Abstract
Human calcitonin (CT) gene transcription is regulated by proximal 5' flanking sequences which mediate cAMP-induced expression, and by a distal basal enhancer region. Using transient expression of CT-CAT constructs, we showed that the basal enhancer is active in a CT-producing small cell lung cancer cell line (DMS53) and the thyroid C cell derived tumor line, TT, but is inactive in non-CT-producing cell lines. In deletional and direct mutational analyses of the distal enhancer region, disruption of two elements resembling recognition sequences for the helix-loop-helix (HLH) family of transcriptional regulatory proteins resulted in a significant loss of basal transcriptional enhancer action. These results suggest that HLH recognition motifs may mediate a significant portion of constitutive CT gene transcriptional activity in these cells. Nuclear protein extracts from DMS53 cells formed specific binding complexes with oligonucleotides containing two of these candidate enhancer sequences. However, proteins capable of binding to these CT gene HLH consensus recognition sites were not restricted to CT-producing cells. We conclude that members of the HLH protein family, some expressed ubiquitously and some expressed or activated in a tissue-restricted fashion, may combine to enhance CT gene transcription in tumor cells of neuroendocrine derivation.
Full text
PDF


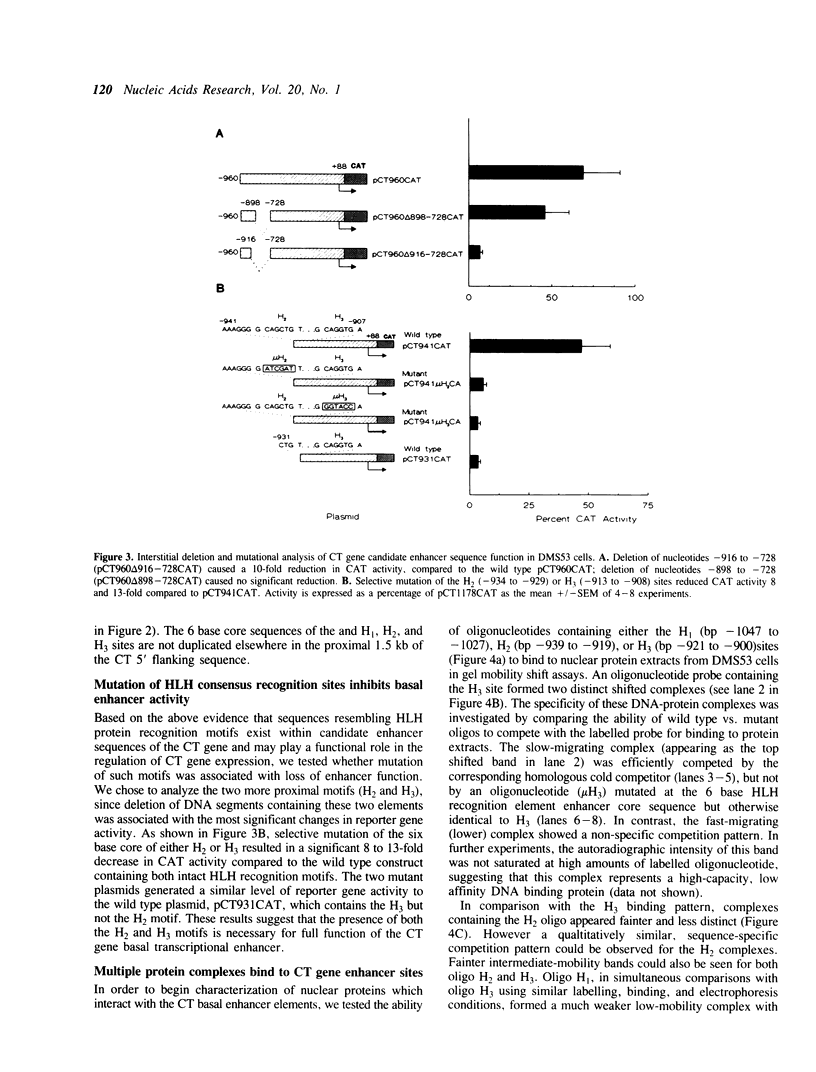
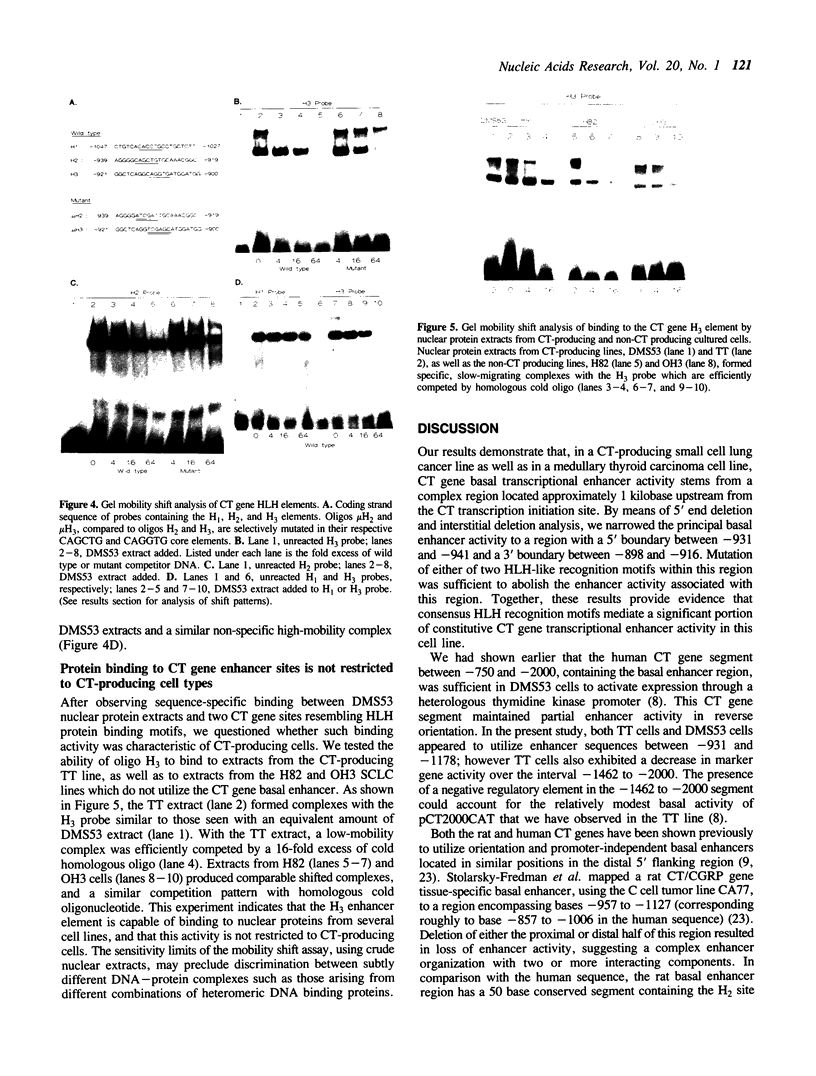


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara S. G., Jonas V., Rosenfeld M. G., Ong E. S., Evans R. M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982 Jul 15;298(5871):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Broad P. M., Symes A. J., Thakker R. V., Craig R. K. Structure and methylation of the human calcitonin/alpha-CGRP gene. Nucleic Acids Res. 1989 Sep 12;17(17):6999–7011. doi: 10.1093/nar/17.17.6999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Ephrussi A., Gilbert W., Tonegawa S. Cell-type-specific contacts to immunoglobulin enhancers in nuclei. 1985 Feb 28-Mar 6Nature. 313(6005):798–801. doi: 10.1038/313798a0. [DOI] [PubMed] [Google Scholar]
- Cordle S. R., Henderson E., Masuoka H., Weil P. A., Stein R. Pancreatic beta-cell-type-specific transcription of the insulin gene is mediated by basic helix-loop-helix DNA-binding proteins. Mol Cell Biol. 1991 Mar;11(3):1734–1738. doi: 10.1128/mcb.11.3.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Cheng P. F., Lassar A. B., Weintraub H. The MyoD DNA binding domain contains a recognition code for muscle-specific gene activation. Cell. 1990 Mar 9;60(5):733–746. doi: 10.1016/0092-8674(90)90088-v. