Abstract
Current abstainers from alcohol have been identified as an inadequate reference group in epidemiologic studies of the effects of alcohol, because inclusion of former drinkers might lead to overestimation of the protective effects and underestimation of the detrimental effects of drinking alcohol. The authors’ objective in the current study was to quantify this association for ischemic heart disease (IHD). Electronic databases were systematically searched for relevant case-control or cohort studies published from 1980 to 2010. Thirty-eight articles fulfilled the inclusion criteria, contributing a total of 5,613 IHD events and 12,097 controls among case-control studies and 1,387 events with combined endpoints and 7,183 events stratified by endpoint among 232,621 persons at risk among cohort studies. Pooled estimates for the subset stratified by sex and endpoint showed a significantly increased risk among former drinkers compared with long-term abstainers for IHD mortality ( among men; relative risk = 1.25, 95% confidence interval: 1.15, 1.36; among women relative risk = 1.54, 95% confidence interval: 1.17, 2.03). For IHD morbidity, the estimates for both sexes were close to unity and not statistically significant. Results were robust in several sensitivity analyses. In future studies, researchers should separate former drinkers from the reference category to obtain unbiased effect estimates. Implications for the overall beneficial and detrimental effects of alcohol consumption on IHD are discussed below.
Keywords: alcohol drinking, alcoholic beverages, case-control studies, cohort studies, coronary artery disease, coronary disease, meta-analysis
The commonly reported J-curve for the relation between average alcohol consumption and the risk of ischemic heart disease (IHD) (1) has been challenged for many reasons, most of which apply to all epidemiologic research. Most importantly, researchers have noted problems with selection bias, because subpopulations with high alcohol intake or detrimental drinking patterns were missed in many cohort studies (2), with unresolved issues of residual confounding (3), and with choice of reference group (4). In this report, we focused on the latter. There has been a longstanding debate about the cardioprotective effects of alcohol based on the selection of reference groups. Shaper et al. (4, 5) argued that the appearance of a cardioprotective effect could be mainly due to the “sick quitter effect,” that is, the fact that some people stop drinking for health reasons and thus artificially inflate the risk of IHD among abstainers, even though other reasons for stopping drinking are plausible and have been reported (e.g., loss of control, social consequences, and religious reasons (6)). Although many have reported that there were independent risks for former drinkers (7–9) that did not obviate a protective association, there is no doubt that the selection of the reference group determines the shape of the dose-response relation between average volume of alcohol consumption and IHD risk. In many high-income countries, however, this reference group is relatively small, which makes inferences problematic because of low power. We conducted a systematic review and quantified the risk of former drinkers by separating it from the risk of long-term abstainers. The analyses were stratified by sex and endpoint (mortality vs. morbidity), as several recent epidemiologic studies showed a more pronounced effect of alcohol on mortality rates than on morbidity rates (10, 11).
MATERIALS AND METHODS
Search strategy
We systematically searched for potentially relevant original articles written from January 1980 to the second week of April 2010 in the following electronic databases: MEDLINE, EMBASE, Web of Science (Science Citation Index Expanded, Social Sciences Citation Index, Arts and Humanities Citation Index), ETOH (Alcohol and Alcohol Problems Science Database, National Institute on Alcohol Abuse and Alcoholism (January 1980–December 2003)), and AIM (Alcohol in Moderation, alcohol industry database). Reference lists of identified papers, relevant reviews (12–20), and meta-analyses (1, 21–26) were scrutinized for additional articles. Because of resource limitations, we did not search the gray literature. Excluding letters, editorials, conference abstracts, reviews, and comments, we used the following free-text keywords and subject headings to identify relevant articles: (alcohol drinking OR alcoholic beverages OR beverages OR (alcohol AND (drinking or intake or consumption) OR (ethanol AND drinking or intake or consumption)) AND (myocardial ischemia OR myocardial infarct* OR coronary disease OR heart diseases OR coronary artery disease OR coronary heart disease OR angina OR cardiac death* OR ischaemic heart disease OR ischemic heart disease OR cardiac event* OR coronary event*) AND (cohort studies OR epidemiologic studies OR follow-up studies OR longitudinal studies OR prospective studies OR case-control studies OR retrospective studies) AND (ratio* OR risk*). No language restrictions were applied. Inclusion criteria were: 1) being a case-control or cohort study; 2) reporting IHD as a separate outcome (International Classification of Diseases (ICD), Ninth Revision, codes 410–414 and ICD, Tenth Revision, codes I20–I25); 3) using an exposure measurement that referred to overall alcohol consumption and not only to selected beverages; 4) reporting a measure of risk and its corresponding measure of variability for former drinkers compared with abstainers (or sufficient data to calculate those risks); and 5) containing estimates that were at least age-adjusted. Exclusion criteria were: 1) self-reporting of IHD morbidity or cardiovascular outcomes combined (i.e., including stroke); 2) being a cross-sectional study; 3) not reporting effect estimates for former drinking; and 4) containing estimates that were not age-adjusted. One author performed the search and excluded studies at the first exclusion pass based on title and abstract. Studies identified for a more detailed assessment (any reported measure of alcohol consumption and IHD as an outcome) were discussed and agreed upon by both authors.
Definition of former drinkers
Measurement error is a common issue in alcohol research. Many different definitions for former drinker have been used in primary studies. Generally, those definitions could be divided into 2 groups. First were studies that classified drinking groups by asking the respondents if they were never, past, or current drinkers (27–30). This type of assessment separates abstainers from former drinkers in a qualitative form. Then there were studies that asked about abstention with an upper quantitative limit, sometimes frequency of drinking days per time period only or frequency of drinks per time period. Examples for those definitions included never or less than once a month (31), or the question, “Did you ever drink 12 or more drinks in your lifetime?” (9, 32, 33).
A recent discussion examined the most suitable reference group for and adequate operationalization of lifetime abstention (22, 34, 35). For example, should somebody who answered “never or almost never” to the question, “Did you drink alcohol in the past?” be classified as a lifetime abstainer? In most studies, this would be considered lifetime abstention, even if researchers could not exclude the possibility that the person did consume some alcohol in the past. As no specific limit was given for the amount of alcohol consumed, we classified such an assessment as qualitative rather than quantitative. Given these operationalizations, our reference group for assessing the effects of former drinking should be labeled as “long-term abstainers or very light drinkers.” Because of the various operationalizations used in the selected studies, we tested potential changes in pooled relative risk estimates caused by different approaches to measurement of abstention in our analyses.
Data extraction
Hazard ratios, relative risks, and odds ratios were treated as equivalent measures of relative risk. In case the reference category included not only long-term abstainers but also, for example, moderate drinkers, we recalculated the effect size measure to reflect abstainers as the reference category. In cases where no confidence interval, standard error, or variance for a risk estimate was reported, we estimated the corresponding standard error from the raw numbers of cases and controls (or persons at risk) (36, 37). We abstracted information on age, number of cases and controls or persons at risk, study design, endpoint, country, and adjustment for confounder. We used maximally adjusted risk estimates (adjusted at least for age) where possible; however, we avoided estimates adjusted for blood pressure because blood pressure represented a mediator on the causal pathway between alcohol consumption and IHD rather than a confounder (38–41), which could result in underestimation of the true relation (42). When estimates stratified by endpoint (mortality and morbidity) were available, sex, and race, we chose those and prepooled the estimates in cases in which >1 estimate was reported within those categories (43).
