Abstract
Obstructive sleep apnea (OSA) is a breathing disorder during sleep that has implications beyond disrupted sleep. It is increasingly recognized as an independent risk factor for cardiac, neurologic, and perioperative morbidities. Yet this disorder remains undiagnosed in a substantial portion of our population. It is imperative for all physicians to remain vigilant in identifying patients with signs and symptoms consistent with OSA. This review focuses on updates in the areas of terminology and testing, complications of untreated OSA, perioperative considerations, treatment options, and new developments in this field.
AASM = American Academy of Sleep Medicine; AHI = apnea-hypopnea index; APAP = automatically adjusting positive airway pressure; CMS= Center for Medicaid and Medicare Services; CompSAS = complex sleep apnea syndrome; CPAP = continuous positive airway pressure; OSA = obstructive sleep apnea; PAP = positive airway pressure; PSG = polysomnography; RDI = respiratory disturbance index
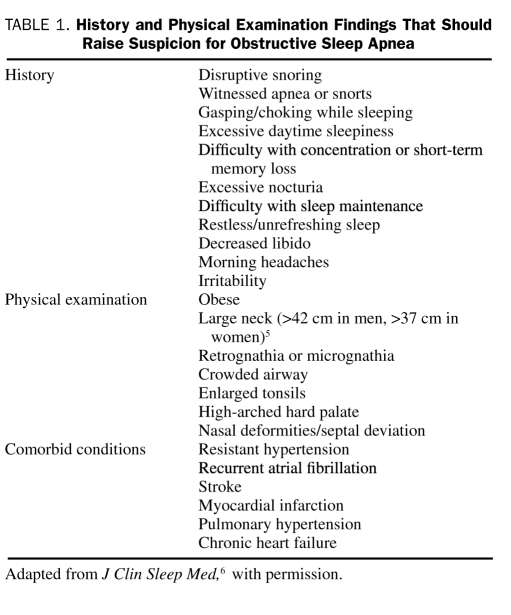
Obstructive sleep apnea (OSA) is a disorder in which a person frequently stops breathing during his or her sleep. It results from an obstruction of the upper airway during sleep that occurs because of inadequate motor tone of the tongue and/or airway dilator muscles. In the United States, the prevalence of OSA is estimated to be 3% to 7% in men and 2% to 5% in women.1 Among patients with a body mass index (calculated as the weight in kilograms divided by height in meters squared) greater than 28, OSA is present in 41%.2 The prevalence of OSA can be as high as 78% in morbidly obese patients who present for bariatric surgery.3 Up to 93% of women and 82% of men may have undiagnosed moderate to severe OSA,4 emphasizing the importance of vigilant evaluations for signs and symptoms of OSA. These may include a spouse's report of disruptive snoring, daytime sleepiness, obesity, and large neck circumference (>42 cm in men)5 (Table 1).6 Not all patients, however, present with typical findings. For example, patients with heart failure and OSA may not present with daytime sleepiness.7 Likewise, a patient may not be aware of snoring or apneic episodes. Thus, it is important to obtain collateral sleep history and recognize associated medical comorbid conditions that may implicate OSA as an underlying diagnosis. This article is intended as an update to the 2003 Concise Review for Clinicians covering OSA.8
TABLE 1.
