Abstract
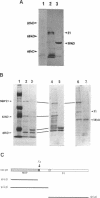
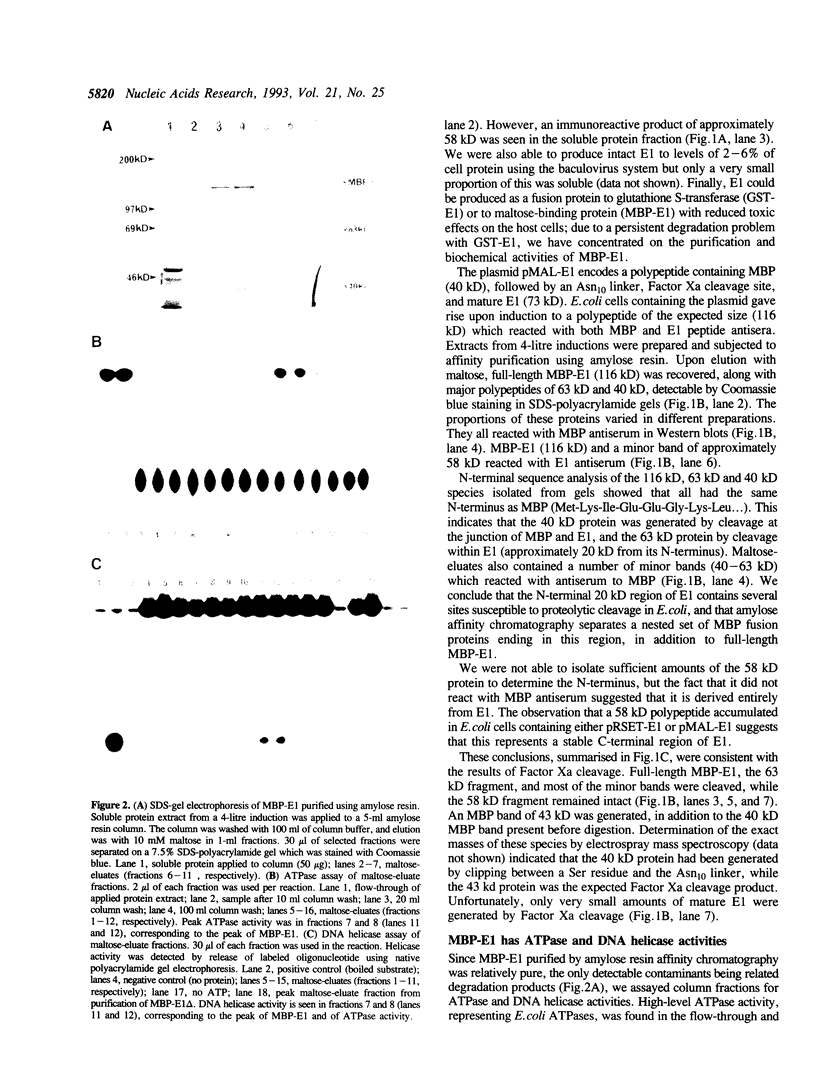
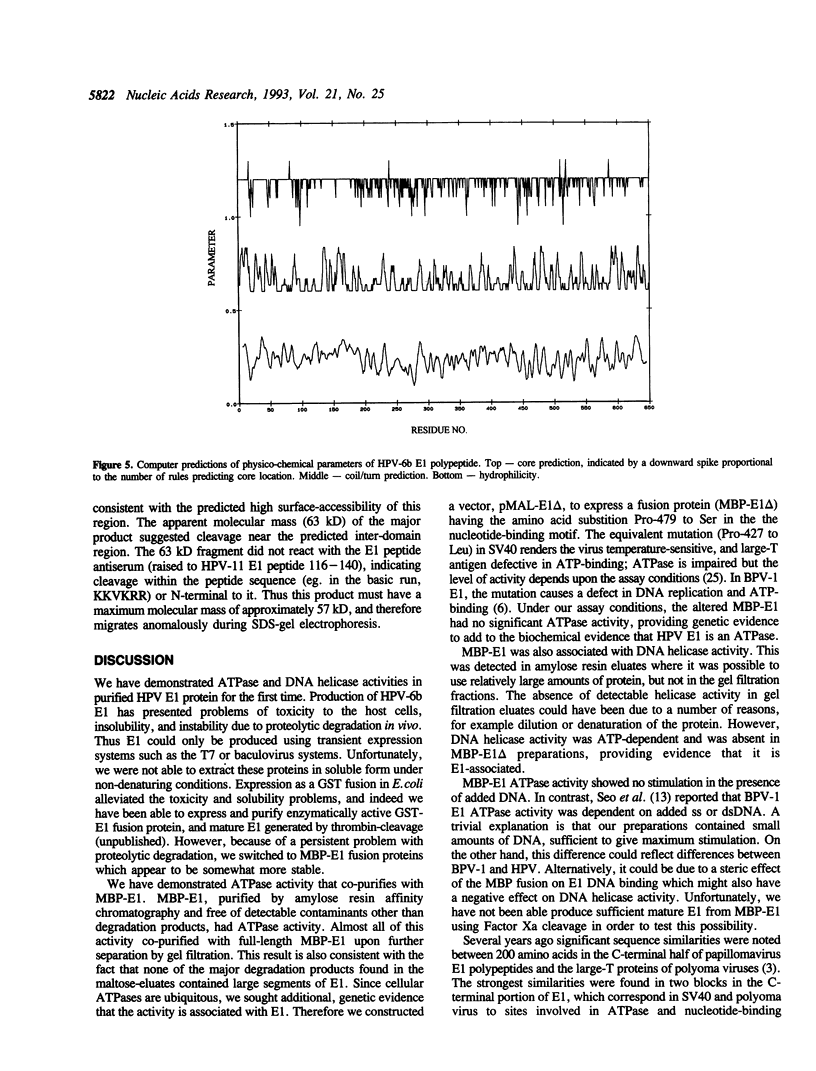
Replication of human papillomavirus (HPV) DNA requires the viral proteins E1 and E2. Amino acid similarities to SV40 large-T antigen had suggested that E1 is a DNA helicase/ATPase involved in initiating viral DNA replication, and this has recently been shown for bovine papillomavirus type 1 (BPV-1) E1 protein. However, in vitro analysis of HPV E1 has been hampered by the inability to produce purified protein using heterologous expression systems. We have succeeded in demonstrating ATPase and DNA helicase activities in purified HPV E1, expressed in E. coli as a maltose-binding protein fusion (MBP-E1), for the first time. As further confirmation that the ATPase and DNA helicase activities are due to E1 and not contaminating E. coli enzymes, we have shown that a fusion protein containing an amino acid change (E1 Pro-479 to Ser), predicted to inactivate ATP-binding, has impaired activities. We have carried out a structure prediction analysis which suggests that E1 may form two domains: a relatively open N-terminal domain (residues 1-125), and a highly structured C-terminal domain (170-649), with an intermediate region (125-170) predicted to form an inter-domain linker. This is consistent with the proteolytic susceptibility of MBP-E1 at a site 15-20 kD from the N-terminus of E1, and the accumulation of a 58 kD C-terminal fragment of E1. We speculate that the N-terminal domain is involved in DNA-binding, while the C-terminal 58 kD may constitute a distinct enzymatic domain. HPV E1 is of interest as a therapeutic target and the availability of pure enzyme will be invaluable in the search for antiviral compounds.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blitz I. L., Laimins L. A. The 68-kilodalton E1 protein of bovine papillomavirus is a DNA binding phosphoprotein which associates with the E2 transcriptional activator in vitro. J Virol. 1991 Feb;65(2):649–656. doi: 10.1128/jvi.65.2.649-656.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bream G. L., Ohmstede C. A., Phelps W. C. Characterization of human papillomavirus type 11 E1 and E2 proteins expressed in insect cells. J Virol. 1993 May;67(5):2655–2663. doi: 10.1128/jvi.67.5.2655-2663.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C. M., Dong G., Broker T. R., Chow L. T. Control of human papillomavirus type 11 origin of replication by the E2 family of transcription regulatory proteins. J Virol. 1992 Sep;66(9):5224–5231. doi: 10.1128/jvi.66.9.5224-5231.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C. M., Ustav M., Stenlund A., Ho T. F., Broker T. R., Chow L. T. Viral E1 and E2 proteins support replication of homologous and heterologous papillomaviral origins. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5799–5803. doi: 10.1073/pnas.89.13.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clertant P., Seif I. A common function for polyoma virus large-T and papillomavirus E1 proteins? Nature. 1984 Sep 20;311(5983):276–279. doi: 10.1038/311276a0. [DOI] [PubMed] [Google Scholar]
