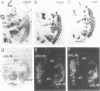
Abstract
Myogenin, as well as other MyoD-related skeletal muscle-specific transcription factors, regulate a large number of skeletal muscle genes during myogenic differentiation. During later development, innervation suppresses myogenin expression in the fetal hind limb musculature. Denervation of skeletal muscle reverses the effects of the nerve, and results in the reactivation of myogenin expression, as well as of other embryonic muscle proteins. Here we report that myogenin upstream sequences confer tissue- and developmental-specific expression in transgenic mice harboring a myogenin/chloramphenicol acetyltransferase (CAT) reporter construct. Using in situ hybridization to analyze serial sections of E12.5 embryos, we found colocalization of CAT and endogenous myogenin transcripts in the primordial muscle of the head and limbs, in the intercostal muscle masses, and in the most caudal somites. Later in development, we observed that the expression of the transgene and endogenous myogenin gene continued to be restricted to skeletal muscle but decreased shortly after birth; a period that coincides with the innervation of secondary myotubes. Furthermore, denervation of the mouse hind limbs induced a 10-fold accumulation of CAT and endogenous myogenin transcripts by 1 day after sciatic nerve resection; a 25-fold increase was observed by 4 days after denervation. Interestingly, we observed that the accumulation of CAT enzyme activity lagged considerably with respect to the increase in CAT transcripts. Our results indicate that the cis-acting elements that temporally and spatially confine transcription of the gene during embryonic development, and that mediate the responses to innervation and denervation of muscle, lie within the upstream sequences analyzed in these studies.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun T., Bober E., Winter B., Rosenthal N., Arnold H. H. Myf-6, a new member of the human gene family of myogenic determination factors: evidence for a gene cluster on chromosome 12. EMBO J. 1990 Mar;9(3):821–831. doi: 10.1002/j.1460-2075.1990.tb08179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T., Buschhausen-Denker G., Bober E., Tannich E., Arnold H. H. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. EMBO J. 1989 Mar;8(3):701–709. doi: 10.1002/j.1460-2075.1989.tb03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T., Rudnicki M. A., Arnold H. H., Jaenisch R. Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell. 1992 Oct 30;71(3):369–382. doi: 10.1016/0092-8674(92)90507-9. [DOI] [PubMed] [Google Scholar]
- Brennan T. J., Olson E. N. Myogenin resides in the nucleus and acquires high affinity for a conserved enhancer element on heterodimerization. Genes Dev. 1990 Apr;4(4):582–595. doi: 10.1101/gad.4.4.582. [DOI] [PubMed] [Google Scholar]
- Buckingham M. E., Lyons G. E., Ott M. O., Sassoon D. A. Myogenesis in the mouse. Ciba Found Symp. 1992;165:111–131. doi: 10.1002/9780470514221.ch7. [DOI] [PubMed] [Google Scholar]
- Buonanno A., Apone L., Morasso M. I., Beers R., Brenner H. R., Eftimie R. The MyoD family of myogenic factors is regulated by electrical activity: isolation and characterization of a mouse Myf-5 cDNA. Nucleic Acids Res. 1992 Feb 11;20(3):539–544. doi: 10.1093/nar/20.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buskin J. N., Hauschka S. D. Identification of a myocyte nuclear factor that binds to the muscle-specific enhancer of the mouse muscle creatine kinase gene. Mol Cell Biol. 1989 Jun;9(6):2627–2640. doi: 10.1128/mcb.9.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine K. G., Baracchini E., Goldman D. Coupling muscle electrical activity to gene expression via a cAMP-dependent second messenger system. J Biol Chem. 1993 Feb 5;268(4):2893–2898. [PubMed] [Google Scholar]