History and Physical Examination Findings That Should Raise Suspicion for Obstructive Sleep Apnea
TERMINOLOGY AND TESTING
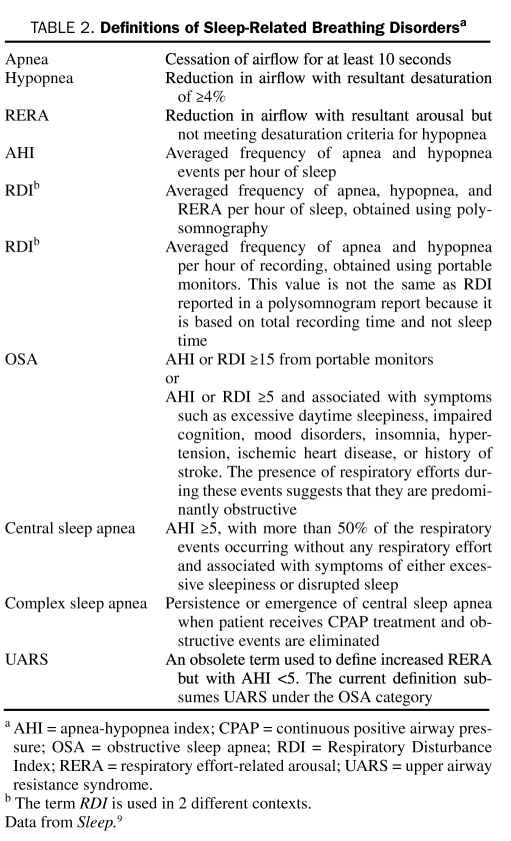
Diagnosis of OSA usually requires overnight polysomnography (PSG) to detect the frequency of apneic and hypopneic events9 (Table 2). Traditionally, this is done as a standardized, facility-based PSG, with multichannel recordings that help determine sleep time, sleep stages, respiratory effort, airflow, cardiac rhythm, oximetry, and limb movements.9 The apnea-hypopnea index (AHI) is the average number of disordered breathing events per hour. Other definitions of sleep-related breathing disorders are highlighted in Table 2. Typically, OSA syndrome is defined as an AHI of 5 or greater with associated symptoms (eg, excessive daytime sleepiness, fatigue, or impaired cognition) or an AHI of 15 or greater, regardless of associated symptoms.9
TABLE 2.
Definitions of Sleep-Related Breathing Disordersa
Overnight, facility-based, and attended PSG remains the criterion standard for diagnosis of OSA. Recently, the Center for Medicaid and Medicare Services (CMS) approved the use of portable PSG to diagnose OSA.10 The American Academy of Sleep Medicine (AASM) recommends considering this route in patients with a high pretest likelihood for moderate to severe OSA without other substantial comorbid conditions.11 The portable monitors include at least 3 sensors that detect respiratory events in the home setting. Because these monitors cannot determine the actual sleep time, AHI cannot be determined (because AHI is an index of apnea and hypopnea per hour of sleep). Rather, the resultant index is known as the respiratory disturbance index (RDI), which represents the frequency of apnea and hypopnea per hour of recording time. Because the total recording time often exceeds the actual sleep time of the patient, RDI from portable monitors often underrepresents the severity of OSA. Hence, a negative result from a portable monitor does not necessarily rule out OSA. Although any treating physician involved in the patient's care can order the study, CMS requires that an AASM-accredited laboratory conduct the study and that a board-certified sleep specialist interpret the results.10
Overnight oximetry is being considered as a surrogate for PSG.12,13 Sensitivity of an overnight oximetry for at least moderately severe OSA can be as high as 98%; however, the specificity can be as low as 40%, depending on how the data are interpreted.12 Therefore, overnight oximetry should only be used as a screening tool or for follow-up after initiation of continuous positive airway pressure (CPAP) therapy when coupled with formal clinical evaluation. In fact, CMS does not recognize the use of overnight oximetry alone in diagnosing OSA. Because of its low specificity, further testing is required for sleepy or otherwise symptomatic patients with a negative oximetry result.
Similarly, several prediction models and questionnaires have been proposed for diagnosing OSA,14 one of the most commonly used of which is the Berlin Questionnaire.15 Although the sensitivity of these questionnaires ranges from 80% to 90%, specificity can be as low as 34%, limiting the utility of this approach.14,16
CONSEQUENCES OF UNTREATED OSA
Untreated OSA is currently recognized as an independent risk factor for the development of certain comorbid conditions and mortality. Therefore, the primary care physician needs to consider OSA when assessing the cause and/or manageability of these diseases.
