The odor of acids has a distinct quality that is perceived as sharp, pungent and often irritating1. How acidity is sensed and translated into an appropriate behavioral response is poorly understood. Here, we describe a functionally segregated population of olfactory sensory neurons (OSNs) in the fruit fly, Drosophila melanogaster, that are highly selective for acidity. These OSNs express IR64a, a member of the recently identified Ionotropic Receptor (IR) family of putative olfactory receptors2. In vivo calcium imaging showed that IR64a+ neurons projecting to the DC4 glomerulus in the antennal lobe are specifically activated by acids. Flies in which the function of IR64a+ neurons or the IR64a gene is disrupted had defects in acid-evoked physiological and behavioral responses, but their responses to non-acidic odorants remained unaffected. Furthermore, artificial stimulation of IR64a+ neurons elicited avoidance responses. Together, these results identify cellular and molecular substrates for acid detection in the Drosophila olfactory system and support a labeled-line mode of acidity coding at the periphery.
Many aversive odorants activate combinations of olfactory sensory neurons (OSNs)3,4, complicating the dissection of the circuits that translate odor recognition into behavior. By contrast, carbon dioxide (CO2), an odorant that is salient for many insect behaviors5-7, activates a single population of dedicated sensory neurons expressing GR21a and GR63a receptors7-9. These neurons are essential for mediating avoidance behavior of Drosophila to CO2 at concentrations lower than ~2%7,8,10. However, we found that flies in which GR21a/GR63a+ neurons were inactivated still avoided CO2, concentrations higher than ~5% (Fig. 1a). Avoidance of high CO2 concentrations required the antennae (Fig. 1a), indicating that another population of antennal neurons mediates avoidance to high CO2.
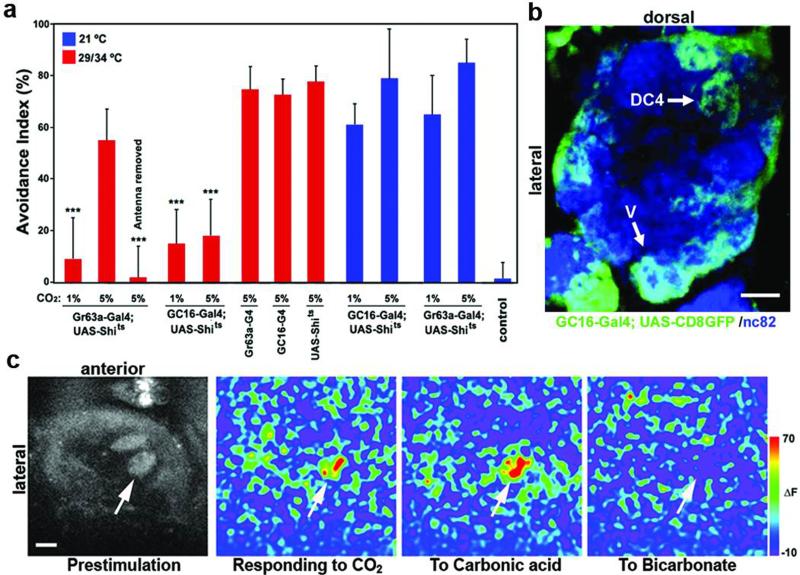
Figure 1. Identification of a glomerulus, DC4, activated by the CO2 metabolite, carbonic acid.
a, Behavioral testing in a T-maze at permissive (21°C) and non-permissive temperatures (29/34°C). ‘Control’ in this and all subsequent figures refer to responses of flies given a choice between two blank tubes. N=6-8. Error bars: s.e.m. ***P<0.001 ANOVA tukey-test. b, A single optical plane of the AL illustrates that the DC4- and V-glomeruli, among others, are labeled by GC16-GAL4. c, In vivo calcium imaging of the AL of a fly carrying GC16-GAL4 and UAS-GCaMP. Arrow indicates DC4. Peak ΔF responses are shown here. Scale bars: 10μm.
