Abstract
Three-dimensional gel matrices provide specialized microenvironments that mimic native tissues and enable stem cells to grow and differentiate into specific cell types. Here, we show that collagen three-dimensional gel matrices prepared in combination with adhesive proteins, such as fibronectin (FN) and laminin (LN), provide significant cues to the differentiation into neuronal lineage of mesenchymal stem cells (MSCs) derived from rat bone marrow. When cultured within either a three-dimensional collagen gel alone or one containing either FN or LN, and free of nerve growth factor (NGF), the MSCs showed the development of numerous neurite outgrowths. These were, however, not readily observed in two-dimensional culture without the use of NGF. Immunofluorescence staining, western blot and fluorescence-activated cell sorting analyses demonstrated that a large population of cells was positive for NeuN and glial fibrillary acidic protein, which are specific to neuronal cells, when cultured in the three-dimensional collagen gel. The dependence of the neuronal differentiation of MSCs on the adhesive proteins containing three-dimensional gel matrices is considered to be closely related to focal adhesion kinase (FAK) activation through integrin receptor binding, as revealed by an experiment showing no neuronal outgrowth in the FAK-knockdown cells and stimulation of integrin β1 gene. The results provided herein suggest the potential role of three-dimensional collagen-based gel matrices combined with adhesive proteins in the neuronal differentiation of MSCs, even without the use of chemical differentiation factors. Furthermore, these findings suggest that three-dimensional gel matrices might be useful as nerve-regenerative scaffolds.
Keywords: three-dimensional collagen gel, mesenchymal stem cells, neuronal differentiation, adhesive proteins, focal adhesion kinase activation
1. Introduction
The regulation of stem cell functions including cell adhesion and migration, mitosis and differentiation into specific tissue cells is now considered a crucial strategy to improve the regenerative potential of scaffolding matrices used for tissue engineering [1–7]. The stem cell niche, which is a pool of microenvironmental cues that control the fate of stem cells, is highly specified depending on the tissue types in terms of physical, chemical and mechanical characteristics [8]. Therefore, providing a physiological situation that mimics the native tissue environment may switch on the action of stem cells to enter into an appropriate stage and undergo tissue differentiation [9–13].
Mesenchymal stem cells (MSCs) are known to have multipotency to develop into a series of cell lineages, including osteoblast, adipocyte, chondrocyte and neural cells, in response to appropriate chemical and physical cues [12–15]. When compared with embryonic stem cells, MSCs are accessible without the possible concerns regarding ethical issues and can be readily obtained from adults, allowing their clinical application [4,6]. Recent studies have demonstrated the potential usefulness of MSCs for the treatment of defective and diseased tissues, including bone, cartilage and nerves [6]. Above all, the importance of microenvironmental control has been highlighted to enable complete use of MSCs in tissue engineering and regenerative therapy [16,17].
Pioneering studies of stem cells and their possible differentiation into nerve cells have recently spurred the regenerative approach of neural tissues [18]. MSCs have also been studied to a great level in attempts to identify biochemical conditions that induce their differentiation into neural lineages [19–22]. MSCs derived from bone marrow may provide an alternative source of neurons [4,6,23]. Indeed, in addition to their differentiation into osteoblasts, chondrocytes, adipocytes and haematopoiesis-supporting stromal cells, recent in vitro studies have demonstrated that both human and rodent MSCs derived from bone marrow have the ability to differentiate into neuron-like cells [20,21,24]. However, few of these studies have included functional assays, and the mechanisms involved in MSC neuronal differentiation are still poorly understood [4,10].
Recent studies have shown the importance of substrate conditions upon which MSCs behave differently in terms of adherence, migration and differentiation [12,18,25]. Indeed, there is accumulating evidence that extracellular matrix (ECM) is involved in the development of neurons by regulating neuronal migration and outgrowth [18,26,27]. Furthermore, stem cell differentiation can be directed only by physical interactions such as the mechanical stiffness of the cell surrounding matrices [2,11,12,27].
Collagen gel has been a model for culturing cells in three-dimensional environments, which is a condition much more similar to native tissue ECM than two-dimensional culture dishes [28–30]. Specifically, the collagen fibrous network and surrounding medium fluid constitute a soft and flexible gel matrix that allows cells to freely reach out and migrate in three dimensions [1,16,31–33]. Although many groups have used collagen gel matrix to investigate the behaviour and differentiation of MSCs into specific cell lineages, such as chondrocytes, osteoblasts and endothelial cells [2,29,34], there have been no reports on the development of neuronal cells.
Therefore, the role of collagen gel three-dimensional matrix in the development of MSCs derived from rat bone marrow into neuronal cells was evaluated in this study in terms of neuronal outgrowth and phenotypic expressions. Specifically, we introduced cell-adhesive proteins such as fibronectin (FN) and laminin (LN) into the collagen matrix, which are known to be crucial adhesion ligands in neuronal cells. Moreover, a key chemical factor, nerve growth factor (NGF), was not added to the culture medium to better investigate the effects of the matrix. The collagen-adhesive protein complexes are considered to provide specialized three-dimensional substrate conditions which regulate and activate signalling pathways that are similar to or at least compensate for those stimulated by growth factors and cytokines through the ECM–integrin interactions.
2. Material and methods
2.1. Isolation, culture and identification of rat bone marrow mesenchymal stem cells
MSCs were isolated from the bone marrow of five-week-old male Sprague–Dawley rats. Rats were sacrificed and the bone marrow was aspirated from the tibiae and femurs into Hank's buffered salt solution (Gibco) containing 0.1 per cent collagenase type I and 0.2 per cent dispase II, and the mononuclear cells were then obtained using the enzyme solution in conjunction with centrifugation at 1500 r.p.m. Next, the cells were plated at a density of 2 × 103 cells cm−2 in two parallel culture dishes (one for proteomic analysis and the other for subculturing) and cultured in α-minimal essential medium (α-MEM; Gibco) supplemented with 10 per cent foetal bovine serum (FBS; Hyclone, Thermo), 2 mM l-glutamine, 100 U ml−1 penicillin and 100 µl ml−1 streptomycin (all from Sigma) at 37°C under a humidified atmosphere containing 5 per cent CO2/95 per cent air for 7 days. Cells subcultured for three passages were used for the experiment. In addition, some MSC subcultures were collected as cell samples for the identification of stem cells by immunocytochemistry with Oct4 and SSEA-1, which are adult MSC markers [34,35]. To accomplish this, cultured cells were fixed and co-immunostained with anti-polyclonal Oct4 antibody (Cell Signaling Tech Inc., no. 2750) and anti-mouse SSEA-1 antibody (Chemicon International, MAB4301) using a standard immunostaining protocol. The cells positively stained for Oct4 or SSEA or Oct4–SSEA were counted from fluorescence images. Ten different fields of image were considered and averaged.
2.2. Preparation of collagen solutions with adhesive proteins
The tails of the sacrificed Sprague–Dawley rats (more than seven weeks old) were removed, skinned and placed in distilled water. A dissecting probe was then used to pull individual tendon fibres through the surrounding fascia and out of the tail. Next, the isolated collagen tendons were dried, sterilized with 0.2 mg ml−1 low molecular weight chitosan overnight in 70 per cent ethanol, and then washed with double-distilled water, after which the collagen fibre was placed in acetic acid (0.01%), diluted in pure water and incubated at 4°C for 72 h for dissolution. This viscous mixture (2 mg ml−1) was then centrifuged at 15 000 r.p.m. for 30 min, after which the supernatant was stored at 4°C as the collagen solution.