Cardiovascular System
A landmark study published in 2000 revealed OSA as an independent, dose-dependent risk factor for hypertension (adjusted odds ratio for even mild OSA, 2).17 Importantly, treatment of OSA with CPAP improved blood pressure control, even in those with resistant hypertension.18
Similarly, a large 10-year prospective study found that untreated severe OSA independently increased the odds of fatal and nonfatal cardiovascular events by 2.87 and 3.17, respectively.19 Lee et al20 reported the prevalence of OSA (defined by AHI ≥15) to be 65.7% among those admitted for an acute myocardial infarction. Severe OSA was associated with a 3- to 4-fold higher odds of having complex tachyarrhythmia, including atrial fibrillation and nonsustained ventricular tachycardia.21,22 If severe OSA was not treated, patients had a higher recurrence rate of atrial fibrillation, which was mitigated by CPAP use.23 For most of these associations, cardiovascular morbidity and mortality were strongest in those younger than 65 years.21 Use of CPAP mitigated these and other cardiovascular risks,19,24 emphasizing the importance of not only recognizing OSA but also maintaining close follow-up to ensure adherence to CPAP therapy.
Central Nervous System
Although the effect of OSA on cognitive impairment, hypersomnolence, and fatigue has long been known,25 emerging data strengthen the relationship between OSA and stroke. Moderately severe OSA (AHI >15) was associated with a higher incidence of stroke, independent of confounders (hazard ratio, 2.86-3.56).26,27 Sahlin et al28 found that the risk of early death increased by 75%, independent of confounders, in those who survived stroke and had moderately severe OSA vs those who did not have OSA. Fortunately, CPAP adherence can also mitigate this excess mortality in those who have had a stroke.29
An association between sleep apnea and epilepsy has been recognized since the early 1980s and appears to be the strongest among older adults who present with new-onset epilepsy.30-32 Prevalence of OSA among those with epilepsy can range from 10% to 45%.31 Seizure control can be improved with adequate treatment of coexisting OSA.32
A recent meta-analysis confirmed at least a 2-fold increased risk of motor vehicle accidents among those with OSA.33 As few as 2 days of CPAP use resulted in significant risk reduction.34 Therefore, recent recommendations to the regulators of commercial drivers include immediate disqualification if the driver has untreated OSA, is nonadherent to therapy, has experienced excessive sleepiness while driving, or has experienced a crash associated with falling asleep while driving.35 The Expert Panel also recommended that commercial drivers who are at increased risk of OSA should only be given conditional certification until they can be evaluated by a sleep specialist.35 Primary care physicians should be aware of these recommendations and inform patients who may be affected.
Endocrine System
Obstructive sleep apnea also appears to be an independent risk factor for insulin resistance and the development of diabetes. The Sleep Heart Health Study, which enrolled 2656 patients, showed that those with moderate to severe OSA were more likely to have an elevated fasting glucose level and 2-hour glucose tolerance (adjusted odds ratio, 1.46 and 1.44, respectively).36 Similarly, the Wisconsin Sleep Cohort, which enrolled 1387 patients, revealed that those with moderately severe OSA were more likely to have diabetes (odds ratio, 2.30).37 The benefits of CPAP treatment in patients with diabetes have not been extensively studied.
Perioperative Complicatons
Unrecognized OSA may lead to perioperative complications. These complications include difficult intubations,38 exaggerated respiratory depressions from anesthetics and analgesics,39 increased postoperative reintubations,40 cardiac dysrhythmias,41 and longer hospital stays.40 Therefore, the American Society of Anesthesiologists recommends early identification and appropriate preparation for perioperative management of patients with suspected OSA.42 Although it is unclear whether complications are decreased by preoperative and postoperative use of CPAP, the American Society of Anesthesiologists recommends its use during recovery if feasible.42
TREATMENT OPTIONS
The CPAP device is still the criterion standard for the treatment of OSA. It uses pressure to provide a pneumatic splint to maintain airway patency. Suboptimal patient adherence to CPAP has led to a number of advances in CPAP-related equipment and in other treatment options. Although CPAP use can decrease morbidity and mortality, close follow-up to ensure adherence is crucial in the management of not only OSA but also other comorbid conditions.
