Abstract
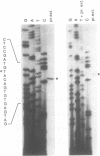
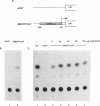
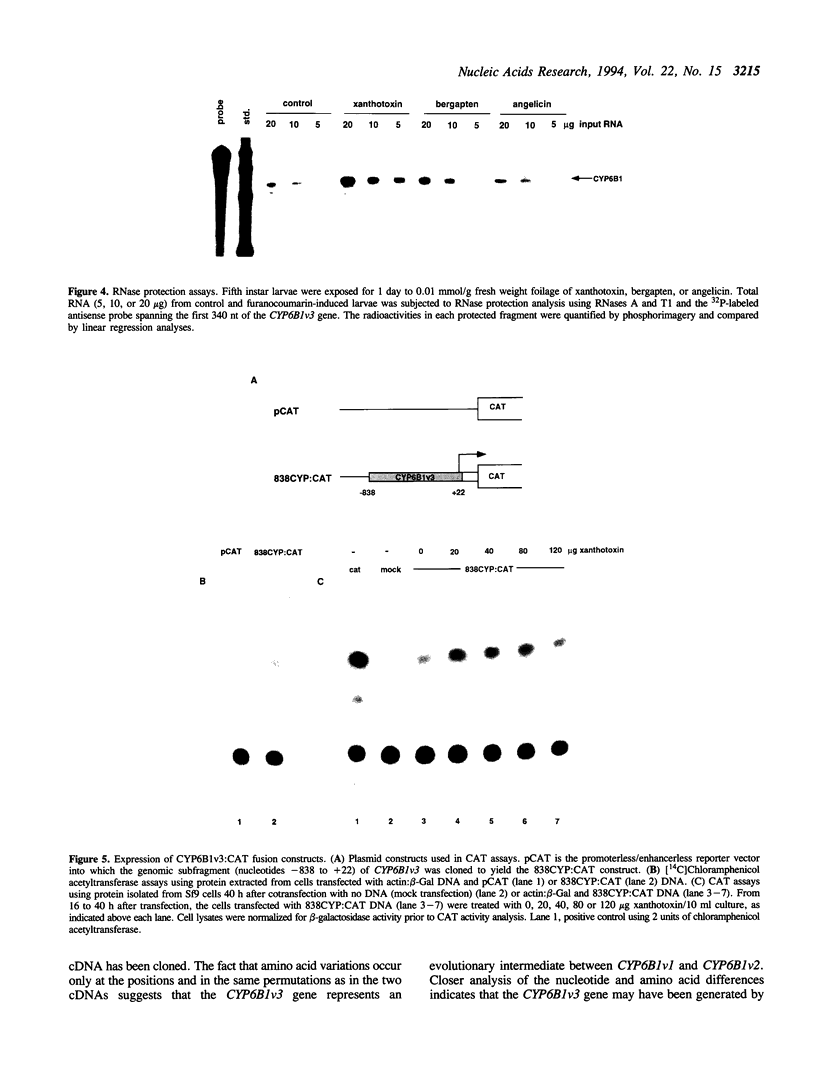
Detoxification of host plant defensive compounds by larval Lepidoptera is mediated by cytochrome P450 monooxygenases (P450s) such as CYP6B1, which is expressed in Papilio polyxenes (black swallowtail) larvae in response to xanthotoxin, a linear furanocoumarin. Baculovirus-mediated expression of two cloned CYP6B1 cDNAs in lepidopteran cell lines has demonstrated that CYP6B1 isozymes primarily metabolize the linear furanocoumarins, xanthotoxin and bergapten, and not angular furanocoumarins. To characterize the regulatory features of the CYP6B1 transcription unit, we have isolated the first full-length CYP6B1v3 genomic DNA clone from P. polyxenes. The open reading frame of this gene is interrupted by a single intron and is virtually identical to the previously characterized CYP6B1 cDNAs. Primer extension and ribonuclease protection analyses have localized the transcription initiation site to a point 28 nucleotides upstream from the AUG initiation codon. RNase protection analyses on RNA from larvae induced by linear and angular furanocoumarins indicate that transcription of the CYP6B1 gene is induced in insects significantly in response to xanthotoxin and only slightly in response to bergapten. Angular furanocoumarins, such as angelicin, which are not appreciably metabolized by the CYP6B1 gene product, do not significantly induce transcription of this gene. We conclude that this P450 gene is transcriptionally regulated in vivo by at least one of the substrates which the encoded protein metabolizes. Transient expression of CAT fusion constructs in transfected Sf9 lepidopteran cells demonstrates that nucleotides -1 to -838 upstream from the CYP6B1v3 transcription initiation site retain basal and xanthotoxin-inducible transcriptional activities in this heterologous cell line. These data clearly indicate that P. polyxenes has adapted to the presence of furanocoumarins in its host plants by evolving P450 isozymes and regulatory cascades which respond to specific toxins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cherbas L., Lee K., Cherbas P. Identification of ecdysone response elements by analysis of the Drosophila Eip28/29 gene. Genes Dev. 1991 Jan;5(1):120–131. doi: 10.1101/gad.5.1.120. [DOI] [PubMed] [Google Scholar]
