Abstract
Transient Receptor Potential (TRP) channels are polymodal cellular sensors involved in a wide variety of cellular processes, mainly by changing membrane voltage and increasing cellular Ca2+. This review outlines in detail the history of the founding member of the TRP family, the Drosophila TRP channel. The field began with a spontaneous mutation in the trp gene that led to a blind mutant during prolonged intense light. It was this mutant that allowed for the discovery of the first TRP channels. A combination of electrophysiological, biochemical, Ca2+ measurements, and genetic studies in flies and in other invertebrates pointed to TRP as a novel phosphoinositide-regulated, and Ca2+ permeable channel. The cloning and sequencing of the trp gene provided its molecular identity. These seminal findings led to the isolation of the first mammalian homologues of the Drosophila TRP channels. We now know that TRP channel proteins are conserved through evolution and are found in most organisms, tissues, and cell-types. The TRP channel superfamily is classified into seven related subfamilies: TRPC, TRPM, TRPV, TRPA, TRPP, TRPML and TRPN. A great deal is known today about participation of TRP channels in many biological processes including initiation of pain, thermoregulation, salivary fluid secretion, inflammation, cardiovascular regulation, smooth muscle tone, pressure regulation, Ca2+ and Mg2+ homeostasis and lysosomal function. The native Drosophila photoreceptor cells, where the founding member of the TRP channels superfamily was found is still a useful preparation to study basic features of this remarkable channel.
Introduction
Channel proteins are of prime importance for the survival and function of virtually every cell. Ca2+permeable channels are of particular importance since Ca2+ is not only a charge carrier but also one of the most important second messengers. Before the discovery of TRP channels, two main classes of Ca2+ permeable channels were known: voltage gated, and ligand gated (Hille, 1992). The TRP channel superfamily represents a new class of Ca2+ permeable channels. Although these channels have six transmembrane segments, S1-S6 and the pore region loop between transmembrane segments S5 and S6, which is typical of voltage gated channels, the positively charge residues in S4 are replaced with uncharged amino acid residues (Phillips et al., 1992). Also, mammalian TRPs, like TRPV1, bind specific “ligands” such as capsaicin (Caterina et al., 1997), still, this channel is not considered as a typical ligand gated channel (Bohlen et al., 2010). Therefore, TRP channels do not strictly belong to either of the above channel categories, when classified by their activation and regulation mechanisms. The TRP channel superfamily constitutes a large and diverse class of proteins that are expressed in many tissues and cell types. The pioneering study of Colbert and Bargmann discovered the first member of the TRPV subfamily in C. elegans on the basis of defective response to odorants, high osmotic strength, or light touch to the nose, thus showing that TRP is conserved from nematodes to humans (Colbert & Bargmann, 1995). Based on amino acid sequence homology, the TRP superfamily can be classified into seven subfamilies: TRPC, TRPM, TRPV, TRPA, TRPP, TRPML, TRPN (Fig. 1, (Clapham, 2003; Corey, 2003; Delmas, 2004; Montell et al., 2002)). Except for TRPN, all of the subfamilies can be found in mammals. Many TRPs have been found to participate in sensory transduction pathways, including thermosensation, mechanosensation, taste perception, perception of pungent compounds, pheromone sensing and osmolarity regulation (for reviews see (Clapham, 2003; Julius, 2005; Minke & Cook, 2002; Montell, 2001; Nilius & Mahieu, 2006; Nishida et al., 2006)). In particular, TRP channel subfamilies have drawn increasing interest because they underlie perception of pain and temperature (e.g. TRPV1, (Jordt et al., 2003; Julius, 2005; Julius & Basbaum, 2001)) Apart from sensory perception, the involvement of TRP channels was demonstrated in many other processes, including salivary fluid secretion, inflammation, cardiovascular regulation, smooth muscle tone, pressure regulation, Ca2+ and Mg2+ homeostasis and lysosomal function (for reviews see (Clapham, 2003; Minke & Cook, 2002; Montell, 2001; Nilius & Voets, 2005)). In addition, TRP channels are also shown to be involved in many cellular functions, including cell adhesion, control of growth and differentiation, proliferation, cell death and cell polarity (for reviews see (Abramowitz & Birnbaumer, 2009; Dadon & Minke, 2010; Miller, 2006; Nishida et al., 2006)). Because of the involvement of TRP channels in many physiological processes, it is not surprising that some of the subfamilies are involved in human diseases (Nilius et al., 2007).
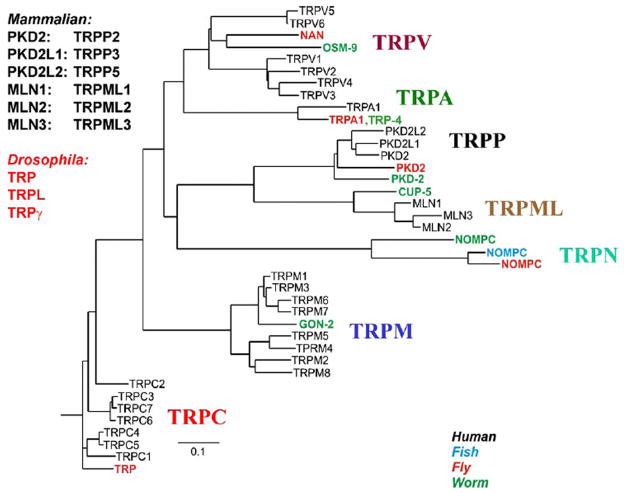
Figure 1. The phylogenetic tree of TRP channels superfamily.
Depicted are the 7 subfamilies that constitute the TRP family. The 4 different species are indicated by different colors. Only some of the Drosophila and C. elegans (worm) members are included. For more details see (Venkatachalam & Montell, 2007). (From Nilius and Mahieu, (Nilius & Mahieu, 2006)).
The founding member of the TRP channel superfamily is the Drosophila TRP channel. Later on, the TRP-like (TRPL) channel was also identified in the Drosophila eye. Both TRP and TRPL channels play a key role in phototransduction, the process in which absorbed light quanta are transformed into an electrical signal in the photoreceptor cell (for reviews see (Hardie & Raghu, 2001; Katz & Minke, 2009)). The seminal findings in Drosophila phototransduction, together with the important discovery by Julius and colleagues of the capsaicin receptor, TRPV1, as a heat-activated ion channel in the pain pathway (Caterina et al., 1997) led to an explosion of interest in TRP channels. It has become apparent that TRP channels are involved in a large variety of important biological mechanisms. This review will provide a detailed and documented outline of the history of the Drosophila TRP channels.
The trp mutant
In 1969, Cosens and Manning identified a spontaneously formed Drosophila melanogaster mutant on the basis of a behavioral phenotype. They mapped this mutation to the third chromosome of the Drosophila genome. They further found that under bright illumination the mutant flies behave as though blind. “We are working with a mutant strain of D. melanogaster which, though behaving phototactically positive in a T-maze under low ambient light, is visually impaired and behaves as though blind in a simple optomotor apparatus “(Cosens & Manning, 1969). They also measured the electroretinogram (ERG), which is the summed electrical response of the entire eye, to light. The ERG was recorded from the compound eye of wild type Drosophila (WT) and the “blind” mutant (see Fig. 2), which they designated later on the ‘A’ mutant. They described the ERG phenotype of this mutant as follows: “ the light response decays to baseline in 10 to 15 s. At this point the mutant eye is apparently blind, for it will not respond to a second test light; and mutant flies exposed to continual bright light ceased to make any phototactic choice in the T-maze but recover their responsiveness only after a time in low ambient light”. “This slow recovery of the mutant eye and its brief ERG response characteristics suggest an explanation in the turn-over of the photopigment” (Cosens & Manning, 1969).
Figure 2.
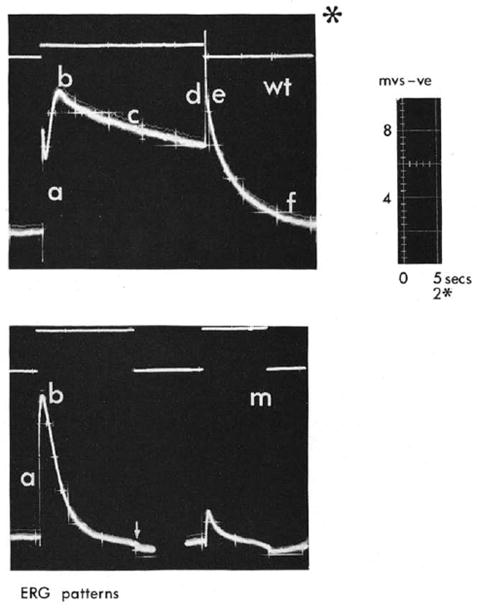
The original electroretinogram (ERG) comparing the response to a light pulse between wild type Drosophila (wt) and a spontaneously formed mutant (m) that was designated later trp by Minke and colleagues. The traces were photographed from an oscilloscope and the different characters indicate various phases in the ERG response. Note that phases c,d,e,f are missing in the mutant. The upper traces represent the light monitor. The * at the upper panel indicates 2.5 faster timescale. Also note that all the traces are presented in an unconventional manner so that negative voltage (corneal negative) is presented upward (From (Cosens, 1971)).
