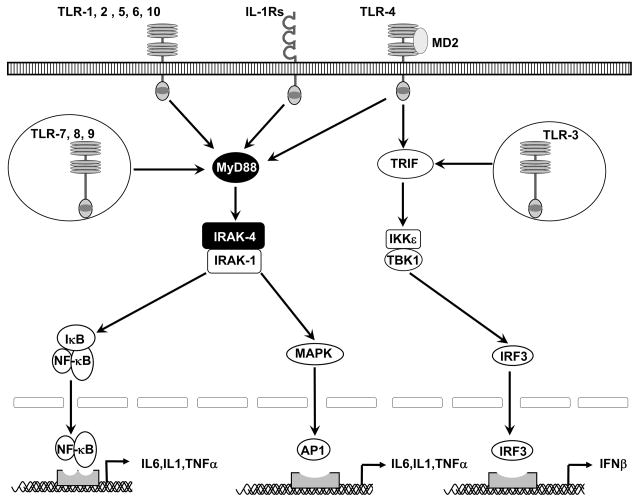
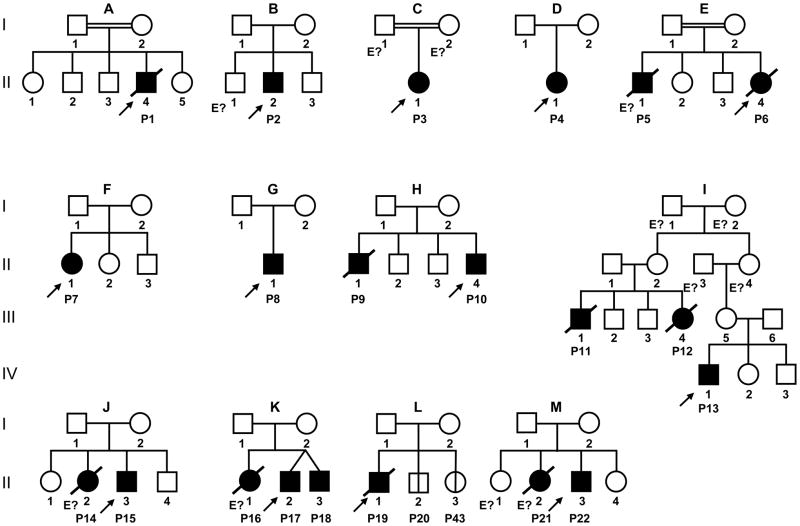
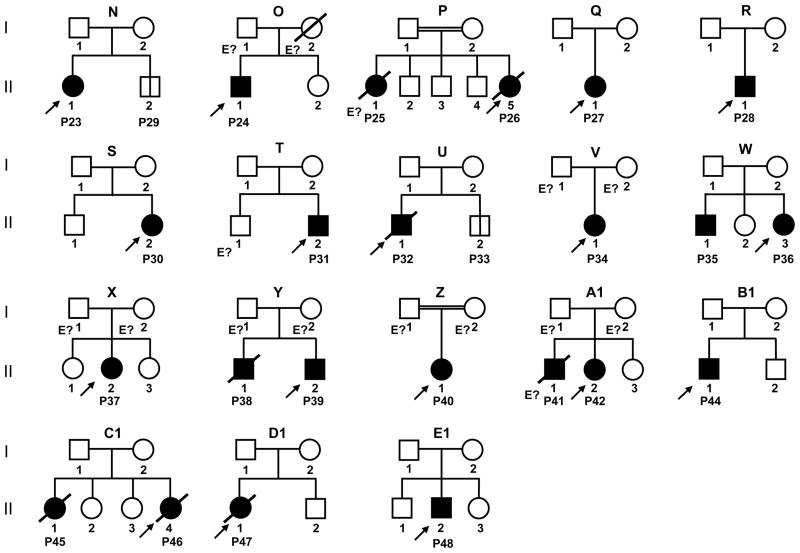
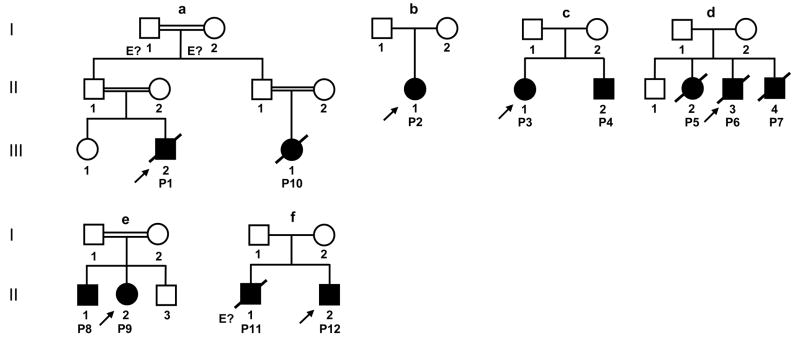
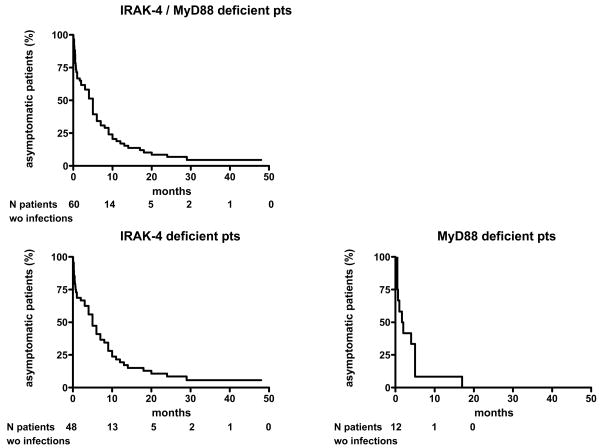
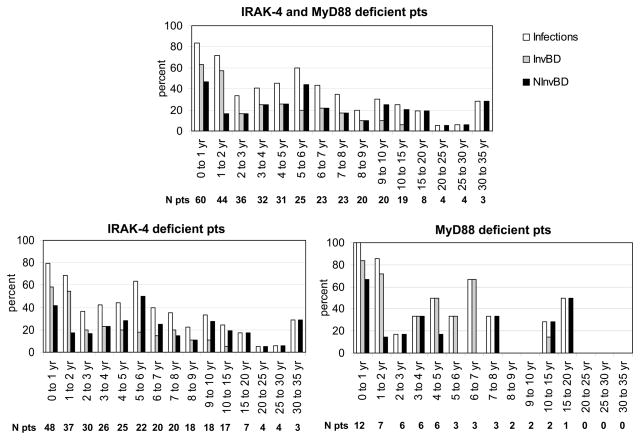
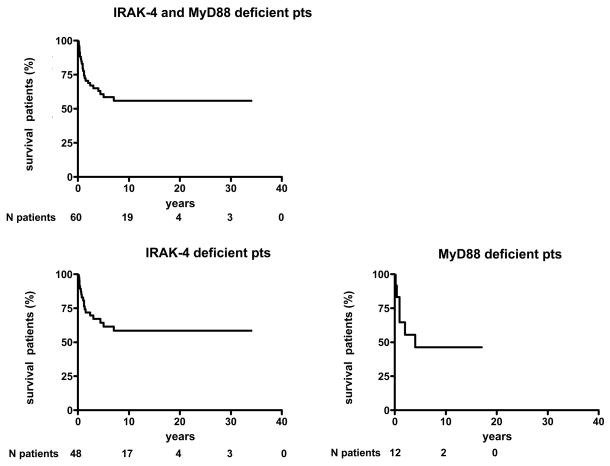
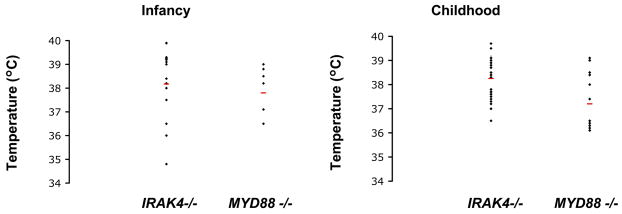
Capucine Picard
Capucine Picard, MD, PhD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,*,
Horst von Bernuth
Horst von Bernuth, MD, PhD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,*,
Pegah Ghandil
Pegah Ghandil, PhD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Maya Chrabieh
Maya Chrabieh
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Ofer Levy
Ofer Levy, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Peter D Arkwright
Peter D Arkwright, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Douglas McDonald
Douglas McDonald, MD, PhD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Raif S Geha
Raif S Geha, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Hidetoshi Takada
Hidetoshi Takada, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Jens C Krause
Jens C Krause, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
C Buddy Creech
C Buddy Creech, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Cheng-Lung Ku
Cheng-Lung Ku, PhD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Stephan Ehl
Stephan Ehl, MD, PhD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Ĺaszĺo Maŕodi
Ĺaszĺo Maŕodi, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Saleh Al-Muhsen
Saleh Al-Muhsen, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Sami Al-Hajjar
Sami Al-Hajjar, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Abdulaziz Al-Ghonaium
Abdulaziz Al-Ghonaium, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Noorbibi K Day-Good
Noorbibi K Day-Good, PhD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Steven M Holland
Steven M Holland, MD, PhD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
John Gallin
John Gallin, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Helen Chapel
Helen Chapel, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
David P Speert
David P Speert, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Carlos Rodriguez-Gallego
Carlos Rodriguez-Gallego, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Elena Colino
Elena Colino, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Ben-Zion Garty
Ben-Zion Garty, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Chaim Roifman
