Abstract
Long-term depression at parallel fiber-Purkinje cell synapses (PF-PC LTD) has been proposed to be required for cerebellar motor learning. To date, tests of this hypothesis have sought to interfere with receptors (mGluR1) and enzymes (PKC, PKG, or αCamKII) necessary for induction of PF-PC LTD and thereby determine if cerebellar motor learning is impaired. Here, we tested three mutant mice that target the expression of PF-PC LTD by blocking internalization of AMPA receptors. Using three different cerebellar coordination tasks (adaptation of the vestibulo-ocular reflex, eyeblink conditioning, and locomotion learning on the Erasmus Ladder), we show that there is no motor learning impairment in these mutant mice that lack PF-PC LTD. These findings demonstrate that PF-PC LTD is not essential for cerebellar motor learning.
INTRODUCTION
Persistent use-dependent changes in synaptic function, including long-term depression (LTD) and long-term potentiation (LTP), have been widely suggested to underlie learning. The theory of PF-PC LTD was originally based on models by Marr (1969), later elaborated by Albus (1971), which suggested that the cerebellar matrix consisting of the parallel fibers (PFs) and orthogonally oriented climbing fibers is optimally designed for entraining and modifying Purkinje cell (PC) output. Recordings obtained by Ito and coworkers confirmed this concept by showing that combined activation of these two inputs resulted in a persistent depression of PF-evoked excitatory postsynaptic currents (EPSCs) in PCs (Ito, 1982; Linden and Connor, 1995). Moreover, their findings indicated that induction of LTD during visuo-vestibular training could, in principle, persistently modify the gain and phase of the simple spike activity of the floccular PCs that drive the vestibulo-ocular reflex (VOR) (Nagao, 1989) (for underlying circuitry see Figure 1A). The potential correlation between LTD induction and cerebellar motor learning was subsequently supported by a series of studies in mouse mutants in which both processes were affected concomitantly (Aiba et al., 1994; Boyden et al., 2006; De Zeeuw et al., 1998; Feil et al., 2003; Hansel et al., 2006; Kim and Thompson, 1997; Koekkoek et al., 2003). For example, blockade of LTD induction by interference with the mGluR1/PKC, PKG, or αCamKII pathways all resulted in impairment of VOR adaptation (Aiba et al., 1994; De Zeeuw et al., 1998; Feil et al., 2003; Hansel et al., 2006). Still, these studies were not conclusive, because pharmacological blocking of LTD did not affect eyeblink conditioning (Welsh et al., 2005), and training without instructive signals from the climbing fibers partially allowed VOR adaptation (Ke et al., 2009). In principle the positive correlations found in the mouse mutants in which induction of LTD was affected could be attributed to the fact that the affected receptors and kinases mediate upstream signaling in a highly divergent fashion. Each kinase has many substrates, most of which are not involved in PF-PC LTD and could affect both baseline function of the cerebellar network and other forms of synaptic and nonsynaptic plasticity in the cerebellum (Chen and Tonegawa, 1997; Hansel et al., 2006; Kano et al., 1996).
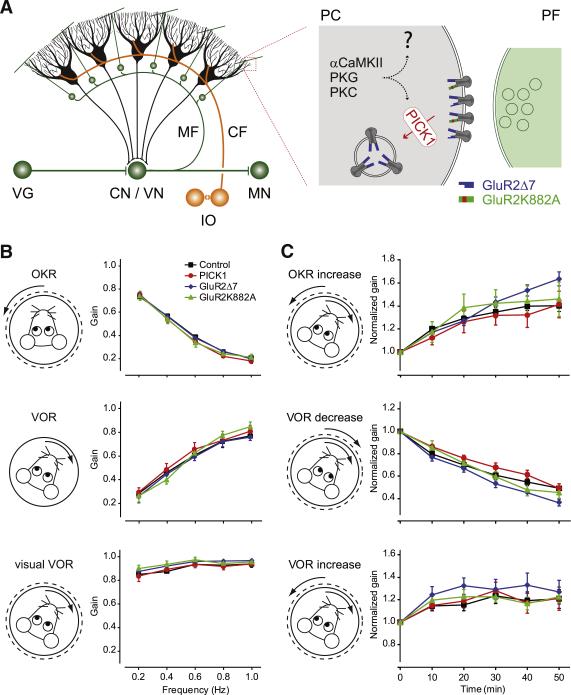
Figure 1. Cerebellar Circuitry, Basic Motor Performance, and Short-Term Motor Learning.
(A) The vestibulo-cerebellum receives excitatory input from the inferior olive (IO, orange) and vestibular ganglion cells (VG, green) and sends an inhibitory projection (black) back to the cerebellar and vestibular nuclei (CN/VN), and from there onward to motor nuclei (MN). According to the Marr-Albus-Ito hypothesis, the climbing fibers (CF) originating in the IO carry the error signal and the mossy fiber-parallel fiber system (MF-PF) relays motor activity signals. The inset shows how, after concomitant activity in CF and PF, AMPA receptors are internalized through interaction of PICK1 (red) with the C-terminal region of AMPA-type glutamate receptor subunit GluR2 (dark gray), resulting in LTD of the PF to Purkinje cell synapse (PF-PC LTD). In the mutant mice used herein, PF-PC LTD expression is blocked either by deletion of PICK1, a knockin in which GluR2 is replaced with a truncated form involving deletion of the last seven amino acids of GluR2 (GluR2Δ7; blue), or within a knockin harboring a point mutation in the PKC recognition motif of GluR2 (GluR2K882A; green). (B) Gain values of the PICK1 KO, GluR2Δ7 KI, and GluR2K882A KI mutants during the optokinetic reflex (OKR), vestibulo-ocular reflex (VOR) in the dark, and VVOR are not significantly different from those of wild-type controls (black). (C) Short-term visuo-vestibular, out-of-phase mismatch training resulted in significant (p < 0.005 for all groups) gain increases of PICK1 KO, GluR2Δ7 KI, and GluR2K882A KI mutants during the OKR (top panel) and VOR (bottom panel). Short-term visuo-vestibular, in-phase mismatch training resulted in significant (p < 0.001 for all groups) gain decreases of PICK1 KO, GluR2Δ7 KI, and GluR2K882A KI mutants during the VOR (middle panel). All these changes were not significantly different from those of wild-type controls. For clarity of presentation the wild-types are presented as a pooled group. For number of mice per group, see Table S1. The p values for individual mutants versus all controls and versus littermates are listed in Table S2. Error bars denote SEM.
