Abstract
Background
Dietary intake of fiber, carbohydrate, glycemic index (GI), and glycemic load (GL) may influence breast cancer survival, but consistent and convincing evidence is lacking.
Methods
We investigated associations of dietary fiber, carbohydrates, GI, and GL with breast cancer prognosis among n=688 stage 0 to IIIA breast cancer survivors in the Health, Eating, Activity, and Lifestyle (HEAL) study. Pre- and postmenopausal women from Western Washington State, Los Angeles County, and New Mexico participated. Usual diet was assessed with a food frequency questionnaire. Total mortality, breast cancer mortality, non-fatal recurrence and second occurrence data were obtained from SEER registries and medical records. Cox proportional hazards regression estimated multivariate-adjusted hazard ratios and 95% CIs.
Results
During a median of 6.7 years follow-up after diagnosis, n= 106 total deaths, n=83 breast cancer-specific deaths and n=82 non-fatal recurrences were confirmed. We observed an inverse association between fiber intake and mortality. Multivariate-adjusted HRRs comparing high to low intake were 0.53 (95% CI 0.23-1.23) and 0.75 (95% CI 0.43-1.31). A threshold effect was observed whereby no additional benefit was observed for intakes >9 g/day. Fiber intake was suggestively inversely associated with breast-cancer specific mortality (HRR=0.68, 95% CI 0.27-1.70) and risk of non-fatal recurrence or second occurrence (HRR=0.68, 95% CI 0.27-1.70), but results were not statistically significant.
Conclusion
Dietary fiber was associated with a non-significant inverse association with breast cancer events and total mortality. Further studies to assess and confirm this relationship are needed in order to offer effective dietary strategies for breast cancer patients.
Impact
Increasing dietary fiber may an effective lifestyle modification strategy for breast cancer survivors.
Keywords: Breast cancer survival, fiber, carbohydrates, glycemic index, glycemic load
Introduction
Increasing evidence suggests that dietary fiber intake may play a role in breast cancer etiology by lowering circulating estrogen levels and modulating inflammation, both of which influence breast cancer risk (1, 2). For example, data from the Women's Health Initiative Observational Study demonstrated that a high fiber diet is associated with lower plasma levels of the proinflammatory cytokines interleukin-6 (IL-6) and tumor necrosis factor-α receptor-2 (TNF-α-R2) and may lower glycemia (2).
In contrast, a diet rich in other carbohydrates, such as sugars and refined carbohydrates, may increase breast cancer risk possibly by inducing metabolic syndrome associated altered endocrine and inflammatory responses (3, 4). High-carbohydrate diets, and particularly diets high in glycemic index (GI) or glycemic load (GL), increase postprandial glucose and insulin concentrations (5-7). This metabolic dysfunction, as well as frank diabetes, has been associated with poor breast cancer survival (8, 9). Glycemic index (GI) is a classification system proposed to quantify the glycemic response to carbohydrates. It is defined as the relative increase in blood glucose level per gram of carbohydrate intake of reference carbohydrate: glucose or white bread (10). Glycemic load (GL) is a similar metric based on carbohydrate quality and quantity. GL is the product of GI and the carbohydrate content (g) of a food item divided by 100 (10-13). When insulin levels are elevated for a prolonged period of time, it may lead to insulin resistance, which is associated with type II diabetes, obesity, cardiovascular disease, and cancer, including breast cancer (13-15).
Besides affecting breast cancer risk, a diet high in fiber, and low in carbohydrates, glycemic index, and glycemic load, might be associated with improved survival and breast cancer recurrence (16). Dietary fiber intake and its relationship to breast cancer prognosis was recently investigated in three studies. A breast cancer survivor cohort study (n=516 survivors) by McEligot et al. observed an inverse association between dietary fiber intake and overall mortality (17). The Nurses Health Study (n=3,846) observed a decreased risk of overall mortality after an initial breast cancer diagnosis only for cereal fiber (18), whereas the Women's Healthy Eating and Living trial among women with early stage breast cancer found no effect between a diet high in fiber intake and breast cancer events or mortality (19). Two cohort studies investigated dietary carbohydrate intake with breast cancer survival and found contradicting results. One reported no association for breast cancer-specific mortality (20), while, as noted above, McEligot reported an inverse association for overall mortality after breast cancer diagnosis (17). To our knowledge, few prospective studies have investigated the association between GI, GL, and breast cancer prognosis. However, previous studies examining these measures in relation to breast cancer incidence have produced inconsistent outcomes (11, 21-23).
Given the increasing numbers of long-term breast cancer survivors, research investigating mortality after diagnosis and prevention of recurrence is of considerable public health importance. By identifying the role of dietary fiber, carbohydrates, GI, and GL, strategies to adapt the diet can be developed so as to reduce risk of second primary or recurrent breast cancer cases and prolong survival. We therefore investigated the association between dietary fiber and carbohydrate intake, glycemic index, and glycemic load, and the outcomes of overall survival, breast cancer-specific survival, and breast cancer recurrence or second occurrence, in the Health, Eating, Activity, and Lifestyle (HEAL) study.
Materials and Methods
Study Design, Population, and Recruitment
The Health, Eating, Activity and Lifestyle (HEAL) study is a multicenter, multiethnic prospective cohort study of 1,183 breast cancer patients designed to determine whether weight, physical activity, diet, sex hormones, and other exposures influence breast cancer prognosis and survival. Details of the study design and procedures have been previously published (24, 25). Briefly, we utilized the National Cancer Institute's Surveillance, Epidemiology, End Results (SEER) registries in New Mexico, Los Angeles County (CA), and Western Washington State to ascertain and recruit English speaking women diagnosed with in situ to Stage IIIA breast cancer. In New Mexico, we recruited 615 women; aged 18 yr or older; diagnosed between July 1996 and March 1999; and living in Bernalillo, Santa Fe, Sandoval, Valencia, or Taos Counties. In Western Washington State, we recruited 202 women between the ages of 40 and 64 years; diagnosed between September 1997 and September 1998; and living in King, Pierce, or Snohomish Counties. In Los Angeles County, we recruited 366 African-American women who had previously participated in other breast cancer case-control studies. These women were diagnosed with breast cancer between May 1995 and May 1998, and were aged 35 to 64 years at diagnosis. Written informed consent was obtained from all participants at each study site. All HEAL procedures were approved by the institutional review boards of the participating centers (Fred Hutchinson Cancer Research Center, University of Southern California, and University of New Mexico) in accord with an assurance filed with and approved by the U.S. Department of Health and Human Services (24).