Chaim Roifman, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Toshiro Hara
Toshiro Hara, MD, PhD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Hideto Yoshikawa
Hideto Yoshikawa, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Shigeaki Nonoyama
Shigeaki Nonoyama, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Joseph Domachowske
Joseph Domachowske, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Andrew C Issekutz
Andrew C Issekutz, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Mimi Tang
Mimi Tang, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Joanne Smart
Joanne Smart, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Simona Eva Zitnik
Simona Eva Zitnik, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Cyrille Hoarau
Cyrille Hoarau, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Dinakantha Kumararatne
Dinakantha Kumararatne, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Adrian Thrasher
Adrian Thrasher, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
E Graham Davies
E Graham Davies, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Claire Bethune
Claire Bethune, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Nicolas Sirvent
Nicolas Sirvent, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Dominique de Ricaud
Dominique de Ricaud, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Yildiz Camcioglu
Yildiz Camcioglu, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
J́ulia Vasconcelos
J́ulia Vasconcelos, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Margarida Guedes
Margarida Guedes, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Artur Bonito Vitor
Artur Bonito Vitor, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Carlos Rodrigo
Carlos Rodrigo, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Francisco AlmaŸan
Francisco AlmaŸan, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Maria Ḿendez
Maria Ḿendez, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Juan Ignacio Aŕostegui
Juan Ignacio Aŕostegui, MD, PhD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Laia Alsina
Laia Alsina, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Claudia Fortuny
Claudia Fortuny, MD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Janine Reichenbach
Janine Reichenbach, MD, PhD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
James W Verbsky
James W Verbsky, MD, PhD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Xavier Bossuyt