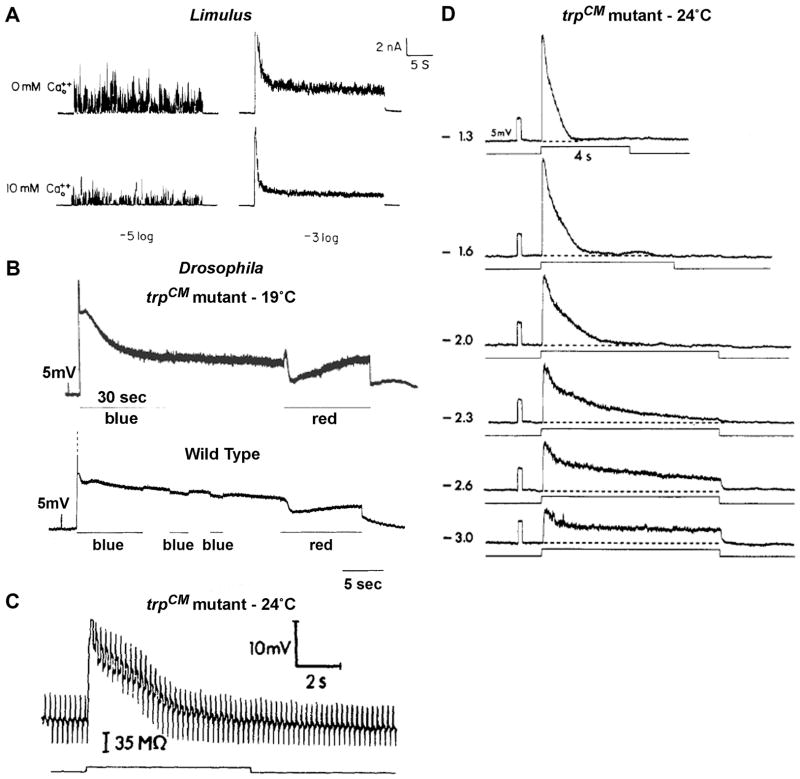
In 1975, while I was a post-doctoral student, together with Chun-Fang Wu, a graduate student, in the lab of William L. Pak at Purdue University, I was fascinated by the phenotype of the Cosens-Manning mutant. In addition to ERG recordings, we applied intracellular recordings from single photoreceptor cells to analyze the mutant phenotype in great detail. At that time, studies in the Limulus ventral photoreceptor cells already indicated that in response to intense prolonged light, the receptor potential is composed of an initial large transient phase that declines to a lower steady state phase due to Ca2+-mediated light adaptation (Lisman & Brown, 1972), see Fig 3A)). Accordingly, we assumed that the large decline of the ERG measurement from the Drosophila mutant during intense light (Fig. 2) is due to abnormally strong light adaptation. In Limulus, stationary fluctuation analysis (a method that derives the mean amplitude, duration and number of the unitary events that compose current or voltage noise) of the steady state response to dim and intense lights indicated that the macroscopic response to light is a linear summation of single photon responses (quantum bumps, (Yeandle & Spiegler, 1973)) which produce the macroscopic receptor potential (Dodge, Jr. et al., 1968; Wong & Knight, 1980). The decline of the initial transient to a lower steady state level during intense light (light adaptation) was explained by a decrease in the amplitudes of the individual bumps that compose the light response. The decrease in bump amplitude was manifested by a highly reduced noise level at steady state in response to intense lights (Fig. 3A, (Dodge, Jr. et al., 1968; Wong & Knight, 1980)). The same principles derived from results in Limulus turned out to be valid for Drosophila photoreceptors (Wu & Pak, 1975). Intracellular recordings from the Cosens-Manning mutant (raised at 19°C, see below) revealed only a small amplitude steady state phase with amplitude that varied among individual mutants in response to intense lights (see below). Strikingly, this small amplitude steady state phase was composed of a large bump noise (Fig. 3B, top), in contrast to the minimal noise amplitude observed in WT flies in response to intense light (Fig. 3B, bottom). Stationary fluctuation analysis confirmed that the mean bump amplitude of the mutant, calculated from the steady state response to intense light was similar to that of 100 fold dimmer light. This means that the intense light becomes equivalent to dim light and then to darkness in these mutant flies where the response declined to baseline (Fig 2D upper traces, (Minke et al., 1975). The conclusion from these experiments was that the decay of the trp mutant’s response during light did not arise from a decrease in bump size (light adaptation) but rather from a decrease in the number of bumps composing the response (reduction in excitation efficiency (Minke et al., 1975)). If the phenotype of the mutant would arise from abnormally strong light adaptation, one would expect a reduction in bump noise during steady state, contrary to our observation (Fig. 3B top). Importantly, the individual bumps of the mutant at both dim and intense lights (Minke et al., 1975) were similar to those of WT during dim lights (Wu & Pak, 1975). In this publication (Minke et al., 1975), the authors designated the mutant (with the agreement of Cosens) ‘Transient Receptor Potential’ or in short TRP because of the transient response to sustained intense lights. Later on, TRP was adapted as the name of the entire TRP superfamily by an international committee (Montell et al., 2002). To test the hypothesis of Cosens; Minke, Wu, and Pak also measured the photopigment level of the trp mutant during illumination with various light intensities and found that the photopigment does not fail to regenerate and that it is indistinguishable from that of WT flies (Minke et al., 1975). This results were strongly supported by a later study, which carefully compared the photopigment of the trp mutant and WT by two independent methods (Minke, 1982). In this later study, light adaptation was also compared between WT and the trp mutant. It was concluded that strong light adaptation cannot explain the mutant phenotype because light adaptation was much weaker in the mutant relative to WT flies (Minke, 1982). An additional possibility to explain the phenotype of the trp mutant was that, in the mutant, prolonged light was suggested to activate a channel, which reduces the amplitude of the light response when activated, due to its negative reversal potential (i.e. which drives the membrane potential towards the negative resting potential, e.g. K+ channel). However, this possibility was ruled out by intracellular bridge measurements showing that the decline of the mutant response to light is accompanied by a decrease in conductance (i.e. by closures of channels, (Fig. 3C, (Minke, 1982)). As mentioned above, the rate of the decay and the final steady state level attained in the receptor potential of the trp mutant vary among individual flies, showing responses that often decay to the dark resting potential level, during light (Fig. 3D). Later on, I resolved this phenomenon by demonstrating that the original Cosens-Manning trp mutant (now designated trpCM) is a developmental temperature-sensitive mutant, showing faster decay to baseline and slower dark recovery kinetics when raised at room temperature (24°C) relative to 19°C (Minke, 1983). The conclusion from all these early studies was that the trp phenotype arises from a reduction in excitation efficiency (i.e. that intense light becomes equivalent to dim light) during constant illumination, due to an unknown defect at intermediate stage of the phototransduction cascade (Minke, 1977; Minke, 1982). All that period, I did not have a clue as to the function of the trp gene product. This period extended up to the time of the La3+ experiments (see below).
Figure 3. The waveforms and light-induced bump noise of wild type and trp mutant during intense lights.
A. The effect of lowering external Ca2+ on light responses of Limulus ventral photoreceptor in response to dim (−log I/I0=5) and intense (−log I/I0=3) lights, recorded by two electrode voltage clamp. Lowering external Ca2+ concentration increased the bump noise (left) and the steady state noise of the macroscopic response to light (right, from (Wong et al., 1982)).
B, top. Intracellular recordings of the receptor potential and the following Prolonged Depolarizing Afterpotential (PDA) in response to intense blue lights in the trpCM mutant raised at 19°C (top) and in WT Drosophila (B bottom). In the trpCM mutant a large increase of bump noise is observed during the steady state phase of the light response and during the PDA of the mutant (top), but not in WT flies (bottom). Suppression of the PDA by red light resulted it a prompt suppression of the bump noise (B, top, red light) (from (Minke et al., 1975).
C. The decay of the response to light of the trpCM mutant raised at 24°C is accompanied by a conductance decrease. The figure shows intracellular bridge measurement made in the trp mutant before, during and after green light stimulus. The bridge, which was balanced in the dark, shows during light an initial conductance increase that then decreases in parallele with the decrease in voltage, showing that the decay of the response is accompanied by closure of channels (from (Minke, 1982).
D. The decay time of the intracellularly recorded response to light of the trpCM mutant raised at 24°C depends on light intensity. At very dim light the response does not decay to baseline (from (Minke, 1982).
The light sensitive channels are the target of a light activated inositol-lipid signaling pathway
The first experiments showing that invertebrate photoreceptors may use the phosphoinositide cascade to activate the light sensitive channels (see summary cartoon in Fig. 4) came from pharmacological studies in Limulus photoreceptors, showing that the light sensitive channels can be activated in the dark by inositol 1,4,5 trisphosphate (InsP3, (Brown et al., 1984; Fein et al., 1984)). In these pioneering experiments, exogenous application of InsP3 in the dark mimicked the effects of light. Previous experiments in Limulus revealed that application of chemicals, specific for activation of G-proteins such as GTPγS, produce bumps -like unitary events in the dark, strongly suggesting that a G-protein mediates light excitation in Limulus (Fein & Corson, 1981). In general, it was established in photoreceptors of several invertebrate species that photoexcited rhodopsin activates a heterotrimeric G protein (Fein, 1986). In fly photoreceptors, Minke and Stephenson showed for the first time that when pharmacological agents known to activate G proteins were applied to the housefly Musca photoreceptors, they mimicked the light dependent activation of the photoreceptor cells (Minke & Stephenson, 1985). Biochemical measurements of light activated GTP hydrolyzing activity (GTPase) in both Musca and Drosophila conducted by Minke, Selinger and colleagues revealed that fly photoreceptors are endowed with a robust light activated G-protein (Blumenfeld et al., 1985; Devary et al., 1987). Later studies conducted by Hyde and colleagues and by Zuker and colleagues used genetic screens, which led to the isolation of two genes encoding visual specific G-protein subunits. These genes, dgq and gβe, encode a Gqα and Gqβ subunit, respectively (Lee et al., 1990; Yarfitz et al., 1991). The most direct demonstration that DGqα participates in the phototransduction cascade came from studies of mutants defective in Gqα which also showed highly reduced sensitivity to light. In the Gαq1 mutant, isolated by Scott Zuker and colleagues (Scott et al., 1995), DGqα protein levels are reduced to ~1%, while Gβ, PLC or rhodopsin protein levels are virtually normal. The Gαq1 mutant exhibits a more than 1000 fold reduced sensitivity to light, thus strongly suggesting that there is no parallel pathway mediated by the G protein, as suggested for Limulus eye (Dorloechter & Stieve, 1997). Manipulations of the DGqα protein level by the inducible heat-shock promoter made it possible to show a strong correlation between the sensitivity to light and DGqα protein levels (Scott et al., 1995).
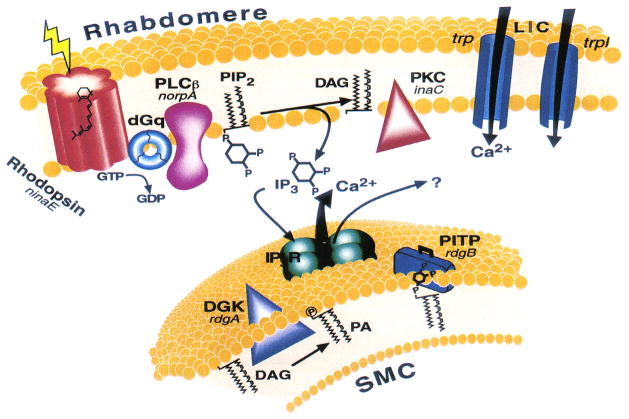
Figure 4.
The phosphoinositide cascade of vision. Cloned genes (for all of which mutants are available) are shown in italics, alongside their corresponding proteins. Upon absorption of light, rhodopsin (ninaE gene) is converted to the active metarhodopsin state, which activates a heterotrimeric G protein (dGq). This leads to activation of phospholipase C (PLCβ, norpA gene) and subsequent opening of two classes of light-sensitive channels encoded at by trp and trpl genes. PLC catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into the soluble inositol 1,4,5-trisphosphate (IP3) and the membrane-bound diacylglycerol (DAG). DAG is recycled to PIP2 by the phosphatidylinositol (PI) cycle shown in an extension of the smooth endoplasmic reticulum called submicrovillar cisternae (SMC, shown on the bottom). DAG is converted to phosphatidic acid (PA) via DAG kinase (DGK, rdgA gene). After conversion to PI, PI is presumed to be transported back to the microvillar membrane by the PI transfer protein (PITP encoded by the rdgB gene). The InsP3 receptor (IP3R), which is an internal Ca2+ channel that opens and releases Ca2+ upon binding of InsP3. (From (Minke & Cook, 2002)).
