Abstract
The herpes simplex virus protein VP16 interacts with cellular factors, including the protein Oct-1, to activate viral immediate early (IE) gene transcription. We have reproduced this effect by addition of purified, full-length VP16 and the DNA-binding 'POU' domain of Oct-1 (Oct-1/POU) to a HeLa cell in vitro transcription system. Stimulation of transcription was dependent on the IE-specific element, TAATGARAT. In agreement with earlier observations from electrophoretic mobility shift assays, activation was not observed when Oct-2/POU, the DNA-binding domain from the Oct-2 protein, was substituted for Oct-1/POU. Single round transcription assays revealed that, together, VP16 and Oct-1/POU facilitate the assembly of pre-initiation complexes at target gene promoters.
Full text
PDF
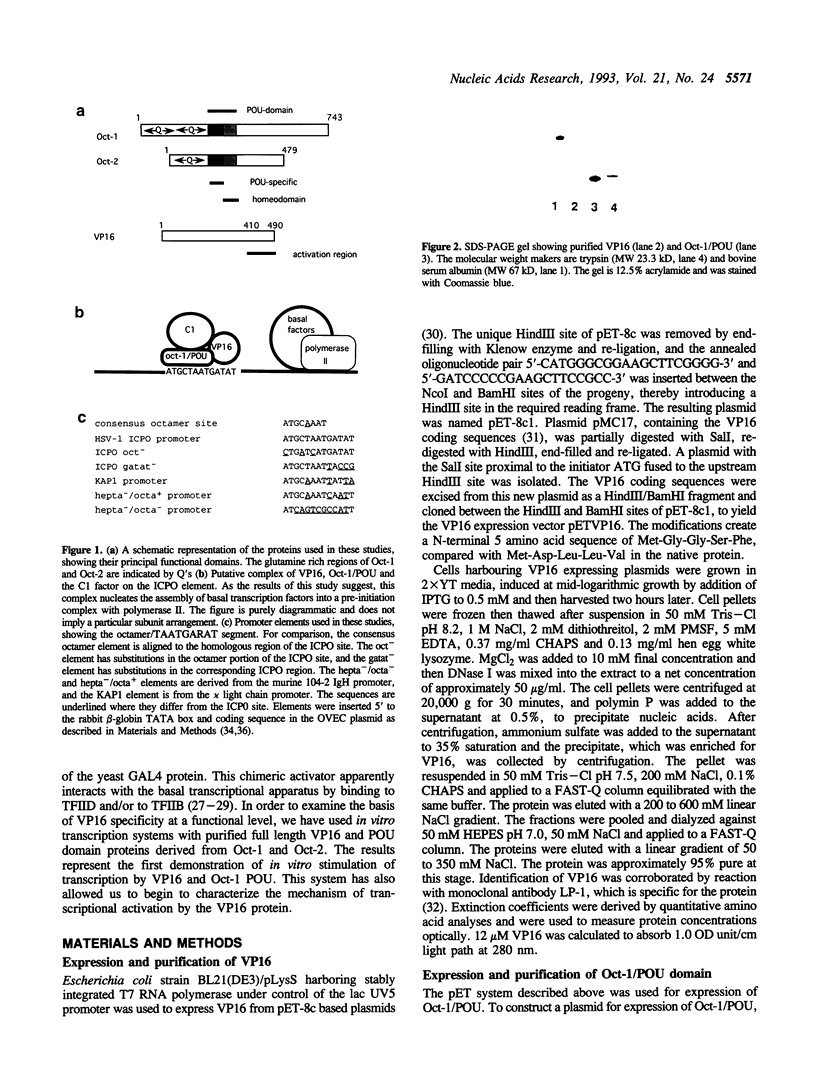





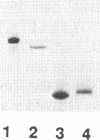
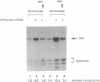
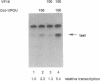
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ace C. I., Dalrymple M. A., Ramsay F. H., Preston V. G., Preston C. M. Mutational analysis of the herpes simplex virus type 1 trans-inducing factor Vmw65. J Gen Virol. 1988 Oct;69(Pt 10):2595–2605. doi: 10.1099/0022-1317-69-10-2595. [DOI] [PubMed] [Google Scholar]
- Arnosti D. N., Merino A., Reinberg D., Schaffner W. Oct-2 facilitates functional preinitiation complex assembly and is continuously required at the promoter for multiple rounds of transcription. EMBO J. 1993 Jan;12(1):157–166. doi: 10.1002/j.1460-2075.1993.tb05641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assa-Munt N., Mortishire-Smith R. J., Aurora R., Herr W., Wright P. E. The solution structure of the Oct-1 POU-specific domain reveals a striking similarity to the bacteriophage lambda repressor DNA-binding domain. Cell. 1993 Apr 9;73(1):193–205. doi: 10.1016/0092-8674(93)90171-l. [DOI] [PubMed] [Google Scholar]
- Brugnera E., Xu L., Schaffner W., Arnosti D. N. POU-specific domain of Oct-2 factor confers 'octamer' motif DNA binding specificity on heterologous Antennapedia homeodomain. FEBS Lett. 1992 Dec 21;314(3):361–365. doi: 10.1016/0014-5793(92)81506-h. [DOI] [PubMed] [Google Scholar]
- Campbell M. E., Palfreyman J. W., Preston C. M. Identification of herpes simplex virus DNA sequences which encode a trans-acting polypeptide responsible for stimulation of immediate early transcription. J Mol Biol. 1984 Nov 25;180(1):1–19. doi: 10.1016/0022-2836(84)90427-3. [DOI] [PubMed] [Google Scholar]
- Cleary M. A., Stern S., Tanaka M., Herr W. Differential positive control by Oct-1 and Oct-2: activation of a transcriptionally silent motif through Oct-1 and VP16 corecruitment. Genes Dev. 1993 Jan;7(1):72–83. doi: 10.1101/gad.7.1.72. [DOI] [PubMed] [Google Scholar]
- Dekker N., Cox M., Boelens R., Verrijzer C. P., van der Vliet P. C., Kaptein R. Solution structure of the POU-specific DNA-binding domain of Oct-1. Nature. 1993 Apr 29;362(6423):852–855. doi: 10.1038/362852a0. [DOI] [PubMed] [Google Scholar]