To identify these sensory neurons, we performed a functional screen for neurons required for responsiveness to CO2 by crossing a collection of GAL4 enhancer traps to UAS-Shibirets11. We isolated a line, GC16-GAL4, that failed to avoid 1% and 5% CO2 (Fig. 1a). GC16-GAL4 is expressed in OSNs that project to the V glomerulus among others, consistent with its defect in avoidance to 1% CO2 (Fig. 1b and Supplementary Fig. 1). To test whether other glomeruli labeled by GC16-GAL4 besides V are activated by CO2, we conducted in vivo calcium imaging12 of the antennal lobe (AL) of flies carrying GC16-GAL4 and UAS-GCaMP, a calcium sensitive GFP13. Using this approach, we identified an additional pair of dorsal glomeruli, termed DC414, that were activated by ~5% CO2 (Fig. 1c).
Since CO2, when dissolved in the lymph fluid inside the antennal sensilla that harbor OSNs, can generate metabolites, such as carbonic acid and bicarbonate ions, we tested whether DC4 could be activated by CO2 metabolites. As shown in Figure 1c, DC4 was stimulated by carbonic acid, but not by bicarbonate, suggesting that these neurons detect acidosis produced by increased CO2 concentrations, rather than CO2 itself.
Axonal projections to DC4 originate from a population of OSNs that reside in coeloconic sensilla and express neither insect Odorant Receptors (ORs) nor Gustatory Receptors (GRs). Instead, we found that these neurons express a novel receptor, IR64a, a member of the chemosensory ionotropic glutamate receptor family2. The IR64a promoter, IR64a-GAL4, driving UAS-CD8GFP labeled the DC4 glomerulus and another glomerulus, DP1m (Fig. 2a). Anti-IR64a immunohistochemistry demonstrated that the IR64a-GAL4 driver recapitulated the endogenous IR64a expression (Supplementary Fig. 2a). We detected ~16±0.9 IR64a+ cells (Supplementary Fig. 2b) surrounding the 3rd chamber of the sacculus15, which is a 3-chamber pit organ that opens to the posterior surface of the antenna (Fig. 2b). These IR64a+ cells send their dendrites to grooved sensilla that project to the interior of the sacculus (Fig. 2b and c).
Figure 2. DC4 is innervated by coeloconic sensillar neurons expressing IR64a.
a, Two different optical planes of the AL- DC4 (left) and DP1m (right)- of a fly bearing IR64a-GAL4; UAS-CD8GFP (a, b, c). b, IR64a+ cells in green (arrows) extend their dendrites to the 3rd chamber of sacculus. Red autofluorescence fortuitously depicts the outline of the antenna and sacculus. I, II and III represent the 1st, 2nd and 3rd chamber, respectively. c, An optical plane of the antenna across the 3rd chamber (arrow) immunostained with α-Elav (red) and α-GFP (green). Arrowheads: dendritic terminals. Double arrowhead: axonal bundles. Scale bars: 10μm.
Since IR64a+ neurons project to the DC4 and DP1m glomeruli, we determined whether only DC4, or both DC4 and DP1m were activated by acids by calcium imaging on flies carrying IR64a-GAL4 and UAS-GCaMP. All acids examined, but not non-acidic odorants, activated DC4 (Fig. 3a and b, and Supplementary Table 1). In contrast, DP1m was activated by acidic and non-acidic odorants (Fig. 3b and Supplementary Fig. 3). We wondered whether DP1m and DC4 might be activated by the functional side chains of some organic acids, rather than by the protons. We therefore tested whether inorganic acids such as hydrochloric acid (HCl) and nitric acid (HNO3), which dissociate completely in water and generate protons without an organic moiety, could activate DP1m and DC4. These inorganic acids, likely free protons in water vapor, activated DC4 in a dosage-dependent manner, but did not activate DP1m (Fig. 3a and b). This is consistent with the observation that only DC4 is activated by CO2, which contains no associated side chains. Furthermore, the strength of the DC4 activation inversely correlated with the pH of an odorant, sodium acetate (Fig. 3c). These results demonstrate that the neurons projecting to the DC4 glomerulus are highly dedicated to the detection of acidity.