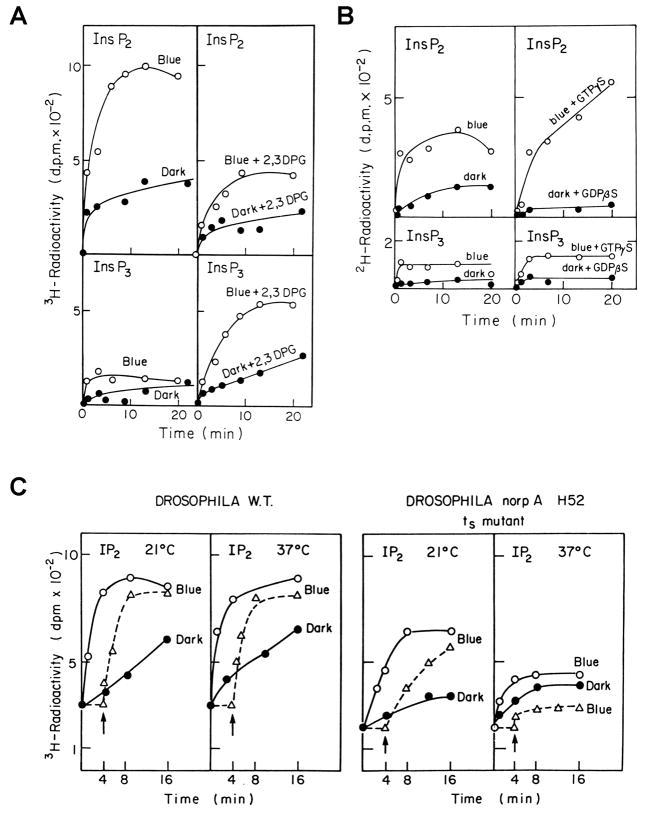
Evidence for light-activated and G-protein-dependent PLC activity in fly photoreceptors came from combined biochemical and electrophysiological experiments. These experiments were conducted by Selinger, Minke and colleagues (Devary et al., 1987), in membrane preparations of Musca and Drosophila eyes. The eye membrane preparations responded to illumination with a G-protein dependent accumulation of InsP3 and inositol-bisphosphate (InsP2), derived from phosphatidylinositol 4,5 bisphosphate (PIP2) hydrolysis by PLC (Fig. 5A, B). The critical role of PIP2 hydrolysis in light excitation was revealed in the biochemical experiments of Selinger and Minke using a reversible temperature sensitive mutant, previously characterized by Deland and Pak. In this mutant, designated ‘no receptor potential A’ (norpA), a temperature sensitive allele, norpA H52 at the permissive temperature of 19°C showed normal electroretinogram (ERG) light response. However, at 37°C the ERG is abolished reversibly (Deland & Pak, 1973). Membrane preparation of norpA H52 heads revealed light activated PIP2 hydrolysis similar to WT at the permissive temperature, which was blocked at 37°C in the membrane preparation of the mutant but not of WT heads ((Selinger & Minke, 1988) Fig 5C). These experiments indicated that PIP2 hydrolysis is critical for light excitation in Drosophila. However, later on, genetic elimination of the single InsP3 receptor, performed by Zuker and colleagues and by Hardie and colleagues (Acharya et al., 1997; Raghu et al., 2000a), had no effect on light excitation, thus putting in question the role of InsP3 in phototransduction.
Figure 5. Biochemical measurements of light-induced and G-protein-dependent hydrolysis of PIP2 and its absence by the norpA mutant which abolishes reversibly the response to light.
A. Light-induced hydrolysis of phosphoinositides in Musca eye membrane preparation. Equivalents of 100 eyes were cut into halves and were incubated in the dark for 4 h at 30°C with [3H]inositol. Preparation of eye membranes and measurements of light dependent phosphoinositide hydrolysis were carried out as described previously (Devary et al., 1987). The upper panels show production of InsP2 and the lower panels show production of InsP3, which is a precursor of InsP2. The right column (top and bottom) shows the effect of the InsP3 phosphatase inhibitor (2,3 DPG) that causes an increase in InsP3 (right bottom) and concurrent reduction in InsP2 right top. The figure shows light dependent PLC activation leading to production of InsP3 and subsequent production of InsP2.
B. Light-induced PIP2 hydrolysis is G-protein dependent. As in A, the figure shows enhancement of InsP2 production by GTPγS (10μM) and suppression of InsP2 production by GDPβS (100μM, right top as compared to left top) on inositol phosphate production. Due to the fast conversion of InsP3 to InsP2 only a small increase in InsP3 could be measured without 2,3 DPG.
C. Light-induced PIP2 hydrolysis in eye membrane preparation of wild type (W.T., left panels) and its absence at elevated temperatures in the norp H52 temperature sensitive mutant of Drosophila. The experimental system was similar to that of panel A except that Drosophila heads were used. Systems depicted by dotted lines were pre-incubated for 4 min at the indicated temperatures in medium lacking ATP and ATP regenerating system which does not allow the biochemical reaction. The latter components were subsequently added (arrow) to initiate the reaction.
The key evidence for the participation of PLC in visual excitation of the fly was provided by Pak and colleagues, who isolated and analyzed the PLC gene of Drosophila, which turned out to be the norpA gene (Bloomquist et al., 1988). The norpA mutant has long been a strong candidate for a transduction defective mutant because of its drastically reduced receptor potential. The norpA gene encodes a β-class PLC, predominately expressed in the rhabdomeres, which has extensive amino acid homology to a PLC extracted from bovine brain (Bloomquist et al., 1988). Transgenic Drosophila, carrying a wild-type construct of the norpA gene on null norpA background, rescued the transformant flies from all the physiological, biochemical and morphological defects, which are associated with the norpA mutants (McKay et al., 1995). The norpA mutant thus provides essential evidence for the critical role of inositol-lipid signaling in activation of the light sensitive channels, by showing that no activation of the channels takes place in the absence of functional PLC. However, the events required for light excitation downstream of PLC activation remain unresolved. Nevertheless, evidence in favor of the diacylglycerol (DAG) branch of the phosphoinositide cascade has accumulated (see Fig 5). Accordingly, studies by Hardie and colleagues showed that exogenous application of polyunsaturated fatty acids (PUFAs), activated the light sensitive channels in the dark (Chyb et al., 1999). In addition, in a mutant without DAG kinase (which inactivates DAG by converting DAG to phosphatidic acid) called rdgA (Masai et al., 1993) the TRP and TRPL channels are constitutively active, presumably because of DAG accumulation (Raghu et al., 2000b).
The cloning and sequencing of the trp gene
In 1985, Montell, Johns and Hafen, then at the Rubin lab at Berkley, applied a differential screen of a library of cloned Drosophila genomic DNA segments with polyadenylated RNA prepared from fly heads and bodies. This screen yielded 20 cloned sequences that were expressed more abundantly in the head than in the body. Among these 20 clones, one was mapped cytogenetically by in situ hybridization to the same position as the trp mutation, 99C, at the tip of the third chromosome. The position of the trp locus was previously mapped by Manning and colleagues (Levy et al., 1982) and by Wong and colleagues (Wong et al., 1985). Accordingly, the cytogenetic location of the trp mutation on the third chromosome was known but the identity of the gene was not. The cytogenetic position, tissue specificity and timing of expression were consistent with the notion that the above clone encodes some DNA portion containing the trp gene. To confirm that this is indeed the case, Montell and colleagues complemented the mutant trpCM by P-element mediated germline transformation of a 7.1-kilobase DNA fragment. Indeed, the trpCM flies carrying the 7.1-kilobase DNA fragment were completely rescued from the trp phenotype and a WT ERG phenotype was observed (see Fig. 2). This was the first isolation of a DNA portion containing the trp gene (Montell et al., 1985), which was followed by cloning and sequencing of the trp gene 4 years later (Montell & Rubin, 1989; Wong et al., 1989).
In 1989, Montell and Rubin cloned, sequenced and presented a molecular characterization of the Drosophila trp gene; a 4.1kb trp RNA transcript, which encodes a 1275 amino acid protein showing no significant similarity with any previously described protein. Their analysis of the deduced amino acid sequence suggested that the trp gene encodes an integral membrane protein that contains 8 transmembrane segments with the C terminus containing a very hydrophilic 8 amino acid sequence that is repeated in tandem 9 times (Montell & Rubin, 1989). Montell and Rubin did note that the overall structure of the trp sequence shared general features with many receptor/transport proteins “This structure shares a number of general features with many receptor/transport proteins. For example, the Ca2+ channel has an even number of multiple transmembrane domains, one of which displays amphipathic character, and no hydrophobic N-terminal signal sequence (Tanable et al. 1987)”. Immunolocalization indicated that trp is expressed in the rhabdomeres, the signaling compartment that is composed of tightly packed microvilli, which contain the proteins required for phototransduction in the photoreceptor cells. Western blot analysis revealed that the trp encoded protein appears to be missing in each of the mutant alleles analyzed (Montell & Rubin, 1989). As described by Montell and Rubin: “Thus, the phenotype arises from the absence of the protein rather than expression of a defective gene product” (Montell & Rubin, 1989). At this stage historically, it is relevant to mention that Pak’s lab previously generated several alleles of the original trpCM mutant and these trp alleles with identified genetic background (unlike the trpCM mutant) were used later on to resolve the role of TRP in phototransduction. Two of these alleles, trpP301 and trpP343 were tested in addition to trpCM by Montell and Rubin. The observation that the trpCM and other trp alleles express no protein biochemically, led Montell and Rubin to the following conclusion: “An alternative proposal, consistent with the protein structure and localization, is that trp is the structural gene for the light sensitive channels. However, electrophysiological analyses suggested that trp does not encode the light-sensitive channel (Minke, 1982) “, (Montell & Rubin, 1989). At that time, there were several observations that led Montell and Rubin to the logical conclusion that the trp gene product cannot be the light sensitive channel: “This conclusion is strongly supported by the protein immunoblot analysis of three trp alleles presented in the current report. Since the behavioral and electrophysiological response of trp flies is normal under conditions of dim light, the light-sensitive channels must be present. Therefore, if trp encodes the light sensitive channel, then the protein must be defective rather than absent. The demonstration that the trp protein is completely missing in each mutant allele examined, indicated that trp is not the structural gene for the light-sensitive channel” (Montell & Rubin, 1989). The data that led to the above conclusion was that the trpCM mutant has normal ERG and normal behavior in response to dim light (Cosens, 1971) as well as normal bumps during both dim and intense lights (Minke et al., 1975). In addition, like the case of vertebrate photoreceptors, they probably assumed that there exists only one type of light sensitive channel. Together, the data and assumption implicated that light sensitive channels exist in the trpCM mutant. This logical conclusion together with the immunoblot measurements showing no expression of TRP in the mutant led Montell and Rubin to conclude that TRP is not a channel.
