Abstract
A diverging diversity-oriented synthesis (DOS) strategy using an allene-containing tryptophan as a key starting material was investigated. An allene-yne substituted derivative of tryptophan 12 gave indolylmethylazabicyclooctadiene 17 when subjected to a microwave-assisted allenic [2 + 2] cycloaddition reaction. This same tryptophan-derived precursor afforded an indolylmethyldihydrocyclopentapyridinone 14 when subjected to a rhodium(I)-catalyzed cyclocarbonylation reaction and an indolylmethylpyrrolidinocyclopentenones 16 when reacted with molybdenum hexacarbonyl. Construction of allenic tetrahydro-β-carboline scaffolds via a Pictet-Spengler reaction and subsequent silver(I)-catalyzed cycloisomerization afforded tetrahydroindolizinoindoles (21). Attachment of allene and alkyne groups to the tetrahydro-β-carboline followed by a microwave-assisted allenic [2 + 2] cycloaddition reaction provided tetrahydrocyclobutaindoloquinolizinones 24 and the tetrahydrocyclopentenone indolizinoindolone 26 when reacted with molybdenum hexacarbonyl. These six scaffolds were used as a template for the construction of a virtual library of 11,748 compounds employing 44 indoles, 12 aldehydes, and 51 alkynes. Diversity analyses using a combination of cell-based chemistry space computations using BCUT (Burden (B) CAS (C) Pearlman at the University of Texas (UT)) metrics and Tanimoto coefficient (Tc) similarity calculations using two-dimensional (2D) fingerprints showed that the compounds in the virtual library occupied new chemical space when compared to the 327,000 compounds in the molecular libraries small molecule repository (MLSMR). A subset of fifty-three compounds was identified from the virtual library using the DVS package of Sybyl 8.0; this subset represents the most diverse compounds within the chemical space defined by these compounds and will be synthesized and screened for biological activity.
Keywords: Thermal [2 + 2] cycloaddition, allene, allene-yne, cyclocarbonylation, cycloisomerization, microwave-assisted, Pictet-Spengler, rhodium(I)-catalyzed, tetrahydro-β-carboline, tryptophan, virtual library, Pauson-Khand, BCUT, Tanimoto, MLSMR, chemical space
Introduction
Chemical synthesis of complex organic molecules has recently undergone some revolutionary transformations, including the emerging area of diversity-oriented synthesis (DOS); the synthesis of a diverse set of small molecules for the identification of new ligands for a variety of biological targets.1 The DOS synthetic strategy is used to access structurally unique compounds and employs a combination of building block-, appendage-, stereochemical-, and skeletal-diversity.2 Of these, skeletal diversity is the most challenging to achieve,3 and at the same time is considered the most powerful because it provides molecularly distinct scaffolds that occupy separate regions of chemical space.4
Skeletal diversity is commonly achieved by a substrate-based approach that employs a common set of reagents to elaborate functionally and structurally unique precursors.5 A substrate-based approach can be time intensive for a variety of reasons, such as understanding the wide range of chemistries employed during substrate preparation. Alternatively, a reagent-based approach utilizes a common intermediate that when subjected to a variety of reaction conditions or reagents, affords different scaffolds.6 The challenge associated with the reagent-based approach, especially when operating under transition metal catalysis conditions, are that small structural changes in a precursor can render an otherwise high yielding reaction, unsuccessful.
In 2004, one of the authors reported a reagent-based approach to skeletal diversity whereby an allene-yne was subjected to three different transition metal catalysts to afford three unique scaffolds.7 This overall strategy was likened to nature’s construction of the carbon skeletons of secondary metabolites from unsaturated substrates such as farnesyl pyrophosphate. The power of this reagent-based approach as a DOS strategy has subsequently been demonstrated by the preparation of multiple libraries of compounds possessing scaffolds and compounds with interesting biological activity.8
