Abstract
Our current study aims to evaluate the mechanisms of tranylcypromine (TCP)-mediated enhancement of nicotine self-administration. We replicated our previous findings which demonstrate that 1-hr pretreatment with TCP (3mg/kg, i.p.) enhances nicotine self-administration (7.5 μg/kg/inj, i.v.) when compared with vehicle-treated rodents. We tested whether TCP-mediated enhancement of nicotine self-administration was due to MAO inhibition or off-target effects by (i) extending the TCP pretreatment time from 1 to 20 hr, and (ii) evaluating the role of the individual TCP stereoisomers in nicotine self-administration studies. While 20-hr and (−)TCP pretreatment induced significant inhibition of MAO (60–90%), animals found nicotine only weakly reinforcing. Furthermore, while both (+) and (±)TCP treatment induced nearly 100% MAO inhibition, (+)TCP pretreated animals took longer to acquire nicotine self-administration compared to (±)TCP pretreated animals. Stable nicotine self-administration in (+)TCP pretreated animals was influenced by nicotinic receptor activation but not nicotine-paired cues. The opposite was found in (±)TCP pretreated animals. Treatment with (−) or (±)TCP increased dopamine and serotonin overflow, while the (+) and (±)TCP treatment enhanced monoamine overflow subsequent to nicotine. Together, our data suggests TCP-enhancement of nicotine self-administration are mediated through mechanisms independent of MAO inhibition, including nicotine-paired cues and monoamine uptake inhibition.
1. INTRODUCTION
Tobacco use is a major cause of premature death in the United States, an effect primarily attributable to carcinogenic properties of cigarette smoke (ACS, 2010; CDC, 2008; Mokdad et al., 2004). Whereas many of the addictive effects of smoking have been associated with nicotine (Henningfield et al., 1985) replacement therapies (e.g. nicotine: gum, inhaler, intranasal spray, transdermal patch, sublingual tablet) have limited success in supporting long-term cessation, with over 80% of users relapsing within one year (Bohadana et al., 2000; Nides, 2008). Furthermore, denicotinized cigarettes provide greater satisfaction than intravenous nicotine in healthy smokers, implicating a role for non-nicotine components in cigarette smoke (Rose et al., 2000). These results parallel those in the animal literature suggesting that nicotine is a weak reinforcer (Clemens et al., 2009), but when co-administered with other constituents in tobacco smoke, like acetaldehyde, can potentiate self-administration behavior with age and sex specific effects (Belluzzi et al., 2005; Park et al., 2007). Taken together, the highly addictive nature of cigarettes may reflect the influence of other tobacco constituents interacting with nicotine to increase reinforcement and reward (Belluzzi et al., 2004; Belluzzi et al., 2005; Clemens et al., 2009; O’Dell and Khroyan, 2009; Villégier et al., 2007a; Villégier et al., 2007b; Villégier et al., 2006a).
Tobacco smoke contains thousands of constituents, some that are known to inhibit monoamine oxidase (MAO) (Khalil et al., 2000), a mitochondrial enzyme that catalyzes the oxidative degradation of many biogenic amines, including serotonin (5-HT), dopamine (DA) and norepinephrine (NE). MAO consists of two isozymes, A and B, which are reduced in activity by 30–40% in the brains of smokers as a result of reversible and irreversible inhibition by tobacco smoke constituents (Fowler et al., 1996a; Fowler et al., 1996b). Since DA, in particular, is a key regulator of limbic reward mechanisms, it has been proposed that this long-lasting inhibition of MAO activity increases synaptic DA levels and enhances nicotine reinforcement (Berlin and Anthenelli, 2001; Fowler et al., 1996a; Fowler et al., 1996b; Lewis et al., 2007).
Whereas several competitive reversible MAO inhibitors in tobacco smoke have been structurally characterized, the irreversible MAO inhibitor(s) have not (Lewis et al., 2007). As a result, animal studies have used clinically available drugs to model the effects of nicotine in combination with irreversible MAO inhibition (Villégier et al., 2007a; Villégier et al., 2007b; Villégier et al., 2006a). We and others (Guillem et al., 2005) have shown that pretreatment with (±) tranylcypromine (TCP), an irreversible, non-selective MAO inhibitor, increases locomotor responses to nicotine (Villégier et al., 2006b), and enhances both nicotine-induced locomotor sensitization (Villégier et al., 2003) and nicotine self-administration (Villégier et al., 2007a; Villégier et al., 2007b; Villégier et al., 2006b). Mechanisms mediating these effects have been linked, at least in part, to 5-HT, NE and DA neurotransmitter systems (Villégier et al., 2010; Villégier et al., 2007a; Villégier et al., 2007b). Other irreversible MAO inhibitors, including the non-selective MAO-A and −B inhibitor, phenelzine, and the modestly MAO-A selective inhibitor, clorgyline, also enhance nicotine self-administration (Guillem et al., 2005, 2006). Furthermore, phenelzine enhances the discriminative stimulus effect of nicotine (Wooters and Bardo, 2007), and greatly prolongs the duration of nicotine withdrawal, as measured by conditioned place aversion (Guillem et al., 2008). Although these findings suggest a profound effect of MAO inhibition on nicotine-induced behavior, all of the enzyme inhibitors that have been used experimentally have additional, acute pharmacological actions (Baker et al., 1992; Baker et al., 2007). (±)TCP stimulates release and blocks the reuptake of monoamines (Baker et al., 1992; Villégier et al., 2007a; Villégier et al., 2007b), and inhibits CYP-2A6 (Ki=0.08 μM) and CYP-2B6 (Ki=5 μM), enzymes associated with the metabolism of nicotine to cotinine (Zhang et al., 2001). Thus, the acute pharmacological effects of (±)TCP complicate interpretation of the actions of this drug, especially when combined with nicotine.
The aim of the present study was therefore to determine whether MAO inhibition and/or the acute effects of (±)TCP, such as monoamine uptake inhibition, influence the enhancement of nicotine self-administration. In order to minimize acute drug effects, we extended the (±)TCP pretreatment time from 1 to 20 hr (which produces 70–90% inhibition of MAO A and B), as well as individually evaluated the effects of the (+) and (−) stereoisomers of TCP. Our findings provide a mechanistic analysis of (±)TCP enhancement of nicotine self-administration. The data demonstrates that mechanisms independent of MAO inhibition, including acute pharmacological effects of TCP, such as the blockade of DA and 5-HT uptake, as well as nicotine-paired cues, represent the primary mechanisms responsible for the enhancement of nicotine self-administration behavior. The work has previously been published in the form of an abstract (Leslie et al., 2009; Lotfipour et al., 2007).
2. MATERIALS AND METHODS
2.1. Animals
Adult (280–300 g) male Sprague-Dawley rats were purchased from Charles River. Rodents were maintained under a normal light dark cycle (12 hrs) in an AAALAC-accredited vivarium having continuous access to food and water. The UCI Institutional Animal Care and Use Committee provided ethical approval to our experiments, which were in compliance with the NIH Guide for Care and Use of Laboratory Animals (NIH No 85–23, rev. 1985). A total of 191 animals were used for this study.
2.2. Resolution of trans-2-phenylcyclopropylamine (TCP)
The positive and negative stereoisomers were purified from (±)TCP as a tartrate salt following established methods previously described (Kaiser et al., 1962). In short, we combined equal amounts of D-tartaric acid (0.1 moles) with the racemic form of TCP in ethanol. We obtained crystals at room temperature, which were then filtered and underwent an additional five subsequent recrystalizations to enhance the purity of the product. We obtained 6.62 grams of (+)TCP D-tartrate, with an optical activity of [α]25D + 30.5. The remaining products in solution were then exposed to L-tartaric acid and crystals were obtained. After three recrystalizations, we obtained 7.4 grams of (−)TCP L-tartrate with the optical activity of [α]25D −31.8. Optical activity was determined by sending samples for analysis to Robertson Microlite laboratories. While the purity of the isomers was not specifically tested, the optical activity of the separated isomers were nearly identical to that of Kaiser et al., which was reported for the (−) and (+) isomers to be: −30.5 and +31.0 (Kaiser et al., 1962).
