Abstract
Tuberous sclerosis (TSC) is a single-gene disorder caused by heterozygous mutations in the TSC1 or TSC2 gene. TSC is often associated with neurological (e.g., epilepsy) and cognitive (intellectual disabilities, specific neuropsychological impairments) dysfunction, as well as neurodevelopmental disorders (e.g., autism, ADHD). In addition, there is a high prevalence of psychiatric problems in TSC populations, including anxiety and mood disorders. To date, little is known about the pathogenetic bases of these associated psychiatric symptoms; for instance, it is unclear whether they are rooted in TSC-associated neurobiological alterations or whether they are secondary psychological phenomena (e.g., because individuals have to cope with the burden of the disease). Here, we report elevated levels of anxiety-related behaviors and mild deficits in two hippocampal-dependent learning tasks in a Tsc2 dominant-negative transgenic mouse model of tuberous sclerosis. These findings establish a mouse model for TSC-related anxiety phenotypes and suggest that anxiety disorders in tuberous sclerosis have a biological foundation.
Keywords: Tuberous sclerosis, TSC, mTOR, anxiety, learning and memory, behavior, mouse
Introduction
Tuberous sclerosis (TSC) is a single-gene disorder, which is inherited in an autosomal-dominant fashion and is caused by mutations in either the TSC1 or the TSC2 gene. TSC belongs to the group of phakomatoses (neurocutaneous disorders) and is characterized by tumor growths and tissue malformations that affect various organ systems, including brain, kidney, heart, lung, skin and liver (Crino et al., 2006; Curatolo et al., 2008).
Neurological, neuropsychiatric, cognitive and behavioral symptoms as well as learning disabilities are common in this disorder. Epilepsy is an important clinical problem in TSC and affects 70 – 80% of individuals over their lifetime (Joinson et al., 2003; Webb et al., 1996). Intelligence quotients (IQ) are distributed bi-modally in TSC populations (de Vries and Howe, 2007; de Vries and Prather, 2007; Joinson et al., 2003; Prather and de Vries, 2004; Winterkorn et al., 2007). Approximately 20 – 30% have very low IQs and require life-long assistance with daily living and personal care tasks. About 50% have an IQ in the normal range (IQ > 70). Nevertheless, specific neuropsychological impairments are common in this normal IQ group and include deficits in attentional-executive skills, memory and other functions (de Vries et al., 2005; de Vries et al., 2009; Harrison et al., 1999; Prather and de Vries, 2004; Ridler et al., 2007). Neurodevelopmental disorders are also frequently associated with TSC. Autism affects approximately 20 – 60% of individuals (Bolton et al., 2002; Smalley, 1998). Attention deficit hyperactivity disorder (ADHD) is associated with TSC in approximately 50% of individuals and attention deficits are noted in most TSC individuals (de Vries et al., 2009; Prather and de Vries, 2004).
In addition to the manifestations mentioned above, psychiatric co-morbidities are also very common in TSC populations (de Vries et al., 2007; Lewis et al., 2004; Muzykewicz et al., 2007; Pulsifer et al., 2007; Raznahan et al., 2006). The most frequent psychiatric manifestations include mood, anxiety and adjustment disorders. The pathophysiological basis of these TSC-related neuropsychiatric symptoms is poorly understood.
Of note, the expressivity of TSC-related phenotypes is highly variable across individuals; while some individuals suffer from severe disabilities, TSC remains subclinical in others. It is possible that the specific nature of the mutation, genetic modifiers or modifying environmental factors contribute to the variable expression of TSC-related phenotype, including neuropsychiatric phenotypes (Dabora et al., 2002; de Vries and Howe, 2007; Kikuchi et al., 2004; Onda et al., 1999; Yeung et al., 2001).
Several mouse models of TSC have been generated which are all based on the genetic etiology of the disorder (i.e., mutations in the TSC1 or TSC2 genes). Heterozygous Tsc1 and Tsc2 mutants have been found to capture some of the TSC-related cognitive deficits (deficits in learning and memory) (Ehninger et al., 2008; Goorden et al., 2007). The Eker rat, which carries a spontaneous mutation in the Tsc2 gene, did not show impairments, but rather improvements, on learning and memory tasks (Waltereit et al., 2006), suggesting that the type of mutation or modifier genes in the genetic background may play a role in modulating TSC-related disease phenotypes. Other animal models, based on cell-type specific homozygous deletions of Tsc1 or Tsc2 in neurons, astrocytes or radial glia, were reported to show seizures and/or pronounced neuropathology (Ehninger et al., 2008; Meikle et al., 2007; Uhlmann et al., 2002; Way et al., 2009). No animal model has so far been reported for the psychiatric symptoms associated with TSC (such as anxiety disorders); this may in part relate to the fact that available models have not been tested in these domains. Certain TSC models, however, such as heterozygous Tsc2 mutant mice, were tested and did not show obvious alterations (Ehninger et al., 2008). Here, we report elevated levels of anxiety-related behaviors in a Tsc2 dominant negative transgenic mouse model of TSC (Tsc2-DN; (Govindarajan et al., 2005)).