HEAL participants completed extensive interviews within their first year after diagnosis (on average 7.5 months postdiagnosis). Information on health habits, medical history, history of breast disease, and reproductive and menstrual history were collected via in-person interviews and self administered questionnaires. Approximately 24 months later (within their third year after diagnosis; (on average 31.5 months postdiagnosis), clinic or in-home visits were conducted to measure height and weight and to collect self-reported data on physical activity, diet, dietary supplements, alcohol, tobacco and other exposures that may influence breast cancer prognosis. Of the 1,223 women initially enrolled at study, 39 (3.2%) women who were later found to have had a prior diagnosis of breast cancer and one woman (<1.0%) who had metastatic disease at initial diagnosis were subsequently excluded. Of the remaining 1,183 women, 239 (20.2%) women did not return for the 24-month follow-up visit. Reasons for nonparticipation in the 24-month interview were death (n=44), too ill (n=2), refusal (n=104), spouse disallowed contact (n=1), moved (n=16), or unable to contact (n=72). A total of 944 women completed the 24-month follow-up visit. For these analyses, we excluded women who had an initial breast cancer diagnosis of in situ disease (n=206); so as to include only invasive disease (26). We further excluded women with missing dietary data (n=17), unreasonable report of dietary intake (> 2 SD greater than the mean intake of our primary dietary exposures) (n=1), women whose non-fatal breast cancer event date or interview date was unknown (n=5) and women whose first event was prior to the 24 month interview when diet was assessed (n=27). Our final sample included n=688 women who had an initial diagnosis of stage I to IIIA breast carcinoma, no breast cancer events before the 24 month interview and who had complete 24-month follow-up questionnaires.
Dietary Assessment
Diet was assessed at the 24-month interview using a Food Frequency Questionnaire (FFQ) developed for the Women's Health Initiative (WHI) (27). This FFQ was designed for use in multiethnic populations of women and asked about usual dietary intake over the past month for participants in Washington State and Los Angeles County and over the previous year for participants in New Mexico (24). The WHI FFQ consisted of 122 line items, 19 adjustment questions, and 4 summary questions. Intake of energy, dietary fiber (includes soluble and insoluble), and carbohydrate were estimated using the University of Minnesota Nutrition Coordinating Center's Nutrition Data Systems for Research food and nutrient database (version 2005). GI values for individual foods and food groups on the FFQ were calculated with the ‘International Tables of Glycemic Index and Glycemic Load Values: 2002’ and a website of the University of Sidney (28, 29). The overall dietary GL for each participant was calculated by multiplying the GI value of each food with the carbohydrate content per serving, dividing by 100, and multiplying that product with the average frequency of intake, and summing the values from all foods. GL values were calculated for total and available (total carbohydrate minus dietary fiber) intake of carbohydrate in grams (28, 29). In this report we used the dietary GL values based on total carbohydrate (vs. available carbohydrate), since our previous observations suggested no differences in GL estimates using total vs. available carbohydrate (28).
Outcomes Ascertainment
Breast cancer recurrences, second breast cancer occurrences, and date and cause of death were assessed from medical records, SEER registry data and self-reported 24-month and 5-years interviews (24). We investigated three outcomes: overall mortality, breast cancer-specific mortality, and non-fatal breast cancer recurrence or second breast cancer occurrence. Overall disease-free and breast cancer-free person-time was defined as the date of diagnosis until first non-fatal breast cancer recurrence or second occurrence, date of death (death from breast cancer or any cause), or end of follow-up period (September, 2004), whichever came first. Recurrence-free person-time was calculated from date of diagnosis until first non-fatal breast cancer recurrence or second breast cancer occurrence, or end of follow-up period (September, 2004), whichever came first.
Statistical Analyses
We divided participants into quartiles of fiber, carbohydrate, GI and GL based on the distribution of intake for the entire study group. All nutrient intakes, except alcohol and GI, were adjusted for total energy intake using the regression-residual method and results were examined with and without these adjustments (30, 31). These adjustments were performed because our initial inspection of the data revealed moderate to strong correlation of total carbohydrate intake, GL and dietary fiber intake with total energy intake, (r2=0.82, r2=0.78, and r2=0.51, respectively). However, residual energy adjustment was not done for alcohol and GI, since these initial data explorations revealed no correlation with total energy intake, r2=0.01 and r2=0.03, respectively. Cox proportional hazard models with age as the time metric estimated hazard rate ratios (HRR) and their corresponding 95% confidence intervals (95% CI) for overall mortality, breast cancer mortality, and non-fatal breast cancer recurrence or second occurrence according to the dietary intake exposure levels. In the energy adjusted models, the HRRs represents the change in overall (or breast cancer) mortality or non-fatal breast cancer recurrence or second occurrence associated with substitution of nutrient intake for an equivalent amount of energy from other nutrients. The proportional hazards assumption was tested with a plot of the negative log of the estimated survivor function against log time (LLS), and with the goodness of fit test with Schoenfeld residuals (GOF) (32). No adverse effect was seen, as the survival curves were parallel and had similar shapes between the different strata, and the correlations were not statistically significant (p>0.10).
All models included cancer treatment (surgery, chemotherapy, radiation), stage (localized, regional, distant) and tamoxifen use (yes, no). A priori Potential confounding factors were indentified based on previous literature and included: age at menarche, age at menopause, age at first birth, height, BMI, BMI at age 18 years, level of education, race, geographic location of residence, physical activity, menopausal status, smoking status, family history of breast cancer, oral contraceptive use, postmenopausal hormone therapy (HT) use, parity, total intake of energy, dietary fiber (only for analyses of carbohydrate, GL and GI), alcohol, folate, and dietary supplement use (1, 6, 21, 22, 33-36). These potential confounders were included in the final multivariable Cox regression model if they changed the crude estimates by more than 10%. Using this approach, the final models were adjusted for total energy intake (kcal/day), (energy adj) dietary fiber intake (g/day) (except for models where fiber was the primary exposure), (energy adj) dietary folate intake (μg/day), physical activity (MET h/week), treatment (surgery, chemotherapy, radiation), stage (localized, regional, distant), and tamoxifen use (yes, no). The same statistical approach was employed for all three outcomes examined. Tests for linear trend were conducted using the median value of each quartile as a continuous variable. The Kaplan-Meier method was used to generate survival probability curves.
