Abstract
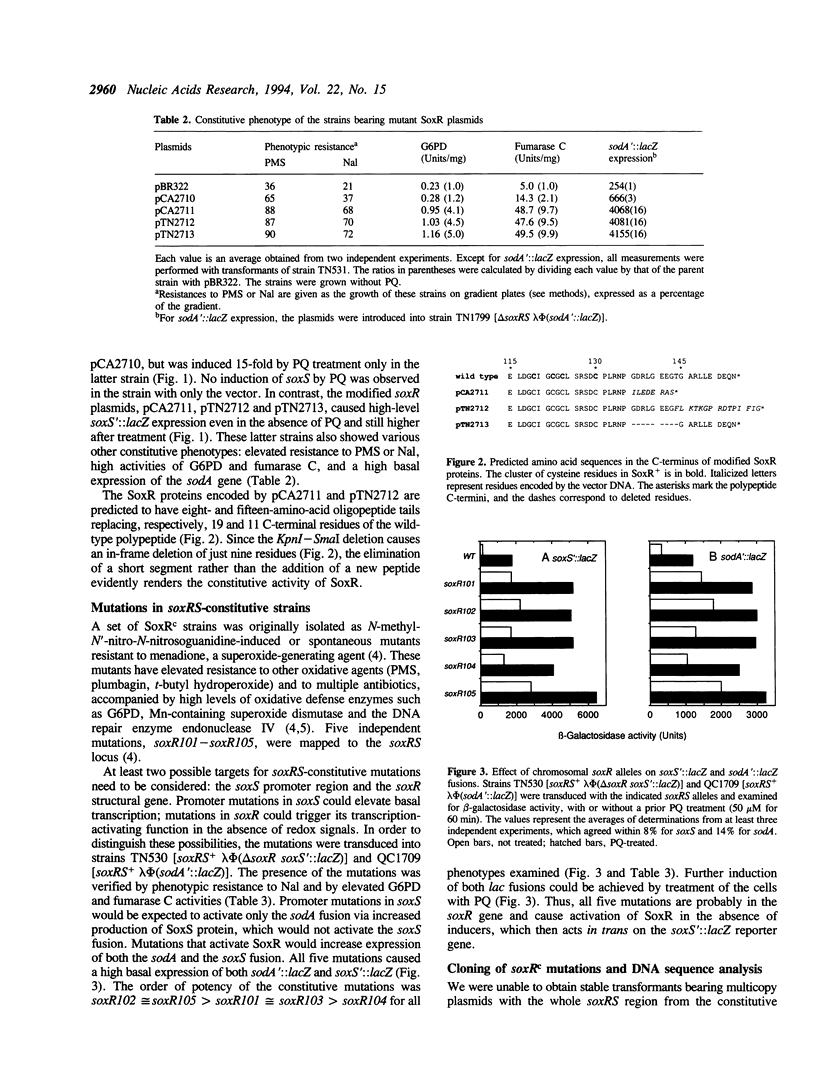
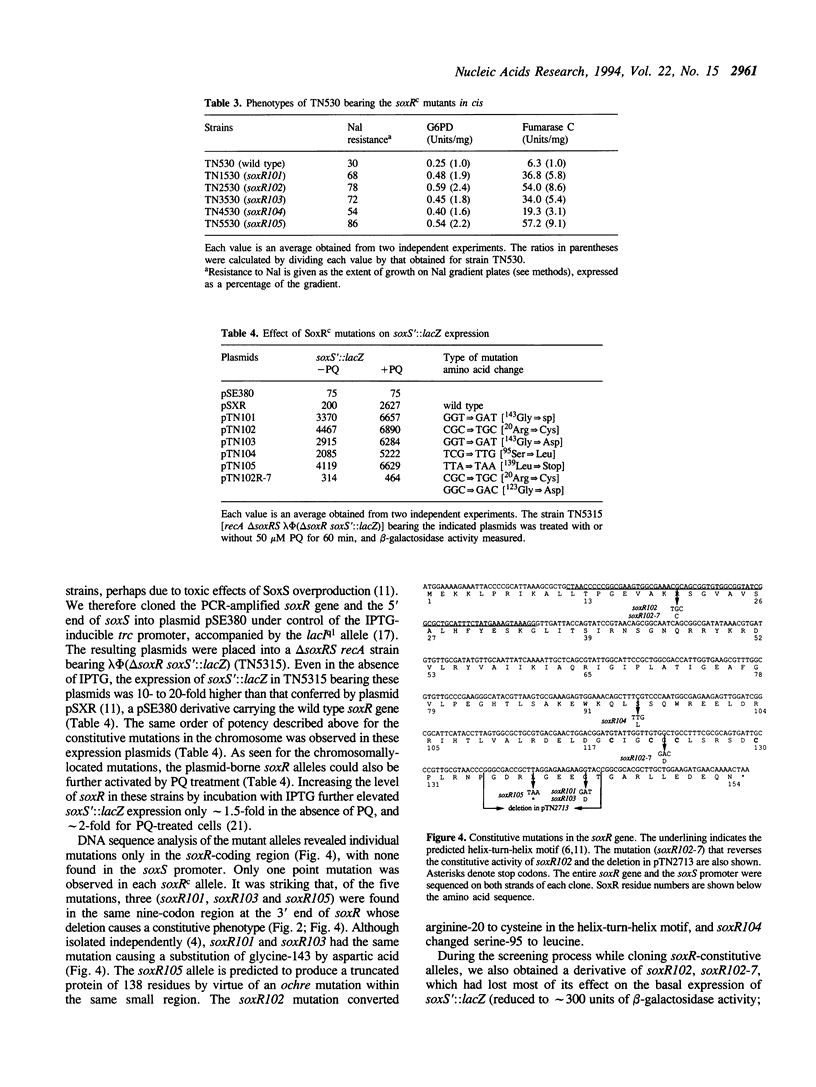
Activation of Escherichia coli oxidative stress regulon genes (sodA, zwf, fumC, nfo, etc.) is mediated by a two-stage regulatory system: the redox-sensitive SoxR protein transcriptionally activates the soxS gene, whose product then stimulates transcription of the regulon genes. Previous experiments showed that limited 3' truncation of soxR gene causes constitutive soxRS expression. DNA sequence analysis of the soxR genes from the soxRS-constitutive strains isolated originally (Greenberg et al. (1990) Proc. Natl. Acad. Sci. USA 87, 6181-6185) revealed that three alleles encode amino acid substitutions or a chain termination clustered near the C-terminus of SoxR. Two other single-amino-acid substitutions in constitutive alleles mapped to the helix-turn-helix motif and to a region of unknown function in the center of the polypeptide, respectively. No constitutive mutation was found within the region encoding the cysteines of the SoxR FeS center, in the soxR or soxS promoters, or in the soxS structural gene. Since an in-frame deletion of just nine SoxR residues (136-144; full-length SoxR = 154 residues) gave rise to a powerful constitutive allele, it appears that a small segment of the SoxR C-terminus maintains the protein in the inactive state. Conservely, an intact C-terminus is evidently not required for gene activation by SoxR.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amábile-Cuevas C. F., Demple B. Molecular characterization of the soxRS genes of Escherichia coli: two genes control a superoxide stress regulon. Nucleic Acids Res. 1991 Aug 25;19(16):4479–4484. doi: 10.1093/nar/19.16.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compan I., Touati D. Interaction of six global transcription regulators in expression of manganese superoxide dismutase in Escherichia coli K-12. J Bacteriol. 1993 Mar;175(6):1687–1696. doi: 10.1128/jb.175.6.1687-1696.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B., Amábile-Cuevas C. F. Redox redux: the control of oxidative stress responses. Cell. 1991 Nov 29;67(5):837–839. doi: 10.1016/0092-8674(91)90355-3. [DOI] [PubMed] [Google Scholar]