Material and Methods
Mice
Tsc2 dominant negative (Tsc2-DN) mice were generated as previously described (Govindarajan et al., 2005) and were maintained on a C57BL/6J genetic background. To generate experimental animals, male Tsc2-DN mice were mated with female C57BL/6J wild-type animals. We used a 1:1 mating scheme in which male mutant mice were left in the cage with a female and her litter. Pups were weaned and tail biopsies for genotyping were taken at postnatal day 21. Genotyping was performed by PCR as previously described (Govindarajan et al., 2005). Mice were housed in groups of 2 – 4 animals per cage. Animals were kept on a 12 h light/dark cycle and received food and water ad libitum. The experiments were performed during the light period of the cycle. For the behavioral experiments, 3 – 6 months male and female mice (at an approximately balanced ratio) were used. One cohort of mice was used for the elevated plus maze and the open field test (in this order with a two week interval between tests; Tsc2-DN: n = 14 mice; WT: n = 16 mice). A separate cohort of animals was used to study spatial learning in the Morris water maze and context discrimination (tasks were run in this order, two-week interval between tests; Tsc2-DN: n = 10 mice; WT: n = 10 mice). Separation calls were studied in yet another batch of animals (Tsc2-DN: n = 18 pups; WT: n = 13 pups). All local and federal regulations regarding animal welfare were followed.
Elevated plus maze
The elevated plus maze contained four arms (each arm 29 cm long and 8 cm wide), two of which were open, while the other two arms had walls on the sides (16.5 cm high). The maze was mounted 1 m above the floor. For the behavioral session, mice were placed in the center part of the elevated plus maze and were allowed to explore the maze for 5 min. The behavior was videotaped for offline analysis. Data from one Tsc2-DN mouse was not available for analysis because it was accidently not recorded. We scored the time spent exploring the open arms. Animals were considered to be exploring the open arms when all four paws were on one of the open arms. This assay has good face, construct and predictive validity with respect to measuring anxiety (Hogg, 1996; Walf and Frye, 2007). Low scores in the measure “time spent in open arms” indicate high levels of anxiety. An unpaired t-test was used to statistically compare the time spent exploring the open arms across genotypes.
Open field
Mice were placed for 10 min in a square open field made of acrylic (footprint 27.5 cm × 27.5 cm) and activity was recorded by an automated system (Med Associates). Zone analysis was performed to determine inner sector occupancy of mice; low scores in this measure can indicate high levels of anxiety.
Context discrimination
On day 1, animals were given a 5 min fear conditioning session in context A; mice received three 0.75 mA shocks (duration 2 s), initiated at 2 min of the session and spaced 1 min apart. Context fear was tested one-day later (5 min test session). In half of the animals, testing took place in the training context (context A). The other half was tested in a novel context (context B), which shared some features with the training context (horizontal floors, ethanol scent, white background noise), but was otherwise distinct (plastic instead of metal grid floor, angled instead of rectangular walls, red instead of white light, different spatial cues in the room). We determined the percentage of time spent freezing in context A and B. These measures were compared by unpaired t-tests to evaluate if the conditioned fear response of Tsc2-DN mice and WT mice, respectively, showed significant context discrimination.
Morris water maze
To habituate animals to investigator contact and procedural elements of subsequent water maze training, mice were handled for 7-days prior to training on the Morris water maze (for approximately 2 min/animal/day). This included repeatedly picking up the animals by the tail and holding them on the hand. Subsequently, mice were trained on the hidden version of the water maze; the escape platform was hidden underneath the water surface in a constant location of the pool. The water in the pool (diameter: 1.2 m) was made opaque with white non-toxic paint (water temperature: 22 – 24°C). Animal behavior was monitored with an automated system (HVS water). Animals were entered into the pool from 1 of 7 randomly assigned starting positions. Trials were completed when the animal climbed on the escape platform or when 60 s had elapsed, which ever came first. Animals remained on the escape platform for 15 s after completion of trials. We gave 4 daily training trials for 5 consecutive days. The inter-trial interval between trials 1 and 2 and trials 3 and 4 was approximately 1 min, between trials 2 and 3 approximately 90 min. To assess how accurately animals had learned the position of the escape platform, we gave a probe trial after completion of training. During the probe trial, the escape platform was removed from the pool and we determined the proportion of time that the animal spent searching in the target quadrant (which previously contained the escape platform) or the other quadrants. Probe trial data were analyzed in two ways. To evaluate if the quadrant occupancy pattern differed across genotypes, we performed a two-way repeated-measures ANOVA with genotype as between-subjects factor and quadrant as within-subjects-factor. To determine if Tsc2-DN or WT animals spent more time in the target quadrant than the other quadrants (which is indicative of preferential searching in the target quadrant), we compared target quadrant occupancy to the average occupancy of the other quadrants by paired t-test. Training escape latencies were analyzed by one-way ANOVA with genotype as between-subjects factor.
Separation calls
Tsc2-DN and WT pups for separation call analyses were derived from crosses of Tsc2-DN males with WT female mice. Tsc2-DN fathers were taken out of the breeding cages prior to birth of the litters to exclude possible parental genotype effects on pup vocalizations. Separation calls were measured at postnatal day 7. Pups were separated from the dam and transferred into a 7.5 cm × 10 cm glass beaker, which was placed under an ultrasound detector located in a sound-attenuating chamber. Ultrasonic vocalizations at 70 kHz were quantified for 13 min using an automated system (Noldus). Tail samples for genotyping were taken after completion of the recording session. We used an unpaired t-test to compare the number of vocalizations (separation calls) emitted during the recording session across genotype.
Statistics
All statistical tests were chosen a priori and are described in more detail in the corresponding sections of Material & Methods and Results, respectively.