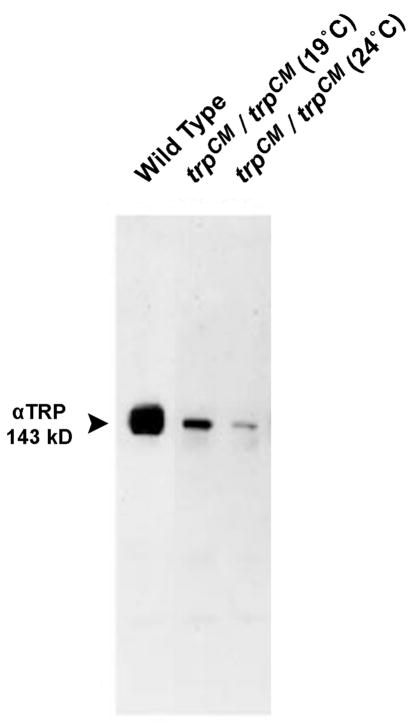
Later analyses of the above 3 trp alleles revealed that trpP343 is indeed a null allele (Scott et al., 1997) and trpP301 is a nearly null allele and both of them show bumps with highly reduced amplitude ((Niemeyer et al., 1996), see below). The trpCM mutant was the only allele on which the behavioral and electrophysiological ERG was carried out (Cosens, 1971). In addition, the bump analyses at dim and intense light were carried out only in trpCM mutant raised at 19°C, which has steady state receptor potential required for the stationary fluctuation analysis (Minke et al., 1975). However, the trpCM expresses 15.1±5.9 % TRP protein when raised at 24°C and 30.4±8.8% TRP protein relative to WT, when raised at 19°C (see Fig. 6 (Yoon et al., 2000)). Later experiments showed that the significant TRP protein that is expressed in the trpCM flies raised at 19°C produces a robust light induced current (Reuss et al., 1997). The later experiments showing that the trpCM flies do express functional TRP protein, allow concluding that TRP can be the light activated channel (see below).
Figure 6. The trpCM mutant is not a null allele and expresses reduced amount of TRP.
Western blot analysis of the homozygote trpCM mutant, raised at 24°C and at 19°C (as indicated) using momoclonal antibody against the TRP protein (Pollock et al., 1995). The TRP protein of WT and the mutant appears on the gel. (from (Yoon et al., 2000)).
Shortly after the sequencing of trp by Montell and Rubin, Wong and colleagues also cloned and sequenced the trp gene and analyzed this gene and its expression in normal and mutant flies. Similar to Montell and Rubin, they concluded that “Our results suggest that the trp protein is not needed for the occurrence of the bumps, since mutants lacking the trp protein such as the trpCM and the trp302 alleles, respond normally to dim light (Cosens and Manning, 1969)”. In addition, they concluded that “Therefore, functions of the elements in the primary pathway of excitation, including those of rhodopsin, G protein, PLC, second messengers, and the ionic channels that makeup the bump, as well as those involved in the prolonged depolarizing afterpotential, are not dependent on the trp protein.” (Wong et al., 1989)
In retrospect, what hampered the understanding of the TRP protein function at that time was the sole use of the trpCM mutant that reveals response inactivation during intense light and yet shows normal bumps amplitude. In addition, based on the already known vertebrate phototransduction, a single type of light sensitive channel was expected, contrary to later findings that at least two types of light sensitive channels exist in Drosophila photoreceptors.
Evidence that TRP is a light sensitive channel
The experiments that constitute a breakthrough in understanding the function of the TRP gene product began in 1989. Hochstrate at the Hamdorf’s lab in Bochum, Germany showed that application of the non-specific Ca2+ channel blocker lanthanum (La3+) to the extracellular space of the retina of the blowfly Calliphora caused a drastic decline of the receptor potential to the dark baseline during a step of light. Accordingly, the receptor potential of WT Calliphora in the presence of La3+ resemble the receptor potential of the trp mutant (Hochstrate, 1989). In a detailed study by Minke and colleagues, these observations were verified in 3 species of flies (Fig. 7A (Suss Toby et al., 1991)). Furthermore, a stationary fluctuation analysis showed that La3+ mimicked the phenotype of the trp mutant in WT flies but had virtually no effect on a mutant homologue of trp in the large fly (the nss mutant, Fig. 7B). Since La3+ is known to block Ca2+ permeable channels, these results allowed for the first time to link the TRP protein to a specific function namely, the results implied that TRP constitutes a route for Ca2+ entry into the photoreceptor cell. It was concluded that the affect of La3+ is as follows: “This effect may arise from an inhibition of a Ca2+ transporter protein located in the surface membrane that normally replenishes Ca2+ pools in the photoreceptors, a process essential for light excitation” (Suss Toby et al., 1991). Shortly thereafter, Minke and Selinger published two review articles that summarized all the evidence supporting the notion that “the trp protein is a plasma membrane component (or part of it) which oscillates between Ca2+ transporting and non-transporting states via conformational changes of the InsP3 receptor” (summarized in Fig. 8, (Minke and Selinger, 1991; Minke and Selinger, 1992). This conclusion was based on the following indirect evidence: 1. In insects (i.e. the honeybee drone) light-induced current is accompanied by a large Ca2+ influx (Minke & Tsacopoulos, 1986). 2. Fly photoreceptor cells contained tiny pigmented granules (pigment granules) that scatter all over the cell body in the dark and move close the rhabdomere during light (Franceschini & Kirschfeld, 1971). It was found by Lo and Pak that movement of the pigment granules during prolonged light in the trp mutant is transient and show dark localization during prolonged lights, unlike WT flies (Lo & Pak, 1981). It was also shown that pigment granule migration is Ca2+ dependent and the granules move in response to Ca2+ elevation in the photoreceptor cell during light (Kirschfeld & Vogt, 1980) to serve as a “pupil mechanism” for controlling light flux (Franceschini & Kirschfeld, 1971). These facts were interpreted by Minke and Selinger as evidence that in the trp mutant Ca2+ level is abnormally low during prolonged lights. 3. The ability to mimic the trp phenotype in WT flies by application of a Ca2+ channel blocker, La3+ was also interpreted as evidence that the trp protein determines intracellular Ca2+ level during light (Minke & Selinger, 1991; Minke & Selinger, 1992), Fig. 8)). 4. Studies in the fly showed that the light sensitive channels are the target of the inositol-lipid signaling pathway (Bloomquist et al., 1988; Devary et al., 1987; Selinger & Minke, 1988). 5. The “conformational coupling” model of Irvine and Berridge (Berridge, 1995; Berridge & Irvine, 1984), suggesting that physical coupling of the InsP3 receptor (InsP3R) to a still unknown surface membrane Ca2+ channel, activates the Ca2+ channel following Ca2+ store depletion.The ensuing Ca2+ influx refilled the empty InsP3 -sensitive Ca2+ stores. Minke and Selinger proposed that TRP constitutes the unknown surface membrane Ca2+ channel/transporter (Fig. 8, for comparison of this old version of phototransduction to a more recent one see also Fig. 4 above). Since there were no concrete data to directly test the conformational coupling hypothesis it was formulated in vague terms and used later on by Hardie and Minke (Hardie & Minke, 1993) and by Hardie (Hardie, 1996a) as a model to explain the gating mechanism of TRP channels “A popular model for store-operated channel activation invokes conformational-coupling, in which channel is gated by interaction with the IP3 receptor, which in turn senses Ca2+ concentration in the store lumen” (Hardie, 1996a). Later studies by Zuker and colleagues and by Hardie showed that the store operated model cannot explain the activation of the light sensitive channels (Hardie, 1996b; Ranganathan et al., 1994)
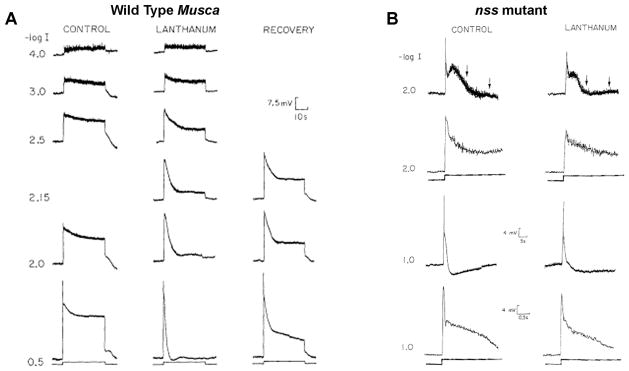
Figure 7. Lanthanum (La3+) mimics the trp phenotype in wild type fly but has no effect on a trp homologue mutant.
Intracellular recordings from single photoreptor cell of white-eyed Musca domestica (A) and from white-eyed nss mutant of Lucilia cuprina (Howard, 1984), which is a mutant homologue of the Drosophila trp (B). Responses to increasing intensities of orange lights are shown. The left columns show responses before application of La3+ (CONTROL). La3+ was applied by pressure injection into the extracellular space. Partial recovery of WT phenotype was observed 20 min after injection (A, right). Injection of La3+ to the extracellular space of the nss mutant had no effect on the rate of decline, but induced a small reduction in the amplitude of the initial peak response (from (Suss Toby et al., 1991)).
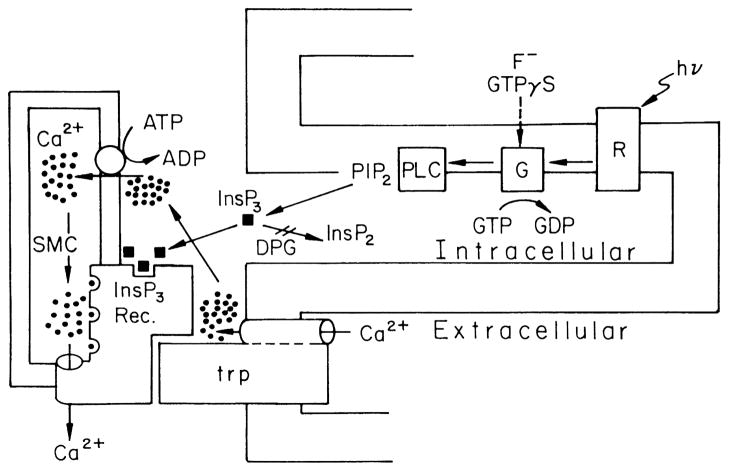
Figure 8. Model scheme that summarizes fly phototransduction according to the “conformational coupling” hypothesis.