In addition, we considered whether the influence of dietary fiber, carbohydrate, GI, and GL, on overall survival, breast cancer specific survival, and non-fatal breast cancer recurrence or second occurrence was modified by body mass index (BMI) (18.5 kg/m2≥ BMI ≤25.0 kg/m2, BMI>25.0 kg/m2), waist circumference (≤88.8 cm, >88.8 cm), menopausal status, physical activity (tertiles; MET h/wk), smoking status (never, former, current), alcohol use (non-users, users), estrogen receptor status of the breast cancer (ER-, ER+), study site (Western Washington State, New Mexico, Los Angeles County), and baseline medical history that may influence dietary changes: history of diabetes mellitus (yes, no), hypertension (yes, no) and tumor grade (grade I, grade II, grade III). To examine if the association between energy-adjusted dietary fiber intake and the risk of death from any cause, breast cancer death, or breast cancer recurrence or second occurrence was modified by physical activity, we included both cross-products expressed as continuous variables, in our regression models. P for the tests for interaction was obtained from a likelihood ratio test with 3 degrees of freedom. All tests of statistical significance were two sided and the significance level was set at α=0.05. The Statistical Analysis Software (SAS version 9.1, SAS Institute, Gary, NC) was used for all analyses.
Results
Median follow-up time for the HEAL cohort was 6.7 years (4,615 person-years), during which the following endpoints were confirmed: non-fatal recurrence or second breast cancer occurrence (n=82), breast cancer-specific death (n=83; 78.3% of all deaths) or any death (n=106). HEAL is a multiethnic cohort: 57.7% of participants are non-Hispanic White, 28.5% are African-American, 11.9% are Hispanic, and the remainders are Asian or mixed race (1.9%). The mean age at study entry was 55.3 ± 10.6 years (Table 1). Compared to the rest of the cohort, women with a non-fatal breast cancer recurrence or second occurrence, or those who died from any cause during follow-up period were more likely to be African-American, smoked more, were less physical active, were less likely to have ever used oral contraceptives and hormone replacement therapy, were more likely to have been diagnosed with hypertension, and consumed less total sugar, dietary fiber, and alcohol (data not shown).
Table I. Characteristics of breast cancer survivors in the Health, Eating, Activity, and Lifestyle (HEAL) study (n=688).
| Demographic and reproductive characteristics1 | |
|---|---|
| Years of follow-up [median (IQR)] | 6.7 (6.1; 7.4) |
| Age (y) | 55.3 ± 10.6 |
| Study site [n (%)] | |
| Western Washington State | 118 (17.2%) |
| New Mexico | 374 (54.4%) |
| Los Angeles County | 196 (28.5%) |
| BMI (kg/m2) | 27.3 ± 1.2 |
| Education [n (%)] | |
| High school only | 188 (27.3%) |
| College | 376 (54.7%) |
| Graduate school | 124 (18.0%) |
| Race | |
| Non-Hispanic White | 397 (57.7%) |
| African-American2 | 196 (28.5%) |
| Hispanic | 82 (11.9%) |
| Other | 13 (1.9%) |
| Current smoker [n (%)] | 92 (13.4%) |
| Physical activity (MET h/week), median (IQR)3 | 5.0 (0.4; 16.5) |
| Postmenopausal at baseline [n (%)] | 419 (60.9%) |
| Age at first birth (y)4 | 22.8 ± 1.2 |
| Number of live births | 1.7 ± 0.5 |
| First-degree relative with breast cancer [n (%)] | 146 (21.2%) |
| Oral contraceptive user [n (% ever)] | 495 (72.0%) |
| HRT user [n (% ever)] | 300 (43.6%) |
|
| |
| Dietary intake | |
|
|
|
| Total energy intake (kcal/ day) | 1293.2 ± 1.6 |
| Glycemic index (daily)5 | 50.9 ± 4.5 |
| Glycemic load (daily)6 | 78.2 ± 1.7 |
| Carbohydrate intake (g/day) | 154.2 ± 1.6 |
| Folate intake (μg/day) | 288.1 ± 1.6 |
| Total sugars (g/day) | 73.5 ± 1.8 |
| Total fiber (g/day)7 | 13.2 ± 0.8 |
| Alcohol user (g/day), median (IQR)8 | 3.6 (1.2; 11.6) |
| Dietary supplement user [n (%)]9 | 604 (87.8%) |
All variables are presented as mean ± SD, unless stated otherwise;
All African-American women are from Los Angeles County.
Moderate/vigorous sport & recreational activities year prior to 24 month interview;
Includes only women that gave birth (n=575 subjects);
Glycemic index: (GL*100)/total carbohydrate (g), GI is unit less;
Glycemic load is based on total carbohydrates, GL is unit less;
Fiber intake: sum of total soluble and insoluble fibers;
Alcohol user (n=337): >0.20g/day;
Dietary supplement user: ≥ 1X p/month use of multivitamins, vitamin A, C, D, E and/or beta carotene.
We found no associations between carbohydrate intake, GL, and risk of death from any cause (Table 2). We observed a suggested linear association between GI and mortality, but after multivariate-adjustment, the linear trend was no longer significant. Dietary fiber showed stronger differences with survival between non-energy-adjusted en energy-adjusted models (Figures 1 and 2). A non-statistically significant inverse association between dietary fiber intake and overall mortality was observed: women with higher vs. lower fiber intake had a 47% reduced risk of death from any cause. After energy adjustment, the hazard ratio was attenuated to a non-significant 25% reduced risk. In both multivariable-adjusted fiber models, the HRRs were similar across the second, third and fourth quartiles compared to the reference group, suggesting a possible threshold effect (table 2).
Table 2. Crude and adjusted Hazard Rate Ratios (HRRs) and 95% confidence intervals (95% CI) for the association between (energy adj) dietary carbohydrates, glycemic index, (energy adj) glycemic load, and (energy adj) dietary fiber intake and overall mortality1, breast cancer mortality2, and non-fatal recurrence and second occurence3.