- Danos O., Giri I., Thierry F., Yaniv M. Papillomavirus genomes: sequences and consequences. J Invest Dermatol. 1984 Jul;83(1 Suppl):7s–11s. doi: 10.1111/1523-1747.ep12281115. [DOI] [PubMed] [Google Scholar]
- Del Vecchio A. M., Romanczuk H., Howley P. M., Baker C. C. Transient replication of human papillomavirus DNAs. J Virol. 1992 Oct;66(10):5949–5958. doi: 10.1128/jvi.66.10.5949-5958.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissmann L. Human papillomaviruses and genital cancer. Semin Cancer Biol. 1992 Oct;3(5):253–261. [PubMed] [Google Scholar]
- Lambert P. F. Papillomavirus DNA replication. J Virol. 1991 Jul;65(7):3417–3420. doi: 10.1128/jvi.65.7.3417-3420.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh J. L., Erfle M., Wykes E. J. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene. 1984 Dec;32(3):481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- Mohr I. J., Clark R., Sun S., Androphy E. J., MacPherson P., Botchan M. R. Targeting the E1 replication protein to the papillomavirus origin of replication by complex formation with the E2 transactivator. Science. 1990 Dec 21;250(4988):1694–1699. doi: 10.1126/science.2176744. [DOI] [PubMed] [Google Scholar]
- Ray S., Anderson M. E., Loeber G., McVey D., Tegtmeyer P. Functional characterization of temperature-sensitive mutants of simian virus 40 large T antigen. J Virol. 1992 Nov;66(11):6509–6516. doi: 10.1128/jvi.66.11.6509-6516.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remm M., Brain R., Jenkins J. R. The E2 binding sites determine the efficiency of replication for the origin of human papillomavirus type 18. Nucleic Acids Res. 1992 Nov 25;20(22):6015–6021. doi: 10.1093/nar/20.22.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riveros-Moreno V., Beddell C., Moncada S. Nitric oxide synthase. Structural studies using anti-peptide antibodies. Eur J Biochem. 1993 Aug 1;215(3):801–808. doi: 10.1111/j.1432-1033.1993.tb18095.x. [DOI] [PubMed] [Google Scholar]
