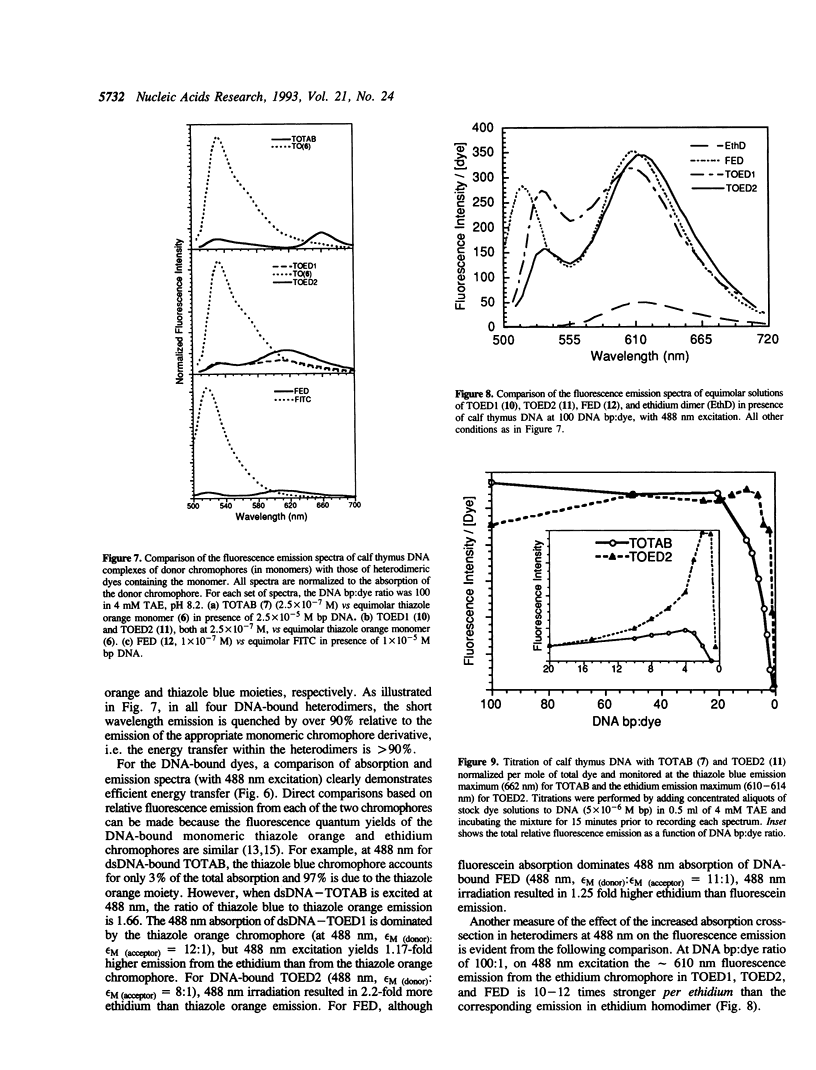
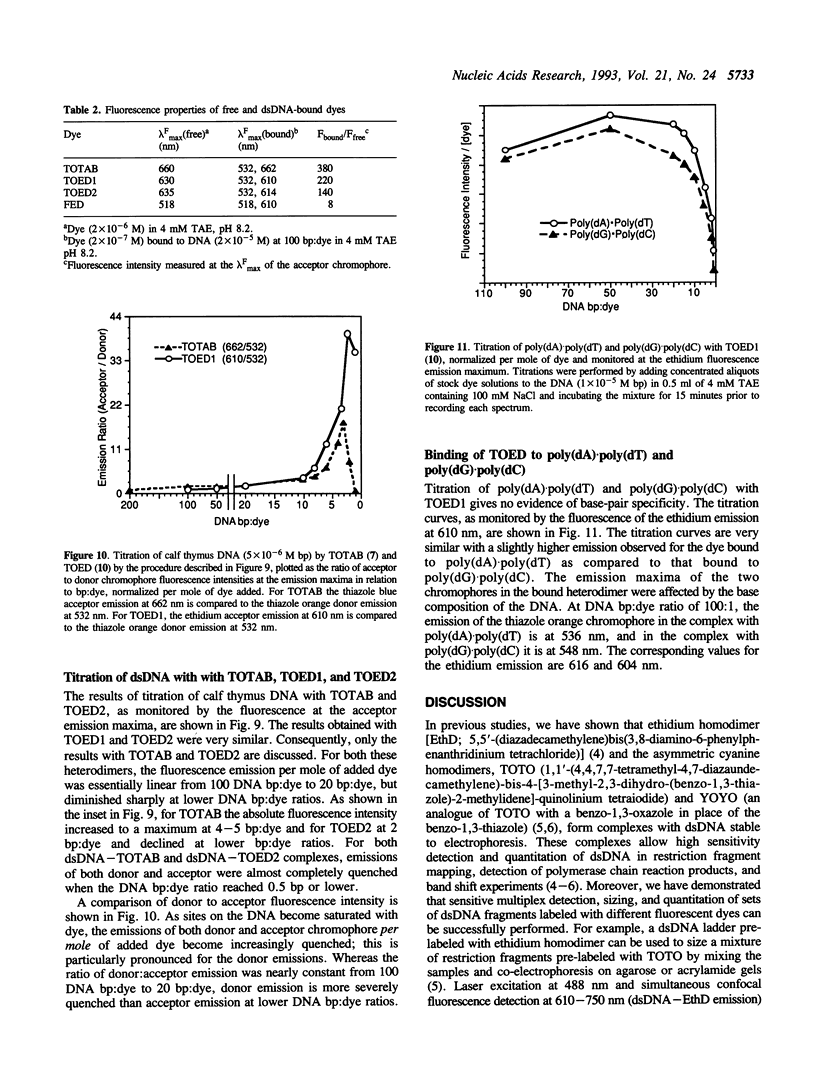
Abstract
Heterodimeric dyes are described which bind tightly to double-stranded (dsDNA) with large fluorescence enhancements. These dyes are designed to exploit energy transfer between donor and acceptor chromophores to tune the separation between excitation and emission wavelengths. The dyes described here absorb strongly at the 488 nm argon ion line, but emit at different wavelengths, and can be applied to multiplex detection of various targets. The chromophores in these dyes, a thiazole orange-thiazole blue heterodimer (TOTAB), two different thiazole orange-ethidium heterodimers (TOED1 and TOED2), and a fluorescein-ethidium heterodimer (FED), are in each case linked through polymethyleneamine linkers. The emission maxima of the DNA-bound dyes lie at 662 (TOTAB), 614 (TOED 2), and 610 nm (FED). The dyes showed a > 100 fold enhancement of the acceptor chromophore fluorescence on binding to dsDNA and no sequence selectivity. In comparison with direct 488 nm excitation of the constituent monomeric dyes, in the heterodimers the fluorescence of the acceptor chromophores was greatly enhanced and the emission of the donor chromophores quenched by over 90%. The acceptor emission per DNA-bound dye molecule was constant from 100 DNA bp:dye to 20 bp:dye and decreased sharply at higher dye:DNA ratios.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assa-Munt N., Denny W. A., Leupin W., Kearns D. R. 1H NMR study of the binding of Bis(acridines) to d(AT)5.d(AT)5. 1. Mode of binding. Biochemistry. 1985 Mar 12;24(6):1441–1449. doi: 10.1021/bi00327a024. [DOI] [PubMed] [Google Scholar]
