Abstract
TNFα signaling and cytokine levels play a crucial role in cervical immunity and the host response to infections. We investigated the role of liganded and unliganded glucocorticoid receptor (GR) in IL-6 and IL-8 gene regulation in response to TNFα in the End1/E6E7 immortalized human endocervical epithelial cell line. In the absence of glucocorticoids, both decreasing GR protein levels by an siRNA strategy and results with the GR antagonist RU486 suggest a role for the unliganded GR in reduction of TNFα-induced IL-6 and IL-8 mRNA levels in End1/E6E7 cells. Moreover, GR-dependent repression of endogenous IL-6 mRNA as well as a minimal IL-6 promoter-reporter gene is also demonstrated in COS-1 cells in the absence of GR ligand, suggesting a transcriptional mechanism that is not cell-specific. TNFα induced recruitment of both the unliganded GR and GR-interacting protein type 1 (GRIP-1) to the IL-6 promoter. This, together with GRIP-1 overexpression studies, suggests a function for GRIP-1 as a GR co-repressor in this context. TNFα was shown to induce phosphorylation of the unliganded human GR at Ser-226 but not Ser-211, unlike dexamethasone, which induced hyperphosphorylation at both serine residues. Ser-226 is shown to be required for the ligand-independent GR-mediated repression of IL-6 in response to TNFα. Taken together, these results support a model whereby the unliganded GR attenuates TNFα-stimulated IL-6 transcription by a mechanism involving selective phosphorylation and recruitment of the unliganded GR and GRIP-1 to the IL-6 promoter. These findings suggest the presence of a novel autoregulatory mechanism that may prevent overproduction of IL-6 in the endocervix, possibly protecting against negative effects of excessive inflammation.
Keywords: Co-repressor transcription, Gene Regulation, Inflammation, Steroid Hormone Receptor, Tumor Necrosis Factor (TNF), Glucocorticoid Receptor, Interleukin-6, Interleukin-8, Ligand-independent Activation, Phosphorylation
Introduction
TNFα is a pleiotropic regulatory pro-inflammatory cytokine that mediates its inflammatory effects via binding to the membrane-bound TNFα receptor. It is responsible for the induction of various cytokines including IL-6 (1, 2). IL-6 is a multifunctional cytokine with potent pro-inflammatory properties. It is responsible for T-cell and lymphocyte activation, B-cell differentiation, leukocytosis, and acute phase protein synthesis (3). IL-6 plays an important role in the host defense against pathogen infections, as well as in cell proliferation and cell differentiation (4). IL-6 has also been associated with various autoimmune diseases and the progression of cervical cancer in the cervix in response to pathogen infections (5).
Epithelial cells lining the female reproductive tract serve as a physical barrier against microbial infection (6–8). Several chemokines and cytokines, including IL-6 and IL-8, are expressed in both primary and immortalized vaginal and cervical epithelial cells, aiding in both innate and adaptive immunity (6, 9–13). In the cervix, IL-6 gene expression can be induced by pathogen infection (14, 15) and exposure to pro-inflammatory cytokines such as TNFα (16, 17). IL-6 activates T-cells, causing them to differentiate, and also plays a role in cervical dilation and tumor angiogenesis (18, 19). In the cervix, TNFα signaling and IL-6 and IL-8 levels are relevant to viral infections such as HIV, where pro-inflammatory and anti-inflammatory cytokines play bidirectional roles in HIV-1 pathogenesis, transmission, susceptibility, and resistance (20). In addition, HIV infection has been proposed to be a TNFα-driven disease (21). HIV-1 decreases cervical epithelial cell barrier function, most likely because of the increased production of inflammatory cytokines produced directly by the epithelial cells, including TNFα and IL-6 (8).
Bacterial and viral infections and other stressors increase TNFα expression and binding to its receptor, triggering a cascade of signaling events that leads to the activation of the transcription factors NFκB2 and AP-1 (22–26). The IL-6 promoter contains numerous regulatory elements within the region 300 bp upstream of the transcriptional start site. These include a NFκB-binding element between positions −73 and −63, CCAAT enhancer-binding protein (C/EBPβ)-binding sites at positions −173 and −145, and an AP-1-binding site located between −283 and −277 relative to the transcriptional start site (1, 27).
Like TNFα, glucocorticoid levels are increased by inflammatory stress through the activation of the hypothalamic-pituitary-adrenal axis (28). Lipophilic glucocorticoids elicit a biological response via binding to their cognate receptor, the glucocorticoid receptor (GR). The GR belongs to the steroid hormone receptor subfamily, part of the broader nuclear receptor superfamily. As an inducible transcription factor, it is found predominately in its inactive state in the cytoplasm of target cells (29). In the absence of ligand, the GR is bound to a protein complex, which consists of heat shock proteins hsp90 and hsp70, immunophilins, and other factors (30). The classical mechanism of glucocorticoid action entails binding of the ligand to the ligand-binding domain of the GR, resulting in a conformational change of the receptor followed by the dissociation of the GR from the chaperone protein complex (31). The ligand-bound GR translocates rapidly to the nucleus, where it recognizes specific palindromic DNA sequences known as glucocorticoid response elements in the promoters of target genes to positively regulate transcription (32). Glucocorticoids can also negatively regulate gene expression via direct binding of the liganded GR to so-called negative glucocorticoid response element sequences or regulate transcription positively or negatively by interacting with other transcription factors (33–40). Ligand-activated GR has been shown to interact with NFκB, AP-1, and C/EBP transcription factors, thereby repressing a variety of immune function genes (33, 34, 39, 41, 42).
In addition to activation by steroidal ligands, the GR has also been reported to be activated by various stimuli in the absence of glucocorticoid ligands (43–45). Ursodeoxycholic acid and the β-adrenergic receptor agonists salmeterol and salbutamol were shown to induce GR-dependent transcriptional activity (43, 44). We have recently shown that gonadotropin-releasing hormone (GnRH) results in a selective glucocorticoid-independent increase of mGR phosphorylation and transcriptional activity on an endogenous promoter (45). It was demonstrated that GnRH-induced activation of the GR is dependent on the presence of the GnRH receptor and involves MAPKs (45). From the above-mentioned studies, it is clear that the unliganded GR can be activated by the GnRH receptor and possibly by other plasma membrane receptors. Whether the unliganded GR can be activated by TNFα has not been previously investigated. The present study explores the roles of liganded and unliganded GR in the response to TNFα on the IL-6 promoter in the human End1/E6E7 cell line, a model for the endocervix.
EXPERIMENTAL PROCEDURES
Cell Culture
End1/E6E7 cells (human endocervical cells immortalized with the human papillomavirus 16/E6E7) (46) and COS-1 cells (monkey kidney cells) were purchased from the American Type Culture Collection. The End1/E6E7 cells have been validated as a model for vaginal epithelial immune function by comparisons with primary cell cultures, tissues, animal models, and clinical findings and show similar toll-like receptor and cytokine profiles to primary cells (11, 47, 48). End1/E6E7 cells were grown in 175-cm2 culture flasks (Greiner Bio-One International) in keratinocyte serum-free medium (Sigma-Aldrich) supplemented with CaCl2 (final concentration, 0.4 mm), 100 IU/ml penicillin, 100 μg/ml streptomycin, the provided bovine pituitary extract, and 0.1 ng/ml recombinant EGF.
End1/E6E7 cells were subcultured at 60% confluency. The cells were passaged with prewarmed 0.25% trypsin, 0.1% EDTA in calcium- and magnesium-free PBS (Highveld Biologicals). For End1/E6E7 only, trypsinization was terminated by adding 10 ml of neutralization medium (DMEM/Ham's F-12 medium (1:1), 1% penicillin-streptomycin, and 10% (v/v) fetal calf serum (Delta Bioproducts)).
COS-1 cells were cultured in 175-cm2 culture flasks (Greiner Bio-One International) in DMEM from Sigma-Aldrich, supplemented with 10% (v/v) FCS from Delta Bioproducts and a penicillin (100 IU/ml) and streptomycin (100 μl/ml) mixture (penicillin-streptomycin) from Invitrogen. All of the cells were maintained at 37 °C in a 5% CO2 humidified incubator. The cells were regularly tested for mycoplasma infection by means of Hoechst staining, and only mycoplasma-negative cell lines were used in experiments.
Test Compounds and Antibodies
11β,16α-9-Fluoro-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione (dexamethasone; DEX), 11β-(4-dimethylamino)phenyl-17β-hydroxy-17-(1-propynyl)estra-4,9-dien-3-one (mifepristone; RU486), and recombinant mouse TNFα (T7539) (at least 95% pure) were obtained from Sigma-Aldrich.
Antibodies
The anti-GR (H-300, sc-8992) and anti-GR-interacting protein 1 (GRIP-1) (M-343, sc-8996) antibodies, used in ChIP assays, were obtained from Santa Cruz Biotechnology. The anti-GRIP-1 antibody (G8970-10) used in Western blotting was from Abcam (US Biological). Anti-GAPDH (14C10) antibody was purchased from Cell Signaling. The anti-histone H3 antibody (ab1791) and anti-rabbit HRP conjugate (NA934VS) were purchased from Abcam and AEC Amersham Biosciences, respectively.
Plasmids
The wild type HA-tagged human GR (pCMV-HA-human GR) as well as the Ser-226 (pCMV-HA-S226A) phosphorylation-deficient hGR mutant were a kind gift from M. J. Garabedian ((New York University School of Medicine). The synthetic reporter promoter construct (IL-6NFκB)3-luc has been described elsewhere (49). Briefly, it contains the proximal 50-bp minimal IL-6 promoter with a concatenated trimer of the wild type NFκB sequence and was kindly provided by G. Haegeman (University of Ghent, Ghent, Belgium). The pGL2-basic empty vector was purchased from Promega (Madison, WI). HA-GRIP was a gift from M. R. Stallcup (University of Southern California).