Results
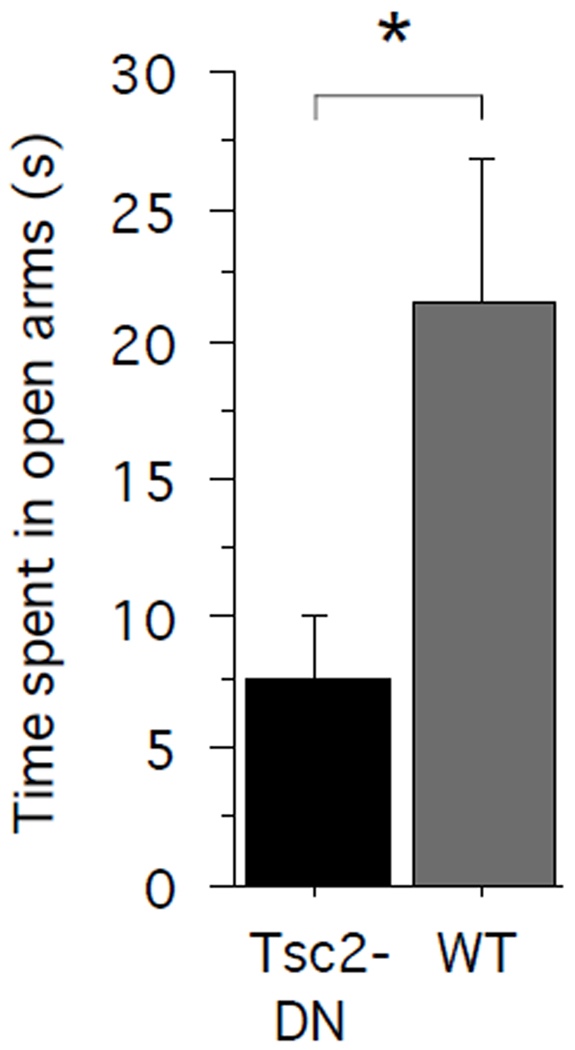
To assess anxiety-related behaviors in Tsc2-DN mice and WT littermate controls, we tested them on the elevated plus maze. We compared the time spent in the open arms across genotypes (Fig. 1). Tsc2-DN mice spent significantly less time in the open arms than wild-type littermate controls (unpaired two-tailed t-test: P = 0.0295; power = 0.546; Cohen’s d = 0.8936; Tsc2-DN: n = 13 mice; WT: n = 16 mice), indicating higher levels of anxiety in Tsc2-DN mice than in WT controls.
Fig. 1.
Tsc2-DN mice spent significantly less time on the open arms of an elevated plus maze than WT controls. The graph shows the time spent (s) on the arms of an elevated plus maze. SEM is shown as error bars. * P < 0.05
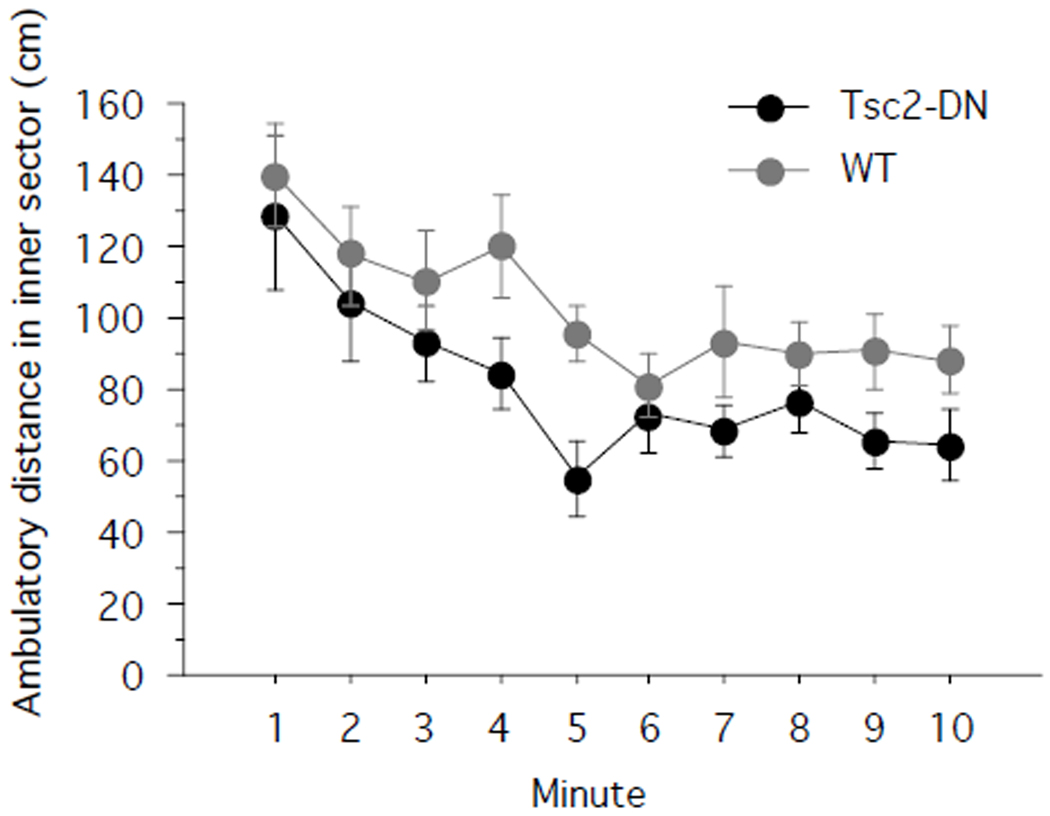
Next, we used the open field assay to assess exploration. In the open field, low center zone occupancy can be indicative of high levels of anxiety. Accordingly, we compared the distance traveled in the inner sector of the open field across genotypes (Fig. 2). Tsc2-DN mice showed a trend towards decreased exploration of the inner sector (one-way repeated-measures ANOVA with genotype as between-subjects factor: F(1,28) = 3.316; P = 0.0793; power = 0.405; Tsc2-DN: n = 14 mice; WT: n = 16 mice). In contrast, there was no trend towards differing ambulatory distances in the outer zone of the open field (One-way repeated-measures ANOVA with genotype as between-subjects factor: F(1,28) = 0.262; P = 0.6129; power = 0.077; Tsc2-DN: n = 14 mice; WT: n = 16 mice). Total ambulatory distance in both inner and outer sector did not differ between genotypes (unpaired two-tailed t-test: P = 0.1466; power = 0.51; Cohen’s d = 0.5481; Tsc2-DN: n = 14 mice; WT: n = 16 mice). Taken together, the results from the elevated plus maze and open field show higher levels of anxiety-related behaviors in Tsc2-DN mice than in controls.