The doses of (±)TCP and its isomers were calculated to take into account the tartrate salt. We chose the dose of TCP at 1.5 mg/kg for the (+) isomer and 1.5 mg/kg for the (−) isomer, based on the evidence of Aspeslet et al., which demonstrated that (±)TCP comes in a 1:1 ratio of the individual isomers of TCP (Aspeslet et al., 1992). The dose represents the predicted ratios of the individual isomers at the 3 mg/kg dose, as used in our previous studies (Villégier et al., 2007a; Villégier et al., 2007b). We have confirmed that the Sigma purchased racemate demonstrates similar behavioral (self-administration) and neurochemical (microdialysis) profiles as obtained from the reconstituted mixture of the individual isomers of TCP at the 1.5 mg/kg dose (equaling the 3 mg/kg racemate dose of TCP).
2.3. MAO Assay
MAO Assay was performed as previously described by (Villégier et al., 2007b). The only differences were the pretreatment schedule and individual isomer administration. Adult male rats were pretreated with either one or three exposure of TCP isomers ((+) or (−)) or 3 exposure of (±)TCP or saline at approximately 11 AM- 12PM (Supplementary Fig. 1a). Pretreatments were timed so that they would occur 1 hr prior to sacrifice (12-1 PM) and were staggered ever 5 min to allow time for tissue homogenization. We administered 1 and 3 days of exposure of the individual isomers to rule out possible differences in their ability to induce MAO inhibition based on the number of days of exposure. We only administered 3 days of exposure for (±)TCP or saline, given our previous results that the number of days of pretreament do not further influence MAO inhibition (Villégier et al., 2007b). The 3 days of exposure was initially chosen based on evidence that 1hr (±)TCP pretreated animals showed enhanced responding on that day, as previously explained (Villégier et al., 2007b). Data is also included from animals receiving 20 hr TCP pretreatments (1, 3, 5 exposures at 4–6 PM, with MAO assay performed 20 hours after final exposure at 12PM -2 PM; Supplementary Fig. 1b). This data was previously published in (Villégier et al., 2007b) and is included for the purpose of comparison to that of the individual stereoisomers. For an extended description of tissue preparation and data acquisition, please refer to the work of (Villégier et al., 2007b).
In brief, rodent brains were extracted, homogenized and centrifuged in sodium phosphate buffer (Villégier et al., 2007b). Enzymatic analysis was performed on tissue supernatants with [14C] serotonin or [14C] phenylethylamine at a concentration of 1 mM. The reaction was terminated with HCl followed by benzene/ethyl acetate exposure for [14C] serotonin or toluene exposure for [14C] phenylethylamine. After centrifugation, the organic phase was mixed with scintillation fluid and quantified with a scintillation counter. Results were calculated as pmol/mg of protein/min and represented in table format as percent over baseline values, similar to previous reports (Villégier et al., 2007b).
2.4. Self-Administration
Animals received (±)TCP (3 mg/kg, i.p.) 1 hr or 20 hr before nicotine (7.5 μg/kg/inj, i.v.) self-administration (Supplementary Fig. 2). Separate animals received either (+)TCP or (−)TCP at (1.5 mg/kg, i.p.) 1 hr prior to nicotine self-administration. The dose of nicotine was chosen based on our previous work that demonstrated peak responding in 1 hr (±)TCP treated animals (Villégier et al., 2007b). Self-administration tests were performed during the times of 9 AM-3 PM. The 1 hr pretreatment time for TCP prior to self-administration was initially based on the enhanced locomotor activity found after 1 hr exposure in (Villégier et al., 2003).
Self-administration procedures, including catheter construction and surgical procedures, were performed as previously described (Villégier et al., 2007b). In brief, animals were fitted with chronic jugular vein catheters and tested in a self-administration chamber using a two nose-poke apparatus, as described (Villégier et al., 2007b). We made two minor modifications to the procedure, shortening the timeout period from 60 sec to 20 sec and reducing the time in the self-administration chamber from 3 hours to 2 hours. These modifications were made in order to reduce the time animals spent in the self-administration chamber while maximizing their responding. Our previous studies had demonstrated that maximal responding occurred within the first 2 hours of self-administration after 1 hr (±)TCP pretreatment. Therefore, we believed that a full behavioral assessment is attained within this shorter 2-hr time frame.
For controls, our study included four separate groups: (i) saline pretreated rats self-administering i.v. saline, (ii) saline pretreated rats self-administering i.v. nicotine (7.5 μg/kg in 20 μl), (iii) 1 hr (±)TCP pretreated animals self-administering i.v. saline and (iv) 20 hr (±)TCP pretreated animals self-administering i.v. saline. Data from control groups is not shown as animals did not acquire stable nicotine self-administration behavior. Stable self-administration is defined as animals exhibiting less than 20% variability in responding across two days, with reinforced responding ≥15 and two times greater than non-reinforced responding, as developed from (Belluzzi et al., 2005; Yoshimura et al., 2007). The schedule of reinforcement was set at a fixed ratio (FR)-1 for 5 days, FR-2 for days 6 and 7, and FR-5 for days 8 and 9 (Supplementary Fig. 2). On the 10th day, the schedule of reinforcement was set to a progressive ratio with the schedule of ratios as follows: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603… based on the formula of Richardson and Roberts, (1995): [5e(number of injections × 0.2))] − 5. The session was terminated if the animal did not reach the next ratio within 30 min or after 4 hrs of the initiation of experimentation, as used previously in our laboratory (Franke et al., 2008; Yoshimura et al., 2007). Catheter patency was tested with Propofol (0.1 mL) and retained in over 70% of animals by day 10 (day of progressive ratio). We observed no differences between groups due to animals failing to finish the entire experiment.
2.5. Mechanistic studies
To assess the differential mechanisms mediating (+) and (±)TCP mediated enhancement of nicotine self-administration, we performed a series of mechanistic behavioral studies (Supplementary Fig. 3). Animals pretreated with (+) and (±)TCP (1.5 and 3 mg/kg, i.p., respectively) were trained to acquire stable nicotine self-administration (FR1). Subsequently, we performed the following four types of manipulations in a counterbalanced design (with at least three days separating each manipulation):
To test the role of nicotine and non-pharmacological stimuli in maintaining nicotine self-administration, the day after steady nicotine self-administration (FR1) had been achieved, animals received (±)TCP or (+)TCP pretreatment and 1 hr later either the nicotine (7.5 μg/kg/inj, i.v.) solution was replaced with saline (i.v.) or the house and cue lights were inactivated during the self-administration sessions.
To test the role of nicotinic receptors in maintaining nicotine self-administration, the day after steady nicotine self-administration (FR1) had been achieved, animals received (±)TCP or (+)TCP pretreatment and 45 min later either a saline (0.5 ml/kg, s.c.) or mecamylamine injection (1, 3 or 5 mg/kg, s.c.). Fifteen minutes later animals were allowed to self-administer nicotine (7.5 μg/kg/inj.).
To test the role of 5-HT 2A/C receptors in maintaining nicotine self-administration animals received (±)TCP or (+)TCP pretreatment and 45 min later a ketanserin (1 mg/kg, s.c.) injection. Fifteen minutes later animals were allowed to self-administer nicotine (7.5 μg/kg/inj.).
After each exposure session, catheter patency was tested with Propofol (0.1 mL). If immediate anesthesia was not observed, data was discarded from the session and the animal was euthanized. Virtually all animals eventually became Propofol negative by the end of these experiments. Responding on the test day was reported as a percentage of baseline responding on the prior day. Animals given (−)TCP were unable to maintain stable nicotine self-administration at higher order schedules of reinforcement, therefore this treatment group was not included in the mechanistic studies.