Statistical analysis
Within each sex and endpoint stratum, we pooled estimates to derive 1 set per article by using fixed-effects estimates weighted by the inverse of their variance. When only estimates using combined endpoints or sex were reported, those estimates were included in all respective strata. All analyses concerning risk were performed on the natural log scale. To account for possible significant between-study heterogeneity, we used DerSimonian-Laird random-effect models (44) to derive a pooled effect across studies in which the between-study variance was estimated in addition to the specified within-variance component. We quantified inconsistencies across studies and their impact on the analysis by using Cochrane's Q (45) and the I2 statistic (46). I2 can be interpreted as the proportion of the total variation in the estimated slopes for each study that is due to heterogeneity between studies. Possible publication bias was explored by using funnel plots of the (log-transformed) effect sizes against their standard error and a formal regression-based test by Peters et al. (47). We tested adjustment for age only as well as adjustment for social class or educational level in separate meta-regression models to investigate their influence on the pooled effect size.
We performed several sensitivity analyses using the following methods of inclusion or exclusion: 1) excluding studies that reported estimates for combined sex and endpoint; 2) excluding studies that reported estimates for combined sex and endpoint or combined endpoint, but stratified by sex; 3) excluding studies that reported estimates for combined sex and endpoint or combined sex, but stratified by endpoint; 4) excluding all studies that were not sex- and endpoint-specific; 5) excluding studies that used current abstention at 2 time points or assessed alcohol intake for ≤20 years; 6) including only studies that used a qualitative measure of long-term alcohol intake (including all studies that did not specify an upper quantitative limit for lifetime alcohol intake); and 7) including only studies that defined abstention as <1 drinking occasion per month or <1 drink per month during a person's lifetime. Sensitivity analyses 1–4 investigated the accuracy of endpoint classification and sex, whereas sensitivity analyses 5–7 investigated the definition of abstention used in the respective studies. All analyses were conducted using Stata statistical software, version 10.1 (48).
RESULTS
The search revealed 1,538 unique citations (Figure 1). Of those, 1,146 were excluded on the first exclusion pass based on the title and abstract. We retrieved 392 full papers and scanned them to determine whether the authors should be included. Of those, 113 were excluded because the authors did not report any estimates for alcohol consumption or enough data to allow us to calculate those estimates, 45 were excluded because IHD was not reported as an outcome, and 15 were excluded because of the study design. Out of the remaining 219 articles, authors in 175 did not assess or report an estimate for former drinkers (26 of these were duplicate reports from studies that were selected), leaving 44 original articles for inclusion. After removing duplicate reports (n = 6) of studies already included in the meta-analysis, 38 unique articles remained for a quantitative analysis (8, 9, 27–33, 49–77). Overall, we considered our analysis a good selection of alcohol-related studies, in particular considering that the studies excluded simply did not assess former drinking and were of lesser quality with respect to alcohol exposure in general, particularly abstention.
Figure 1.
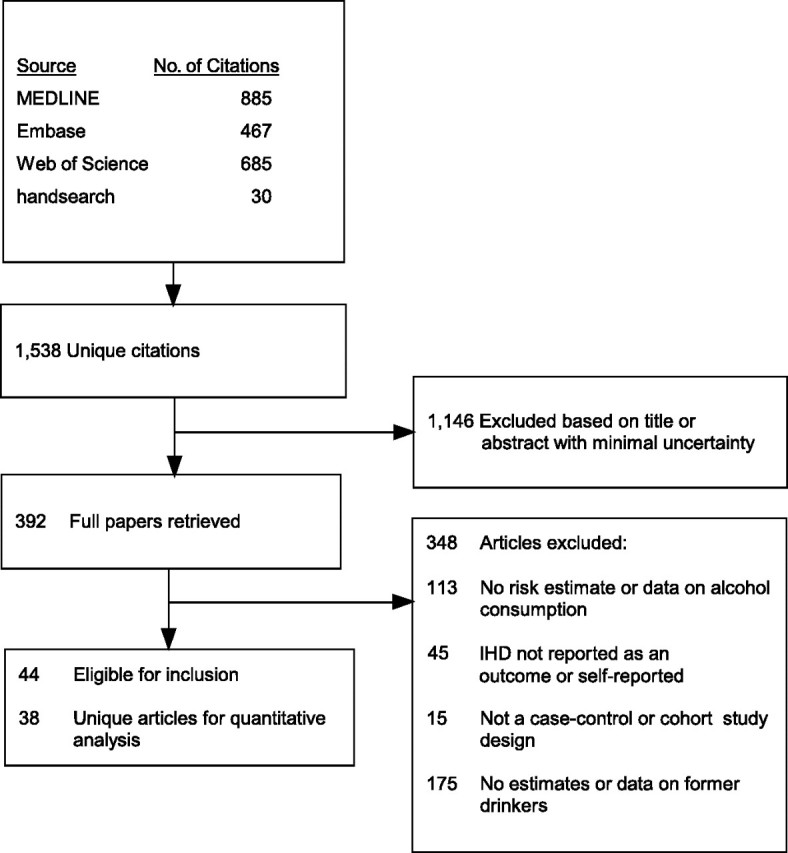
Selection process used in a study of the effect of former drinking on ischemic heart disease risk, 1980–2010.
Among the articles selected for a quantitative analysis, 3 reported only 1 estimate for sex and endpoint combined, 8 reported estimates for mortality and morbidity combined, and 7 reported estimates for both sexes by endpoint (Table 1). These estimates were used in any of the respective analyses labeled as all available estimates, whereas the 20 articles reporting sex- and endpoint-specific estimates were used in the analyses labeled as estimates stratified by sex and endpoint.
Table 1.