The work described herein is an extension of this diversification strategy with a focus on the cyclocarbonylation and carbocyclization reactions of allene and/or alkyne groups attached to an indole nucleus.9 The indole nucleus was selected as an ideal starting point due to its presence in many naturally occurring, bioactive molecules.10 Furthermore, the cyclocarbonylation and carbocyclization reactions described within offer a novel approach to the preparation of terpene indole alkaloids.11 Underscoring the importance of this class of compounds is the recent discovery of a β-carboline-containing compound from a library of 12,000 natural products and synthetic compounds, possessing nanomolar activity against plasmodium falciparum.12
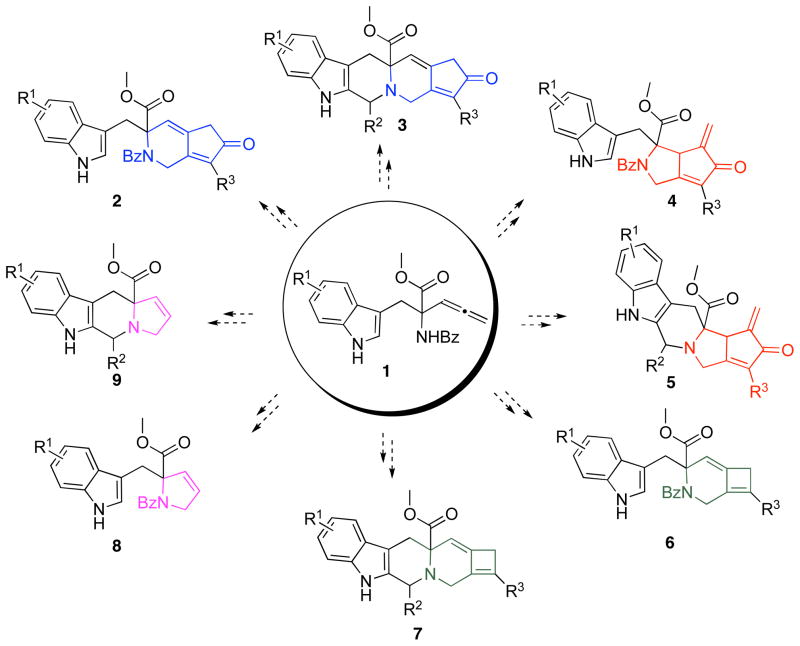
The diversification protocol delineated within (Scheme 1), involves functionalization of the conformationally mobile indolylaminopentadienoate 1 with an alkyne followed by: 1) a Rh(I)-catalyzed cyclocarbonylation reaction,13 2) a thermal [2 + 2] cycloaddition reaction,14 or 3) a molybdenum mediated Pauson-Khand-type reaction15 4) to give 2, 4 and 6, respectively. The Ag(I)-catalyzed cycloisomerization reaction of 1 to give 8 was not performed since a library of structurally similar compounds has already been prepared in our laboratories.16 Alternatively, a conformationally locked tetrahydro-β-carboline can be generated by subjecting a deprotected 1 to a Pictet-Spengler reaction. This allene-containing tetrahydro-β-carboline can then be subjected to a Ag(I)-catalyzed cycloisomerization reaction to give 9.17,18 Alternatively, functionalization of the allene-containing tetrahydro-β-carboline with an alkyne and reaction with molybdenum hexacarbonyl gives the Pauson-Khand product 5; reaction of the same allene-yne to the thermal [2 + 2] cycloaddition reaction gives 7. Attempts to effect the Rh(I)-catalyzed cyclocarbonylation reaction to give 3 under the standard conditions were not successful. The details for accessing these skeletally unique compounds are delineated within.
Scheme 1.
Diverging DOS Strategy for an Allene-containing Tryptophan
Results and Discussion
Methodology Development
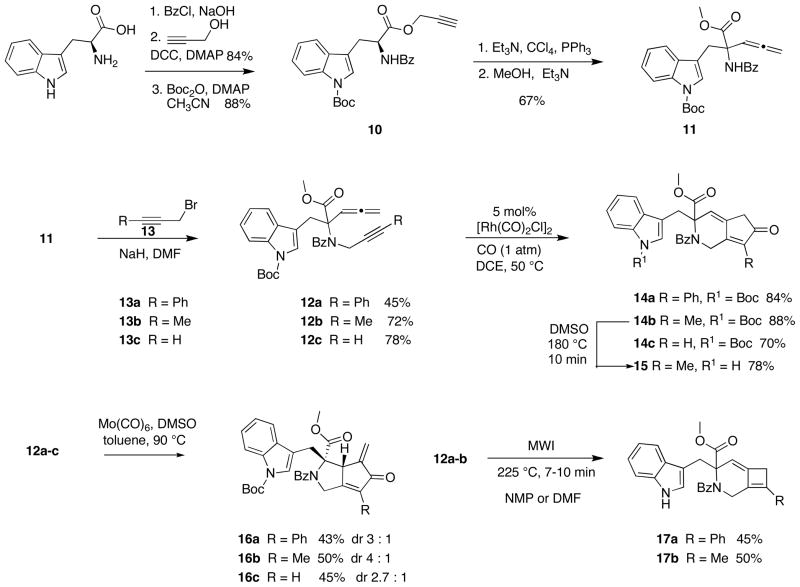
To obtain the requisite allene-yne for the indolylmethyl-containing scaffolds 2, 3 and 4, tryptophan was benzoyl protected and then converted to corresponding propargyl ester using dicyclohexylcarbodiimide and N,N-dimethylaminopyridine in 84% yield over two steps. The Boc group was then appended to the indolyl nitrogen to afford 10 in 88% yield.19 Propargyl ester 10 was subjected to triphenylphosphine, carbon tetrachloride, and triethylamine to effect a dehydrative Claisen rearrangement followed by treatment with methanol and triethylamine provided allenic amino-ester 11 in 67% yield.20 Allene-ynes 12a–c were then obtained via reaction of 11 with propargyl bromides 13a–c in the presence of sodium hydride in DMF in 45–78% yield (Scheme 2).
Scheme 2.
Synthesis, Cyclocarbonylation and Carbocyclization Reactions of Allene-ynes 12a–c
Next, the allene-ynes were subjected to the cyclocarbonylation reaction conditions. Reaction of allene-ynes 12a–c with 5 mol% rhodium biscarbonylchloride dimer [Rh(CO)2Cl]2 under one atmosphere of carbon monoxide at 50 °C in DCE for 1–2 h afforded 84%, 88% and 70% yields of 14a, 14b and 14c, respectively (Scheme 2). Attempts to effect the cyclocarbonylation in the absence of the Boc protecting group afforded only products resulting from decomposition and no recovered starting material. However, it was subsequently shown that the Boc group could be removed from 14b by heating in DMSO at 180 °C for 10 min to give 15 in 78% yield.