- Gerster T., Roeder R. G. A herpesvirus trans-activating protein interacts with transcription factor OTF-1 and other cellular proteins. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6347–6351. doi: 10.1073/pnas.85.17.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves R., O'Hare P. Separation of requirements for protein-DNA complex assembly from those for functional activity in the herpes simplex virus regulatory protein Vmw65. J Virol. 1989 Apr;63(4):1641–1650. doi: 10.1128/jvi.63.4.1641-1650.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., Roeder R. G. Functional steps in transcription initiation and reinitiation from the major late promoter in a HeLa nuclear extract. J Biol Chem. 1987 Mar 15;262(8):3452–3461. [PubMed] [Google Scholar]
- Herr W., Sturm R. A., Clerc R. G., Corcoran L. M., Baltimore D., Sharp P. A., Ingraham H. A., Rosenfeld M. G., Finney M., Ruvkun G. The POU domain: a large conserved region in the mammalian pit-1, oct-1, oct-2, and Caenorhabditis elegans unc-86 gene products. Genes Dev. 1988 Dec;2(12A):1513–1516. doi: 10.1101/gad.2.12a.1513. [DOI] [PubMed] [Google Scholar]
- Hisatake K., Roeder R. G., Horikoshi M. Functional dissection of TFIIB domains required for TFIIB-TFIID-promoter complex formation and basal transcription activity. Nature. 1993 Jun 24;363(6431):744–747. doi: 10.1038/363744a0. [DOI] [PubMed] [Google Scholar]
- Katan M., Haigh A., Verrijzer C. P., van der Vliet P. C., O'Hare P. Characterization of a cellular factor which interacts functionally with Oct-1 in the assembly of a multicomponent transcription complex. Nucleic Acids Res. 1990 Dec 11;18(23):6871–6880. doi: 10.1093/nar/18.23.6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler I., Bucher E., Seipel K., Müller-Immerglück M. M., Schaffner W. Promoters with the octamer DNA motif (ATGCAAAT) can be ubiquitous or cell type-specific depending on binding affinity of the octamer site and Oct-factor concentration. Nucleic Acids Res. 1991 Jan 25;19(2):237–242. doi: 10.1093/nar/19.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler I., Schaffner W. Octamer transcription factors and the cell type-specificity of immunoglobulin gene expression. FASEB J. 1990 Mar;4(5):1444–1449. doi: 10.1096/fasebj.4.5.2407588. [DOI] [PubMed] [Google Scholar]
- Kemler I., Schreiber E., Müller M. M., Matthias P., Schaffner W. Octamer transcription factors bind to two different sequence motifs of the immunoglobulin heavy chain promoter. EMBO J. 1989 Jul;8(7):2001–2008. doi: 10.1002/j.1460-2075.1989.tb03607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristie T. M., LeBowitz J. H., Sharp P. A. The octamer-binding proteins form multi-protein--DNA complexes with the HSV alpha TIF regulatory protein. EMBO J. 1989 Dec 20;8(13):4229–4238. doi: 10.1002/j.1460-2075.1989.tb08608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristie T. M., Sharp P. A. Interactions of the Oct-1 POU subdomains with specific DNA sequences and with the HSV alpha-trans-activator protein. Genes Dev. 1990 Dec;4(12B):2383–2396. doi: 10.1101/gad.4.12b.2383. [DOI] [PubMed] [Google Scholar]
- Kristie T. M., Sharp P. A. Purification of the cellular C1 factor required for the stable recognition of the Oct-1 homeodomain by the herpes simplex virus alpha-trans-induction factor (VP16). J Biol Chem. 1993 Mar 25;268(9):6525–6534. [PubMed] [Google Scholar]