Here we investigated the role of PF-PC LTD in cerebellar motor learning by testing three different mutant mice in which blockade of PF-PC LTD expression is achieved by the targeting of late events in the LTD signaling cascade, i.e., downstream at the level of the GluRs and the related proteins that control their trafficking (Steinberg et al., 2006). The mutants are the PICK1 knockout (KO) mouse, the GluR2Δ7 knockin (KI) mouse, and the GluR2K882A KI mouse (Figure 1A). The homozygous PICK1 KO mouse lacks PICK1, an essential intermediary between PKC activation and internalization of the AMPA receptor (Xia et al., 2000). The GluR2Δ7 KI mouse lacks the last seven amino acids of the C-terminal tail; this mutation eliminates the C-terminal type II PDZ ligand and disrupts the interaction of GluR2 with PICK1 and GRIP1/2 (Steinberg et al., 2006; Xia et al., 2000). Finally, and most specifically, the GluR2K882A KI mouse contains a mutated form of GluR2 that incorporates a single lysine mutation in the consensus recognition motif for PKC (S/T-X-K/R) and thereby prevents phosphorylation at S880 by PKC and internalization of the AMPA receptor while leaving the PDZ ligand and phosphorylation by other kinases functionally intact (Chung et al., 2003; Kemp and Pearson, 1990; Steinberg et al., 2006; Wang and Linden, 2000; Xia et al., 2000). Thus, all three types of mutant mice lack expression of cerebellar LTD, although their upstream induction pathways are not directly affected (Steinberg et al., 2006). All three types of mutant mice were subjected to VOR adaptation, eyeblink conditioning, and locomotion learning on the Erasmus Ladder to cover a wide range of cerebellar learning behaviors (De Zeeuw et al., 1998; Koekkoek et al., 2003; Van Der Giessen et al., 2008).
RESULTS
To determine whether the LTD-expression-deficient mutants can be used to uncover specific phenotypes relevant to vestibulo-cerebellar learning, we first ascertained whether they had gross deficits in their basic motor performance (Figure 1B). Basic eye movement tests showed that both amplitude (gain) and timing (phase) of the optokinetic reflex (OKR), VOR in the dark, and visual VOR (VVOR) in the mutants were not significantly different from those in their wild-type littermates, over a range of stimulus frequencies varying from 0.2 Hz to 1.0 Hz (p > 0.4 for all values; ANOVA for repeated measures; for n and p values, see Tables S1 and S2 available online). These data were comparable to those obtained in the mutant mice in which the induction of LTD was impaired by blockade or deletion of one of the kinases PKC, PKG, or αCamKII (De Zeeuw et al., 1998; Feil et al., 2003; Hansel et al., 2006). Subsequently, we subjected the PICK1 KO, GluRΔ7 KI, and GluR2K882A KI mice to various short-term adaptation tests, including OKR gain-up, VOR gain-down, and VOR gain-up training (Figure 1C). After being exposed for 50 min to different forms of visuo-vestibular training, all mutants showed significant adaptation for all three protocols (p < 0.005 for all protocols, paired Student's t test), and none of the mutants showed any sign of impairment compared to wild-types (p > 0.5 for all parameters, ANOVA for repeated measures; for numerical details, see Tables S1 and S2). The outcomes of these tests stand in marked contrast to those of the LTD-induction-deficient kinase mutants (Boyden et al., 2006; De Zeeuw et al., 1998; Feil et al., 2003; Hansel et al., 2006), in which clear deficits of motor learning are found.
In theory, differences among the PICK1 KO, GluRΔ7 KI, and GluR2K882A KI mutants and their wild-type littermates could become apparent when they are subjected to a longer, more robust training paradigm (see also Blazquez et al., 2004; De Zeeuw and Yeo, 2005). To address this point, we also employed a 6 day in-phase visuo-vestibular training paradigm, which results in very prominent gain and phase learning changes in wild-types (Wulff et al., 2009), but to not as great a degree in the LTD-induction-deficient kinase mutants (e.g., van Alphen and De Zeeuw, 2002). With this long-term training, both VOR gain and VOR phase values of all PICK1 KO, GluR2Δ7 KI, and GluR2K882A KI mutants are also adapted significantly (p < 0.001 for all mutants; ANOVA for repeated measures), and this form of adaptation also occurred at levels that were comparable to those of their wild-type littermates (p > 0.4 for all comparisons; ANOVA; Figure 2A).
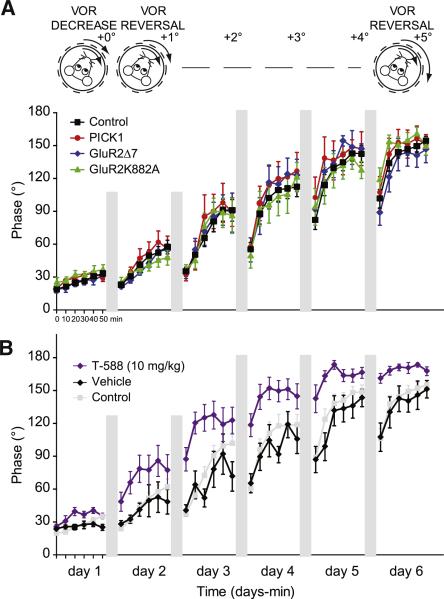
Figure 2. Long-Term VOR Phase Adaptation Is Normal.