Characteristics of 38 Articles Selected for Quantitative Analysis of the Effect of Former Drinking on Ischemic Heart Disease Risk, 1980–2010
| Study | Sex | Study Design | Endpoint | No. of Cases | Total Sample Sizea | Country | Adjustment |
| Rosenberg et al., 1981 (51) | Female | Case-control | Morbidity | 149 | 337 | United States | Age, hospital, religion, educational level, menopausal status, physician contacts, smoking, hypertension, diabetes, abnormal blood lipids, obesity, year of admission, and contraceptive use |
| Kaufman et al., 1985 (59) | Male | Case-control | Morbidity | 220 | 339 | United States | Age and smoking |
| Kono et al., 1986 (49) | Male | Cohort | Mortality | 86 | 1,570 | Japan | Age and smoking |
| Klatsky et al., 1986 (61) | Both (combined) | Cohort | Morbidity | 184 | 11,076 | United States | Age, sex, race, smoking, coffee intake, and educational level |
| Lazarus et al., 1991 (62) | Both (separate) | Cohort | Mortality | 64 | 903 | United States | Age |
| Jackson et al., 1991 (31) | Both (separate) | Case-control | Mortality and morbidity (stratified) | 153 | 455 | New Zealand | Age, smoking, hypertension, social class, exercise level, and recent change in drinking |
| Kono et al., 1991 (72) | Both (combined) | Case-control | Morbidity | 32 | 111 | Japan | Age |
| Cullen et al., 1993 (53) | Both (separate) | Cohort | Mortality | 124 | 739 | Australia | Age, occupation, smoking, blood pressure, probable or suspected coronary heart disease, forced expiratory volume, diabetes, cholesterol, uric acid, and treatment for blood pressure |
| Iso et al., 1995 (57) | Male | Cohort | Mortality and morbidity (combined) | 11 | 744 | Japan | Age |
| Wannamethee et al., 1997 (68) | Male | Cohort | Mortality and morbidity (combined) | 63 | 583 | United Kingdom | Age, social class, physical activity, BMI, diabetes, angina, smoking, and medication |
| Rehm et al., 1997 (9) | Both (separate) | Cohort | Mortality and morbidity (stratified) | 805 | 4,244 | United States | Age and smoking |
| McElduff and Dobson, 1997 (64) | Both (separate) | Case-control | Mortality and morbidity (combined) | 973 | 1,447 | Australia | Age, smoking, blood pressure, cholesterol, angina, stroke, previous myocardial infarction, and diabetes |
| Kitamura et al., 1998 (60) | Male | Cohort | Mortality and morbidity (combined) | 20 | 1,493 | Japan | Age, serum total cholesterol, smoking, BMI, left ventricular hypertrophy, and diabetes |
| Liao et al., 2000 (32) | Both (separate) | Cohort | Mortality | 749 | 17,133 | United States | Age |
| Miyake et al., 2000 (50) | Both (combined) | Case-control | Morbidity | 247 | 545 | Japan | Age and sex |
| Sempos et al., 2002 (33) | Both (separate) | Cohort | Mortality and morbidity (combined) | 126 | 493 | United States | Age |
| Wannamethee and Shaper, 2002 (69) | Male | Cohort | Mortality | 69 | 591 | United Kingdom | Age, social class, smoking, BMI, physical activity level, employment, prior stroke, diabetes, medication, and self-reported health status |
| Romelsjö et al., 2003 (66) | Both (separate) | Case-control | Morbidity | 141 | 294 | Sweden | Age, hospital, marital status, socioeconomic status, smoking, physical activity, cardioatherosclerotic disease, job strain, social anchorage, and life control |
| Klatsky et al., 2003 (8) | Both (separate) | Cohort | Mortality | 606 | 19,692 | United States | Age, race, BMI, education, marital status, smoking, and ischemic heart disease risk index |
| Fuchs et al., 2004 (55) | Male | Cohort | Mortality and morbidity (combined) | 174 | 2,168 | United States | Age, smoking (cigarette-years), BMI, low density lipoprotein level, WHR, educational level, income, sport index, and diabetes |
| Wells et al., 2004 (70) | Both (separate) | Case-control | Mortality and morbidity (combined) | 307 | 606 | New Zealand | Age |
| Trevisan et al., 2004 (67) | Male | Case-control | Morbidity | 146 | 354 | United States | Age, education, smoking, saturated fat, dietary fiber, and physical activity |
| Grønbæk et al., 2004 (74) | Both (combined) | Cohort | Mortality | 228 | 4,104 | Denmark | Age, sex, smoking, educational level, and BMI |
| Negri et al., 2005 (75) | Both (combined) | Case-control | Morbidity | 150 | 278 | Italy | Age and sex |
| Mäkelä et al., 2005 (71) | Male | Cohort | Mortality and morbidity (combined) | 94 | 413 | Finland | Age, cohort period, marital status, educational level, and smoking |
| Kabagambe et al., 2005 (58) | Both (combined) | Case-control | Morbidity | 1,070 | 2,055 | Costa Rica | Age and smoking |
| Doll et al., 2005 (73) | Male | Cohort | Mortality | 149 | 989 | United Kingdom | Age |
| Mukamal et al., 2006 (65) | Both (combined) | Cohort | Mortality and morbidity (combined) | 402 | 2,162 | United States | Age, sex, race, educational level, marital status, smoking, exercise, depression, aspirin use, BMI, and diabetes |
| Maraldi et al., 2006 (63) | Both (combined) | Cohort | Mortality and morbidity (combined) | 216 | 1,756 | United States | Age, sex, and race |
| Harriss et al., 2007 (56) | Both (separate) | Cohort | Mortality | 92 | 12,262 | Australia | Age, country of birth, smoking, and daily energy and fruit intake |
| Dorn et al., 2007 (54) | Female | Case-control | Morbidity | 202 | 938 | United States | Age, BMI, educational level, race, smoking, and menopausal status |
| Burke et al., 2007 (52) | Both (combined) | Cohort | Mortality and morbidity (combined) | 130 | 249 | Australia | Age, sex, and accessibility to alcohol |
| Ikehara et al., 2008 (29) | Both (separate) | Cohort | Mortality | 445 | 51,909 | Japan | Age |
| Schooling et al., 2008 (28) | Both (separate) | Cohort | Mortality | 344 | 45,227 | China | Age, smoking, education, housing, monthly expenditure, BMI, and physical activity level |
| Schooling et al., 2009 (30) | Both (separate) | Case-control | Mortality | 1,823 | 9,951 | China | Age, sex, education, physical activity level, occupational physical activity level, and smoking |
| Ikehara et al., 2009 (27) | Male | Cohort | Mortality | 104 | 4,888 | Japan | Age |
| Mukamal et al., 2010 (76) | Both (combined) | Cohort | Mortality | 3,134 | 65,563 | United States | Age, sex, race, smoking, marital status, educational level, region, urbanization, BMI, and general health status |
| Arriola et al., 2010 (77) | Both (separate) | Cohort | Mortality and morbidity (combined) | 151 | 12,584 | Spain | Age, center, smoking, height, educational level, physical activity level, WHR, vitamin E intake, antithrombotic and antihemorrhagic drug use, and energy intake |
Abbreviations: BMI, body mass index; WHR, waist-to-hip ratio.
Current abstainers.
A total of 5,613 IHD events with 12,097 controls among case-control studies and 1,387 events with combined endpoints and 7,183 events stratified by endpoint among 232,621 persons at risk among cohort studies (taking into account multiple articles per study) were used in this analysis. Selected articles originated in the United States (n = 14), Japan (n = 7), Australia (n = 4), the United Kingdom (n = 3), New Zealand (n = 2), and China (n = 2), with an additional 1 study each from Denmark, Sweden, Spain, Finland, Italy, and Costa Rica (Table 1). Only 3 articles based on 2 studies (9, 33, 55) provided estimates stratified by race. We therefore refrained from analyzing those separately and included each estimate in the respective sex and endpoint strata.
The proportion of former drinkers among current abstainers varied considerably across the primary studies and strata examined. The mean percentages of former drinkers among current abstainers were between 16% and 90% among men and between 1% and 74% among women. Although the percentage of former drinkers covered a wide range across studies, the percentage was not associated with the effect size regardless of whether all estimates or estimates stratified by sex and endpoint were considered in any of the strata in our analyses, as all correlation coefficients were small in magnitude and not significant (Table 2).
Table 2.
Proportion of Former Drinkers Among Current Abstainers, by Sex and EndPoint, 1980–2010
| Sex, Endpoint, and Model | No. of Studies | % of Former Drinkersa |
rb | P Value | ||
| Weighted Mean | Minimum | Maximum | ||||
| Men | ||||||
| Mortality | ||||||
| All available estimates (combined sex or endpoint included) | 27 | 31 | 16 | 83 | −0.15 | 0.57 |
| Stratified by sex and endpoint | 14 | 32 | 16 | 70 | −0.36 | 0.20 |
| Morbidity | ||||||
| All available estimates (combined sex or endpoint included) | 23 | 37 | 16 | 90 | 0.03 | 0.91 |
| Stratified by sex and endpoint | 5 | 61 | 31 | 90 | 0.38 | 0.54 |
| Women | ||||||
| Mortality | ||||||
| All available estimates (combined sex or endpoint included) | 18 | 16 | 1 | 47 | −0.15 | 0.56 |
| Stratified by sex and endpoint | 10 | 8 | 2 | 33 | 0.20 | 0.58 |
| Morbidity | ||||||
| All available estimates (combined sex or end point included) | 17 | 25 | 1 | 74 | 0.04 | 0.87 |
| Stratified by sex and endpoint | 5 | 38 | 14 | 74 | 0.46 | 0.44 |
Among all current abstainers.