| HRR (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Overall mortality (n=106) | Breast cancer mortality (n=83) | Non-fatal recurrence or new occurrence (n=82) | ||||
| Crude HRR (95% CI) | Multivariate adjusted HRR (95% CI) | Crude HRR (95% CI) | Multivariate adjusted HRR (95% CI) | Crude HRR (95% CI) | Multivariate adjusted HRR (95% CI) | |
| Carbohydrate intake (g/day) | ||||||
| Q1: <110.9 | 1.00 (ref) | 1.00 (ref)9 | 1.00 (ref) | 1.00 (ref)9 | 1.00 (ref) | 1.00 (ref)9 |
| Q2: 110.9-161.8 | 0.84 (0.50; 1.40) | 0.99 (0.54; 1.80) | 0.94 (0.54; 1.64) | 1.02 (0.53; 1.98) | 0.90 (0.51; 1.58) | 0.98 (0.50; 1.92) |
| Q3: 161.8-212.9 | 0.58 (0.33; 1.00) | 0.70 (0.33; 1.49) | 0.51 (0.26; 0.97) | 0.52 (0.22; 1.24) | 0.51 (0.26; 0.97) | 0.53 (0.22; 1.26) |
| Q4: >212.9 | 0.66 (0.39; 1.13) | 0.99 (0.39; 2.50) | 0.66 (0.36; 1.21) | 0.76 (0.27; 2.17) | 0.66 (0.36; 1.21) | 0.77 (0.27; 2.19) |
| P for trend: | 0.06 | 0.78 | 0.06 | 0.38 | 0.07 | 0.40 |
|
| ||||||
| Energy adj carbohydrate intake(g/day)5 | ||||||
| Q1: <137.5 | 1.00 (ref) | 1.00 (ref)10 | 1.00 (ref) | 1.00 (ref)10 | 1.00 (ref) | 1.00 (ref)10 |
| Q2: 137.5-157.1 | 0.54 (0.31; 0.96) | 0.54 (0.30; 0.96) | 0.48 (0.25; 0.93) | 0.45 (0.23; 0.88) | 0.50 (0.26; 0.97) | 0.47 (0.24; 0.92) |
| Q3: 157.1-175.7 | 0.78 (0.46; 1.31) | 0.76 (0.44; 1.32) | 0.79 (0.45; 1.40) | 0.73 (0.40; 1.34) | 0.82 (0.46; 1.46) | 0.76 (0.42; 1.40) |
| Q4: >175.7 | 0.68 (0.40; 1.17) | 0.70 (0.38; 1.29) | 0.66 (0.36; 1.20) | 0.59 (0.30; 1.17) | 0.68 (0.38; 1.25) | 0.62 (0.31; 1.23) |
| P for trend:4 | 0.29 | 0.35 | 0.29 | 0.21 | 0.36 | 0.26 |
|
| ||||||
| Glycemic index (GI)6 | ||||||
| Q1: <48.3 | 1.00 (ref) | 1.00 (ref)11 | 1.00 (ref) | 1.00 (ref)11 | 1.00 (ref) | 1.00 (ref)11 |
| Q2: 48.3-51.2 | 1.05 (0.56; 1.95) | 0.96 (0.51; 1.79) | 1.32 (0.63; 2.75) | 1.22 (0.58; 2.55) | 1.31 (0.63; 2.74) | 1.22 (0.58; 2.55) |
| Q3: 51.2-53.8 | 1.65 (0.94; 2.87) | 1.52 (0.87; 2.68) | 2.22 (1.15; 4.28) | 2.05 (1.05; 3.99) | 2.23 (1.16; 4.30) | 2.07 (1.06; 4.03) |
| Q4: >53.8 | 1.58 (0.90; 2.79) | 1.40 (0.78; 2.50) | 1.81 (0.92; 3.56) | 1.60 (0.80; 3.21) | 1.73 (0.87; 3.42) | 1.56 (0.77; 3.13) |
| P for trend: | 0.05 | 0.14 | 0.04 | 0.10 | 0.05 | 0.12 |
|
| ||||||
| Glycemic load (GL)7 | ||||||
| Q1: <55.9 LOG | 1.00 (ref) | 1.00 (ref)12 | 1.00 (ref) | 1.00 (ref)12 | 1.00 (ref) | 1.00 (ref)12 |
| Q2: 55.9-83.8 | 1.11 (0.67; 1.84) | 1.40 (0.76; 2.59) | 1.24 (0.71; 2.17) | 1.53 (0.77; 3.01) | 1.19 (0.68; 2.10) | 1.48 (0.75; 2.93) |
| Q3: 83.8-109.8 | 0.60 (0.34; 1.07) | 0.82 (0.36; 1.87) | 0.62 (0.32; 1.20) | 0.81 (0.33; 2.00) | 0.62 (0.33; 1.20) | 0.82 (0.33; 2.04) |
| Q4: >109.8 | 0.73 (0.42; 1.27) | 1.23 (0.46; 3.31) | 0.72 (0.38; 1.35) | 1.11 (0.37; 3.34) | 0.72 (0.39; 1.35) | 1.14 (0.38; 3.44) |
| P for trend: | 0.09 | 0.89 | 0.13 | 0.95 | 0.14 | 1.00 |
|
| ||||||
| Energy adj GL5 | ||||||
| Q1: <69.7 | 1.00 (ref) | 1.00 (ref)13 | 1.00 (ref) | 1.00 (ref)13 | 1.00 (ref) | 1.00 (ref)13 |
| Q2: 69.7-80.6 | 1.07 (0.62; 1.85) | 1.09 (0.63; 1.90) | 0.99 (0.55; 1.81) | 1.00 (0.55; 1.83) | 0.95 (0.52; 1.73) | 0.95 (0.52; 1.76) |
| Q3: 80.6-92.0 | 0.83 (0.47; 1.47) | 0.84 (0.47; 1.53) | 0.91 (0.49; 1.67) | 0.89 (0.47; 1.67) | 0.90 (0.49; 1.66) | 0.88 (0.47; 1.67) |
| Q4: >92.0 | 0.91 (0.52; 1.60) | 0.95 (0.53; 1.70) | 0.78 (0.42; 1.47) | 0.75 (0.39; 1.45) | 0.78 (0.42; 1.47) | 0.75 (0.39; 1.45) |
| P for trend: | 0.59 | 0.70 | 0.42 | 0.37 | 0.44 | 0.38 |
|
| ||||||
| Dietary fiber intake (g/day)8 | ||||||
| Q1: <8.8 | 1.00 (ref) | 1.00 (ref)14 | 1.00 (ref) | 1.00 (ref)14 | 1.00 (ref) | 1.00 (ref)14 |