According to this model, TRP (trp) is a new type of channel/transporter, which is activated by light-induced depletion of the InsP3-sensitive Ca2+ stores (SMC), following production of InsP3 by G-protein (G) activated PLC and binding of InsP3 to the InsP3 Receptor. According to this model depletion of Ca2+ from the stores, couples TRP to the InsP3 receptor and opens the TRP Ca2+ channel (from (Minke & Selinger, 1991)).
Evidence that directly linked TRP to the light activated channel came after the introduction of the patch clamping technique to Drosophila photoreceptors by Hardie (Hardie, 1991). In the Hardie’s 1991 paper he showed for the first time that the light activated channels of Drosophila are Ca2+ permeable (Hardie, 1991). In the discussion of this paper Hardie raised the possibilities that “One intriguing hypothesis is that in Drosophila, the light-sensitive channels might actually be Ins(1,4,5)P3 receptor”. In addition, he discussed the possibility that “Based on the effects of lanthanum (Hochstrate 1990; Suss-Toby et al. 1991) and the trp and nss mutations (in Drosophila and Lucilia respectively), Minke and coworkers have recently stressed the importance of a putative transmembrane Ca2+ transporter in fly photoreceptors (Minke & Selinger 1991; Suss-Toby et al. 1991). Possibly, the light sensitive calcium conductance shown in the present study actually represents this ‘Ca2+ transporter’, hypothesized by Minke & Selinger (1991) to represent the trp gene product “.
The strong compelling evidence demonstrating that TRP is a light activated and Ca2+ permeable channel came from the discovery of Hardie and Minke that TRP is indeed a Ca2+ permeable channel that is missing in the trp mutant (Hardie & Minke, 1992). Hardie and Minke summarized the results as follows “Using whole-cell recordings from Drosophila photoreceptors, we show that the wild-type response is mediated by at least two functionally distinct classes of light-sensitive channels and that both the trp mutation and a Ca2+channel blocker (La3+) selectively abolished one class of channel with high Ca2+ permeability” (Hardie & Minke, 1992). They also wrote “We conclude that the recently sequenced trp protein represents a class of light-sensitive channel required for inositide-mediated Ca2+ entry and suggest that this process is necessary for maintained excitation during intense illumination in fly photoreceptors”.
At the time of the Hardie and Minke publication (1992), Kelly and colleagues were searching for Drosophila genes encoding for calmodulin (CaM) binding proteins (Phillips et al., 1992). They discovered a new membrane protein with overall 39% amino acid identity to TRP and with 74% identity within the trans-membrane domains. Because of this similarity, they designated this protein as TRP-like (TRPL). A detailed analysis of the amino acid sequence of this protein revealed two CaM binding domains at the C-terminal, ankyrin-like repeats at the N-terminal, 6 transmembrane segments (S1-S6) and a putative pore region between S5 and S6, typical for voltage gated channels. They also identified the ankyrin repeats in the known amino acid sequence of TRP. Importantly, comparison of the S5-S6 region of TRPL with that of the α1 subunit of the brain voltage gated Ca2+ channel revealed several short sequences of amino acids identity (Phillips et al., 1992). They concluded that “The identification of a protein similar to the trp gene product, yet also able to bind Ca2+/calmodulin, allows for a reinterpretation of the phenotype of the trp mutation and suggest that both genes encode light-sensitive ion channels” (Phillips et al., 1992). Furthermore, they showed that TRPL is expressed in the rhabdomere in addition to TRP channel. The discovery of the TRPL channel agreed nicely with the evidence and conclusion from the patch clamp recordings (Hardie & Minke, 1992). The two articles were published side-by-side in Neuron, indicating together that the wild-type response is mediated by at least two functionally distinct classes of light-sensitive channels, TRP and TRPL.
Direct evidence that the TRP and TRPL channels constitute the major route of Ca2+ influx into the photoreceptor cells came from simultaneous measurements of light-induced whole cell currents and fluorescence of Ca2+ indicators, by Minke and colleagues ((Peretz et al., 1994), Fig. 9A, B)). Importantly, these studies showed that removal of external Ca2+ abolished the increase in cellular Ca2+ during illumination indicating that most of the light-induced increase in cellular Ca2+ arises from Ca2+ influx. Furthermore, they showed that Ca2+ influx is significantly reduced in the trp mutant (Fig. 7C, D) and that genetic elimination of the light activated phospholipase C (PLC, in the norpA mutants, see above) also abolished the light-induced increase in cellular Ca2+ together with the light-induced current (Fig. 9E). Together, these studies indicated that the phosphoinositide pathway is necessary for both light excitation and increase in cytosolic Ca2+ (Peretz et al., 1994). Shortly thereafter, independent studies by Zuker and colleagues (Ranganathan et al., 1994) and later on by Hardie (Hardie, 1996c) obtained similar results and conclusions. The notion that the trp gene encodes a Ca2+ permeable channel has been recently confirmed by Hardie and colleagues by using a mutant with a point mutation at the suspected pore region of the channel, altering its Ca2+ permeability properties (Liu et al., 2007).
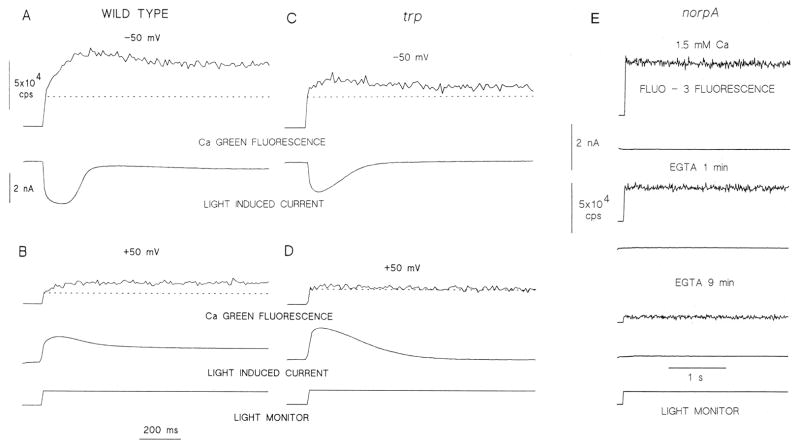
Figure 9. Calcium indicator fluorescence reveals light-induced large elevation in intracellular Ca2+, which is smaller in the trp mutant relative to WT and it is totally abolished in a PLC null mutant.
A and B: Calcium green-5N fluorescence (Kd< 30 μM) measured in WT at negative (−50 mV, A) and positive (+50 mV, B) membrane potentials. The reduced Ca2+ signal at positive membrane potential is due to reduced driving force for Ca2+ influx. The dotted lines indicate resting Ca2+ level in all panels.
C and D: Measurements similar to A and B performed in the trpCM mutant (raised at 24°C) showing a large reduction of Ca2+ influx in the mutant.
E: Both the light-induced current (lower trace in each pair) and increase in cellular Ca2+ are absent in the norpAP24, a virtually null PLC mutant. Fluo 3 (Kd< 1μM) fluorescence revealed only the resting Ca2+ level that was largely reduced by prolonged exposure to EGTA. (modified from (Peretz et al., 1994))
Additional independent evidence that the light-induced current of WT flies is mediated by the TRP and TRPL channels, came from the isolation of a null trpl mutant (which lacks TRPL) by Zuker and colleagues (Niemeyer et al., 1996). Importantly, in the double mutant trpl302;trpP301 the response to light was largely reduced. The light response was not abolished since the trpP301 allele is not null. However, the residual response was abolished by La3+, which blocks TRP but not TRPL at μM concentrations (Niemeyer et al., 1996). Consistent with this observation, application of La3+ to the trpl mutant completely abolished the response to light (Niemeyer et al., 1996). They wrote “We demonstrated that the light-activated conductance is composed of TRP and TRPL ion channels and that each can be activated on its own” (Niemeyer et al., 1996). This interpretation was strongly supported by a subsequent study of Zuker and colleagues showing that the double mutant trpl302;trpP343 completely abolished the response to light (Scott et al., 1997). Together, these experiments indicated that TRP and TRPL are required to produce all forms of the response to light and no additional channel is required to produce the light response.
A third TRP homologue channel of Drosophila with similarity to TRP and TRPL was discovered by Montell and colleagues and was designated TRPγ (Xu et al., 2000). Since the double null mutant trpl;trp has no response to light, TRPγ cannot form an independent channel like TRP or TRPL in the Drosophila eye. Nevertheless, heterologous expression of TRPγ in tissue culture cells did produce a functional channel (Jors et al., 2006; Xu et al., 2000). Since TRPL expressed in some tissue culture cells is constitutively active contrary to the situation in vivo, it was suggested that the main physiological function of TRPγ is to prevent this constitutive activity of TRPL in the eye (Xu et al., 2000). The whole issue of TRPγ and its role in phototransduction, if any, is not clear because the initial study has not been continued.
Heterologous expression of the TRP and TRPL channels
Important and direct evidence that a specific gene encodes for a functional channel protein is obtained by expressing the putative channel in a heterologous system and demonstrating that the expressed protein functions as a channel. This criterion proved valid when applied to the TRPL channel but not to TRP. Indeed, expression of trpl cDNA in a variety of expression systems has provided important support for the notion that the trpl gene product functions as a channel (see below). For the TRP channel the situation is very different. Heterologous TRP expressing cells included two insect cell lines (Spodoptera Sf9 cells and Drosophila Schneider 2, S2 cells) and two mammalian cell lines (HEK293T and CHO cells) as well as Xenopus oocytes. However, it is doubtful whether the heterologously expressed TRP reaches the surface membrane and functions as a channel; all reported conductances measured in cells transfected with TRP cDNA can be explained by enhancement of leak current or by enhancement of host cells endogenous channel activity. HEK cells and and Xenopus oocytes are known to express endogenous TRP channels which may contribute to the observed currents. In these studies Schilling and colleagues first reported heterologous expression of TRP (Vaca et al., 1994). These investigators found that expression of trp cDNA in Sf9 cells led to the appearance of a novel conductance that could be activated by Ca2+ store depletion using the Ca2+ pump inhibitor, thapsigargin. However, there is no similarity between the properties of the heterologously expressed TRP dependent conductance and the light-induced conductance of Drosophila. The TRP-dependent currents in the Sf9 cells markedly differed from the light-activated current of Drosophila in several main aspects (see Fig. 10 for the I–V curves of the native channels): The current-voltage relationship (I–V curves) is approximately linear in Sf9 cells expressing TRP (Vaca et al., 1994), while native Drosophila TRP shows inward and outward rectification (as measured in the trpl mutant), suggesting that leak current was measured in the tissue culture cells. Also, the TRP-dependent current of Drosophila shows positive and negative feedback effects of Ca2+ on the light induced current, which are completely absent in the TRP expressing Sf9 cells. Moreover, the TRP-dependent conductance in Sf9 cells has low permeability to Ba2+ and is not blocked by Mg2+ in concentrations that have a strong blocking effect in Drosophila (Reuss et al., 1997). The difficulty in functional expression of the Drosophila TRP channel may arise from the requirement of specific proteins to the transport of TRP to the plasma membrane (Cheng & Nash, 2007; Li et al., 1999).