Recognizing that the standard CPAP mask, which covers the nose or the nose and mouth, may be the most cumbersome part of the treatment, manufacturers of the device currently offer more than 100 different mask options to customize the treatment. Nasal pillows, which insert into the nostrils only, are preferred by some patients with claustrophobia. Other available masks include those that have no attached straps, cover the mouth only, or cover the entire face, the last of which are counterintuitively preferred by some claustrophobic patients. Other styles can be worn like a hat, which may be helpful for those with finger dexterity limitations. For patients who require a full face mask (that covers the nose and mouth) but have problems with skin breakdown at the bridge of the nose, a mask that combines a mouth mask with nasal pillows is an option. Cloth masks made without any plastic are available for patients who are extremely sensitive to plastic material. With such a variety of mask options, a referral back to the sleep specialist is warranted if the patient is struggling with adherence.
Advances have also been made in the design of CPAP machines. Some systems have a heated wire integrated into the tubing to maximize humidity without causing excessive condensation. Machines are much smaller and quieter. The latest model is merely 1.4 kg (3 lbs) and generates less than 24 dB of noise, which is less than whisper-quiet conversation.
To help patients who struggle with the pressure generated by CPAP, machines can now temporarily decrease the pressure at the beginning of exhalation to facilitate easier respiratory cycles. These devices automatically increase the pressure back up to the preset pressure before exhalation is completed, thus allowing sufficient pressure to maintain airway patency. Similarly, automatically adjusting positive airway pressure (APAP), using a proprietary algorithm, will automatically increase and decrease pressure on the basis of identified respiratory events. The APAP device is particularly useful for patients who require higher pressures in the supine than in the nonsupine position. Rather than using 1 pressure, such devices automatically adjust the pressure to meet the patient's pressure requirements during any given body position. This often results in lower average pressure throughout the night, which may contribute to better tolerance. Although most postbariatric patients may not require CPAP, a large minority (>44%) have residual AHI greater than 10.43 In such patient groups, APAP may be beneficial because the required pressure will most likely decrease with weight loss. Bilevel positive airway pressure (PAP) devices allow for separate inspiratory and expiratory pressure settings that may be more comfortable for some patients. Although studies have not shown improvements in adherence,44 select patients seem to tolerate bilevel PAP better than CPAP, particularly when high pressures are required or if patients had a prolonged period of poor initial tolerance to CPAP treatment.45 In fact, AASM suggests bilevel PAP be used when CPAP pressure exceeds 15 cm H2O.46
Primary care physicians may be asked to certify CPAP adherence for patients covered by CMS. By CMS rules, adherence is defined as use of the CPAP device for at least 4 hours per night for at least 70% of the nights during any given consecutive 30-day period.10 Local vendors can download the integrated adherence data from the CPAP device. Patients have 90 days after CPAP issuance to meet these criteria. Therefore, we recommend a follow-up visit within the first 30 to 60 days to allow for additional intervention if a patient is not meeting adherence criteria.
Recognizing that PAP adherence can still be problematic, several investigators studied the use of a soporific. Adherence may be improved with the use of a benzodiazepine receptor agonist such as eszopiclone, particularly if baseline adherence is 4 hours or less.47 Other means of improving adherence include the addition of humidity, intensive education, close follow-up, and treatment of nasal congestion.
For persistently nonadherent patients with lower AHI, predominantly supine OSA, lower body mass index, or with certain facial and airway features (quantifiable by a qualified dental specialist), an oral appliance may be an option.48 These custom-made devices protrude the lower jaw forward to thrust the tongue base forward, thereby enlarging the retropharyngeal region. Success rates of oral appliances are 30% to 80%, depending on the selection criteria, definition of success, and the device used.49 Of the many devices available, we recommend types that are adjustable. Positional therapy is another option for those who experience mild OSA predominantly while sleeping on their back. This approach uses a barrier, such as a body pillow or tennis balls in a t-shirt, to prevent supine sleep. Although such an approach may be effective temporarily, overall adherence has been disappointing.50
Surgical options are also available, including removal of tissue from the posterior pharyngeal region (eg, uvulopalatopharyngoplasty) and maxillary-mandibular advancement, in which both the maxilla and the mandible are surgically advanced, thereby permanently enlarging the posterior pharyngeal region. Success rates of upper airway surgery vary from 24% to 86%, depending on severity, patient selection, definition of success, and type of surgery performed.51,52 Typically, a higher success rate is achieved if multilevel surgery is performed (eg, uvulopalatopharyngoplasty followed by maxillary-mandibular advancement).52 The last surgical option is a tracheostomy.