RNA Isolation and cDNA Synthesis
RNA was isolated from End1/E6E7 cells using TRI® reagent (Sigma-Aldrich) as per the manufacture's protocol. Briefly, End1/E6E7 cells were plated in 12-well culture plates (Nunc) at a density of 1 × 105 cells/well. At 70–80% confluency, the cells were treated with test compounds and incubated as described in the figure legends. Induction medium was removed, and 400 μl of TRI® reagent was added to cells and incubated for 5 min at room temperature to allow lysis. RNA (0.5 μg) was reverse transcribed using the Transcriptor first strand cDNA synthesis kit (Roche Applied Science), primed with anchored 2.5 μm oligo(dT)18 as per the manufacturer's instructions in a total reaction volume of 10 μl.
Real Time PCR
Equal volumes of synthesized cDNA were used for semi-quantitative real time PCR using the SensiMix dT kit (Quantace Ltd.) and the Corbett real time PCR machine. IL-6 mRNA levels were measured using the following primer set: IL-6 forward, 5′-TCTCCACAAGCGCCTTCG-3′ and IL-6 reverse, 5′-CTCAGGGCTGAGATGCCG-3′. IL-8 mRNA levels were measured using the following primer set: IL-8 forward, 5′-TGCCAAGGAGTGCTAAAG-3′ and IL-8 reverse, 5′-CTCCACAACCCTCTGCAC-3′. GAPDH served as an internal control using the following primer set: GAPDH forward, 5′-TGAACGGGAAGCTCACTGG-3′ and GAPDH reverse, 5′-TCCACCACCCTGTTGCTGTA-3′. PCR conditions for all of the primer sets were as follows: 95 °C for 10 min followed by 40 cycles of 95 °C for 10 s, 60 °C for 10 s, and 72 °C for 10 s. Melting curve analysis was performed to confirm amplification of a single product in each sample. Relative transcript levels were calculated using the method described by Pfaffl (50) and were normalized to the relative GAPDH transcript levels.
siRNA Transfections
End1/E6E7 cells plated at a density of 1 × 105 cells/well in a 12-well culture plate were transfected with 10 nm validated GR HS_NR3C1_5 (catalog number SI02654757) siRNA directed against the human GR or validated scrambled nonsilencing sequence control (NSC) siRNA (catalog number 1027310) (Qiagen) using HiPerfect transfection reagent (Qiagen) as per the manufacturer's instructions. Briefly, specific or NSC siRNA was diluted in prewarmed Optimem medium with GlutaMAXTM (Invitrogen), to which 3.5 μl of transfection reagent was added. The transfection mixture was incubated at room temperature for 10 min and then added dropwise to the cells to a final concentration of 10 nm. The cells were incubated for 24 h before being treated for 24 h with compounds. RNA was then harvested, and IL-6 and IL-8 mRNA levels were analyzed by quantitative real time PCR, as described above. The cells that were transfected in parallel were analyzed by Western blotting as described below to verify the protein knockdown.
Transient Transfections
For GRIP-1 co-factor overexpression studies, 12-well plates were used. End1/E6E7 cell were plated at a density of 2 × 105 cells/well, and 24 h after seeding, the cells were transfected with 500 ng of pHA-GRIP-1 or pGL2-basic empty vector. Transfected cells were incubated overnight in culture medium at 37 °C in a humidified incubator. The cells were treated 24 h after transfection and incubated for 24 h with various compounds, as described in the figure legends. RNA was isolated as described elsewhere.
For investigation of endogenous IL-6 mRNA expression in the absence and presence of overexpressed GR, COS-1 cells were seeded in 12-well plates at a density of 2 × 105 cells/well. Twenty-four hours after seeding, COS-1 cells were transiently transfected with 1 μg of pCMV-HA-human wild type GR. The cells were treated with compounds 24 h after transfection as described in the figure legends. RNA was isolated as described elsewhere.
For investigation of IL-6 promoter-reporter activity in the absence and presence of overexpressed GR, COS-1 cells were seeded in 24-well plates at a density of 5 × 104 cells/well. The following day cells were transfected with 125 ng of pCMV-HA-hGR or pGL2-basic and 250 ng of (IL-6NFκB)3-luc plasmids. Twenty-four hours after transfection, the cells were treated as described in the figure legends.
To investigate the involvement of phosphorylation at Ser-226 of the unliganded GR in regulating endogenous IL-6 mRNA levels in response to TNFα, the following protocol was followed. COS-1 cells in 12-well plates were seeded at a density of 1 × 105 cells/well. Twenty-four hours after seeding, COS-1 cells were transfected with 1 μg of pCMV-HA-S226A mutant GR, pCMV-HA-hGR, or pGL2-basic (empty vector). Transfected cells were treated 24 h after transfection as described in the figure legends. RNA was isolated as described elsewhere.
Promoter Reporter Luciferase Assays
Luciferase assay reagent (Promega Corp.) was used to quantify luciferase activity in accordance with the manufacturer's instructions. Briefly, 10 μl of cell lysate was allowed to react with 50 μl of luciferase assay reagent. The relative light units were measured using the Veritas luminometer (Turner Biosystems). A further 5 μl of cell lysate for each sample was used to measure protein concentration in each well using the Bradford protein determination method. Luciferase relative light units were normalized to protein concentration, and the results were expressed as fold induction compared with vehicle EtOH control (0.1% EtOH) set as 1.
Subcellular Nuclear Fractionation Assay
End1/E6E7 cells, plated at a density of 2 × 105 cells/well in a 6-well culture dish (Nunc, Denmark), were grown to 80% confluency, after which culture medium was aspirated and replaced with keratinocyte serum-free medium not supplemented with bovine pituitary extract, EGF, and CaCl2, followed by incubation for 24 h. The cells were pretreated with steroid or vehicle (EtOH) for 1 h before TNFα stimulation (20 ng/ml) and further incubated for 2 h, after which they were washed with ice-cold PBS. Buffer A (10 mm HEPES, pH 7.9, 1.5 mm MgCl2 10 mm KCl, 0.5 mm DTT, 0.05% (v/v) Nonidet P-40, 1 proteinase minitablet (Roche Applied Science)) was added to cells (100 μl/well), and cell lysates were harvested by scraping. Lysates were placed in 1.5-ml microcentrifuge tubes, and tubes were placed on ice for 10 min. The lysates were centrifuged for 5 min at 3000 rpm at 4 °C (Eppendorf 5417R). The nuclear pellet was washed with 1 ml of PBS and centrifuged at 3000 rpm for 5 min. The pellet was resuspended in 80 μl of DNase I buffer (40 mm Tris, pH 7.9, 10 mm NaCl, 6 mm MgCl2, 10 mm CaCl2), and 5 μl of DNase I enzyme was added, followed by incubation for 10 min at 37 °C. To 80-μl nuclear fractions, 20 μl of 5× SDS-PAGE loading buffer (100 mm Tris-HCl, pH 6.8, 5% (w/v) SDS, 20% (v/v) glycerol, 2% β-mercaptoethanol, and 0.1% (w/v) bromphenol blue) (51) was added. For SDS-PAGE and Western blot analysis, samples were incubated at 100 °C for 10 min followed by SDS-PAGE as described below. For Western blotting, hybridization was performed with antibodies raised against GR and H3. H3 served as a control for nuclear fractions, as well as loading control.
Western Blot Analysis
For Western blot analysis, protein samples were separated by SDS-PAGE at 200 V in running buffer (25 mm Tris-HCl, pH 6.8, 250 mm glycine, and 0.1% SDS) (51) using a Bio-Rad Mini Protean® II electrophoresis cell. The proteins were electroblotted onto HybondTM ECLTM nitrocellulose membrane (AEC Amersham Biosciences) for 90 min at 180 mA with Bio-Rad Mini Trans-blot® cell in ice-cold transfer buffer (25 mm Tris, 200 mm glycine, 10% (v/v) methanol). The membranes were blocked in 10% (w/v) fat-free milk powder in Tris-buffered saline (50 mm Tris, 150 mm NaCl) containing 0.1% (v/v) Tween (TBS-T), unless otherwise stated, for 1 h at room temperature, followed by incubation with primary antibody overnight at 4 °C. After incubation with primary antibody, the membranes were washed for 15 min and twice for 5 min in TBS-T at room temperature and incubated with the appropriate secondary HRP-conjugated antibody at room temperature for 1 h. The membranes were subsequently washed as before.