- Cohen M. B., Schuler M. A., Berenbaum M. R. A host-inducible cytochrome P-450 from a host-specific caterpillar: molecular cloning and evolution. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10920–10924. doi: 10.1073/pnas.89.22.10920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambor J. E., Mennone J., Coon M. E., Hanke J. H., Kavathas P. Identification and characterization of an Alu-containing, T-cell-specific enhancer located in the last intron of the human CD8 alpha gene. Mol Cell Biol. 1993 Nov;13(11):7056–7070. doi: 10.1128/mcb.13.11.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgen J. C., Fallon A. M. Polybrene-mediated transfection of cultured lepidopteran cells: induction of a Drosophila heat shock promoter. In Vitro Cell Dev Biol. 1990 Jul;26(7):731–736. doi: 10.1007/BF02624430. [DOI] [PubMed] [Google Scholar]
- Lindberg R., Burkhart B., Ichikawa T., Negishi M. The structure and characterization of type I P-450(15) alpha gene as major steroid 15 alpha-hydroxylase and its comparison with type II P-450(15) alpha gene. J Biol Chem. 1989 Apr 15;264(11):6465–6471. [PubMed] [Google Scholar]
- Lou H., McCullough A. J., Schuler M. A. 3' splice site selection in dicot plant nuclei is position dependent. Mol Cell Biol. 1993 Aug;13(8):4485–4493. doi: 10.1128/mcb.13.8.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R., Cohen M. B., Berenbaum M. R., Schuler M. A. Black swallowtail (Papilio polyxenes) alleles encode cytochrome P450s that selectively metabolize linear furanocoumarins. Arch Biochem Biophys. 1994 May 1;310(2):332–340. doi: 10.1006/abbi.1994.1175. [DOI] [PubMed] [Google Scholar]
- Mäenpä J., Sigusch H., Raunio H., Syngelmä T., Vuorela P., Vuorela H., Pelkonen O. Differential inhibition of coumarin 7-hydroxylase activity in mouse and human liver microsomes. Biochem Pharmacol. 1993 Mar 9;45(5):1035–1042. doi: 10.1016/0006-2952(93)90247-t. [DOI] [PubMed] [Google Scholar]
- Papadopoulos N., Crow M. T. Transcriptional control of the chicken cardiac myosin light-chain gene is mediated by two AT-rich cis-acting DNA elements and binding of serum response factor. Mol Cell Biol. 1993 Nov;13(11):6907–6918. doi: 10.1128/mcb.13.11.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N. V., Omer C. A. Phenobarbital and sulfonylurea-inducible operons encoding herbicide metabolizing cytochromes P-450 in Streptomyces griseolus. Gene. 1992 Mar 1;112(1):67–76. doi: 10.1016/0378-1119(92)90304-8. [DOI] [PubMed] [Google Scholar]
- Shaw G. C., Fulco A. J. Inhibition by barbiturates of the binding of Bm3R1 repressor to its operator site on the barbiturate-inducible cytochrome P450BM-3 gene of Bacillus megaterium. J Biol Chem. 1993 Feb 5;268(4):2997–3004. [PubMed] [Google Scholar]
- Upadhya P., Rao M. V., Venkateswar V., Rangarajan P. N., Padmanaban G. Identification and functional characterization of a cis-acting positive DNA element regulating CYP 2B1/B2 gene transcription in rat liver. Nucleic Acids Res. 1992 Feb 11;20(3):557–562. doi: 10.1093/nar/20.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]