Two representative adhesive proteins, LN and FN, were added to the collagen solution. LN was obtained from Invitrogen (Carlsbad, CA) while FN was produced in our laboratory. Specifically, FN is a recombinant protein that contains key cell-binding domains from the ninth to the tenth type III domains (FNIII9–10). The FN used in this study was produced as previously described [36]. Briefly, the following polymerase chain reaction (PCR) primers were designed to recognize the ninth to the tenth type III domains of FN: FN9F 5′-GCTGGTACCGATACCATCATCCCAGCTG-3′, FN10R 5′-CCAAGCTTATGGTTTGTCAATTTC-3′. The PCR products were then cloned into pBAD/His A in-frame with the C-terminal 6× His tag. The FN fragment containing the poly-His tag was expressed and purified using a Ni2+ affinity column under denaturing conditions. The recombinant FN has previously been shown to maintain a biological activity similar to that of human plasma FN in terms of cell adhesion and proliferation. The quantity of adhesive proteins added into the collagen gel was 10 ng ml−1 for FNIII9–10 and 100 ng ml−1 for LN.
2.3. Reconstitution of mesenchymal stem cells within collagen-based gel matrix for three-dimensional cell culture
For the three-dimensional collagen gel-based culture groups (collagen only, collagen with LN and collagen with FN), 2 ml of the collagen-based solutions was poured into Petri dishes (60 mm; Falcon, USA). These solutions were then mixed with 0.4 ml FBS and 0.8 ml 3× concentrated α-MEM, after which they were mixed with 0.8 ml of the MSC suspension in α-MEM (1–2 × 106 ml−1). After gentle agitation, the solutions were allowed to form gels over 10 min during incubation at 37°C under 5 per cent CO2/95 per cent air. The cell–gel constructs were cultured in the medium consisting of α-MEM supplemented with 10 per cent FBS and 10 per cent antibiotic–antimycotic solution (Gibco). Moreover, two-dimensional culture was prepared for comparison by culturing MSCs directly on top of the collagen gel-coated culture dish.
After culturing, the collagen-based gels were subjected to enzymatic treatment with 2 mg ml−1 collagenase (type I) solution followed by centrifugation. The cell pellets were then collected and the cell numbers proliferated for 3 and 6 days were counted using a haemocytometer after trypan blue staining. The cell viability level was also measured by an MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulphophenyl)-2H-tetrazolium) assay and compared with that measured on tissue culture plastic.
2.4. Mechanical characterization of collagen-based gels
The mechanical stiffness of the viscoelastic collagen gel matrices was measured using a dynamic mechanical analyser (DMA25, Metravib, France). For the confined compression measurement, a cup–plate testing geometry was used in which the diameter of the cup container and the compressive plate were 18 and 15 mm, respectively. The gap between the cup and the plate edge allows the interstitial fluid of collagen gels to flow out. The collagen solution used for the three-dimensional cell culture condition was placed in the cylindrical cup (18 mm diameter × 15 mm height) and then allowed to form a gel, after which a dynamic compression load was applied to the gel at a frequency of 1 Hz while increasing the force from 0.001 to 0.01 N. The storage modulus of the samples was recorded and presented in table 1.
Table 1.
Young's modulus of the collagen-based gel matrices as measured by means of dynamic mechanical analysis.
| col | col–FN | col–LN | |
|---|---|---|---|
| Young's modulus (kPa) | 1.5 (0.15) | 1.5 (0.17) | 1.7 (0.12) |
2.5. Immunocytochemistry with NeuN and glial fibrillary acidic protein
Cell differentiation was revealed by co-immunostaining with the specific neuronal markers, rabbit polyclonal anti-glial fibrillary acidic protein (GFAP) (Dako, Denmark 1 : 500) and anti-NeuN (Chemicon, Temecula, CA, 1 : 500). After 3 and 6 days of culture, the cells were harvested from the three-dimensional collagen-based gels or two-dimensional culture dish, fixed with 4 per cent paraformaldehyde, and made permeable with 0.2 per cent Triton X-100. The samples were then blocked with 10 per cent goat serum, after which they were probed with primary antibodies, washed with phosphate-buffered saline (PBS) three times, and incubated with secondary antibodies conjugated with Cy3 for anti-mouse primary antibody and with fluorescein isothiocyanate (FITC) for anti-rabbit primary antibody. The nuclei were stained with 6-diamidino-2-phenylindole (DAPI). The images were captured using a ZEISS M700 confocal laser scanning microscope (CLSM) and the percentage of cells positively stained for GFAP or NeuN was calculated. Ten different fields of image were considered and averaged.
2.6. Western blots with glial fibrillary acidic protein and nestin-1
Development into neuronal cells was confirmed by western blot analysis. Briefly, the extracts of the harvested cells were prepared with RIPA buffer, after which protein samples were resolved on 10 per cent sodium dodecyl sulphate–polyacrylamide gels, transblotted to nitrocellulose (NC) membranes blocked with 2.5 per cent bovine serum albumin (BSA) in Tris-buffered saline with 0.1 per cent Tween-20 and probed with primary antibodies (anti-rabbit GFAP antibody and anti-mouse nestin-1 antibody; Chemicon, 1:10 000). The blots were then incubated with horseradish peroxidase-conjugated secondary IgG and immunoreactive bands were detected using ECL detection reagent (Pierce, Rockford, IL). The band intensities were also quantified by means of an intensitometer (Quantity I, BD Science) after normalizing to the band intensity of β-actin. The western blot analysis was performed five times, and the intensities measured were presented as the mean ± s.d.
2.7. Immunocytochemical study with focal adhesion kinase and F-actin
After culturing, the three-dimensional collagen-based gel matrices were fixed with 4 per cent paraformaldehyde (PFA), mounted on a coated slide and then air-dried at room temperature. Next, the samples were subjected to a regular immunostaining procedure with anti-rabbit focal adhesion kinase (FAK) antibody (A-17, Santa Cruz, 1 : 1000) and anti-rabbit secondary conjugated FITC with phalloidin staining for F-actin. A Zeiss M700 CLSM was used for image processing, and the positively stained cells were counted.
2.8. Fluorescence-activated cell sorting analysis
Cells were disaggregated with 2 mg ml−1 collagenase, washed twice with PBS and then resuspended to 1 × 106 cells ml−1. The harvest cells were fixed with 4 per cent PFA for 15 min, after which they were treated with 0.2 per cent Triton X-100. Blocking was conducted using 5 per cent BSA and the cells were probed with primary antibodies against GFAP and NeuN for 1–2 h at 4°C. The cells were then incubated with secondary antibodies conjugated to green for 45 min, after which they were washed with PBS. Data were acquired for at least 1000 cells using the BD FACS calibur flow cytometry system (BD Biosciences, Franklin Lakes, NJ) and then analysed using the Cell Quest-Pro software (BD Biosciences).