- Lai J. S., Cleary M. A., Herr W. A single amino acid exchange transfers VP16-induced positive control from the Oct-1 to the Oct-2 homeo domain. Genes Dev. 1992 Nov;6(11):2058–2065. doi: 10.1101/gad.6.11.2058. [DOI] [PubMed] [Google Scholar]
- Lin Y. S., Green M. R. Mechanism of action of an acidic transcriptional activator in vitro. Cell. 1991 Mar 8;64(5):971–981. doi: 10.1016/0092-8674(91)90321-o. [DOI] [PubMed] [Google Scholar]
- Lin Y. S., Ha I., Maldonado E., Reinberg D., Green M. R. Binding of general transcription factor TFIIB to an acidic activating region. Nature. 1991 Oct 10;353(6344):569–571. doi: 10.1038/353569a0. [DOI] [PubMed] [Google Scholar]
- Mackem S., Roizman B. Structural features of the herpes simplex virus alpha gene 4, 0, and 27 promoter-regulatory sequences which confer alpha regulation on chimeric thymidine kinase genes. J Virol. 1982 Dec;44(3):939–949. doi: 10.1128/jvi.44.3.939-949.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean C., Buckmaster A., Hancock D., Buchan A., Fuller A., Minson A. Monoclonal antibodies to three non-glycosylated antigens of herpes simplex virus type 2. J Gen Virol. 1982 Dec;63(2):297–305. doi: 10.1099/0022-1317-63-2-297. [DOI] [PubMed] [Google Scholar]
- O'Hare P., Goding C. R. Herpes simplex virus regulatory elements and the immunoglobulin octamer domain bind a common factor and are both targets for virion transactivation. Cell. 1988 Feb 12;52(3):435–445. doi: 10.1016/s0092-8674(88)80036-9. [DOI] [PubMed] [Google Scholar]
- Pomerantz J. L., Kristie T. M., Sharp P. A. Recognition of the surface of a homeo domain protein. Genes Dev. 1992 Nov;6(11):2047–2057. doi: 10.1101/gad.6.11.2047. [DOI] [PubMed] [Google Scholar]
- Post L. E., Mackem S., Roizman B. Regulation of alpha genes of herpes simplex virus: expression of chimeric genes produced by fusion of thymidine kinase with alpha gene promoters. Cell. 1981 May;24(2):555–565. doi: 10.1016/0092-8674(81)90346-9. [DOI] [PubMed] [Google Scholar]
- Preston C. M., Frame M. C., Campbell M. E. A complex formed between cell components and an HSV structural polypeptide binds to a viral immediate early gene regulatory DNA sequence. Cell. 1988 Feb 12;52(3):425–434. doi: 10.1016/s0092-8674(88)80035-7. [DOI] [PubMed] [Google Scholar]
- Rigoni P., Xu L., Harshman K., Schaffner W., Arnosti D. N. Conserved cysteine residues of Oct-2 POU domain confer sensitivity to oxidation but are dispensable for sequence-specific DNA binding. Biochim Biophys Acta. 1993 May 28;1173(2):141–146. doi: 10.1016/0167-4781(93)90174-c. [DOI] [PubMed] [Google Scholar]
- Roberts S. G., Ha I., Maldonado E., Reinberg D., Green M. R. Interaction between an acidic activator and transcription factor TFIIB is required for transcriptional activation. Nature. 1993 Jun 24;363(6431):741–744. doi: 10.1038/363741a0. [DOI] [PubMed] [Google Scholar]