Xavier Bossuyt, MD, PhD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Rainer Doffinger
Rainer Doffinger, PhD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Laurent Abel
Laurent Abel, MD, PhD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Anne Puel
Anne Puel, PhD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1,
Jean-Laurent Casanova
Jean-Laurent Casanova, MD, PhD
1Study Center of Primary Immunodeficiencies, Assistance Publique Ĥopitaux de Paris, Necker Hospital, Paris, France (CP). Laboratory of Human Genetics of Infectious Diseases, Necker Branch, INSERM U550, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Paris Descartes University, Paris, France (CP, HvB, PG, MC, CLK, L. Abel, AP, JLC). Department of Pediatric Pneumology and Immunology, Charit́e Hospital-Humboldt University, Berlin, Germany (HvB). Novel Primary Immunodeficiency and Infectious Diseases Program, College of Medicine, King Saud University, Riyadh, Kingdom of Saudi Arabia (PG, SAM, SAH, AAG, JLC). Division of Infectious Diseases (OL); and Division of Immunology (DM, RSG), Children’s Hospital Boston, Boston, Massachusetts (OL). Harvard Medical School, Boston, Massachusetts (OL, DM, RSG). University of Manchester, Royal Manchester Children’s Hospital, Manchester, United Kingdom (PDA). Department of Pediatrics, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan (HT, TH). Department of Pediatrics, Division of Pediatric Infectious Diseases, Vanderbilt University, Nashville, Tennessee (JCK, CBC). Centre of Chronic Immunodeficiency, University of Freiburg, Freiburg, Germany (SE). Department of Infectious and Pediatric Immunology, Medical and Health Science Center, University of Debrecen, Debrecen, Hungary (LM). Department of Pediatrics, King Faisal Specialist Hospital and Research Centre, Riyadh, Kingdom of Saudi Arabia (SAM, SAH, AAG). Department of Pediatrics, Division of Allergy and Immunology, University of South Florida and All Children’s Hospital, St. Petersburg, Florida (NKDG). Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland (SMH, JG). University of Oxford and Oxford Radcliffe Hospital, Oxford, United Kingdom (HC). Division of Infectious and Immunological Diseases, Department of Pediatrics, University of British Columbia, Vancouver, British Columbia, Canada (DPS). Department of Immunology, Dr Negrin University Hospital of Gran Canaria, Las Palmas de Gran Canaria, Spain (CRG). Department of Pediatrics, Unit of Infectious Diseases, Insular-Materno-Infantil