Fig. 2.
Tsc2-DN mice showed a trend towards less exploration of the center sector of the open field. Shown is the distance travelled (cm) in the center sector of the open field. SEM is shown as error bars.
Next, we looked at hippocampus-dependent learning and memory in Tsc2-DN and WT control mice. We started by probing spatial learning and memory using the hidden version of the Morris water maze. In this task, mice learn to locate an escape platform hidden underneath the water surface in a pool of water. As the escape platform is not directly visible, animals have to use distal spatial cues available in the testing room to learn to navigate to the escape platform. We gave 4 daily training trials over 5 consecutive days.
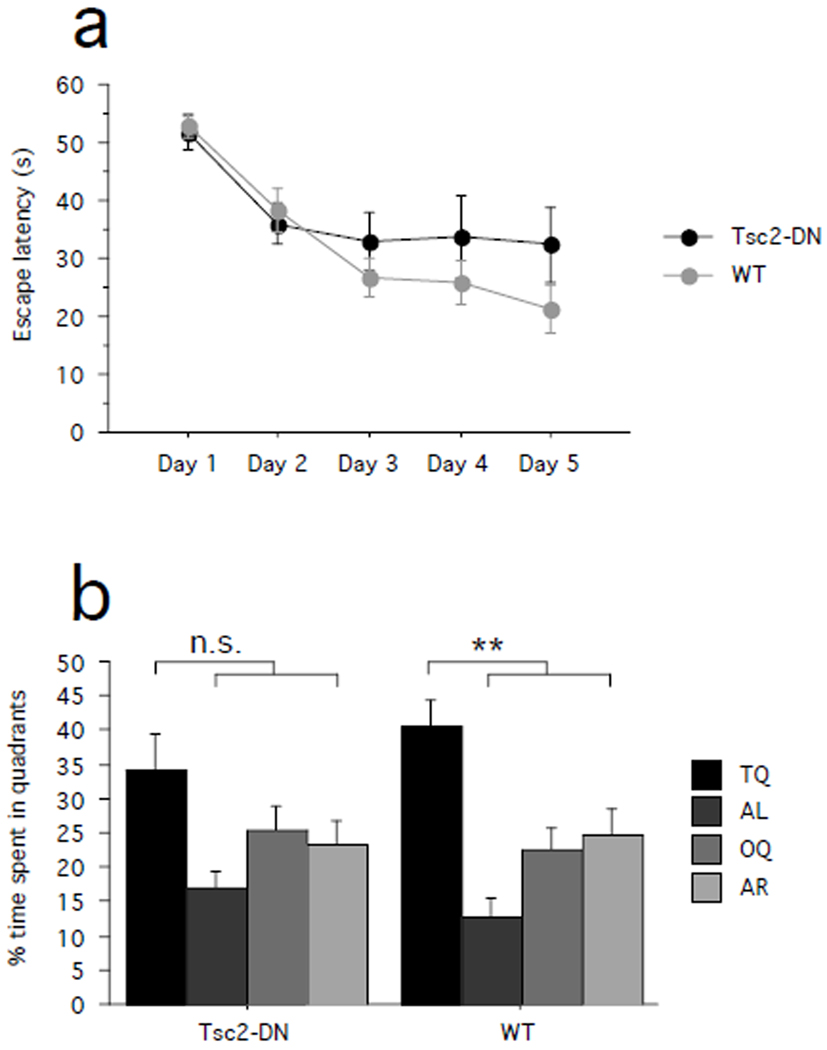
Escape latencies decreased over the course of training in both Tsc2-DN and WT mice, showing that mice of both genotypes improved on the task. There was no significant difference between genotypes (Fig. 3a; one-way ANOVA with genotype as between-subjects factor: effect of training day, F(4,72) = 12.835, P < 0.0001; power = 1.000; genotype effect, F(1,18) = 1.365, P = 0.2579; power = 0.188; Tsc2-DN: n = 10 mice; WT: n = 10 mice).
Fig. 3.
Spatial learning in a hippocampus-dependent version of the Morris water maze. (a) Escape latencies (s) are plotted by training day. (b) Shown is quadrant occupancy during the probe trial given after completion of training (quadrants: T – target, AL –adjacent left, O – Opposite, AR – adjacent right). SEM is shown as error bars. n.s. P > 0.05, ** P < 0.01.
To assess how accurately the location of the escape platform was learned during training, we gave a probe trial after completion of training. During the probe trial, we removed the escape platform from the pool and determined the proportion of time that the animals spent searching in either the target quadrant (which previously contained the escape platform) or the other quadrants. The proportion of time the animals spent in each of the quadrants did not significantly differ between genotypes (Fig. 3b; two-way ANOVA with genotype as between-subjects factor and quadrant as within-subjects factor: genotype × quadrant interaction, F(3,72) = 0.881, P = 0.4550; power = 0.227). Further analyses revealed that WT mice spent significantly more time in the target quadrant than the other quadrants (Fig. 3b; paired one-tailed t-test, target quadrant vs. average of the other quadrants: WT, P = 0.0030; power = 0.966; Cohen’s d = 2.2800; n = 10 mice), suggesting preferential searching in the target quadrant. In contrast, while there also was a trend towards higher quadrant occupancy in the target quadrant in Tsc2-DN mice, this did not reach statistical significance (paired one-tailed t-test, target quadrant vs. average of the other quadrants: Tsc2-DN, P = 0.1211, power = 0.836; Cohen’s d = 0.9684; n = 10 mice). Swimming speed during the probe trial did not differ between genotypes (paired two-tailed t-test: P = 0.4777, power = 0.513; Cohen’s d = 0.3243; Tsc2-DN: n = 10 mice; WT: n = 10 mice).