2.9. Transfection of mesenchymal stem cells with micro-RNA focal adhesion kinase
Knockdown of FAK in MSCs was conducted using micro-ribonucleic acid (miRNA). Cultured MSCs (1 × 105 cells ml−1) were transfected with FAK-specific miRNA plasmid (0.1 µg µl−1) or scramble plasmid as a control using 100 µl of Lonza transfection reagent according to the manufacturer's instructions. FAK–miRNA and scramble miRNA as a control plasmid were kindly provided by Wen Cheng Xiong at the Medical College of Georgia. The transfected MSCs were cultured within the three-dimensional collagen-based gels (collagen, collagen–LN, collagen–FN) or on a two-dimensional culture dish. After 3 days of culture, cells were fixed with 4 per cent PFA and analysed for changes in the neurite outgrowth pattern using a CLSM. Cells with neurite outgrowth were calculated from five different fields of images and the percentage changes during the knockdown study were compared.
2.10. Real-time RT–PCR for integrin β1 and sodium channel gene expression
First-strand cDNA was reverse-transcribed in a final volume of 10 µl using 1 µl of purified DNA-free total RNA, 1 mM random hexamer (Roche, Basel, Switzerland), 40 U of SuperScript II reverse transcriptase (Life Technologies, Gaithersburg, MD), and 40 U of RNase Inhibitor (Roche). The buffer consisted of 50 mM Tris–HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM DTT and 5 mM dNTPs. The reaction was allowed to proceed at 37°C for 90 min and then at 42°C for 30 min, after which it was terminated by heating to 65°C for 10 min. A similar reaction mixture without the reverse transcriptase enzyme was prepared and used as a template to demonstrate the absence of contaminating genomic DNA. Real-time PCR was conducted using a Sensimixplus SYBR kit (Quantace QT605-550). The 10 µl PCR included 50 ng RT product, 2× Sensimixplus and 1 µl of primers. The reactions were incubated in a 96-well optical plate at 95°C for 10 min, after which they were subjected to 40 cycles of 95°C for 15 s and 60°C for 10 min. The threshold cycle Ct values were determined using default threshold settings. The primer used for amplification of the sodium channel was prepared based on [37], while that used for integrin β1 was designed according to Yamamoto et al. [38]. The primer sequences are presented in table 2. The real-time PCR experiments were carried out five times and data were averaged.
Table 2.
Primer sequences used to amplify the sodium channel and integrin β1 genes by real-time RT–PCR.
| Gene | primers | |
|---|---|---|
| integrin β1 | forward | 5′-ACGTGCATGTTGTGGAGACT-3′ |
| backward | 5′-ACAGTTGTCACGGCACTCTT-3′ | |
| sodium channel | forward | 5′-TGCCCTACCCACCTCACAAC-3′ |
| backward | 5′-CCGGGCTAGTGAGCTGCTT-3′ |
2.11. Statistical analysis
Data were obtained from replicate tests and then presented as the mean ± s.d. Statistical analysis was carried out by means of Student's t-test. The significance level was considered to be p < 0.05.
3. Results
3.1. Identification of mesenchymal stem cells
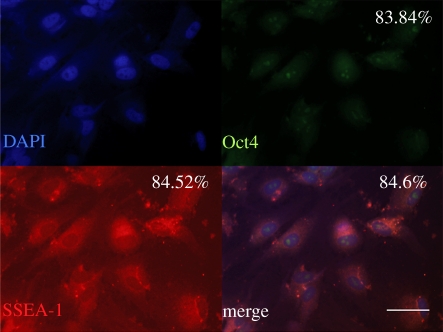
The MSCs isolated from rats and expanded in culture medium were identified by co-immunostaining with the stem cell markers, Oct4 and SSEA. As shown in figure 1, most cells are positive for both Oct4 and SSEA-1. The cells positively stained for either Oct4 or SSEA-1 or Oct4–SSEA-1 were counted to be about 84–85% (presented in the inset of images).
Figure 1.
Identification of MSCs used in this study through immunostaining. Most cells were positive for anti-Oct4 and anti-SSEA, the MSC-specific markers. Samples were co-immunostained with DAPI (nuclei), Oct4 and SSEA. Scale bar, 30 µm. The percentage of cells positively stained for Oct4 or SSEA or Oct4–SSEA is presented in the inset of each picture. Ten different fields of image were considered and averaged.
3.2. Mesenchymal stem cells growth and morphological changes
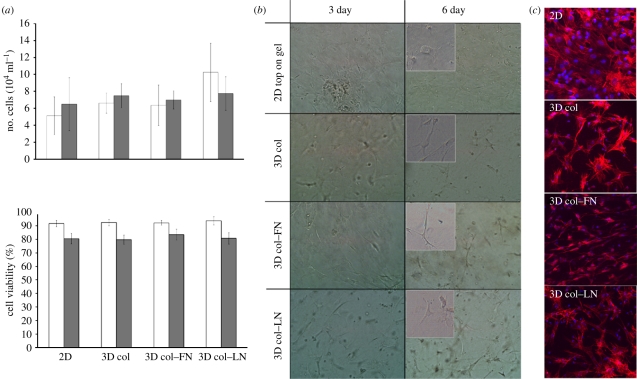
The proliferation level and viability of the cells cultured under the two- and three-dimensional conditions were shown to be similar (figure 2a). However, the morphology of cells grown under two- and three-dimensional gel conditions differed greatly (figure 2b). Under two-dimensional conditions, the cell shape was predominantly fibroblast-like throughout the culture period, while many cells appeared to show neuron-like morphology with much more elongated cytoplasmic processes under three-dimensional culture conditions. Interestingly, within the collagen gel matrix containing FNIII9–10, cells in many parts were adjacent, in direct contact and lined up in a row (enlarged in inset). In the three-dimensional collagen–LN gel, cells exhibited a highly elongated star shape.
Figure 2.
(a) Cell proliferation and viability measurement during culture under two- or three-dimensional conditions at days 3 (unfilled bars) and 6 (filled bars). The cell proliferation level was counted using trypan blue exclusion and haemocytometer counting. The cell viability on each sample was measured by an MTS assay and was presented with respect to that on tissue culture plastic. (b) Morphology of cells grown under two- and three-dimensional conditions, as revealed by optical microscopy (enlarged shown in insets at day 6). (c) Cytoskeletal arrangement of cells under CLSM. Cells were cultured either on top of a two-dimensional collagen-coated culture dish (two-dimensional conditions) or within the three-dimensional collagen-based gels (three-dimensional conditions; collagen, collagen–FN and collagen–LN). While two-dimensional cultured cells showed typical fibroblastic morphology, those grown on three-dimensional gels primarily exhibited directional elongation. Many cells in the three-dimensional collagen–FN gel were lined up in a row. Scale bars: (b,c) 100 µm.
The cytoskeletal arrangement of cells cultured under two- and three-dimensional conditions was more clearly demonstrated by F-actin staining (figure 2c). Cells cultured on two dimensions exhibited active cytoskeletal processes without any directional growth; however, the three-dimensional cultured cells appeared to present directional growth, a form of neurite outgrowth found in neuronal cells, and this growth was most conspicuous in cells cultured within the collagen–FN gel.