Pearson's correlation coefficient of proportion of former drinkers with effect size.
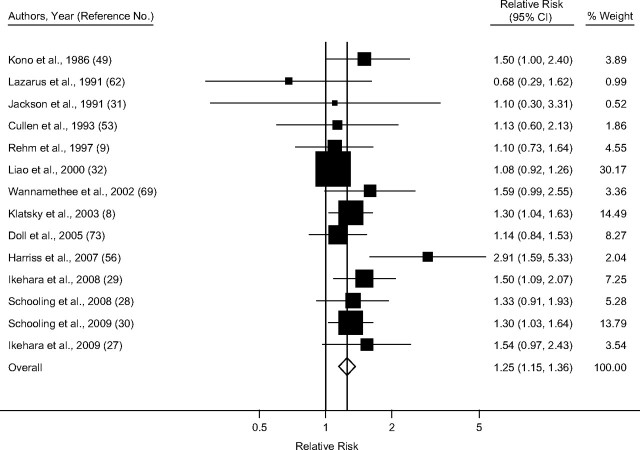
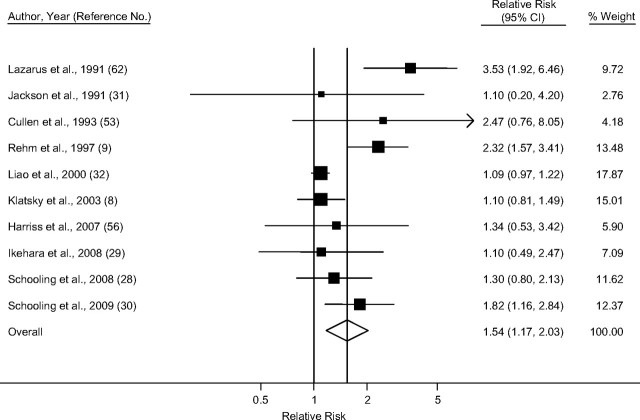
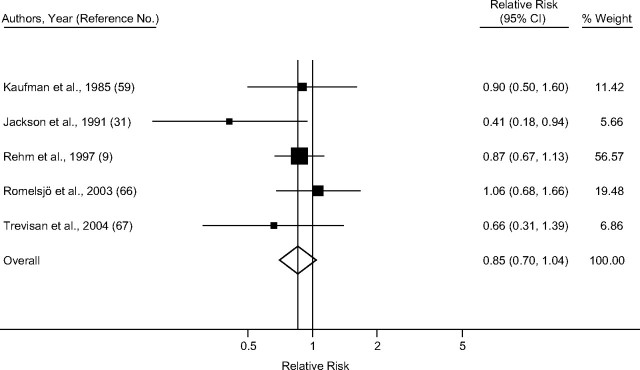
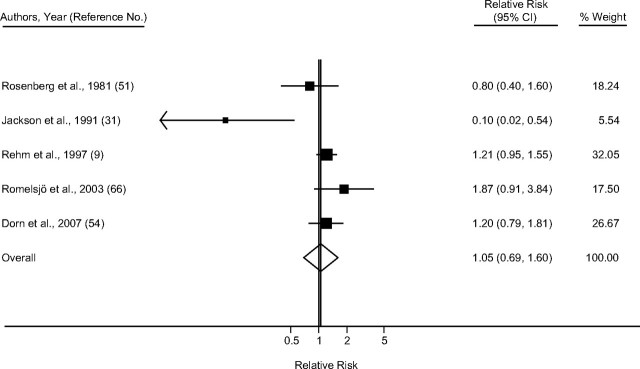
Taking into account only studies that reported primary estimates stratified by sex and endpoint, the pooled random-effect relative risk estimate for IHD mortality among men (Figure 2) was 1.25 (95% confidence interval (CI): 1.15, 1.36), with little heterogeneity (I2 = 26.4%; χ2 (13 df) = 17.67, P = 0.17) (Table 3). Among women (Figure 3), the summary random-effects relative risk estimate for mortality was 1.54 (95% CI: 1.17, 2.03), with substantial heterogeneity (I2 = 71.1%; χ2 (9 df) =31.12, P < 0.001). The effect for IHD morbidity (Figures 4 and 5) as an endpoint was slightly >1 among women (relative risk = 1.05, 95% CI: 0.69, 1.60), and <1 for men (relative risk = 0.85, 95% CI: 0.70, 1.04). Neither estimate, however, was statistically significant. Significantly elevated relative risks for IHD-related death but not for IHD-related morbidity remained in the analysis after estimates for combined sex or endpoint were included (Table 3). Adjustment for neither age only (where applicable) nor social class was statistically significantly related to the pooled effect estimate for male mortality (P = 0.16 and P = 0.73, respectively), male morbidity (social class: P = 0.99), female mortality (P = 0.97 and P = 0.78), and female morbidity (social class: P = 0.75). Results were similar when estimates for combined sex or endpoint were included.
Figure 2.
Pooled relative risk of ischemic heart disease mortality among former drinkers compared with abstainers in men, stratified by sex and endpoint, 1980–2010. CI, confidence interval.
Table 3.
Pooled Relative Risks for Ischemic Heart Diseasea in Former Drinkers Compared With Long-Term Abstainers, by Sex and EndPoint, 1980–2010
| Sex, EndPoint, and Model | No. of Studies | Pooled Relative Risk | 95% Confidence Interval | I2, % |
| Men | ||||
| Mortality | ||||
| All available estimates (combined sex or endpoint included) | 27 | 1.21b | 1.11, 1.33 | 33.3 |
| Stratified by sex and endpoint | 14 | 1.25 | 1.15, 1.36 | 26.4 |
| Morbidity | ||||
| All available estimates (combined sex or endpoint included) | 23 | 0.97 | 0.89, 1.06 | 5.2 |
| Stratified by sex and endpoint | 5 | 0.85 | 0.70, 1.04 | 10.6 |
| Women | ||||
| Mortality | ||||
| All available estimates (combined sex or endpoint included) | 18 | 1.36b | 1.16, 1.60 | 63.3 |
| Stratified by sex and endpoint | 10 | 1.54b | 1.17, 2.03 | 71.1 |
| Morbidity | ||||
| All available estimates (combined sex or endpoint included) | 17 | 1.08b | 0.93, 1.24 | 42.0 |
| Stratified by sex and endpoint | 5 | 1.05b | 0.69, 1.60 | 64.9 |
Includes International Classification of Diseases, Ninth Revision, codes 410–414 and International Classification of Diseases, Tenth Revision, codes I20–I25.
Random-effects models.
Figure 3.
Pooled relative risk of ischemic heart disease mortality among former drinkers compared with abstainers in women, stratified by sex and endpoint. Weights are from random-effects models, 1980–2010. CI, confidence interval.
Figure 4.
Pooled relative risk of ischemic heart disease morbidity among former drinkers compared with abstainers in men, stratified by sex and endpoint, 1980–2010. CI, confidence interval.
Figure 5.
Pooled relative risk of ischemic heart disease morbidity among former drinkers compared with abstainers in women, stratified by sex and endpoint. Weights are from random-effects models, 1980–2010. CI, confidence interval.