Chromatin Immunoprecipitation Assay
To evaluate the association of the GR and the co-factor, GRIP-1, with the IL-6 promoter, the protocol described by Ma et al. (52) was followed with a few modifications. Briefly, End1/E6E7 cells were plated at a density of 5 × 106 cells/15-cm2 culture dish and allowed to reach 80% confluency, after which culture medium was replaced with keratinocyte serum-free medium not supplemented with bovine pituitary extract, EGF, CaCl2, and PenStrep, followed by incubation for 24 h. The cells were treated with steroid for 1 h prior to the addition of 20 ng/ml TNFα and then incubated at 37 °C for a further 2 h. The proteins were cross-linked with 1% formaldehyde for 10 min at 37 °C. Cross-linking was stopped by the addition of 0.125 m glycine, and the mixture was incubated for 5 min at room temperature, while shaking. The cells were washed twice with ice-cold PBS. Thereafter, the cells were scaped and harvested in PBS containing protease inhibitors tablet (Complete Mini protease inhibitor mixture (Roche Applied Science)) followed by centrifugation for 10 min at 1200 × g. The pelleted cells were resuspended in 500 μl of nuclear lysis buffer (1% (w/v) SDS, 50 mm Tris-HCl, pH 8.0, 10 mm EDTA plus 1 tablet 1× Complete Mini protease inhibitor mixture/10 ml). The cell lysates were placed on ice and sonicated to allow for fragmentation of DNA to fragments of between 150 and 500 bp. The cells were sonicated on Power 3 for 10 cycles at 20 s/cycle, with 40-s intervals between pulses, using the Misonix Sonicator® 3000 sonicator. Sonicated chromatin was centrifuged for 10 min at 15,000 × g at 4 °C to pellet cell debris, and the supernatant was transferred to a clean microcentrifuge tube followed by spectrophotometry of the sonicated lysate to measure the amount of A260 units/μl. Nuclear lysis buffer was used to dilute samples to equal chromatin concentration. Sonicated chromatin was either stored at −80 °C or prepared immediately for immunoprecipitation. An aliquot of 30 μg was set aside and served as input sample, whereas 100 μg of sonicated chromatin was diluted with immunoprecipitation dilution buffer (0.01% (w/v) SDS, 20 mm Tris-HCl, pH 8.0, 1.1% (v/v) Triton X-100, 167 mm NaCl, 1.2 mm EDTA plus 1× Complete Mini protease inhibitor mixture (1 tablet/10 ml)) and precleared for 1 h. Chromatin was precleared with 20 μl of 50:50 (v/v) preblocked protein A/G Plus beads (sc-2003; Santa Cruz Biotechnology) for 1 h on a rotating wheel at 4 °C to reduce nonspecific binding. 2 μg of primary antibody (anti-GR, anti-GRIP-1, or anti-IgG antibody) was added to precleared chromatin. This mixture was incubated overnight at 4 °C on a rotating wheel. The following day, 40 μl of precleared protein A/G Plus beads were added, and the mixture was incubated for 6 h at 4 °C on a rotating wheel to allow the capture of the immunosample complexes. The samples were then centrifuged at 5000 × g for 1 min at 4 °C, and the pellet was washed sequentially with 1 ml each of wash buffers I, II, and III (52) to remove DNA and proteins nonspecifically associated with the protein A/G Plus beads. This was followed by three washes with 1 ml of TE buffer (10 mm Tris-HCl, pH 8.0, 1 mm EDTA). The immunoprecipitated DNA-protein complexes were eluted from the protein A/G Plus beads twice with 150 μl of elution buffer (52, 53). The eluates were pooled, and the eluted DNA-protein complexes, as well as input samples, were incubated at 65 °C overnight after the addition of 5 m NaCl to a final concentration of 300 nm to reverse the cross-linking. This was followed by a further incubation at 45 °C for 1 h in the presence of 15 nm EDTA, 125 nm Tris-HCl, and 60 ng/ml proteinase K (Roche Applied Science). Both immunoprecipitated and input DNA were purified using the QIAquick® PCR purification kit (Qiagen) according to the manufacturer's instructions. The purified immunoprecipitated and input DNA were analyzed by means of real time PCR using primers specific for the human IL-6 promoter (hIL-6 sense, 5′-GCGCTAGCCTCAATGACGACCTAAG-3′ and hIL-6 antisense, 5′-GAGCCTCAGACATCTCCAGTCCTAT-3′) (53). Conditions for the real time PCRs were as follows: 95 °C for 10 min followed by 40 cycles of 95 °C for 10 s, 50 °C for 10 s, and 72 °C for 10 s. Both melting curve analysis and agarose gel electrophoresis were performed to confirm specific product amplification in each sample. Relative protein recruitment was determined using real time PCR and calculated by the method described by Pfaffl (50) with slight modifications (50) because the primer efficiency was assumed to be 2 and normalized relative to input, which was set as 1.
Data and Statistical Analysis
GraphPad Prism® version 5.00 for Windows (GraphPad Software) was used for graphical representations and statistical analysis. One-way ANOVA was performed with Dunnett's multiple comparison's test as post-test (when comparing treatment conditions to control (EtOH) only) or Tukey's post-test (when comparing all values to each other). For grouped analysis, i.e. how the response is affected by two factors, two-way ANOVA was used for statistical analysis with Bonferroni as post-test. p values for comparison of two samples were obtained by using the paired t test. The p values are represented as follows: *, p < 0.05; **, p < 0.01; and ***, p < 0.001. Where all of the values were compared with each other, different lowercase letters indicate statistically significant difference; therefore, conditions with the same letter are not statistically significantly different from each other (p > 0.05), whereas those having different letters are statistically significantly different from each other (p < 0.05). For all of the experiments, unless otherwise indicated, the error bars represent the S.E. of three independent experiments.
RESULTS
TNFα Is a Potent Inducer of IL-6 mRNA Expression in End1/E6E7 Cells
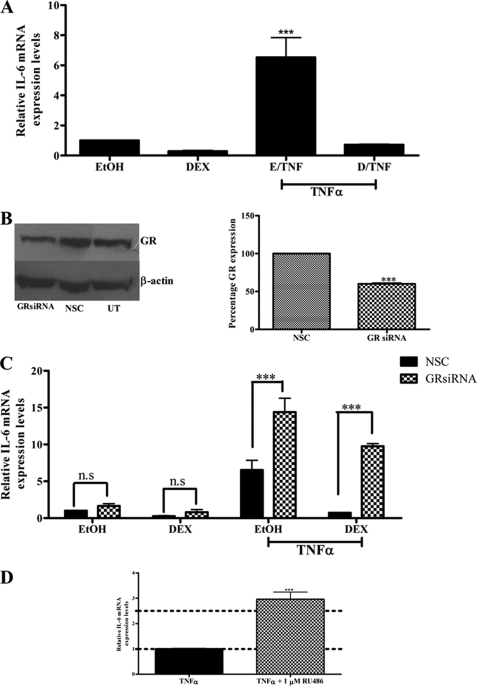
To investigate the effects of TNFα and DEX on IL-6 gene expression, End1/E6E7 cells were treated with 1 μm DEX in the absence and presence of 20 ng/ml TNFα for 24 h, and IL-6 mRNA levels were measured by means of quantitative real time PCR (qPCR), and normalized to GAPDH mRNA levels. As shown in Fig. 1A, IL-6 mRNA expression is significantly (p < 0.001) increased in response to TNFα compared with vehicle control. Additionally, 1 μm DEX strongly attenuates TNFα induction of IL-6 gene expression, whereas DEX alone showed a slight repression of basal IL-6 expression, although this was not significant (Fig. 1A).
FIGURE 1.
Reduced GR protein expression and the GR antagonist RU486 result in increased TNFα-mediated induction of IL-6 mRNA expression in End1/E6E7 cells. A, End1/E6E7 cells were treated with vehicle control or 1 μm DEX in the absence or presence of 20 ng/ml TNFα. Relative interleukin-6 mRNA levels were normalized to GAPDH mRNA levels, which served as an internal control. Relative IL-6 mRNA expression was calculated relative to vehicle control, which was set as 1. The graph shows results of three independent experiments. For statistical analysis, one-way ANOVA and Tukey's multiple comparison post-test were used. B, in parallel, for verification of GR knockdown, End1/E6E7 cells were transfected with 10 nm NSC or GR siRNA oligonucleotides. Forty-eight hours after transfection, the cells were harvested, and whole cell lysates were separated by 8% SDS-PAGE and transferred to nitrocellulose membrane. GR-specific antibody was used for Western blotting analysis and β-actin-specific antibody as loading control. A representative blot is shown for untransfected cells and NSC and GR siRNA transfected cells. Western blots of four independent experiments were pooled and quantified to determine the percentage GR protein expression. For statistical analysis, Student's t test was used. C, End1/E6E7 cells were transfected with either nonspecific siRNA (NSC (black bar)) or with siRNA specific for the human GR (checked bar). Twenty-four hours after transfection, the cells were treated with 20 ng/ml TNFα in the absence or presence of 1 μm DEX. Total RNA was isolated 24 h after induction and reverse transcribed, and relative levels of expression of IL-6 mRNA were measured by qPCR and normalized to relative GAPDH mRNA expression. Relative fold induction of IL-6 mRNA expression was normalized to EtOH, NSC. The graph shows pooled results of three independent experiments. For statistical analysis, two-way ANOVA was used with Bonferroni's post-test. n.s., not significant. D, End1/E6E7 cells were induced with 20 ng/ml TNFα in the absence or presence of 1 μm RU486. The graph represents pooled results of three independent experiments. For statistical analysis, Student's t test was used. For all panels, *** denotes p value < 0.001.
Decreased GR Protein Levels and the GR Antagonist RU486 Both Increase TNFα-induced IL-6 Gene Expression in End1/E6E7 Cells
To investigate the involvement of the GR in TNFα-induced IL-6 mRNA expression, End1/E6E7 cells were transfected with 10 nm validated GR-specific siRNA oligonucleotides or NSC siRNA. The efficiency of the GR siRNA was determined by Western blotting, and GR protein expression was found to decrease by ∼40% as compared with the NSC (Fig. 1B). The siRNA transfection reagent alone as well as the NSC had no effect on IL-6 gene expression as shown in supplemental Fig. S1.
The GR siRNA-transfected End1/E6E7 cells were stimulated with 20 ng/ml TNFα in the absence and presence of 1 μm DEX for 24 h, after which IL-6 mRNA levels were determined by qPCR. Reduced GR levels significantly (p < 0.001) reversed DEX-mediated repression of TNFα-induced IL-6 mRNA levels, confirming a requirement of the GR in DEX-mediated repression of IL-6 mRNA expression (Fig. 1C). Interestingly, qPCR analysis revealed that diminished GR protein levels resulted in an ∼2.6-fold increase in TNFα-induced IL-6 mRNA levels in the absence of GR ligand (Fig. 1C). As expected, the liganded GR inhibits the TNFα response in the presence of DEX, whereas the results suggest that the unliganded GR reduces the TNFα response in the absence of DEX on the endogenous IL-6 gene in End1/E6E7 cells (Fig. 1C).
RU486 is a well known GR antagonist with a high binding affinity for this steroid receptor (54). Consistent with this established effect, we show that RU486 antagonizes DEX-mediated repression of IL-6 mRNA levels in the End1/E6E7 cells (supplemental Fig. S2). To further establish the involvement of the GR in the TNFα-mediated response on IL-6 expression, End1/E6E7 cells were treated with 20 ng/ml TNFα in the absence and presence of 1 μm RU486. It was found that TNFα-induced IL-6 mRNA expression was significantly increased ∼2.5-fold in the presence of 10 μm RU486 (Fig. 1D). This is consistent with the result seen in Fig. 1C, showing an increase in the TNFα response in the presence of GR siRNA.