(A) Long-term visuo-vestibular in-phase mismatch training resulted in phase reversals of PICK1 KO (red), GluR2Δ7 KI (blue), and GluR2K882A KI (green) mutants during the VOR, but these reversals were not significantly different from those of wild-type controls (black). For clarity of presentation the wild-types are presented as a pooled group. Number of mice per group is listed in Table S1, and p values, in Table S2. (B) VOR phase reversal in C57BL/6 mice injected with LTD-blocking T-588 (purple, 10 mg/kg, i.p.) and subjected to the same visuo-vestibular mismatch training paradigm was significantly faster (p < 0.003 for day 3–5) compared with that of C57BL/6 mice injected with vehicle (black). Data from wild-type control mice without injection (gray) are added for comparison. Error bars denote SEM.
One could argue that the PICK1 KO, GluR2Δ7 KI, and GluR2K882A KI mice had sufficient time to develop compensatory mechanisms that bypass the requirement for LTD. To test this, we injected C57BL/6 wild-type mice with T-588 (10 mg/kg i.p.), a cognitive enhancer, shown to block LTD both in vitro and in vivo (Kimura et al., 2005; Welsh et al., 2005). In line with the data obtained in the PICK1 KO, GluR2Δ7 KI, and GluR2K882A KI mice, the learning behavior was not impaired following injections with T-588. In fact, surprisingly, the injections resulted in a faster VOR phase reversal (p < 0.003 on days 3, 4, and 5; ANOVA for repeated measures; Figure 2B) and higher gain values on day 6 (p < 0.001; ANOVA for repeated measures; data not shown). Thus, when we blocked LTD either chemically or by genetically targeting the late events in its signaling cascade, deficits in cerebellar motor learning could not be observed following either three different types of short-term, visuo-vestibular training or an extremely strong and sensitive form of long-term, visuo-vestibular training.
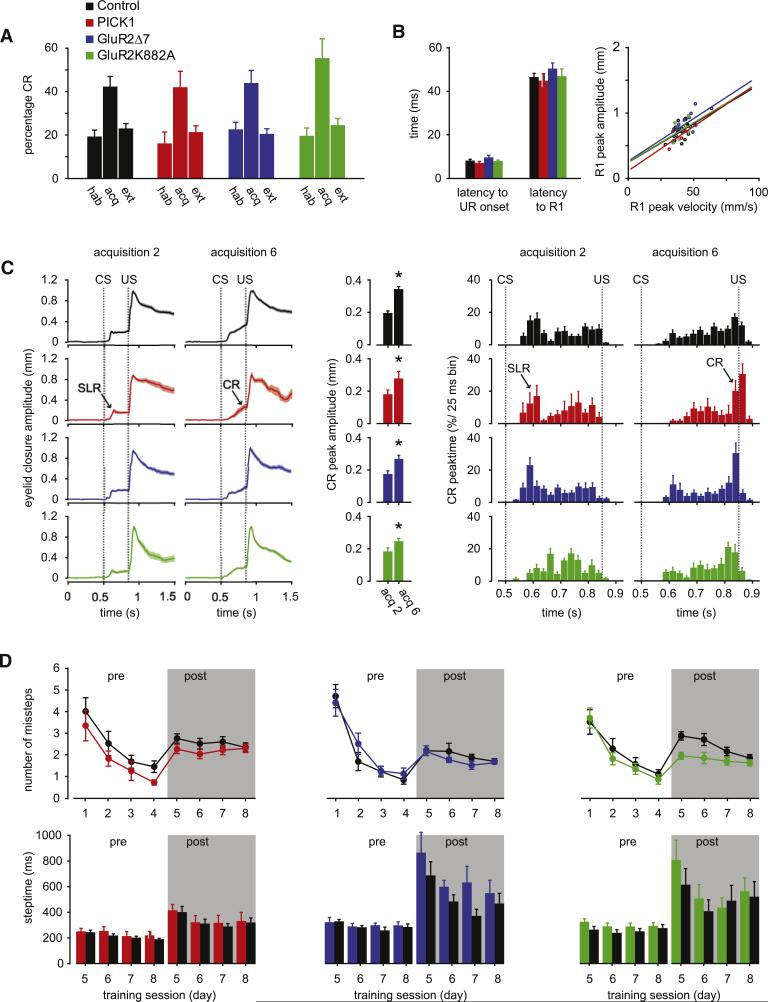
To find out whether the absence of a phenotype in the LTD-expression-deficient mutants is specific for the vestibulo-cerebellum, or whether it can be extrapolated to other parts of the cerebellum, we subjected them to eyeblink conditioning tests using a tone and an airpuff as the conditioned stimulus (CS) and unconditioned stimulus (US), respectively. Eyeblink conditioning has previously been demonstrated to require mGluR1 (Aiba et al., 1994; Kishimoto et al., 2002) and PKC (Koekkoek et al., 2003), which are both necessary for the induction of LTD. Similar to that in controls, the percentage of conditioned responses (CRs) in the PICK1 KO, GluR2Δ7 KI, and GluR2K882A KI mice increased significantly (all p < 0.05; t test, between animals p > 0.2; ANOVA for repeated measures) (Figure 3A; Tables S1 and S2). In addition, the timing and amplitude of the CRs in the PICK1 KO, GluR2Δ7 KI, and GluR2K882A KI mutants were indistinguishable from those in control mice (Figure 3C; Tables S1 and S2). Moreover, the kinetics of the unconditioned eyelid responses in all three types of mutants did not differ significantly from those of control mice, suggesting that the performances of their eyelid responses were also normal (Figure 3B). Subsequently, we subjected the LTD-expression-deficient mutants to locomotion conditioning tests on the Erasmus Ladder using a tone and a rising rung as the CS and US, respectively. Conditioning on the Erasmus Ladder has previously been demonstrated to require intact inferior olivary neurons and PCs (Van Der Giessen et al., 2008; Renier et al., 2010), the climbing fiber activity of which facilitates the induction of LTD (Albus, 1971; Marr, 1969). The PICK1 KO, GluR2Δ7 KI, and GluR2K882A KI mutants demonstrated a normal basic performance in locomotion in that their baseline steptimes and numbers of missteps were not significantly different from those of controls (Figure 3D, “pre” panels indicate pretraining; Tables S1 and S2). The introduction of a perturbation, preceded by a 15 kHz tone at a fixed time interval so as to condition their locomotion patterns, caused a significant increase in steptimes in all groups (all p < 0.01, t test; Figure 3D, “post” panels indicate posttraining). In all three types of mutants these changes were not significantly different from those in their equivalent controls (all p > 0.14, t test; Tables S1 and S2).