| Q2: 8.8-12.8 | 0.44 (0.26; 0.77) | 0.45 (0.24; 0.83) | 0.49 (0.26; 0.91) | 0.52 (0.26; 1.04) | 0.46 (0.24; 0.86) | 0.48 (0.23; 0.98) |
| Q3: 12.8-18.3 | 0.49 (0.29; 0.83) | 0.50 (0.25; 0.99) | 0.54 (0.30; 0.98) | 0.61 (0.28; 1.32) | 0.54 (0.30; 0.98) | 0.61 (0.28; 1.32) |
| Q4: >18.3 | 0.53 (0.32; 0.89) | 0.53 (0.23; 1.23) | 0.61 (0.34; 1.07) | 0.68 (0.27; 1.70) | 0.61 (0.34; 1.07) | 0.68 (0.27; 1.70) |
| P for trend: | 0.02 | 0.27 | 0.11 | 0.62 | 0.12 | 0.66 |
|
| ||||||
| Energy adj dietary fiber intake (g/day)5 | ||||||
| Q1: <10.3 | 1.00 (ref) | 1.00 (ref)15 | 1.00 (ref) | 1.00 (ref)15 | 1.00 (ref) | 1.00 (ref)15 |
| Q2: 10.3-12.9 | 0.75 (0.44; 1.26) | 0.76 (0.45; 1.29) | 0.76 (0.41; 1.39) | 0.78 (0.42; 1.43) | 0.72 (0.39; 1.33) | 0.74 (0.40; 1.37) |
| Q3: 12.9-16.3 | 0.76 (0.45; 1.30) | 0.86 (0.50; 1.48) | 0.91 (0.50; 1.63) | 1.03 (0.57; 1.87) | 0.91 (0.50; 1.63) | 1.02 (0.56; 1.85) |
| Q4: >16.3 | 0.66 (0.38; 1.14) | 0.75 (0.43; 1.31) | 0.75 (0.41; 1.39) | 0.85 (0.46; 1.59) | 0.75 (0.41; 1.39) | 0.84 (0.45; 1.57) |
| P for trend: | 0.81 | 0.94 | 0.79 | 0.55 | 0.74 | 0.53 |
Women who developed breast cancer recurrence or second breast cancer occurrence, or died (any cause) during follow-up period;
Women who developed (non-)breast cancer recurrence or new primary breast cancer occurrence during follow-up period;
Women who developed non-breast cancer recurrence or new primary breast cancer occurrence during follow-up period;
Test for linear trend used median quartile values as a continous variable;
Energy-adjusted values are obtained by using the residual regression method;
Glycemic index: (GL*100)/total carbohydrate (g);
Glycemic load is based on total carbohydrates;
Fiber intake: sum of total soluble and insoluble fibers;
Adjusted for fiber (g/day), folate (μg/day) intake, tumor stage, treatment, and tamoxifen use;
Adjusted for energy adjusted folate intake (μg/day), energy adjusted fiber intake (g/day), tumor stage, treatment, and tamoxifen use;
Adjusted for physical activity (M ET h/week), tumor stage, treatment, and tamoxifen use;
Adjusted for total energy intake (kcal/day), folate intake (μg/day), fiber intake (g/day), tumor stage, treatment, and tamoxifen use;
Adjusted for energy adjusted fiber intake (g/day), tumor stage, treatment, and tamoxifen use;
Adjusted for total energy intake (kcal/day), folate intake (μg/day), tumor stage, treatment, and tamoxifen use;
Adjusted for physical activity (MET h/week), tumor stage, treatment, and tamoxifen use.
Figure 1.
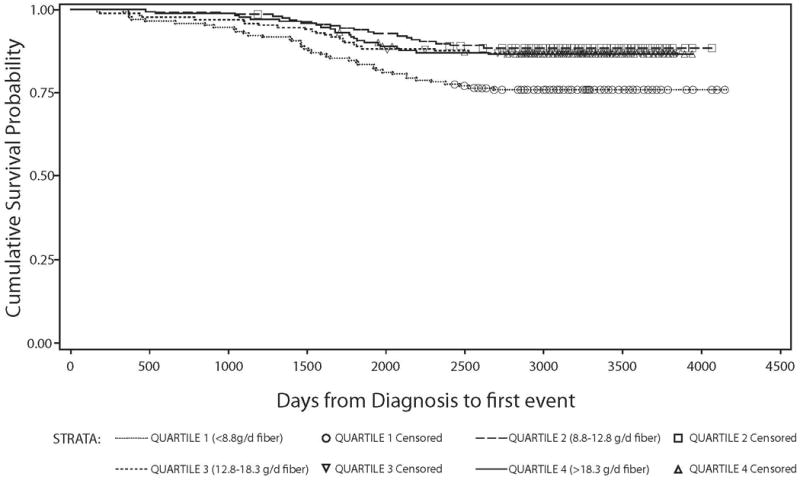
Kaplan Meier survival curve stratified by quartiles of dietary fiber intake (g/day) among all HEAL breast cancer survivors:days from diagnosis to first event (recurrence, second occurence, breast cancer specific mortality, total mortality) against overall survival probability.
Figure 2.
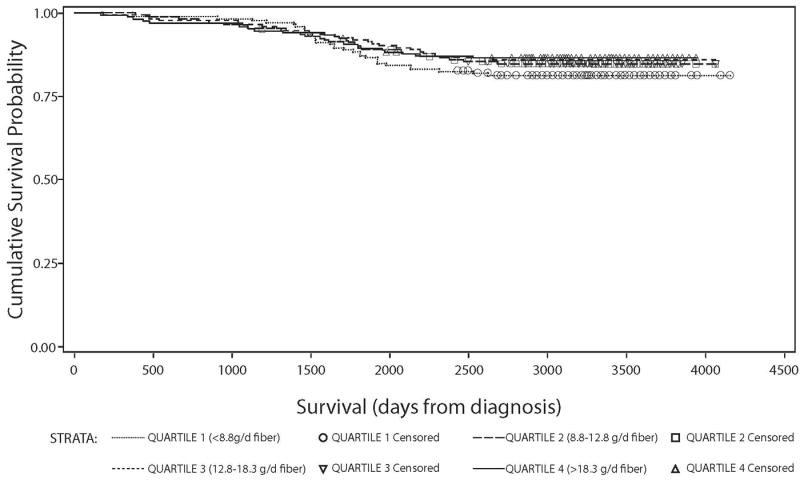
Kaplan Meier survival curve stratified by quartiles of dietary energy adjusted fiber intake (g/day) among all HEAL breast cancer survivors: survival time against overall survival probability.
For the 83 women with breast cancer-specific mortality, the results were similar to those observed for overall mortality. Similar to total mortality, GI was positively associated and dietary fiber intake was inversely, but not significantly, associated with breast cancer-specific mortality. With the exception of high vs. low GI being suggestively associated with increased risk of recurrence, none of the dietary exposures were significantly associated with risk of non-fatal recurrence or second breast cancers in either non-energy-adjusted or energy-adjusted models.