Figure 3. Activation of DC4 by acidity requires IR64a.
a, In vivo calcium imaging of IR64a-GAL4; UAS-GCaMP flies with different genotypes. For each genotype, pre-stimulation (left), peak ΔF responses (middle) and traces for glomerular activation (right) are shown. Arrow: DC4; Arrowhead: DP1m. b, Integration of the GCaMP signals during glomerular activation. N=4-12. c, The GCaMP signal of DC4 responding to sodium acetate solution with different pH. The dotted line is a nonlinear regression fit of the data. d, α-IR64a (green) immunohistochemistry on sectioned antennae. Red auto-fluorescence outlines the antenna. Scale bars in a and d: 10μm.
To determine whether the IR64a gene is required for acid detection, we obtained a mutation (IR64ami) that has a transposable Minos element16 inserted in the 3rd intron of the IR64a locus. Flies homozygous for the IR64ami allele had significantly reduced IR64a mRNA transcript (Supplementary Fig. 4) and IR64a protein (Fig. 3d) in the antennae compared to wild type flies. Therefore, IR64ami is a strong loss-of-function mutation. This mutation abrogated glomerular activation of DC4 by acids (Fig. 3a and Supplementary Table 2). IR64ami also attenuated the activation of DP1m to acidic and non-acidic odorants. Two different IR64a transgenes- the genomic IR64a-HA driven by its own regulatory elements and a UAS-IR64a cDNA driven by IR64a-GAL4- rescued the odor sensing defects of IR64ami mutants (Fig. 3a and Supplementary Table 2). These results demonstrated that IR64a has a cell autonomous function as a component of the acid-sensing machinery required for DC4 activation and the machinery through which other odorants activate DP1m.
IR64a protein is localized in the cell bodies and dendrites, but not in axonal processes in the AL (Fig. 3d and 4e). It is highly enriched in the tip of the dendritic terminals that innervate coeloconic sensilla protruding the lumen of the 3rd chamber of the sacculus. The subcellular localization of IR64a is consistent with its direct involvement in acid detection. To determine the role of IR64a as a putative acid receptor, we ectopically expressed IR64a in another population of sensory neurons that are normally insensitive to acids and asked whether IR64a is capable of conferring acid sensitivity in these neurons. We expressed IR64a by using the IR76a-GAL4 driver2, which is expressed in coeloconic sensory neurons that project to the VM4 glomerulus in the AL2. Calcium imaging experiment showed that ectopic expression of IR64a induces odor sensitivity in VM4 to organic acids and 3-octanol, which normally activate DP1m (Supplementary Fig. 5), substantiating that IR64a is the direct determinant in odor detection. However, IR64a alone was not capable of conferring sensitivity to DC4-specific stimuli such as inorganic acids or CO2 (Supplementary Fig. 5 and Supplementary Table 2). This result suggests that although IR64a alone can induce responsiveness to a number of odorants that activate DP1m, it likely requires a co-receptor in DC4 neurons to mediate the specificity to acidity.
Figure 4. IR64a+ neurons and IR64a are necessary and sufficient for avoidance behavior.
a, Avoidance responses of flies expressing TNT or inactivated (Imp) TNT by IR64a-GAL4 in a T-maze. N=13-37. b, Calcium imaging of GR63a1;IR64ami mutant carrying IR64a-GAL4, UAS-GR21a, UAS-GR63a and UAS-GCaMP in response to 5% CO2. c, Avoidance of GR63a1;IR64ami mutant expressing CO2 receptors to 5% CO2. d, Avoidance of flies with different genotypes blindly tested in a T-maze. N=12-27. e, α-HA immunohistochemstry on a fly harboring IR64a-HA genomic transgene. IR64a-HA protein (green) is localized in dendrites (left), but not in axons (right). Scale bars: 10μm. *** P<0.001; * P<0.05 ANOVA tukey-test.