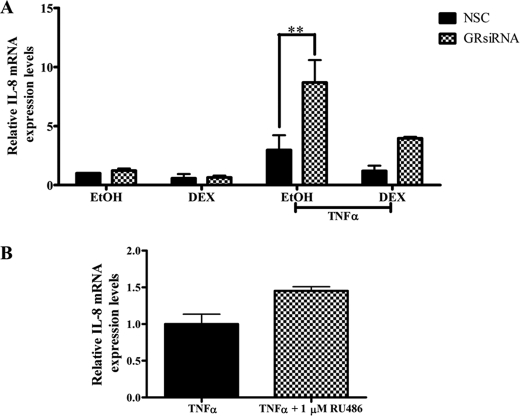
Decreased GR Protein Levels Potentiate, whereas the GR Antagonist RU486 Augments TNFα-induced IL-8 Gene Expression in End1/E6E7 Cells
To determine whether the effects observed for the IL-6 gene in End1/E6E7 cells are gene-specific, similar experiments were performed as described above, probing for endogenous IL-8 mRNA levels. As shown for IL-6 mRNA expression, TNFα-induced IL-8 mRNA levels are significantly increased when GR levels are reduced by means of siRNA (Fig. 2A). Furthermore, although not statistically significant as for IL-6 mRNA expression, the TNFα-induced IL-8 mRNA levels increased in the presence of 1 μm RU486, consistent with an increase in the TNFα response in the presence of GR siRNA (Fig. 2B). This suggests that these effects of the unliganded GR on the TNFα response are not IL-6 gene-specific.
FIGURE 2.
Reduced GR protein expression and the GR antagonist RU486 result in an increased TNFα-mediated induction of IL-8 mRNA expression in End1/E6E7 cells. A, End1/E6E7 cells were transfected with either nonspecific siRNA (NSC (black bars)) or with siRNA specific for the human GR (checked bars). Twenty-four hours after transfection, the cells were treated with 20 ng/ml TNFα in the absence or presence of 1 μm DEX. Total RNA was isolated 24 h after induction and reverse transcribed, and the relative levels of expression of IL-8 mRNA were measured by qPCR and normalized to relative GAPDH mRNA expression. Relative fold induction of IL-8 mRNA expression was normalized to vehicle control, NSC. The graph shows pooled results of three independent experiments. For statistical analysis, two-way ANOVA was used with Bonferroni's post-test. The p values are represented as follows: **, p < 0.01. B, End1/E6E7 cells were induced with 20 ng/ml TNFα in the absence or presence of 1 μm RU486. The graph represents pooled results of three independent experiments. For statistical analysis, Student's t test was used, with no statistical difference found.
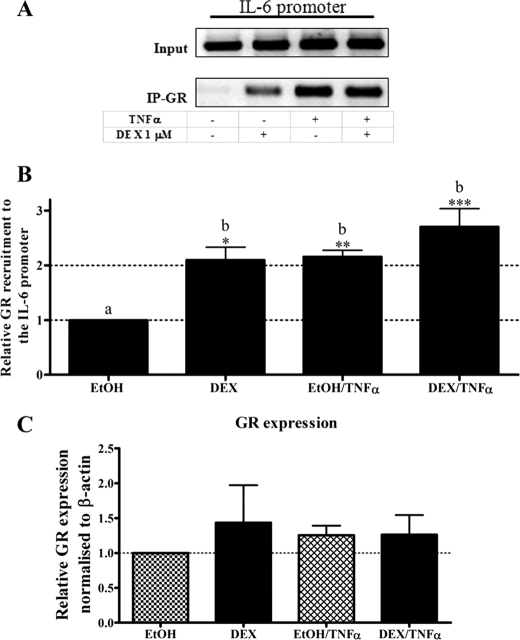
The GR Is Recruited to the IL-6 Promoter in Response to TNFα in the Absence of Glucocorticoid in End1/E6E7 Cells
Having established that decreasing GR protein levels result in a significant (p < 0.001) increase of TNFα-induced IL-6 mRNA expression, recruitment of the endogenous GR to the endogenous IL-6 promoter in the presence of TNFα was next investigated in intact End1/E6E7 cells. This was done by ChIP assay, using an anti-GR antibody for immunoprecipitation, and primers spanning 296 bp of the IL-6 promoter encompassing the NFκB site. Intact End1/E6E7 cells were treated with vehicle control or DEX in the absence or presence of TNFα. As shown in Fig. 3 (A and B), DEX treatment resulted in significant recruitment of the GR to the endogenous IL-6 promoter (p < 0.05). Similarly, DEX treatment in the presence of TNFα also induced significant (p < 0.001) GR recruitment (Fig. 3, A and B). Surprisingly, in the absence of DEX, TNFα caused recruitment of the endogenous GR to the IL-6 promoter to a similar extent as that of DEX in the presence and absence of TNFα (p < 0.01; Fig. 3, A and B). Recruitment of GR to the IL-6 promoter by TNFα alone was not significantly different from that induced by TNFα plus DEX. It could be argued that TNFα affects GR levels; however, as shown in Fig. 3C, TNFα does not alter GR levels compared with vehicle (Fig. 3C). The IgG-negative control confirmed the specificity of the GR antibody (Fig. 4A). Furthermore, agarose gel analysis showed that a single, specific product of the expected size is amplified by PCR and that input samples result in amplification of PCR products of similar intensity (Fig. 3A).
FIGURE 3.
The GR is recruited to the IL-6 promoter in response to TNFα in the absence and presence of DEX in End1/E6E7 cells. End1/E6E7 cells were pretreated with EtOH or 1 μm DEX for 1 h and subsequently treated with 20 ng/ml TNFα for an additional 2 h, followed by ChIP. Immunoprecipitated GR protein bound to the endogenous IL-6 promoter was detected using primers specific for the IL-6 promoter encompassing the NFκB, C/EBPβ, and AP-1 regulatory elements. The co-immunoprecipitated DNA fragments and input DNA were analyzed by qPCR. A, the qPCR products were analyzed on a 2% agarose gel, and a representative result is shown. B, the graph is representative of pooled quantified results of at least four independent experiments and is shown normalized to input and expressed as the fold response relative to EtOH control, which was set as 1. For statistical analysis, one-way ANOVA was used, and Tukey's multiple comparison post-test was performed. Different lowercase letters indicate statistically significant differences; therefore, conditions with the same letter are not statistically significantly different from each other (p > 0.05), whereas those having different letters are statistically significantly different from each other (p < 0.05). The p values are represented as follows: *, p < 0.05; **, p < 0.01; and ***, p < 0.001. The p values represent statistical significance compared with vehicle control (EtOH). C, equal volume of whole cell extracts were separated via SDS-PAGE followed by Western blotting with anti-GR and anti-GAPDH antibodies. The graph is representative of pooled results from three independent experiments with vehicle control (EtOH) set as 1. For statistical analysis, one-way ANOVA and Tukey's multiple comparison post-test were used. The blots were quantified and analyzed relative to vehicle control using Alphaease densitometry software.
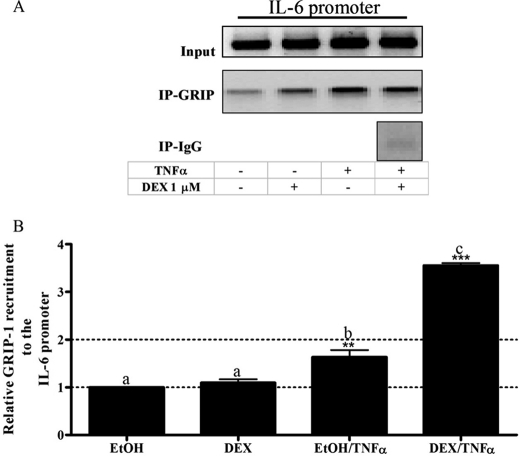
FIGURE 4.
The co-factor GRIP-1 is recruited to the IL-6 promoter in response to both TNFα and DEX/TNFα in End1/E6E7 cells. A, End1/E6E7 cells were pretreated with EtOH or 1 μm DEX for 1 h before the addition of 20 ng/ml TNFα for 2 h. Immunoprecipitated (IP) GRIP-1 protein bound to the endogenous IL-6 promoter was detected using primers specific for the IL-6 promoter. A, the co-immunoprecipitated DNA fragments and input DNA were analyzed by qPCR with the PCR product analyzed on a 2% agarose gel. B, the graph is representative of pooled, quantified results of three independent experiments and are shown normalized to input and expressed as fold response relative to EtOH control. For statistical analysis, one-way ANOVA with Tukey's multiple comparison post-test was performed. Different lowercase letters indicate statistically significant difference; therefore, conditions with the same letter are not statistically significantly different from each other (p > 0.05), whereas those having different letters are statistically significantly different from each other (p < 0.05). The p values are represented as follows: **, p < 0.01; and ***, p < 0.001. The p values represent statistical significance compared with vehicle control (EtOH).
Increased GRIP-1 Co-factor Recruitment to the IL-6 Promoter Occurs in Response to TNFα in the Absence and Presence of DEX, but Not with DEX Alone in End1/E6E7 Cells
Because the co-factor GRIP-1 has been shown to be involved in repression of both AP-1- and NFκB-driven promoters in a GR ligand-dependent manner (55, 56), the involvement of GRIP-1 in GR-mediated repression of IL-6 was next investigated in the absence and presence of DEX and TNFα.
As shown in Fig. 4, DEX treatment alone did not induce recruitment of GRIP-1 to the endogenous IL-6 promoter (p > 0.05), whereas treatment with TNFα significantly (p < 0.01) recruited GRIP-1 to the promoter. DEX in the presence of TNFα caused the most recruitment of GRIP-1 to the IL-6 promoter (p < 0.001). These results suggest that GRIP-1 plays a role in the negative regulation of IL-6 expression in response to TNFα alone and to co-treatment with DEX plus TNFα, in addition to other positively acting factor(s) recruited by TNFα.
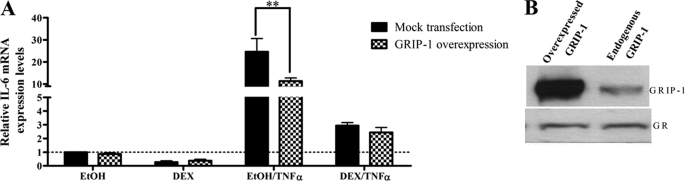
Overexpression of GRIP-1 Protein in End1/E6E7 Cells Attenuates TNFα-induced IL-6 mRNA Levels
Because the previous results suggest that GRIP-1 is involved in negative regulation of IL-6 mRNA levels, the role of GRIP-1 in TNFα-induced IL-6 gene expression in End1/E6E7 cells was further investigated by overexpression of GRIP-1. End1/E6E7 cells were transiently transfected with a GRIP-1 expression vector. Twenty-four hours after transfection, the cells were treated with DEX in the absence and presence of TNFα for 24 h. Total RNA was isolated, and IL-6 mRNA expression was measured by qPCR and was normalized to GAPDH mRNA levels.