- Greenberg J. T., Demple B. A global response induced in Escherichia coli by redox-cycling agents overlaps with that induced by peroxide stress. J Bacteriol. 1989 Jul;171(7):3933–3939. doi: 10.1128/jb.171.7.3933-3939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. T., Monach P., Chou J. H., Josephy P. D., Demple B. Positive control of a global antioxidant defense regulon activated by superoxide-generating agents in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6181–6185. doi: 10.1073/pnas.87.16.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo E., Demple B. An iron-sulfur center essential for transcriptional activation by the redox-sensing SoxR protein. EMBO J. 1994 Jan 1;13(1):138–146. doi: 10.1002/j.1460-2075.1994.tb06243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao S. M., Hassan H. M. Biochemical characterization of a paraquat-tolerant mutant of Escherichia coli. J Biol Chem. 1985 Sep 5;260(19):10478–10481. [PubMed] [Google Scholar]
- Kappus H., Sies H. Toxic drug effects associated with oxygen metabolism: redox cycling and lipid peroxidation. Experientia. 1981 Dec 15;37(12):1233–1241. doi: 10.1007/BF01948335. [DOI] [PubMed] [Google Scholar]
- Liochev S. I., Fridovich I. Fumarase C, the stable fumarase of Escherichia coli, is controlled by the soxRS regulon. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5892–5896. doi: 10.1073/pnas.89.13.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunoshiba T., Hidalgo E., Amábile Cuevas C. F., Demple B. Two-stage control of an oxidative stress regulon: the Escherichia coli SoxR protein triggers redox-inducible expression of the soxS regulatory gene. J Bacteriol. 1992 Oct;174(19):6054–6060. doi: 10.1128/jb.174.19.6054-6060.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunoshiba T., Hidalgo E., Li Z., Demple B. Negative autoregulation by the Escherichia coli SoxS protein: a dampening mechanism for the soxRS redox stress response. J Bacteriol. 1993 Nov;175(22):7492–7494. doi: 10.1128/jb.175.22.7492-7494.