In additional analyses, we investigated whether the associations of the dietary variables of interest with the specified outcomes were modified by physical activity, BMI, waist circumference, menopausal status, smoking status, alcohol intake, history of high blood pressure, estrogen receptor status, and tumor grade. Evidence to support effect modification by variables was not observed (data not shown).
Discussion
Post-cancer diagnosis lifestyle changes, including dietary changes, are an attractive option for improving breast cancer prognosis because of their relatively low costs and minimal patient burden. The principal finding from this report is that compared to lower fiber intakes, moderate to high intake of dietary fiber may lead to a better clinical outcome after a breast cancer diagnosis. Women with a dietary fiber intake of >8.8 g/day had a 47% lower risk of death from any cause than women with lower intake. The findings were similar in direction and magnitude for breast cancer mortality and non-fatal breast recurrence or second occurrence, although the results for those outcomes were not statistically significant. Still, the findings suggest an increase in dietary fiber may be a useful addition to the breast cancer treatment repertoire.
Our results are consistent with a cohort study conducted by McEligot et al. (17) among postmenopausal women diagnosed with breast cancer. This cohort study showed an inverse association of dietary fiber intake with overall mortality (total deaths=91) when comparing the highest tertile with the lowest tertile of intake: HRR=0.48 (95% CI: 0.27, 0.86, p for trend=0.01). Subgroup analysis with breast cancer-specific mortality (n=41) showed similar, but not statistically significant, results. The McEligot et al. study was similar to ours in length of follow-up, but included more non-Hispanic Whites (92.3% vs. 58.4%), and the intake of dietary fiber was also lower on average than that which we observed (tertile 2: 8.7 g/day vs. 9.9 g/day, tertile 3: 13.3 g/day vs. 15.8 g/day) (17). No association was found for total fiber or several fiber types with death from any cause, breast cancer death or recurrence in the Nurses' Health Study (n=3,846). However, cereal fiber was associated with a decreased risk of death from any cause, HRR=0.71 (95% CI: 0.53, 0.96) (18). In a previous analysis with a smaller sample size (n=1,982) and shorter follow-up time, the Nurses' Health Study reported that higher intake of total fiber was associated with decreased risk of overall mortality (HRR: 0.69, 95% CI: 0.50, 0.97). Analyses using breast cancer-specific death as the outcome were comparable (17). Conversely, a cohort study among 603 pre- and postmenopausal breast cancer survivors found no association with breast cancer-specific mortality for the highest vs. the lowest quartile intake (HRR=0.70, 95% CI: 0.4, 1.3, p for trend=0.34) (20). The Women's Healthy Eating and Living (WHEL) randomized trial (n=3088) among survivors of early stage breast cancer (stage I-IIIA), found also no effect of a diet that was very high in vegetables, fruit, and fiber and low in fat on all-cause mortality and breast cancer recurrence or second occurrence during a 7.3-year follow-up period (19).
Only two other recent cohort studies investigated total carbohydrate intake with breast cancer survival. One, consistent with our results, observed no association for breast cancer-specific mortality for the highest intake of total carbohydrates (>224 g/day) vs. the lowest quartile (<146 g/day) (HRR=1.5, 95% CI: 0.7, 3.4, p for trend=0.69) (20), whereas a cohort study including only postmenopausal women observed a strong and significant inverse association between overall survival and carbohydrate intake (when assessed as percentage of total energy). The HRR comparing the highest tertile (≥51.8% energy) to the lowest tertile (<42.7% energy) for carbohydrates was 0.32 (95% CI: 0.18, 0.56, p for trend<0.0001) (17). All of these studies used self-reported measures of diet where the associations are typically interpreted in relative vs. absolute measures. While the analytic procedures in these studies do not characterize absolute intakes, consistency was observed with regard to the quantile cutpoints. The observed differences between these studies and our outcomes can be possibly explained by differences in sample size, in-person interviews and measured height and weight vs. self-reported, mailed questionnaires, methods for energy adjustment in dietary exposures, and time points and methods of diet assessment (i.e., differing assessment instruments). In addition, all outcomes in HEAL used both the SEER cancer registries and medical records whereas the McEligot study used only the local cancer registry.
Several biological mechanisms support a role for dietary fiber and favorable breast cancer outcomes. Dietary fiber intake may have the potential to alter inflammatory processes in the tumor microenvironment, as there are already well-known associations of fiber and decreased plasma levels of the inflammatory cytokines IL-6 and TNF-α-R2 and decreased concentrations of high sensitivity C-reactive protein (CRP) (2, 37). Elevations of these markers of inflammation are related to reduced survival among breast cancer patients, as they have several tumor-promoting effects (37, 38). Furthermore, evidence suggests that dietary fiber intake improves breast cancer prognosis through reduced estrogen levels (39). Biological mechanisms to support this mechanism include increased fecal excretion and inhibition of intestinal re-absorption of estrogens, beneficial changes in estrogen metabolism, interference of estrogen bio-availability by phytoestrogens, and stimulation of the sex hormone-binding globulin (SHBG) production (39, 40). In addition, dietary fiber can play a role in modulating insulin resistance through controlling postprandial glucose levels and improving insulin sensitivity; additional mechanisms that have been related to breast cancer survival (41). Taken together, the potential mechanisms, the results presented in this study plus the results from other studies with sufficient sample size that focused on survival, all suggest that dietary fiber intake can improve breast cancer prognosis.
The strengths of our study include the prospective design, the use of medical records and ability to control for breast cancer treatments, detailed information on diet, and complete follow-up data on 92.3% of the cohort. In addition, we were able to assess a comprehensive list of potential confounders and effect modifiers in relation to dietary fiber, carbohydrate, GI, and GL, and breast cancer prognosis. This study is not without limitations. First, dietary assessment is limited by a single measurement and the reference time period for dietary assessment differed across the three study sites. Women may have changed their dietary intake through follow-up, which may induce misclassification of exposure. Secondly, results may have been affected by the misclassification of GI and GL. Previous studies observed high inter and intra-individual coefficients of variations (CV's) for GI and GL in response to identical foods, depending on food preparation conditions, such as cooking time and degree of food ripeness (29, 42, 43). These classification systems may therefore not accurately reflect the insulinemic and glycemic effects of consumption and metabolism of foods. Thirdly, there may be residual confounding by energy intake. All exposures of interest, except for GI, were adjusted for energy consumption to correct some of the underreporting bias (44, 45). However, we may have introduced an over-adjustment for the dietary fiber model as the correlation for dietary fiber and energy intake was rather modest (r2=0.51). Because fiber and energy are weakly to modestly correlated, energy-adjusted models may artificially create a relationship between energy and fiber that does not, in fact, exist. Furthermore, as observed in our research, women with higher dietary fiber consumption have healthier lifestyles compared to those with a higher energy intake. Adjustment for total energy intake may therefore have led to misclassification, which weakens, rather than strengthens, the observed relationship between dietary fiber intake and breast cancer prognosis. Therefore, non-energy adjusted models show the primary results from which our inferences were drawn. Further, we may have been somewhat underpowered for our analyses (including analyses to test for effect modification) since we had only n=106 total deaths and n=82 recurrences. Finally, we cannot rule out the possibility of residual confounding, as is the case in any observational study.