2.6. In vivo Microdialysis-HPLC-ECD
In vivo microdialysis experiments followed the same procedure previously described (Villégier et al., 2007a; Villégier et al., 2007b). The only modifications included a change in drug treatments. In brief, we performed dual surgeries on adult male rats to implant a jugular intravenous catheter and a cranial guide cannula. Using a stereotaxic apparatus, guide cannula were directed above the nucleus accumbens shell with the following coordinates relative to bregma (anterior/posterior: +2.0, Medial/Lateral: ±1.2; Dorsal/Ventral: 5.8 mm) (Paxinos and Watson, 1986). Animals received two daily injections of (±)TCP (3 mg/kg, i.p.), (+)- or (−)TCP (1.5 mg/kg, i.p.) or saline (Supplementary Fig. 4). Microdialysis experiments were performed on the third treatment day (Supplementary Fig. 4). The guide cannula was replaced with a 2 mm microdialysis probe and animals were given a 4 hour equilibration. Subsequently, neurotransmitters were quantified every 20 min for 60 min followed by a 3rd treatment exposure. After two hours of collection, half of the animals were given either intravenous saline (2 × 100 μl) or nicotine (2 × 100 μl of 30 μg/kg/inj, 1 min apart). Nicotine was administered two hours after tranylcypromine, based on evidence that peak responding during nicotine self-administration behavior occurs 2hr post-(±)TCP administration (i.e. 1 hr after being placed into the self-administration chamber), as previously reported (Villégier et al., 2007b). Neurotransmitters were quantified with ESA 580 HPLC-5600 electrochemical chemical detector using a 5014B microdialysis cell.
2.7. Statistics
Data from Table 1, 2 and 3 were analyzed with a 1-way ANOVA with Bonferroni-corrected post hoc comparisons. Data from Fig. 1–2 were analyzed with a 2 and 3-way ANOVA with repeated measures. Data from Fig. 3 were analyzed with a 2-way ANOVA with repeated measures and Bonferroni-corrected t-tests. Data from Fig. 4 were analyzed with a 2 and 4-way ANOVA with repeated measures and Bonferroni-corrected paired t-tests. Data from Fig. 5 were analyzed with a 2 and 3-way ANOVA and Bonferroni-corrected t-tests. Data from Fig. 6 were analyzed with a 2-way ANOVA with repeated measures and Bonferroni-corrected t-tests. All statistical analyses were performed using SYSTAT 10, JMP 7.02 or SPSS 17 statistical software. Statistical significance was set at p ≤ 0.05.
Table 1.
Inhibitory effect of TCP pretreatments on brain MAO-A and -B activities.
| Pretreatment | MAO Activity Mean % of saline control (± SEM) | |
|---|---|---|
| MAO-A | MAO-B | |
| Saline | 100.0 ± 18.9aaa | 100.0 ± 3.9aaa |
| 1 hr (−) TCP | 41.5 ± 3.5bb | 29.2 ± 4.0bbb |
| 20 hr (±) TCP | 27.1 ± 3.6b | 13.9 ± 2.7 |
| 1 hr (+) TCP | 1.7 ± 0.1 | 2.6 ± 0.6 |
| 1 hr (±) TCP | 1.5 ± 0.3 | 1.7 ± 0.2 |
p<0.001 vs. all 4 TCP groups;
p<0.05,
p<0.002 vs. 1-hr (±) and (+) TCP;
p≤0.01 vs. other 3 TCP groups n = 3–12/group.
Table 2.
Effect of TCP pretreatments on nicotine self-administration breakpoint values.
| Pretreatment | Breakpoint value (Mean ± SEM) |
|---|---|
| 1 hr (±) TCP | 122.1 ± 35.3 |
| 1 hr (+) TCP | 57.8 ± 17.2 |
| 1 hr (−) TCP | 19.4 ± 7.6* |
| 20 hr (±) TCP | 34.8 ± 26.6 |
p<0.05 vs. 1-hr (±) TCP; n=9–13/group.
Table 3.
TCP pretreatments effects on baseline levels for DA and 5-HT at t= 60 min (Mean ± SEM).
| Pretreatment | Baseline DA Levels | Baseline 5-HT Levels |
|---|---|---|
| pg/20 μl | pg/20 μl | |
| Saline | 4.9 ± 0.9aa, c | 8.8 ± 2.9 |
| 1 hr (−) TCP | 11.3 ± 2.7 | 8.3 ± 2.7 |
| 20 hr (±) TCP | 34.0 ± 8.8b | 16.5 ± 4.0 |
| 1 hr (+) TCP | 29.2 ± 5.0 | 25.2 ± 4.8d |
| 1 hr (±) TCP | 31.5 ± 4.9b | 20.4 ± 3.5 |
p<0.01 vs. 20-hr (±) TCP, 1 hr (±) TCP
p<0.05 vs. 1-hr (−)TCP,
p<0.05 vs. (+) TCP,
p≤0.05 vs. 1 hr (−) TCP. n = 4–11/group. Data for the individual pretreatments at t=60 min are pooled from animals later given either saline or nicotine, i.v. at t= 180 min.
Figure 1. Influence of TCP pretreatment on acquisition time course of nicotine self-administration.
Mean (+ SEM) reinforced and non-reinforced responding was plotted for days 1–5 at FR1. Animals received (±)TCP (3 mg/kg, i.p.) 1 hr (A) or 20 hr (B) before nicotine (7.5 μg/kg/inj, i.v.) self-administration. The 1-hr pretreatment group acquired by day 2 and maintained responding through day 5 (***p<0.001 vs. Non-Reinforced, n = 21), but the 20-hr pretreated group showed weaker acquisition (**p<0.01 5-day total Reinforced vs. Non-Reinforced, n = 12). Separate animals received either (+)TCP (C) or (−)TCP (D) at (1.5 mg/kg, i.p.) 1 hr prior to nicotine self-administration. The (+)TCP-pretreated animals acquired by day 4 (*p<0.05, **p<0.01 vs. Non-Reinforced, n=12) whereas the (−)-pretreated animals acquired by day 3 (n=12).
Figure 2. Influence of TCP pretreatment on nicotine self-administration under different reinforcement ratios.

Mean (+SEM) reinforced and non-reinforced responding was plotted for days 5–9 for the three reinforcement ratios. Animals received (±) TCP (3 mg/kg, i.p.) 1 hr (A) or 20 hr (B) before nicotine (7.5 μg/kg/inj, i.v.) self-administration. The 1-hr pretreatment group maintained self-administration at all ratios, but the 20-hr group did not (*p<0.05, ***p<0.001 vs. Non-Reinforced; ++p<0.01 vs. previous day, n=21). Tests of (+)TCP (C) and (−)TCP (D) at (1.5 mg/kg, i.p.) 1 hr prior to nicotine self-administration showed that the (+)TCP-pretreated animals maintained self-administration at all ratios, but the (−)TCP-pretreated rats did not (*p<0.05, **p<0.01 vs. Non-Reinforced, n=12/group).
Figure 3. DA and 5-HT overflow in the nucleus accumbens shell after TCP and nicotine treatment.
Animals received two daily injections of (±)TCP (3 mg/kg, i.p.), (+)- or (−)TCP (1.5 mg/kg, i.p.) or saline. On the third day, after 60 min of basal overflow, all animals received a third identical injection, except a subset of the (±)TCP-treated group received saline (20-hr (±)TCP) (A–D). Subsequently, animals received either i.v. saline or nicotine at t=180. Time course data is presented for DA (A & C) and 5-HT (B & D) for the 240 min collection period. Black bars over the individual time-points represent data presented in E–H. Mean (+ SEM) overflow difference from baseline (t=80 – t=60, black bars in A–D) scores are shown for DA (E) and 5-HT (G). Both DA and 5-HT overflow was significantly increased by (±) and (−)TCP, but only DA overflow was increased by (+)TCP (*p<0.05, **p<0.01 t=80 vs. t=60; n=4–11/group). Mean (+SEM) overflow difference from baseline (t=200 – t=180) following i.v. nicotine (30 μg/kg × 2 inj, i.v.) is shown for DA (F) and 5-HT (H). Nicotine significantly elevated DA levels in the (±)- and (+)TCP-pretreated group, with similar trends for 5-HT overflow, although the results did not pass Bonferroni correction for multiple comparisons (*p<0.05, **p<0.01 t=200 vs. t=180; n=2–7/group). For 5-HT, the groups that have n=2 are the saline (i.p.) pretreated animals given saline (i.v) and the saline (i.p.) pretreated animals given nicotine (i.v.). Mean (+SEM) overflow difference from baseline (t=200 – t=180) following i.v. saline are not shown.
Figure 4. Effects of elimination of nicotine or cues during nicotine self-administration.