Overexpression of GRIP-1 had no effect on basal (vehicle control) IL-6 mRNA levels (Fig. 5A). Increased GRIP-1 protein levels appeared to moderately increase DEX-mediated repression of TNFα-induced IL-6 mRNA expression as shown in Fig. 5, suggesting that the IL-6 promoter may already be saturated with GRIP-1 protein. However, in the absence of DEX, TNFα-induced IL-6 transcription was significantly (p < 0.01) reduced when GRIP-1 protein was overexpressed. Western blotting showed that the overexpression of GRIP-1 did not affect the levels of endogenous GR (Fig. 5B). Taken together, the results in Figs. 3–5 suggest that in the presence of TNFα, GRIP-1 recruited to the IL-6 promoter reduces the levels of transcription of endogenous IL-6 in End1/E6E7 cells, via GR bound to the promoter, both in the absence and in the presence of DEX. The results suggest that in the absence of DEX, but in the presence of TNFα, the unliganded GR occupies the promoter and recruits some GRIP-1, which functions in this context as a co-repressor to dampen the TNFα response. In the presence of DEX and TNFα, more GRIP-1 is recruited, resulting in more repression, via the liganded GR.
FIGURE 5.
Overexpression of GRIP-1 attenuates TNFα induction of IL-6 gene expression in End1/E6E7 cells. Twenty-four hours after transfection with 500 ng of mock plasmid (black bars) or 500 ng of GRIP-1 plasmid DNA (checked bars), End1/E6E7 cells were treated with vehicle control (EtOH) or 1 μm DEX in the absence or presence of 20 ng/ml TNFα for 24 h. Total RNA was isolated, and 500 ng of mRNA was reverse-transcribed. Relative IL-6 mRNA expression was measured by qPCR and normalized to relative GAPDH gene expression, which served as internal control. Relative IL-6 gene expression of treated samples was calculated relative to vehicle control. A, the graph represents pooled results of three independent experiments. For statistical analysis, two-way ANOVA was used with Bonferroni as post-test. The p values are represented as follows: **, p < 0.01. B, in parallel, whole cell lysates were prepared from End1/E6E7 cells transfected with HA-GRIP and separated by 6% SDS-PAGE. GRIP-1 and GR protein levels were probed for as described under “Experimental Procedures” to verify overexpression of GRIP-1 and unchanged total GR levels.
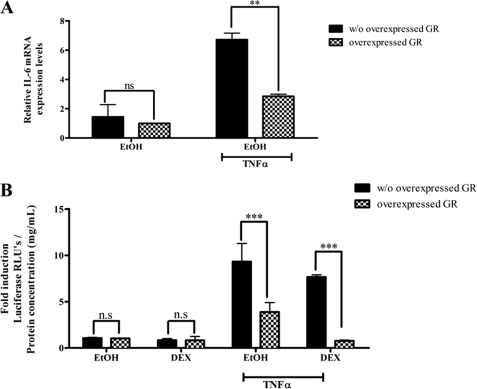
The Unliganded GR Dampens TNFα Induction of Endogenous IL-6 mRNA and an IL-6 Minimal Promoter Reporter Construct in COS-1 Cells
To investigate whether the effect of the unliganded GR on the IL-6 promoter is cell-specific, experiments were also performed in COS-1 cells. Endogenous IL-6 mRNA levels in COS-1 cells were significantly reduced in the presence of overexpressed GR (Fig. 6A), suggesting that these effects of the unliganded GR on IL-6 gene expression are not cell-specific. In addition, TNFα-induced luciferase activity was significantly reduced in the presence of overexpressed GR (Fig. 6B) in COS-1 cells transfected with a minimal IL-6 promoter plasmid construct containing three intact NFκB sites. This result is consistent with a mechanism involving regulation of transcription via the unliganded GR and further suggests that the reduction of the TNFα response by the unliganded GR on the endogenous IL-6 promoter occurs via the NFκB sites.
FIGURE 6.
Overexpression of the GR attenuates TNFα-induced IL-6 mRNA expression as well as IL-6 minimal promoter-reporter gene activity in COS-1 cells. A, the GR was overexpressed in COS-1 cells as described under “Experimental Procedures.” Twenty-four hours after transfection, the transfected cells were treated with 20 ng/ml TNFα or vehicle control for 24 h. Total RNA was isolated, and 500 ng of mRNA was reverse-transcribed. Relative IL-6 mRNA expression was measured by qPCR and normalized to relative GAPDH gene expression, which served as an internal control. Relative IL-6 gene expression of treated samples was calculated relative to vehicle control. The graph represents pooled results of two independent experiments. For statistical analysis, two-way ANOVA was used with Bonferroni as a post-test. The p values are represented as follows: **, p < 0.01. B, the GR was overexpressed in COS-1 cells together with a minimal IL-6 promoter containing three NFκB-binding sites. Transfected cells were treated with 20 ng/ml TNFα in the absence or presence of 1 μm DEX. The graph represents pooled results of three independent experiments. For statistical analysis, two-way ANOVA was used with Bonferroni's post-test. The p values are represented as follows: ***, p < 0.001. n.s., not significant.
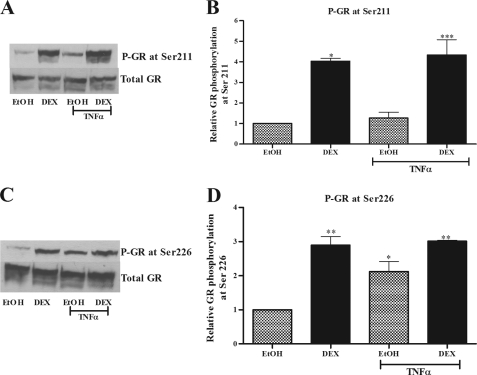
TNFα Induces Phosphorylation of Unliganded GR at Ser-226
Because unliganded GR-mediated repression of IL-6 gene expression in response to TNFα was observed in both COS-1 and End1/E6E7 cells, COS-1 cells were used to further elucidate the mechanism involved, because of their low levels of endogenous GR. Agonist-induced phosphorylation of the GR has been shown to play an important role in GR-mediated cellular responses (57). A recent study also showed that ligand-induced phosphorylation is required for maximal GR activation of a glucocorticoid response element-containing promoter to facilitate GRIP-1 co-factor recruitment (58). Thus TNFα-induced phosphorylation of the GR in the absence of ligand could be a mechanism whereby TNFα activates the unliganded GR. COS-1 cells were treated with TNFα in the absence or presence of DEX for 1 h. The phosphorylation status of overexpressed GR was determined by Western blotting using specific anti-Ser(P)-211 and anti-Ser(P)-226 GR antibodies (Fig. 7, A and C). DEX treatment resulted in a significant (p < 0.01) induction of ligand-dependent phosphorylation at both Ser-211 and Ser-226 of the overexpressed GR in COS-1 cells (Figs. 7, B and D). Furthermore, TNFα in the absence of GR ligand also significantly (p < 0.05) induced GR phosphorylation at Ser-226 but not at Ser-211, showing that TNFα selectively induces phosphorylation of the GR in COS-1 cells. A similar result was also obtained for the endogenous GR in End1/E6E7 cells (supplemental Fig. S3), although the lower GR levels made it difficult to establish statistical significance from Western blotting. Taken together, these results suggest that TNFα activates the GR in the absence of glucocorticoids to result in GR phosphorylation at Ser-226, unlike the phosphorylation pattern observed with DEX, in both COS-1 and End1/E6E7 cells.
FIGURE 7.
TNFα induces phosphorylation of overexpressed GR at Ser-226 and not Ser-211 in COS-1 cells. COS-1 cells were treated with 1 μm DEX in the absence and presence of 20 ng/ml TNFα for 1 h. The cells were harvested, and whole cell lysates were separated by 8% SDS-PAGE. A and C, phospho-GR-specific antibodies raised against serine 211 (A) and serine 226 (C), respectively, were used for Western blotting. Phospho-GR protein levels were normalized to total GR expressed. Total GR protein levels were measured after membrane was stripped and reprobed with an anti-GR specific antibody. B and D, graphs are representative of pooled results from three independent experiments. The relative amounts of phosphorylation at the specific serine residues were quantified and expressed as amounts of phosphorylated GR relative to total GR with vehicle control (EtOH) set as 1. For statistical analysis, one-way ANOVA with Tukey's multiple comparison post-test was performed. The p values are represented as follows: *, p < 0.05; **, p < 0.01; ***, p < 0.001.
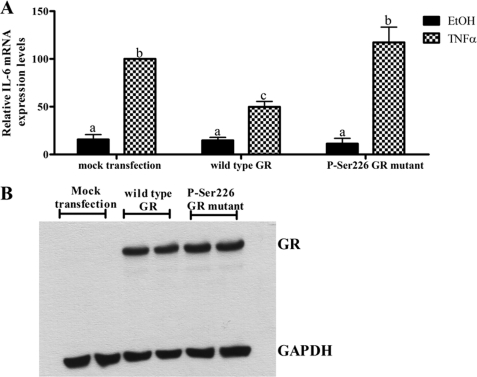
hGR Mutant Experiments Suggest That Repression of TNFα-induced IL-6 Gene Expression by the Unliganded GR Requires Phosphorylation of the GR at Ser-226
COS-1 cells were transfected with wild type hGR or the S226A hGR mutant constructs, after which cells were treated with TNFα or vehicle. Overexpression of wild type hGR inhibits (p < 0.05) TNFα-mediated IL-6 gene expression (Fig. 8A), as shown previously (Fig. 6A). Interestingly, the overexpression of a S226A hGR mutant did not inhibit TNFα-induced IL-6 mRNA expression, unlike wild type GR (Fig. 8A). The observed effects were not due to differences in GR expression levels (Fig. 8B). This suggests that TNFα-induced phosphorylation of the GR at Ser-226 is required for the ligand-independent repression of IL-6 transcription by the GR.