We did not find any evidence of publication bias among men (P = 0.44 and P = 0.49 for mortality and morbidity, respectively) or women (P = 0.70 and P = 0.42 for mortality and morbidity, respectively). Omitting each study one by one and reestimating the models did not reveal any substantial divergence from the overall pooled estimates. Although all of these studies showed a reasonable distinction between long-term abstainers and former drinkers, we also conducted several sensitivity analyses with regard to stratification of reported estimates by endpoint and sex and to definition of abstention. These sensitivity analyses did not reveal any substantial differences in pooled effect estimates. Three studies stood out in their measurements of alcohol exposure. Two reported on current nondrinking at 2 points in time (62, 74), and 1 (49) assessed nondrinking status (and all other drinking categories) for the past 20 years at baseline. Excluding those 3 studies resulted in only marginally different pooled estimates among male and female mortality rates, for which those studies reported estimates.
DISCUSSION
Correct identification of drinking groups is crucial for determining the dose–response relation between alcohol consumption and IHD risk. In this article, we summarized current epidemiologic evidence for a risk difference between former drinkers and long-term abstainers. Our results showed that there was a substantial difference in IHD risk depending on whether the endpoint was mortality or morbidity. Former drinkers were at an increased risk for IHD when mortality was considered as an endpoint for both sexes, and pooled estimates were statistically significant with similar effect sizes. However, we did not find evidence for an effect of former drinking on morbidity rates in our analysis. In other words, in our meta-analysis, we found evidence for the “sick quitter effect” as an outcome measurement for mortality but not for morbidity. The reasons behind this difference by endpoint are unclear. Aside from assuming a real biologic effect of former drinking, methodological properties, such as differences in outcome ascertainment or study design in general, could also explain this effect. When comparing pooled relative risks for IHD-related mortality and morbidity using only primary estimates that were stratified by sex and endpoint, it is important to consider that this effectively represents a comparison of study design, because all but 1 estimate for mortality were from cohort studies, and all but 2 estimates for morbidity were from case-control studies. However, there were fewer studies available for a stratified analysis of morbidity as an endpoint, thus limiting the conclusions about this relation. Although we cannot rule out residual confounding as an explanation for our results, meta-regression models did not reveal a significant influence of differential adjustment across studies. Nevertheless, this issue deserves further study, and we encourage researchers to report more detailed results of any type of regression modeling to allow better judgment of the effect of adjusting for potential confounders of the alcohol–heart disease relation.
Several other limitations apply as well. Although the cardioprotective association of regular, low-to-moderate alcohol consumption is well-established (1), with convincing experimental evidence regarding plausible biologic pathways (78), alcohol consumption in relation to IHD risk is more complex in both biologic mechanisms and drinking behavior over the lifetime than we were able to investigate with the current data. For example, we were unable to examine former drinking behavior (length and variability of past alcohol exposure) in detail because of the lack of data. Compared with long-term abstainers, former drinkers could show a lower IHD risk when former drinking was mostly regular moderate drinking and an increased risk when former drinking was mostly characterized by irregular heavy drinking or regular heavy drinking. There is some evidence that the proportion of occasional (infrequent) drinkers among current abstainers can be much higher than that of true lifetime abstainers (79). Again, it will depend on the operationalization of the word occasional. To give an example, it is inconceivable that drinking alcohol on 20 occasions during one's lifetime could have a biologic effect on IHD (34, 35). On the other hand, occasional drinking once a month, and often in binges, may have an effect on IHD (80). Reports have shown that it is almost impossible to differentiate between lifetime abstainers and very infrequent drinkers in retrospective studies, as large fractions of self-identified lifetime abstainers report at least some drinking (35, 79). This implies that repeated measures of exposure would be good epidemiologic practice in studies on alcohol as a risk factor. In addition, the proportion of current abstainers in a population typically varies across and within countries, more so among women than among men (81, 82). Although the proportion may vary, the relative risk should not be biased (83). One might also argue that the large variability of the proportion of former drinkers among current abstainers warrants cautious interpretation and limits the generalizability of the results of our study; however, we found no evidence of such an effect.
When considering measurement of IHD incidence and IHD death, differences in classification of the outcome across countries and studies might explain our results from a methodological point of view. Recent studies have shown that a further stratification within the ICD codes for IHD might be necessary, because these studies have shown differences in coding of the underlying cause of death across countries (84–86). In addition, the exposure-outcome relation may contain more complexities than has been appreciated thus far. In a Russian study, Zaridze et al. (87) reported that acute IHD other than myocardial infarction (ICD-10) code I24, the most common cause of heart disease in that study) showed a particularly strong detrimental relation to average alcohol intake without any cardioprotective associations. Nevertheless, their study also found that a substantial fraction of subjects who were originally classified as having IHD in fact had alcohol poisoning, although the typically detrimental pattern of irregular heavy drinking occasions in Russia also needs to be considered in this case. However, little data exist to confirm this phenomenon in other countries and different drinking cultures.
Although the sick quitter effect assumes that former drinkers stopped drinking for health reasons, we could not be sure that this was the case in the studies included in this analysis. A recent methodological study in the United States found that slightly more than half of former drinkers who lost control of their drinking also reported serious health effects because of their drinking (88). Regardless of the reason why they stopped drinking, a former drinker's increased risk of death is unlikely to explain away the cardioprotective association commonly found for moderate regular drinking. Adjusting a relative risk of 0.80 (the nadir of the J-shaped dose-response curve described by Corrao et al. (1)) among men with our pooled relative risk using only estimates stratified by sex and endpoint for former drinking (relative risk = 1.25, assuming 32% of current abstainers were former drinkers) showed that the corrected relative risk was 0.86 when the effect of former drinking was taken into account. In other words, the cardioprotective association of alcohol might be slightly overestimated when the wrong comparison group is selected, but this factor cannot be used as an argument to doubt the cardioprotective association per se.
Our results underline the importance of correct identification of the reference group if the J-curve commonly found in epidemiologic studies on alcohol consumption and IHD risk is to be further refined. This has ultimately important implications for the choice of the reference group in any epidemiologic study on alcohol consumption in relation to IHD. However, explanations for this difference are speculative thus far. The difference in risk among former drinkers seems to be dependent on either the type of outcome measurement, namely mortality and morbidity, or the study design (cohort studies showed a significant effect, whereas case-control studies did not). Although it was beyond the scope of this analysis to determine potential reasons for differential effects, they may also include outcome assessment, which might be more valid when disease incidence rather than death is concerned, or exposure measurement because the length and type (light, regular, irregular, or heavy regular alcohol consumption) of former drinking behavior should determine the lifetime IHD risk. All those groups might be associated with differential risk based on their former drinking pattern and the length of such a drinking pattern. To have a meaningful reference group, future studies should investigate not only the reason subjects stopped drinking but also some details about former drinking behavior.
Acknowledgments
Author affiliations: Centre for Addiction and Mental Health, Toronto, Ontario, Canada (Michael Roerecke, Jürgen Rehm); Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (Michael Roerecke, Jürgen Rehm); Department of Psychiatry, Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada (Jürgen Rehm); and Technische Universität Dresden, Dresden, Germany (Jürgen Rehm).
This work was financially supported by a small contribution from the Global Burden of Disease Study and by the grant, “Drinking Patterns & Ethnicity: Impact on Mortality Risks” (National Institute on Alcohol Abuse and Alcoholism no. 1R01AA016644-01A1) to the second author.