Figure 3. Eyeblink Conditioning and Locomotion Conditioning Are Not Impaired.
(A) Eyeblink conditioning increased the percentage of conditioned responses (CRs) significantly (all p < 0.05; habituation session versus acquisition session 6) in PICK1 KO, GluR2Δ7 KI, and GluR2K882A KI mutants, as well as their littermate controls. (B) Kinetics of unconditioned responses (URs) are unaffected in all groups. Latency to UR onset, R1 during unconditioned stimulus (US)-only trials, and R1 peak amplitude versus UR peak velocity (right) also did not differ between PICK1 KO, GluR2Δ7 KI, GluR2K882A KI, and control mice. Each dot represents the mean value of one animal. (C) Amplitude (left three panels) and timing (right two panels) of CRs are normal in all three mutant mice compared to that of control mice. Left two panels: Means of all eyeblink CR raw data traces for each group in paired trials during training sessions 2 and 6. Middle panel: Averaged CR amplitude for sessions 2 and 6. Right two panels: Mean percentage of CR peak times (25 ms bin) for each group during training session 2 and 6. At the end of the training (session 6), in all groups the majority of CR peak times are clustered around US onset at 850 ms. Short-latency responses (SLRs), which occur frequently during mouse eyeblink conditioning, are indicated. CS onset is set at 500 ms, US onset at 850 ms, interstimulus interval (ISI) = 350 ms, US duration = 30 ms. (D) Analysis of locomotion conditioning on the Erasmus Ladder revealed no significant differences in number of missteps and steptime between mutant and control mice. Both motor performance values (left panels without background) and motor learning values (right panels with gray transparent background) were not affected. During locomotion conditioning, the US, i.e. a rising rung, occurs 250 ms after the onset of the CS (a 15 kHz tone). All mutants show an acquired change in steptime after (post) the CS compared to before (pre). This change is not different from that seen in littermate controls (Table S2). For number of mice per group, see Table S1. The p values for individual mutants versus all controls and versus littermates are listed in Table S2. Error bars denote SEM.
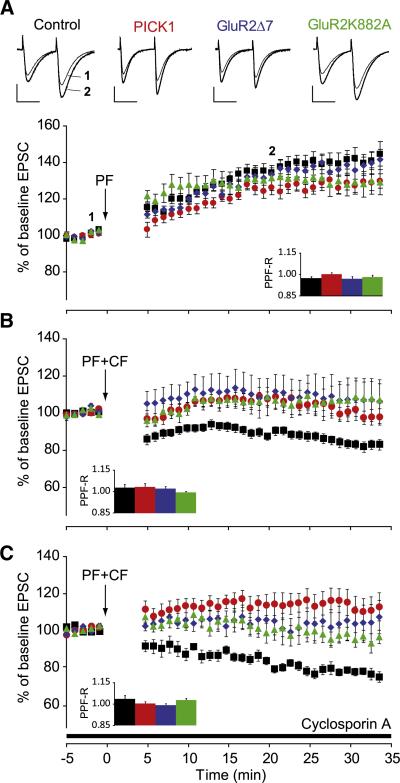
Together, these data argue against the Marr-Albus-Ito hypothesis, which predicts that PF-PC LTD is essential for cerebellar motor learning. However, certain questions must be addressed. First, do the PICK1 KO, GluR2Δ7 KI, and GluR2K882A KI mutants show compensations that may rescue the behavioral phenotype even with LTD impaired? For example, changes in PF-PC LTP induction might partially compensate for impaired LTD induction (Coesmans et al., 2004; Lev-Ram et al., 2003; Schonewille et al., 2010). To address this possibility we investigated induction of postsynaptic LTP at the PF to PC synapse in slices derived from mutant mice (Figure 4A). No significant difference in LTP (p > 0.2; ANOVA for repeated measures) was found among the PICK1 KO, GluR2Δ7 KI, and GluR2K882A KI mutants and wild-type littermates. This suggests that the blockade of PF-PC LTD induction is not compensated for by alterations in postsynaptic PF-PC LTP. Second, is LTD impaired in adult PICK1 KO, GluR2Δ7 KI, and GluR2K882A KI mutants? To date, impaired LTD has only been shown in young PICK1 KO, GluR2Δ7 KI, and GluR2K882A KI mutants (Steinberg et al., 2006), whereas all the behavioral experiments described herein have been performed in adults. We therefore assessed LTD in slices of 3- to 6-month-old PICK1 KO, GluRΔ7 KI, and GluR2K882A KI mutants. In these older mutants too, LTD was impaired compared to that of controls (p < 0.04 for all comparisons; ANOVA for repeated measures) (Figure 4B; Table S2). Even when we repeated the LTD protocol in the presence of the postsynaptic LTP blocker cyclosporin A, a calcineurin inhibitor (Belmeguenai and Hansel, 2005), LTD was not revealed (p < 0.05 for all comparisons; ANOVA for repeated measures) (Figure 4C). Third, is there presynaptic compensation? Paired pulse facilitation was not affected in the mutant mice (data not shown; all p > 0.10; one-way ANOVA), arguing against compensation for the deletion through major presynaptic changes. The ratio of paired pulse facilitation (calculated as PPF-R, ratio of before to after) did not differ significantly from 1 in any of the induction paradigms (all p > 0.05; one-sample t test), affirming the postsynaptic nature of the types of plasticity tested here. Finally, is LTD induction required during motor learning in younger animals only, meaning that adult ones might depend on other forms of plasticity? To address this concern, we performed the same VOR and OKR adaptation tests in 4- to 6-week-old mice. Similar to adults, these animals did not show any deficit in their motor learning capabilities (p > 0.7 for all comparisons) (Figure S1).