3.3. Neuronal specific differentiation of mesenchymal stem cells: immunocytochemical study
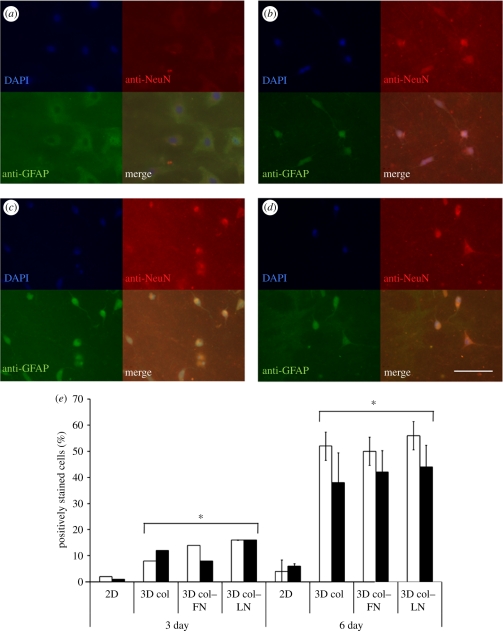
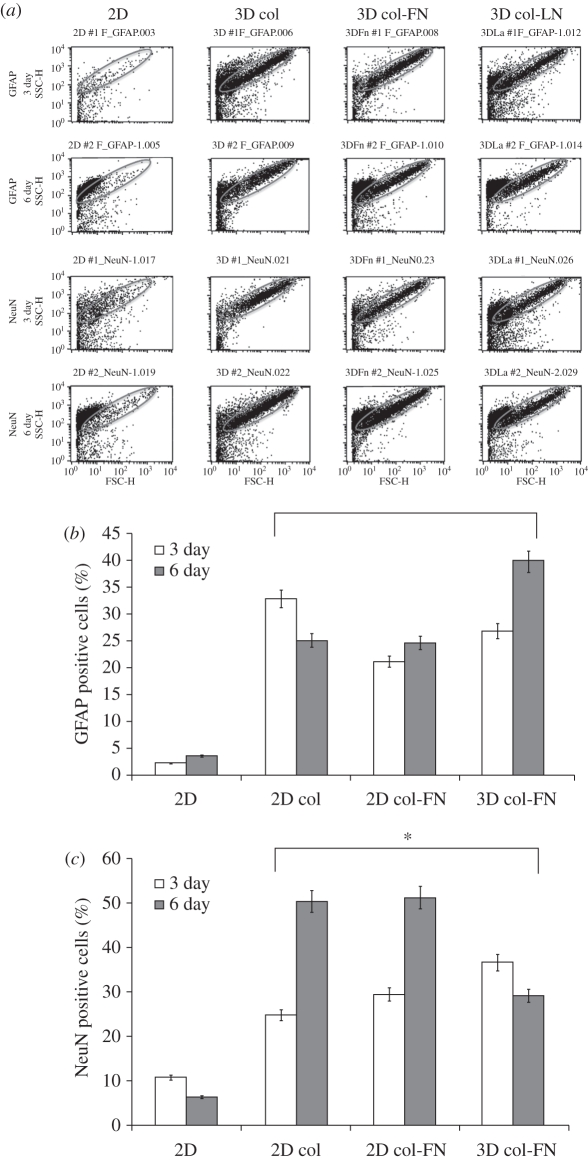
The neuronal differentiation of MSCs cultured under two- or three-dimensional conditions for 6 days was assessed by immunocytochemical staining with anti-NeuN and anti-GFAP, which are neuronal-specific markers. Co-staining showed no positive signals in the cells cultured under two-dimensional conditions (figure 3a). However, those cultured under three-dimensional gel conditions (collagen (b), collagen–FN (c) and collagen–LN (d)) were generally positive for both NeuN (nuclei stains) and GFAP (cytoplasm stains). The cells positively stained for NeuN or GFAP were counted and the percentage is presented (figure 3e). Results showed that a significantly higher percentage of cells was positive either for GFAP or for NeuN under the three-dimensional gel conditions than under the two-dimensional conditions for both culture periods (p < 0.05), and the significance was even greater at day 6.
Figure 3.
Immunostaining of cells with anti-NeuN and anti-GFAP showing the neural-specific differentiation of MSCs. Anti-NeuN and anti-GFAP stain nuclei and cytoplasm, respectively. While many cells cultured under three-dimensional conditions (collagen (b), collagen–FN (c) and collagen–LN (d)) were positive to both NeuN and GFAP, those cultured under two-dimensional conditions (a) were rarely so. Scale bar: 100 µm. The cells positively stained for GFAP (unfilled bars) or NeuN (filled bars) are shown in (e). A statistically significant difference was found between the two- and three-dimensional conditions as well as between day 3 and day 6 (*p < 0.05 by Student's t-test; 10 fields of images were considered and data are presented as the mean ± s.d.).
3.4. Western blot analysis with glial fibrillary acidic protein and nestin-1
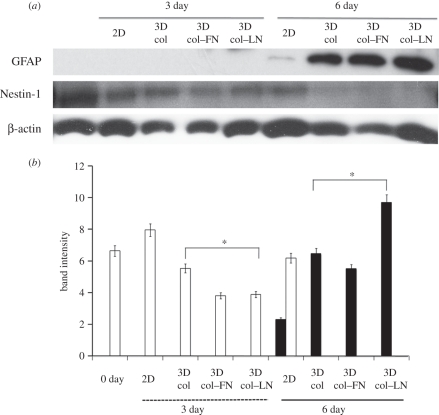
The expression of GFAP and nestin-1 in the cells cultured (for days 3 and 6) on either two- or three-dimensional gel matrices was examined at the protein level by western blot analysis (figure 4). The GFAP protein band was clear at day 6 for all three-dimensional collagen-based gel matrices (figure 4a). However, a very weak signal of GFAP was detected in the two-dimensional cultured cells at day 6. Nestin-1, which is a typical immature neuron cell marker [39], was already highly expressed at day 0, and was still present at high levels in all the cultured cells (both two- and three-dimensional) at day 3, although a slight decrease was observed. At day 6, while the protein band was still preserved in the two-dimensional cultured cells, the expression was dramatically decreased (almost disappeared) in the three-dimensional cultured cells. The band intensities were quantified by means of an intensitometer. The average value and standard deviation from five different experimental sets are presented in figure 4b. There were clear trends in the expressions between the two- and three-dimensional culture conditions, with a statistically significant difference (p < 0.05). Nestin-1 was downregulated at day 3 and disappeared at day 6 under the three-dimensional gel conditions, while GFAP was upregulated at day 6 under the three-dimensional gel conditions. Among the three-dimensional gel matrices, those trends were most significant in collagen–LN gel.
Figure 4.
Western blot analyses of the expression of GFAP and nestin-1 proteins in cells cultured on two- and three-dimensional collagen-based gel matrices for 3 and 6 days. (a) Representative bands were revealed: GFAP at 50 kDa and nestin-1 at 85 kDa. β-actin was used as a reference. (b) Quantification of the band intensities of GFAP (filled bars) and nestin-1 (unfilled bars) as measured by an intensitometer when normalized to the intensity of β-actin. Statistical significance in the expressions was noticed between two- versus three-dimensional conditions (nestin-1 downregulation at day 3 and GFAP upregulation at day 6; *p < 0.05 by Student's t-test for five different experimental sets. Nestin-1 disappeared in the three-dimensional gel samples at day 6).