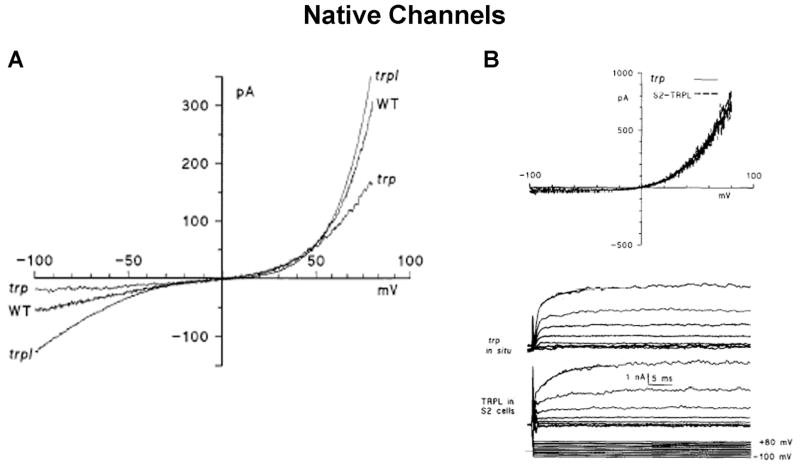
Figure 10. Current Voltage relationships of the native TRPL and TRP in WT and in the trp and trpl mutants.
A. Current Voltage (I–V) relationships determined from voltage ramps in photoreceptors of wild type (WT), trpP301 and trpl302 mutants during whole cell recordings from ommatidia bathed in physiological Ringer’s solution containing nominal 0 Ca2+ and 4mM Mg2+. The current in trp shows simple exponential outward rectification (similar to that of panel A), however, in both WT and trpl flies there is a conspicuous S shape inward and outward rectification, which are markedly different than the I–V curve of TRP in pannel A (from (Reuss et al., 1997).
B. Comparison of the I–V curves recorded by whole cell measurements from a photoreceptor of the trp mutant (expressing only TRPL) and the S2 cell expressing heterologously the TRPL channel. The top panel shows I–V curves derived from voltage ramps, while the bottom traces show voltage clamped currents in response to voltage steps between −100 mV and +80 mV in 20 mV increments (bottom traces). A striking similarity is observed between the data obtained from native and the heterologously expressed TRPL channels (from (Hardie et al., 1997).
Three years later, Montell and colleagues expressed TRP in HEK cells and showed current which could be activated by thapsigargin presumably due to Ca2+ store depletion (Xu et al., 1997). However, the properties of this current, like those of the Sf9 cells, showed considerable discrepancies from the Drosophila TRP current suggesting that it can arise from non-specific leak current. Also, Ca2+ permeability was considerable smaller relative to the native TRP channels and sensitivity to block by either La3+ or Mg2+ was also much smaller. Finally, a linear current voltage relationship without rectification (characteristic of the endogenous TRP channels) was also reported. It should be pointed out that other studies on the native Drosophila light activated channels showed that the TRP and TRPL channels cannot be activated by thapsigargin that was used to activate the heterologously expressed channels (Cook & Minke, 1999; Hardie, 1996b; Ranganathan et al., 1994).
Expression of TRP in Xenopus oocytes has provided inconsistent data, while one study showed enhancement of the endogenous Ca2+ activated Cl− current in TRP expressing oocytes (Petersen et al., 1995), another study failed to see any significant effect when TRP was expressed alone without TRPL (Gillo et al., 1996). In summary, the above results strongly suggest that the measured currents in heterologous systems expressing TRP did not arise from activation of Drosophila TRP channels.
The situation is very different when TRPL is expressed heterologously. The appearance of a non-selective cation conductance with properties of the native TRPL channel has been reported after expression of TRPL in Sf9 cells (Harteneck et al., 1995; Hu et al., 1994; Hu & Schilling, 1995), S2 cells (Hardie et al., 1997; Parnas et al., 2009), HEK293 cells (Xu et al., 1997), CHO cells (Harteneck et al., 1995), and Xenopus oocytes (Gillo et al., 1996; Lan et al., 1996). In several cases, constitutive activity of the TRPL channels were largely enhanced by co-expressing a receptor, like the muscarinic M1 receptor (Hardie et al., 1997; Parnas et al., 2009), which activates endogenous G-protein coupled phosphoinositide pathways. A common feature of the TRPL expression studies is a constitutive spontaneous activity, which increases with time during whole-cell recording. The channel properties of TRPL when expressed in Drosophila S2 cells were similar to the TRPL-dependent light sensitive conductance as determined in the native cells of trp mutants during whole cell recordings (Hardie et al., 1997). A variety of properties including single channel conductance and open times, ionic selectivity for monovalent and divalent ions, block by Mg2+ and current voltage relationship were found to be indistinguishable between native and heterologously expressed TRPL (Hardie et al., 1997; Parnas et al., 2007). The properties of TRPL channels expressed in other expression systems appear similar (Kunze et al., 1997; Obukhov et al., 1998). Thus, in contrast to the apparent failure to heterologously express functional TRP, heterologous expression of TRPL appears to form a conductance similar to that found in the native tissue, except that some conditions are required to prevent constitutive activity which does not occur under normal conditions in vivo.
Concluding remarks
The Drosophila TRP channel is the founding member of the TRP channel superfamily, which has an immense contribution to cellular signaling. The first report that TRP-related proteins might also be found outside invertebrate photoreceptors came from Petersen and colleagues (Petersen et al., 1995) who identified partial sequences of TRP homologues from Xenopus oocyte and murine brain cDNA libraries. Shortly thereafter the full sequence of a human homologue (TRPC1) was reported following homology searches of EST databases by Birnbaumer and colleagues and by Montell and colleagues (Wes et al., 1995; Zhu et al., 1995). The following studies identified the TRPC subfamily members, based on the of amino acid sequence homology with the Drosophila TRP and TRPL channels. Subsequently, several groups cloned and sequenced the other TRP superfamilies, independently of the Drosophila TRP and TRPL channels (Fig. 1). The functional role of most members of mammalian TRPC subfamily in the native tissues is largely unknown. Many of them show very widespread tissue distribution but their properties have been mainly inferred from heterologous expression studies. The expression pattern of various members of the TRP superfamily in specific cells and tissues provided clues as to their specific functions, which seem to be diverse. Unfortunately, the absence of selective antagonists and the difficulty in analyzing many members of the TRP family in native tissues, impose great difficulty in understanding the function, mechanism of activation and properties under physiological conditions. The detailed studies in Drosophila that combine genetic dissection with powerful electrophysiological and single cell-Ca2+ measuring techniques, may provide important clues regarding the activation mechanism of at least the mammalian TRPC channels that share a relatively high structural similarity with Drosophila TRP and TRPL. In addition, there is steadily growing research on mammalian members of the TRP superfamily that have been conducted in the native tissue, using knockout mice. It thus seems that studies in the native tissue are indispensable and are likely to shed new light and reveal important functions of mammalian TRP channels. There are several examples of TRP channels which are involved in diseases (Nilius et al., 2005) and many examples of TRP channels that are related to disease states (Abramowitz & Birnbaumer, 2009; Nilius et al., 2005). Therefore, the importance of understanding TRP channels in native tissue has wide implications in future treatment of pathological states.
The Drosophila TRP channel has contributed uniquely to the field of sensory neurobiology by identifying the founding member of the TRP superfamily. An additional important contribution to the Drosophila TRP channel research has been provided by studies of phototransduction as a model system for the role of the ubiquitous inositol-lipid signaling in TRP channel regulation and activation. The great advantage of using Drosophila photoreceptors is the accessibility of the preparation, the ease of light stimulation, the robust expression of key molecular components and most importantly, the ability to apply the great power of the Drosophila molecular genetics. Therefore, the native Drosophila photoreceptor cells, is still a useful preparation to study basic features of this remarkable channel. This was recently demonstrated by studies of Hardie and colleagues showing light-induced pH changes, which strongly affected the Drosophila TRP and TRPL, thus having important implications on TRP gating in general (Huang et al., 2010).
Acknowledgments
The experimental part of this review was supported by grants from the National Institute of Health (RO1 EY 03529), the Israel Science Foundation (ISF), the US-Israel Binational Science Foundation (BSF), The German Israel Foundation (GIF) and the Minerva Foundation. I thank Nansi J. Colley, Ben Katz, Shaya Lev, David Zeevi and Maximilian Peters for critical reading of the manuscript.
