Abstract
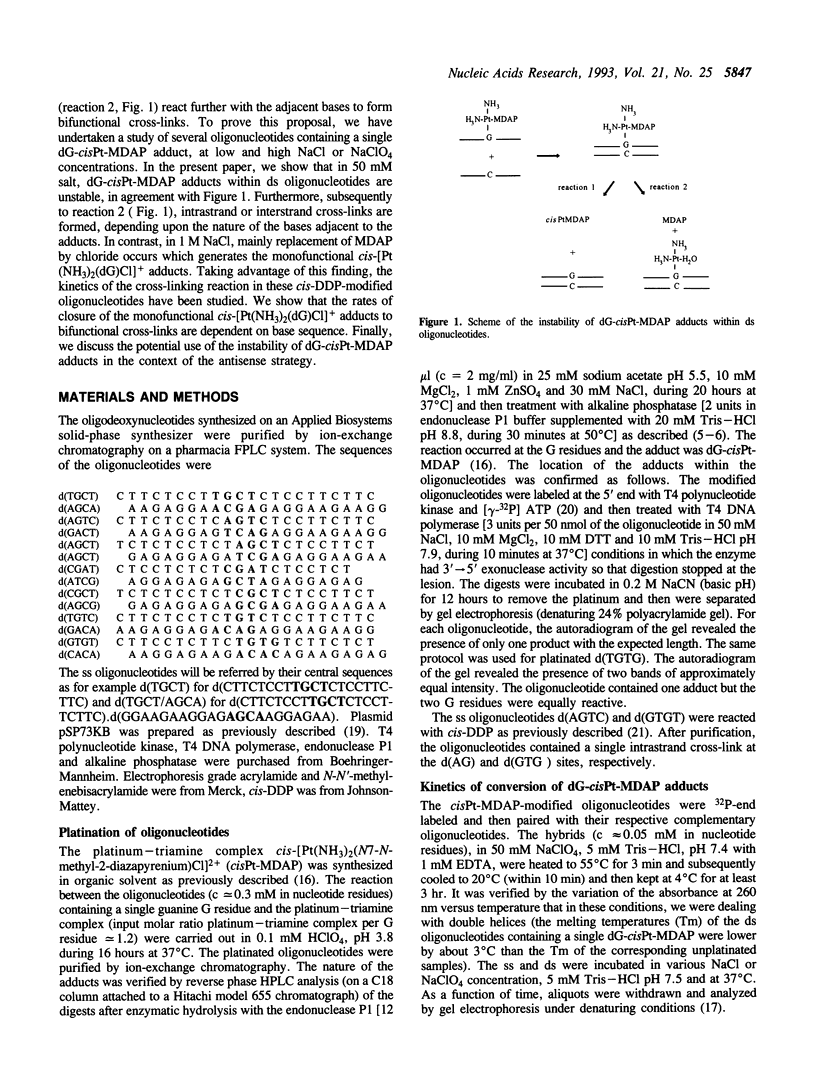
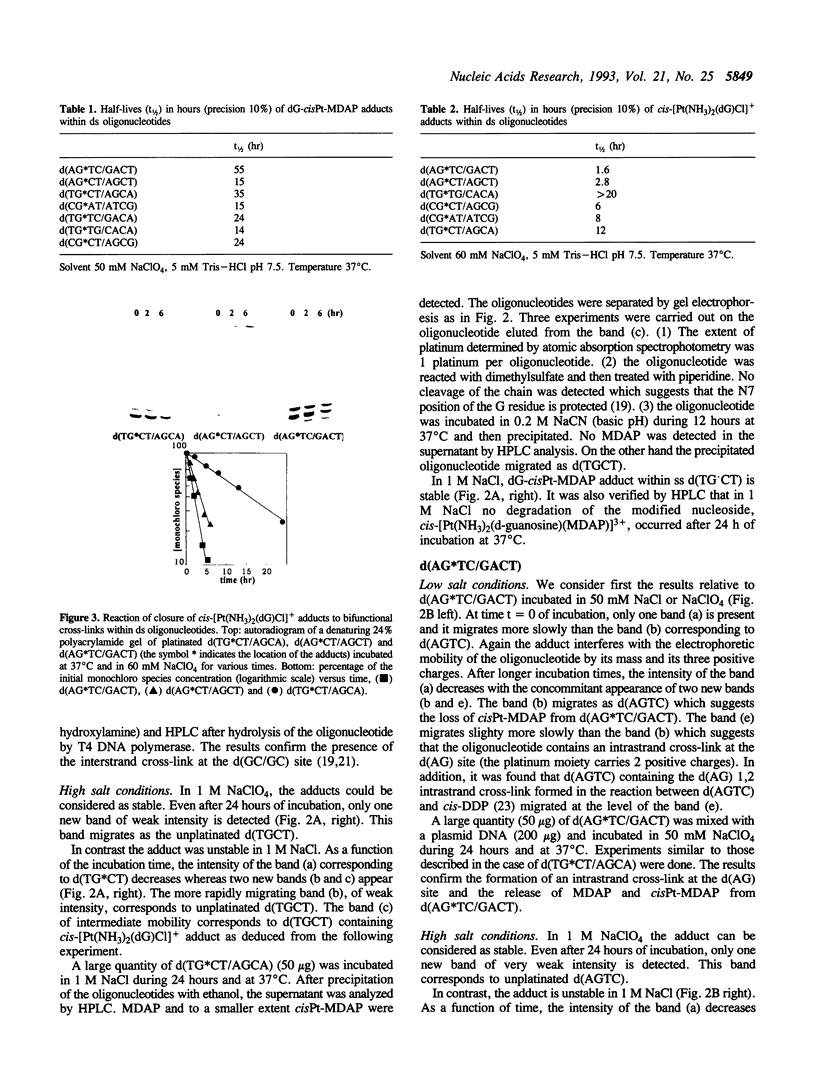
Single- and double-stranded oligonucleotides containing a single monofunctional cis-[Pt(NH3)2(dG)(N7-N-methyl-2-diazapyrenium)]3+ adduct have been studied at two NaCl concentrations. In 50 mM and 1 M NaCl, the adducts within the single-stranded oligonucleotides are stable. In contrast, they are unstable within the corresponding double-stranded oligonucleotides. In 50 mM NaCl, the bonds between platinum and guanine or N-methyl-2,7-diazapyrenium residues are cleaved and subsequently, intra- or interstrand cross-links are formed as in the reaction between DNA and cis-DDP. In 1 M NaCl, the main reaction is the replacement of N-methyl-2,7-diazapyrenium residues by chloride which generates double-stranded oligonucleotides containing a single monofunctional cis-[Pt(NH3)2(dG)Cl]+ adduct. The rates of closure of these monofunctional adducts to bifunctional cross-links have been studied in 60 mM NaClO4. Within d(TG.CT/AGCA), d(CG.CT/AGCG) and d(AG.CT/AGCT) (the symbol.indicates the location of the adducts in the central sequences of oligonucleotides), the half-lifes (t1/2) of the cis-[Pt(NH3)2(dG)Cl]+ adducts are respectively 12, 6 and 2.8 hr and the cross-linking reactions occur between guanine residues on the opposite strands. Within d(AG.TC/GACT), d(CG.AT/ATCG) and d(TGTG./CACA) or d(TG.TG/CACA) t1/2 are respectively 1.6, 8 and larger than 20 hr and the intrastrand cross-links are formed at the d(AG), d(GA) and d(GTG) sites, respectively. The conclusion is that the rates of conversion of cis-platinum-DNA monofunctional adducts to minor bifunctional cross-links are dependent on base sequence. The potential use of the instability of cis-[Pt(NH3)2(dG)(N7-N-methyl-2-diazapyrenium)]3+ adducts is discussed in the context of the antisense strategy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anin M. F., Gaucheron F., Leng M. Lability of monofunctional cis-platinum adducts: role of DNA double helix. Nucleic Acids Res. 1992 Sep 25;20(18):4825–4830. doi: 10.1093/nar/20.18.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernges F., Holler E. The reaction of platinum(II) complexes with DNA. Kinetics of intrastrand crosslink formation in vitro. Nucleic Acids Res. 1991 Apr 11;19(7):1483–1489. doi: 10.1093/nar/19.7.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabretta B. Inhibition of protooncogene expression by antisense oligodeoxynucleotides: biological and therapeutic implications. Cancer Res. 1991 Sep 1;51(17):4505–4510. [PubMed] [Google Scholar]