- Chahine K. G., Walke W., Goldman D. A 102 base pair sequence of the nicotinic acetylcholine receptor delta-subunit gene confers regulation by muscle electrical activity. Development. 1992 May;115(1):213–219. doi: 10.1242/dev.115.1.213. [DOI] [PubMed] [Google Scholar]
- Cheng T. C., Hanley T. A., Mudd J., Merlie J. P., Olson E. N. Mapping of myogenin transcription during embryogenesis using transgenes linked to the myogenin control region. J Cell Biol. 1992 Dec;119(6):1649–1656. doi: 10.1083/jcb.119.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T. C., Wallace M. C., Merlie J. P., Olson E. N. Separable regulatory elements governing myogenin transcription in mouse embryogenesis. Science. 1993 Jul 9;261(5118):215–218. doi: 10.1126/science.8392225. [DOI] [PubMed] [Google Scholar]
- Covault J., Sanes J. R. Neural cell adhesion molecule (N-CAM) accumulates in denervated and paralyzed skeletal muscles. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4544–4548. doi: 10.1073/pnas.82.13.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Dennis M. J., Ziskind-Conhaim L., Harris A. J. Development of neuromuscular junctions in rat embryos. Dev Biol. 1981 Jan 30;81(2):266–279. doi: 10.1016/0012-1606(81)90290-6. [DOI] [PubMed] [Google Scholar]
- Donoghue M. J., Merlie J. P., Rosenthal N., Sanes J. R. Rostrocaudal gradient of transgene expression in adult skeletal muscle. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5847–5851. doi: 10.1073/pnas.88.13.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclert A., Piette J., Changeux J. P. Induction of acetylcholine receptor alpha-subunit gene expression in chicken myotubes by blocking electrical activity requires ongoing protein synthesis. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1391–1395. doi: 10.1073/pnas.87.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclert A., Piette J., Changeux J. P. Influence of innervation of myogenic factors and acetylcholine receptor alpha-subunit mRNAs. Neuroreport. 1991 Jan;2(1):25–28. doi: 10.1097/00001756-199101000-00006. [DOI] [PubMed] [Google Scholar]
- Dutton E. K., Simon A. M., Burden S. J. Electrical activity-dependent regulation of the acetylcholine receptor delta-subunit gene, MyoD, and myogenin in primary myotubes. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2040–2044. doi: 10.1073/pnas.90.5.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D. G., Cheng T. C., Cserjesi P., Chakraborty T., Olson E. N. Analysis of the myogenin promoter reveals an indirect pathway for positive autoregulation mediated by the muscle-specific enhancer factor MEF-2. Mol Cell Biol. 1992 Sep;12(9):3665–3677. doi: 10.1128/mcb.12.9.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D. G., Olson E. N. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989 May;3(5):628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- Eftimie R., Brenner H. R., Buonanno A. Myogenin and MyoD join a family of skeletal muscle genes regulated by electrical activity. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1349–1353. doi: 10.1073/pnas.88.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fambrough D. M. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979 Jan;59(1):165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- Fontaine B., Sassoon D., Buckingham M., Changeux J. P. Detection of the nicotinic acetylcholine receptor alpha-subunit mRNA by in situ hybridization at neuromuscular junctions of 15-day-old chick striated muscles. EMBO J. 1988 Mar;7(3):603–609. doi: 10.1002/j.1460-2075.1988.tb02853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V., Upson L. M., Hayes W. P., Vyklicky L., Jr, Winters C. A., Buonanno A. Molecular cloning and development analysis of a new glutamate receptor subunit isoform in cerebellum. J Neurosci. 1992 Mar;12(3):1010–1023. doi: 10.1523/JNEUROSCI.12-03-01010.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour B. P., Fanger G. R., Newton C., Evans S. M., Gardner P. D. Multiple binding sites for myogenic regulatory factors are required for expression of the acetylcholine receptor gamma-subunit gene. J Biol Chem. 1991 Oct 25;266(30):19871–19874. [PubMed] [Google Scholar]