The molybdenum-mediated cyclocarbonylation of 12c was previously demonstrated in our group;21 the yield and diastereoselectivity for the formation of 16c are shown in Scheme 2. To determine whether the groups on the terminus of the alkyne affected the yield and/or the diastereoselectivity of the cyclocarbonylation reaction, substrates 12a–b were reacted molybdenum hexacarbonyl and DMSO in toluene to afford α-alkylidene cyclopentenones 16a–b (Scheme 2). Unfortunately, the cyclocarbonylation of the aryl and alkyl substituted alkynes (12a–b) afforded the major diastereomers of 16a and 16b in about the same yield as terminal alkyne 43–50% yield. There was only a marginal increase in the diastereoslectivity observed for the reaction of 12b. The diastereomeric ratios were determined by the 1H NMR of the crude sample, and the major diastereomer was separated via column chromatography. The stereochemical assignments were made by analogy to compounds reported previously.
Next, we were interested in examining the thermal [2 + 2] cycloaddition of the tryptophan-substituted allene-ynes. Heating allene-yne 12a and 12b at 225 °C under microwave irradiation for 7–10 min afforded bicyclo[4.2.0]octa-1,6-dienes 17a {1, 0, 29} and 17b{1, 0, 37} in 45% and 50% yields respectively; with the Boc-group removed during these high reaction temperatures (Scheme 2). Structurally similar terminal alkynes have previously been shown to decompose under these reaction conditions, thus substrate 12c was not subjected to the thermal cycloaddiiton.
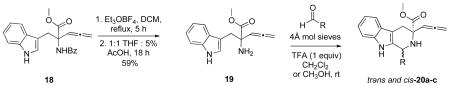
Conformationally constrained compounds have a number of advantages over flexible ones, including the orientation of functional groups in very specific ways that can allow for more rapid determination of the pharmacophore conformation. Thus strategies to inhibit the conformational mobility of allene-ynes 12 were considered. Emerging as an interesting option was an allene containing tetrahydro-β-carboline via a Pictet-Spenger reaction. Interestingly, no examples of a Pictet-Spengler reaction performed in the presence of an allene could be identified in the literature, probably due to the strong acids and elevated reaction temperatures typically required for the cyclization.22 We reasoned that hydrolysis of the allene of 19 during the Pictet-Spengler reaction could be avoided if the reaction was performed at ambient temperature and in the presence of an organic acid and molecular sieves.
Preparation of the Pictet-Spengler precursor 19 was accomplished from benzamide protected tryptophan via the same strategy as that described for the synthesis of allene 11 (see supporting information). Removal of the benzoyl-protecting group of the α-allenic amino-ester 18 with Meerwein’s reagent to provide amine 19 in 59% yield (Table 1).20,23 With amine 19 in hand, we investigated the cyclization reaction via Pictet-Spengler reaction conditions. Amine 19 was subjected to aqueous formaldehyde and trifluoroacetic acid in the presence of 4 Å molecular sieves in methanol at room temperature to afford allene-containing tetrahydro-β-carboline 20a{1, 2, 0} in 71% yield (Table 1, entry 1). Reaction of 19 with p-fluorobenzaldehyde under similar conditions in CH2Cl2 gave 20b{1, 5, 0} in 70% yield as a 2 : 1 trans : cis isomeric mixture (Table 1, entry 2). Reacting 19 with n-butanal under the same conditions gave tetrahydro-β-carboline 20c{1, 9, 0} in 68% yield, also as a 2 : 1 trans : cis isomeric mixture (Table 1, entry 3). The trans-isomer was determined to be the major product based upon calculations of the products of the subsequent allenic cycloisomerization reaction (see supporting information). 24,25
Table 1.
Pictet-Spengler Reaction Affording Allenic Tetrahydro-β-Carbolines 20a–c
 | ||||||
|---|---|---|---|---|---|---|
| Entry | R | Product | Solvent | Reaction Time | Yield (%) | dr |
| 1 | H | 20a{1, 2, 0} | CH3OH | 5 h | 71 | N/A |
| 2 | p-C6H4F | 20b{1, 5, 0} | CH2Cl2 | 2.5 h | 70 | 2 : 1 |
| 3 | n-Pr | 20c{1, 9, 0} | CH2Cl2 | 50 min | 68 | 2 : 1 |
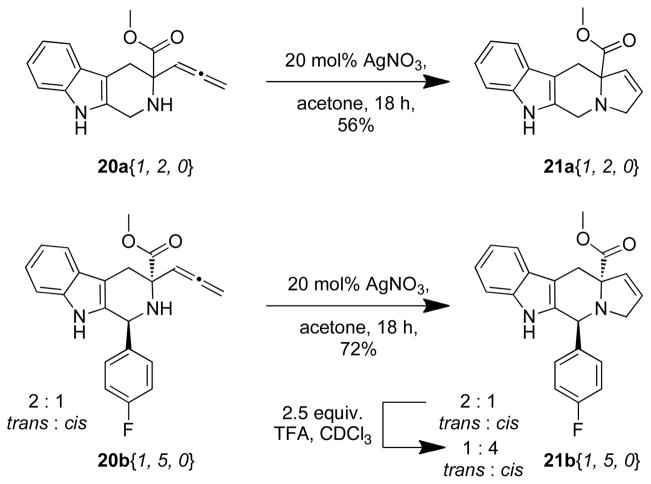
Allene-containing tetrahydro-β-carbolines 20a–b were next subjected to a Ag(I)-catalyzed cycloisomerization reaction (Scheme 3).22 Reaction of tetrahydro-β-carboline derivative 20a{1, 2, 0} with 20 mol% silver nitrate in acetone in a vial wrapped in aluminum foil for 18 h afforded 21a{1, 2, 0} in 56% yield. Treatment of a 2 : 1 trans : cis mixture of tetrahydro-β-carboline 20b{1, 5, 0} to the same conditions gave 21b{1, 5, 0} in 72% yield as a 2 : 1 trans : cis isomeric mixture (Scheme 3). Equilibration of the 2 : 1 trans : cis isomeric mixture of 21b{1, 5, 0} to a 1 : 4 trans : cis isomeric mixture was accomplished by via reaction of a solution of 21b{1, 5, 0} in CDCl3 to 2.5 equiv. of TFA for 2.5 h in quantitative yield (see supporting information). The isomers were separated via column chromatography on silica gel and characterized. Due to the lack of diastereocontrol observed in the silver catalyzed cyclization of 21b, the cyclization of 21c was not examined
Scheme 3.