3.5. Fluorescence-activated cell sorting analysis
The extent of neural differentiation of the cultured cells was assessed by fluorescence-activated cell sorting (FACS) analysis. Figure 5a shows a histogram of FACS of the two- and three-dimensional cultured cells. The results revealed that a higher population of cells expressed GFAP and NeuN under the three-dimensional culture conditions than under the two-dimensional culture conditions. The population of cells positive for GFAP and NeuN is quantified in figure 5b,c, respectively. While less than 5–10% of cells were positive for GFAP and NeuN under two-dimensional conditions, large populations of cells cultured under three-dimensional conditions expressed GFAP and NeuN. Specifically, NeuN-positive cells were increased by approximately 50 per cent after 6 days of culture in the three-dimensional collagen and collagen–FN. There was a statistically significant difference between the two- and three-dimensional culture conditions in both GFAP and NeuN (p < 0.05).
Figure 5.
FACS analysis: (a) FACS histogram of cells cultured under two- or three-dimensional conditions for 3 and 6 days. (b) GFAP- and (c) NeuN-positive cell population depending on the culture conditions. A statistically significant difference was found between the culture conditions (two- versus three-dimensional) at all the culture periods (*p < 0.05 for n = 3, by Student's t-test).
3.6. Effect of focal adhesion kinase knockdown on mesenchymal stem cell neurite outgrowth
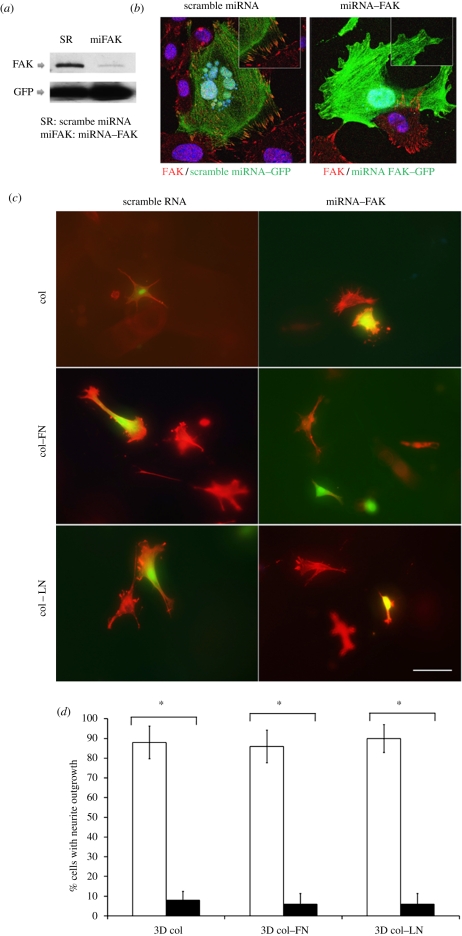
The effect of FAK on the neuronal differentiation of MSCs was investigated by an miRNA–FAK knockdown study. The experimental design of the miRNA–FAK knockdown was confirmed in TM4 cells, as shown in figure 6a,b. When the scramble miRNA (as a control) was used, good expression of the FAK signal was evident upon western blot analysis (figure 6a, left column band) and immunostaining (figure 6b, left image; based on the clear FAK red signals around the green fluorescence protein (GFP)-stained cell). However, the FAK signal was almost completely absent when miRNA–FAK-transfected cells were evaluated by western blot (figure 6a, right column band) and immunostaining (figure 6b, right image; based on no FAK red signal in the GFP-positive cell). Based on these findings, the scramble miRNA and FAK–miRNA were transfected to the MSCs, which were then cultured in three dimensions within the collagen-based gel matrices for 3 days. As shown in figure 6c,d, the FAK knockdown cells presented significantly decreased neurite outgrowth when compared with the scramble miRNA-transfected cells.
Figure 6.
(Overleaf.) Study of the effects of FAK on neurite outgrowth of MSCs cultured on a three-dimensional collagen–gel matrix. (a,b) Design of the FAK knockdown gene, FAK–miRNA, transiently transfected into TM4 cells effectively blocked the selective expression of FAK (scramble miRNA was used as a control) based on western blot (a) and immunostaining assay (b). While the representative cells exhibited FAK-positive signals when transfected with scramble miRNA (left in (b)), no FAK signal was observed in cells transfected with the FAK knockdown gene, as indicated by the absence of FAK staining around the GFP-stained cell (right in (b)). (c) FAK knockdown gene (FAK–miRNA) was transfected into MSCs that were cultured on three-dimensional collagen-based gel matrices. Observation by CLSM revealed a significant reduction in the neurite outgrowth patterns in the GFP-stained cells when treated with miRNA–FAK with respect to those treated with transcramble miRNA. Scale bars: (b,c) 100 µm. (d) Quantification of the cells with neurite outgrowth after the FAK knockdown study. Data were obtained from five different fields of images and presented as the mean ± s.d. Neurite outgrowth was significantly decreased by the FAK knockdown for all cases (*p < 0.05, by Student's t-test; from five different fields of images; unfilled bars, scramble RNA; filled bars, miRNA–FAK).
3.7. Integrin β1 and sodium channel gene expressions
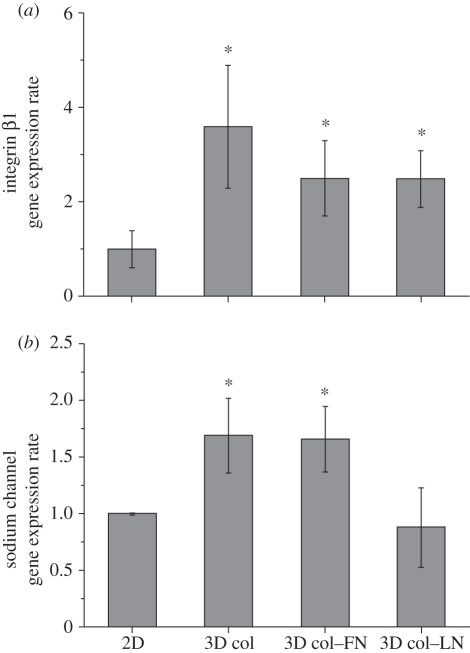
The mRNA levels of integrin β1 and the sodium channel were examined by real-time RT–PCR (figure 7). Integrin β1 is known to mediate the initial cell adhesion and internal signal transduction through FAK. Significantly higher levels of integrin β1 were expressed in the three-dimensional cultured cells than in the two-dimensional cultured cells. The sodium channel expression was also enhanced in the three-dimensional collagen and collagen–FN cultured cells.
Figure 7.
mRNA levels of (a) integrin β1 and (b) sodium channels, as determined by real-time RT–PCR. Integrin β1 is a key cell-matrix receptor that mediates the initial cell adhesion and internal signal transduction through FAK. Note the significantly higher expression of integrin β1 in the three-dimensional cultured cells than in the two-dimensional culture (a). Sodium channel expression was also greatly enhanced in the MSCs cultured within the three-dimensional collagen and collagen–FN (b). Gene levels are presented with respect to the two-dimensional control. Statistical significance was observed between samples (two- versus three-dimensional) (*p < 0.05 for n = 5 by Student's t-test).