Mean reinforced responses as a percentage of baseline (+ SEM) were plotted for each treatment group. The day after steady nicotine self-administration (FR1) had been achieved, either the nicotine (7.5 μg/kg/inj, i.v.) solution was replaced with saline (i.v.) (white bars) or the house and cue lights were inactivated (gray bars) during the self-administration sessions. A. (±)TCP-trained animals showed no change in responding after saline was substituted for nicotine (Sal + Cues), but significantly decreased responding after cues were eliminated (Nic + No Cues; *p<0.05 vs. Baseline (Nic + Cues), n=6/group). B. Conversely, (+)TCP-trained animals decreased responding following saline (i.v.) substitution (**p<0.01 vs. Baseline (Nic + Cues), n=7–9/group), but not after cue elimination.
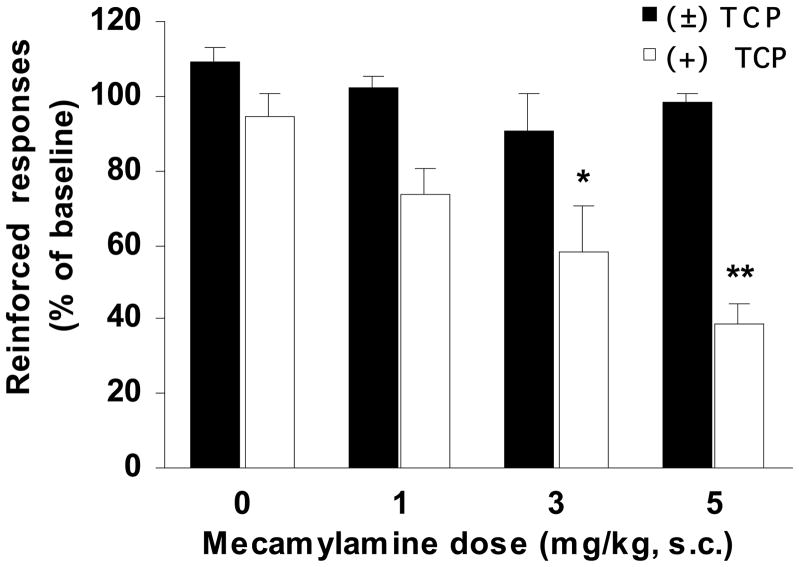
Figure 5. Antagonism of nicotinic receptors.
The day after steady nicotine self-administration (FR1) had been achieved, animals received (±)TCP (3 mg/kg, i.p.; black bars) or (+)TCP (1.5 mg/kg, i.p.; white bars) pretreatment and 45 min later either a saline (0.5 ml/kg, s.c.) or mecamylamine injection (1, 3 or 5 mg/kg, s.c.). Fifteen minutes later animals were allowed to self-administer nicotine (7.5 μg/kg/inj.). Mecamylamine had no affect in the (±)TCP-trained group (n=6/group), but caused a significant drop in responding in the (+)TCP-group (*p<0.05; **p<0.005 vs. baseline, n=6–7/group). Mean reinforced responses (+SEM) were plotted for each treatment group.
Figure 6. Antagonism of serotonergic receptors.
The day after steady nicotine self-administration (FR1) had been achieved, animals received (±)TCP (3 mg/kg, i.p.; black bars) or (+)TCP (1.5 mg/kg, i.p.; white bars) pretreatment and 45 min later a ketanserin (1 mg/kg, s.c.) injection. Ketanserin caused a drop in responding in the (±)TCP-treated group (*p=0.008 vs. baseline, n=6), but not in the (+)TCP-trained group (n=5). Mean reinforced responses (+SEM) were plotted for each treatment group.
2.8. Materials
Materials used in our current studies were obtained as previously published (Villégier et al., 2007a; Villégier et al., 2007b).
3. RESULTS
3.1. All TCP treatments significantly inhibit MAO activity
Adult male rats were pretreated with either one or three exposure of TCP isomers ((+) or (−)) or 3 exposure of (±)TCP or saline and were sacrificed 1 hr after the last injection (Supplementary Fig. 1). Our data for the 20 hr study was previously published in (Villégier et al., 2007b), and is included for the purpose of comparison. As previously reported, the number of days of pretreatment after 20 hr (±)TCP administration did not influence MAO inhibition, thus the data was combined across days of exposure (Villégier et al., 2007b). We observed no main effect for number of pretreatments by MAO A [F1,11 = 0.27, p = n.s.] and B [F1,11 = 0.73, p = n.s.] inhibition for both stereoisomers, (+) and (−)TCP, thus the data for the 1 and 3 day exposure were pooled.
There was an overall effect of pretreatment on both MAO-A [F4,25 = 33.79, p < 0.0001] and MAO-B activity [F4,25 = 83.21, p < 0.0001], with all TCP pretreatments producing significant inhibition (Table 1). The 1-hr pretreatment with (±)- or (+)TCP resulted in almost complete (≈ 100%) inhibition of both MAO-A and –B activities, and although still substantial, less enzyme inhibition was observed following 20-hr (±)TCP (70–90%) and 1-hr (−)TCP pretreatments (60–70%) (Table 1).
3.2. Differential effects of TCP pretreatment on nicotine self-administration
In comparing treatment groups at FR1, there were significant effects of TCP pretreatment [F3,53 = 2.72, p ≤ 0.05], Day [F4,212 = 13.19, p < 0.001], Reinforced/Non-Reinforced responses [F1,53 = 47.34, p < 0.001], and interactions of TCP × Day [F12,212 = 1.86, p < 0.05] and Reinforced/Non-Reinforced × Day [F4,212 = 11.20, p < 0.001]. Following 1-hr pretreatment with (±)TCP (Fig. 1A), there was rapid acquisition of nicotine self-administration as indicated by significant effects of Day [F4,80 = 9.98, p < 0.001] and Reinforced/Non-Reinforced [F1,20 = 31.04, p < 0.001], and a significant interaction of Day × Reinforced/Non-Reinforced [F4,80 = 7.71, p < 0.001]. Post-hoc analysis showed significant acquisition of self-administration, as defined by a significant difference between reinforced and non-reinforced responding, on Day 2. In contrast, 20-hr pretreatment with (±)TCP (Fig. 1B) resulted in much weaker nicotine self-administration, as indicated by only a significant effect of Reinforced/Non-Reinforced [F1,11 = 10.32, p < 0.01], but not Day [F4,44 = 0.87, p = n.s.] or Day × Reinforced/Non-Reinforced [F4,44 = 1.62, p = n.s.].
The 1-hr pretreatment with (+)TCP (Fig. 1C) induced significant nicotine self-administration, although with a slower onset than that of (±)TCP. ANOVA showed a significant effect of Day [F4,44 = 9.92, p < 0.001] and Reinforced/Non-Reinforced [F1,11 = 12.83, p < 0.01], and a significant interaction of Day × Reinforced/Non-Reinforced [F4,44 = 5.02, p < 0.01]. Significant acquisition of self-administration was not seen until day 4. Nicotine self-administration following 1-hr pretreatment with (−)TCP (Fig. 1D) was weaker than that following the (+) isomer, but was still significant. There were significant effects of Day [F4,44 = 2.52, p ≤ 0.05], Reinforced/Non-Reinforced [F1,11 = 6.05, p < 0.05] and a significant interaction of Day × Reinforced/Non-Reinforced [F4,44 = 2.69, p < 0.05]. Significant self-administration was observed on day 3, although the overall number of responses was lower than that in animals pretreated with (±)- or (+)TCP.
Fig. 2 shows the effect of TCP pretreatments on nicotine self-administration as task difficulty increased for all animals that completed ratios of FR1 (Day 1–5), FR2 (Days 6 & 7) and FR5 (Days 8 & 9). There were significant overall effects of TCP pretreatment [F3,37 = 4.64, p < 0.01], Day [F4,148 = 11.17, p < 0.001], Reinforced/Non-Reinforced [F1,37 = 46.26, p < 0.001], and a significant interaction of Day × Reinforced/Non-Reinforced [F4,148 = 2.94, p < 0.05]. Following 1-hr pretreatment with (±)TCP (Fig. 2A), animals maintained nicotine self-administration as the reinforcement ratio was increased, as indicated by a significant difference between reinforced and non-reinforced responding [F1,13 = 21.94, p < 0.001], but there was a decrease in the number of infusions following introduction of the FR2 schedule. In contrast, the 20-hr pretreatment of (±)TCP (Fig. 2B) did not support nicotine self-administration at higher reinforcement schedules [Reinforced/Non-Reinforced: F1,7 = 4.10, p = n.s.] and there was a significant decline in infusions with increasing ratios [Day: F4,28 = 3.86, p < 0.05]. Pretreatment with (+)TCP (Fig. 2C) maintained nicotine self-administration at all ratios, much as (±)TCP did, but the decrease in infusions taken on higher ratios was not statistically significant. The number of infusions after (−) TCP (Fig. 2D), while generally lower than other pretreatments, were still significant [Reinforced/Non-Reinforced: F1,9 = 6.58, p < 0.05; Day: F4,36 = 6.83, p < 0.001; Day × Reinforced/Non-Reinforced: F4,36 = 2.92, p < 0.05] but did not exhibit stable nicotine self-administration at higher order schedules of reinforcement.