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edbrooke M. R., Parker D., McVey J. H., Riley J. H., Sorenson G. D., Pettengill O. S., Craig R. K. Expression of the human calcitonin/CGRP gene in lung and thyroid carcinoma. EMBO J. 1985 Mar;4(3):715–724. doi: 10.1002/j.1460-2075.1985.tb03688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Nau M. M., Minna J. D. Characterization of variant subclasses of cell lines derived from small cell lung cancer having distinctive biochemical, morphological, and growth properties. Cancer Res. 1985 Jun;45(6):2924–2930. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin C. A., Baylin S. B. Expression of the c-myb oncogene in human small cell lung carcinoma. Cancer Res. 1985 Jan;45(1):272–275. [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Jonas V., Lin C. R., Kawashima E., Semon D., Swanson L. W., Mermod J. J., Evans R. M., Rosenfeld M. G. Alternative RNA processing events in human calcitonin/calcitonin gene-related peptide gene expression. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1994–1998. doi: 10.1073/pnas.82.7.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauweryns J. M., van Ranst L. Calcitonin gene related peptide immunoreactivity in rat lung: light and electron microscopic study. Thorax. 1987 Mar;42(3):183–189. doi: 10.1136/thx.42.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff S. E., Evans R. M., Rosenfeld M. G. Splice commitment dictates neuron-specific alternative RNA processing in calcitonin/CGRP gene expression. Cell. 1987 Feb 13;48(3):517–524. doi: 10.1016/0092-8674(87)90202-9. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Moss L. G., Moss J. B., Rutter W. J. Systematic binding analysis of the insulin gene transcription control region: insulin and immunoglobulin enhancers utilize similar transactivators. Mol Cell Biol. 1988 Jun;8(6):2620–2627. doi: 10.1128/mcb.8.6.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Nelkin B. D., Rosenfeld K. I., de Bustros A., Leong S. S., Roos B. A., Baylin S. B. Structure and expression of a gene encoding human calcitonin and calcitonin gene related peptide. Biochem Biophys Res Commun. 1984 Sep 17;123(2):648–655. doi: 10.1016/0006-291x(84)90278-x. [DOI] [PubMed] [Google Scholar]
- Peleg S., Abruzzese R. V., Cote G. J., Gagel R. F. Transcription of the human calcitonin gene is mediated by a C cell-specific enhancer containing E-box-like elements. Mol Endocrinol. 1990 Nov;4(11):1750–1757. doi: 10.1210/mend-4-11-1750. [DOI] [PubMed] [Google Scholar]
