Abstract
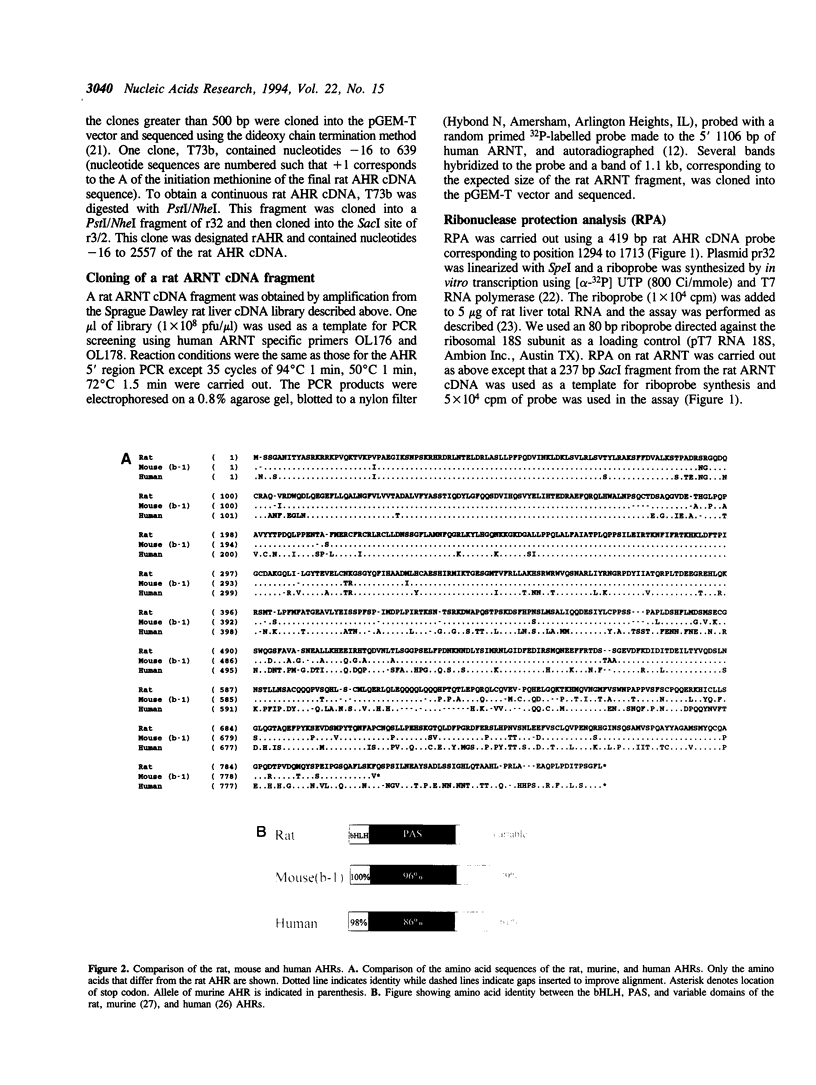
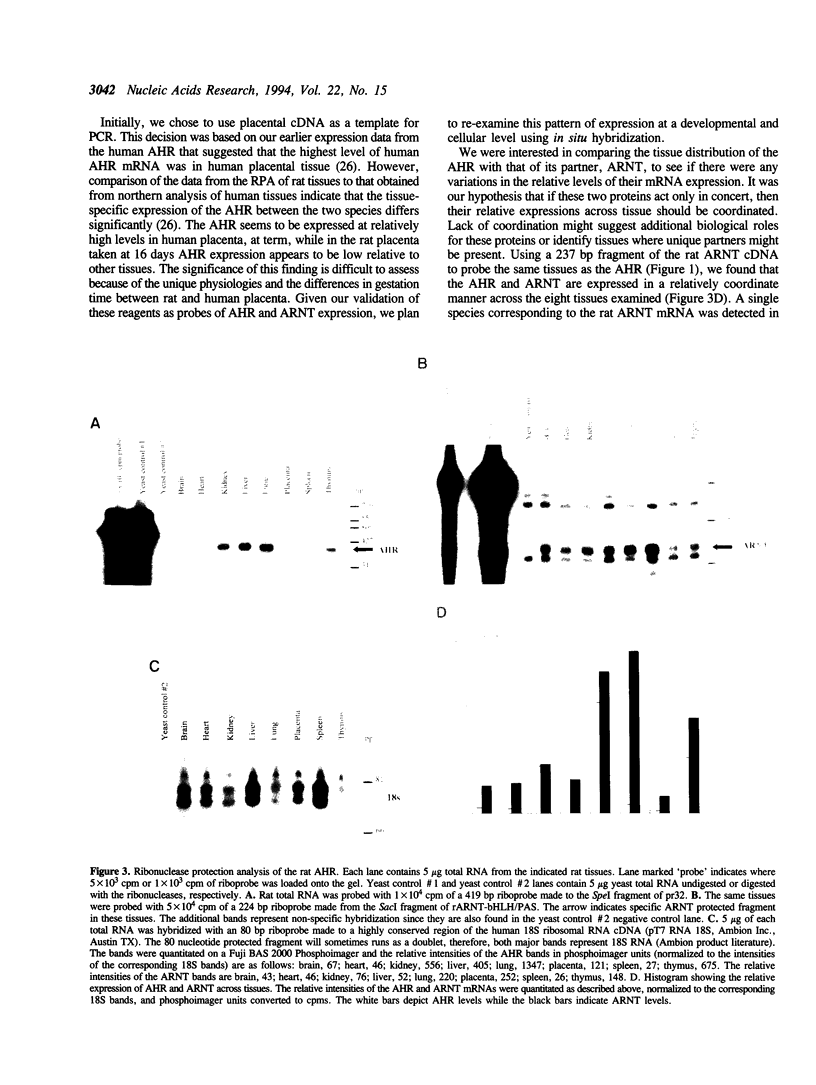
The Ah-receptor (AHR) is a ligand activated transcription factor that mediates the biological effects of agonists such as 2,3,7,8-tetrachlorodibenzo-p-dioxin. Upon binding agonists, the AHR dimerizes with a structurally related protein known as ARNT and this heterodimer then binds cognate enhancer elements and activates the expression of target genes. In this report we describe the cloning of the rat AHR cDNA and a fragment of the rat ARNT cDNA for use as probes in ribonuclease protection analysis. Ribonuclease protection analysis indicated that the rat AHR mRNA is expressed at the highest levels in the lung > thymus > kidney > liver while lower levels were expressed in heart and spleen. The rat AHR and ARNT mRNAs were expressed in a largely coordinate manner across the eight tissues examined with the exception of the placenta where AHR levels were relatively low compared to ARNT. In these experiments, a rare splice variant of the AHR was cloned that encoded a protein with a deletion in the ligand binding domain. In vitro expression studies demonstrated that in contrast to the full length AHR, the splice variant did not bind ligand nor did it bind to a cognate enhancer element in the presence of ARNT.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burbach K. M., Poland A., Bradfield C. A. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstedt-Duke J. M. Tissue distribution of the receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin in the rat. Cancer Res. 1979 Aug;39(8):3172–3176. [PubMed] [Google Scholar]
- Dolwick K. M., Schmidt J. V., Carver L. A., Swanson H. I., Bradfield C. A. Cloning and expression of a human Ah receptor cDNA. Mol Pharmacol. 1993 Nov;44(5):911–917. [PubMed] [Google Scholar]
- Dolwick K. M., Swanson H. I., Bradfield C. A. In vitro analysis of Ah receptor domains involved in ligand-activated DNA recognition. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8566–8570. doi: 10.1073/pnas.90.18.8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ema M., Sogawa K., Watanabe N., Chujoh Y., Matsushita N., Gotoh O., Funae Y., Fujii-Kuriyama Y. cDNA cloning and structure of mouse putative Ah receptor. Biochem Biophys Res Commun. 1992 Apr 15;184(1):246–253. doi: 10.1016/0006-291x(92)91185-s. [DOI] [PubMed] [Google Scholar]
