Abstract
In the dry or frozen states, macromolecules are damaged directly by interactions with ionizing radiation. Since γ-rays and high-energy electrons randomly ionize orbital electrons in their path, larger molecules are more likely to suffer an interaction with these radiations. In each interaction, energy is transferred to the struck molecule, resulting in irreversibly broken covalent bonds. There is an extensive literature describing these radiation modifications in both synthetic and biopolymers. Although many different properties are measured, there emerges a similar picture of the nature of radiation damage that is common to all macromolecules. The techniques used in study of one species may be used to resolve questions raised in the other class of macromolecules.
There is considerable literature concerning the effects of ionizing radiation on matter. In general, the irreversible effects of radiation are due to the ionization of valence electrons. In all cases, irradiation of macromolecules in liquid (usually aqueous) solution resulted in much greater effects than irradiation of the same material when dry or frozen. This is due to the diffusible radiation products, primarily from the solvent, in which the specific radiation effect depends on the particular radiolytic product as well as the particular reactive ligand on the macromolecule.
Dry samples obviously have no solvent radiation products. In frozen samples, the same initial radiation products are created as in the liquid state. However, the diffusion constant of these products differs by a factor greater than 105. In the frozen state these products still react (albeit more slowly because of the lower temperature), but are restricted to only nearby molecules. All the neighboring molecules essentially are other solvent molecules. Therefore, in both the frozen and dry states, virtually all the radiation damage to biopolymers and to synthetic polymers is that due to the interaction of the radiation directly on that molecule – this is the direct effect of radiation.
Studies of these direct effects of ionizing radiations on macromolecules have been widely reported and many remarkable findings were published many years ago, most notably by Charlesby, Chapiro and Dole (1-3) in synthetic macromolecules and Pollard (4) in biological macromolecules. Many similar observations were reported, such as the fact that radiation sensitivity was dependent on temperature (5). Subsequently a vast literature has appeared in both fields, mostly without awareness of the other field. There has evolved a detailed picture of direct radiation effects identical in both kinds of molecules. Properties described in molecules of one species can therefore be studied further in the other species.
High energy electrons interact randomly with all electrons in their trajectory (6). In each interaction there is a transfer of energy from the radiation to the exposed material. The transfer of energy from radiations to target materials occurs over a wide range of energy. Starting in 1929 (7), an extensive literature concerns the loss of energy (‘straggling’) of high energy electrons. The spectrum of energy losses has been widely described, especially for high energy electrons (6, 8,9). There are several putative mechanisms for these interactions with different amounts of energy transferred, such as the ‘glancing’ and ‘head-on’ described by Fano (9). Less attention was focused on the fate of this energy in irradiated matter.
If the amount of energy transferred is small, the orbital electrons are excited to a higher energy level in the atom. After a period of time, they drop down to a lower energy level and emit excess energy, often as ultraviolet light. The atom and its’ associated molecule are returned to their original state. However, if the energy transferred is greater than the ionization potential of an electron, the electron is ejected from that atom (called a ‘primary ionization’; the emitted electron is called a ‘secondary electron’). Energy depositions larger than the ionization potential would leave ‘excess’ energy which could be donated as kinetic energy to the ejected electron or absorbed by the parent atom or molecule. The energy spectrum of emitted electrons has been determined in many different materials. The ‘excess’ (kinetic) energy of these secondary electrons is very small (9), “a few eV” (10). According to Fano (9) “… the great majority of the secondary electrons have a rather low energy. …. can transfer energy only to the external electrons of atoms, and this only when passing right through or very close to an atom. … Low-energy secondaries dissipate most of their energy within a short distance from their point of origin. This distance is of the order of 10 A in solid or liquid materials ….”. At 25 C. the range of 0.1 eV electrons in water is close to 1 nm, while for 10 eV electrons it is ~10 nm (11). This indicates that (a) the parent molecule is the principal recipient of the additional energy and (b) little further damage is caused by the secondary electrons.
The minimum energy to cause an ionization in a molecule is therefore dependent on the minimum ionization potential of the constituent atoms. Ionization potentials of inner electrons are considerably higher. However, if any of these are ejected, outer electrons will fall into that hole resulting in ionization of the valence electron. Protein molecules are composed of five major elements, H C O N and S, and their lowest ionization potentials range from 10.357 to 14.53 eV. In a typical 50 000 molecular weight protein, the relative abundance of each of these atoms is known. Thus the energy deposition for the minimum ionization can easily be calculated. Similar calculations can also be done for polyethylene and for a typical cell. The latter uses the elemental composition of a dry cell, otherwise oxygen and hydrogen would overwhelm the analysis.