- Goldhamer D. J., Faerman A., Shani M., Emerson C. P., Jr Regulatory elements that control the lineage-specific expression of myoD. Science. 1992 Apr 24;256(5056):538–542. doi: 10.1126/science.1315077. [DOI] [PubMed] [Google Scholar]
- Goldman D., Brenner H. R., Heinemann S. Acetylcholine receptor alpha-, beta-, gamma-, and delta-subunit mRNA levels are regulated by muscle activity. Neuron. 1988 Jun;1(4):329–333. doi: 10.1016/0896-6273(88)90081-5. [DOI] [PubMed] [Google Scholar]
- Goldman D., Staple J. Spatial and temporal expression of acetylcholine receptor RNAs in innervated and denervated rat soleus muscle. Neuron. 1989 Aug;3(2):219–228. doi: 10.1016/0896-6273(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Hall Z. W., Sanes J. R. Synaptic structure and development: the neuromuscular junction. Cell. 1993 Jan;72 (Suppl):99–121. doi: 10.1016/s0092-8674(05)80031-5. [DOI] [PubMed] [Google Scholar]
- Hasty P., Bradley A., Morris J. H., Edmondson D. G., Venuti J. M., Olson E. N., Klein W. H. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993 Aug 5;364(6437):501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]
- Huang C. F., Tong J., Schmidt J. Protein kinase C couples membrane excitation to acetylcholine receptor gene inactivation in chick skeletal muscle. Neuron. 1992 Oct;9(4):671–678. doi: 10.1016/0896-6273(92)90030-h. [DOI] [PubMed] [Google Scholar]
- Huang M. T., Gorman C. M. The simian virus 40 small-t intron, present in many common expression vectors, leads to aberrant splicing. Mol Cell Biol. 1990 Apr;10(4):1805–1810. doi: 10.1128/mcb.10.4.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell T. M., Siegel R. E., Fischbach G. D. Induction of acetylcholine receptors on cultured skeletal muscle by a factor extracted from brain and spinal cord. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5397–5401. doi: 10.1073/pnas.76.10.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarsfeld A., Laufer R., Fontaine B., Devillers-Thiéry A., Dubreuil C., Changeux J. P. Regulation of muscle AChR alpha subunit gene expression by electrical activity: involvement of protein kinase C and Ca2+. Neuron. 1989 Mar;2(3):1229–1236. doi: 10.1016/0896-6273(89)90307-3. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Buskin J. N., Lockshon D., Davis R. L., Apone S., Hauschka S. D., Weintraub H. MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell. 1989 Sep 8;58(5):823–831. doi: 10.1016/0092-8674(89)90935-5. [DOI] [PubMed] [Google Scholar]
- Li L., Heller-Harrison R., Czech M., Olson E. N. Cyclic AMP-dependent protein kinase inhibits the activity of myogenic helix-loop-helix proteins. Mol Cell Biol. 1992 Oct;12(10):4478–4485. doi: 10.1128/mcb.12.10.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Zhou J., James G., Heller-Harrison R., Czech M. P., Olson E. N. FGF inactivates myogenic helix-loop-helix proteins through phosphorylation of a conserved protein kinase C site in their DNA-binding domains. Cell. 1992 Dec 24;71(7):1181–1194. doi: 10.1016/s0092-8674(05)80066-2. [DOI] [PubMed] [Google Scholar]
- Lin H., Yutzey K. E., Konieczny S. F. Muscle-specific expression of the troponin I gene requires interactions between helix-loop-helix muscle regulatory factors and ubiquitous transcription factors. Mol Cell Biol. 1991 Jan;11(1):267–280. doi: 10.1128/mcb.11.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z. Y., Dechesne C. A., Eldridge J., Paterson B. M. An avian muscle factor related to MyoD1 activates muscle-specific promoters in nonmuscle cells of different germ-layer origin and in BrdU-treated myoblasts. Genes Dev. 1989 Jul;3(7):986–996. doi: 10.1101/gad.3.7.986. [DOI] [PubMed] [Google Scholar]