4. Discussion
In this study, we demonstrated that collagen-based three-dimensional gel matrices are effective in inducing neuronal cell differentiation of MSCs. The principal fate of MSCs is self-renewal without amplification and/or differentiation when cultured on a normal two-dimensional culture dish without a supply of neurogenic medium, such as NGF [18], and this was also observed in the present study. However, there are probably several critical factors present in in vivo marrow microenvironments which are responsible for the maintenance of MSC properties that are missing in normal two-dimensional culture systems. Studies have revealed several microenvironmental factors that regulate MSC differentiation in terms of chemical and physical cues, including growth factors, chemokines, mechanical force and substrate physical properties [39]. Depending on the regulatory conditions, MSCs can undergo differentiation into neurons, adipose cells, osteoblasts and myocytes.
Compared with the chemical factors, the microenvironmental physical or mechanical stimuli have begun to be understood quite recently as necessary cues to regulate stem cell fate, including cell adhesion, movement, growth, differentiation and death [11,30]. Although in vivo stimuli involve a complex combination of chemical and physical cues and are highly dependent on cell type as well as the tissue of origin, the physical/mechanical conditions related to cell matrix properties are considered to be possible stem cell regulatory tools that alleviate the need for stimulation with chemical factors [40].
To gain better insight into the effects of a three-dimensional matrix, that is, to mimic the in vivo microenvironment, here the MSCs were cultured under collagen-based three-dimensional gel matrix conditions, particularly in combination with adhesive proteins, such as FN and LN. The effects of the three-dimensional gel conditions on the differentiation of MSCs into nerve cells were evaluated through observation of the cellular morphology and protein and molecular assays. As shown in figure 2, a neuron-like shape with a long directional cytoplasmic elongation was readily observed in cells cultured within the collagen gel-based three-dimensional matrices, but not in cells cultured under two-dimensional conditions. Additionally, some cells cultured within the FN–collagen gel appeared to undergo synaptic formation between adjacent cells. Furthermore, biochemical assays (immunostaining, flow cytometry and western blot analyses) of the differentiation of cells disclosed that the cells retained characteristics of neural development. Moreover, evidence of the Na channel gene expression revealed by real-time RT–PCR demonstrated that the developed cells had some functional activity of nerve cells. It is further considered that a large population of the differentiated cells grown under the three-dimensional culture conditions were astrocytes, which are GFAP-positive, as evidenced by the FACS and western blot analyses. Indeed, astrocytes, which are specialized glial cells, outnumber neurons by over fivefold in vivo, and are known to closely overlay the entire central nervous system (CNS) and exert many vital functions in the healthy CNS [41,42].
The three-dimensional microenvironmental physical conditions are likely to be the primary factors enabling the neural development of MSCs under collagen-based gel conditions that were observed in the present study. The rigidity of the collagen-based gel should enable MSCs to be switched into the downstream of neuronal development. Here we measured the modulus of the collagen-based gel matrix by dynamic mechanical analysis and found that the values for all three collagen-based gels were in the range of 1.5–1.7 kPa on average (shown in table 1). These values are just within the modulus ranges reported for native nerve tissues, such as brain and spinal cord [43–45]. Indeed, nerve tissue is one of the softest tissues in the body, being in direct contrast to the much stiffer tissues such as skeletal muscle, E ∼ 10 kPa [46]; cartilage, E ∼ 500 kPa [47]; and cortical bone, E ∼ 15–106 kPa [48]. Therefore, the three-dimensional collagen gel conditions provide an appropriate microphysical environment for the multipotent MSCs to recognize and differentiate into the neuronal lineage.
Given the evidence that changes in cell–matrix interactions altered cell shape and neuronal gene expressions, it is important to understand whether there are mechanisms to transmit mechanical signals from the cell surface or cytoplasm to the cell nucleus. In fact, microenvironmental conditions have identified complex molecular and biochemical pathways activated or modified by integrin-mediated adhesion and have provided insight into the mechanisms that regulate adhesion-dependent cellular processes such as cell spreading, proliferation, differentiation and survival [41]. The physical stimulation involved in the three-dimensional collagen gel matrix is also believed to be reflected in the intracellular signalling process though an integrin-mediated reaction. The initial cellular recognition of ECM molecules was found to be superior under the three-dimensional gel culture conditions, as highlighted by the significantly enhanced expression of integrin β1. Generally, integrin signalling involves complex actions of several adhesion molecules. Among these, FAK has been considered to be one of the most important neurite outgrowth regulation factors in neuron cells [18,49]. Our study also demonstrated that MSCs conditioned with FAK knockdown did not show neuronal outgrowth, suggesting that FAK is a key dominant protein in the intracellular signalling pathway to neuronal differentiation of MSCs within the collagen three-dimensional matrix. However, the intracellular signalling pathway of MSCs for lineage differentiation under three-dimensional culture conditions has not been clearly elucidated to date. Only a recent study under two-dimensional culture conditions has reported that the conditional knockdown of FAK disrupted the ECM-stimulated differentiation of MSCs, providing crucial evidence of the involvement of FAK in neurite outgrowth and guidance [49,50]. This finding suggested that FAK plays a role as a possible link between guidance cue receptors and cytoskeleton reorganization. Our results for the three-dimensional collagen gel conditions are in line with those of previous studies with respect to the role of FAK in the regulation of MSC differentiation, particularly into a neuronal lineage.
Based on the findings that the collagen-based three-dimensional gel culture system provides appropriate conditions for MSCs to differentiate into a neuronal lineage, future applications in nerve regeneration and neurological disease models such as spinal cord injury therapy, wound healing and neuronal degeneration therapy through MSCs may be considered. Although more studies in animal models of neurodegenerative diseases and injury are still needed to assess the function and efficacy of the collagen gel matrix system in vivo, the ability of MSCs to undergo neural differentiation in artificial sets indicates their possible differentiation in the CNS microenvironment as well as the potential usefulness of the MSC gel constructs for nerve tissue engineering.
5. Conclusions
The three-dimensional environmental cues provided by collagen-based gel matrices with an appropriate stiffness were shown to trigger neural differentiation of MSCs without the supplement of a chemical differentiation cue, NGF. Compared with the cells cultured under collagen-coated two-dimensional conditions, those cultured on three-dimensional collagen-based gels demonstrated neuron-like morphologies, larger populations positive for neuronal markers and greater expression levels of associated genes and proteins.
Acknowledgements
All protocols involving animals were conducted according to the standards of IRB in the Dankook University regulations.
This work was supported by the Priority Research Centres Programme (grant no. 2009-0093829) and WCU (World Class University) Programme (grant no. R31-10069) through the National Research Foundation (NRF) funded by the Ministry of Education, Science and Technology.