The authors thank the following members of the core group of the Comparative Risk Assessment for Alcohol within the Global Burden of Disease 2005 Study for their support and comments on the general methodology and an earlier version of this paper: G. Borges, G. Gmel, K. Graham, B. Grant, C. Parry, V. Poznyak, and R. Room. The authors also thank D. Baliunas, B. Taylor, H. Irving, S. Mohapatra, and J. Patra for their comments during the preparation of this manuscript and B. Taylor for copy editing.
Conflict of interest: none declared.
Glossary
Abbreviations
- CI
confidence interval
- ICD
International Classification of Diseases
- IHD
ischemic heart disease
References
- 1.Corrao G, Rubbiati L, Bagnardi V, et al. Alcohol and coronary heart disease: a meta-analysis. Addiction. 2000;95(10):1505–1523. doi: 10.1046/j.1360-0443.2000.951015056.x. [DOI] [PubMed] [Google Scholar]
- 2.Rehm J, Gmel G, Sempos CT, et al. Alcohol-related morbidity and mortality. Alcohol Res Health. 2003;27(1):39–51. [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson R, Broad J, Connor J, et al. Alcohol and ischaemic heart disease: probably no free lunch. Lancet. 2005;366(9501):1911–1912. doi: 10.1016/S0140-6736(05)67770-7. [DOI] [PubMed] [Google Scholar]
- 4.Shaper AG, Wannamethee G, Walker M. Alcohol and mortality in British men: explaining the U-shaped curve. Lancet. 1988;2(8623):1267–1273. doi: 10.1016/s0140-6736(88)92890-5. [DOI] [PubMed] [Google Scholar]
- 5.Shaper AG. The unhealthy abstainers question is still important. Addiction. 1995;90(4):488–490. doi: 10.1111/j.1360-0443.1995.tb02181.x. [DOI] [PubMed] [Google Scholar]
- 6.Epler AJ, Sher KJ, Piasecki TM. Reasons for abstaining or limiting drinking: a developmental perspective. Psychol Addict Behav. 2009;23(3):428–442. doi: 10.1037/a0015879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klatsky AL, Armstrong MA, Friedman GD. Risk of cardiovascular mortality in alcohol drinkers, ex-drinkers and nondrinkers. Am J Cardiol. 1990;66(17):1237–1242. doi: 10.1016/0002-9149(90)91107-h. [DOI] [PubMed] [Google Scholar]
- 8.Klatsky AL, Friedman GD, Armstrong MA, et al. Wine, liquor, beer, and mortality. Am J Epidemiol. 2003;158(6):585–595. doi: 10.1093/aje/kwg184. [DOI] [PubMed] [Google Scholar]
- 9.Rehm JT, Bondy SJ, Sempos CT, et al. Alcohol consumption and coronary heart disease morbidity and mortality. Am J Epidemiol. 1997;146(6):495–501. doi: 10.1093/oxfordjournals.aje.a009303. [DOI] [PubMed] [Google Scholar]
- 10.Rehm J, Taylor B, Mohapatra S, et al. Alcohol as a risk factor for liver cirrhosis: a systematic review and meta-analysis. Drug Alcohol Rev. 2010;29(4):437–445. doi: 10.1111/j.1465-3362.2009.00153.x. [DOI] [PubMed] [Google Scholar]
- 11.Rehm J, Baliunas D, Borges G, et al. The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction. 2010;105(5):817–843. doi: 10.1111/j.1360-0443.2010.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grønbaek M. Alcohol, type of alcohol, and all-cause and coronary heart disease mortality. Ann N Y Acad Sci. 2002;957(1):16–20. doi: 10.1111/j.1749-6632.2002.tb02902.x. [DOI] [PubMed] [Google Scholar]
- 13.Grønbaek M. Epidemiologic evidence for the cardioprotective effects associated with consumption of alcoholic beverages. Pathophysiology. 2004;10(2):83–92. doi: 10.1016/j.pathophys.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Poikolainen K. It can be bad for the heart, too—drinking patterns and coronary heart disease. Addiction. 1998;93(12):1757–1759. doi: 10.1046/j.1360-0443.1998.931217571.x. [DOI] [PubMed] [Google Scholar]
- 15.Rehm J, Sempos CT, Trevisan M. Alcohol and cardiovascular disease—more than one paradox to consider. Average volume of alcohol consumption, patterns of drinking and risk of coronary heart disease—a review. J Cardiovasc Risk. 2003;10(1):15–20. doi: 10.1097/01.hjr.0000051961.68260.30. [DOI] [PubMed] [Google Scholar]
- 16.Rehm J, Room R, Graham K, et al. The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease: an overview. Addiction. 2003;98(9):1209–1228. doi: 10.1046/j.1360-0443.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- 17.Britton A, McKee M. The relation between alcohol and cardiovascular disease in Eastern Europe: explaining the paradox. J Epidemiol Community Health. 2000;54(5):328–332. doi: 10.1136/jech.54.5.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marmot MG. Alcohol and coronary heart disease. Int J Epidemiol. 2001;30(4):724–729. doi: 10.1093/ije/30.4.724. [DOI] [PubMed] [Google Scholar]
- 19.Rehm J, Room R, Monteiro M, et al. Alcohol use. In: Ezzati M, Lopez A, Rodgers A, et al., editors. Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Geneva, Switzerland: World Health Organization; 2004. pp. 959–1109. [Google Scholar]
- 20.Shaper AG. Alcohol and mortality: a review of prospective studies. Br J Addict. 1990;85(7):837–847. doi: 10.1111/j.1360-0443.1990.tb03710.x. [DOI] [PubMed] [Google Scholar]
- 21.Bagnardi V, Zatonski W, Scotti L, et al. Does drinking pattern modify the effect of alcohol on the risk of coronary heart disease? Evidence from a meta-analysis. J Epidemiol Community Health. 2008;62(7):615–619. doi: 10.1136/jech.2007.065607. [DOI] [PubMed] [Google Scholar]
- 22.Fillmore KM, Stockwell T, Chikritzhs T, et al. Moderate alcohol use and reduced mortality risk: systematic error in prospective studies and new hypotheses. Ann Epidemiol. 2007;17(suppl 5):S16–S23. doi: 10.1016/j.annepidem.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Gmel G, Gutjahr E, Rehm J. How stable is the risk curve between alcohol and all-cause mortality and what factors influence the shape? A precision-weighted hierarchical meta-analysis. Eur J Epidemiol. 2003;18(7):631–642. doi: 10.1023/a:1024805021504. [DOI] [PubMed] [Google Scholar]
- 24.Rimm EB, Klatsky A, Grobbee D, et al. Review of moderate alcohol consumption and reduced risk of coronary heart disease: is the effect due to beer, wine, or spirits. BMJ. 1996;312(7033):731–736. doi: 10.1136/bmj.312.7033.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Castelnuovo A, Rotondo S, Iacoviello L, et al. Meta-analysis of wine and beer consumption in relation to vascular risk. Circulation. 2002;105(24):2836–2844. doi: 10.1161/01.cir.0000018653.19696.01. [DOI] [PubMed] [Google Scholar]
- 26.English D, Holman C, Milne E, et al. Canberra, Australia: Commonwealth Department of Human Services and Health; 1995. The Quantification of Drug-Caused Morbidity and Mortality in Australia, 1995. [Google Scholar]
- 27.Ikehara S, Iso H, Yamagishi K, et al. Alcohol consumption, social support, and risk of stroke and coronary heart disease among Japanese men: the JPHC Study. Alcohol Clin Exp Res. 2009;33(6):1025–1032. doi: 10.1111/j.1530-0277.2009.00923.x. [DOI] [PubMed] [Google Scholar]