When nicotine self-administration breakpoint values were examined, there was a significant overall effect of TCP pretreatment [F3,37 = 3.436, p < 0.05] and a significant difference between the (±) and (-) isomers (Table 2). These combined results suggest that mechanisms independent of MAO inhibition may be important factors influencing nicotine self-administration behavior at FR1 or higher order schedules of reinforcement.
3.3. Differential effects of TCP pretreatment on monoamine overflow in the nucleus accumbens
We have previously reported the effects of (±)TCP and nicotine on extracellular DA and 5-HT overflow in the brain (Villégier et al., 2007a; Villégier et al., 2007b; Villégier et al., 2006a). We now further demonstrate the distinct differences between the effects of TCP stereoisomers and the 20-hr (±)TCP pretreatment on both DA and 5-HT overflow in the nucleus accumbens shell (Fig. 3, Supplementary Fig. 4).
Daily pretreatments with TCP and its stereoisomers resulted in differing basal levels of DA and 5-HT overflow in the nucleus accumbens (Fig. 3A–D, Table 3). Significant effects of pretreatment were observed for baseline DA levels (F4,40 = 6.43, p < 0.001) and baseline 5-HT levels (F4,30 = 3.13, p < 0.05), confirming that TCP pretreatment significantly enhances extracellular neurotransmitter overflow in reward centers of the brain (Fig. 3A–D, Table 3). Baseline DA levels were significantly elevated in 1-hr (±)TCP, 1-hr (+)TCP, and 20-hr (±)TCP-pretreated groups, as compared to saline, whereas 5-HT levels were only significantly increased by pretreatment with 1 hr (+)TCP (Fig. 3A–D, Table 3).
Analysis of the effects of the third daily pretreatment of TCP at t=60 min are shown in Fig. 3E & G. Given the lack of difference observed between treatment groups at the t=60 time point (Fig. 3A & C), data was pooled for the analysis in Fig. 3E & G. Results represent the difference between neurotransmitter levels 20 min after the third TCP or vehicle control pretreatment injection (t=80 min) subtracted from baseline values (t=60 min) on extracellular levels of DA and 5-HT overflow, as shown in Fig. 3E & G, respectively. For DA overflow (Fig. 3E), there were significant effects of TCP treatment [F4,40 = 6.07, p < 0.001], Time [F1,40 = 34.89, p < 0.001] and an interaction of TCP treatment × Time [F4,40 = 13.52, p < 0.001]. Post-hoc analyses showed that acute injection of both (±)- and (−)TCP induced significant release of DA, whereas (+)TCP had a less prominent effect. For 5-HT overflow (Fig. 3G), there were significant effects of TCP treatment [F4,30 = 3.97, p < 0.05], Time [F1,30 = 7.00, p < 0.05] and an interaction of TCP treatment × Time [F4,30 = 11.83, p < 0.001]. Post-hoc analyses showed that acute injection of both (±)- and (−)TCP induced significant release of 5-HT, while no effects were observed for the other pretreatments.
Acute treatment with TCP and its stereoisomers had differential effects on nicotine-induced overflow of DA and 5-HT overflow, as measured by the difference in extracellular transmitter levels prior to and following nicotine injection (t=200 – t=180). Nicotine induced DA overflow (Fig. 3F) was significantly influenced by TCP treatment [F4,24 = 8.28, p < 0.001], Time [F1,24 = 22.53, p < 0.001] and there was a significant interaction of TCP treatment × Time [F4,24 = 6.31, p < 0.01]. Post-hoc analyses showed significant nicotine-induced DA overflow in animals acutely pretreated with (±)- and (+)TCP (by 50–75%, or 21–33 pg/20 μl), but not in the other treatment groups. Notably, while saline and 20-hr (±) TCP pretreated animals had an increase in nicotine-induced dopamine release (≈30%, 0.89 & 5.56 pg/20μl, respectively, (Fig. 3F)), the increases were not significantly different from baseline. Nicotine-induced 5-HT overflow (Fig. 3H) was significantly influenced by TCP treatment [F4,16 = 13.51, p < 0.001], Time [F1,16 = 8.79, p < 0.01], with a significant interaction of TCP treatment × Time [F4,16 = 3.14, p < 0.05]. Post-hoc analyses revealed that (±)TCP pretreatment enhances nicotine-induced 5-HT release (p ≤ 0.05) with a trend for (+)TCP treated animals (p = n.s.), although the results did not pass Bonferroni correction for multiple comparisons. Importantly, DA and 5-HT-induced release were not observed after i.v. saline control injections in the animals given the various pretreatments (Fig. 3C & D). The finding demonstrates that i.v. nicotine, and not the injection alone, is the mediator of the increase in neurotransmitter release.
Taken together, the findings suggest that the (+) and (−) stereoisomers operate by different mechanisms that combine to produce the overall neurochemical effect of (±)TCP. Whereas (±)TCP-induced acute enhancement of extracellular levels of DA and 5-HT is primarily mediated by the actions of the (−) isomer, with negligible to minor contributions by the (+) isomer, the enhancement of nicotine-induced neurotransmitter release mainly reflects the activity of the (+) isomer (Fig. 3).
3.4. Different mechanisms underlie (±)TCP and (+)TCP enhancement of nicotine self-administration
Additional studies were undertaken to determine whether there were distinct mechanisms underlying the enhancement of nicotine self-administration by (±) and (+)TCP pretreatments (Figs. 4–6). When responding following elimination of drug-associated cues (Nic + No Cues) or i.v. nicotine (Sal + Cues) was compared to the prior day’s baseline response (Nic + Cues), significant effects were seen for Day [F1,24 = 33.94, p < 0.001] and Reinforced/Non-Reinforced responses [F1,24 = 155.837, p < 0.001], with a significant interaction of TCP pretreatment × Treatment × Reinforced/Non-Reinforced × Day [F1,24 = 4.75, p < 0.05]. As shown in Fig. 4, removal of either drug-associated cues (Nic + No Cues) or nicotine from the i.v. injection (Sal + Cues) had different effects on reinforced responding in animals that received (±)TCP pretreatment as compared to (+)TCP. In (±)TCP-pretreated animals (Fig. 4A), cue removal significantly decreased reinforced responding whereas nicotine removal did not. In contrast, the opposite was found for the (+)TCP-pretreated group (Fig. 4B). These findings indicate that animals pretreated with (+)TCP found nicotine more reinforcing than conditioned cues, whereas the opposite was the case for those pretreated with (±)TCP.
Pretreatment with the antagonist, mecamylamine (Mec), provided further evidence for a differential role of nicotinic receptors in maintaining stable responding under the two pretreatment conditions (Fig. 5). When reinforced responding in the presence of Mec (0–5 mg/kg, s.c.) was compared with the prior day baseline response, there were significant effects of TCP Pretreatment [F1,41 = 19.70, p < 0.001], Day [F1,41 = 45.00, p < 0.0001], and interactions of Day × Mec dose [F3,41=7.93, p < 0.001] and Day × TCP pretreatment [F2,41=31.56, p < 0.0001]. Whereas Mec dose-dependently reduced self-administration in (+)TCP pretreated animals, it had no significant effect at any dose tested in animals pretreated with (±)TCP (Fig. 5). These findings confirm that nicotinic receptors are critical for maintaining self-administration in animals pretreated with (+)TCP, but not in those pretreated with (±)TCP.