The minimum transfer that causes an ionization is described above. From these data, the low energy spectrum can be determined [Fig 1]. This shows why the spectrum has a maximum (the most probable energy deposition) near 20 eV. Above 20 eV there are contributions due both to energy transfers in excess of the ionization potential and also interactions with inner electrons. At the high end of the energy depositions, the distribution losses follow the inverse square law (as E−2) (8,12). In these spectra, the “most probable” energy loss is 20 eV and the average energy deposition is near 60 ev - which has been experimentally verified (13) and theoretically predicted (14).
Figure 1.
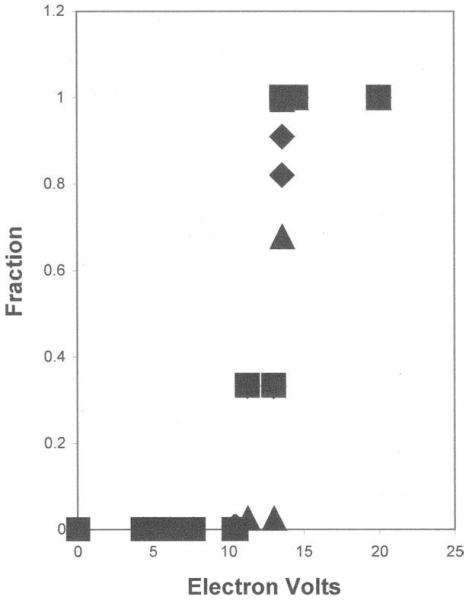
Fraction of ionizations due to each element versus low energy depositions for a typical protein (squares), polyethylene (triangles) and a typical dry cell (diamonds).
Radiation Target Theory
Since gamma rays and high energy electrons ionize randomly throughout the volume of the exposed material, the greater the number of electrons in an atom, the greater will be the chance of a radiation interaction; The greater the number of atoms in a molecule, the greater the chance that molecule will be ‘hit’. Thus the direct effects of radiation are directly proportional to the mass of the irradiated molecules. These facts lead to the exponential dependence of radiation effects on the radiation exposure: the surviving molecules decrease exponentially with radiation dose at a rate that depends directly on the mass of the molecule. Using this ‘target analysis’, this rate of disappearance of molecular properties in irradiated matter revealed the mass of the required structures. This has been used extensively in radiation studies of biological molecules.
The radiation sensitivity of all macromolecules – whether biological or synthetic – was known to depend on the irradiation temperature. Other observations of radiation effects on macromolecules have come from electron microscopy and from X-ray crystallography. In both of these observations, the detected patterns were found to degrade if the sample were exposed for extended periods. Using both techniques, samples were reported to suffer much less damage if the sample was at reduced temperature during exposure. Quantitatively the same temperature dependence is observed in all biomolecules (15) Furthermore, the same temperature dependence found in biomolecules also exists in synthetic polymers and is due to the production of free radicals (5).
Alexander, Black and Charlesby indicated in 1955 (16) that direct radiation effects seemed to be common among all macromolecules. Thus the fundamental nature of radiation sensitivity is identical in all macromolecules. Most radiation studies of biomolecules concerned proteins or DNA, while the principal synthetic polymer that was studied was polyethylene. The different chemical nature of these macromolecules required measurement of different properties. In synthetic polymers, “the different chemical effects of radiation are: 1. cross-linking, 2. main chain scission and 3. changes in unsaturation.” (17). In polyethylene, cross-linking is the predominant effect. Among the biomolecules, DNA (a large molecule composed of two chains twisted in a helix) revealed main chain scissions in one or both chains. The repeating monomer in DNA is a phosphate-ribose-base; there are four different bases polymerized in a precise sequence. Radiation effects on the bases and the sugar have been widely reported, as well as the biological activity of the molecule. Cross-linking of the chains is apparently very rare. Proteins are composed of eighteen different amino acids, also polymerized in a precise sequence. Previous radiation studies showed that all the amino acids had approximately the same radiation sensitivity. However, a recent report indicated that X-ray diffraction patterns of crystalline lysozyme revealed a specific radiation damage in the loss of certain amino acids, specifically cysteine. When the same experiment was repeated using different wavelength X-rays the specific effects on these amino acids disappeared (18). Unfortunately there has been no further confirmation of this observation.