- Fujisawa-Sehara A., Yamane M., Fujii-Kuriyama Y. A DNA-binding factor specific for xenobiotic responsive elements of P-450c gene exists as a cryptic form in cytoplasm: its possible translocation to nucleus. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5859–5863. doi: 10.1073/pnas.85.16.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasiewicz T. A., Rucci G. Cytosolic receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin. Evidence for a homologous nature among various mammalian species. Mol Pharmacol. 1984 Jul;26(1):90–98. [PubMed] [Google Scholar]
- Gielen J. E., Goujon F. M., Nebert D. W. Genetic regulation of aryl hydrocarbon hydroxylase induction. II. Simple Mendelian expression in mouse tissues in vivo. J Biol Chem. 1972 Feb 25;247(4):1125–1137. [PubMed] [Google Scholar]
- Greenlee W. F., Poland A. Nuclear uptake of 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J and DBA/2J mice. Role of the hepatic cytosol receptor protein. J Biol Chem. 1979 Oct 10;254(19):9814–9821. [PubMed] [Google Scholar]
- Hod Y. A simplified ribonuclease protection assay. Biotechniques. 1992 Dec;13(6):852–854. [PubMed] [Google Scholar]
- Hoffman E. C., Reyes H., Chu F. F., Sander F., Conley L. H., Brooks B. A., Hankinson O. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science. 1991 May 17;252(5008):954–958. doi: 10.1126/science.1852076. [DOI] [PubMed] [Google Scholar]
- Huang Z. J., Edery I., Rosbash M. PAS is a dimerization domain common to Drosophila period and several transcription factors. Nature. 1993 Jul 15;364(6434):259–262. doi: 10.1038/364259a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers E. W., Miller W. Optimal alignments in linear space. Comput Appl Biosci. 1988 Mar;4(1):11–17. doi: 10.1093/bioinformatics/4.1.11. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Gelboin H. V. The in vivo and in vitro induction of aryl hydrocarbon hydroxylase in mammalian cells of different species, tissues, strains, and developmental and hormonal states. Arch Biochem Biophys. 1969 Oct;134(1):76–89. doi: 10.1016/0003-9861(69)90253-7. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Gonzalez F. J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- Neuhold L. A., Shirayoshi Y., Ozato K., Jones J. E., Nebert D. W. Regulation of mouse CYP1A1 gene expression by dioxin: requirement of two cis-acting elements during induction. Mol Cell Biol. 1989 Jun;9(6):2378–2386. doi: 10.1128/mcb.9.6.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdew G. H. Association of the Ah receptor with the 90-kDa heat shock protein. J Biol Chem. 1988 Sep 25;263(27):13802–13805. [PubMed] [Google Scholar]
- Poland A., Glover E. 2,3,7,8,-Tetrachlorodibenzo-p-dioxin: segregation of toxocity with the Ah locus. Mol Pharmacol. 1980 Jan;17(1):86–94. [PubMed] [Google Scholar]