FIGURE 8.
Phosphorylation of the GR at Ser-226 is required for the ligand-independent GR-mediated repression of IL-6 in response to TNFα. A, COS-1 cells were transfected with 1 μg of wild type hGR or Ser(P)-226 hGR mutant. Twenty-four hours after transfection, the cells were treated with vehicle control or 20 ng/ml TNFα. Total RNA was isolated, and 500 ng of mRNA was reverse-transcribed. Relative IL-6 mRNA expression was measured by qPCR and normalized to relative GAPDH gene expression, which served as internal control. Relative IL-6 gene expression of treated samples was calculated relative to vehicle control. The graph represents pooled results of three independent experiments. TNFα-treated mock transfected samples were set as 100% IL-6 expression. For statistical analysis, one-way ANOVA and Tukey's multiple comparison post-test were used. Different lowercase letters indicate statistically significant differences; therefore, conditions with the same letter are not statistically significantly different from each other (p > 0.05), whereas those having different letters are statistically different from each other (p < 0.05). B, COS-1 cells transfected in parallel as described above were prepared for Western blot analysis. The cells were harvested, and whole cell lysates were separated by 8% SDS-PAGE. Total GR protein levels were probed for, and GAPDH levels served as loading control. This served to verify equal expression of wild type hGR versus Ser(P)-226 hGR mutant expression.
DISCUSSION
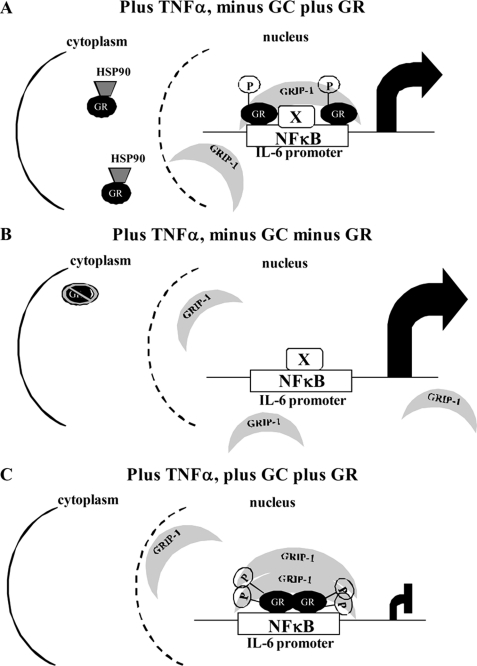
In the present study, the role of the GR in transcriptional regulation of endogenous IL-6 in response to TNFα and DEX in End1/E6E7 cells was investigated. We provide evidence that the unliganded-GR is involved in dampening TNFα-induced IL-6 transcription. Furthermore, this affect is not IL-6 gene-specific, because similar results were obtained on another pro-inflammatory gene, the IL-8 gene, in End1/E6E7 cells. We show in these cells that the GR is recruited to the IL-6 promoter in response to TNFα in both the absence and the presence of DEX. Furthermore, our results suggest that GRIP-1 recruitment to the IL-6 promoter plays a role in the negative regulation of IL-6 expression in response to TNFα alone and to co-treatment with DEX plus TNFα, in addition to other positively acting factor(s) recruited by TNFα. Experiments with overexpressed hGR in COS-1 cells suggest that the ligand-independent GR-mediated repression of IL-6 gene expression in response to TNFα is not cell-specific and is mediated by binding of unliganded GR to NFκB sites on the IL-6 promoter. Further mechanistic studies show that TNFα selectively induces hyperphosphorylation of both endogenous GR in End1/E6E7 cells and overexpressed GR in COS cells, at Ser-226 but not Ser-211, in contrast to DEX, which induces GR hyperphosphorylation at both Ser-211 and Ser-226. Results with the GR S226A mutant suggest that GR phosphorylation at Ser-226 is required for the ligand-independent GR-mediated repression of IL-6 in response to TNFα. Fig. 9 summarizes these results in the form of a model for the role of the GR in modulation of the IL-6 response in response to TNFα.
FIGURE 9.
Schematic model for GR cross-talk with TNFα signaling on the IL-6 promoter in endocervical epithelial cells. A, TNFα stimulated IL-6 promoter in the presence of GR but the absence of glucocorticoids. B, TNFα stimulated IL-6 promoter in the absence of GR and glucocorticoids. C, TNFα stimulated IL-6 promoter in the presence of GR and glucocorticoids. “X” denotes an unidentified transcription factor, while “p” denotes a phosphorylated serine residue.
Whether or not TNF induces GR nuclear translocation in the absence of glucocorticoids is unclear. Extensive nuclear translocation experiments were performed by both biochemical fractionation and immunofluorescence on expressed GR in COS-1 cells and endogenous GR in End1/E6E7 cells (data not shown and supplemental Fig. S4). Our results suggest that TNFα may result in a small degree of nuclear translocation, as compared with DEX, in both cell types. For example, we found by biochemical fractionation that TNFα appeared to induce some nuclear translocation of the unliganded GR in the End1/E6E7 cells, albeit not as efficiently as DEX (supplemental Fig. S4), but no statistical significance could be established for this result, possibly because of the small percentage changes involved. We find that both cell types contain some nuclear GR in the absence of GR ligand, consistent with the literature (59), even after serum starvation. Thus from these results we cannot exclude the possibility that the effects of TNFα do not involve GR nuclear translocation but rather target unliganded nuclear GR.
The finding that TNFα increases IL-6 gene expression is consistent with previous reports in End1/E6E7 cells (11). The repressive action of DEX on TNFα-induced IL-6 expression is also in accordance with previous studies in murine endothelial heart (TC10s) and mouse fibroblast (L929A) cells (34, 60). This is, however, the first study to show GR ligand-independent IL-6 gene repression by recruitment of the GR to the IL-6 promoter in response to TNFα. For both treatment with TNFα alone and TNFα in the presence of DEX, GR acts as a repressor, indicating that although TNFα induces IL-6 expression (Fig. 1), it concurrently recruits the GR to dampen gene expression, possibly thereby preventing overproduction of IL-6 mRNA.
Numerous studies have reported ligand-independent activation of steroid receptors including the estrogen receptor (ER) (61–63), progesterone receptor (PR) (64), and androgen receptor (65, 66). Ligand-independent activation of a steroid receptor by TNFα has also been previously demonstrated (61) in U2OS cells stably transfected with the ER. The unliganded ER as been shown to be recruited to the TNFα promoter in response to TNFα, and a decrease in ER protein attenuates TNFα gene expression (61). However, unlike the present study showing a repressive role for the unliganded GR on the IL-6 and IL-8 promoters, the unliganded ER was shown to act as a co-activator on the TNFα promoter (61), suggesting receptor-, cell-, or promoter-specific effects. Only a few studies have investigated ligand-independent activation of the GR (43–45). All three studies investigating activation of the unliganded GR reported increased transactivation of glucocorticoid-inducible promoters (43–45), whereas the present study is the first to report ligand-independent transrepression by the GR on an endogenous target gene. Ligand-independent repression by nuclear receptors has been shown for the thyroid hormone receptor (67), retinoic acid receptor (68), and the peroxisome proliferator-activated receptor γ (69). However, the ligand-independent repression induced by these nuclear receptors is reversed in the presence of agonists, in contrast to findings the present study, which show increased repression of IL-6 expression in the presence of GR agonist. The above-mentioned studies showed fewer co-repressors recruited in response to ligand, unlike the finding of the present study, whereby increased GRIP-1 is recruited in the presence of ligand, further supporting the hypothesis that GRIP-1 acts as a co-repressor for both the unliganded and liganded GR in this context. The ability of GRIP-1 to act as a GR co-repressor via a tethering mechanism is consistent with the literature (55, 56).
The GR contains a number of phosphorylation sites with Ser-203, Ser-211, and Ser-226 conserved between species. These residues become hyperphosphorylated on ligand binding (57, 70). Hyperphosphorylation at one or more of these sites was shown to be required for promoter-specific increased transcriptional activation efficacy (58). As shown in Fig. 7 and supplemental Fig. S3, DEX induced phosphorylation of both serine residues, Ser-211 and Ser-226, in the absence and presence of TNFα, whereas TNFα selectively increases phosphorylation at Ser-226. Furthermore, we show using the S226A GR mutant (Fig. 7B) that GR phosphorylation at Ser-226 is required for ligand-independent GR-mediated repression of IL-6 expression in response to TNFα, consistent with the Western blot results. Interestingly, basal phosphorylation of both serine residues investigated was high in the End1/E6E7 cell line, which might suggest a relatively high level of endogenous unliganded GR present in the nucleus. Nuclear GR in the absence of DEX stimulation is unlikely to be due to GR agonist present in the medium, because the selective phosphorylation induced by TNFα in both End1/E6E7 and COS-1 cells argues against this phenomenon, as well as the lack of GR recruitment to the IL-6 promoter in the absence of TNFα in the End1/E6E7 cells. Our recent study (45) investigating ligand-independent activation of the GR by GnRH, also reported selective GR phosphorylation at Ser-226 and not Ser-211 in a mouse pituitary cell line (LβT2) (45). Taken together, these results suggest that Ser-226 plays an important role in ligand-independent activation of the GR in several cell types, in response to several membrane receptor ligands other than the GR.
The exact signaling pathways by which TNFα results in phosphorylation of the unliganded GR at Ser-226 still remain to be established. The PKC pathway in combination with the MAPK pathways were reported to be involved in the GnRH-induced ligand-independent phosphorylation of the GR in LβT2 cells (45). Because TNFα has been shown to activate JNK (71) and JNK has been shown to interact with and phosphorylate the GR in other cells (72), it is possible that TNF could induce GR phosphorylation at Ser-226 via JNK in End1/E6E7 cells. However, results from our laboratory (45) suggest that the GR is not phosphorylated by JNK in LβT2 cells, indicating that the kinases involved may be cell-specific (45). In addition, using MAPK pathway inhibitors, we find that the MAPK pathways ERK, JNK, and p38 do not appear to be involved in TNFα-induced IL-6 mRNA expression in the absence of DEX in the End1/E6E7 cells,3 suggesting that MAPKs are not involved in phosphorylation of the unliganded GR at Ser-226 in these cells. Previous results from our laboratory suggest that TNFα-induced GR phosphorylation of the GR at Ser-226 might be required for the interaction of GRIP-1 with the unliganded GR in End1/E6E7 cells, because we have previously shown that ligand-induced phosphorylation of transfected human GR at one or more serine residues (at positions 211, 226, or 203) is required for GRIP-1 interaction in transfected COS-1 cells (58).