Figure 4. Postsynaptic LTP Is Normal, whereas Postsynaptic LTD Is Blocked.
(A) EPSC amplitudes were significantly (p < 0.005 for all groups) increased following LTP induction (arrow) in PICK1 KO (red), GluR2Δ7 KI (blue), and GluR2K882A KI (green) mutants, but these increases were not significantly different from those in controls (black). (B) LTD induction in slices of 3- to 6-month-old PICK1 KO, GluR2Δ7 KI, and GluR2K882A KI mutants was significantly impaired compared to that of controls. (C) Even after application of the LTP blocker cyclosporin A, LTD induction did not occur in the mutants. Insets show that the ratio of paired pulse facilitation after induction of plasticity to that before induction (PPF-R) did not change in control, PICK KO, GluR2Δ7 KI, and GluR2K882A KI mice, suggesting that plasticity occurred post-synaptically. For clarity of presentation the wild-types are presented as a pooled group. Number of mice per group is listed in Table S1, and p values, in Table S2. Scale bars: horizontal, 30 ms; vertical, 100 pA. Error bars denote SEM.
DISCUSSION
Against our expectations, we did not find any deficit in various forms of cerebellar motor learning when we tested three different types of mutant mice that lack expression of cerebellar LTD. Together, these data argue against an essential role for LTD in cerebellar motor learning. Still, despite the absence of a compensatory change in PF-PC LTP induction or presynaptic PF plasticity, we cannot exclude the development of other compensatory mechanisms that might contribute to cerebellar motor learning in the three types of LTD-expression-deficient mutants tested here. These compensations could take the form of changes in basal electrophysiological function, use-dependent neuronal plasticity, or both. Perhaps the cerebellar PCs and/or the neurons that feed into them are sufficiently enriched with various forms of plasticity such that deletion of PF-PC LTD alone does not result in a behavioral deficit (D'Angelo et al., 1999; Jörntell and Ekerot, 2003; Salin et al., 1996). If the compensatory mechanisms indeed play a role, they may in fact operate rather fast, because even application of T-588, which blocks LTD by acutely reducing calcium release from intracellular stores, does not lead to deficits in cerebellar motor learning (current study; Welsh et al., 2005). However, the potential occurrence of compensatory mechanisms does not undermine the conclusion that the data presented here challenge the classical Marr-Albus-Ito hypothesis, because the ability to adjust the PF input to PCs was proposed to be the fundamental and essential requirement for motor learning (Albus, 1971; Marr, 1969). Our data demonstrate that motor learning can occur completely normally in the absence of PF-PC LTD, or at least in the absence of the form of PF-PC LTD that has been investigated intensely with a wide range of stimulus protocols over the past decades (Ito, 1982; Linden and Connor, 1995; De Zeeuw et al., 1998; Hansel et al., 2006).
Why can the general impairments in cerebellar motor learning that occur in the PKC, PKG, and αCamKII mutants (Boyden et al., 2006; De Zeeuw et al., 1998; Feil et al., 2003; Hansel et al., 2006) not be compensated for? In these kinase mutants the blockades may, in contrast to those in the PICK1 KO, GluR2Δ7 KI, and GluR2K882A KI mutants, not only affect LTD at their PF synapses, but also other forms of cerebellar plasticity. For example, inhibition of PKC may affect the efficacy of GABA receptors at the molecular layer interneuron to PC synapses by influencing GABA receptor surface density and sensitivity to positive allosteric modulators, modifying chloride conductance (Song and Messing, 2005), or both, while inhibition of αCamKII may directly affect LTP at these GABAergic inputs (Kano et al., 1996). Interestingly, plasticity at both the PF to molecular layer interneuron synapse and at the molecular layer interneuron to PC synapse have, just like PF-PC LTD, been reported to depend on climbing fiber activity (Jörntell et al., 2010). Indeed, recent evidence demonstrates that loss of instructive climbing fiber signals results in impaired VOR adaptation (Ke et al., 2009), supporting the possibility that climbing fibers may play an important role here. Thus, this disynaptic plasticity in the feedforward inhibition onto PCs provides a possible answer to the emerging question of what the role of the climbing fibers might be when climbing-fiber-induced PF-PC LTD is not essential. Similarly, PCs also display intrinsic plasticity (Belmeguenai et al., 2010), and protein kinases may well be required for persistent use-dependent modulation of one or more of the ion channels involved. Finally, the kinases might also play a role in presynaptic plasticity at the PC to cerebellar nuclei neuron synapse (Pedroarena and Schwarz, 2003) and/or postsynaptic plasticity at the mossy fiber or climbing fiber collateral to cerebellar nuclei neuron synapse (Pugh and Raman, 2008; Zhang and Linden, 2006). Thus, combined deficits in plasticity at the PF to PC synapse, the molecular layer interneuron to PC synapse, the PC to cerebellar nuclei neuron synapse, and the collateral to cerebellar nuclei neuron synapse, and in the intrinsic plasticity of PCs, provide interesting alternative explanations for the behavioral phenotypes observed in the PC-specific PKC, PKG, and αCamKII mutants (De Zeeuw et al., 1998; Feil et al., 2003; Hansel et al., 2006).