The AASM has published practice parameters for additional medical therapies.53 Neither medication nor oxygen therapy is recommended for primary treatment of OSA. Exceptions are adjunctive uses of a stimulant therapy with modafinil in those who remain adherent to OSA treatment but have residual sleepiness without any other identifiable cause and topical nasal corticosteroids in those with concurrent rhinitis. Positional therapy (in which some barrier is used to minimize supine sleep) may be acceptable as an adjunctive or secondary therapy option in those who have respiratory events predominantly in the supine position only.
NEW DEVELOPMENTS
Some patients who appear to have OSA during the diagnostic test develop central sleep apnea on CPAP initiation (Table 2). The incidence of this form of atypical apnea, known as Complex Sleep Apnea Syndrome (CompSAS), is 10% to 20%.54 Patients with CompSAS tolerate CPAP very poorly because of increased sleep disruptions resulting from central sleep apnea events. Although some of those with CompSAS can eventually be treated with CPAP, up to 50% will require the use of a new PAP device known as the adaptive servo-ventilator.55 Because CompSAS is difficult to diagnose and treat, patients with suspected CompSAS should be referred to a sleep center for further evaluation and treatment.
Recently, a new treatment device has undergone a multicenter trial to assess efficacy. Rather than using a machine to generate PAP, Provent uses a 1-way valve to maintain a constant pressure in the posterior pharyngeal region.56 The attractive feature of this device is its simplicity: it is a tape-like device worn over the nostrils nightly and does not require any tubing or electricity. This product has been in the European market and has received approval by the US Food and Drug Administration; however, the results of a recently completed double-blind, prospective, multicenter trial have not yet been published.
Other novel therapy devices are currently being tested but are not yet ready to be marketed.
CONCLUSION
The diagnosis and treatment of OSA have been facilitated by continuous and ongoing advances in this evolving field. It is important to recognize that OSA independently affects morbidity and mortality if left untreated. Primary care physicians can assist in ensuring that OSA is properly diagnosed and treated by being more aware of this disorder and its effect on the overall health of their patients. This disorder should be suspected in obese or somnolent patients or in those who snore. Patients with OSA may also present with resistant hypertension, recurrent atrial fibrillation, or stroke or may have elevated fasting glucose values. Screening with overnight oximetry may indicate severity but is insufficient to diagnose OSA. Overnight PSG, whether facility-based or portable, is required for appropriate diagnosis. Once diagnosed, CPAP initiation is the criterion standard of treatment; however, in those with mild OSA, an oral appliance may be acceptable. Addition of humidity, nasal corticosteroids, different interface options, and/or different devices may be helpful for patients who struggle with adherence. To facilitate these interventions, patients may need referral back to the vendor or a sleep center, especially because 10% to 20% may have CompSAS, which requires an entirely different approach to treatment. Some patients may need an ear, nose, and throat or an orthodontic consultation for an evaluation of a surgical intervention if other attempts have failed. Regardless of the treatment approach, obese patients should be counseled on weight loss. Patients who are struggling to lose weight and whose obesity is causing medical complications may be considered for bariatric intervention. Novel treatment options offer alternatives to improve adherence and thus reduce the burden of OSA.
Supplementary Material
On completion of this article, you should be able to (1) identify patients who may have obstructive sleep apnea (OSA), (2) recognize the implications of untreated OSA, and (3) review and apply different treatment options for management of OSA.
CME Questions About Obstructive Sleep Apnea
-
A 67-year-old man presents for a follow-up of difficult-to-control hypertension. He does not have a bed partner and is unaware if he snores. He denies snort arousals. His medical history includes recent myocardial infarction and stroke. He admits to some daytime sleepiness, readily falling asleep when he sits down to read or watch television. He is obese (body mass index, 42). His oral pharynx is very crowded, and his neck circumference is 46 cm. Which one of the following is the next best step in the diagnostic evaluation of this patient?