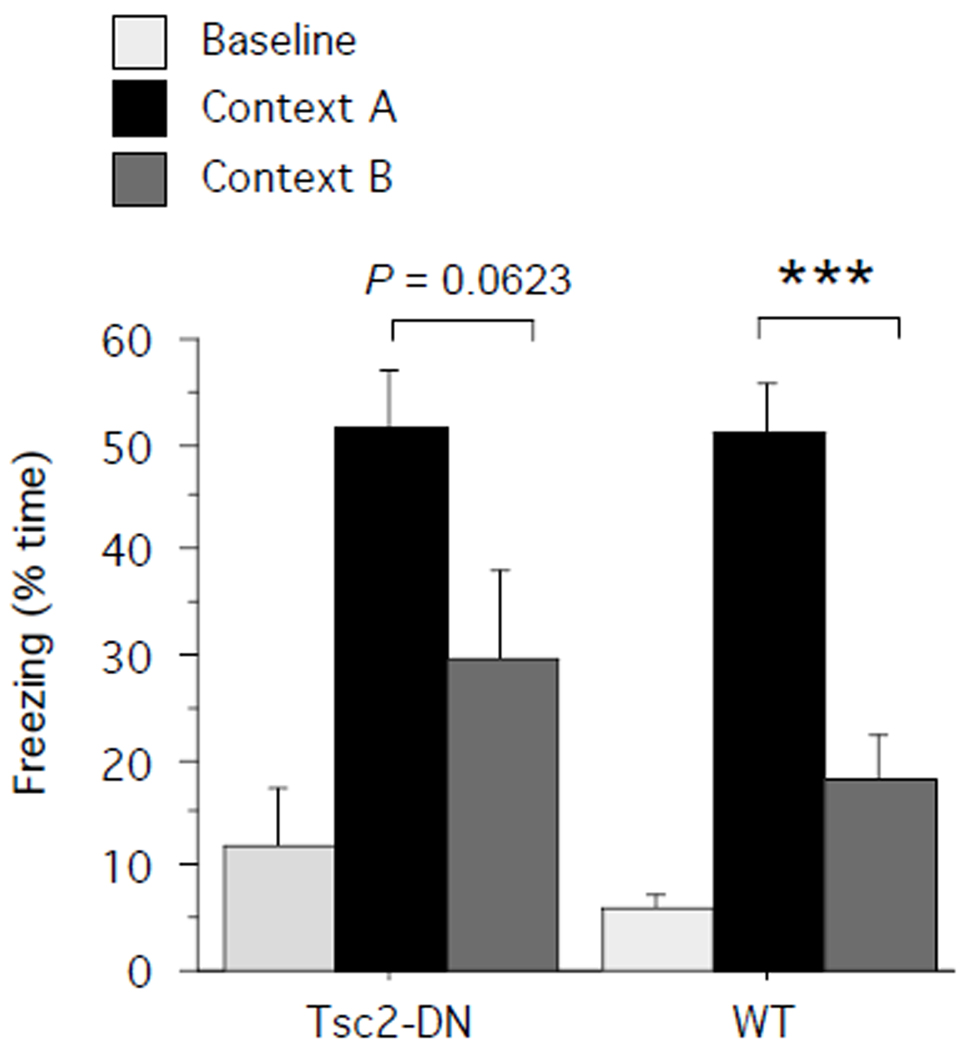
Discriminating between similar contexts also depends on intact hippocampal function. To assess context discrimination, Tsc2-DN mice and WT controls were given a training session in context A (5 min session, 3 × 0.75-mA-2-s shocks, spaced 1 min apart, first shock after 2 min). One day later, half of the mice from each group were tested for 5 min in the training context (context A), the other half was tested in a novel context (context B), which shared some features with the training context but was otherwise distinct (for details, see Material and Methods section). Freezing to the training context was of similar magnitude in Tsc2-DN mice and WT controls (Fig. 4; unpaired two-tailed t-test: P = 0.9683; power = 0.503; Cohen’s d = 0.0259; WT: n = 5 mice, Tsc2-DN: n = 5 mice). Comparison of freezing levels in the training and novel context revealed that the conditioned fear response in WT mice clearly discriminated between the two contexts (Fig. 4; unpaired one-tailed t-test: P = 0.0007; power = 0.788; Cohen’s d = 3.3423; training context: n = 5 mice, novel context: n = 5 mice). Although in Tsc2-DN mice there was a clear trend for higher freezing levels in the training context than the novel context, this did not reach significance (Fig. 4; Tsc2-DN, unpaired one-tailed t-test: P = 0.0623; power = 0.686; Cohen’s d = 1.3691; training context: n = 5 mice, novel context: n = 5 mice). These provisional findings suggest that the contextual discrimination of Tsc2-DN mutants is worse than that of WT mice.
Fig. 4.