Having shown that IR64a is part of the acid-sensing machinery, we next determined whether IR64a+ neurons are necessary for the flies’ behavioral response to acids. We engineered flies in which IR64a+ cells are silenced by targeted expression of tetanus toxin (TNT)17. In a T-maze, these flies showed significant reductions in avoidance to several acids, while responses to non-acidic odorants such as benzaldehyde and 3-octanol were unaffected (Fig. 4a). This experiment, however, could not distinguish whether DC4 or DP1m is required for acid avoidance because IR64a-GAL4 is expressed in both populations of sensory neurons. Nonetheless, DP1m is unlikely to be important because it is not activated by acidity (Fig. 3a-c). To confirm the importance of DC4 in acid avoidance, we generated a GAL80 transgene under the control of the IR64a promoter and crossed this line to flies carrying GC16-GAL4 and UAS-Shibirets. Because GC16-GAL4 is expressed in DC4 neurons, but not in DP1m neurons, the IR64a-GAL80 transgene selectively relieves neuronal inhibition only in DC4 neurons. We confirmed that IR64a-GAL80 suppresses GC16-GAL4 activity only in DC4 neurons using a UASCD8GFP transgene (Supplementary Fig. 6a). The IR64a-GAL80 transgene rescued the behavioral defects of flies carrying GC16-GAL4 and UAS-Shibirets, supporting the specific role of DC4 mediating behavioral responses to acids (Supplementary Fig. 6b).
We next examined whether artificial activation of IR64a+ neurons is sufficient to trigger avoidance responses. We generated GR63a1; IR64ami double mutants expressing the CO2 receptors, UAS-GR21a and UAS-GR63a, and UAS-GCaMP by the IR64a-GAL4 driver. Expression of the two CO2 receptors in CO2 insensitive sensory neurons was previously shown to be sufficient to confer ectopic sensitivity to CO28,9. Indeed, the DC4 glomerulus in these flies was artificially stimulated by 5% CO2 (Fig. 4b). However, we could not detect activation of DP1m by CO2 in these flies (Fig. 4b), possibly because GR21a and GR63a receptors do not function properly in DP1m neurons. Behavioral experiments demonstrated that GR63a1; IR64ami double mutant flies failed to distinguish ambient air from 5% CO2. The CO2-blind flies with CO2 receptors expressed in IR64a+ neurons, however, exhibited robust avoidance to CO2 (Fig. 4c). This suggests that avoidance behavior is hardwired into the olfactory circuitry that detects acidity. Because DP1m in these flies does not appear to be activated by CO2, we reason that activation of DC4 neurons alone is sufficient for generating avoidance responses. These data, together with the observation that acidity evoked calcium responses only in DC4, firmly establish that IR64a+ neurons projecting to the DC4 glomerulus are necessary for acid sensation and sufficient for avoidance behavior. These results provide strong evidence for functional segregation of acid sensing at the periphery that drives innate avoidance behavior.
Consistent with the physiological defects, IR64ami flies had impaired avoidance to acids, but had normal responses to an unrelated odorant (Fig. 4d). Conversely, flies in which the Minos element was precisely excised from the IR64a locus (IR64a revertants) and those carrying an IR64a transgene in the IR64ami mutant background displayed robust avoidance to acids (Fig. 4d). Although the average avoidance indices of IR64ami and IR64a-GAL4xUAS-TNT flies were significantly different from those of wild type, they still exhibited moderate avoidance responses to acids (avoidance index of 20-25%). This residual response is unlikely to be mediated by the olfactory system, because antennaless flies had avoidance responses similar to IR64ami (Fig. 4d). Thus, additional acid sensors likely exist elsewhere in the fly.