Overnight oximetry
Berlin Questionnaire
Overnight polysomnography (PSG)
Empirical initiation of automatically adjusting positive airway pressure (APAP)
Initiation of therapy with a stimulant such as modafinil
-
Which one of the following is not associated with obstructive sleep apnea (OSA)?
Myocardial infarction
Pericardial effusion
Hypertension
Stroke
Insulin resistance
-
Which one of the following perioperative complications is not associated with untreated or unrecognized OSA?
Cardiac dysrhythmias
Difficult endotracheal intubation
Increased hospital length of stay
Increased postoperative endotracheal reintubations
Resistance to anesthetics
-
Which one of the following is the most efficacious treatment for OSA?
Continuous positive airway pressure (CPAP)
Bariatric surgery
Uvulopalatopharyngoplasty
Maxillary-mandibular advancement
Oral appliance
-
Which one of the following is not an option when a patient is having difficulty with CPAP adherence?
Supplemental nocturnal oxygen
Heated humidity
Changing to a different style of mask
Consideration of different machine options
Referral back to the local sleep medicine clinic
This activity was designated for 1 AMA PRA Category 1 Credit(s).™
Because the Concise Review for Clinicians contributions are now a CME activity, the answers to the questions will no longer be published in the print journal. For CME credit and the answers, see the link on our Web site at mayoclinicproceedings.com.
REFERENCES
- 1. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vgontzas AN, Tan TL, Bixler EO, Martin LF, Shubert D, Kales A. Sleep apnea and sleep disruption in obese patients. Arch Intern Med. 1994;154:1705-1711 [PubMed] [Google Scholar]
- 3. Lopez PP, Stefan B, Schulman CI, Byers PM. Prevalence of sleep apnea in morbidly obese patients who presented for weight loss surgery evaluation: more evidence for routine screening for obstructive sleep apnea before weight loss surgery. Am Surg. 2008;74:834-838 [PubMed] [Google Scholar]
- 4. Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705-706 [DOI] [PubMed] [Google Scholar]
- 5. Dancey DR, Hanly PJ, Soong C, Lee B, Shepard J, Jr, Hoffstein V. Gender differences in sleep apnea: the role of neck circumference. Chest. 2003;123:1544-1550 [DOI] [PubMed] [Google Scholar]
- 6. Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276 [PMC free article] [PubMed] [Google Scholar]
- 7. Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Arch Intern Med. 2006;166:1716-1722 [DOI] [PubMed] [Google Scholar]
- 8. Olson EJ, Moore WR, Morgenthaler TI, Gay PC, Staats BA. Obstructive sleep apnea-hypopnea syndrome. Mayo Clin Proc. 2003;78:1545-1552 [DOI] [PubMed] [Google Scholar]
- 9. American Academy of Sleep Medicine. European Respiratory Society. Australasian Sleep Association. American Thoracic Society Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research: the report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667-689 [PubMed] [Google Scholar]
- 10. National Government Services, Inc. LCD for positive airway pressure (PAP) devices for the treatment of obstructive sleep apnea (L27230): American Medical Association; Center for Medicaid and Medicare; 2010. http://www.nationwidemedical.com/wp-content/uploads/2010/06/LCD-for-Positive-Airway-Pressure-doc-region-b.pdf Accessed March 22, 2011 [Google Scholar]
- 11. Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients: Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737-747 [PMC free article] [PubMed] [Google Scholar]
- 12. Magalang UJ, Dmochowski J, Veeramachaneni S, et al. Prediction of the apnea-hypopnea index from overnight pulse oximetry. Chest. 2003;124:1694-1701 [DOI] [PubMed] [Google Scholar]
- 13. Lin CL, Yeh C, Yen CW, Hsu WH, Hang LW. Comparison of the indices of oxyhemoglobin saturation by pulse oximetry in obstructive sleep apnea hypopnea syndrome. Chest. 2009;135:86-93 [DOI] [PubMed] [Google Scholar]