- Decoville M., Schwartz A., Locker D., Leng M. Detection of minor adducts in cisplatin-modified DNA by transcription footprinting. FEBS Lett. 1993 May 24;323(1-2):55–58. doi: 10.1016/0014-5793(93)81447-8. [DOI] [PubMed] [Google Scholar]
- Eastman A. Characterization of the adducts produced in DNA by cis-diamminedichloroplatinum(II) and cis-dichloro(ethylenediamine)platinum(II). Biochemistry. 1983 Aug 2;22(16):3927–3933. doi: 10.1021/bi00285a031. [DOI] [PubMed] [Google Scholar]
- Eastman A. Reevaluation of interaction of cis-dichloro(ethylenediamine)platinum(II) with DNA. Biochemistry. 1986 Jul 1;25(13):3912–3915. doi: 10.1021/bi00361a026. [DOI] [PubMed] [Google Scholar]
- Eastman A. The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacol Ther. 1987;34(2):155–166. doi: 10.1016/0163-7258(87)90009-x. [DOI] [PubMed] [Google Scholar]
- Fichtinger-Schepman A. M., van der Veer J. L., den Hartog J. H., Lohman P. H., Reedijk J. Adducts of the antitumor drug cis-diamminedichloroplatinum(II) with DNA: formation, identification, and quantitation. Biochemistry. 1985 Jan 29;24(3):707–713. doi: 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- Gaucheron F., Malinge J. M., Blacker A. J., Lehn J. M., Leng M. Possible catalytic activity of DNA in the reaction between the antitumor drug cis-diamminedichloroplatinum(II) and the intercalator N-methyl-2,7-diazapyrenium. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3516–3519. doi: 10.1073/pnas.88.9.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis L. S., Amundsen A. R., Stern E. W. Chemical and biological properties of a new series of cis-diammineplatinum(II) antitumor agents containing three nitrogen donors: cis-[Pt(NH3)2(N-donor)Cl]+. J Med Chem. 1989 Jan;32(1):128–136. doi: 10.1021/jm00121a024. [DOI] [PubMed] [Google Scholar]
- Hollis L. S., Sundquist W. I., Burstyn J. N., Heiger-Bernays W. J., Bellon S. F., Ahmed K. J., Amundsen A. R., Stern E. W., Lippard S. J. Mechanistic studies of a novel class of trisubstituted platinum(II) antitumor agents. Cancer Res. 1991 Apr 1;51(7):1866–1875. [PubMed] [Google Scholar]