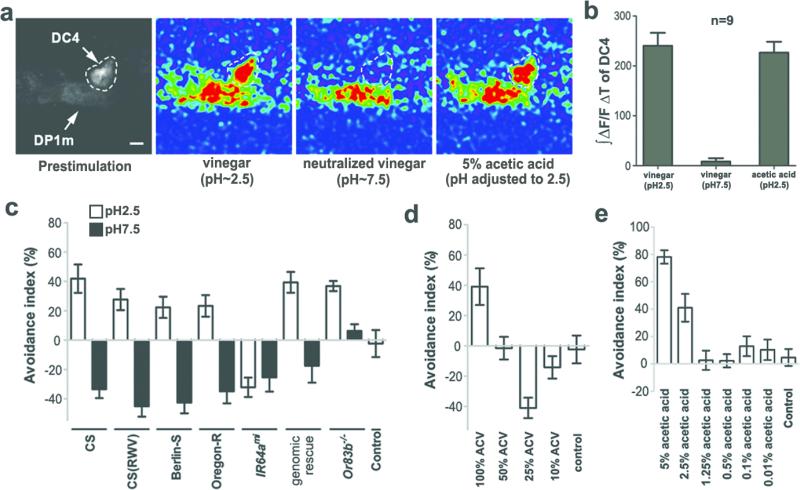
Fruit flies are often called “vinegar flies” because of their attraction to vinegar. Indeed, flies were attracted to certain concentrations of vinegar in a T-maze (Fig. 5d). However, a major ingredient of vinegar is acetic acid, which flies avoid (Fig. 5e). It is possible that flies are not repelled by vinegar because other constituents in vinegar inhibit DC4 activation by acetic acid. Alternatively, constituents other than acetic acid in vinegar might elicit an attraction response that overrides DC4-mediated avoidance. To distinguish between these possibilities, we performed in vivo calcium imaging to measure activation of DC4 following exposure to vinegar. As shown in Figure 5a and b, apple cider vinegar (ACV) that contains ~5% acetic acid activated DC4 as effectively as pure 5% acetic acid. These results suggest that vinegar contains attractants capable of overcoming DC4-mediated avoidance by activating other olfactory receptors. We predicted that neutralized vinegar would not activate DC4 and should be more attractive to flies because it still contains attractants. Consistent with this prediction, calcium imaging showed that DC4 was not stimulated by neutralized vinegar (Fig. 5a and 5b). Moreover, wild type (CS) flies avoided a high concentration of vinegar (pH=2.5) in a T-maze, but became attracted to neutralized vinegar (pH=7.5) at the same concentration (Fig. 5c). A similar behavioral switch was observed in other D. melanogaster strains such as Berlin and Oregon R, and with another type of vinegar (Fig. 5c). Furthermore, flies were attracted to 25% ACV (Fig. 5d), but not to acetic acid that had been diluted to the same concentration of acidity (Fig. 5e). This further supports the model that flies are attracted to components other than acid in vinegar. Avoidance of vinegar requires a functional IR64a+ circuit, since IR64ami mutants were equally attracted to regular and neutralized vinegar (Fig. 5c).
Figure 5. Fruit flies are attracted to components other than acetic acid in vinegar.
a, Calcium imaging of flies bearing IR64a-GAL4 and UAS-GCaMP. b, Integration of the GCaMP signals during odor presentation. c, Responses of starved flies to regular (pH~2.5) and neutralized (pH~7.5) vinegar in a T-maze. RWV: red wine vinegar. N=8-22. Positive avoidance index indicates an avoidance whereas negative index shows an attraction. d, Starved flies are attracted to diluted ACV (25%). N=6-11. e, Starved flies are not attracted to diluted acetic acid. The acidity of 25% ACV is same as that of 1.25% acetic acid. N=5-8.
Animals across the phylogeny exhibit innate aversion to a plume of acid18-20, often emanating from spoiled food or unripe fruit. Our characterization of Drosophila IR64a provides a cellular and molecular mechanism that can explain the distinct olfactory sensation of acidity. In the mammalian taste system, acid detection is mediated by a unique cell type, independent of other taste modalities21. This labeled-line organization is similar to those of acid and CO2 receptors in the fly olfactory system. Both acid and CO2 sensors are highly specific to their ligands and mediate similar avoidance behavior. This further raises a question- where are these two similar aversive stimuli represented in the brain? The identification of neural substrates in the CNS mediating acid and CO2 sensation will facilitate future mapping of the avoidance circuitry.
Method Summary
Transgenic flies and fly stocks
IR64a-GAL4 was made by cloning the 5’ sequence of IR64a into pCasper4-AUG-GAL4X24. IR64a-HA genomic transgene was constructed in pCasper4 with in-frame HA coding sequence at the C-terminus of IR64a. UAS-IR64a was made by cloning IR64a cDNA into pUAST vector. IR64ami and deficiency flies uncovering the IR64a locus were obtained from the Bloomington Drosophila Stock Center.
Antibody and immunohistochemistry
Rabbit α-IR64a polyclonal antibody was generated against a peptide antigen (SGKRDDGEMEEEEPPGQQ). Immunostaining of the fly brain and cryo-sectioning of the antennae were performed as previously described7,23.