GRIP-1 is part of the p160 family of co-factors, which interacts with the conserved LXXLL sequence motif situated in the AF-2 region of steroid receptors (73). This family of co-factors is generally recognized as playing a role as co-activators (74). However, GRIP-1 has been reported to be involved in DEX-mediated repression of the AP-1-driven collegenase-3 gene (55), and a subsequent study also showed GRIP-1 acting as a co-repressor on a NFκB-driven promoter construct in response to DEX (56). TNFα induction of IL-6 expression has been reported to occur mainly via NFκB (27), which recruits co-factors such as SRC-1 and CBP/p300 to the promoter region of the IL-6 gene (1, 27, 75). The present study shows that GRIP-1 is recruited to the IL-6 promoter in response to TNFα, not in the capacity of co-activator, but as co-repressor (Fig. 4). This is supported by the finding that overexpression of GRIP-1 attenuated TNFα-induced IL-6 mRNA expression (Fig. 5A). Co-factor interaction with other steroid receptors such as the PR, ER, and androgen receptor in the absence of ligand (in response to a nonsteroid hormone stimulus) has been previously reported (66, 76–83). GnRH treatment of mouse pituitary αT3–1 cells induced progesterone response element reporter promoter activity, which was dependent on both PR and steroid receptor co-activator (SRC)-3 expression (81). Both PR and SRC-3 are recruited to the progesterone response element and gonadotropin α-subunit promoter in response to GnRH (81). Furthermore, cylin-D1 and increased cAMP levels induced the interaction of SRC-1 with ERα in a ligand-independent manner (84). Increased cAMP levels have also been shown to facilitate interaction between SRC-1 and chicken PR in a ligand-independent manner (83), and SRC-1 and androgen receptor protein-protein interaction is induced in response to IL-6 in the absence of androgens (66). Furthermore, phosphorylation of both PR and ER increases their association with co-factors, namely SRC-1, GRIP-1, and CBP (77, 83). Phosphorylation of the ER at Ser-104/106/118 has also been shown to regulate SRC-1, GRIP-1, and CBP interaction with unliganded ER (77). Thus TNFα-induced GR phosphorylation, activation, and modulation of co-factor interaction may also occur for other steroid receptors.
The finding in the present study that GRIP-1 acts in a repressor mode on the IL-6 promoter suggests that positively acting factors other than GRIP-1 mediate the increase in transcription of the IL-6 gene relative to vehicle, in response to TNFα. Because TNFα has been shown to be a potent activator of both NFκB and AP-1, both of which are required for maximal induction of IL-6 gene expression in murine fibrosarcoma L929sA, human embryonic kidney HEK293T, and primary fibroblast cells (1, 85), both of these factors are likely to be involved. In addition, they may also be involved in interaction with the unliganded GR, because both the p65 subunit of NFκB (33, 86–88) and c-Jun have been shown to interact with the liganded GR (89).
To conclude, from the present study and other recent findings regarding the GR (45), it is clear that a paradigm shift concerning the mechanism of action of the GR is developing. The present study proposes a model whereby TNFα significantly increases pro-inflammatory IL-6 gene expression, the extent of which depends on the expression levels of the unliganded GR, by concurrently recruiting unliganded GR and GRIP-1 to the IL-6 promoter. This suggests that any factors that modulate GR protein levels will also affect the inflammatory response in the absence of glucocorticoids. This mechanism could be designed to protect the cervix from an excessive pro-inflammatory response. Uncontrolled inflammation could have negative effects on the female reproductive tract, such as increased susceptibility to disease (90). These mechanisms may be particularly relevant to pathogen infections such as HIV-1 in the cervix, where TNFα, IL-6, and GR levels are likely to play a key role in pathogenesis. Physiological relevance might also apply to other mucosal surfaces such as the respiratory and intestinal mucosa.
Supplementary Material
Acknowledgments
We thank members of the Hapgood laboratory for helpful discussions, especially Katie Hadley. We thank Calvin Kemp and Lance Wehmeyer for the immunofluorescence experiments.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
N. J. D. Verhoog and J. P. Hapgood, unpublished data.
- NFκB
- nuclear factor κB
- GR
- glucocorticoid receptor
- hGR
- human GR
- GRIP-1
- GR-interacting protein type 1
- DEX
- dexamethasone
- C/EBP
- CCAAT enhancer-binding protein
- AP-1
- activator protein-1
- GnRH
- gonadotropin-releasing hormone
- NSC
- nonsilencing sequence control
- ANOVA
- analysis of variance
- qPCR
- quantitative real time PCR
- ER
- estrogen receptor
- PR
- progesterone receptor
- SRC
- steroid receptor co-activator
- CBP
- cAMP-responsive element-binding protein-binding protein.
REFERENCES
- 1. Vanden Berghe W., De Bosscher K., Boone E., Plaisance S., Haegeman G. (1999) J. Biol. Chem. 274, 32091–32098 [DOI] [PubMed] [Google Scholar]
- 2. Cromwell O., Hamid Q., Corrigan C. J., Barkans J., Meng Q., Collins P. D., Kay A. B. (1992) Immunology 77, 330–337 [PMC free article] [PubMed] [Google Scholar]
- 3. Kishimoto T. (1989) Blood 74, 1–10 [PubMed] [Google Scholar]
- 4. Iglesias M., Plowman G. D., Woodworth C. D. (1995) Am. J. Pathol. 146, 944–952 [PMC free article] [PubMed] [Google Scholar]
- 5. Rasmussen S. J., Eckmann L., Quayle A. J., Shen L., Zhang Y. X., Anderson D. J., Fierer J., Stephens R. S., Kagnoff M. F. (1997) J. Clin. Invest. 99, 77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wira C. R., Fahey J. V., Sentman C. L., Pioli P. A., Shen L. (2005) Immunol. Rev. 206, 306–335 [DOI] [PubMed] [Google Scholar]
- 7. Kaushic C., Ferreira V. H., Kafka J. K., Nazli A. (2010) Am. J. Reprod. Immunol. 63, 566–575 [DOI] [PubMed] [Google Scholar]
- 8. Nazli A., Chan O., Dobson-Belaire W. N., Ouellet M., Tremblay M. J., Gray-Owen S. D., Arsenault A. L., Kaushic C. (2010) PLoS Pathog. 6, e1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Woodworth C. D., Simpson S. (1993) Am. J. Pathol. 142, 1544–1555 [PMC free article] [PubMed] [Google Scholar]
- 10. Barclay C. G., Brennand J. E., Kelly R. W., Calder A. A. (1993) Am. J. Obstet. Gynecol. 169, 625–632 [DOI] [PubMed] [Google Scholar]
- 11. Fichorova R. N., Anderson D. J. (1999) Biol. Reprod. 60, 508–514 [DOI] [PubMed] [Google Scholar]
- 12. Oakeshott P., Hay P., Hay S., Adefowora A., Shattock R. (2003) Int. J. STD AIDS 14, 289–290 [DOI] [PubMed] [Google Scholar]
- 13. Miller C. J., Shattock R. J. (2003) Microbes Infect. 5, 59–67 [DOI] [PubMed] [Google Scholar]
- 14. Malejczyk J., Malejczyk M., Urbanski A., Köck A., Jablonska S., Orth G., Luger T. A. (1991) Cell Immunol. 136, 155–164 [DOI] [PubMed] [Google Scholar]
- 15. Partridge M., Chantry D., Turner M., Feldmann M. (1991) J. Invest. Dermatol. 96, 771–776 [DOI] [PubMed] [Google Scholar]
- 16. Yoshizaki K., Nishimoto N., Matsumoto K., Tagoh H., Taga T., Deguchi Y., Kuritani T., Hirano T., Hashimoto K., Okada N. (1990) Cytokine 2, 381–387 [DOI] [PubMed] [Google Scholar]
- 17. Zhang Y. H., Lin J. X., Vilcek J. (1990) Mol. Cell Biol. 10, 3818–3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hirano T., Akira S., Taga T., Kishimoto T. (1990) Immunol. Today 11, 443–449 [DOI] [PubMed] [Google Scholar]
- 19. Jones R. L., Morison N. B., Hannan N. J., Critchley H. O., Salamonsen L. A. (2005) Hum. Reprod. 20, 2724–2735 [DOI] [PubMed] [Google Scholar]
- 20. Fichorova R. N., Bajpai M., Chandra N., Hsiu J. G., Spangler M., Ratnam V., Doncel G. F. (2004) Biol. Reprod. 71, 761–769 [DOI] [PubMed] [Google Scholar]
- 21. Herbein G., Khan K. A. (2008) Trends Immunol. 29, 61–67 [DOI] [PubMed] [Google Scholar]
- 22. Rothe M., Sarma V., Dixit V. M., Goeddel D. V. (1995) Science 269, 1424–1427 [DOI] [PubMed] [Google Scholar]
- 23. Hsu H., Shu H. B., Pan M. G., Goeddel D. V. (1996) Cell 84, 299–308 [DOI] [PubMed] [Google Scholar]
- 24. Liu Z. G., Hsu H., Goeddel D. V., Karin M. (1996) Cell 87, 565–576 [DOI] [PubMed] [Google Scholar]
- 25. Natoli G., Costanzo A., Ianni A., Templeton D. J., Woodgett J. R., Balsano C., Levrero M. (1997) Science 275, 200–203 [DOI] [PubMed] [Google Scholar]
- 26. Lee S. Y., Reichlin A., Santana A., Sokol K. A., Nussenzweig M. C., Choi Y. (1997) Immunity 7, 703–713 [DOI] [PubMed] [Google Scholar]