- Hélène C., Toulmé J. J. Specific regulation of gene expression by antisense, sense and antigene nucleic acids. Biochim Biophys Acta. 1990 Jun 21;1049(2):99–125. doi: 10.1016/0167-4781(90)90031-v. [DOI] [PubMed] [Google Scholar]
- Lemaire M. A., Schwartz A., Rahmouni A. R., Leng M. Interstrand cross-links are preferentially formed at the d(GC) sites in the reaction between cis-diamminedichloroplatinum (II) and DNA. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1982–1985. doi: 10.1073/pnas.88.5.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonetti J. P., Degols G., Clarenc J. P., Mechti N., Lebleu B. Cell delivery and mechanisms of action of antisense oligonucleotides. Prog Nucleic Acid Res Mol Biol. 1993;44:143–166. doi: 10.1016/s0079-6603(08)60219-6. [DOI] [PubMed] [Google Scholar]
- Malinge J. M., Leng M. Reaction of nucleic acids and cis-diamminedichloroplatinum(II) in the presence of intercalating agents. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6317–6321. doi: 10.1073/pnas.83.17.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinge J. M., Schwartz A., Leng M. Characterization of the ternary complexes formed in the reaction of cis-diamminedichloroplatinum (II), ethidium bromide and nucleic acids. Nucleic Acids Res. 1987 Feb 25;15(4):1779–1797. doi: 10.1093/nar/15.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinge J. M., Sip M., Blacker A. J., Lehn J. M., Leng M. Formation of a DNA monofunctional cis-platinum adduct cross-linking the intercalating drug N-methyl-2,7-diazapyrenium. Nucleic Acids Res. 1990 Jul 11;18(13):3887–3891. doi: 10.1093/nar/18.13.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrot L., Leng M. Chemical probes of the conformation of DNA modified by cis-diamminedichloroplatinum(II). Biochemistry. 1989 Feb 21;28(4):1454–1461. doi: 10.1021/bi00430a005. [DOI] [PubMed] [Google Scholar]
- Marshall W. S., Caruthers M. H. Phosphorodithioate DNA as a potential therapeutic drug. Science. 1993 Mar 12;259(5101):1564–1570. doi: 10.1126/science.7681216. [DOI] [PubMed] [Google Scholar]
- Sip M., Schwartz A., Vovelle F., Ptak M., Leng M. Distortions induced in DNA by cis-platinum interstrand adducts. Biochemistry. 1992 Mar 10;31(9):2508–2513. doi: 10.1021/bi00124a010. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Cheng Y. C. Antisense oligonucleotides as therapeutic agents--is the bullet really magical? Science. 1993 Aug 20;261(5124):1004–1012. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]