- 14. Rowley JA, Aboussouan LS, Badr MS. The use of clinical prediction formulas in the evaluation of obstructive sleep apnea. Sleep. 2000;23:929-938 [DOI] [PubMed] [Google Scholar]
- 15. Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485-491 [DOI] [PubMed] [Google Scholar]
- 16. Strauss RS, Browner WS. Risk for obstructive sleep apnea. Ann Intern Med. 2000;132:758-759 [DOI] [PubMed] [Google Scholar]
- 17. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378-1384 [DOI] [PubMed] [Google Scholar]
- 18. Dernaika TA, Kinasewitz GT, Tawk MM. Effects of nocturnal continuous positive airway pressure therapy in patients with resistant hypertension and obstructive sleep apnea. J Clin Sleep Med. 2009;5:103-107 [PMC free article] [PubMed] [Google Scholar]
- 19. Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046-1053 [DOI] [PubMed] [Google Scholar]
- 20. Lee CH, Khoo SM, Tai BC, et al. Obstructive sleep apnea in patients admitted for acute myocardial infarction: prevalence, predictors, and effect on microvascular perfusion. Chest. 2009;135:1488-1495 [DOI] [PubMed] [Google Scholar]
- 21. Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the risk of incident atrial fibrillation. J Am Coll Cardiol. 2007;49:565-571 [DOI] [PubMed] [Google Scholar]
- 22. Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2006;173:910-916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589-2594 [DOI] [PubMed] [Google Scholar]
- 24. Abe H, Takahashi M, Yaegashi H, et al. Efficacy of continuous positive airway pressure on arrhythmias in obstructive sleep apnea patients. Heart Vessels. 2010;25:63-69 [DOI] [PubMed] [Google Scholar]
- 25. Verstraeten E. Neurocognitive effects of obstructive sleep apnea syndrome. Curr Neurol Neurosci Rep. 2007;7:161-166 [DOI] [PubMed] [Google Scholar]
- 26. Valham F, Mooe T, Rabben T, Stenlund H, Wiklund U, Franklin KA. Increased risk of stroke in patients with coronary artery disease and sleep apnea: a 10-year follow-up. Circulation. 2008;118:955-960 [DOI] [PubMed] [Google Scholar]
- 27. Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269-277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sahlin C, Sandberg O, Gustafson Y, et al. Obstructive sleep apnea is a risk factor for death in patients with stroke: a 10-year follow-up. Arch Intern Med. 2008;168:297-301 [DOI] [PubMed] [Google Scholar]
- 29. Martinez-Garcia MA, Soler-Cataluna JJ, Ejarque-Martinez L, et al. Continuous positive airway pressure treatment reduces mortality in patients with ischemic stroke and obstructive sleep apnea: a 5-year follow-up study. Am J Respir Crit Care Med. 2009;180:36-41 [DOI] [PubMed] [Google Scholar]
- 30. Wyler AR, Weymuller EA., Jr Epilepsy complicated by sleep apnea. Ann Neurol. 1981;9:403-404 [DOI] [PubMed] [Google Scholar]
- 31. Manni R, Terzaghi M, Arbasino C, Sartori I, Galimberti CA, Tartara A. Obstructive sleep apnea in a clinical series of adult epilepsy patients: frequency and features of the comorbidity. Epilepsia. 2003;44:836-840 [DOI] [PubMed] [Google Scholar]
- 32. Malow BA, Levy K, Maturen K, Bowes R. Obstructive sleep apnea is common in medically refractory epilepsy patients. Neurology. 2000;55:1002-1007 [DOI] [PubMed] [Google Scholar]
- 33. Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5:573-581 [PMC free article] [PubMed] [Google Scholar]
- 34. Tregear S, Reston J, Schoelles K, Phillips B. Continuous positive airway pressure reduces risk of motor vehicle crash among drivers with obstructive sleep apnea: systematic review and meta-analysis. Sleep. 2010;33:1373-1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ancoli-Israel S, Czeisler CA, George CFP, Guilleminault C, Pack AI. Expert Panel Recommendations: Obstrucitve Sleep Apnea and Commercial Motor Vehicle Driver Safety. Washington, DC: US Department of Transportation; 2008. http://www.fmcsa.dot.gov/rules-regulations/TOPICS/mep/report/Sleep-MEP-Panel-Recommendations-508.pdf Accessed March 22, 2011 [Google Scholar]