In conclusion, results from this multiethnic cohort of breast cancer survivors demonstrated that a modest dietary fiber intake (at least 9 g/day) improved overall survival and may also improve breast cancer-specific outcomes. This is approximately equivalent to three slices of whole grain bread daily. Clinicians may wish to consider this modest dietary change for their breast cancer patients.
Acknowledgments
We would like to thank the HEAL research team: University of Southern California and the City of Hope Cancer Center: Leslie Bernstein, Frank Gilliland, Kathleen Meeske, Roberta McKean-Cowdin, Carol Koprowski, Jane Sullivan-Halley; Fred Hutchinson Cancer Research Center: Anne McTiernan, Cornelia Ulrich, Marian Neuhouser, Catherine Duggan; University of New Mexico/University of Louisville: Charles Wiggins, Richard Baumgartner, Kathy Baumgartner, Lorna Marchand, Josela Fetherolf, Sharon Wayne; National Cancer Institute: Rachel Ballard-Barbash, Linda Harlan, Ashley Smith, Todd Gibson, Anita Ambs, Bryce Reeve, Catherine Alfano. Furthermore the authors would like to thank all the HEAL participants for their ongoing dedication to this study.
Grant Support: Funding for this work was provided by National Cancer Institute contracts N01-CN-75036-20, N01-PC-67010/N01-PC-35139, N01-PC-67007/N01-PC-35138, and N01-PC-67009/N01-PC-35142 and National Institutes of Health training grant T32 CA09661. A portion of this work was conducted through the Clinical Research Center at the University of Washington and supported by National Institutes of Health grant M01-RR-00037. Data collection for the Women's Contraceptive and Reproductive Experiences Study (CARE) at the University of Southern California was supported by the National Institute of Child Health and Human Development contract N01-HD-3-3175. Patient identification was supported in part by the California Department of Health Services grant 050Q-8709-S1528.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Holmes MD, Liu S, Hankinson SE, Colditz GA, Hunter DJ, Willett WC. Dietary carbohydrates, fiber, and breast cancer risk. Am J Epidemiol. 2004;159:732–9. doi: 10.1093/aje/kwh112. [DOI] [PubMed] [Google Scholar]
- 2.Ma Y, Hebert JR, Li W, et al. Association between dietary fiber and markers of systemic inflammation in the Women's Health Initiative Observational Study. Am J Clin Nutr. 2008;24:941–9. doi: 10.1016/j.nut.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xue F, Michels KB. Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. Am J Clin Nutr. 2007;86:823S–35s. doi: 10.1093/ajcn/86.3.823S. [DOI] [PubMed] [Google Scholar]
- 4.Zhao G, Etherton TD, Martin KR, G PJ, West SG, Kris-Etherton P. Dietary alpha-linoleic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am J Clin Nutr. 2007;85:385–91. doi: 10.1093/ajcn/85.2.385. [DOI] [PubMed] [Google Scholar]
- 5.Lof M, Weiderpass E. Impact of diet on breast cancer risk. Current Opinion in Obstetrics and Gynecology. 2009;21:80–5. doi: 10.1097/GCO.0b013e32831d7f22. [DOI] [PubMed] [Google Scholar]
- 6.Augustin LSA, Maso LD, Vecchia CL, et al. Dietary glycemic index and glycemic load, and breast cancer risk: a case-control study. Ann Oncol. 2001;12:1533–8. doi: 10.1023/a:1013176129380. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins DJA, Kendall CWC, Augustin LSA, et al. Glycemic index: overview of implications in health and disease. Am J Clin Nutr. 2002;76:266S–73S. doi: 10.1093/ajcn/76/1.266S. [DOI] [PubMed] [Google Scholar]
- 8.Patterson R, Flatt S, Saquib N, et al. Medical comorbidities predict mortality in women with a history of early stage breast cancer. Breast Cancer Research and Treatment. 2010;122:859–65. doi: 10.1007/s10549-010-0732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson K, Patterson RE, Flatt SW, et al. Clinically defined type 2 diabetes mellitus and prognosis in early-stage breast cancer. J Clin Oncol. 2011;29:7–10. doi: 10.1200/JCO.2010.29.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolever TM, Jenkins DJ. The use of the glycemic index in predicting the blood glucose response to mixed meals. Am J Clin Nutr. 1986;43:167–72. doi: 10.1093/ajcn/43.1.167. [DOI] [PubMed] [Google Scholar]
- 11.Barclay AW, Petocz P, McMillan-Price J, et al. Glycemic index, glycemic load, and chronic disease risk--a meta-analysis of observational studies. Am J Clin Nutr. 2008;87:627–37. doi: 10.1093/ajcn/87.3.627. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins DJ, Wolever TM, Collier GR, et al. Metabolic effects of a low-glycemic-index diet. Am J Clin Nutr. 1987;46:968–75. doi: 10.1093/ajcn/46.6.968. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins DJ, Kendall CW, Augustin LS, et al. Glycemic index: overview of implications in health and disease. Am J Clin Nutr. 2002;76:266S–73S. doi: 10.1093/ajcn/76/1.266S. [DOI] [PubMed] [Google Scholar]
- 14.Pollak MN. Insulin, insulin-like growth factors, insulin resistance, and neoplasia. Am J Clin Nutr. 2007;86:820s–1s. doi: 10.1093/ajcn/86.3.820S. [DOI] [PubMed] [Google Scholar]
- 15.Parekh N, Okada T, Lu-Yao G. Obesity, insulin resistance and cancer prognosis: Implications for practice for providing care among cancer survivors. J Am Diet Assoc. 2009;109:1346–53. doi: 10.1016/j.jada.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dossus L, Kaaks R. Nutrition, metabolic factors and cancer risk. Best Practice & Research Clinical Endocrinology & Metabolism. 2008;22:551–71. doi: 10.1016/j.beem.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 17.McEligot AJ, Largent J, Ziogas A, Peel D, Anton-Culver H. Dietary fat, fiber, vegetable, and micronutrients are associated with overall survival in postmenopausal women diagnosed with breast cancer. Nutr Cancer. 2006;55:132–40. doi: 10.1207/s15327914nc5502_3. [DOI] [PubMed] [Google Scholar]