Calcium imaging
In vivo calcium imaging was conducted with a live, behaving fly preparation that was subjected to a minimally invasive surgical procedure12. Briefly, flies were glued down to a custom-made plastic slide. Head cuticle was removed to expose the dorsal side of the brain, which was submerged in adult hemolymph like buffer4. The glomerular responses of the antennal lobe were detected by two-photon microscopy. Odor from the headspace of odorant-containing vials was delivered to the fly antenna by a puffing device.
Behavioral test
Testing acid-evoked behavioral responses of flies were conducted in a T-maze as previously described7. For the experiments using vinegars, flies were starved for 23 hours prior to behavior testing.
Supplementary Material
Acknowledgements
We thank M. Dus for helpful discussions and technical support for making anti-IR64a antibody; M. Kim for technical assistance; J. Treisman, N. Ringstad, C. Desplan, M. Warman, G. Fishell, M. Chesler, A. Wong, L. Vosshall and D. Anderson for discussions and comments on the manuscript; R. Lehmann for gratefully sharing their two-photon microscope. This work was initiated in David Anderson's laboratory at Caltech. G.S. expresses gratitude to Hae-Ri Song for her support. Financial support was provided by the NRSA fellowship (M.A), Boehringer Ingelheim Foundation (R. Bell), the Centre National de la Recherche Scientifique (Y.G), European Research Council Starting Independent Researcher Grant (R. Benton), the Swiss National Science Foundation (R. Benton), Alfred P. Sloan Foundation (G.S), Whitehall Foundation (G.S), Whitehead President Award (G.S), and NIH 1RO1GM089746 (G.S).
Methods
Fly stocks
Flies were raised on standard cornmeal medium at 25°C. IR64ami (stock number: 24610) and deficiency flies (stock number: 25119) uncovering the IR64a locus were obtained from the Bloomington stock center. IR64a revertant flies were generated by mobilizing the Minos element using Minos transposase as previously described22. Precise excision lines were identified by a loss of GFP signal in the eyes and confirmed by PCR genotyping and sequencing. Other flies were previously described: UAS-GCaMP(1.3)4; Or83b-/-23; Or83b-GAL44; Gr63a18; UAS-Shits11; UAS-TNT and UAS-ImpTNT17.
Transgenic constructs and flies
IR64a-GAL4 was made by cloning 2.5kb or 8kb DNA sequence upstream of IR64a into pCasper4-AUG-GAL4X24. The 8kb IR64a upstream sequence was subcloned into pCasper-GAL80 vector to generate IR64a-GAL80. IR64a-HA genomic duplication construct was made in pCasper4 with 8kb IR64a upstream sequence, 4kb IR64a genomic coding sequence (including introns), and in-frame HA coding sequence followed by 1.4kb IR64a downstream genomic sequence. UAS-IR64a was made by cloning IR64a cDNA sequence into pUAST vector. Transgenic flies were generated by the Bestgene Inc. (CA).
Antibodies and immunohistochemistry
Rabbit α-IR64a polyclonal antibody was generated against a peptide antigen (SGKRDDGEMEEEEPPGQQ) corresponding to 202-220 amino acid residues of IR64a protein by the Yenzyme Inc. (CA). This peptide is located within the predicted N-terminal extracelluar domain. Anti-IR64a serum was affinity purified and used at 1:1000 dilutions for immunohistochemistry. Other antibodies used in immunohistochemistry were: monoclonal α-HA (Covance) 1:1000; Rabbit α-GFP (Invitrogen) 1:500; monoclonal α-Elav (Hybridoma Bank) 1:100; nc82 (Hybridoma Bank) 1:50. Immunostaining of the fly brain and cryo-sectioning of the antennae were performed as previously described7,23.
Odorant preparation and delivery
Odorants and acids were dissolved in water or paraffin oil (v/v%). Odorants/acids that were diluted in water include hydrochloric acid, nitric acid, sulfuric acid, vinegar, formic acid, acetic acid, propionic acid, butyric acid, isobutyric acid, hexanoic acid, sodium hydroxide, methanol, ethanol, formaldehyde, paraformaldehyde, acetaldehyde, propionaldehyde, butyraldehyde and valeraldehyde. Carbonic acid was freshly made from sodium bicarbonate solution (1M) and adjusted to pH 2.0 by adding hydrochloric acid. Sodium acetate solution (100mM) was made in water and was adjusted to desired pH by adding acetic acid. Other odorants were dissolved in paraffin oil. Diluted odorants (300ul) or control solvent were placed in 16 ml glass vials with sealed rubber cap (Supelco) and was allowed to equilibrate with the headspace for at least one hour before usage.