Biological activity and main chain scission are the principal reported radiation effects in proteins. Cross-linking has been observed, but only rarely. Some proteins (‘glycoproteins’) have oligosaccharides covalently bound at specific positions along the polymer chain, but radiation damage to the sugar moieties does not perturb the polypeptide portion of the molecule (19). Von Sonntag (20) suggested that a primary ionization in a sugar ring resulted in severe structural damage to that ring, but little effect further down the oligosaccharide polymer. There is evidence that a single primary ionization can cause multiple damages randomly throughout a protein polymer, while in RNA (21) and DNA (22), the radiation damage in localized, presumably to the site of the primary ionization. This latter observation was suggested to be due to the presence of a sugar, ribose (23).
Since the physical bases are the same, this should not be surprising. The comparison is hampered because of the different properties that are usually measured. The temperature-dependence of radiation sensitivity is the most detailed comparison presented so far. Chain scission may now be considered, although almost all the available data will come from polyethylene and from proteins.
Proteins contain charged groups that enable them to be separated in an electric field. The technique – called SDS PAGE (Sodium Dodecyl Sulfate PolyAcrylamide Gel Electrophoresis) – involves the detergent SDS to unfold the polymer which is then placed on a sheet of polyacrylamide gel and subjected to an electric field. The protein moves in this field (electrophoresis) at a rate inversely related to its molecular weight. Irradiated proteins that have suffered one or more chain scissions will move as separate fragments; these can be detected by a variety of techniques including staining, antibody reactions or ligand binding. The relative movement reveals the molecular weight of the fragment and the intensity of the stain (or other detection technique) yields the amount of material.
When dry or frozen proteins are irradiated, there is a progressive decrease in stain intensity of the original band with increasing radiation dose, indicating scission(s) of the polymer chain. Staining reactions are noted along the gel below the original position, indicating a variety of radiolytic products with different molecular weights. If the scissions are random, it is expected that the stain intensity along the gel will be a Poisson distribution. If there were specific scission points (“weak spots”?), specific new bands would appear. If there were cross-linked molecules (which had not suffered scissions), new bands would appear at positions comparable to two or more times the original molecular weight. The vast majority of published reports show that proteins are randomly scissioned by radiation. In a very few reports, weak bands have been seen at higher molecular weights in certain proteins, while in some proteins, smaller fragments were detected if the protein were irradiated in the absence of oxygen. Surprisingly, such fragments were shown to some from only one end of the polypeptide chain (24). Nevertheless, the general conclusion is that after a single radiation interaction, proteins are randomly scissioned with little cross-linking. This seems to be true for every protein that has been studied. Very few reports of other irradiated biomolecules have appeared, but the limited data do not indicate anything different.
Synthetic polymers differ in that the SDS PAGE technique is not applicable if the molecule is uncharged, so other techniques have to be used. Among these would be size-exclusion column chromatography. The principal radiation effects reported in synthetic polymers are chain scission and unsaturation; hydrogen gas evolution is often detected. The most widely studied compound, polyethylene, clearly shows that cross-linking is a predominant effect. Crystalline portions of irradiated polyethylene efficiently trap radicals. When these radicals decay in vacuo they result largely in cross-links; in the presence of O2 the reaction products also include carbonyls that occur via a variety of steps, also involving main chain scission. This suggests a possible mechanism for the oxygen effect in proteins quoted above and further indicates a commonality of ionizing radiation effects in all macromolecules.
Results indicate that a single radiation interaction is responsible for radiation effects seen in synthetic polymers. If cross-linking were due to two independent interactions (one in each polymer), then the formation of dimers would be expected to depend on the square of the radiation dose. The most-commonly measured property is the formation of a gel, but this requires at least two cross-links per molecule – at least two ionizations in one polymer. In a protein, this could occur from a single primary ionization, but is it possible or reasonable in polyethylene? Extensive cross-linking in synthetic polymers leads to formation of a gel. In proteins and other biomolecules separation by SDS PAGE permits detection of some cross-linked species: dimers, trimers, etc. But extensively cross-linked complexes would not enter nor move in the electrophoretic gel; they would be detected as a stained band at the top of the gel. These have not been reported. This does not mean that cross-linking has not occurred, since individual molecules could be cross-linked AND scissioned. The extensive smaller molecular weight fragments and the paucity of larger complexes in biomolecules indicates that chain scission is much more common than cross-linking.