Context discrimination was assessed by first training mice in context conditioning and testing half of the population in the training context (context A), the other half in a novel context (context B). Percent time freezing before shock (baseline) and on test in the training context or a novel context is shown. SEM is shown as error bars. *** p < 0.001
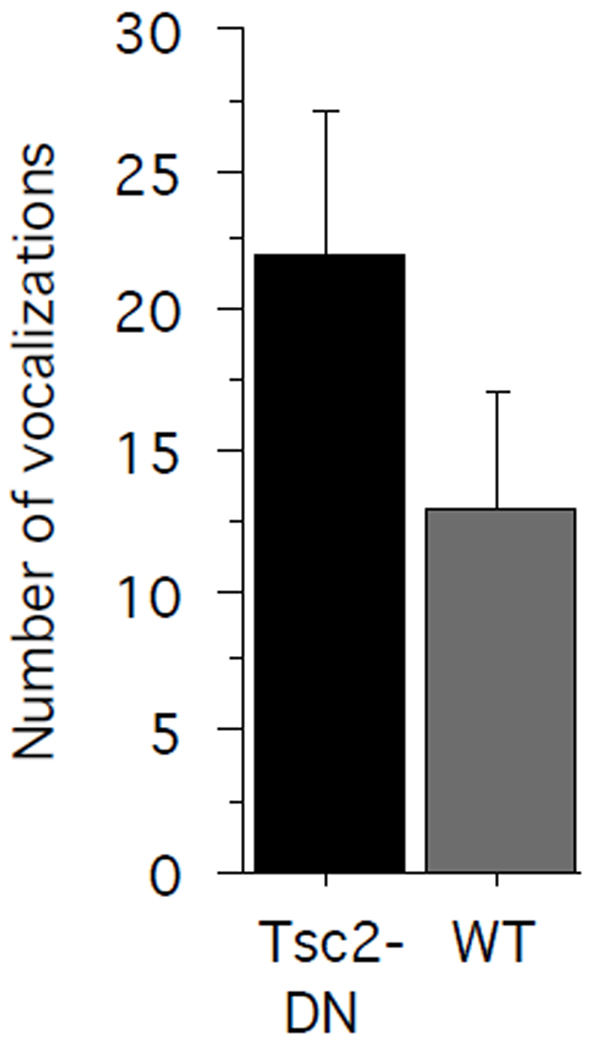
We also wanted to assess neurological function at an early postnatal stage in Tsc2-DN mice. When separated from their mothers, pups emit vocalizations in the ultrasonic range (separation calls), which induces retrieval behavior in the mother. To assess separation calls in Tsc2-DN pups and WT control pups, we separated P7 pups from their mother and recorded ultrasonic vocalizations. The number of ultrasonic vocalizations emitted by WT pups and Tsc2 dominant negative pups did not significantly differ between genotypes (Fig. 5; unpaired two-tailed t-test: P = 0.2076; power = 0.530; Cohen’s d = 0.4835; WT, n = 13 pups; Tsc2-DN, n = 18 pups).
Fig. 5.
Separation calls were tested in pups at postnatal day 7. The graph shows the number of ultrasonic vocalizations during a 13 min test period in Tsc2-DN mice and WT controls. There was no significant difference between genotypes. SEM is shown as error bars.
Discussion
Here, we report increased levels of anxiety-related behaviors in Tsc2-DN mice in a mouse model of tuberous sclerosis. Tsc2-DN mice spent less time on the open arms of an elevated plus maze. Additionally, our provisional results also suggest that these mice may have deficits in hippocampal learning and memory tasks.
These findings are interesting in light of reports that show a considerable prevalence of anxiety disorders in human TSC populations (de Vries et al., 2007; Lewis et al., 2004; Muzykewicz et al., 2007; Pulsifer et al., 2007; Raznahan et al., 2006). TSC-related anxiety disorders tend to overlap with mood disorders and may be more common in cognitively less impaired individuals (Muzykewicz et al., 2007) although this association of cognitive ability and anxiety has not been found in other studies (de Vries et al., 2007). Conceivably, the ability to perceive and/or report anxiety symptoms may be dependent on the general level of cognitive functioning, which could at least in part explain such an association. Despite the high prevalence in TSC populations, the pathogenesis of TSC-related mental illness, including anxiety disorders, is poorly understood. It is possible that TSC-related anxiety phenotypes directly result from neurobiological changes due to loss of function of TSC proteins. Alternatively, they may represent a psychological response to the difficult circumstances associated with having to live with the burden of this disorder. Our finding of increased anxiety-related behaviors in a mouse model of tuberous sclerosis is consistent with the idea that the anxiety phenotype associated with TSC is caused by the mutation.
Prior research showed that Tsc2-DN mice display signs of perturbed cerebellar development (Govindarajan et al., 2005). Tsc2-DN mice showed normal ambulatory distances in the open field assay and normal swimming speed in the Morris water maze, suggesting that cerebellar pathologies may not translate into gross motor dysfunction in this model. These findings suggest that the behavioral results described above were not confounded by substantial genotype differences in motor function. Nevertheless, it may be useful to also test Tsc2-DN mice on the accelerating rotarod to more specifically look at motor coordination in these animals.
The behavioral phenotypic profile of Tsc2-DN mice appears to differ in some respects from that of other mouse models of this disorder. Increased levels of anxiety-related behaviors, as assessed with the elevated plus maze, were not present in Tsc2+/− mice (Ehninger et al., 2008). Interestingly, however, Tsc2+/− mice showed increased generalization of learned fear in a contextual discrimination paradigm (Ehninger et al., 2008), a finding that may also be of relevance for tuberous sclerosis-related anxiety phenotypes. Like Tsc2+/− and Tsc1+/− mice, which showed impairments in hippocampus-dependent learning and memory tasks (Ehninger et al., 2008; Goorden et al., 2007), our provisional experiments indicate that Tsc2-DN mice also appear to show some hippocampus-dependent learning and memory deficits.