Synthesis of Tetrahydroindolizinoindoles
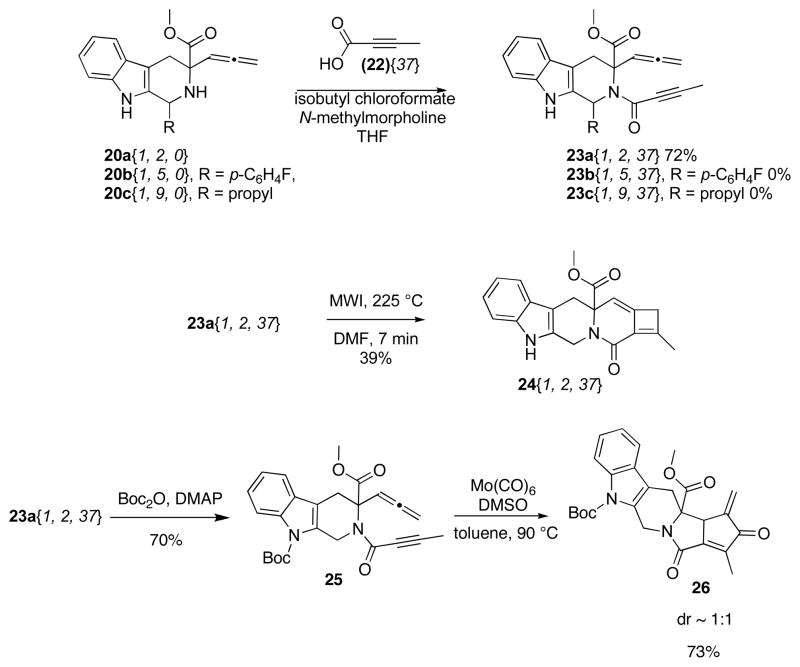
With a more conformationally constrained allene-containing β-carboline in hand, functionalization with an alkyne group was examined. Reacting tetrahydro-β-carboline 20a{1, 2, 0} with 2-butynoic acid (22) and isobutyl chloroformate at room temperature for 18 h afforded allene-yne 23a{1, 2, 37} in 72% yield after flash chromatography (Scheme 4). Reacting 20b{1, 5, 0} or 20c{1, 9, 0} under similar conditions afforded no product even after refluxing in THF; suggesting that the steric bulk surrounding the N-2 position of tetrahydro-β-carbolines impedes this condensation reaction.
Scheme 4.
Synthesis, Cyclocarbonylation and Carbocyclization of Allene-yne 23a{1, 2, 37}
With allene-yne 23a{1, 2, 37} in hand, we proceeded with the allenic [2 + 2] cycloaddition reaction. Heating a DMF solution of 23a{1, 2, 37} in the microwave at 225 °C for 7 min afforded tetrahydrocyclobutaindoloquinolizinone 24{1, 2, 37} in a 39% yield (Scheme 4). Attempts to effect a Rh(I)-catalyzed cyclocarbonylation reaction of 23a{1, 2, 37} afforded an unidentifiable brown precipitate. However, protecting the nitrogen on the indole 23a with a Boc group to give 25 enabled the reaction of propiolamide 25 in presence of molybdenum hexacarbonyl and DMSO in toluene afforded the desired α-alkylidene cyclopentenone 26 in 73% yield as a 1:1 mixture of diastereomers (Scheme 4). Interestingly, the Rh(I)-catalyzed cyclocarbonylation reaction of 25 afforded a 45% yield of a ~ 1 : 3 mixture of regioisomeric products with the major product being the α-alkylidene cyclopentenone 26, the same product that was obtained for the molybdenum mediated process. The minor product was the desired 4-alkylidene cyclopentenone, resulting from the reaction with the distal double bond of the allene. We have observed regioselective discrepancies on occasion in our lab, and it nearly always involves precursors with coordinating heteroatoms near the reacting alkyne or allene. The low yield of this reaction is attributed to the long reaction times (15 h).
The scope and limitation studies described above support the premise that the allene and allene-yne containing tryptophans provide entry into a multiple skeletally unique scaffolds. Below we have delineated a virtual library development exemplifying the uniqueness of this scaffold and its occupation of new chemical space when compared to the compounds in the NIH molecular repository.