The mutations in the PICK1 KO, GluR2Δ7 KI, and GluR2K882A KI mutants were global, i.e., not cell specific. Thus, it was remarkable that both cerebellar motor performance and motor learning were normal, despite the fact that the mutations affect multiple cell types in both the cerebellum and its supportive systems. The global character of the mutations even further strengthens the implications of the general absence of a necessary and sufficient correlation between our cell physiological and behavioral findings. One would expect more deficits in general, and it raises the possibility that the affected protein and receptors, as well as the correlated cell physiological deficit in LTD, can be readily compensated for in general. The same argument may hold for the specific concept that was put forward by the Marr-Albus-Ito hypothesis, i.e., the idea that climbing fiber activity during motor learning weakens the PF influence onto PCs and thereby reduces their output. As explained above, there may be different climbing-fiber-driven mechanisms in place that can act simultaneously under normal conditions and that can compensate for each other's absence in particular mutant mice. For example, the climbing fibers might be able to both depress the PF to PC synapse and potentiate the molecular layer interneuron to PC synapse (Jörntell et al., 2010), and both could ultimately lead to a depression of PCs’ simple spike activity. Thus, in principle a climbing-fiber-driven reduction in simple spikes may still occur during learning in the PICK1 KO, GluR2Δ7 KI, and GluR2K882A KI mutants, despite a blockade of LTD at the PF to PC synapse. Such a combined action of different mechanisms might also explain why blocking the GABAergic input from the molecular layer interneurons onto the PCs still allows a substantial level of motor learning (Wulff et al., 2009); i.e., in that case PF-PC LTD may be enhanced to compensate. It might thus be useful to use the current LTD-expression-deficient mice in combination with others to identify the combination of plasticities that may be essential for cerebellar motor learning.
EXPERIMENTAL PROCEDURES
All experiments were conducted in accordance with The Dutch Ethical Committee for animal experiments.
Eye Movement Recordings
Mice aged 4–6 (young) or 12–30 (adult) weeks were prepared for experiments under isoflurane anesthesia by receiving a construct on the skull allowing their immobilization. After 5 days of recovery, mice were placed in a restrainer, which was fixed onto the center of a turntable that was surrounded by a cylindrical screen. Baseline OKR and VVOR were evoked by rotating the screen and turntable, respectively. Short-term adaptation was evoked by drum and table rotation out of phase or in phase with an amplitude of 5° at 0.6 Hz for 5 × 10 min. Long-term adaptation was induced by in-phase training with equal amplitude on day 1 (5° at 0.6 Hz, 5 × 10 min) and an increase in drum amplitude by 1° each subsequent day. Gain (eye velocity/stimulus velocity) and phase (eye to stimulus in degrees) values were calculated offline. Chemical block of LTD was induced by i.p. injections of 10.0 mg/kg T-588 (provided by Toyama, Japan), dissolved in sterile saline (1.0 mg/ml), heated to ~37°, and injected 30 min prior to the start of the experiment.
Eyeblink Conditioning
Mice aged 12–30 weeks were anesthetized, surgically prepared, and investigated with the use of MDMT as described before (Koekkoek et al., 2003) (Neurasmus B.V., http://www.neurasmus.com). After a recovery period of 4 days, mice were subjected to two habituation sessions, six training sessions, and four extinction sessions A training session consisted of eight blocks, each consisting of six paired trials, one US only trail, and one CS only trial. For the US we used a mild corneal airpuff (30 ms), and for the CS, an auditory tone (interstimulus interval, 350 ms, CS and US coterminate). Eyelid responses in paired trials were categorized into auditory startle responses (latency to peak, 5–50 ms), short-latency responses (latency to onset, 50–70 ms, and latency to peak, ~115 ms), or cerebellar CRs (latency to onset, 50–350 ms, and latency to peak, 360 ms). For CS only trials we used the same values, except that the latency to peak amplitude of the CR was smaller than 400 ms instead of 360 ms.
The Erasmus Ladder
Mice aged 12–30 weeks were subjected to the Erasmus Ladder (Neurasmus B.V., http://www.neurasmus.com/), which consists of two, single-opening black boxes equipped with a bright white light. These shelters are connected by a ladder consisting of 37 double rungs placed 15 mm apart, with alternate rungs in a descended position, so as to create an alternating stepping pattern with 30 mm gaps. Mice were subjected to four motor performance sessions followed by four associative motor learning sessions, each consisting of 72 trials. On days 5–8, mice were trained to avoid an obstacle by the presentation of a tone (90 dB, 15 Hz tone; CS) 285 ms before a rung rose (12 mm; US) in the swing phase of their right paws. Steptime is defined as the time needed to place one of the front paws from one rung to the other; and missteps, as the number of touches on the descended rungs. A decrease in post steptime (steptime directly after the CS) over the sessions, implying that mice learn to adjust their stepping patterns to the obstacle, is taken as a measure of associative motor learning.
Cell Physiological Recordings
Patch-clamp experiments were performed as recently published (Schonewille et al., 2010). In short, sagittal slices of the cerebellar vermis (250 μm) from adult mice were made in ice-cold oxygenated “slicing” solution containing (in mM) 2.5 KCl, 1 CaCl2, 3 MgCl2, 25 NaHCO3, 1.25 NaH2PO4, 240 sucrose, and 25 D-glucose. Slices were kept at room temperature (23°C ± 1°C) in oxygenated ACSF containing (in mM) 124 NaCl, 5 KCl, 1.25 Na2HPO4, 2 MgSO4, 2 CaCl2, 26 NaHCO3, 20 D-glucose, and 100 μM picrotoxin. Cyclosporin A (bath applied, 5 μM in 0.5% EtOH) was added where indicated. Whole-cell patch-clamp recordings were performed using an EPC-10 amplifier (HEKA, Lambrecht) and patch pipettes filled with (in mM) 120 K-Gluconate, 9 KCl, 10 KOH, 3.48 MgCl2, 4 NaCl, 10 HEPES, 4 Na2ATP, 0.4 Na3GTP, and 17.5 sucrose (at pH 7.25). PF-PC LTD was induced by pairing PF and CF stimulation at 1 Hz for 5 min, and PF-PC LTP was induced by PF stimulation alone at 1 Hz for 5 min. Test responses (two pulses at 50 ms interval) were evoked every 20 s in voltage-clamp mode to prevent spontaneous spiking. In all experiments, cells were switched to current-clamp mode for tetanization.