- Benson S. C., Mathies R. A., Glazer A. N. Heterodimeric DNA-binding dyes designed for energy transfer: stability and applications of the DNA complexes. Nucleic Acids Res. 1993 Dec 11;21(24):5720–5726. doi: 10.1093/nar/21.24.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugain B., Barbet J., Capelle N., Roques B. P., Le Pecq J. B. DNA Bifunctional intercalators. 2. Fluorescence properties and DNA binding interaction of an ethidium homodimer and an acridine ethidium heterodimer. Biochemistry. 1978 Nov 28;17(24):5078–5088. doi: 10.1021/bi00617a002. [DOI] [PubMed] [Google Scholar]
- Gaugain B., Barbet J., Oberlin R., Roques B. P., Le Pecq J. B. DNA bifunctional intercalators. I. Synthesis and conformational properties of an ethidium homodimer and of an acridine ethidium heterodimer. Biochemistry. 1978 Nov 28;17(24):5071–5078. doi: 10.1021/bi00617a001. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Peck K., Mathies R. A. A stable double-stranded DNA-ethidium homodimer complex: application to picogram fluorescence detection of DNA in agarose gels. Proc Natl Acad Sci U S A. 1990 May;87(10):3851–3855. doi: 10.1073/pnas.87.10.3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N., Rye H. S. Stable dye-DNA intercalation complexes as reagents for high-sensitivity fluorescence detection. Nature. 1992 Oct 29;359(6398):859–861. doi: 10.1038/359859a0. [DOI] [PubMed] [Google Scholar]
- LePecq J. B., Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J Mol Biol. 1967 Jul 14;27(1):87–106. doi: 10.1016/0022-2836(67)90353-1. [DOI] [PubMed] [Google Scholar]
- Ledbetter D. H. The 'colorizing' of cytogenetics: is it ready for prime time? Hum Mol Genet. 1992 Aug;1(5):297–299. doi: 10.1093/hmg/1.5.297. [DOI] [PubMed] [Google Scholar]
- Lee L. G., Chen C. H., Chiu L. A. Thiazole orange: a new dye for reticulocyte analysis. Cytometry. 1986 Nov;7(6):508–517. doi: 10.1002/cyto.990070603. [DOI] [PubMed] [Google Scholar]