The air within the head space of the odorant-containing vials was delivered to the fly antennae by using a puffing device, which includes a pump generating 22ml/min flow of humidified air-stream and an electronic valve controller. The puffing device was programmed to precisely control the on and off switch between valves connected to the odorant-containing vials. At the resting state, air constantly flows through the control vial and is delivered to the fly antennae. Upon odor stimulation, the valve connected to an odorant-containing vial opens for 1 second to allow odorant in the head space of the vial to be delivered to the fly antennae. After odorant delivery, the valve quickly shuts down and redirects the air to flow through the control vial again. This valve switching delivery system allows fast clearance of odorants and produces minimal mechanical disturbance.
Live fly preparation and calcium imaging
Flies were anesthetized and glued to a custom-made plastic slide by their wings using UV convertible glue (Kemxert Corp, PA). The proboscis was glued down to the chest to restrain head movement. The head and thorax were pushed through an opening (0.8mm wide x 1.6mm long) and exposed to the upper side of the slide. The fly was carefully oriented such that the antennae point downward. Small drops of wax (~55°C) were applied around the eyes and thorax to immobilize the fly. Head cuticle was carefully removed to expose the dorsal side of the brain, which was submerged in adult hemolymph like buffer4. This protocol is more sensitive than the previously described dissected brain preparation, which was used to show that CO2 solely activated the V glomerulus7.
Glomerular responses to odor stimulations were recorded using a two-photon microscope with a 40x water immersion objective lens. Real-time images were acquired at 7.57 frames per second with a resolution of 128x128 pixels. Imaging data were processed using image J with custom plug-in to generate pseudo color intensity images. ΔF/F was calculated as described in reference25. ∫ΔF/F Δt was computed as total area under activation peak divided by the width of the peak.
Flies used in the imaging experiments were 10~15 days old with two exceptions: First, 5-day old UAS-GCaMP(1.3); GC16-GAL4 flies were used in Fig. 1c because GC16-GAL4 expression in the DC4 glomerulus becomes very weak in older flies. Second, the presence of many UAS elements significantly reduced the efficiency of IR64a-GAL4 to drive UAS-GCaMP expression. Thus, older (30-day old) flies carrying UAS-GCaMP; IR64a-GAL4/UAS-Gr21a; Gr63a1, IR64ami, UAS-Gr63a were used in Fig. 4b. Genotypes of flies described in Fig. 3 are: UAS-GCaMP; IR64a-GAL4; IR64ami, IR64a-GAL4/TM6B (IR64ami/+), UAS-GCaMP; IR64a-GAL4; IR64ami, IR64a-GAL4 (IR64ami/IR64ami), UAS-GCaMP; UAS-IR64a/IR64a-GAL4; IR64ami, IR64a-GAL4 (UAS-IR64a rescue), and UAS-GCaMP; IR64a-GAL4; IR64ami, IR64a-HA (genomic IR64a-HA rescue).
Behavioral tests
Seven to twelve-day old flies were used for most of the behavioral tests except that 5-day old GC16-GAL4 flies were used in Figure 1a. For antennaless flies, behavioral tests were performed 24 hours after the surgery. All behavioral tests were carried out at 23~25°C with the exception of experiments using UAS-Shits flies. Flies carrying UAS-Shits were heat shocked in a 34°C water bath for 4-5 minutes, and subsequent behavioral testing was done at 29°C. T-maze experiments were performed as previously described7. Odorants for T-maze tests were diluted in water or paraffin oil to the following concentrations (v/v): acetic acid (10%), propionic acid (2.5%), isobutyric acid (1.25%), butyric acid (5%), hexanoic acid (5%), benzaldehyde (1.25%) and 3-octanol (5%). Apple cider vinegar (ACV) and red wine vinegar (RWV) were used without dilution. Neutralized vinegars were obtained by adding sodium hydroxide to vinegars until the pH reached 7.5. Flies previously starved for 23 hours were used for behavioral testing with vinegar.