- Piette J., Bessereau J. L., Huchet M., Changeux J. P. Two adjacent MyoD1-binding sites regulate expression of the acetylcholine receptor alpha-subunit gene. Nature. 1990 May 24;345(6273):353–355. doi: 10.1038/345353a0. [DOI] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Sorenson G. D., Pettengill O. S., Brinck-Johnsen T., Cate C. C., Maurer L. H. Hormone production by cultures of small-cell carcinoma of the lung. Cancer. 1981 Mar 15;47(6):1289–1296. doi: 10.1002/1097-0142(19810315)47:6<1289::aid-cncr2820470610>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Steenbergh P. H., Höppener J. W., Zandberg J., Van de Ven W. J., Jansz H. S., Lips C. J. Calcitonin gene related peptide coding sequence is conserved in the human genome and is expressed in medullary thyroid carcinoma. J Clin Endocrinol Metab. 1984 Aug;59(2):358–360. doi: 10.1210/jcem-59-2-358. [DOI] [PubMed] [Google Scholar]
- Stolarsky-Fredman L., Leff S. E., Klein E. S., Crenshaw E. B., 3rd, Yeakley J., Rosenfeld M. G. A tissue-specific enhancer in the rat-calcitonin/CGRP gene is active in both neural and endocrine cell types. Mol Endocrinol. 1990 Mar;4(3):497–504. doi: 10.1210/mend-4-3-497. [DOI] [PubMed] [Google Scholar]
- Travis W. D., Linnoila R. I., Tsokos M. G., Hitchcock C. L., Cutler G. B., Jr, Nieman L., Chrousos G., Pass H., Doppman J. Neuroendocrine tumors of the lung with proposed criteria for large-cell neuroendocrine carcinoma. An ultrastructural, immunohistochemical, and flow cytometric study of 35 cases. Am J Surg Pathol. 1991 Jun;15(6):529–553. doi: 10.1097/00000478-199106000-00003. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Lockshon D., Lassar A. MyoD binds cooperatively to two sites in a target enhancer sequence: occupancy of two sites is required for activation. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5623–5627. doi: 10.1073/pnas.87.15.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- de Bustros A., Lee R. Y., Compton D., Tsong T. Y., Baylin S. B., Nelkin B. D. Differential utilization of calcitonin gene regulatory DNA sequences in cultured lines of medullary thyroid carcinoma and small-cell lung carcinoma. Mol Cell Biol. 1990 Apr;10(4):1773–1778. doi: 10.1128/mcb.10.4.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bustros A., Nelkin B. D., Silverman A., Ehrlich G., Poiesz B., Baylin S. B. The short arm of chromosome 11 is a "hot spot" for hypermethylation in human neoplasia. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5693–5697. doi: 10.1073/pnas.85.15.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]