natl. Acad. Sci. U. S. A. 2001;98:2622–2627. doi: 10.1073/pnas.051629498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhrzadeh L, Laskin JD, Gardner CR, Laskin DL. Superoxide dismutaseoverexpressing mice are resistant to ozone-induced tissue injury and increases in nitric oxide and tumor necrosis factor-alpha. Am. J. Respir. Cell Mol. Biol. 2004;30:280–287. doi: 10.1165/rcmb.2003-0044OC. [DOI] [PubMed] [Google Scholar]
- Gosselin D, Rivest S. Role of IL-1 and TNF in the brain: twenty years of progress on a Dr. Jekyll/Mr. Hyde duality of the innate immune system. Brain Behav. Immun. 2007;21:281–289. doi: 10.1016/j.bbi.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Gowdy K, Krantz QT, Daniels M, Linak WP, Jaspers I, Gilmour MI. Modulation of pulmonary inflammatory responses and antimicrobial defenses in mice exposed to diesel exhaust. Toxicol. Appl. Pharmacol. 2008;229:310–319. doi: 10.1016/j.taap.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Haczku A, Atochina EN, Tomer Y, Cao Y, Campbell C, Scanlon ST, Russo SJ, Enhorning G, Beers MF. The late asthmatic response is linked with increased surface tension and reduced surfactant protein B in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;283:L755–765. doi: 10.1152/ajplung.00062.2002. [DOI] [PubMed] [Google Scholar]
- Heffner JE, Repine JE. Antioxidants in the lung. In: Crystal RG, West JB, editors. The Lung. Raven Press; New York: 1991. pp. 1811–1820. [Google Scholar]
- Himi T, Yoshioka I, Kataura A. Influence of age on the production of interleukin-8-like chemokine (GRO/CINC-1) in rat nasal mucosa. Eur. Arch. Otorhinolaryngol. 1997;254:101–104. doi: 10.1007/BF01526189. [DOI] [PubMed] [Google Scholar]
- Hiura TS, Kaszubowski MP, Li N, Nel AE. Chemicals in diesel exhaust particles generate reactive oxygen radicals and induce apoptosis in macrophages. J. Immunol. 1999;163:5582–5591. [PubMed] [Google Scholar]
- Ischiropoulos H, Nadziejko CE, Kikkawa Y. Effect of aging on pulmonary superoxide dismutase. Mech. Ageing Dev. 1990;52:11–26. doi: 10.1016/0047-6374(90)90141-2. [DOI] [PubMed] [Google Scholar]
- Ito Y, Betsuyaku T, Nasuhara Y, Nishimura M. Lipopolysaccharide-induced neutrophilic inflammation in the lungs differs with age. Exp. Lung Res. 2007;33:375–384. doi: 10.1080/01902140701634843. [DOI] [PubMed] [Google Scholar]
- Kelley J. Cytokines of the lung. Am. Rev. Respir. Dis. 1990;141:765–788. doi: 10.1164/ajrccm/141.3.765. [DOI] [PubMed] [Google Scholar]
- Kelly FJ, Dunster C, Mudway I. Air pollution and the elderly: oxidant/antioxidant issues worth consideration. Eur. Respir. J. Suppl. 2003;40:70s–75s. doi: 10.1183/09031936.03.00402903. [DOI] [PubMed] [Google Scholar]
- Kumar R, Burns EA. Age-related decline in immunity: implications for vaccine responsiveness. Expert Rev. Vaccines. 2008;7:467–479. doi: 10.1586/14760584.7.4.467. [DOI] [PubMed] [Google Scholar]
- Lang JD, McArdle PJ, O'Reilly PJ, Matalon S. Oxidant-antioxidant balance in acute lung injury. Chest. 2002;122:314S–320S. doi: 10.1378/chest.122.6_suppl.314s. [DOI] [PubMed] [Google Scholar]
- Lehmann W, Edgar CM, Wang K, Cho TJ, Barnes GL, Kakar S, Graves DT, Rueger JM, Gerstenfeld LC, Einhorn TA. Tumor necrosis factor alpha (TNF-alpha) coordinately regulates the expression of specific matrix metalloproteinases (MMPS) and angiogenic factors during fracture healing. Bone. 2005;36:300–310. doi: 10.1016/j.bone.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Li N, Wang M, Oberley TD, Sempf JM, Nel AE. Comparison of the prooxidative and proinflammatory effects of organic diesel exhaust particle chemicals in bronchial epithelial cells and macrophages. J. Immunol. 2002;169:4531–4541. doi: 10.4049/jimmunol.169.8.4531. [DOI] [PubMed] [Google Scholar]