- Poland A., Glover E., Kende A. S. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem. 1976 Aug 25;251(16):4936–4946. [PubMed] [Google Scholar]
- Poland A., Glover E. Variation in the molecular mass of the Ah receptor among vertebrate species and strains of rats. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1439–1449. doi: 10.1016/0006-291x(87)90811-4. [DOI] [PubMed] [Google Scholar]
- Poland A., Knutson J. C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Pollenz R. S., Sattler C. A., Poland A. The aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator protein show distinct subcellular localizations in Hepa 1c1c7 cells by immunofluorescence microscopy. Mol Pharmacol. 1994 Mar;45(3):428–438. [PubMed] [Google Scholar]
- Safe S. Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and related compounds: environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs). Crit Rev Toxicol. 1990;21(1):51–88. doi: 10.3109/10408449009089873. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. V., Carver L. A., Bradfield C. A. Molecular characterization of the murine Ahr gene. Organization, promoter analysis, and chromosomal assignment. J Biol Chem. 1993 Oct 15;268(29):22203–22209. [PubMed] [Google Scholar]
- Shen E. S., Whitlock J. P., Jr Protein-DNA interactions at a dioxin-responsive enhancer. Mutational analysis of the DNA-binding site for the liganded Ah receptor. J Biol Chem. 1992 Apr 5;267(10):6815–6819. [PubMed] [Google Scholar]
- Swanson H. I., Bradfield C. A. The AH-receptor: genetics, structure and function. Pharmacogenetics. 1993 Oct;3(5):213–230. doi: 10.1097/00008571-199310000-00001. [DOI] [PubMed] [Google Scholar]
- Swanson H. I., Perdew G. H. Detection of the Ah receptor in rainbow trout: use of 2-azido-3-[125I]iodo-7,8-dibromodibenzo-p-dioxin in cell culture. Toxicol Lett. 1991 Sep;58(1):85–95. doi: 10.1016/0378-4274(91)90194-b. [DOI] [PubMed] [Google Scholar]
- Telakowski-Hopkins C. A., King R. G., Pickett C. B. Glutathione S-transferase Ya subunit gene: identification of regulatory elements required for basal level and inducible expression. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1000–1004. doi: 10.1073/pnas.85.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw M. L., Göttlicher M., Gustafsson J. A., Poellinger L. Definition of a novel ligand binding domain of a nuclear bHLH receptor: co-localization of ligand and hsp90 binding activities within the regulable inactivation domain of the dioxin receptor. EMBO J. 1993 Nov;12(11):4169–4179. doi: 10.1002/j.1460-2075.1993.tb06101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]