- 27. Vanden Berghe W., Plaisance S., Boone E., De Bosscher K., Schmitz M. L., Fiers W., Haegeman G. (1998) J. Biol. Chem. 273, 3285–3290 [DOI] [PubMed] [Google Scholar]
- 28. Newton R. (2000) Thorax 55, 603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Picard D., Kumar V., Chambon P., Yamamoto K. R. (1990) Cell Regul. 1, 291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pratt W. B., Toft D. O. (1997) Endocr. Rev. 18, 306–360 [DOI] [PubMed] [Google Scholar]
- 31. Griekspoor A., Zwart W., Neefjes J., Michalides R. (2007) Nucl. Recept. Signal. 5, e003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou J., Cidlowski J. A. (2005) Steroids 70, 407–417 [DOI] [PubMed] [Google Scholar]
- 33. Scheinman R. I., Gualberto A., Jewell C. M., Cidlowski J. A., Baldwin A. S., Jr. (1995) Mol. Cell Biol. 15, 943–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De Bosscher K., Schmitz M. L., Vanden Berghe W., Plaisance S., Fiers W., Haegeman G. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 13504–13509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Bosscher K., Vanden Berghe W., Haegeman G. (2000) J. Neuroimmunol. 109, 16–22 [DOI] [PubMed] [Google Scholar]
- 36. Adcock I. M., Caramori G. (2001) Immunol. Cell Biol. 79, 376–384 [DOI] [PubMed] [Google Scholar]
- 37. Reichardt H. M., Tuckermann J. P., Göttlicher M., Vujic M., Weih F., Angel P., Herrlich P., Schütz G. (2001) EMBO J. 20, 7168–7173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. De Bosscher K., Vanden Berghe W., Haegeman G. (2006) Oncogene 25, 6868–6886 [DOI] [PubMed] [Google Scholar]
- 39. Jonat C., Rahmsdorf H. J., Park K. K., Cato A. C., Gebel S., Ponta H., Herrlich P. (1990) Cell 62, 1189–1204 [DOI] [PubMed] [Google Scholar]
- 40. Nissen R. M., Yamamoto K. R. (2000) Genes Dev. 14, 2314–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. De Bosscher K., Haegeman G. (2009) Mol. Endocrinol. 23, 281–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beck I. M., Vanden Berghe W., Vermeulen L., Yamamoto K. R., Haegeman G., De Bosscher K. (2009) Endocr. Rev. 30, 830–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tanaka H., Makino Y., Miura T., Hirano F., Okamoto K., Komura K., Sato Y., Makino I. (1996) J. Immunol. 156, 1601–1608 [PubMed] [Google Scholar]
- 44. Eickelberg O., Roth M., Lörx R., Bruce V., Rüdiger J., Johnson M., Block L. H. (1999) J. Biol. Chem. 274, 1005–1010 [DOI] [PubMed] [Google Scholar]
- 45. Kotitschke A., Sadie-Van Gijsen H., Avenant C., Fernandes S., Hapgood J. P. (2009) Mol. Endocrinol. 23, 1726–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fichorova R. N., Rheinwald J. G., Anderson D. J. (1997) Biol. Reprod. 57, 847–855 [DOI] [PubMed] [Google Scholar]
- 47. Fichorova R. N., Desai P. J., Gibson F. C., 3rd, Genco C. A. (2001) Infect. Immun. 69, 5840–5848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Trifonova R. T., Doncel G. F., Fichorova R. N. (2009) Antimicrob. Agents Chemother. 53, 1490–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Plaisance S., Vanden Berghe W., Boone E., Fiers W., Haegeman G. (1997) Mol. Cell Biol. 17, 3733–3743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pfaffl M. W. (2001) Nucleic Acids Res. 29, 2002–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sambrook J., Maniatus T., Fritsch E. F. (1989) Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 52. Ma H., Shang Y., Lee D. Y., Stallcup M. R. (2003) Methods Enzymol. 364, 284–296 [DOI] [PubMed] [Google Scholar]
- 53. Vanden Berghe W., Dijsselbloem N., Vermeulen L., Ndlovu N., Boone E., Haegeman G. (2006) Cancer Res. 66, 4852–4862 [DOI] [PubMed] [Google Scholar]
- 54. Cadepond F., Ulmann A., Baulieu E. E. (1997) Annu. Rev. Med. 48, 129–156 [DOI] [PubMed] [Google Scholar]
- 55. Rogatsky I., Zarember K. A., Yamamoto K. R. (2001) EMBO J. 20, 6071–6083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rogatsky I., Luecke H. F., Leitman D. C., Yamamoto K. R. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 16701–16706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ismaili N., Garabedian M. J. (2004) Ann. N.Y. Acad. Sci. 1024, 86–101 [DOI] [PubMed] [Google Scholar]
- 58. Avenant C., Kotitschke A., Hapgood J. P. (2010) Biochemistry 49, 972–985 [DOI] [PubMed] [Google Scholar]
- 59. Sarabdjitsingh R. A., Meijer O. C., de Kloet E. R. (2010) Brain Res. 1331, 1–11 [DOI] [PubMed] [Google Scholar]
- 60. Koubovec D., Vanden Berghe W., Vermeulen L., Haegeman G., Hapgood J. P. (2004) Mol. Cell. Endocrinol. 221, 75–85 [DOI] [PubMed] [Google Scholar]
- 61. Cvoro A., Tzagarakis-Foster C., Tatomer D., Paruthiyil S., Fox M. S., Leitman D. C. (2006) Mol. Cell 21, 555–564 [DOI] [PubMed] [Google Scholar]
- 62. Bunone G., Briand P. A., Miksicek R. J., Picard D. (1996) EMBO J. 15, 2174–2183 [PMC free article] [PubMed] [Google Scholar]
- 63. El-Tanani M. K., Green C. D. (1997) Mol. Endocrinol. 11, 928–937 [DOI] [PubMed] [Google Scholar]
- 64. Pierson-Mullany L. K., Lange C. A. (2004) Mol. Cell Biol. 24, 10542–10557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ueda T., Bruchovsky N., Sadar M. D. (2002) J. Biol. Chem. 277, 7076–7085 [DOI] [PubMed] [Google Scholar]
- 66. Ueda T., Mawji N. R., Bruchovsky N., Sadar M. D. (2002) J. Biol. Chem. 277, 38087–38094 [DOI] [PubMed] [Google Scholar]
- 67. Lin H. M., Zhao L., Cheng S. Y. (2002) J. Biol. Chem. 277, 28733–28741 [DOI] [PubMed] [Google Scholar]
- 68. Chen J. D., Evans R. M. (1995) Nature 377, 454–457 [DOI] [PubMed] [Google Scholar]
- 69. Yu C., Markan K., Temple K. A., Deplewski D., Brady M. J., Cohen R. N. (2005) J. Biol. Chem. 280, 13600–13605 [DOI] [PubMed] [Google Scholar]
- 70. Weigel N. L., Moore N. L. (2007) Nucl. Recept. Signal. 5, e005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Reinhard C., Shamoon B., Shyamala V., Williams L. T. (1997) EMBO J. 16, 1080–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bruna A., Nicolàs M., Muñoz A., Kyriakis J. M., Caelles C. (2003) EMBO J. 22, 6035–6044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McKenna N. J., O'Malley B. W. (2002) Endocrinology 143, 2461–2465 [DOI] [PubMed] [Google Scholar]
- 74. McKenna N. J., Lanz R. B., O'Malley B. W. (1999) Endocr. Rev. 20, 321–344 [DOI] [PubMed] [Google Scholar]
- 75. De Bosscher K., Vanden Berghe W., Haegeman G. (2001) Mol. Endocrinol. 15, 219–227 [DOI] [PubMed] [Google Scholar]
- 76. Tremblay A., Giguère V. (2001) J. Steroid Biochem. Mol. Biol. 77, 19–27 [DOI] [PubMed] [Google Scholar]
- 77. Dutertre M., Smith C. L. (2003) Mol. Endocrinol. 17, 1296–1314 [DOI] [PubMed] [Google Scholar]
- 78. Smith C. L., Conneely O. M., O'Malley B. W. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 6120–6124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Deblois G., Giguère V. (2003) J. Steroid Biochem. Mol. Biol. 85, 123–131 [DOI] [PubMed] [Google Scholar]
- 80. Zwijsen R. M., Buckle R. S., Hijmans E. M., Loomans C. J., Bernards R. (1998) Genes Dev. 12, 3488–3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. An B. S., Selva D. M., Hammond G. L., Rivero-Muller A., Rahman N., Leung P. C. (2006) J. Biol. Chem. 281, 20817–20824 [DOI] [PubMed] [Google Scholar]
- 82. Lavinsky R. M., Jepsen K., Heinzel T., Torchia J., Mullen T. M., Schiff R., Del-Rio A. L., Ricote M., Ngo S., Gemsch J., Hilsenbeck S. G., Osborne C. K., Glass C. K., Rosenfeld M. G., Rose D. W. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 2920–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rowan B. G., Garrison N., Weigel N. L., O'Malley B. W. (2000) Mol. Cell Biol. 20, 8720–8730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kalkhoven E., Wissink S., van der Saag P. T., van der Burg B. (1996) J. Biol. Chem. 271, 6217–6224 [DOI] [PubMed] [Google Scholar]
- 85. Ng S. B., Tan Y. H., Guy G. R. (1994) J. Biol. Chem. 269, 19021–19027 [PubMed] [Google Scholar]
- 86. McKay L. I., Cidlowski J. A. (2000) Mol. Endocrinol. 14, 1222–1234 [DOI] [PubMed] [Google Scholar]
- 87. Caldenhoven E., Liden J., Wissink S., Van de Stolpe A., Raaijmakers J., Koenderman L., Okret S., Gustafsson J. A., Van der Saag P. T. (1995) Mol. Endocrinol. 9, 401–412 [DOI] [PubMed] [Google Scholar]
- 88. Ray A., Prefontaine K. E. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 752–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Brogan I. J., Murray I. A., Cerillo G., Needham M., White A., Davis J. R. (1999) Mol. Cell Endocrinol. 157, 95–104 [DOI] [PubMed] [Google Scholar]
- 90. Lin W. W., Karin M. (2007) J. Clin. Invest. 117, 1175–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.