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunoshiba T., deRojas-Walker T., Wishnok J. S., Tannenbaum S. R., Demple B. Activation by nitric oxide of an oxidative-stress response that defends Escherichia coli against activated macrophages. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9993–9997. doi: 10.1073/pnas.90.21.9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhill J., Ansari A. Z., Wright J. G., Brown N. L., O'Halloran T. V. Construction and characterization of a mercury-independent MerR activator (MerRAC): transcriptional activation in the absence of Hg(II) is accompanied by DNA distortion. EMBO J. 1993 Feb;12(2):413–421. doi: 10.1002/j.1460-2075.1993.tb05673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross W., Park S. J., Summers A. O. Genetic analysis of transcriptional activation and repression in the Tn21 mer operon. J Bacteriol. 1989 Jul;171(7):4009–4018. doi: 10.1128/jb.171.7.4009-4018.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O. Untwist and shout: a heavy metal-responsive transcriptional regulator. J Bacteriol. 1992 May;174(10):3097–3101. doi: 10.1128/jb.174.10.3097-3101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsaneva I. R., Weiss B. soxR, a locus governing a superoxide response regulon in Escherichia coli K-12. J Bacteriol. 1990 Aug;172(8):4197–4205. doi: 10.1128/jb.172.8.4197-4205.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkup L. K., Kogoma T. Escherichia coli proteins inducible by oxidative stress mediated by the superoxide radical. J Bacteriol. 1989 Mar;171(3):1476–1484. doi: 10.1128/jb.171.3.1476-1484.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Weiss B. Two divergently transcribed genes, soxR and soxS, control a superoxide response regulon of Escherichia coli. J Bacteriol. 1991 May;173(9):2864–2871. doi: 10.1128/jb.173.9.2864-2871.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Weiss B. Two-stage induction of the soxRS (superoxide response) regulon of Escherichia coli. J Bacteriol. 1992 Jun;174(12):3915–3920. doi: 10.1128/jb.174.12.3915-3920.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]