Virtual Library Development and Diversity Analysis
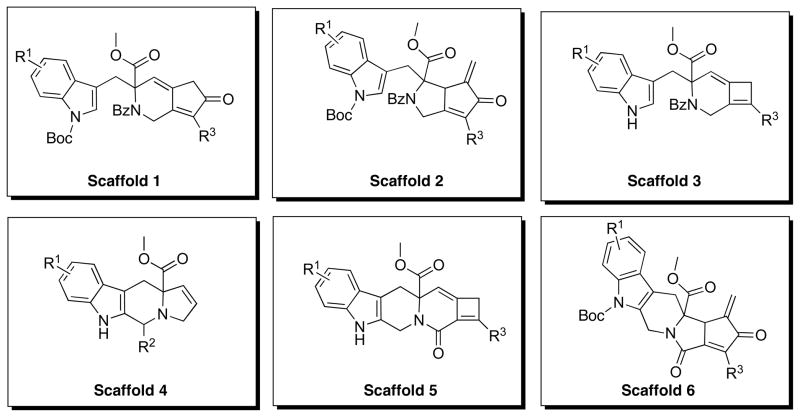
With the synthetic methodology for the synthesis of six molecularly distinct scaffolds in place, a large virtual library composed of these scaffolds was created and data–mining methods were used to identify which and how many compounds should be synthesized to provide a representative subset of the chemical space. The ultimate goal of the data-mining task was to maximize biologically relevant chemical space with the minimum number of compounds in an effort to optimize resources. The compounds in the virtual library were assembled from commercially available building blocks from Aldrich and because very few tryptophan, propiolic acid, and propargyl bromide derivatives were commercially available, indoles and alkynes were used for the virtual library design. This substitution is plausible based upon the known syntheses of tryptophan derivatives from indoles and serine,26 propiolic acids from alkynes and carbon dioxide, and propargyl bromides from alcohols derived from alkynes and formaldehyde. The library consists of Scaffolds 1–6 provided by the synthetic methodology (Scheme 5).
Scheme 5.
Library Scaffold Assignment
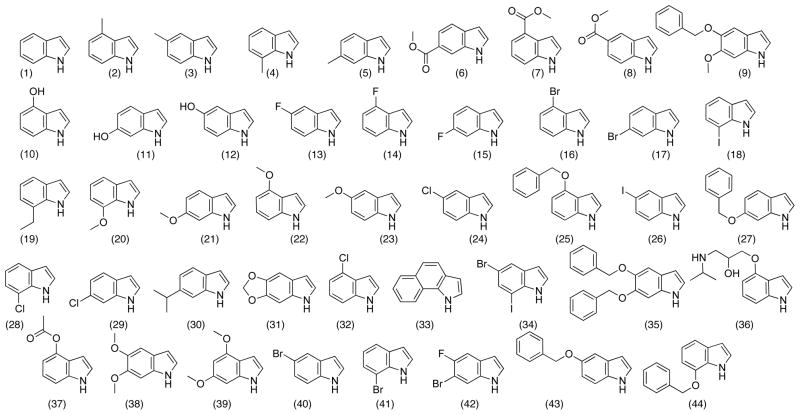
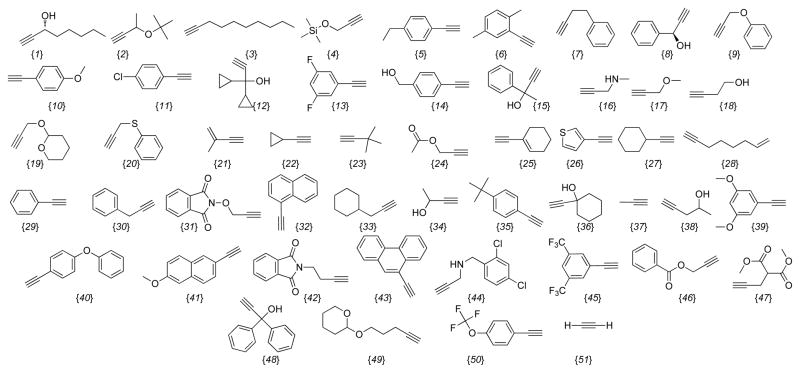
The Aldrich chemical database was mined for commercially available indoles, aldehydes and alkynes using a generic substructure search. The structures were downloaded as SD files and converted into a Microsoft Excel format and the undesired reactants were filtered out manually. For example, compounds possessing isotopes or incompatible reactive functionalities were removed from the database; although compounds containing reactive functional groups that we deemed could be easily protected were left in the list to give the best representation of potentially accessible chemical space. The aldehyde database was further refined by imposing a molecular weight constraint of < 250. The complete database consisted of 44 indoles (Chart 1A), 12 aldehydes (Chart 1B), and 51 alkynes (Chart 1C). Next, the virtual library was constructed using the Legion package of Sybyl 8.0 to generate 528 compounds of the Scaffold 4 and 2,244 compounds of each of the scaffolds 1, 2, 3, 5 and 6 for a total of 11,748 compounds.
Chart 1A.
Indole Building Blocks
Chart 1B.
Aldehyde Building Blocks
Chart 1C.