A surprising result of radiation damage in proteins was observed by size-exclusion chromatography (25-27): irradiated proteins eluted at the same position as native proteins. Since elution position depends on hydrodynamic radii, proteins with scissioned backbones do not suffer alteration of this hydrodynamic property. Thus no peptide fragments are released under non-denaturing conditions. On denturation, random fragments are released. Catalytic function is lost in enzymes, although in one report (28) enzyme activity survived in a scissioned polypeptide.
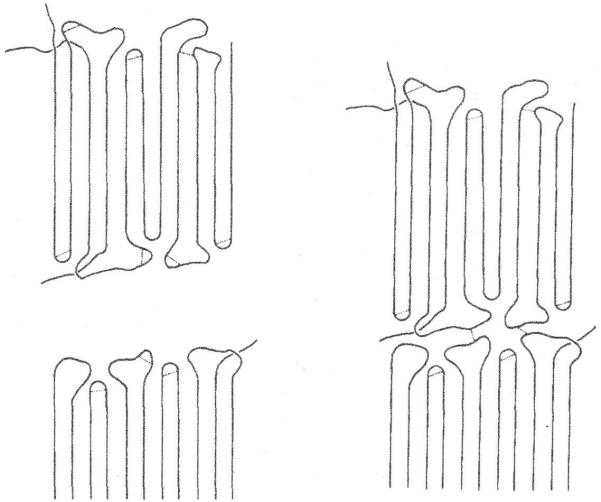
In crystallline polyethylene the polymer chain folds so that long stretches of the chain lie parallel to adjacent linear regions. The folds are short non-crystalline regions. On exposure to ionizing radiation, “Radicals form more or less randomly throughout the crystal. They cannot combine to yield cross-links within the lattice but are capable to migrate along the straight stems. They will have a chance to form a link in the fold surface region” and “If another layer is nearby they will link, amongst others, different layers together, hence create molecules contributing to a network.” (29). [Figure 2] On a macro scale this is a gel.
Figure 2.
Formation of cross-links in irradiated crystalline polyethylene. From (29) with permission.
Protein molecules have complex tertiary structures containing regions of ‘alpha helices’ and ‘beta sheets’. These regions fold and sometimes come in close contact with other regions. If cross-links form at these loci after radiation exposure, this would explain the unchanged hydrodynamic properties described above. However the release of fragments on denaturation shows that random scissions of the polymer backbone are also occurring. This offers further evidence that the detailed nature of radiation damage is common to both synthetic and biological polymers.
The information derived from the biological and synthetic polymer studies embellish our understanding of radiation effects on macromolecules. Realization of the commonality of these effects indicates that unsolved issues (such as why sugar rings block energy transfer, or how cross-links form between nearby polymer chains) may be studied by use of both kinds of macromolecules using the techniques uniquely appropriate to each.
Experimental techniques to study ionizing radiation effects differ in biological and synthetic polymers, but the evolving description is the same as shown by the effect of temperature. The physics of radiation interactions is identical in both, resulting in random electron ionizations. In all polymers, direct action of ionizing radiation ruptures covalent bonds in backbones and also in side groups. Charged residues are neutralized by reaction with free radicals and small ions or by reaction with charged groups on the same or nearby polymers. If the backbone is scissioned, the polymer mass is decreased. However, reaction with other polymer fragments (cross-linking) is more complex. Depending on the size of the reactive species, the molecular mass of the product may be less or greater than the parent molecules. In certain situations (such as in crystalline polymers) multiple cross links can form on a polymer leading to a gel-like network of very high molecular mass. Clearly these radiation effects are common to all macromolecules.
ACKNOWLEDGEMENT
Figure 2 from (29) is reprinted with permission from Elsevier.
Author biography
Ellis S. Kempner obtained a PhD in biophysics from Yale for radiation studies involving target theory. Subsequent positions were at the Carnegie Institution of Washington, Institut Pasteur in Paris and the National Institutes of Health in Bethesda where radiation studies were resumed to help Rodbell (Nobel Prize 1994) in his study of g-proteins. For the next thirty years, radiation target analysis was further developed and utilized to study many biological problems such as nucleic acids (RNA and DNA), enzymes (lipases, cholesterol enzymes), receptors and infectious agents (prions, with Prusiner (Nobel Prize 1997)) as well as synthetic polymers (with Salovey).