References
- Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23(2):297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya JK, Jalink K, Hardy RW, Hartenstein V, Zuker CS. InsP3 receptor is essential for growth and differentiation but not for vision in Drosophila. Neuron. 1997;18(6):881–887. doi: 10.1016/s0896-6273(00)80328-1. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Capacitive calcium entry. Biochem J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bloomquist BT, Shortridge RD, Schneuwly S, Perdew M, Montell C, Steller H, et al. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988;54:723–733. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- Blumenfeld A, Erusalimsky J, Heichal O, Selinger Z, Minke B. Light-activated guanosinetriphosphatase in Musca eye membranes resembles the prolonged depolarizing afterpotential in photoreceptor cells. Proc Natl Acad Sci U S A. 1985;82(20):7116–7120. doi: 10.1073/pnas.82.20.7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen CJ, Priel A, Zhou S, King D, Siemens J, Julius D. A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain. Cell. 2010;141(5):834–845. doi: 10.1016/j.cell.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JE, Rubin LJ, Ghalayini AJ, Tarver AP, Irvine RF, Berridge MJ, et al. myo-Inositol polyphosphate may be a messenger for visual excitation in Limulus photoreceptors. Nature. 1984;311:160–163. doi: 10.1038/311160a0. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Nash HA. Drosophila TRP channels require a protein with a distinctive motif encoded by the inaF locus. Proc Natl Acad Sci U S A. 2007;104(45):17730–17734. doi: 10.1073/pnas.0708368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chyb S, Raghu P, Hardie RC. Polyunsaturated fatty acids activate the Drosophila light-sensitive channels TRP and TRPL. Nature. 1999;397(6716):255–259. doi: 10.1038/16703. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426(6966):517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Colbert HA, Bargmann CI. Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron. 1995;14(4):803–812. doi: 10.1016/0896-6273(95)90224-4. [DOI] [PubMed] [Google Scholar]
- Cook B, Minke B. TRP and calcium stores in Drosophila phototransduction. Cell Calcium. 1999;25(2):161–171. doi: 10.1054/ceca.1998.0018. [DOI] [PubMed] [Google Scholar]
- Corey DP. New TRP channels in hearing and mechanosensation. Neuron. 2003;39(4):585–588. doi: 10.1016/s0896-6273(03)00505-1. [DOI] [PubMed] [Google Scholar]
- Cosens D. Blindness in a Drosophila mutant. J Insect Physiol. 1971;17:285–302. [Google Scholar]
- Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285–287. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- Dadon D, Minke B. Cellular functions of Transient Receptor Potential channels. Int J Biochem Cell Biol. 2010 doi: 10.1016/j.biocel.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deland MC, Pak WL. Reversibly temperature sensitive phototransduction mutant of Drosophila melanogaster. Nat New Biol. 1973;244(136):184–186. doi: 10.1038/newbio244184a0. [DOI] [PubMed] [Google Scholar]
- Delmas P. Polycystins: from mechanosensation to gene regulation. Cell. 2004;118(2):145–148. doi: 10.1016/j.cell.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Devary O, Heichal O, Blumenfeld A, Cassel D, Suss E, Barash S, et al. Coupling of photoexcited rhodopsin to inositol phospholipid hydrolysis in fly photoreceptors. Proc Natl Acad Sci U S A. 1987;84:6939–6943. doi: 10.1073/pnas.84.19.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge FA, Jr, Knight BW, Toyoda J. Voltage noise in Limulus visual cells. Science. 1968;160:88–90. doi: 10.1126/science.160.3823.88. [DOI] [PubMed] [Google Scholar]
- Dorloechter M, Stieve H. The Limulus ventral photoreceptor: light response and the role of calcium in a classic preparation. Prog Neurobiol. 1997;53:451–515. doi: 10.1016/s0301-0082(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Fein A. Blockade of visual excitation and adaptation in Limulus photoreceptor by GDP-b-S. Science. 1986;232(4757):1543–1545. doi: 10.1126/science.3487116. [DOI] [PubMed] [Google Scholar]
- Fein A, Corson DW. Excitation of Limulus photoreceptors by vanadate and by a hydrolysis-resistant analog of guanosine triphosphate. Science. 1981;212(4494):555–557. doi: 10.1126/science.6782676. [DOI] [PubMed] [Google Scholar]
- Fein A, Payne R, Corson DW, Berridge MJ, Irvine RF. Photoreceptor excitation and adaptation by inositol 1,4,5- trisphosphate. Nature. 1984;311:157–160. doi: 10.1038/311157a0. [DOI] [PubMed] [Google Scholar]
- Franceschini N, Kirschfeld K. Pseudopupil phenomena in the compound eye of Drosophila. Kybernetik. 1971;9(5):159–182. doi: 10.1007/BF02215177. [DOI] [PubMed] [Google Scholar]
- Gillo B, Chorna I, Cohen H, Cook B, Manistersky I, Chorev M, et al. Coexpression of Drosophila TRP and TRP-like proteins in Xenopus oocytes reconstitutes capacitative Ca2+ entry. Proc Natl Acad Sci U S A. 1996;93(24):14146–14151. doi: 10.1073/pnas.93.24.14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC. Whole-cell recordings of the light induced current in dissociated Drosophila photoreceptors: evidence for feedback by calcium permeating the light-sensitive channels. Proc R Soc Lond B. 1991;245:203–210. [Google Scholar]
- Hardie RC. Calcium signalling: setting store by calcium channels. Curr Biol. 1996a;6(11):1371–1373. doi: 10.1016/s0960-9822(96)00733-6. [DOI] [PubMed] [Google Scholar]
- Hardie RC. Excitation of Drosophila photoreceptors by BAPTA and ionomycin: evidence for capacitative Ca2+ entry? Cell Calcium. 1996b;20(4):315–327. doi: 10.1016/s0143-4160(96)90037-8. [DOI] [PubMed] [Google Scholar]
- Hardie RC. INDO-1 measurements of absolute resting and light-induced Ca2+ concentration in Drosophila photoreceptors. J Neurosci. 1996c;16:2924–2933. doi: 10.1523/JNEUROSCI.16-09-02924.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Minke B. Novel Ca2+ channels underlying transduction in Drosophila photoreceptors: implications for phosphoinositide-mediated Ca2+ mobilization. Trends Neurosci. 1993;16:371–376. doi: 10.1016/0166-2236(93)90095-4. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Raghu P. Visual transduction in Drosophila. Nature. 2001;413(6852):186–193. doi: 10.1038/35093002. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Reuss H, Lansdell SJ, Millar NS. Functional equivalence of native light-sensitive channels in the Drosophila trp301 mutant and TRPL cation channels expressed in a stably transfected Drosophila cell line. Cell Calcium. 1997;21(6):431–440. doi: 10.1016/s0143-4160(97)90054-3. [DOI] [PubMed] [Google Scholar]
- Harteneck C, Obukhov AG, Zobel A, Kalkbrenner F, Schultz G. The Drosophila cation channel trpl expressed in insect Sf9 cells is stimulated by agonists of G-protein-coupled receptors. FEBS Lett. 1995;358:297–300. doi: 10.1016/0014-5793(94)01455-a. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. 2. Sunderland, MA: Sinauer Associate, Inc; 1992. [Google Scholar]
- Hochstrate P. Lanthanum mimicks the trp photoreceptor mutant of Drosophila in the blowfly Calliphora. J Comp Physiol A. 1989;166:179–187. doi: 10.1007/BF00193462. [DOI] [PubMed] [Google Scholar]
- Howard J. Calcium enables photoreceptor pigment migration in a mutant fly. J Exp Biol. 1984;113:471–475. [Google Scholar]
- Hu Y, Schilling WP. Receptor-mediated activation of recombinant Trpl expressed in Sf9 insect cells. Bichem J. 1995;305:605–611. doi: 10.1042/bj3050605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Vaca L, Zhu X, Birnbaumer L, Kunze DL, Schilling WP. Appearance of a novel Ca2+ influx pathway in Sf9 insect cells following expression of the transient receptor potential-like (trpl) protein of Drosophila. Biochem Biophys Res Commun. 1994;201:1050–1056. doi: 10.1006/bbrc.1994.1808. [DOI] [PubMed] [Google Scholar]
- Huang J, Liu CH, Hughes SA, Postma M, Schwiening CJ, Hardie RC. Activation of TRP channels by protons and phosphoinositide depletion in Drosophila photoreceptors. Curr Biol. 2010;20(3):189–197. doi: 10.1016/j.cub.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Jordt SE, McKemy DD, Julius D. Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr Opin Neurobiol. 2003;13(4):487–492. doi: 10.1016/s0959-4388(03)00101-6. [DOI] [PubMed] [Google Scholar]
- Jors S, Kazanski V, Foik A, Krautwurst D, Harteneck C. Receptor-induced activation of Drosophila TRP gamma by polyunsaturated fatty acids. Journal of Biological Chemistry. 2006;281(40):29693–29702. doi: 10.1074/jbc.M602215200. [DOI] [PubMed] [Google Scholar]
- Julius D. From peppers to peppermints: natural products as probes of the pain pathway. Harvey Lect. 2005;101:89–115. [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413(6852):203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Katz B, Minke B. Drosophila photoreceptors and signaling mechanisms. Front Cell Neurosci. 2009;3:2. doi: 10.3389/neuro.03.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschfeld K, Vogt K. Calcium ions and pigment migration in fly photoreceptors. Naturwissenschaften. 1980;67:516–517. [Google Scholar]
- Kunze DL, Sinkins WG, Vaca L, Schilling WP. Properties of single Drosophila Trpl channels expressed in Sf9 insect cells. Am J Physiol. 1997;272:C27–C34. doi: 10.1152/ajpcell.1997.272.1.C27. [DOI] [PubMed] [Google Scholar]
- Lan L, Bawden MJ, Auld AM, Barritt GJ. Expression of Drosophila trpl cRNA in Xenopus laevis oocytes leads to the appearance of a Ca2+ channel activated by Ca2+ and calmodulin, and by guanosine 5′[gamma-thio]triphosphate. Biochem J. 1996;316(Pt 3):793–803. doi: 10.1042/bj3160793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Dobbs MB, Verardi ML, Hyde DR. dgq: a Drosophila gene encoding a visual system-specific G alpha molecule. Neuron. 1990;5:889–898. doi: 10.1016/0896-6273(90)90349-k. [DOI] [PubMed] [Google Scholar]
- Levy LS, Ganguly R, Ganguly N, Manning JE. The selection, expression, and organization of a set of head-specific genes in Drosophila. Dev Biol. 1982;94(2):451–464. doi: 10.1016/0012-1606(82)90362-1. [DOI] [PubMed] [Google Scholar]
- Li C, Geng C, Leung HT, Hong YS, Strong LL, Schneuwly S, et al. INAF, a protein required for transient receptor potential Ca2+ channel function. Proc Natl Acad Sci U S A. 1999;96(23):13474–13479. doi: 10.1073/pnas.96.23.13474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Brown JE. The effects of intracellular Ca2+ on the light response and on light adaptation in Limulus ventral photoreceptors. Adv Exp Med Biol. 1972;24(0):23–33. doi: 10.1007/978-1-4684-8231-7_2. [DOI] [PubMed] [Google Scholar]