References
- 1.Clause K. C., et al. 2010. A three-dimensional gel bioreactor for assessment of cardiomyocyte induction in skeletal muscle derived stem cells. Tissue Eng. Part C Methods 16, 375–385 10.1089/ten.tec.2009.0098 (doi:10.1089/ten.tec.2009.0098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X. D. 2010. Extracellular matrix provides an optimal niche for the maintenance and propagation of mesenchymal stem cells. Birth Defect Res. C 90, 45–54 10.1002/bdrc.20171 (doi:10.1002/bdrc.20171) [DOI] [PubMed] [Google Scholar]
- 3.Kim N. R., et al. 2009. Discovery of a new and efficient small molecule for neuronal differentiation from mesenchymal stem cell. J. Med. Chem. 52, 7931–7933 10.1021/jm9015558 (doi:10.1021/jm9015558) [DOI] [PubMed] [Google Scholar]
- 4.Sadan O., Melamed E., Offen D. 2009. Bone-marrow-derived mesenchymal stem cell therapy for neurodegenerative diseases. Expert Opin. Biol. Ther. 9, 1487–1497 10.1517/14712590903321439 (doi:10.1517/14712590903321439) [DOI] [PubMed] [Google Scholar]
- 5.Tabata Y. 2009. Biomaterial technology for tissue engineering applications. J. R. Soc. Interface 6, S311–S324 10.1098/rsif.2008.0448.focus (doi:10.1098/rsif.2008.0448.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hipp J., Atala A. 2008. Sources of stem cells for regenerative medicine. Stem Cell Rev. 4, 3–11 10.1007/s12015-008-9010-8 (doi:10.1007/s12015-008-9010-8) [DOI] [PubMed] [Google Scholar]
- 7.Pittenger M. F., et al. 1999. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 10.1126/science.284.5411.143 (doi:10.1126/science.284.5411.143) [DOI] [PubMed] [Google Scholar]
- 8.Pierret C., Morrison J. A., Rath P., Zigler R. E., Engel L. A., Fairchild C. L., Shi H., Maruniak J. A., Kirk M. D. 2010. Developmental cues and persistent neurogenic potential within an in vitro neural niche. BMC Dev. Biol. 10, 5. 10.1186/1471-213X-10-5 (doi:10.1186/1471-213X-10-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Titushkin I., Sun S., Shin J., Cho M. 2010. Physicochemical control of adult stem cell differentiation: shedding light on potential molecular mechanisms. J. Biomed. Biotechnol. 2010, 743476. 10.1155/2010/743476 (doi:10.1155/2010/743476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Discher D. E., Mooney D. J., Zandstra P. W. 2009. Growth factors, matrices, and forces combine and control stem cells. Science 324, 1673–1677 10.1126/science.1171643 (doi:10.1126/science.1171643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guilak F., Cohen D. M., Estes B. T., Gimble J. M., Liedtke W., Chen C. S. 2009. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5, 17–26 10.1016/j.stem.2009.06.016 (doi:10.1016/j.stem.2009.06.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leipzig N. D., Shoichet M. S. 2009. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials 30, 6867–6878 10.1016/j.biomaterials.2009.09.002 (doi:10.1016/j.biomaterials.2009.09.002) [DOI] [PubMed] [Google Scholar]
- 13.Engler A. J., Sen S., Sweeney H. L., Discher D. E. 2006. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 10.1016/j.cell.2006.06.044 (doi:10.1016/j.cell.2006.06.044) [DOI] [PubMed] [Google Scholar]
- 14.Barnabé G. F., et al. 2009. Chemically-induced rat mesenchymal stem cells adopt molecular properties of neuronal-like cells but do not have basic neuronal functional properties. PLoS ONE 4, e5222. 10.1371/journal.pone.0005222 (doi:10.1371/journal.pone.0005222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim M. R., Jeon E. S., Kim Y. M., Lee J. S., Kim J. H. 2009. Thromboxane A2 induces differentiation of human mesenchymal stem cells to smooth muscle-like cells. Stem Cells 27, 191–199 10.1634/stemcells.2008-0363 (doi:10.1634/stemcells.2008-0363) [DOI] [PubMed] [Google Scholar]
- 16.Assoian R. K., Klein E. A. 2008. Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol. 18, 347–352 10.1016/j.tcb.2008.05.002 (doi:10.1016/j.tcb.2008.05.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zajac A. L., Discher D. E. 2008. Cell differentiation through tissue elasticity-coupled, myosin-driven remodeling. Curr. Opin. Cell Biol. 20, 609–615 10.1016/j.ceb.2008.09.006 (doi:10.1016/j.ceb.2008.09.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mruthyunjaya S., Manchanda R., Godbole R., Pujari R., Shiras A., Shastry P. 2010. Laminin-1 induces neurite outgrowth in human mesenchymal stem cells in serum/differentiation factors-free conditions through activation of FAK–MEK/ERK signaling pathways. Biochem. Biophys. Res. Commun. 391, 43–48 10.1016/j.bbrc.2009.10.158 (doi:10.1016/j.bbrc.2009.10.158) [DOI] [PubMed] [Google Scholar]
- 19.Hu B. Y., Du Z. W., Zhang S. C. 2009. Differentiation of human oligodendrocytes from pluripotent stem cells. Nat. Protocol 4, 1614–1622 10.1038/nprot.2009.186 (doi:10.1038/nprot.2009.186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi C. B., et al. 2006. Analysis of neuron-like differentiation of human bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 350, 138–146 10.1016/j.bbrc.2006.09.010 (doi:10.1016/j.bbrc.2006.09.010) [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Ramos J., et al. 2000. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp. Neurol. 164, 247–256 10.1006/exnr.2000.7389 (doi:10.1006/exnr.2000.7389) [DOI] [PubMed] [Google Scholar]
- 22.Woodbury D., Schwarz E. J., Prockop D. J., Black I. B. 2000. Adult rat and human bone marrow stromal cells differentiate into neurons. J. Neurosci. Res. 61, 364–370 (doi:10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C) [DOI] [PubMed] [Google Scholar]
- 23.Tuan R. S., Boland G., Tuli R. 2003. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res. Ther. 5, 32–45 10.1186/ar614 (doi:10.1186/ar614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox L., Shen J., Ma K., Liu Q., Shi G., Pappas G. D., Qu T., Cheng J. 2010. Membrane properties of neuron-like cells generated from adult human bone marrow-derived mesenchymal stem cells. Stem Cells Dev. 19, 1831–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNamara L. E., McMurray R. J., Biggs M. J. P., Kantawong F., Oreffo R. O. C., Dalby M. J. 2010. Nanotopographical control of stem cell differentiation. J. Tissue Eng. 2010, 120623. 10.4061/2010/120623 (doi:10.4061/2010/120623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooke M. J., Zahir T., Phillips S. R., Shah D. S., Athey D., Lakey J. H., Shoichet M. S., Przyborski S. A. 2009. Neural differentiation regulated by biomimetic surfaces presenting motifs of extracellular matrix proteins. J. Biomed. Mater. Res. 93, 824–832 10.1002/jbm.a.32585 (doi:10.1002/jbm.a.32585) [DOI] [PubMed] [Google Scholar]