There are several reasons that could account for the different behavioral profile of Tsc2-DN and Tsc2+/− mice. First, the generation of Tsc2+/− mice involved the insertion of a neomycin resistance cassette into the second coding exon of Tsc2 (Onda et al., 1999). Consequently, Tsc2+/− mice showed reduced tuberin abundance (reduced by approximately 30%) and disinhibited mTOR signaling in the hippocampus (Ehninger et al., 2008). In Tsc2-DN mice, a modified Tsc2 transgene was expressed (the Tsc2-DN transgene) that carries mutations in two structural motifs of tuberin (Govindarajan et al., 2005; Pasumarthi et al., 2000): Amino acid residues 1617 through 1655 were deleted, impacting on the structural integrity of the GTPase activating protein (GAP) domain of Tsc2. As a consequence, the mutant protein is not able to interact with and accelerate the inactivation of small G-proteins (Govindarajan et al., 2005; Pasumarthi et al., 2000), such as Rheb and rap1. In addition, the transgene included a splice variant in which amino acid residues 1679 through 1742 were substituted. This renders the rabaptin-5 binding motif of tuberin non-functional. Taken together, the mutant protein is thought to interfere with the GAP function of Tsc2 and its rabaptin-5 binding through displacement of endogenous tuberin (Govindarajan et al., 2005; Pasumarthi et al., 2000). This alters mTOR signaling (Govindarajan et al., 2005) and vesicle trafficking (Pasumarthi et al., 2000), the latter one presumably through Rab5/rabaptin-5. Differing biochemical consequences of the Tsc2-DN mutation and the inactivating Tsc2 mutation in Tsc2+/− mice may contribute to the different behavioral profiles in these mouse models of tuberous sclerosis. Comparison of biochemical profiles in brain tissue of Tsc2-DN mice and Tsc2+/− mice may provide a useful starting point for future studies aimed at establishing mechanisms for genotype-behavioral phenotype relationships in TSC. Conceivably, different TSC-related mutations may contribute to the variability of neuropsychiatric phenotypes in human TSC populations as well (de Vries and Howe, 2007).
Another variable that may contribute to behavioral profiles is cells affected by mutation: Tsc2+/− mice carry a germ-line mutation, which is present in all cells of the body. In Tsc2-DN mice, random integration transgenesis was employed (Govindarajan et al., 2005) and, hence, the Tsc2-DN transgene could be expressed in a subpopulation of cells, even though the ubiquitous CMV promoter was used to drive the transgene. Accordingly, different cell populations could be affected by loss of Tsc2 function. Finally, genetic background is known to potently modify genotype-phenotype relationships in genetic disorders, including TSC (Dabora et al., 2002; Kikuchi et al., 2004; Onda et al., 1999; Yeung et al., 2001). It is possible that genetic modifiers contribute to different behavioral profiles in Tsc2+/− mice and Tsc2-DN mice.
Taken together, Tsc2-DN mice showed elevated levels of anxiety-related behaviors and therefore provide a useful model system to better understand TSC-related anxiety phenotypes. We believe that comparative studies of available TSC animal models with different behavioral phenotypic profiles will provide a fruitful approach to gain insights into genotype-phenotype relationships relevant to tuberous sclerosis.
Acknowledgements
Tsc2-DN mice were kindly provided by Jack L. Arbiser. This work was supported by DFG grant EH223/2-1 and funds of the German Centre for Neurodegenerative Diseases (DZNE) to D.E. and NIH R01 MH084315 to A.J.S.
References
- Bolton PF, Park RJ, Higgins JN, Griffiths PD, Pickles A. Neuro-epileptic determinants of autism spectrum disorders in tuberous sclerosis complex. Brain. 2002;125:1247–1255. doi: 10.1093/brain/awf124. [DOI] [PubMed] [Google Scholar]
- Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372:657–668. doi: 10.1016/S0140-6736(08)61279-9. [DOI] [PubMed] [Google Scholar]
- Dabora SL, Roberts P, Nieto A, Perez R, Jozwiak S, Franz D, Bissler J, Thiele EA, Sims K, Kwiatkowski DJ. Association between a high-expressing interferon-gamma allele and a lower frequency of kidney angiomyolipomas in TSC2 patients. Am J Hum Genet. 2002;71:750–758. doi: 10.1086/342718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries P, Humphrey A, McCartney D, Prather P, Bolton P, Hunt A. Consensus clinical guidelines for the assessment of cognitive and behavioural problems in Tuberous Sclerosis. Eur Child Adolesc Psychiatry. 2005;14:183–190. doi: 10.1007/s00787-005-0443-1. [DOI] [PubMed] [Google Scholar]
- de Vries PJ, Gardiner J, Bolton PF. Neuropsychological attention deficits in tuberous sclerosis complex (TSC) Am J Med Genet A. 2009;149A:387–395. doi: 10.1002/ajmg.a.32690. [DOI] [PubMed] [Google Scholar]
- de Vries PJ, Howe CJ. The tuberous sclerosis complex proteins - a GRIPP on cognition and neurodevelopment. Trends Mol Med. 2007 doi: 10.1016/j.molmed.2007.06.003. [DOI] [PubMed] [Google Scholar]
- de Vries PJ, Hunt A, Bolton PF. The psychopathologies of children and adolescents with tuberous sclerosis complex (TSC): a postal survey of UK families. Eur Child Adolesc Psychiatry. 2007;16:16–24. doi: 10.1007/s00787-006-0570-3. [DOI] [PubMed] [Google Scholar]