Alkyne Building Blocks
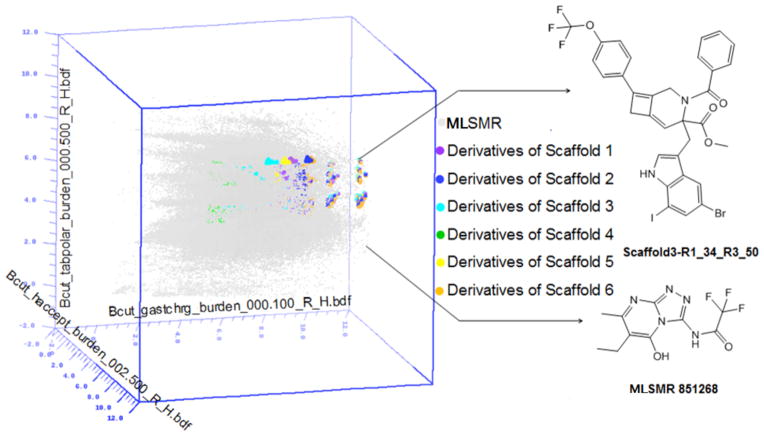
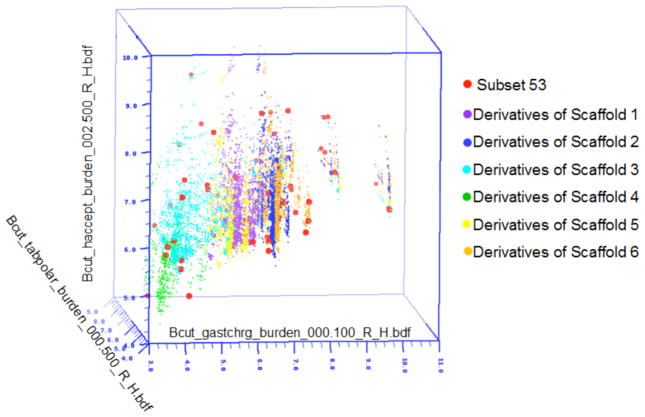
A Diversity analysis was performed using a combination of cell partition based chemistry space matrix computations using BCUT (Burden (B) CAS (C) Pearlman at the University of Texas (UT)) metrics and Tanimoto coefficient (Tc) similarity calculations using two-dimensional (2D) fingerprints.27 NIH Molecular Libraries Small Molecule Repository (MLSMR) collects samples for high throughput biological screening and distributes them to the NIH Molecular Libraries Probe Production Center Network. As our group is in this network, we applied 3D chemistry space BCUT metrics calculations to analyze the structural diversity of the virtual library and to determine whether the virtual library contributes chemical diversity value to the existing MLSMR library. The calculations showed that compounds found within this virtual library fill new chemical space or void regions when compared to the 327,000+ compounds from NIH small molecule repository (MLSMR).28 The BCUT matrix considers physical properties including atomic Gasteiger-Huckel charges, polarizabilities, H-bond donor (HBD), and H-bond acceptor (HBA) descriptors. These four descriptors correspond to electrostatic, dispersion, and H-bonding modes of the important bimolecular interactions. For the three-dimensional plot shown in Figure 1, we selected the three molecular property descriptors that gave the most diverse representation of our library of compounds when compared to comparison to the MLSMR. Each scaffold is color-coded and each dot represents a compound in the chemistry space matrix. All compounds from the scaffolds occupy the void regions except for scaffold 4 that is imbedded among the grey regions of MLSMR chemical space (Fig. 1).
Figure 1.
3D chemistry-space matrix plots of a virtual library of 11,748 compounds constructed from Scaffolds 1–6 (Scaffold 1 = purple dots, Scaffold 2 = blue dots, Scaffold 3 = cyan dots, Scaffold 4 = green dots, Scaffold 5= yellow dots, Scaffold 6 = light orange dots) and the 327,000+ compound MLSMR (gray dots) by Sybyl diversity analysis based on the BCUT metrics calculation. The new compounds clearly fill voids in chemical space when compared to the MLSMR database.
A pairwise similarity comparison was also performed for each core in order to evaluate the similarity properties of the new virtual library versus the entire MLSMR database by using a 2D fingerprint Tanimoto coefficient (Tc) calculation. In this step, the most similar compound in the MLSMR database was identified for each individual molecule in the virtual library and the corresponding Tanimoto coefficient (Tc) value of these two compounds was recorded. The compound similarity results shown in Table 2 indicated that all the compounds derived from Scaffolds 1, 3, 5 and 6 have Tc values less than 0.75 and, none of the compounds in the virtual library have Tc value larger than 0.8, when compared to the compounds in the MLSMR database; all six scaffolds have a mean Tc value well below 0.75 (0.63, 0.74, 0.64, 0.73, 0.67 and 0.67 for Scaffolds 1–6, respectively). Moreover, the largest Tc value is 0.71 for compounds derived from Scaffold 3, which means none of these compounds has Tc score more than 0.71 when compared with any of the MLSMR compounds.
Table 2.
2D Fingerprint similarity results for comparison of the derivatives of six scaffolds with the MLSMR compounds
| Scaffold | Mean Tc (stdev) | % Compounds < Tc 0.75 | Tc Max. |
|---|---|---|---|
| 1 | 0.66 (0.02) | 100 | 0.73 |
| 2 | 0.74 (0.03) | 61.5 | 0.85 |
| 3 | 0.64 (0.02) | 100 | 0.71 |
| 4 | 0.73 (0.03) | 79.7 | 0.80 |
| 5 | 0.67 (0.02) | 100 | 0.74 |
| 6 | 0.67(0.02) | 100 | 0.73 |
Because synthesis of a compound subset from this virtual library of 11,748 compounds is the final goal of these calculations, fifty-three representative compounds were extracted from the library by cell-based diverse subset selection using the DVS package of Sybyl 8.0. This subset consisted of nine compounds from Scaffold 1, seven compounds from Scaffold 2, thirteen compounds from Scaffold 3, seven compounds from Scaffold 4, five compounds from Scaffold 5 and twelve compounds from Scaffold 6. For comparison purposes, this fifty-three compound subset has been highlighted within the overall virtual library (Fig. 2).
Figure 2.
3D chemical space comparison plot of the 53 representative compounds (red dots) vs. the 11,748 compound virtual combinatorial library built from six scaffolds (color coded).