- Li N, Alam J, Venkatesan MI, Eiguren-Fernandez A, Schmitz D, Di Stefano E, Slaughter N, Killeen E, Wang X, Huang A, Wang M, Miguel AH, Cho A, Sioutas C, Nel AE. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: protecting against the proinflamma-tory and oxidizing effects of diesel exhaust chemicals. J. Immunol. 2004;173:3467–3481. doi: 10.4049/jimmunol.173.5.3467. [DOI] [PubMed] [Google Scholar]
- Linke MJ, Harris CE, Korfhagen TR, McCormack FX, Ashbaugh AD, Steele P, Whitsett JA, Walzer PD. Immunosuppressed surfactant protein A-deficient mice have increased susceptibility to Pneumocystis carinii infection. J. Infect. Dis. 2001;183:943–952. doi: 10.1086/319252. [DOI] [PubMed] [Google Scholar]
- Liu B, Kimura Y. Local immune response to respiratory syncytial virus infection is diminished in senescence-accelerated mice. J. Gen. Virol. 2007;88:2552–2558. doi: 10.1099/vir.0.83089-0. [DOI] [PubMed] [Google Scholar]
- Liu Q, Nilsen-Hamilton M. Identification of a new acute phase protein. J. Biol. Chem. 1995;270:22565–22570. doi: 10.1074/jbc.270.38.22565. [DOI] [PubMed] [Google Scholar]
- Lloberas J, Celada A. Effect of aging on macrophage function. Exp. Gerontol. 2002;37:1325–1331. doi: 10.1016/s0531-5565(02)00125-0. [DOI] [PubMed] [Google Scholar]
- Lykkesfeldt J, Ames BN. Ascorbic acid recycling in rat hepatocytes as measurement of antioxidant capacity: decline with age. Methods Enzymol. 1999;299:83–88. doi: 10.1016/s0076-6879(99)99011-0. [DOI] [PubMed] [Google Scholar]
- Matsuzaki T, Amakawa K, Yamaguchi K, Ishizaka A, Terashima T, Matsumaru A, Morishita T. Effects of diesel exhaust particles on human neutrophil activation. Exp. Lung Res. 2006;32:427–439. doi: 10.1080/01902140601047641. [DOI] [PubMed] [Google Scholar]
- McClintock D, Zhuo H, Wickersham N, Matthay MA, Ware LB. Biomarkers of inflammation, coagulation and fibrinolysis predict mortality in acute lung injury. Crit. Care. 2008;12:R41. doi: 10.1186/cc6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra J, Ma Q, Kelly C, Mitsnefes M, Mori K, Barasch J, Devarajan P. Kidney NGAL is a novel early marker of acute injury following transplantation. Pediatr. Nephrol. 2006;21:856–863. doi: 10.1007/s00467-006-0055-0. [DOI] [PubMed] [Google Scholar]
- Missiaglia E, Blaveri E, Terris B, Wang YH, Costello E, Neoptolemos JP, Crnogorac-Jurcevic T, Lemoine NR. Analysis of gene expression in cancer cell lines identifies candidate markers for pancreatic tumorigenesis and metastasis. Int. J. Cancer. 2004;112:100–112. doi: 10.1002/ijc.20376. [DOI] [PubMed] [Google Scholar]
- Murciano C, Yanez A, O'Connor JE, Gozalbo D, Gil ML. Influence of aging on murine neutrophil and macrophage function against Candida albicans. FEMS Immunol. Med. Microbiol. 2008;53:214–221. doi: 10.1111/j.1574-695X.2008.00418.x. [DOI] [PubMed] [Google Scholar]
- Murtaugh MP, Baarsch MJ, Zhou Y, Scamurra RW, Lin G. Inflammatory cytokines in animal health and disease. Vet. Immunol. Immunopathol. 1996;54:45–55. doi: 10.1016/s0165-2427(96)05698-x. [DOI] [PubMed] [Google Scholar]
- Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut. 1996;38:414–420. doi: 10.1136/gut.38.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pride NB. Ageing and changes in lung mechanics. Eur. Respir. J. 2005;26:563–565. doi: 10.1183/09031936.05.00079805. [DOI] [PubMed] [Google Scholar]