- Logan C., Khoo W. K., Cado D., Joyner A. L. Two enhancer regions in the mouse En-2 locus direct expression to the mid/hindbrain region and mandibular myoblasts. Development. 1993 Mar;117(3):905–916. doi: 10.1242/dev.117.3.905. [DOI] [PubMed] [Google Scholar]
- Lomo T., Rosenthal J. Control of ACh sensitivity by muscle activity in the rat. J Physiol. 1972 Mar;221(2):493–513. doi: 10.1113/jphysiol.1972.sp009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J. P., Isenberg K. E., Russell S. D., Sanes J. R. Denervation supersensitivity in skeletal muscle: analysis with a cloned cDNA probe. J Cell Biol. 1984 Jul;99(1 Pt 1):332–335. doi: 10.1083/jcb.99.1.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J. P., Kornhauser J. M. Neural regulation of gene expression by an acetylcholine receptor promoter in muscle of transgenic mice. Neuron. 1989 Apr;2(4):1295–1300. doi: 10.1016/0896-6273(89)90067-6. [DOI] [PubMed] [Google Scholar]
- Merlie J. P., Sanes J. R. Concentration of acetylcholine receptor mRNA in synaptic regions of adult muscle fibres. Nature. 1985 Sep 5;317(6032):66–68. doi: 10.1038/317066a0. [DOI] [PubMed] [Google Scholar]
- Miner J. H., Wold B. Herculin, a fourth member of the MyoD family of myogenic regulatory genes. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1089–1093. doi: 10.1073/pnas.87.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Vaessin H., Caudy M., Jan L. Y., Jan Y. N., Cabrera C. V., Buskin J. N., Hauschka S. D., Lassar A. B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989 Aug 11;58(3):537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y., Hanaoka K., Hayasaka M., Esumi E., Li S., Nonaka I., Nabeshima Y. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature. 1993 Aug 5;364(6437):532–535. doi: 10.1038/364532a0. [DOI] [PubMed] [Google Scholar]
- Noden D. M. Vertebrate craniofacial development: the relation between ontogenetic process and morphological outcome. Brain Behav Evol. 1991;38(4-5):190–225. doi: 10.1159/000114388. [DOI] [PubMed] [Google Scholar]
- Numberger M., Dürr I., Kues W., Koenen M., Witzemann V. Different mechanisms regulate muscle-specific AChR gamma- and epsilon-subunit gene expression. EMBO J. 1991 Oct;10(10):2957–2964. doi: 10.1002/j.1460-2075.1991.tb07846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N. Interplay between proliferation and differentiation within the myogenic lineage. Dev Biol. 1992 Dec;154(2):261–272. doi: 10.1016/0012-1606(92)90066-p. [DOI] [PubMed] [Google Scholar]
- Olson E. N. MyoD family: a paradigm for development? Genes Dev. 1990 Sep;4(9):1454–1461. doi: 10.1101/gad.4.9.1454. [DOI] [PubMed] [Google Scholar]
- Ontell M., Hughes D., Bourke D. Morphometric analysis of the developing mouse soleus muscle. Am J Anat. 1988 Mar;181(3):279–288. doi: 10.1002/aja.1001810306. [DOI] [PubMed] [Google Scholar]
- Ontell M., Kozeka K. The organogenesis of murine striated muscle: a cytoarchitectural study. Am J Anat. 1984 Oct;171(2):133–148. doi: 10.1002/aja.1001710202. [DOI] [PubMed] [Google Scholar]
- Ordahl C. P., Le Douarin N. M. Two myogenic lineages within the developing somite. Development. 1992 Feb;114(2):339–353. doi: 10.1242/dev.114.2.339. [DOI] [PubMed] [Google Scholar]
- Parry D. J., Parslow H. G. Fiber type susceptibility in the dystrophic mouse. Exp Neurol. 1981 Sep;73(3):674–685. doi: 10.1016/0014-4886(81)90204-1. [DOI] [PubMed] [Google Scholar]
- Patapoutian A., Miner J. H., Lyons G. E., Wold B. Isolated sequences from the linked Myf-5 and MRF4 genes drive distinct patterns of muscle-specific expression in transgenic mice. Development. 1993 May;118(1):61–69. doi: 10.1242/dev.118.1.61. [DOI] [PubMed] [Google Scholar]
- Piette J., Bessereau J. L., Huchet M., Changeux J. P. Two adjacent MyoD1-binding sites regulate expression of the acetylcholine receptor alpha-subunit gene. Nature. 1990 May 24;345(6273):353–355. doi: 10.1038/345353a0. [DOI] [PubMed] [Google Scholar]