- Liu CH, Wang T, Postma M, Obukhov AG, Montell C, Hardie RC. In vivo identification and manipulation of the Ca2+ selectivity filter in the Drosophila transient receptor potential channel. J Neurosci. 2007;27(3):604–615. doi: 10.1523/JNEUROSCI.4099-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo MV, Pak WL. Light-induced pigment granule migration in the retinular cells of Drosophila melanogaster. Comparison of wild type with ERG- defective mutants. J Gen Physiol. 1981;77:155–175. doi: 10.1085/jgp.77.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai I, Okazaki A, Hosoya T, Hotta Y. Drosophila retinal degeneration A gene encodes an eye-specific diacylglycerol kinase with cysteine-rich zinc-finger motifs and ankyrin repeats. Proc Natl Acad Sci U S A. 1993;90:11157–11161. doi: 10.1073/pnas.90.23.11157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay RR, Chen DM, Miller K, Kim S, Stark WS, Shortridge RD. Phospholipase C rescues visual defect in norpA mutant of Drosophila melanogaster. Journal of Biological Chemistry. 1995;270(22):13271–13276. doi: 10.1074/jbc.270.22.13271. [DOI] [PubMed] [Google Scholar]
- Miller BA. The role of TRP channels in oxidative stress-induced cell death. J Membr Biol. 2006;209(1):31–41. doi: 10.1007/s00232-005-0839-3. [DOI] [PubMed] [Google Scholar]
- Minke B. Drosophila mutant with a transducer defect. Biophys Struct Mechanism. 1977;3:59–64. doi: 10.1007/BF00536455. [DOI] [PubMed] [Google Scholar]
- Minke B. Light-induced reduction in excitation efficiency in the trp mutant of Drosophila. J Gen Physiol. 1982;79:361–385. doi: 10.1085/jgp.79.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minke B. The trp is a Drosophila mutant sensitive to developmental temperature. J Comp Physiol A. 1983;151:483–486. [Google Scholar]
- Minke B, Cook B. TRP channel proteins and signal transduction. Physiol Rev. 2002;82(2):429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- Minke B, Selinger Z. Inositol lipid pathway in fly photoreceptors: excitation, calcium mobilization and retinal degeneration. In: Osborne NA, Chader GJ, editors. Progress in retinal research. Pergamon Press; Oxford: 1991. pp. 99–124. [Google Scholar]
- Minke B, Selinger Z. Intracellular messengers in invertebrate photoreceptors studied in mutant flies. In: Boulton A, Baker G, Taylor C, editors. Neuromethods. Clifton, N.J.: The Humana Press Inc; 1992. pp. 517–563. [Google Scholar]
- Minke B, Stephenson RS. The characteristics of chemically induced noise in Musca photoreceptors. J Comp Physiol. 1985;156:339–356. [Google Scholar]
- Minke B, Tsacopoulos M. Light induced sodium dependent accumulation of calcium and potassium in the extracellular space of bee retina. Vision Res. 1986;26:679–690. doi: 10.1016/0042-6989(86)90082-9. [DOI] [PubMed] [Google Scholar]
- Minke B, Wu C, Pak WL. Induction of photoreceptor voltage noise in the dark in Drosophila mutant. Nature. 1975;258:84–87. doi: 10.1038/258084a0. [DOI] [PubMed] [Google Scholar]
- Montell C. Physiology, phylogeny, and functions of the TRP superfamily of cation channels. 2001:1–17. doi: 10.1126/stke.2001.90.re1. http://stkesciencemag.org/cgi/content/full/OC_sigtrans2001/90/rel. [DOI] [PubMed]
- Montell C, Birnbaumer L, Flockerzi V, Bindels RJ, Bruford EA, Caterina MJ, et al. A unified nomenclature for the superfamily of TRP cation channels. Mol Cell. 2002;9(2):229–231. doi: 10.1016/s1097-2765(02)00448-3. [DOI] [PubMed] [Google Scholar]
- Montell C, Jones K, Hafen E, Rubin G. Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science. 1985;230:1040–1043. doi: 10.1126/science.3933112. [DOI] [PubMed] [Google Scholar]
- Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85(5):651–659. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- Nilius B, Mahieu F. A road map for TR(I)Ps. Mol Cell. 2006;22(3):297–307. doi: 10.1016/j.molcel.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87(1):165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Nilius B, Voets T. TRP channels: a TR(I)P through a world of multifunctional cation channels. Pflugers Arch. 2005;451(1):1–10. doi: 10.1007/s00424-005-1462-y. [DOI] [PubMed] [Google Scholar]
- Nilius B, Voets T, Peters J. TRP channels in disease. Sci STKE. 2005;2005(295):re8. doi: 10.1126/stke.2952005re8. [DOI] [PubMed] [Google Scholar]
- Nishida M, Hara Y, Yoshida T, Inoue R, Mori Y. TRP channels: molecular diversity and physiological function. Microcirculation. 2006;13(7):535–550. doi: 10.1080/10739680600885111. [DOI] [PubMed] [Google Scholar]
- Obukhov AG, Schultz G, Luckhoff A. Regulation of heterologously expressed transient receptor potential-like channels by calcium ions. Neuroscience. 1998;85(2):487–495. doi: 10.1016/s0306-4522(97)00616-7. [DOI] [PubMed] [Google Scholar]
- Parnas M, Katz B, Lev S, Tzarfaty V, Dadon D, Gordon-Shaag A, et al. Membrane lipid modulations remove divalent open channel block from TRP-like and NMDA channels. J Neurosci. 2009;29(8):2371–2383. doi: 10.1523/JNEUROSCI.4280-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas M, Katz B, Minke B. Open channel block by Ca2+ underlies the voltage dependence of Drosophila TRPL channel. J Gen Physiol. 2007;129(1):17–28. doi: 10.1085/jgp.200609659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz A, Suss-Toby E, Rom-Glas A, Arnon A, Payne R, Minke B. The light response of Drosophila photoreceptors is accompanied by an increase in cellular calcium: effects of specific mutations. Neuron. 1994;12:1257–1267. doi: 10.1016/0896-6273(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Petersen CC, Berridge MJ, Borgese MF, Bennett DL. Putative capacitative calcium entry channels: expression of Drosophila trp and evidence for the existence of vertebrate homologues. Biochem J. 1995;311(Pt 1):41–44. doi: 10.1042/bj3110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AM, Bull A, Kelly LE. Identification of a Drosophila gene encoding a calmodulin- binding protein with homology to the trp phototransduction gene. Neuron. 1992;8:631–642. doi: 10.1016/0896-6273(92)90085-r. [DOI] [PubMed] [Google Scholar]
- Pollock JA, Assaf A, Peretz A, Nichols CD, Mojet MH, Hardie RC, et al. TRP a protein essential for inositide-mediated Ca2+ influx is localized adjacent to the calcium stores in Drosophila photoreceptors. J Neurosci. 1995;15:3747–3760. doi: 10.1523/JNEUROSCI.15-05-03747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu P, Colley NJ, Webel R, James T, Hasan G, Danin M, et al. Normal phototransduction in Drosophila photoreceptors lacking an InsP3 receptor gene. Mol Cell Neurosci. 2000a;15(5):429–445. doi: 10.1006/mcne.2000.0846. [DOI] [PubMed] [Google Scholar]
- Raghu P, Usher K, Jonas S, Chyb S, Polyanovsky A, Hardie RC. Constitutive activity of the light-sensitive channels TRP and TRPL in the Drosophila diacylglycerol kinase mutant, rdgA. Neuron. 2000b;26(1):169–179. doi: 10.1016/s0896-6273(00)81147-2. [DOI] [PubMed] [Google Scholar]
- Ranganathan R, Bacskai BJ, Tsien RY, Zuker CS. Cytosolic calcium transients: spatial localization and role in Drosophila photoreceptor cell function. Neuron. 1994;13:837–848. doi: 10.1016/0896-6273(94)90250-x. [DOI] [PubMed] [Google Scholar]
- Reuss H, Mojet MH, Chyb S, Hardie RC. In vivo analysis of the Drosophila light-sensitive channels, TRP and TRPL. Neuron. 1997;19:1249–1259. doi: 10.1016/s0896-6273(00)80416-x. [DOI] [PubMed] [Google Scholar]
- Scott K, Becker A, Sun Y, Hardy R, Zuker C. Gqa protein function in vivo: genetic dissection of its role in photoreceptor cell physiology. Neuron. 1995;15:919–927. doi: 10.1016/0896-6273(95)90182-5. [DOI] [PubMed] [Google Scholar]
- Scott K, Sun Y, Beckingham K, Zuker CS. Calmodulin regulation of Drosophila light-activated channels and receptor function mediates termination of the light response in vivo. Cell. 1997;91(3):375–383. doi: 10.1016/s0092-8674(00)80421-3. [DOI] [PubMed] [Google Scholar]
- Selinger Z, Minke B. Inositol lipid cascade of vision studied in mutant flies. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):333–341. doi: 10.1101/sqb.1988.053.01.040. [DOI] [PubMed] [Google Scholar]
- Suss Toby E, Selinger Z, Minke B. Lanthanum reduces the excitation efficiency in fly photoreceptors. J Gen Physiol. 1991;98:849–868. doi: 10.1085/jgp.98.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaca L, Sinkins WG, Hu Y, Kunze DL, Schilling WP. Activation of recombinant trp by thapsigargin in Sf9 insect cells. Am J Physiol. 1994;267:C1501–C1505. doi: 10.1152/ajpcell.1994.267.5.C1501. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wes PD, Chevesich J, Jeromin A, Rosenberg C, Stetten G, Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci, USA. 1995;92:9652–9656. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F, Hokanson KM, Chang LT. Molecular basis of an inherited retinal defect in Drosophila. Invest Ophthalmol Vis Sci. 1985;26(2):243–246. [PubMed] [Google Scholar]
- Wong F, Knight BW. Adapting-bump model for eccentric cells of Limulus. J Gen Physiol. 1980;76(5):539–557. doi: 10.1085/jgp.76.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F, Knight BW, Dodge FA. Adapting bump model for ventral photoreceptors of Limulus. J Gen Physiol. 1982;79(6):1089–1113. doi: 10.1085/jgp.79.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F, Schaefer EL, Roop BC, LaMendola JN, Johnson Seaton D, Shao D. Proper function of the Drosophila trp gene product during pupal development is important for normal visual transduction in the adult. Neuron. 1989;3:81–94. doi: 10.1016/0896-6273(89)90117-7. [DOI] [PubMed] [Google Scholar]
- Wu CF, Pak WL. Quantal basis of photoreceptor spectral sensitivity of Drosophila melanogaster. J Gen Physiol. 1975;66(2):149–168. doi: 10.1085/jgp.66.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XZ, Chien F, Butler A, Salkoff L, Montell C. TRPg, a Drosophila TRP-related subunit, forms a regulated cation channel with TRPL. Neuron. 2000;26(3):647–657. doi: 10.1016/s0896-6273(00)81201-5. [DOI] [PubMed] [Google Scholar]
- Xu XZS, Li HS, Guggino WB, Montell C. Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell. 1997;89:1155–1164. doi: 10.1016/s0092-8674(00)80302-5. [DOI] [PubMed] [Google Scholar]
- Yarfitz S, Niemi GA, McConnell JL, Fitch CL, Hurley JB. A Gb protein in the Drosophila compound eye is different from that in the brain. Neuron. 1991;7:429–438. doi: 10.1016/0896-6273(91)90295-b. [DOI] [PubMed] [Google Scholar]
- Yeandle S, Spiegler JB. Light-evoked and spontaneous discrete waves in the ventral nerve photoreceptor of Limulus. J Gen Physiol. 1973;61(5):552–571. doi: 10.1085/jgp.61.5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J, Cohen Ben-Ami H, Hong YS, Park S, Strong LLR, Bowman J, et al. Novel mechanism of massive photoreceptor degeneration caused by mutations in the trp gene of Drosophila. J Neurosci. 2000;20:649–659. doi: 10.1523/JNEUROSCI.20-02-00649.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Chu PB, Peyton M, Birnbaumer L. Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett. 1995;373:193–198. doi: 10.1016/0014-5793(95)01038-g. [DOI] [PubMed] [Google Scholar]