Methodology for the synthesis of six tryptophan-derived scaffolds has been developed using the Pictet-Spengler, metal-catalyzed allenic cyclocarbonylation, microwave-assisted allenic [2 + 2] cycloaddition, and Ag(I)-catalyzed allenic cycloisomerization reactions. The scaffolds were used as the basis for a virtual library possessing 11,748 compounds virtual library that occupies new regions of chemical space when compared to the MLSMR using the BCUT metrics and 2-D fingerprint analysis methods. A subset of fifty-three compounds was selected to represent chemical space within the entire virtual library with the aid of the DVS package of Sybyl 8.0, molecular weight filtering, and manual selection of synthetically feasible building blocks. This approach to library synthesis should aid in future endeavors to fill chemical space and expedite the generation of compound diversity. The synthesis of and biological evaluation of these fifty-three compounds will be reported in due course.
Supplementary Material
Acknowledgments
We would like to thank the NIGMS P50GM067082 for generous funding.
Footnotes
Supporting Information Available. Experimentals procedures and characterization data are provided for all new compounds. Computational studies supporting the stereochemical assignment of compound 21b are discussed. Compound selection charts for the virtual library of fifty-three compounds are also included in the supporting information.
References
- 1.Tan DS. Diversity-Oriented Synthesis: Exploring the Intersections Between Chemistry and Biology. Nature Chem Biol. 2005;1:74–84. doi: 10.1038/nchembio0705-74. [DOI] [PubMed] [Google Scholar]
- 2.Dandapani S, Marcaurelle LA. Current Strategies for Diversity-Oriented Synthesis. Curr Opin Chem Biol. 2010;14:362–370. doi: 10.1016/j.cbpa.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 3.A scaffold analysis of the 24 million compounds in the Chemical Abstracts (CAS) database reveals that half of the compounds can be described by a mere 143 molecular frameworks, see: Lipkus AH, Yuan Q, Lucas KA, Funk SA, Bartelt WF, III, Schenck RJ, Trippe AJ. Structural Diversity of Organic Chemistry. A Scaffold Analysis of the CAS Registry. J Org Chem. 2008;73:4443–4451. doi: 10.1021/jo8001276.
- 4.When screening libraries of compounds for biological activity, increasing the occupation of distinct chemical space increases the likelihood of finding biological activity for a wider range of biological targets, see: Sauer WHB, Schwarz MK. Molecular Shape Diversity of Combinatorial Libraries: A Prerequisite for Broad Bioactivity. J Chem Inf Comput Sci. 2003;43:987–1003. doi: 10.1021/ci025599w.
- 5.For recent work demonstrating the rapid preparation of over eighty distinct scaffolds, see: Morton D, Leach S, Cordier C, Warriner S, Nelson A. Synthesis of Natural-Product-Like Molecules with Over Eighty Distinct Scaffolds. Angew Chem Int Ed. 2009;48:104–109. doi: 10.1002/anie.200804486.
- 6.For a recent review of this build/couple/pair strategy, see: Nielsen TE, Schreiber SL. Towards the Optimal Screening Collection: A Synthesis Strategy. Angew Chem Int Ed. 2008;47:48–56. doi: 10.1002/anie.200703073.
- 7.Brummond KM, Mitasev B. Allenes and Transition Metals: A Diverging Approach to Heterocycles. Org Lett. 2004;6:2245–2248. doi: 10.1021/ol0492391. [DOI] [PubMed] [Google Scholar]
- 8.For an example of a library generated using this diverging DOS strategy, see: Werner S, Iyer PS, Fodor MD, Coleman CM, Twining LA, Mitasev B, Brummond KM. Solution-Phase Synthesis of a Tricyclic Pyrrole-2-Carboxamide Discovery Library Applying a Stetter-Paal-Knorr Reaction Sequence. J Comb Chem. 2006;8:368–380. doi: 10.1021/cc050160c.
- 9.Carbocyclization approaches using alkene and enyne metathesis to prepare functionalized heterocycles is common, but less so for other transition metal catalyzed processes. For a leading references, see: Ben-Othman R, Othman M, Coste S, Decroix B. One-pot Enyne Metathesis/Diels-Alder Reaction for the Construction of Highly Functionalized Novel Polycyclic Aza-Compounds. Tetrahedron. 2008;64:559.
- 10.For a recent review of indole-containing natural products: Kawasaki T, Higuchi K. Simple Indole Alkaloids and Those with Nonrearranged Monoterpenoid Unit. Nat Prod Rep. 2005;22:761. doi: 10.1039/b502162f.
- 11.O’Connor SE, Maresh J. Chemistry and Biology of Monoterpene Indole Alkaloid Biosynthesis. Nat Prod Rep. 2006;23:532. doi: 10.1039/b512615k. [DOI] [PubMed] [Google Scholar]
- 12.Rottmann M, McNamara C, Yeung BKS, Lee MCS, Zou B, Russell B, Seitz P, Plouffe DM, Dharia NV, Tan J, Cohen SB, Spencer KR, Gonzalez-Paez GE, Lakshminarayana SB, Goh A, Suwanarusk R, Jegla T, Schmitt EK, Beck HP, Brun R, Nosten F, Renia L, Dartois V, Keller TH, Fidock DA, Winzeler EA, Diangana TT. Spiroindolones, A Potent Compound Class for the Treatment of Malaria. Science. 2010;329:1175–1180. doi: 10.1126/science.1193225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.For leading references, see: Brummond KM, Davis M, Huang C. Rh(I)-Catalyzed Cyclocarbonylation of Allenol Esters to Prepare Acetoxy 4-Alkylidene Cyclopent-3-en-2-ones. J Org Chem. 2009;74:8314–8320. doi: 10.1021/jo901459t.