- 28.Schooling CM, Sun W, Ho SY, et al. Moderate alcohol use and mortality from ischaemic heart disease: a prospective study in older Chinese. PLoS One. 2008;3(6) doi: 10.1371/journal.pone.0002370. e2370. (doi: doi:10.1371/journal.pone.0002370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikehara S, Iso H, Toyoshima H, et al. Alcohol consumption and mortality from stroke and coronary heart disease among Japanese men and women: the Japan Collaborative Cohort Study. Stroke. 2008;39(11):2936–2942. doi: 10.1161/STROKEAHA.108.520288. [DOI] [PubMed] [Google Scholar]
- 30.Schooling CM, Lam TH, Ho SY, et al. Alcohol and cardio-respiratory deaths in Chinese: a population-based case-control study of 32,462 older Hong Kong adults. BMC Public Health. 2009;9:49. doi: 10.1186/1471-2458-9-49. doi: 10.1186/1471-2458-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson R, Scragg R, Beaglehole R. Alcohol consumption and risk of coronary heart disease. BMJ. 1991;303(6796):211–216. doi: 10.1136/bmj.303.6796.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao Y, McGee DL, Cao G, et al. Alcohol intake and mortality: findings from the National Health Interview Surveys (1988 and 1990) Am J Epidemiol. 2000;151(7):651–659. doi: 10.1093/oxfordjournals.aje.a010259. [DOI] [PubMed] [Google Scholar]
- 33.Sempos C, Rehm J, Crespo CJ, et al. No protective effect of alcohol consumption on coronary heart disease (CHD) in African Americans: average volume of drinking over the life course and CHD morbidity and mortality in a U.S. national cohort. Contemp Drug Probl. 2002;29(4):805–820. [Google Scholar]
- 34.Klatsky AL. Invited commentary: never, or hardly ever? It could make a difference. Am J Epidemiol. 2008;168(8):872–875. doi: 10.1093/aje/kwn192. [DOI] [PubMed] [Google Scholar]
- 35.Rehm J, Irving H, Ye Y, et al. Are lifetime abstainers the best control group in alcohol epidemiology? On the stability and validity of reported lifetime abstention. Am J Epidemiol. 2008;168(8):866–871. doi: 10.1093/aje/kwn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenland S, Rothman KJ. Introduction to categorical statistics. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. pp. 238–257. [Google Scholar]
- 37.Bland JM, Altman DG. Statistics notes. The odds ratio. BMJ. 2000;320(7247):1468. doi: 10.1136/bmj.320.7247.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rehm J. Alcohol consumption and mortality. What do we know and where should we go? Addiction. 2000;95(7):989–995. doi: 10.1046/j.1360-0443.2000.9579891.x. [DOI] [PubMed] [Google Scholar]
- 39.Mckee M, Britton A. The positive relationship between alcohol and heart disease in eastern Europe: potential physiological mechanisms. J R Soc Med. 1998;91(8):402–407. doi: 10.1177/014107689809100802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puddey IB, Rakic V, Dimmitt SB, et al. Influence of pattern of drinking on cardiovascular disease and cardiovascular risk factors—a review. Addiction. 1999;94(5):649–663. doi: 10.1046/j.1360-0443.1999.9456493.x. [DOI] [PubMed] [Google Scholar]
- 41.Taylor B, Irving HM, Baliunas D, et al. Alcohol and hypertension: gender differences in dose-response relationships determined through systematic review and meta-analysis. Addiction. 2009;104(12):1981–1990. doi: 10.1111/j.1360-0443.2009.02694.x. [DOI] [PubMed] [Google Scholar]
- 42.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 43.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 44.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 45.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–129. [Google Scholar]
- 46.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 47.Peters JL, Sutton AJ, Jones DR, et al. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295(6):676–680. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 48.Stata Statistical Software, Release 10.1. Stata Corporation. [computer program]. College Station, TX: StataCorp, LP; 2008. [Google Scholar]
- 49.Kono S, Ikeda M, Tokudome S, et al. Alcohol and mortality: a cohort study of male Japanese physicians. Int J Epidemiol. 1986;15(4):527–532. doi: 10.1093/ije/15.4.527. [DOI] [PubMed] [Google Scholar]
- 50.Miyake Y. Risk factors for non-fatal acute myocardial infarction in middle-aged and older Japanese. Fukuoka Heart Study Group. Jpn Circ J. 2000;64(2):103–109. doi: 10.1253/jcj.64.103. [DOI] [PubMed] [Google Scholar]
- 51.Rosenberg L, Slone D, Shapiro S, et al. Alcoholic beverages and myocardial infarction in young women. Am J Public Health. 1981;71(1):82–85. doi: 10.2105/ajph.71.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burke V, Lee AH, Hunter E, et al. Alcohol intake and incidence of coronary disease in Australian Aborigines. Alcohol Alcohol. 2007;42(2):119–124. doi: 10.1093/alcalc/agl102. [DOI] [PubMed] [Google Scholar]
- 53.Cullen KJ, Knuiman MW, Ward NJ. Alcohol and mortality in Busselton, Western Australia. Am J Epidemiol. 1993;137(2):242–248. doi: 10.1093/oxfordjournals.aje.a116665. [DOI] [PubMed] [Google Scholar]
- 54.Dorn JM, Hovey K, Williams BA, et al. Alcohol drinking pattern and non-fatal myocardial infarction in women. Addiction. 2007;102(5):730–739. doi: 10.1111/j.1360-0443.2007.01765.x. [DOI] [PubMed] [Google Scholar]
- 55.Fuchs FD, Chambless LE, Folsom AR, et al. Association between alcoholic beverage consumption and incidence of coronary heart disease in whites and blacks: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2004;160(5):466–474. doi: 10.1093/aje/kwh229. [DOI] [PubMed] [Google Scholar]
- 56.Harriss LR, English DR, Hopper JL, et al. Alcohol consumption and cardiovascular mortality accounting for possible misclassification of intake: 11-year follow-up of the Melbourne Collaborative Cohort Study. Addiction. 2007;102(10):1574–1585. doi: 10.1111/j.1360-0443.2007.01947.x. [DOI] [PubMed] [Google Scholar]
- 57.Iso H, Kitamura A, Shimamoto T, et al. Alcohol intake and the risk of cardiovascular disease in middle-aged Japanese men. Stroke. 1995;26(5):767–773. doi: 10.1161/01.str.26.5.767. [DOI] [PubMed] [Google Scholar]
- 58.Kabagambe EK, Baylin A, Ruiz-Narvaez E, et al. Alcohol intake, drinking patterns, and risk of nonfatal acute myocardial infarction in Costa Rica. Am J Clin Nutr. 2005;82(6):1336–1345. doi: 10.1093/ajcn/82.6.1336. [DOI] [PubMed] [Google Scholar]
- 59.Kaufman DW, Rosenberg L, Helmrich SP, et al. Alcoholic beverages and myocardial infarction in young men. Am J Epidemiol. 1985;121(4):548–554. doi: 10.1093/oxfordjournals.aje.a114032. [DOI] [PubMed] [Google Scholar]
- 60.Kitamura A, Iso H, Sankai T, et al. Alcohol intake and premature coronary heart disease in urban Japanese men. Am J Epidemiol. 1998;147(1):59–65. doi: 10.1093/oxfordjournals.aje.a009367. [DOI] [PubMed] [Google Scholar]