University Hospital, Las Palmas de Gran Canaria, Spain (EC). Schneider Children’s Medical Center, Petah Tiqva, Israel (BZG). Division of Immunology and Allergy, Department of Pediatrics, Hospital for Sick Children, University of Toronto, Toronto, Ontario, Canada (C. Roifman). Department of Neurology, Miyagi Children’s Hospital, Sendai, Japan (HY). Department of Pediatrics, National Defense Medical College, Saitama, Japan (SN). Pediatrics, Microbiology and Immunology, SUNY Upstate Medical University, Syracuse, New York (JD). Dalhousie University, Halifax, Nova Scotia, Canada (ACI). Royal Children’s Hospital, Parkville, Victoria, Australia (MT, JS). University Children Hospital Ljubljana, Ljubljana, Slovenia (SEZ). Unit́e transversale d’Allergologie, Ńephrologie et Immunologie Clinique, Centre hospitalier universitaire de Tours, Tours, France (CH). Department of Clinical Biochemistry and Immunology, Addenbrookes Hospital, Cambridge, United Kingdom (DK, RD). Paediatric Immunology and Molecular Immunology Unit, Institute of Child Health, London, United Kingdom (AT). Department of Clinical Immunology, Great Ormond St Hospital, London, United Kingdom (EGD). Immunology Department, Derriford Hospital, Plymouth, United Kingdom (CB). University Hospital Archet 2, Nice, France (NS). Lenval Foundation, Children’s Hospital, Nice, France (DDR). Cerrahpasa Medical School, Istanbul University, Istanbul, Turkey (YC). Pediatric Department, General Hospital of Santo Ant́onio, Porto, Portugal (JV, MG). Pediatric Department, Hospital S. Jõao, Porto, Portugal (ABV). Pediatric Department, Germans Trias i Pujol Hospital, Barcelona Autonomous University, Barcelona, Spain (C. Rodrigo, FA, MM). Immunology Department-CDB, Hospital Cĺinic-IDIBAPS, Barcelona University, Barcelona, Spain (JIA). Pediatric Department, Hospital Sant Joan de Deu, Barcelona University, Barcelona, Spain (L. Alsina, CF). Immunology Department, Universiẗats-Kinderspital Z̈urich, Z̈urich, Switzerland (JR). Pediatrics and Microbiology and Molecular Genetics, Medical College of Wisconsin, Milwaukee, Wisconsin (JMV). Experimental Laboratory Medicine, Department of Medical Diagnostic Sciences, Biomedical Science Group, Catholic University Leuven, Leuven, Belgium (XB). Laboratory of Human Genetics of Infectious Diseases, Rockefeller Branch, The Rockefeller University, New York, New York (JLC). Pediatric Hematology-Immunology Unit, Necker Hospital, Assistance Publique Ĥopitaux de Paris, Paris, France (JLC)
1