- Sadowski I., Ma J., Triezenberg S., Ptashne M. GAL4-VP16 is an unusually potent transcriptional activator. Nature. 1988 Oct 6;335(6190):563–564. doi: 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- Seipel K., Georgiev O., Schaffner W. Different activation domains stimulate transcription from remote ('enhancer') and proximal ('promoter') positions. EMBO J. 1992 Dec;11(13):4961–4968. doi: 10.1002/j.1460-2075.1992.tb05603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D., Purves F., Roizman B. Role of alpha-transinducing factor (VP16) in the induction of alpha genes within the context of viral genomes. J Virol. 1991 Jul;65(7):3504–3513. doi: 10.1128/jvi.65.7.3504-3513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern S., Herr W. The herpes simplex virus trans-activator VP16 recognizes the Oct-1 homeo domain: evidence for a homeo domain recognition subdomain. Genes Dev. 1991 Dec;5(12B):2555–2566. doi: 10.1101/gad.5.12b.2555. [DOI] [PubMed] [Google Scholar]
- Stern S., Tanaka M., Herr W. The Oct-1 homoeodomain directs formation of a multiprotein-DNA complex with the HSV transactivator VP16. Nature. 1989 Oct 19;341(6243):624–630. doi: 10.1038/341624a0. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Sturm R. A., Das G., Herr W. The ubiquitous octamer-binding protein Oct-1 contains a POU domain with a homeo box subdomain. Genes Dev. 1988 Dec;2(12A):1582–1599. doi: 10.1101/gad.2.12a.1582. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Grossniklaus U., Herr W., Hernandez N. Activation of the U2 snRNA promoter by the octamer motif defines a new class of RNA polymerase II enhancer elements. Genes Dev. 1988 Dec;2(12B):1764–1778. doi: 10.1101/gad.2.12b.1764. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Herr W. Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell. 1990 Feb 9;60(3):375–386. doi: 10.1016/0092-8674(90)90589-7. [DOI] [PubMed] [Google Scholar]
- Triezenberg S. J., Kingsbury R. C., McKnight S. L. Functional dissection of VP16, the trans-activator of herpes simplex virus immediate early gene expression. Genes Dev. 1988 Jun;2(6):718–729. doi: 10.1101/gad.2.6.718. [DOI] [PubMed] [Google Scholar]
- Verrijzer C. P., Alkema M. J., van Weperen W. W., Van Leeuwen H. C., Strating M. J., van der Vliet P. C. The DNA binding specificity of the bipartite POU domain and its subdomains. EMBO J. 1992 Dec;11(13):4993–5003. doi: 10.1002/j.1460-2075.1992.tb05606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrijzer C. P., Van der Vliet P. C. POU domain transcription factors. Biochim Biophys Acta. 1993 Apr 29;1173(1):1–21. doi: 10.1016/0167-4781(93)90237-8. [DOI] [PubMed] [Google Scholar]
- Westin G., Gerster T., Müller M. M., Schaffner G., Schaffner W. OVEC, a versatile system to study transcription in mammalian cells and cell-free extracts. Nucleic Acids Res. 1987 Sep 11;15(17):6787–6798. doi: 10.1093/nar/15.17.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Brou C., Wu J., Lutz Y., Moncollin V., Chambon P. The acidic transcriptional activator GAL-VP16 acts on preformed template-committed complexes. EMBO J. 1992 Jun;11(6):2229–2240. doi: 10.1002/j.1460-2075.1992.tb05282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A. C., LaMarco K., Peterson M. G., Herr W. The VP16 accessory protein HCF is a family of polypeptides processed from a large precursor protein. Cell. 1993 Jul 16;74(1):115–125. doi: 10.1016/0092-8674(93)90299-6. [DOI] [PubMed] [Google Scholar]
- Zawel L., Reinberg D. Initiation of transcription by RNA polymerase II: a multi-step process. Prog Nucleic Acid Res Mol Biol. 1993;44:67–108. doi: 10.1016/s0079-6603(08)60217-2. [DOI] [PubMed] [Google Scholar]