Subsequent studies demonstrated that 5-HT receptors were important for maintaining responding in animals pretreated with (±)TCP (Fig. 6). Following treatment with the 5-HT2a/c receptor antagonist, ketanserin (1 mg/kg, s.c.), there was a significant effect of Day [F1,9 = 10.82, p < 0.01] and a significant interaction of Day × TCP Pretreatment [F2,9 = 8.64, p < 0.05]. Whereas reinforced responding was significantly reduced by ketanserin in animals pretreated with (±)TCP, it had no effect on those pretreated with (+)TCP, providing critical evidence for the role of 5-HT receptors in maintaining nicotine self-administration behavior in (±)TCP treated animals (Fig. 6).
4. DISCUSSION
Growing evidence implicates MAO inhibitors in the mechanisms underlying tobacco addiction. We and others have shown enhanced nicotine self-administration after 1-hr pretreatment with (±)TCP. However, since (±)TCP has a number of acute effects other than the inhibition of MAO, the purpose of this study was to understand the mechanism(s) responsible for (±)TCP enhancement of reinforced responding. Our findings illustrate that the acute pharmacological off-target effects of TCP, such as the blockade of DA and 5-HT uptake, as well as nicotine-paired cues, may act as primary factors mediating the acquisition and maintenance of nicotine self-administration behavior, independent of MAO inhibition (for summary, see Table 4).
Table 4.
Summary of Behavioral and Neurochemical Findings
| Pretreatment | |||||
|---|---|---|---|---|---|
| Experiment | Saline | 20-hr (±)TCP | (−)TCP | (+)TCP | (±)TCP |
| MAO A & B Inhibition (MAO Assay) | − | + | + | + | + |
| Acute TCP-Induced DA Release (Microdialysis) | − | − | + | + Minimal Effect | + |
| Acute TCP-Induced 5-HT Release (Microdialysis) | − | − | + | − | + |
| Nicotine-Induced DA Release (Microdialysis) | − | − | − | + | + |
| Nicotine-Induced 5-HT Release (Microdialysis) | − | − | − | − | − |
| FR1 Nicotine Self-Administration | − | + Minimal Effect | + Minimal Effect | + On Days 4–5 | + |
| FR2–5 Nicotine Self- Administration | − | − | + Minimal Effect | + | + |
| PR Nicotine Self- Administration | − | − | − | − | + |
| nAChR Blockade (Mec) Nicotine Self-Administration | N/A | N/A | N/A | + | − |
| Saline Substitution For i.v. Nicotine Self-Administration | N/A | N/A | N/A | + | − |
| Cue Elimination Nicotine Self-Administration | N/A | N/A | N/A | − | + |
| 5-HT Receptor Blockade Ketanserin Nicotine Self-Administration | N/A | N/A | N/A | − | + |
| Saline i.p. Substitution Nicotine Self-Administration | N/A | N/A | N/A | + | + |
| Norharmane i.p. Substitution Nicotine Self-Administration | N/A | N/A | N/A | N/A | + |
(−) = Not significantly influenced or behavior not observed; (+) = Significant effect observed; N/A = Not Applicable (Experiment Not Performed); FR: Fixed Ratio, PR: Progressive Ratio
4.1. The Role of MAO inhibition In TCP Enhancement of Nicotine Self Administration Behavior
Previous studies have proposed that nicotine self-administration may be enhanced because of the well known MAO inhibiting potency of (±)TCP (ED50=0.18 mg/kg), (+)TCP (ED50=0.15 mg/kg) and (−)TCP (ED50=0.64 mg/kg) (Fuentes et al., 1976; Hampson et al., 1986). However, besides MAO inhibition, TCP has other pharmacological effects. For example, (±)TCP can inhibit the reuptake of norepinephrine (IC50 and ID50=0.43–1.2 μM) and dopamine (IC50 and ID50=1.7–4.78 μM), with (−)TCP (ID50=0.045–1.2 μM) having greater potency than the (+) isomer (ID50=1.5–4 μM) in mediating the effect (Baker et al., 1992; Horn and Snyder, 1972). Others have replicated the relative differences in the (+) and (−) stereoisomers, demonstrating that at 1–10μM concentrations, (−)TCP more potently blocked norepinephrine (73% inhibition), dopamine (74% inhibition) and serotonin (51% inhibition) reuptake than the (+) isomer (60, 49 and 36% inhibition, respectively) (Hampson et al., 1986). These findings are in line with our current results that demonstrate that the (−) isomer of TCP has a significant enhancement of DA and 5-HT overflow 20 min post-injection, while the (+) isomer only modestly influences DA release. Given these results, the enhancement of nicotine self-administration behavior after 1 hr (±)TCP pretreatment appear to be primarily mediated through the acute effects of monoamine reuptake inhibition. As a first step in disentangling the mechanisms responsible for TCP enhancement of nicotine self-administration, we used two distinct paradigms. First, to decrease the influence of the acute effects of (±)TCP, we used a 20 hr pretreatment paradigm to significantly inhibit MAO A and B activity (Villégier et al., 2007b). Second, we compared 1 hr pretreatment with (±)TCP (3mg/kg) to that of its equal concentrations of enantiomeric isomers, 50% (+) (1.5 mg/kg) - and 50% (−) (1.5 mg/kg) TCP, in order to distinguish the role of each isomer in their interactive affects with nicotine. The dose of each isomer (1.5 mg/kg, i.p) was used to specifically evaluate how they individually contribute to the optimal reported dose of (±)TCP (3mg/kg) (Villégier et al., 2007b) in mediating the enhancement of nicotine self-administration and monoamine release in the nucleus accumbens.
In the first approach, following 20 hr pretreatment with (±)TCP, MAO A and B were inhibited by nearly 70–90%, as previously reported (Villégier et al., 2007b). However, this substantial inhibition of MAO, which is greater than that found in smokers (30–40%) (Fowler et al., 1996a; Fowler et al., 1996b), was only associated with weak to negligible: (i) nicotine induced dopamine release in the nucleus accumbens (30%) and (ii) acquisition and maintenance of nicotine self-administration behavior. The findings resemble those of saline pretreated animals (with MAO activity fully functional), which do not acquire nicotine self-administration during, at least, the first 5 days of FR1 responding and weak nicotine-induced dopamine release in the nucleus accumbens (≈30%), as similarly found by our group (Belluzzi et al., 2005; Villégier et al., 2007a; Villégier et al., 2007b) and others (Pontieri et al., 1996). Taken together, the results provide evidence that MAO inhibition, is not involved in the mechanism influencing (±) or (+)TCP-induced enhancement of nicotine self-administration behavior.
In contrast, animals administered the 1 hr pretreatment of (−)TCP induced quicker, albeit weak, acquisition of nicotine self-administration during the first 5 days of FR1 responding, even though this treatment produced similar inhibition of MAO activity (60–70%), as compared with the 20-hr pretreatment paradigm (70–90%). Furthermore, the 1 hr pretreatment with (+)TCP induced slower acquisition of nicotine self-administration than (−) and (±)TCP pretreated animals, even though (+)TCP induced MAO inhibition to a maximal extent (100%). Thus, we found little relationship between the degree of MAO inhibition and the enhanced acquisition of nicotine self-administration at a FR1 schedule. Moreover, both (−)TCP and 20hr TCP treated animals failed to demonstrate stable nicotine self-administration behavior at higher order schedules of reinforcement, in contrast to the (±) or (+)TCP isomer treated animals. Such effects may be driven by the lack of nicotine-induced dopamine release in (−)TCP and 20hr TCP treated animals. Together, the findings provide supportive evidence that mechanisms outside of MAO inhibition mediate TCP enhancement of nicotine self-administration behavior. It is important to note that while we do not observe stable nicotine self-administration in saline pretreated animals, it is well known that animals with MAO activity fully functional can acquire and maintain stable nicotine self-administration behavior if testing conditions are appropriately optimized, including: longer acquisition days and times (i.e. more than 5 days on an FR1 schedule) with the use of higher nicotine doses, food restriction, and prior training, to name a few (for examples, please see (Clemens et al., 2010; De Vries et al., 2005; Diergaarde et al., 2008; Donny et al., 1998). These differences are likely due to the use of our stringent acquisition paradigm that does not include prior training or food restriction and uses a relatively low dose of nicotine. Variability across laboratories performing intravenous nicotine self-administration has been observed. Authors have started to test possible reasons for this variability, please see (Clemens et al., 2010; Donny et al., 1998).