The avoidance index = (number of flies in control tube - number of flies in experimental tube)/ (number of flies in experimental tube + number of flies in control tube). Testing flies were given a choice in a T-maze for 30-40 seconds before counting flies in each tube. A group of 30-35 flies were tested in each trial.
Footnotes
The authors declare no conflict of interests.
References
- 1.Dravnieks A. Odor quality: semantically generated multidimensional profiles are stable. Science. 1982;218(4574):799–801. doi: 10.1126/science.7134974. [DOI] [PubMed] [Google Scholar]
- 2.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136(1):149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng M, et al. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron. 2002;36(3):463–474. doi: 10.1016/s0896-6273(02)00975-3. [DOI] [PubMed] [Google Scholar]
- 4.Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112(2):271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 5.Kellogg FE. Water vapour and carbon dioxide receptors in Aedes aegypti. J Insect Physiol. 1970;16(1):99–108. doi: 10.1016/0022-1910(70)90117-4. [DOI] [PubMed] [Google Scholar]
- 6.Thom C, Guerenstein PG, Mechaber WL, Hildebrand JG. Floral CO2 reveals flower profitability to moths. J Chem Ecol. 2004;30(6):1285–1288. doi: 10.1023/b:joec.0000030298.77377.7d. [DOI] [PubMed] [Google Scholar]
- 7.Suh GS, et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431(7010):854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- 8.Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445(7123):86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 9.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci U S A. 2007;104(9):3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faucher C, Forstreuter M, Hilker M, de Bruyne M. Behavioral responses of Drosophila to biogenic levels of carbon dioxide depend on life-stage, sex and olfactory context. J Exp Biol. 2006;209(Pt 14):2739–2748. doi: 10.1242/jeb.02297. [DOI] [PubMed] [Google Scholar]
- 11.Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47(2):81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 12.Yorozu S, et al. Distinct sensory representations of wind and near-field sound in the Drosophila brain. Nature. 2009;458(7235):201–205. doi: 10.1038/nature07843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat Biotechnol. 2001;19(2):137–141. doi: 10.1038/84397. [DOI] [PubMed] [Google Scholar]
- 14.Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15(17):1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 15.Shanbhag SR, Singh K, Singh RN. Fine structure and primary sensory projections of sensilla located in the sacculus of the antenna of Drosophila melanogaster. Cell Tissue Res. 1995;282(2):237–249. doi: 10.1007/BF00319115. [DOI] [PubMed] [Google Scholar]
- 16.Franz G, Loukeris TG, Dialektaki G, Thompson CR, Savakis C. Mobile Minos elements from Drosophila hydei encode a two-exon transposase with similarity to the paired DNA-binding domain. Proc Natl Acad Sci U S A. 1994;91(11):4746–4750. doi: 10.1073/pnas.91.11.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sweeney ST, Broadie K, Keane J, Niemann H, O'Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14(2):341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 18.Sambongi Y, et al. Caenorhabditis elegans senses protons through amphid chemosensory neurons: proton signals elicit avoidance behavior. Neuroreport. 2000;11(10):2229–2232. doi: 10.1097/00001756-200007140-00033. [DOI] [PubMed] [Google Scholar]
- 19.Joseph RM, Devineni AV, King IF, Heberlein U. Oviposition preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila. Proc Natl Acad Sci U S A. 2009;106(27):11352–11357. doi: 10.1073/pnas.0901419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziemann AE, et al. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139(5):1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang AL, et al. The cells and logic for mammalian sour taste detection. Nature. 2006;442(7105):934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metaxakis A, Oehler S, Klinakis A, Savakis C. Minos as a genetic and genomic tool in Drosophila melanogaster. Genetics. 2005;171(2):571–581. doi: 10.1534/genetics.105.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43(5):703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 24.Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102(2):147–159. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- 25.Asahina K, Louis M, Piccinotti S, Vosshall LB. A circuit supporting concentration-invariant odor perception in Drosophila. J Biol. 2009;8(1):9. doi: 10.1186/jbiol108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.