- 27.Lin P. W., Wu C. C., Chen C. H., Ho H. O., Chen Y. C., Sheu M. T. 2005. Characterization of cortical neuron outgrowth in two- and three-dimensional culture systems. J. Biomed. Mater. Res. B Appl. Biomater. 75, 146–157 [DOI] [PubMed] [Google Scholar]
- 28.Mumaw J. L., Machacek D., Shields J. P., Dodla M. C., Dhara S. K., Stice S. L. 2010. Neural differentiation of human embryonic stem cells at the ultrastructural level. Microsc. Microanal. 16, 80–90 10.1017/S1431927609991279 (doi:10.1017/S1431927609991279) [DOI] [PubMed] [Google Scholar]
- 29.Parenteau-Bareil R., Gauvin R., Berthod F. 2010. Collagen-based biomaterials for tissue engineering applications. Materials 3, 1863–1887 10.3390/ma3031863 (doi:10.3390/ma3031863) [DOI] [Google Scholar]
- 30.Berrier A. L., Yamada K. M. 2007. Cell-matrix adhesion. J. Cell Physiol. 213, 565–573 10.1002/jcp.21237 (doi:10.1002/jcp.21237) [DOI] [PubMed] [Google Scholar]
- 31.Sung K. E., Su G., Pehlke C., Trier S. M., Eliceiri K. W., Keely P. J., Freidl A., Beebe D. J. 2009. Control of 3-dimensional collagen matrix polymerization for reproducible human mammary fibroblast cell culture in microfluidic devices. Biomaterials 30, 4833–4841 10.1016/j.biomaterials.2009.05.043 (doi:10.1016/j.biomaterials.2009.05.043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin P. W., Wu C. C., Chen C. H., Ho H. O., Chen Y. C., Sheu M. T. 2005. Characterization of cortical neuron outgrowth in two- and three-dimensional culture systems. J. Biomed. Mater. Res. 75, 146–157 10.1002/jbm.b.30276 (doi:10.1002/jbm.b.30276) [DOI] [PubMed] [Google Scholar]
- 33.Eslaminejad M. B., Mirzadeh H., Nickmahzar A., Mohamadi Y., Mivehchi H. 2009. Type I collagen gel in seeding medium improves murine mesenchymal stem cell loading onto the scaffold, increases their subsequent proliferation, and enhances culture mineralization. J. Biomed. Mater. Res. 90, 659–667 10.1002/jbm.b.31332 (doi:10.1002/jbm.b.31332) [DOI] [PubMed] [Google Scholar]
- 34.Tai M. H., Chang C. C., Kiupel M., Webster J. D., Olson L. K., Trosko J. E. 2005. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis 26, 495–502 10.1093/carcin/bgh321 (doi:10.1093/carcin/bgh321) [DOI] [PubMed] [Google Scholar]
- 35.Anjos-Afonso F., Bonnet D. 2007. Nonhematopoietic/endothelial SSEA-1+ cells define the most primitive progenitors in the adult murine bone marrow mesenchymal compartment. Blood 109, 1298–1306 10.1182/blood-2006-06-030551 (doi:10.1182/blood-2006-06-030551) [DOI] [PubMed] [Google Scholar]
- 36.Kim T. I., Jang J. H., Chung C. P., Ku Y. 2003. Fibronectin fragment promotes osteoblast-associated gene expression and biological activity of human osteoblast-like cell. Biotech. Lett. 25, 2007–2011 10.1023/B:BILE.0000004393.02839.d8 (doi:10.1023/B:BILE.0000004393.02839.d8) [DOI] [PubMed] [Google Scholar]
- 37.Cummins T. R., Black J. A., Dib-Hajj S. D., Waxman S. G. 2000. Glial-derived neurotrophic factor upregulates expression of functional SNS and NaN sodium channels and their currents in axotomized dorsal root ganglion neurons. J. Neurosci. 20, 8754–8761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto Z., Kanbara K., Nakajima M., Kinoshita M., Abe M. 2004. Effect of suture repair on expression of ß1 integrin subunit in wounded rat patellar tendon. J. Orthop. Sci. 9, 613–618 10.1007/s00776-004-0840-1 (doi:10.1007/s00776-004-0840-1) [DOI] [PubMed] [Google Scholar]
- 39.Horváth S., Prandovszky E., Kis Z., Krummenacher C., Eisenberg R. J., Cohen G. H., Janka Z., Toldi J. 2006. Spatiotemporal changes of the herpes simplex virus entry receptor nestin-1 in murine brain during postnatal development. J. Neurovirol. 12, 161–170 10.1080/13550280600760594 (doi:10.1080/13550280600760594) [DOI] [PubMed] [Google Scholar]
- 40.Kuo C. K., Tuan R. S. 2008. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng. Part A 14, 1615–1627 10.1089/ten.tea.2006.0415 (doi:10.1089/ten.tea.2006.0415) [DOI] [PubMed] [Google Scholar]
- 41.Streuli C. H. 2009. Integrins and cell-fate determination. J. Cell Sci. 122, 171–177 10.1242/jcs.018945 (doi:10.1242/jcs.018945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giannone G., Sheetz M. P. 2006. Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways. Trends Cell Biol. 16, 213–223 10.1016/j.tcb.2006.02.005 (doi:10.1016/j.tcb.2006.02.005) [DOI] [PubMed] [Google Scholar]
- 43.Sofroniew M. V., Vinters H. V. 2010. Astrocytes: biology and pathology. Acta Neuropathol. 119, 7–35 10.1007/s00401-009-0619-8 (doi:10.1007/s00401-009-0619-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta D., Tator C. H., Shoichet M. S. 2006. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials 27, 2370–2379 10.1016/j.biomaterials.2005.11.015 (doi:10.1016/j.biomaterials.2005.11.015) [DOI] [PubMed] [Google Scholar]
- 45.Gefen A., Margulies S. S. 2004. Are in vivo and in situ brain tissues mechanically similar? J. Biomech. 37, 1339–1352 10.1016/j.jbiomech.2003.12.032 (doi:10.1016/j.jbiomech.2003.12.032) [DOI] [PubMed] [Google Scholar]
- 46.Collinsworth A. M., Zhang S., Kraus W. E., Truskey G. A. 2002. Apparent elastic modulus and hysteresis of skeletal muscle cells throughout differentiation. Am. J. Physiol. Cell Physiol. 283, C1219–C1227 [DOI] [PubMed] [Google Scholar]
- 47.Athanasiou K. A., Rosenwasser M. P., Buckwalter J. A., Malinin T. I., Mow V. C. 1991. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. J. Orthop. Res. 9, 330–340 10.1002/jor.1100090304 (doi:10.1002/jor.1100090304) [DOI] [PubMed] [Google Scholar]
- 48.Katsamanis F., Raftopoulos D. D. 1990. Determination of mechanical properties of human femoral cortical bone by the Hopkinson bar stress technique. J. Biomech. 23, 1173–1184 10.1016/0021-9290(90)90010-Z (doi:10.1016/0021-9290(90)90010-Z) [DOI] [PubMed] [Google Scholar]
- 49.Tucker B. A., Rahimtula M., Mearow K. M. 2008. Src and FAK are key early signalling intermediates required for neurite growth in NGF-responsive adult DRG neurons. Cell Signal 20, 241–257 10.1016/j.cellsig.2007.10.014 (doi:10.1016/j.cellsig.2007.10.014) [DOI] [PubMed] [Google Scholar]
- 50.Ren X. R., et al. 2004. Focal adhesion kinase in netrin-1 signaling. Nat. Neurosci. 7, 1204–1212 10.1038/nn1330 (doi:10.1038/nn1330) [DOI] [PubMed] [Google Scholar]