REFERENCES
- 1.Charlesby A. Atomic Radiation and Polymers. Pergamon Press; Oxford: 1960. [Google Scholar]
- 2.Chapiro A. Radiation Chemistry of Polymeric Systems. Interscience; New York: 1962. [Google Scholar]
- 3.Dole M. The Radiation Chemistry of Macromolecules. Academic Press; New York: 1972. [Google Scholar]
- 4.Hutchinson F, Pollard E. In: Mechanisms in Radiobiology. Errera M, Forssberg A, editors. Vol. 1. Academic Press; New York: pp. 71–92. [Google Scholar]
- 5.Kempner ES, Wood R, Salovey R. J. Polym. Sci. B Polym. Phys. 1986;24:2337–2343. [Google Scholar]
- 6.Bethe H. Annalen der Physik. 1930;5:325–400. [Google Scholar]
- 7.Williams EJ. Proc. Roy. Soc. London Ser. A. 1929;125:420–445. [Google Scholar]
- 8.Landau L. Journal of Physics USSR. 1944;VIII(4):201–205. [Google Scholar]
- 9.Fano U. In: Nickson JJ, editor. The Basic Aspects of Radiation Effects on Living Systems; Symposium on Radiobiology; Wiley New York. 1952.pp. 13–24. [Google Scholar]
- 10.Dubus A, Devooght J, Dehaes JC. Scanning Microscopy. 1990;4:1–20. [Google Scholar]
- 11.Meesungnoen J, Jay-Gerin J-P, Filali-Mouhim A, Mankhetkorn S. Radiat. Res. 2002;158:657–660. doi: 10.1667/0033-7587(2002)158[0657:leepri]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Goldwasser EL, Mills FE, Hanson AO. Physical Revw. 1952;88:1137–1141. [Google Scholar]
- 13.Rauth AM, Simpson JA. Radiat. Res. 1964;22:643–661. [Google Scholar]
- 14.Ronan RS, Heinz WF, Kempner ES. Rad. Environ. Biophys. 1996;35:159–162. doi: 10.1007/s004110050025. [DOI] [PubMed] [Google Scholar]
- 15.Kempner ES, Haigler HT. J. Biol. Chem. 1982;257:13297–13299. [PubMed] [Google Scholar]
- 16.Alexander P, Black RM, Charlesby A. Proc. Roy. Soc. 1955;A232:31–48. [Google Scholar]
- 17.Keller A. In: Developments in Crystalline Polymers, part I. Bassett DC, editor. Appl. Sci. Pub.; NY: 1982. pp. 37–113. [Google Scholar]
- 18.Aslantas M, Kendi E, Stojanoff V. Biophys. Soc. 2005 Abstract 702-Pos. [Google Scholar]
- 19.Kempner ES, Miller JH, McCreery JM. Analyt. Biochem. 1986;156:140–146. doi: 10.1016/0003-2697(86)90165-x. [DOI] [PubMed] [Google Scholar]
- 20.von Sonntag C. Adv. Carbohydr. Chem. Biochem. 1980;37:7–77. [Google Scholar]
- 21.Bernstein SL, Kempner ES. Proc. Nat’l. Acad. Sci. USA. 1996;93:6410–6414. doi: 10.1073/pnas.93.13.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anchorodquy TJ, Molina M, Kempner ES. Analyt. Biochem. 2009;385:229–233. doi: 10.1016/j.ab.2008.10.049. deC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kempner ES, Salovey R, Bernstein SL. Radiat. Phys. Chem. 1996;48:577–581. [Google Scholar]
- 24.le Maire M, Thauvette L, de Foresta B, Viel A, Beauregard G, Potier M. Biochem. J. 1990;267:431–439. doi: 10.1042/bj2670431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potier M, Thauvette L, Michaud L, Giroux S, Beauregard G. Biochemistry. 1991;30:8151–8157. doi: 10.1021/bi00247a009. [DOI] [PubMed] [Google Scholar]
- 26.Miller JH, Fedoronko DA, Hass BD, Myint M, Kempner ES. Arch. Biochem. Biophys. 1998;352:281–287. doi: 10.1006/abbi.1998.0604. [DOI] [PubMed] [Google Scholar]
- 27.Osborne JC, Jr., Miller JH, Kempner ES. Biophys. Jour. 2000;78:1698–1702. doi: 10.1016/S0006-3495(00)76721-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kempner ES, Whittaker JW, Miller JH. Prot. Sci. 2010;19:236–241. doi: 10.1002/pro.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keller A, Ungar G. Rad. Phys. Chem. 1983;22:155–181. [Google Scholar]