- de Vries PJ, Prather PA. The tuberous sclerosis complex. N Engl J Med. 2007;356:92. doi: 10.1056/NEJMc062928. author reply 93–94. [DOI] [PubMed] [Google Scholar]
- Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goorden SM, van Woerden GM, van der Weerd L, Cheadle JP, Elgersma Y. Cognitive deficits in Tsc1+/− mice in the absence of cerebral lesions and seizures. Ann Neurol. 2007;62:648–655. doi: 10.1002/ana.21317. [DOI] [PubMed] [Google Scholar]
- Govindarajan B, Brat DJ, Csete M, Martin WD, Murad E, Litani K, Cohen C, Cerimele F, Nunnelley M, Lefkove B, et al. Transgenic expression of dominant negative tuberin through a strong constitutive promoter results in a tissue-specific tuberous sclerosis phenotype in the skin and brain. J Biol Chem. 2005;280:5870–5874. doi: 10.1074/jbc.M411768200. [DOI] [PubMed] [Google Scholar]
- Harrison JE, O'Callaghan FJ, Hancock E, Osborne JP, Bolton PF. Cognitive deficits in normally intelligent patients with tuberous sclerosis. Am J Med Genet. 1999;88:642–646. [PubMed] [Google Scholar]
- Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Joinson C, O'Callaghan FJ, Osborne JP, Martyn C, Harris T, Bolton PF. Learning disability and epilepsy in an epidemiological sample of individuals with tuberous sclerosis complex. Psychol Med. 2003;33:335–344. doi: 10.1017/s0033291702007092. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Sudo A, Mitani H, Hino O. Presence of a modifier gene(s) affecting early renal carcinogenesis in the Tsc2 mutant (Eker) rat model. Int J Oncol. 2004;24:75–80. [PubMed] [Google Scholar]
- Lewis JC, Thomas HV, Murphy KC, Sampson JR. Genotype and psychological phenotype in tuberous sclerosis. J Med Genet. 2004;41:203–207. doi: 10.1136/jmg.2003.012757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, Jensen FE, Kwiatkowski DJ. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007;27:5546–5558. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzykewicz DA, Newberry P, Danforth N, Halpern EF, Thiele EA. Psychiatric comorbid conditions in a clinic population of 241 patients with tuberous sclerosis complex. Epilepsy Behav. 2007;11:506–513. doi: 10.1016/j.yebeh.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Onda H, Lueck A, Marks PW, Warren HB, Kwiatkowski DJ. Tsc2(+/−) mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J Clin Invest. 1999;104:687–695. doi: 10.1172/JCI7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasumarthi KB, Nakajima H, Nakajima HO, Jing S, Field LJ. Enhanced cardiomyocyte DNA synthesis during myocardial hypertrophy in mice expressing a modified TSC2 transgene. Circ Res. 2000;86:1069–1077. doi: 10.1161/01.res.86.10.1069. [DOI] [PubMed] [Google Scholar]
- Prather P, de Vries PJ. Behavioral and cognitive aspects of tuberous sclerosis complex. J Child Neurol. 2004;19:666–674. doi: 10.1177/08830738040190090601. [DOI] [PubMed] [Google Scholar]
- Pulsifer MB, Winterkorn EB, Thiele EA. Psychological profile of adults with tuberous sclerosis complex. Epilepsy Behav. 2007;10:402–406. doi: 10.1016/j.yebeh.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Joinson C, O'Callaghan F, Osborne JP, Bolton PF. Psychopathology in tuberous sclerosis: an overview and findings in a population-based sample of adults with tuberous sclerosis. J Intellect Disabil Res. 2006;50:561–569. doi: 10.1111/j.1365-2788.2006.00828.x. [DOI] [PubMed] [Google Scholar]
- Ridler K, Suckling J, Higgins NJ, de Vries PJ, Stephenson CM, Bolton PF, Bullmore ET. Neuroanatomical correlates of memory deficits in tuberous sclerosis complex. Cereb Cortex. 2007;17:261–271. doi: 10.1093/cercor/bhj144. [DOI] [PubMed] [Google Scholar]
- Smalley SL. Autism and tuberous sclerosis. J Autism Dev Disord. 1998;28:407–414. doi: 10.1023/a:1026052421693. [DOI] [PubMed] [Google Scholar]
- Uhlmann EJ, Wong M, Baldwin RL, Bajenaru ML, Onda H, Kwiatkowski DJ, Yamada K, Gutmann DH. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol. 2002;52:285–296. doi: 10.1002/ana.10283. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltereit R, Welzl H, Dichgans J, Lipp HP, Schmidt WJ, Weller M. Enhanced episodic-like memory and kindling epilepsy in a rat model of tuberous sclerosis. J Neurochem. 2006;96:407–413. doi: 10.1111/j.1471-4159.2005.03538.x. [DOI] [PubMed] [Google Scholar]
- Way SW, McKenna J, 3rd, Mietzsch U, Reith RM, Wu HC, Gambello MJ. Loss of Tsc2 in radial glia models the brain pathology of tuberous sclerosis complex in the mouse. Hum Mol Genet. 2009;18:1252–1265. doi: 10.1093/hmg/ddp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb DW, Fryer AE, Osborne JP. Morbidity associated with tuberous sclerosis: a population study. Dev Med Child Neurol. 1996;38:146–155. doi: 10.1111/j.1469-8749.1996.tb12086.x. [DOI] [PubMed] [Google Scholar]
- Winterkorn EB, Pulsifer MB, Thiele EA. Cognitive prognosis of patients with tuberous sclerosis complex. Neurology. 2007;68:62–64. doi: 10.1212/01.wnl.0000250330.44291.54. [DOI] [PubMed] [Google Scholar]
- Yeung RS, Gu H, Lee M, Dundon TA. Genetic identification of a locus, Mot1, that affects renal tumor size in the rat. Genomics. 2001;78:108–112. doi: 10.1006/geno.2001.6654. [DOI] [PubMed] [Google Scholar]