- Pulsatelli L, Meliconi R, Mazzetti I, Dolzani P, Meneghetti A, Neri S, Silvestri T, Ravaglia G, Forti P, Facchini A, Mariani E. Chemokine production by peripheral blood mononuclear cells in elderly subjects. Mech. Ageing Dev. 2000;121:89–100. doi: 10.1016/s0047-6374(00)00200-1. [DOI] [PubMed] [Google Scholar]
- Ricciardolo FL, Di Stefano A, Sabatini F, Folkerts G. Reactive nitrogen species in the respiratory tract. Eur. J. Pharmacol. 2006;533:240–252. doi: 10.1016/j.ejphar.2005.12.057. [DOI] [PubMed] [Google Scholar]
- Roudkenar MH, Kuwahara Y, Baba T, Roushandeh AM, Ebishima S, Abe S, Ohkubo Y, Fukumoto M. Oxidative stress induced lipocalin 2 gene expression: addressing its expression under the harmful conditions. J. Radiat. Res. (Tokyo) 2007;48:39–44. doi: 10.1269/jrr.06057. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Xi S, Hartsfield CL, Choi AM. Mitogen activated protein kinase (MAPK) pathway regulates heme oxygenase-1 gene expression by hypoxia in vascular cells. Antioxid. Redox Signal. 2002;4:587–592. doi: 10.1089/15230860260220085. [DOI] [PubMed] [Google Scholar]
- Saber A, Jacobson N, Bornholdt J, Kjaer S, Dybdahl M, Risom L, Loft S, Vogel U, Wallin H. Cytokine expression in mice exposed to diesel exhaust particles by inhalation: role of tumor necrosis factor. Part. Fibre Toxicol. 2006;3:4. doi: 10.1186/1743-8977-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagai M, Saito H, Ichinose T, Kodama M, Mori Y. Biological effects of diesel exhaust particles. I. In vitro production of superoxide and in vivo toxicity in mouse. Free Radic. Biol. Med. 1993;14:37–47. doi: 10.1016/0891-5849(93)90507-q. [DOI] [PubMed] [Google Scholar]
- Salvi S, Blomberg A, Rudell B, Kelly F, Sandstrom T, Holgate ST, Frew A. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am. J. Respir. Crit. Care Med. 1999;159:702–709. doi: 10.1164/ajrccm.159.3.9709083. [DOI] [PubMed] [Google Scholar]
- Samet JM, Zeger SL, Dominici F, Curriero F, Coursac I, Dockery DW, Schwartz J, Zanobetti A. The National Morbidity, Mortality, and Air Pollution Study. Part II. Morbidity and mortality from air pollution in the United States. Res. Rep. Health Eff. Inst. 2000;94:5–70. discussion 71–79. [PubMed] [Google Scholar]
- Sasaki M, Kashima M, Ito T, Watanabe A, Izumiyama N, Sano M, Kagaya M, Shioya T, Miura M. Differential regulation of metalloproteinase production, proliferation and chemotaxis of human lung fibroblasts by PDGF, interleukin-1 beta and TNF-alpha. Mediat. Inflamm. 2000;9:155–160. doi: 10.1080/09629350020002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schins RP, Borm PJ. Mechanisms and mediators in coal dust induced toxicity: a review. Ann. Occup. Hyg. 1999;43:7–33. doi: 10.1016/s0003-4878(98)00069-6. [DOI] [PubMed] [Google Scholar]
- Schlesinger RB. The health impact of common inorganic components of fine particulate matter (PM2.5) in ambient air: a critical review. Inhal. Toxicol. 2007;19:811–832. doi: 10.1080/08958370701402382. [DOI] [PubMed] [Google Scholar]
- Schuetzle D, Lee FS, Prater TJ. The identification of polynuclear aromatic hydrocarbon (PAH) derivatives in mutagenic fractions of diesel particulate extracts. Int. J. Environ. Anal. Chem. 1981;9:93–144. doi: 10.1080/03067318108071903. [DOI] [PubMed] [Google Scholar]
- Servais S, Boussouar A, Molnar A, Douki T, Pequignot JM, Favier R. Age-related sensitivity to lung oxidative stress during ozone exposure. Free Radic. Res. 2005;39:305–316. doi: 10.1080/10715760400011098. [DOI] [PubMed] [Google Scholar]
- Squier TC. Oxidative stress and protein aggregation during biological aging. Exp. Gerontol. 2001;36:1539–1550. doi: 10.1016/s0531-5565(01)00139-5. [DOI] [PubMed] [Google Scholar]