Data Analysis
All values are shown as mean ± SEM. All p values were determined for mutants against pooled (values used here) and mutant-specific controls (see Table S2), using two-tailed Student's t test, one-way ANOVA, or ANOVA for repeated measures with a posthoc Tukey test to determine significance between the groups. p < 0.05 was considered statistically significant.
See Supplemental Experimental Procedures for a full description of experiments.
Supplementary Material
ACKNOWLEDGMENTS
We kindly thank R. Avila Freire, M. Rutteman, D. Smeets, J. van der Burg, E. Haasdijk, and E. Goedknegt for their technical assistance, and we kindly thank the Dutch Organization for Medical Sciences (F.E.H., C.I.D.Z.), Life Sciences (M.S., F.E.H., C.I.D.Z.), Erasmus University Rotterdam Fellowship program (M.S., F.E.H.), Senter (Neuro-Bsik, C.I.D.Z.), Prinses Beatrix Fonds (C.I.D.Z.), the SENSOPAC program, C7 and CEREBNET of the European Community (C.I.D.Z.), the United States Public Health Service MH51106 (D.J.L.) and NS36715 (R.L.H.), and the Howard Hughes Medical Institute (R.L.H.) for their financial support. H.J.B. and C.I.D.Z. were supported by Neurasmus B.V. We kindly thank Toyama (Toyama Chemical Co. Ltd., Tokyo, Japan) for providing the T-588.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information for this article includes one figure, two tables, and Supplemental Experimental Procedures and can be found with this article online at doi:10.1016/j.neuron.2011.02.044.
REFERENCES
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- Albus JS. A theory of cerebellar function. Math. Biosci. 1971;10:25–61. [Google Scholar]
- Belmeguenai A, Hansel C. A role for protein phosphatases 1, 2A, and 2B in cerebellar long-term potentiation. J. Neurosci. 2005;25:10768–10772. doi: 10.1523/JNEUROSCI.2876-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmeguenai A, Hosy E, Bengtsson F, De Zeeuw CI, Pedroarena C, Jorntell H, Hansel C. Intrinsic plasticity as a negative regulator of synaptic gain in cerebellar Purkinje cells. J. Neurosci. 2010;30:13630–13643. doi: 10.1523/JNEUROSCI.3226-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazquez PM, Hirata Y, Highstein SM. The vestibulo-ocular reflex as a model system for motor learning: What is the role of the cerebellum? Cerebellum. 2004;3:188–192. doi: 10.1080/14734220410018120. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Katoh A, Pyle JL, Chatila TA, Tsien RW, Raymond JL. Selective engagement of plasticity mechanisms for motor memory storage. Neuron. 2006;51:823–834. doi: 10.1016/j.neuron.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Chen C, Tonegawa S. Molecular genetic analysis of synaptic plasticity, activity-dependent neural development, learning, and memory in the mammalian brain. Annu. Rev. Neurosci. 1997;20:157–184. doi: 10.1146/annurev.neuro.20.1.157. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Steinberg JP, Huganir RL, Linden DJ. Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science. 2003;300:1751–1755. doi: 10.1126/science.1082915. [DOI] [PubMed] [Google Scholar]
- Coesmans M, Weber JT, De Zeeuw CI, Hansel C. Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron. 2004;44:691–700. doi: 10.1016/j.neuron.2004.10.031. [DOI] [PubMed] [Google Scholar]
- D'Angelo E, Rossi P, Armano S, Taglietti V. Evidence for NMDA and mGlu receptor-dependent long-term potentiation of mossy fiber-granule cell transmission in rat cerebellum. J. Neurophysiol. 1999;81:277–287. doi: 10.1152/jn.1999.81.1.277. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Yeo CH. Time and tide in cerebellar memory formation. Curr. Opin. Neurobiol. 2005;15:667–674. doi: 10.1016/j.conb.2005.10.008. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Hansel C, Bian F, Koekkoek SK, van Alphen AM, Linden DJ, Oberdick J. Expression of a protein kinase C inhibitor in Purkinje cells blocks cerebellar LTD and adaptation of the vestibulo-ocular reflex. Neuron. 1998;20:495–508. doi: 10.1016/s0896-6273(00)80990-3. [DOI] [PubMed] [Google Scholar]
- Feil R, Hartmann J, Luo C, Wolfsgruber W, Schilling K, Feil S, Barski JJ, Meyer M, Konnerth A, De Zeeuw CI, Hofmann F. Impairment of LTD and cerebellar learning by Purkinje cell-specific ablation of cGMP-dependent protein kinase I. J. Cell Biol. 2003;163:295–302. doi: 10.1083/jcb.200306148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel C, de Jeu M, Belmeguenai A, Houtman SH, Buitendijk GH, Andreev D, De Zeeuw CI, Elgersma Y. alphaCaMKII Is essential for cerebellar LTD and motor learning. Neuron. 2006;51:835–843. doi: 10.1016/j.neuron.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar control of the vestibulo-ocular reflex—around the flocculus hypothesis. Annu. Rev. Neurosci. 1982;5:275–296. doi: 10.1146/annurev.ne.05.030182.001423. [DOI] [PubMed] [Google Scholar]
- Jörntell H, Ekerot CF. Receptive field plasticity profoundly alters the cutaneous parallel fiber synaptic input to cerebellar interneurons in vivo. J. Neurosci. 2003;23:9620–9631. doi: 10.1523/JNEUROSCI.23-29-09620.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörntell H, Bengtsson F, Schonewille M, De Zeeuw CI. Cerebellar molecular layer interneurons - computational properties and roles in learning. Trends Neurosci. 2010;33:524–532. doi: 10.1016/j.tins.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Kano M, Kano M, Fukunaga K, Konnerth A. Ca(2+)-induced rebound potentiation of gamma-aminobutyric acid-mediated currents requires activation of Ca2+/calmodulin-dependent kinase II. Proc. Natl. Acad. Sci. USA. 1996;93:13351–13356. doi: 10.1073/pnas.93.23.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke MC, Guo CC, Raymond JL. Elimination of climbing fiber instructive signals during motor learning. Nat. Neurosci. 2009;12:1171–1179. doi: 10.1038/nn.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp BE, Pearson RB. Protein kinase recognition sequence motifs. Trends Biochem. Sci. 1990;15:342–346. doi: 10.1016/0968-0004(90)90073-k. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Thompson RF. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends Neurosci. 1997;20:177–181. doi: 10.1016/s0166-2236(96)10081-3. [DOI] [PubMed] [Google Scholar]