- 14.Chen D, Brummond KM. Microwave-Assisted Intramolecular [2 + 2] Allenic Cycloaddition Reaction for the Rapid Assembly of Bicyclo[420]octa-16-dienes and Bicyclo[520]nona-17-dienes. OrgLett. 2005;7:3473–3475. doi: 10.1021/ol051115g. [DOI] [PubMed] [Google Scholar]; Brummond KM, Tantillo DJ. Differentiating Mechanistic Possibilities for the Thermal Intramolecular [2 + 2] Cycloaddition of Allene-ynes. J Am Chem Soc. 2010;132:11952–11966. doi: 10.1021/ja102848z. [DOI] [PubMed] [Google Scholar]
- 15.Brummond KM, Wan H, Kent JL. An Intramolecular Allenic [2 + 2 + 1] Cycloaddition. J Org Chem. 1998;63:6535. doi: 10.1021/jo016305t. [DOI] [PubMed] [Google Scholar]
- 16.For the design and synthesis of a 3,4-dehydroproline amide discovery library, see: Werner S, Kasi D, Brummond KM. Design and Synthesis of a 3,4-Dehydroproline Amide Discovery Library. J Comb Chem. 2007;9:677–683. doi: 10.1021/cc070011p.and PubChem SID #’s 8143029–8143143
- 17.a) Dieter RK, Yu H. Synthesis of 3-Pyrrolines, Annulated 3-Pyrrolines, and Pyrroles from α-Amino Allenes. Org Lett. 2001;3:3855–3858. doi: 10.1021/ol016654+. [DOI] [PubMed] [Google Scholar]; b) Dieter RK, Chen N, Yu H, Nice LE, Gore VK. Reaction of α-(N-Carbamoyl)alkylcuprates with Propargyl Substrates: Synthetic Route to α-Amino Allenes and Δ3-Pyrrolines. J Org Chem. 2005;70:2109–2119. doi: 10.1021/jo0481405. [DOI] [PubMed] [Google Scholar]; c) Dieter RK, Chen N, Gore VK. Reaction of α-(N-Carbamoyl)alkylcuprates with Enantioenriched Propargyl Electrophiles: Synthesis of Enantioenriched 3-Pyrrolines. J Org Chem. 2006;71:8755–8760. doi: 10.1021/jo061442h. [DOI] [PubMed] [Google Scholar]; d) Mitasev B, Brummond KM. Synthesis of Functionalized Δ3-Pyrrolines via a Ag(I)-Catalyzed Cyclization of Amino Acid Derived Allenes. Synlett. 2006:3100–3104. [Google Scholar]
- 18.For a review on the silver-catalyzed allenic cycloisomerization reaction please see: Alvarez-Corral M, Munoz-Dorado M, Rodriguez-Garcia I. Chem Rev. 2008;108:3174–3198. doi: 10.1021/cr078361l.
- 19.Franzén H, Grehn L, Ragnarsson U. Synthesis, Properties and Use of Nin-Boc-Tryptophan Derivatives. J Chem Soc, Chem Comm. 1984:1699–1700. [Google Scholar]
- 20.Castelhano A, Horne S, Taylor G, Billedeu R, Krantz A. Synthesis of α-Amino Acids with β,γ-Unsaturated Side Chains. Tetrahedron. 1988;44:5451–5466. [Google Scholar]
- 21.Brummond KM, Curran DP, Mitasev B, Fischer S. Heterocyclic α-Alkylidene Cyclopentenones Obtained via a Pauson-Khand Reaction of Amino Acid Derived Allenynes. A Scope and Limitation Study Directed Toward the Preparation of a Tricyclic Pyrrole Library. J Org Chem. 2005;70:1745–1753. doi: 10.1021/jo0481607. [DOI] [PubMed] [Google Scholar]
- 22.For a review of the Pictet-Spengler reaction please see: Cox ED, Cook JM. The Pictet-Spengler Condensation: A New Direction for an Old Reaction. Chem Rev. 1995;95:1797–1842.
- 23.Brummond KM, Painter TO, Probst DA, Mitasev B. Rhodium(I)-Catalyzed Allenic Carbocyclization Reaction Affording δ- and ε-Lactams. Org Lett. 2007;9:347–349. doi: 10.1021/ol062842u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ungemach F, DiPierro M, Weber R, Cook JM. Stereospecific Synthesis of Trans-1,3-Disubstituted-1,2,3,4-Tetrahydro-β-Carbolines. J Org Chem. 1981;46:164–168. [Google Scholar]
- 25.Casnati G, Dossena A, Pochini A. Electrophilic Substitution in Indoles: Direct Attack at the 2-Position of 3-Alkylindoles. Tetrahedron Lett. 1972:5277. [Google Scholar]
- 26.For a selected example see: Blaser G, Sanderson JM, Batsanov AS, Howard JAK. The Facile Synthesis of a Series of Trytophan Derivatives. Tetrahedron Lett. 2008;49:2795–2798.
- 27.Xie X-QC, Chen JZ. Data Mining a Small Molecule Drug Screening Representative Subset from NIH PubChem. J Chem Inf and Modelings. 2008;48:465–475. doi: 10.1021/ci700193u. [DOI] [PubMed] [Google Scholar]
- 28.Of the databases that our compounds could be compared to, the NIH Molecular Repository was selected because this will be the physical location of the compounds. NIH Molecular Repository (http://mlsmr.glpg.com/MLSMR_HomePage)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.