4.2. Mechanisms underlying TCP enhancement of self-administration behavior
4.2.1. Role of nAChRs and Cues
In order to better understand the pharmacological, and non-pharmacological, mechanisms mediating (±)TCP induced changes in nicotine self-administration behavior, we have evaluated the effects of blocking nAChRs with mecamylamine (a nonselective and noncompetitive antagonist), the elimination of i.v. nicotine or nicotine-paired cues on sustained self-administration responding. Our results reveal that (+)TCP pretreatment induces stable self-administration behavior that is mediated via activation of nicotinic receptors, as shown by decreased responding following removal of nicotine from the i.v. solution or nicotinic receptor antagonism. In contrast, pretreatment with (±)TCP results in stable reinforced responding that is insensitive to nicotinic receptor blockade. Whether these effects hold true over longer periods of mecamylamine exposure would need to be investigated. For the purpose of this study, however, we chose not to evaluate the mechanisms of extinction in (±)TCP pretreated animals. Indeed, it has previously been shown that nicotine, whether given contingently or non-contingently, can enhance the salient properties of non-nicotine stimuli, such as cue lights or auditory stimuli (Chaudhri et al., 2006). We now show that animals treated with (±)TCP, and trained on nicotine and cues, are sensitive to removal of cue associated stimuli. This decrease in responding is not seen in (+)TCP treated animals. Taken together, the data suggest that after the acquisition of stable nicotine self-administration, non-pharmacological cues, at least in part, have enhanced reinforcing properties over intravenous nicotine in (±)TCP treated animals, with opposite effects observed in animals treated with (+)TCP.
4.2.2. Role of 5-HT
(±)TCP has long been known to act on serotonergic systems (Smith, 1980), which can influence locomotor function in animals (Villégier et al., 2006a) and nicotine self-administration (Villégier et al., 2010). Our data provide further evidence that 5-HT2A/C mechanisms are important in mediating the effects of (±)TCP most likely through the 5-HT releasing properties of the (−) isomer. In contrast, 5-HT2A/C mechanisms were not observed in animals treated with the (+) isomer, thereby suggesting differential mechanisms for increased nicotine self-administration for the individual isomers of TCP. These findings are in line with our laboratory’s recently published data showing that serotonergic depletion, through p-chloroamphetamine (PCA) exposure, can increase nicotine self-administration in MAO inhibited animals (Villégier et al., 2010). These effects could be blocked by both ketanserin and ritanserin, two independent 5-HT receptor antagonists. Therefore, either through serotonergic depletion or through (±)TCP exposure, 5-HT2/C receptors appear to be critical players in mediating nicotine self-administration behavior.
The work of Levin and colleagues has provided important rationale for the use of ketanserin as a smoking cessation therapy (Levin et al., 2005; Levin et al., 2008; Rezvani et al., 2005). While the 5-HT2A and 2C receptors appear to have opposing effects on dopamine activity in reward related centers of the brain (Di Matteo et al., 2002), blockade of both can significantly reduce nicotine self-administration (Levin et al., 2008) and cocaine’s locomotor effects (Filip et al., 2001; McMahon and Cunningham, 2001). Whether 5-HT2A/C receptor blockade can similarly inhibit the reinforcing properties of cigarette smoke constituents interacting with nicotine still needs to be investigated. Such findings have clinical implications for the use of ketanserin as a potential smoking cessation therapy.
Given the significant limitation that tranylcypromine is not found in tobacco smoke and has a series of off-target effects, we propose that the use of this drug, albeit a helpful proof-of concept model, may not represent the optimal method for studying underlying mechanisms tobacco addiction in human smokers.
4.4. Conclusions
Our findings suggest that MAO inhibition is not the mediating factor influencing (±)TCP-induced enhancement of nicotine self-administration behavior. We propose that more complex mechanisms may be involved, where off-target effects including monoamine release (i.e. 5-HT, NE and DA), are involved in the enhancement of nicotine reinforcement. Such effects could influence the reinforcing properties of drug-associated stimuli that are linked with nicotine intake. Future studies aimed at characterizing the pharmacological properties of non-nicotine smoke constituents are needed to better understand the mechanisms underlying tobacco addiction.
Supplementary Material
Acknowledgments
Our research studies were supported by PHS Grant DA19138, DA21267 and a TRDRP fellowship, 13DT-0033. The authors would like to thank Isabel Canto and Yali Yu for their contributions to this study and Alex Kempf for his artistic contributions to the supplementary figure cartoons.
Footnotes
5. Disclosure/Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Monica M. Arnold, Email: marnold@uci.edu.
Derk J. Hogenkamp, Email: dhogenka@uci.edu.
Kelvin W. Gee, Email: kwgee@uci.edu.
James D. Belluzzi, Email: belluzzi@uci.edu.
Frances M. Leslie, Email: fmleslie@rgs.uci.edu.
References
- ACS. Cancer Facts & Figures 2010. American Cancer Society; Atlanta: 2010. [Google Scholar]
- Aspeslet LJ, Baker GB, Coutts RT, Mousseau DD. A gas chromatographic procedure for separation and quantitation of the enantiomers of the antidepressant tranylcypromine. Biochem Pharmacol. 1992;44:1894–1897. doi: 10.1016/0006-2952(92)90088-z. [DOI] [PubMed] [Google Scholar]
- Baker GB, Coutts RT, McKenna KF, Sherry-McKenna RL. Insights into the mechanisms of action of the MAO inhibitors phenelzine and tranylcypromine: a review. J Psychiatry Neurosci. 1992;17:206–214. [PMC free article] [PubMed] [Google Scholar]
- Baker GB, Sowa B, Todd KG. Amine oxidases and their inhibitors: what can they tell us about neuroprotection and the development of drugs for neuropsychiatric disorders? J Psychiatry Neurosci. 2007;32:313–315. [PMC free article] [PubMed] [Google Scholar]
- Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology (Berl) 2004;174:389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Berlin I, Anthenelli RM. Monoamine oxidases and tobacco smoking. Int J Neuropsychopharmacol. 2001;4:33–42. doi: 10.1017/S1461145701002188. [DOI] [PubMed] [Google Scholar]
- Bohadana A, Nilsson F, Rasmussen T, Martinet Y. Nicotine inhaler and nicotine patch as a combination therapy for smoking cessation: a randomized, double-blind, placebo-controlled trial. Arch Intern Med. 2000;160:3128–3134. doi: 10.1001/archinte.160.20.3128. [DOI] [PubMed] [Google Scholar]
- CDC, CfDCaP; CDC. Morbidity and Mortality Weekly Report. 2008. Annual Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity Losses—United States, 2000–2004; pp. 1226–1228. [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Caille S, Cador M. The effects of response operandum and prior food training on intravenous nicotine self-administration in rats. Psychopharmacology (Berl) 2010;211:43–54. doi: 10.1007/s00213-010-1866-z. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Caille S, Stinus L, Cador M. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. Int J Neuropsychopharmacol. 2009;12:1355–1366. doi: 10.1017/S1461145709000273. [DOI] [PubMed] [Google Scholar]