- Ludwig M., Hensel N. F., Hartzman R. J. Calibration of a resonance energy transfer imaging system. Biophys J. 1992 Apr;61(4):845–857. doi: 10.1016/S0006-3495(92)81892-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergny J. L., Slama-Schwok A., Montenay-Garestier T., Rougée M., Hélène C. Fluorescence energy transfer between dimethyldiazaperopyrenium dication and ethidium intercalated in poly d(A-T). Photochem Photobiol. 1991 Apr;53(4):555–558. doi: 10.1111/j.1751-1097.1991.tb03670.x. [DOI] [PubMed] [Google Scholar]
- Quesada M. A., Rye H. S., Gingrich J. C., Glazer A. N., Mathies R. A. High-sensitivity DNA detection with a laser-excited confocal fluorescence gel scanner. Biotechniques. 1991 May;10(5):616–625. [PubMed] [Google Scholar]
- Rye H. S., Quesada M. A., Peck K., Mathies R. A., Glazer A. N. High-sensitivity two-color detection of double-stranded DNA with a confocal fluorescence gel scanner using ethidium homodimer and thiazole orange. Nucleic Acids Res. 1991 Jan 25;19(2):327–333. doi: 10.1093/nar/19.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rye H. S., Yue S., Quesada M. A., Haugland R. P., Mathies R. A., Glazer A. N. Picogram detection of stable dye-DNA intercalation complexes with two-color laser-excited confocal fluorescence gel scanner. Methods Enzymol. 1993;217:414–431. doi: 10.1016/0076-6879(93)17080-o. [DOI] [PubMed] [Google Scholar]
- Rye H. S., Yue S., Wemmer D. E., Quesada M. A., Haugland R. P., Mathies R. A., Glazer A. N. Stable fluorescent complexes of double-stranded DNA with bis-intercalating asymmetric cyanine dyes: properties and applications. Nucleic Acids Res. 1992 Jun 11;20(11):2803–2812. doi: 10.1093/nar/20.11.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelin L. P. Polyfunctional DNA intercalating agents. Med Res Rev. 1986 Jul-Sep;6(3):275–340. doi: 10.1002/med.2610060303. [DOI] [PubMed] [Google Scholar]