- 18.Holmes MD, Chen WY, Hankinson SE, Willett WC. Physical activity's impact on the association of fat and fiber intake with survival after breast cancer. Am J Epidemiol. 2009;170:1250–6. doi: 10.1093/aje/kwp291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pierce JP, Natarajan L, Caan BJ, et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: The Women's Healthy Eating and Living (WHEL) Randomized Trial. JAMA. 2007;298:289–98. doi: 10.1001/jama.298.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borugian MJ, Sheps SB, Kim-Sing C, et al. Insulin, macronutrient intake, and physical activity: are potential indicators of insulin resistance associated with mortality from breast cancer? Cancer Epidemiol Biomarkers Prev. 2004;13:1163–72. [PubMed] [Google Scholar]
- 21.Wen W, Shu XO, Li H, et al. Dietary carbohydrates, fiber, and breast cancer risk in Chinese women. Am J Clin Nutr. 2009;89:283–9. doi: 10.3945/ajcn.2008.26356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linos E, Willett WC, Cho E, Frazier L. Adolescent diet in relation to breast cancer risk among premenopausal women. Cancer Epidemiol Biomarkers Prev. 2010;19:689–96. doi: 10.1158/1055-9965.EPI-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gnagnarella P, Gandini S, La Vecchia C, Maisonneuve P. Glycemic index, glycemic load, and cancer risk: a meta-analysis. Am J Clin Nutr. 2008;87:1793–801. doi: 10.1093/ajcn/87.6.1793. [DOI] [PubMed] [Google Scholar]
- 24.McTiernan A, Rajan B, Tworoger S, et al. Adiposity and sex hormones in postmenopausal breast cancer survivors. J Clin Oncol. 2003;21:1961–6. doi: 10.1200/JCO.2003.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neuhouser ML, Nojomi M, Baumgartner RN, et al. Dietary fat, tamoxifen use and circulating sex hormones in postmenopausal breast cancer survivors. Nutr Cancer. 2010;62:164–74. doi: 10.1080/01635580903305359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prentice RL, Caan B, Chlebowski RT, et al. Low-fat dietary pattern and risk of invasive breast cancer: the Women's Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:629–42. doi: 10.1001/jama.295.6.629. [DOI] [PubMed] [Google Scholar]
- 27.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 28.Neuhouser ML, Tinker L, Thomson C, et al. Development of a glycemic index database for food frequency questionnaires used in epidemiologic studies. J Nutr. 2006;136:1604–9. doi: 10.1093/jn/136.6.1604. [DOI] [PubMed] [Google Scholar]
- 29.Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- 30.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 31.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–8S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 32.Kleinbaum DG, Klein M. Statistics for Biology and Health. New York: Springer; 2005. Survival analysis: a self-learning text. [Google Scholar]
- 33.Jonas CR, McCullough ML, Teras LR, Walker-Thurmond KA, Thun MJ, Calle EE. Dietary glycemic index, glycemic load, and risk of incident breast cancer in postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2003;12:573–7. [PubMed] [Google Scholar]
- 34.Higginbotham S, Zhang ZF, Lee IM, Cook NR, Buring JE, Liu S. Dietary glycemic load and breast cancer risk in the Women's Health Study. Cancer Epidemiol Biomarkers Prev. 2004;13:65–70. doi: 10.1158/1055-9965.epi-03-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCann SE, McCann WE, Hong CC, et al. Dietary patterns related to glycemic index and load and risk of premenopausal and postmenopausal breast cancer in the Western New York Exposure and Breast Cancer Study. Am J Clin Nutr. 2007;86:465–71. doi: 10.1093/ajcn/86.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giles GG, Simpson JA, English DR, et al. Dietary carbohydrate, fibre, glycaemic index, glycaemic load and the risk of postmenopausal breast cancer. Int J Cancer. 2006;118:1843–7. doi: 10.1002/ijc.21548. [DOI] [PubMed] [Google Scholar]
- 37.Ma Y, Griffith JA, Chasan-Taber L, et al. Association between dietary fiber and serum C-reactive protein. Am J Clin Nutr. 2006;83:760–6. doi: 10.1093/ajcn/83.4.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pierce BL, Neuhouser ML, Wener MH, et al. Correlates of circulating C-reactive protein and serum amyloid A concentrations in breast cancer survivors. Breast Cancer Research and Treatment. 2009;114:155–67. doi: 10.1007/s10549-008-9985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rock CL, Flatt SW, Thomson CA, et al. Effects of a high-fiber, low-fat diet intervention on serum concentrations of reproductive steroid hormones in women with a history of breast cancer. J Clin Oncol. 2004;22:2379–87. doi: 10.1200/JCO.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 40.Morisset AS, Blouin K, Tchernof A. Impact of diet and adiposity on circulating levels of sex hormone-binding globulin and androgens. Nutr Rev. 2008;66:506–16. doi: 10.1111/j.1753-4887.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 41.Galisteo M, Duarte J, Zarzuelo A. Effects of dietary fibers on disturbances clustered in the metabolic syndrome. J Nutr Biochem. 2008;19:71–84. doi: 10.1016/j.jnutbio.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 42.Vrolix R, Mensink RP. Variability of the glycemic response to single food products in healthy subjects. Contemporary Clinical Trials. 2010;31:5–11. doi: 10.1016/j.cct.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 43.Brand-Miller JC, Thomas M, Swan V, Ahmad ZI, Petocz P, Colagiuri S. Physiological validation of the concept of glycemic load in lean young adults. J Nutr. 2003;133:2728–32. doi: 10.1093/jn/133.9.2728. [DOI] [PubMed] [Google Scholar]
- 44.Willett WC. Nutritional Epidemiology. 2nd. New York: Oxford University Press; 1998. Correction of effects of measurement error; pp. 302–20. [Google Scholar]
- 45.Willett W. Nutritional Epidemiology. New York: Oxford Universal Press; 1998. [Google Scholar]