- Steerenberg PA, Zonnenberg JA, Dormans JA, Joon PN, Wouters IM, van Bree L, Scheepers PT, Van Loveren H. Diesel exhaust particles induced release of interleukin 6 and 8 by (primed) human bronchial epithelial cells (BEAS 2B) in vitro. Exp. Lung Res. 1998;24:85–100. doi: 10.3109/01902149809046056. [DOI] [PubMed] [Google Scholar]
- Sunil VR, Laumbach RJ, Patel KJ, Turpin BJ, Lim HJ, Kipen HM, Laskin JD, Laskin DL. Pulmonary effects of inhaled limonene ozone reaction products in elderly rats. Toxicol. Appl. Pharmacol. 2007a;222:211–220. doi: 10.1016/j.taap.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunil VR, Patel KJ, Nilsen-Hamilton M, Heck DE, Laskin JD, Laskin DL. Acute endotoxemia is associated with upregulation of lipocalin 24p3/Lcn2 in lung and liver. Exp. Mol. Pathol. 2007b;83:177–187. doi: 10.1016/j.yexmp.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvas S. Advancing age and immune cell dysfunction: is it reversible or not? Expert Opin. Biol. Ther. 2008;8:657–668. doi: 10.1517/14712598.8.5.657. [DOI] [PubMed] [Google Scholar]
- Sydbom A, Blomberg A, Parnia S, Stenfors N, Sandstrom T, Dahlen SE. Health effects of diesel exhaust emissions. Eur. Respir. J. 2001;17:733–746. doi: 10.1183/09031936.01.17407330. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Shank JA, Flanders SE, Soutiere SE, Rabold R, Mitzner W, Wagner EM. Changes in lung permeability and lung mechanics accompany homeostatic instability in senescent mice. J. Appl. Physiol. 2003;95:1681–1687. doi: 10.1152/japplphysiol.00190.2003. [DOI] [PubMed] [Google Scholar]
- Tasat DR, Mancuso R, O'Connor S, Molinari B. Age-dependent change in reactive oxygen species and nitric oxide generation by rat alveolar macrophages. Aging Cell. 2003;2:159–164. doi: 10.1046/j.1474-9728.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Tsagareli ZG, Gogiashvili LE, Topuria ZM, Dzhandieri KN. Morpho-functional estimation of blood-air barrier and lung surfactant in rats of different age. Georgian Med. News. 2008:47–52. [PubMed] [Google Scholar]
- USEPA . Health assessment document for diesel engine exhaust. U.S. Environmental Protection Agency; Washington, DC: 2002. [Google Scholar]
- USEPA . Air quality criteria for particulate matter. U. S. Environmental Protection Agency; Research Triangle Park, NC: 2004. [Google Scholar]
- van der Loo B, Bachschmid M, Spitzer V, Brey L, Ullrich V, Luscher TF. Decreased plasma and tissue levels of vitamin C in a rat model of aging: implications for antioxidative defense. Biochem. Biophys. Res. Commun. 2003;303:483–487. doi: 10.1016/s0006-291x(03)00360-7. [DOI] [PubMed] [Google Scholar]
- Wolters M, Strohle A, Hahn A. Age-associated changes in the metabolism of vitamin B(12) and folic acid: prevalence, aetiopathogenesis and pathophysiological consequences. Z. Gerontol. Geriatr. 2004;37:109–135. doi: 10.1007/s00391-004-0169-6. [DOI] [PubMed] [Google Scholar]
- Yanagisawa R, Takano H, Inoue K, Ichinose T, Yoshida S, Sadakane K, Takeda K, Yoshino S, Yamaki K, Kumagai Y, Yoshikawa T. Complementary DNA microarray analysis in acute lung injury induced by lipopolysaccharide and diesel exhaust particles. Exp. Biol. Med. (Maywood) 2004;229:1081–1087. doi: 10.1177/153537020422901013. [DOI] [PubMed] [Google Scholar]
- Yin XJ, Schafer R, Ma JY, Antonini JM, Weissman DD, Siegel PD, Barger MW, Roberts JR, Ma JK. Alteration of pulmonary immunity to Listeria monocytogenes by diesel exhaust particles (DEPs). I. Effects of DEPs on early pulmonary responses. Environ. Health Perspect. 2002;110:1105–1111. doi: 10.1289/ehp.021101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Urban air pollution in the world's megacities. 1992 [Google Scholar]