- De Vries TJ, de Vries W, Janssen MC, Schoffelmeer AN. Suppression of conditioned nicotine and sucrose seeking by the cannabinoid-1 receptor antagonist SR141716A. Behav Brain Res. 2005;161:164–168. doi: 10.1016/j.bbr.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Cacchio M, Di Giulio C, Esposito E. Role of serotonin(2C) receptors in the control of brain dopaminergic function. Pharmacol Biochem Behav. 2002;71:727–734. doi: 10.1016/s0091-3057(01)00705-5. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology (Berl) 1998;136:83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- Filip M, Nowak E, Papla I. On the role of serotonin2A/2C receptors in the sensitization to cocaine. J Physiol Pharmacol. 2001;52:471–481. [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R, Alexoff D, Shea C, Schlyer D, Wolf AP, Warner D, Zezulkova I, Cilento R. Inhibition of monoamine oxidase B in the brains of smokers. Nature. 1996a;379:733–736. doi: 10.1038/379733a0. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, Shea C, Alexoff D, MacGregor RR, Schlyer DJ, Zezulkova I, Wolf AP. Brain monoamine oxidase A inhibition in cigarette smokers. Proc Natl Acad Sci U S A. 1996b;93:14065–14069. doi: 10.1073/pnas.93.24.14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke RM, Park M, Belluzzi JD, Leslie FM. Prenatal nicotine exposure changes natural and drug-induced reinforcement in adolescent male rats. Eur J Neurosci. 2008;27:2952–2961. doi: 10.1111/j.1460-9568.2008.06253.x. [DOI] [PubMed] [Google Scholar]
- Fuentes JA, Oleshansky MA, Neff NH. Comparison of the apparent antidepressant activity of (−) and (+) tranylcypromine in an animal model. Biochem Pharmacol. 1976;25:801–804. doi: 10.1016/0006-2952(76)90150-7. [DOI] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, Stinus L. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J Neurosci. 2005;25:8593–8600. doi: 10.1523/JNEUROSCI.2139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, Stinus L. Monoamine oxidase A rather than monoamine oxidase B inhibition increases nicotine reinforcement in rats. Eur J Neurosci. 2006;24:3532–3540. doi: 10.1111/j.1460-9568.2006.05217.x. [DOI] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Koob GF, Cador M, Stinus L. Monoamine oxidase inhibition dramatically prolongs the duration of nicotine withdrawal-induced place aversion. Biol Psychiatry. 2008;63:158–163. doi: 10.1016/j.biopsych.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Hampson DR, Baker GB, Coutts RT. A comparison of the neurochemical properties of the stereoisomers of tranylcypromine in the central nervous system. Cell Mol Biol. 1986;32:593–599. [PubMed] [Google Scholar]
- Henningfield JE, Miyasato K, Jasinski DR. Abuse liability and pharmacodynamic characteristics of intravenous and inhaled nicotine. J Pharmacol Exp Ther. 1985;234:1–12. [PubMed] [Google Scholar]
- Horn AS, Snyder SH. Steric requirements for catecholamine uptake by rat brain synaptosomes: studies with rigid analogs of amphetamine. J Pharmacol Exp Ther. 1972;180:523–530. [PubMed] [Google Scholar]
- Kaiser C, Lester BM, Zirkle CL. 2-Substituted Cyclopropylamines. I. Derivatives and Analogs of 2-Phenylcyclopropylamine. J Med Pharm Chem. 1962;91:1243–1265. doi: 10.1021/jm01241a017. [DOI] [PubMed] [Google Scholar]
- Khalil AA, Steyn S, Castagnoli N., Jr Isolation and characterization of a monoamine oxidase inhibitor from tobacco leaves. Chem Res Toxicol. 2000;13:31–35. doi: 10.1021/tx990146f. [DOI] [PubMed] [Google Scholar]
- Leslie FM, Belluzzi JD, Lotfipour S, Arnold, Villégier AS. Positive Effects of Monoamine oxidase inhibitors on the acquisition of nicotine self-administration in rats. SRNT; Dublin, Ireland: 2009. [Google Scholar]
- Levin E, Icenogle L, Farzad A. Ketanserin attenuates nicotine-induced working memory improvement in rats. Pharmacol Biochem Behav. 2005;82:289–292. doi: 10.1016/j.pbb.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Levin ED, Slade S, Johnson M, Petro A, Horton K, Williams P, Rezvani AH, Rose JE. Ketanserin, a 5-HT2 receptor antagonist, decreases nicotine self-administration in rats. Eur J Pharmacol. 2008;600:93–97. doi: 10.1016/j.ejphar.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A, Miller JH, Lea RA. Monoamine oxidase and tobacco dependence. Neurotoxicology. 2007;28:182–195. doi: 10.1016/j.neuro.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Lotfipour S, Villégier AS, Belluzzi JD, Leslie FM. The Role of Monoamine Oxidase Inhibition in Tranylcypromine Enhancement of Nicotine Reward. Society for Neuroscience; San Diego, California: 2007. [Google Scholar]
- McMahon LR, Cunningham KA. Antagonism of 5-hydroxytryptamine(2a) receptors attenuates the behavioral effects of cocaine in rats. J Pharmacol Exp Ther. 2001;297:357–363. [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Nides M. Update on pharmacologic options for smoking cessation treatment. Am J Med. 2008;121:S20–31. doi: 10.1016/j.amjmed.2008.01.016. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Khroyan TV. Rodent models of nicotine reward: what do they tell us about tobacco abuse in humans? Pharmacol Biochem Behav. 2009;91:481–488. doi: 10.1016/j.pbb.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MK, Belluzzi JD, Han SH, Cao J, Leslie FM. Age, sex and early environment contribute to individual differences in nicotine/acetaldehyde-induced behavioral and endocrine responses in rats. Pharmacol Biochem Behav. 2007;86:297–305. doi: 10.1016/j.pbb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: 1986. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Caldwell DP, Levin ED. Nicotinic-serotonergic drug interactions and attentional performance in rats. Psychopharmacology (Berl) 2005;179:521–528. doi: 10.1007/s00213-004-2060-y. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Johnson M. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacol Biochem Behav. 2000;67:71–81. doi: 10.1016/s0091-3057(00)00301-4. [DOI] [PubMed] [Google Scholar]
- Smith DF. Lithium and motor activity of animals: effects and possible mechanism of action. Int Pharmacopsychiatry. 1980;15:197–217. doi: 10.1159/000468440. [DOI] [PubMed] [Google Scholar]
- Villégier AS, Belluzzi JD, Leslie FM. Serotonergic mechanism underlying tranylcypromine enhancement of nicotine self-administration. Synapse. 2010 doi: 10.1002/syn.20864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villégier AS, Blanc G, Glowinski J, Tassin JP. Transient behavioral sensitization to nicotine becomes long-lasting with monoamine oxidases inhibitors. Pharmacol Biochem Behav. 2003;76:267–274. doi: 10.1016/s0091-3057(03)00223-5. [DOI] [PubMed] [Google Scholar]
- Villégier AS, Lotfipour S, Belluzzi JD, Leslie FM. Involvement of alpha1-adrenergic receptors in tranylcypromine enhancement of nicotine self-administration in rat. Psychopharmacology (Berl) 2007a;193:457–465. doi: 10.1007/s00213-007-0799-7. [DOI] [PubMed] [Google Scholar]
- Villégier AS, Lotfipour S, McQuown SC, Belluzzi JD, Leslie FM. Tranylcypromine enhancement of nicotine self-administration. Neuropharmacology. 2007b;52:1415–1425. doi: 10.1016/j.neuropharm.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Villégier AS, Salomon L, Blanc G, Godeheu G, Glowinski J, Tassin JP. Irreversible blockade of monoamine oxidases reveals the critical role of 5-HT transmission in locomotor response induced by nicotine in mice. Eur J Neurosci. 2006a;24:1359–1365. doi: 10.1111/j.1460-9568.2006.05011.x. [DOI] [PubMed] [Google Scholar]
- Villégier AS, Salomon L, Granon S, Changeux JP, Belluzzi JD, Leslie FM, Tassin JP. Monoamine oxidase inhibitors allow locomotor and rewarding responses to nicotine. Neuropsychopharmacology. 2006b;31:1704–1713. doi: 10.1038/sj.npp.1300987. [DOI] [PubMed] [Google Scholar]
- Wooters TE, Bardo MT. The monoamine oxidase inhibitor phenelzine enhances the discriminative stimulus effect of nicotine in rats. Behav Pharmacol. 2007;18:601–608. doi: 10.1097/FBP.0b013e3282eff0d5. [DOI] [PubMed] [Google Scholar]
- Yoshimura RF, Hogenkamp DJ, Li WY, Tran MB, Belluzzi JD, Whittemore ER, Leslie FM, Gee KW. Negative allosteric modulation of nicotinic acetylcholine receptors blocks nicotine self-administration in rats. J Pharmacol Exp Ther. 2007;323:907–915. doi: 10.1124/jpet.107.128751. [DOI] [PubMed] [Google Scholar]
- Zhang W, Kilicarslan T, Tyndale RF, Sellers EM. Evaluation of methoxsalen, tranylcypromine, and tryptamine as specific and selective CYP2A6 inhibitors in vitro. Drug Metab Dispos. 2001;29:897–902. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.