- Santucci S., Androphy E. J., Bonne-Andréa C., Clertant P. Proteins encoded by the bovine papillomavirus E1 open reading frame: expression in heterologous systems and in virally transformed cells. J Virol. 1990 Dec;64(12):6027–6039. doi: 10.1128/jvi.64.12.6027-6039.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E., Dürst M., Demankowski C., Lattermann O., Zech R., Wolfsperger E., Suhai S., zur Hausen H. DNA sequence and genome organization of genital human papillomavirus type 6b. EMBO J. 1983;2(12):2341–2348. doi: 10.1002/j.1460-2075.1983.tb01744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo Y. S., Müller F., Lusky M., Gibbs E., Kim H. Y., Phillips B., Hurwitz J. Bovine papilloma virus (BPV)-encoded E2 protein enhances binding of E1 protein to the BPV replication origin. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2865–2869. doi: 10.1073/pnas.90.7.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo Y. S., Müller F., Lusky M., Hurwitz J. Bovine papilloma virus (BPV)-encoded E1 protein contains multiple activities required for BPV DNA replication. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):702–706. doi: 10.1073/pnas.90.2.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stammers D. K., Tisdale M., Court S., Parmar V., Bradley C., Ross C. K. Rapid purification and characterisation of HIV-1 reverse transcriptase and RNaseH engineered to incorporate a C-terminal tripeptide alpha-tubulin epitope. FEBS Lett. 1991 Jun 3;283(2):298–302. doi: 10.1016/0014-5793(91)80613-8. [DOI] [PubMed] [Google Scholar]
- Sun S., Thorner L., Lentz M., MacPherson P., Botchan M. Identification of a 68-kilodalton nuclear ATP-binding phosphoprotein encoded by bovine papillomavirus type 1. J Virol. 1990 Oct;64(10):5093–5105. doi: 10.1128/jvi.64.10.5093-5105.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustav M., Stenlund A. Transient replication of BPV-1 requires two viral polypeptides encoded by the E1 and E2 open reading frames. EMBO J. 1991 Feb;10(2):449–457. doi: 10.1002/j.1460-2075.1991.tb07967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustav M., Ustav E., Szymanski P., Stenlund A. Identification of the origin of replication of bovine papillomavirus and characterization of the viral origin recognition factor E1. EMBO J. 1991 Dec;10(13):4321–4329. doi: 10.1002/j.1460-2075.1991.tb05010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson V. G., Ludes-Meyers J. A bovine papillomavirus E1-related protein binds specifically to bovine papillomavirus DNA. J Virol. 1991 Oct;65(10):5314–5322. doi: 10.1128/jvi.65.10.5314-5322.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Li R., Mohr I. J., Clark R., Botchan M. R. Activation of BPV-1 replication in vitro by the transcription factor E2. Nature. 1991 Oct 17;353(6345):628–632. doi: 10.1038/353628a0. [DOI] [PubMed] [Google Scholar]
- Yang L., Mohr I., Fouts E., Lim D. A., Nohaile M., Botchan M. The E1 protein of bovine papilloma virus 1 is an ATP-dependent DNA helicase. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5086–5090. doi: 10.1073/pnas.90.11.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]