- Prody C. A., Merlie J. P. The 5'-flanking region of the mouse muscle nicotinic acetylcholine receptor beta subunit gene promotes expression in cultured muscle cells and is activated by MRF4, myogenin and myoD. Nucleic Acids Res. 1992 May 11;20(9):2367–2372. doi: 10.1093/nar/20.9.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reist N. E., Werle M. J., McMahan U. J. Agrin released by motor neurons induces the aggregation of acetylcholine receptors at neuromuscular junctions. Neuron. 1992 May;8(5):865–868. doi: 10.1016/0896-6273(92)90200-w. [DOI] [PubMed] [Google Scholar]
- Rhodes S. J., Konieczny S. F. Identification of MRF4: a new member of the muscle regulatory factor gene family. Genes Dev. 1989 Dec;3(12B):2050–2061. doi: 10.1101/gad.3.12b.2050. [DOI] [PubMed] [Google Scholar]
- Rosenthal N. Muscle cell differentiation. Curr Opin Cell Biol. 1989 Dec;1(6):1094–1101. doi: 10.1016/s0955-0674(89)80056-0. [DOI] [PubMed] [Google Scholar]
- Rudnicki M. A., Braun T., Hinuma S., Jaenisch R. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell. 1992 Oct 30;71(3):383–390. doi: 10.1016/0092-8674(92)90508-a. [DOI] [PubMed] [Google Scholar]
- Salminen A., Braun T., Buchberger A., Jürs S., Winter B., Arnold H. H. Transcription of the muscle regulatory gene Myf4 is regulated by serum components, peptide growth factors and signaling pathways involving G proteins. J Cell Biol. 1991 Nov;115(4):905–917. doi: 10.1083/jcb.115.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoon D., Lyons G., Wright W. E., Lin V., Lassar A., Weintraub H., Buckingham M. Expression of two myogenic regulatory factors myogenin and MyoD1 during mouse embryogenesis. Nature. 1989 Sep 28;341(6240):303–307. doi: 10.1038/341303a0. [DOI] [PubMed] [Google Scholar]
- Simon A. M., Hoppe P., Burden S. J. Spatial restriction of AChR gene expression to subsynaptic nuclei. Development. 1992 Mar;114(3):545–553. doi: 10.1242/dev.114.3.545. [DOI] [PubMed] [Google Scholar]
- Spurway N. C. Objective characterization of cells in terms of microscopical parameters: an example from muscle histochemistry. Histochem J. 1981 Mar;13(2):269–317. doi: 10.1007/BF01006884. [DOI] [PubMed] [Google Scholar]
- Tsay H. J., Neville C. M., Schmidt J. Protein synthesis is required for the denervation-triggered activation of acetylcholine receptor genes. FEBS Lett. 1990 Nov 12;274(1-2):69–72. doi: 10.1016/0014-5793(90)81331-h. [DOI] [PubMed] [Google Scholar]
- Tsay H. J., Schmidt J. Skeletal muscle denervation activates acetylcholine receptor genes. J Cell Biol. 1989 Apr;108(4):1523–1526. doi: 10.1083/jcb.108.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usdin T. B., Fischbach G. D. Purification and characterization of a polypeptide from chick brain that promotes the accumulation of acetylcholine receptors in chick myotubes. J Cell Biol. 1986 Aug;103(2):493–507. doi: 10.1083/jcb.103.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Wentworth B. M., Donoghue M., Engert J. C., Berglund E. B., Rosenthal N. Paired MyoD-binding sites regulate myosin light chain gene expression. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1242–1246. doi: 10.1073/pnas.88.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzemann V., Sakmann B. Differential regulation of MyoD and myogenin mRNA levels by nerve induced muscle activity. FEBS Lett. 1991 May 6;282(2):259–264. doi: 10.1016/0014-5793(91)80490-t. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Sassoon D. A., Lin V. K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989 Feb 24;56(4):607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Yee S. P., Rigby P. W. The regulation of myogenin gene expression during the embryonic development of the mouse. Genes Dev. 1993 Jul;7(7A):1277–1289. doi: 10.1101/gad.7.7a.1277. [DOI] [PubMed] [Google Scholar]