- 61.Klatsky AL, Armstrong MA, Friedman GD. Relations of alcoholic beverage use to subsequent coronary artery disease hospitalization. Am J Cardiol. 1986;58(9):710–714. doi: 10.1016/0002-9149(86)90342-5. [DOI] [PubMed] [Google Scholar]
- 62.Lazarus NB, Kaplan GA, Cohen RD, et al. Change in alcohol consumption and risk of death from all causes and from ischaemic heart disease. BMJ. 1991;303(6802):553–556. doi: 10.1136/bmj.303.6802.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maraldi C, Volpato S, Kritchevsky SB, et al. Impact of inflammation on the relationship among alcohol consumption, mortality, and cardiac events: the Health, Aging, and Body Composition Study. Arch Intern Med. 2006;166(14):1490–1497. doi: 10.1001/archinte.166.14.1490. [DOI] [PubMed] [Google Scholar]
- 64.McElduff P, Dobson AJ. How much alcohol and how often? Population based case-control study of alcohol consumption and risk of a major coronary event. BMJ. 1997;314(7088):1159–1164. doi: 10.1136/bmj.314.7088.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mukamal KJ, Chung H, Jenny NS, et al. Alcohol consumption and risk of coronary heart disease in older adults: the Cardiovascular Health Study. J Am Geriatr Soc. 2006;54(1):30–37. doi: 10.1111/j.1532-5415.2005.00561.x. [DOI] [PubMed] [Google Scholar]
- 66.Romelsjö A, Branting M, Hallqvist J, et al. Abstention, alcohol use and risk of myocardial infarction in men and women taking account of social support and working conditions: the SHEEP case-control study. Addiction. 2003;98(10):1453–1462. doi: 10.1046/j.1360-0443.2003.00488.x. [DOI] [PubMed] [Google Scholar]
- 67.Trevisan M, Dorn J, Falkner K, et al. Drinking pattern and risk of non-fatal myocardial infarction: a population-based case-control study. Addiction. 2004;99(3):313–322. doi: 10.1111/j.1360-0443.2003.00630.x. [DOI] [PubMed] [Google Scholar]
- 68.Wannamethee SG, Shaper AG. Lifelong teetotallers, ex-drinkers and drinkers: mortality and the incidence of major coronary heart disease events in middle-aged British men. Int J Epidemiol. 1997;26(3):523–531. doi: 10.1093/ije/26.3.523. [DOI] [PubMed] [Google Scholar]
- 69.Wannamethee SG, Shaper AG. Taking up regular drinking in middle age: effect on major coronary heart disease events and mortality. Heart. 2002;87(1):32–36. doi: 10.1136/heart.87.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wells S, Broad J, Jackson R. Alcohol consumption and its contribution to the burden of coronary heart disease in middle-aged and older New Zealanders: a population-based case-control study. N Z Med J. 2004;117(1190):U793. [PubMed] [Google Scholar]
- 71.Makelä P, Paljärvi T, Poikolainen K. Heavy and nonheavy drinking occasions, all-cause and cardiovascular mortality and hospitalizations: a follow-up study in a population with a low consumption level. J Stud Alcohol. 2005;66(6):722–728. doi: 10.15288/jsa.2005.66.722. [DOI] [PubMed] [Google Scholar]
- 72.Kono S, Handa K, Kawano T, et al. Alcohol intake and nonfatal acute myocardial infarction in Japan. Am J Cardiol. 1991;68(10):1011–1014. doi: 10.1016/0002-9149(91)90487-6. [DOI] [PubMed] [Google Scholar]
- 73.Doll R, Peto R, Boreham J, et al. Mortality in relation to alcohol consumption: a prospective study among male British doctors. Int J Epidemiol. 2005;34(1):199–204. doi: 10.1093/ije/dyh369. [DOI] [PubMed] [Google Scholar]
- 74.Grønbaek M, Johansen D, Becker U, et al. Changes in alcohol intake and mortality: a longitudinal population-based study. Epidemiology. 2004;15(2):222–228. doi: 10.1097/01.ede.0000112219.01955.56. [DOI] [PubMed] [Google Scholar]
- 75.Negri E, La Vecchia C, Pelucchi C, et al. The risk of acute myocardial infarction after stopping drinking. Prev Med. 2005;40(6):725–728. doi: 10.1016/j.ypmed.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 76.Mukamal KJ, Chen CM, Rao SR, et al. Alcohol consumption and cardiovascular mortality among U.S. adults, 1987–2002. J Am Coll Cardiol. 2010;55(13):1328–1335. doi: 10.1016/j.jacc.2009.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arriola L, Martinez-Camblor P, Larrañaga N, et al. Alcohol intake and the risk of coronary heart disease in the Spanish EPIC cohort study. Heart. 2010;96(2):124–130. doi: 10.1136/hrt.2009.173419. [DOI] [PubMed] [Google Scholar]
- 78.Rimm EB, Williams P, Fosher K, et al. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319(7224):1523–1528. doi: 10.1136/bmj.319.7224.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Caldwell TM, Rodgers B, Power C, et al. Drinking histories of self-identified lifetime abstainers and occasional drinkers: findings from the 1958 British Birth Cohort Study. Alcohol Alcohol. 2006;41(6):650–654. doi: 10.1093/alcalc/agl088. [DOI] [PubMed] [Google Scholar]
- 80.Roerecke M, Rehm J. Irregular heavy drinking occasions and risk of ischemic heart disease: a systematic review and meta-analysis. Am J Epidemiol. 2010;171(6):633–644. doi: 10.1093/aje/kwp451. [DOI] [PubMed] [Google Scholar]
- 81.Mäkelä P, Gmel G, Grittner U, et al. Drinking patterns and their gender differences in Europe. Alcohol Alcohol Suppl. 2006;41(1):I8–I18. doi: 10.1093/alcalc/agl071. [DOI] [PubMed] [Google Scholar]
- 82.Rehm J, Rehn N, Room R, et al. The global distribution of average volume of alcohol consumption and patterns of drinking. Eur Addict Res. 2003;9(4):147–156. doi: 10.1159/000072221. [DOI] [PubMed] [Google Scholar]
- 83.Groves RM. Survey Errors and Survey Costs. New York, NY: John Wiley & Sons, Inc; 1989. [Google Scholar]
- 84.Winkler V, Ott JJ, Becher H. Reliability of coding causes of death with ICD-10 in Germany. Int J Public Health. 2010;55(1):43–48. doi: 10.1007/s00038-009-0053-7. [DOI] [PubMed] [Google Scholar]
- 85.Danesh J, Saracci R, Berglund G, et al. EPIC-Heart: the cardiovascular component of a prospective study of nutritional, lifestyle and biological factors in 520,000 middle-aged participants from 10 European countries. Eur J Epidemiol. 2007;22(2):129–141. doi: 10.1007/s10654-006-9096-8. [DOI] [PubMed] [Google Scholar]
- 86.Lozano R, Murray C, Lopez A, et al. Miscoding and Misclassification of Ischemic Heart Disease Mortality. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 87.Zaridze D, Brennan P, Boreham J, et al. Alcohol and cause-specific mortality in Russia: a retrospective case-control study of 48,557 adult deaths. Lancet. 2009;373(9682):2201–2214. doi: 10.1016/S0140-6736(09)61034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lown EA, Greenfield TK, Rogers JD. Health effects from drinking: type, severity, and associated drinking patterns based on qualitative and quantitative questions in a methodological survey. Subst Use Misuse. 2007;42(5):793–810. doi: 10.1080/10826080701202346. [DOI] [PubMed] [Google Scholar]