- Kimura T, Sugimori M, Llinás RR. Purkinje cell long-term depression is prevented by T-588, a neuroprotective compound that reduces cytosolic calcium release from intracellular stores. Proc. Natl. Acad. Sci. USA. 2005;102:17160–17165. doi: 10.1073/pnas.0508190102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto Y, Fujimichi R, Araishi K, Kawahara S, Kano M, Aiba A, Kirino Y. mGluR1 in cerebellar Purkinje cells is required for normal association of temporally contiguous stimuli in classical conditioning. Eur. J. Neurosci. 2002;16:2416–2424. doi: 10.1046/j.1460-9568.2002.02407.x. [DOI] [PubMed] [Google Scholar]
- Koekkoek SK, Hulscher HC, Dortland BR, Hensbroek RA, Elgersma Y, Ruigrok TJ, De Zeeuw CI. Cerebellar LTD and learning-dependent timing of conditioned eyelid responses. Science. 2003;301:1736–1739. doi: 10.1126/science.1088383. [DOI] [PubMed] [Google Scholar]
- Lev-Ram V, Mehta SB, Kleinfeld D, Tsien RY. Reversing cerebellar long-term depression. Proc. Natl. Acad. Sci. USA. 2003;100:15989–15993. doi: 10.1073/pnas.2636935100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DJ, Connor JA. Long-term synaptic depression. Annu. Rev. Neurosci. 1995;18:319–357. doi: 10.1146/annurev.ne.18.030195.001535. [DOI] [PubMed] [Google Scholar]
- Marr D. A theory of cerebellar cortex. J. Physiol. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao S. Behavior of floccular Purkinje cells correlated with adaptation of vestibulo-ocular reflex in pigmented rabbits. Exp. Brain Res. 1989;77:531–540. doi: 10.1007/BF00249606. [DOI] [PubMed] [Google Scholar]
- Pedroarena CM, Schwarz C. Efficacy and short-term plasticity at GABAergic synapses between Purkinje and cerebellar nuclei neurons. J. Neurophysiol. 2003;89:704–715. doi: 10.1152/jn.00558.2002. [DOI] [PubMed] [Google Scholar]
- Pugh JR, Raman IM. Mechanisms of potentiation of mossy fiber EPSCs in the cerebellar nuclei by coincident synaptic excitation and inhibition. J. Neurosci. 2008;28:10549–10560. doi: 10.1523/JNEUROSCI.2061-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renier N, Schonewille M, Giraudet F, Badura A, Tessier-Lavigne M, Avan P, De Zeeuw CI, Chédotal A. Genetic dissection of the function of hindbrain axonal commissures. PLoS Biol. 2010;8:e1000325. doi: 10.1371/journal.pbio.1000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin PA, Malenka RC, Nicoll RA. Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron. 1996;16:797–803. doi: 10.1016/s0896-6273(00)80099-9. [DOI] [PubMed] [Google Scholar]
- Schonewille M, Belmeguenai A, Koekkoek SK, Houtman SH, Boele HJ, van Beugen BJ, Gao Z, Badura A, Ohtsuki G, Amerika WE, et al. Purkinje cell-specific knockout of the protein phosphatase PP2B impairs potentiation and cerebellar motor learning. Neuron. 2010;67:618–628. doi: 10.1016/j.neuron.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Messing RO. Protein kinase C regulation of GABAA receptors. Cell. Mol. Life Sci. 2005;62:119–127. doi: 10.1007/s00018-004-4339-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg JP, Takamiya K, Shen Y, Xia J, Rubio ME, Yu S, Jin W, Thomas GM, Linden DJ, Huganir RL. Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron. 2006;49:845–860. doi: 10.1016/j.neuron.2006.02.025. [DOI] [PubMed] [Google Scholar]
- van Alphen AM, De Zeeuw CI. Cerebellar LTD facilitates but is not essential for long-term adaptation of the vestibulo-ocular reflex. Eur. J. Neurosci. 2002;16:486–490. doi: 10.1046/j.1460-9568.2002.02094.x. [DOI] [PubMed] [Google Scholar]
- Van Der Giessen RS, Koekkoek SK, van Dorp S, De Gruijl JR, Cupido A, Khosrovani S, Dortland B, Wellershaus K, Degen J, Deuchars J, et al. Role of olivary electrical coupling in cerebellar motor learning. Neuron. 2008;58:599–612. doi: 10.1016/j.neuron.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Wang YT, Linden DJ. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25:635–647. doi: 10.1016/s0896-6273(00)81066-1. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Yamaguchi H, Zeng XH, Kojo M, Nakada Y, Takagi A, Sugimori M, Llinás RR. Normal motor learning during pharmacological prevention of Purkinje cell long-term depression. Proc. Natl. Acad. Sci. USA. 2005;102:17166–17171. doi: 10.1073/pnas.0508191102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff P, Schonewille M, Renzi M, Viltono L, Sassoe-Pognetto M, Badura A, Gao Z, Hoebeek FE, van Dorp S, Wisden W, et al. Synaptic inhibition of Purkinje cells mediates consolidation of vestibulo-cerebellar motor learning. Nat. Neurosci. 2009;12:1042–1049. doi: 10.1038/nn.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28:499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. Long-term depression at the mossy fiber-deep cerebellar nucleus synapse. J. Neurosci. 2006;26:6935–6944. doi: 10.1523/JNEUROSCI.0784-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.