- 36. Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521-530 [DOI] [PubMed] [Google Scholar]
- 37. Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590-1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Siyam MA, Benhamou D. Difficult endotracheal intubation in patients with sleep apnea syndrome [table of contents]. Anesth Analg. 2002;95:1098-1102 [DOI] [PubMed] [Google Scholar]
- 39. Chung SA, Yuan H, Chung F. A systemic review of obstructive sleep apnea and its implications for anesthesiologists. Anesth Analg. 2008;107:1543-1563 [DOI] [PubMed] [Google Scholar]
- 40. Gupta RM, Parvizi J, Hanssen AD, Gay PC. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case-control study. Mayo Clin Proc. 2001;76:897-905 [DOI] [PubMed] [Google Scholar]
- 41. Mooe T, Gullsby S, Rabben T, Eriksson P. Sleep-disordered breathing: a novel predictor of atrial fibrillation after coronary artery bypass surgery. Coron Artery Dis. 1996;7:475-478 [PubMed] [Google Scholar]
- 42. Gross JB, Bachenberg KL, Benumof JL, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2006;104:1081-1093 [DOI] [PubMed] [Google Scholar]
- 43. Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122:535-542 [DOI] [PubMed] [Google Scholar]
- 44. Gay PC, Herold DL, Olson EJ. A randomized, double-blind clinical trial comparing continuous positive airway pressure with a novel bilevel pressure system for treatment of obstructive sleep apnea syndrome. Sleep. 2003;26:864-869 [DOI] [PubMed] [Google Scholar]
- 45. Ballard RD, Gay PC, Strollo PJ. Interventions to improve compliance in sleep apnea patients previously non-compliant with continuous positive airway pressure. J Clin Sleep Med. 2007;3:706-712 [PMC free article] [PubMed] [Google Scholar]
- 46. Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4:157-171 [PMC free article] [PubMed] [Google Scholar]
- 47. Lettieri CJ, Shah AA, Holley AB, Kelly WF, Chang AS, Roop SA. Effects of a short course of eszopiclone on continuous positive airway pressure adherence: a randomized trial. Ann Intern Med. 2009;151:696-702 [DOI] [PubMed] [Google Scholar]
- 48. Schmidt-Nowara W, Lowe A, Wiegand L, Cartwright R, Perez-Guerra F, Menn S. Oral appliances for the treatment of snoring and obstructive sleep apnea: a review. Sleep. 1995;18:501-510 [DOI] [PubMed] [Google Scholar]
- 49. Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29:244-262 [DOI] [PubMed] [Google Scholar]
- 50. Bignold JJ, Deans-Costi G, Goldsworthy MR, et al. Poor long-term patient compliance with the tennis ball technique for treating positional obstructive sleep apnea. J Clin Sleep Med. 2009;5:428-430 [PMC free article] [PubMed] [Google Scholar]
- 51. Khan A, Ramar K, Maddirala S, Friedman O, Pallanch JF, Olson EJ. Uvulopalatopharyngoplasty in the management of obstructive sleep apnea: the Mayo Clinic experience. Mayo Clin Proc. 2009;84:795-800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33:1396-1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Morgenthaler TI, Kapen S, Lee-Chiong T, et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006;29:1031-1035 [PubMed] [Google Scholar]
- 54. Lehman S, Antic NA, Thompson C, Catcheside PG, Mercer J, McEvoy RD. Central sleep apnea on commencement of continuous positive airway pressure in patients with a primary diagnosis of obstructive sleep apnea-hypopnea. J Clin Sleep Med. 2007;3:462-466 [PMC free article] [PubMed] [Google Scholar]
- 55. Kuzniar TJ, Pusalavidyasagar S, Gay PC, Morgenthaler TI. Natural course of complex sleep apnea: a retrospective study. Sleep Breath. 2008;12(2):135-139 [DOI] [PubMed] [Google Scholar]
- 56. Rosenthal L, Massie CA, Dolan DC, Loomas B, Kram J, Hart RW. A multicenter, prospective study of a novel nasal EPAP device in the treatment of obstructive sleep apnea: efficacy and 30-day adherence. J Clin Sleep Med. 2009;5:532-537 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.