Abstract
Disturbed blood flow induces apoptosis of vascular endothelial cells, which causes atherosclerosis. In this issue, Heo et al. (2011. J. Cell Biol. doi:10.1083/jcb.201010051) sheds light on p53’s role in this phenomenon. Disturbed flow induces peroxynitrite production, which activates protein kinase C ζ and it’s binding to the E3 SUMO (small ubiquitin-like modifier) ligase PIASy (protein inhibitor of activated STATy). This leads to p53 SUMOylation and its export to the cytosol, where it binds to the antiapoptotic protein Bcl-2 to induce apoptosis.
Blood flow generates shear stress on vascular endothelial cells, which potently regulates endothelial morphology and function, including cell death and growth as well as inflammatory and thrombotic responses. The importance of shear stress in vascular biology and pathophysiology is highlighted by the protective role of stable flow (unidirectional and high shear stress) against atherosclerosis (atheroprotective) and the contrasting role of unstable flow (low and oscillatory shear stress) in promoting atherosclerosis (proatherogenic; Nam et al., 2009).
Endothelial cells contain numerous mechanosensors that detect local shear stress forces and transduce them into a variety of cell signaling pathways (Fig. 1 A). Via these mechanosensors, stable flow induces cell cycle arrest and inhibits apoptosis and inflammation in endothelial cells through acute and chronic mechanisms. Acute pathways include production of several factors, including nitric oxide (NO) from endothelial nitric oxide synthase (eNOS), which acts as a key mediator of the protective effect of stable flow. Long-term stable flow up-regulates atheroprotective genes, such as Klf2, eNOS, and antioxidant genes, coupled with the down-regulation of proinflammatory and proatherogenic genes (Ni et al., 2010).
Figure 1.
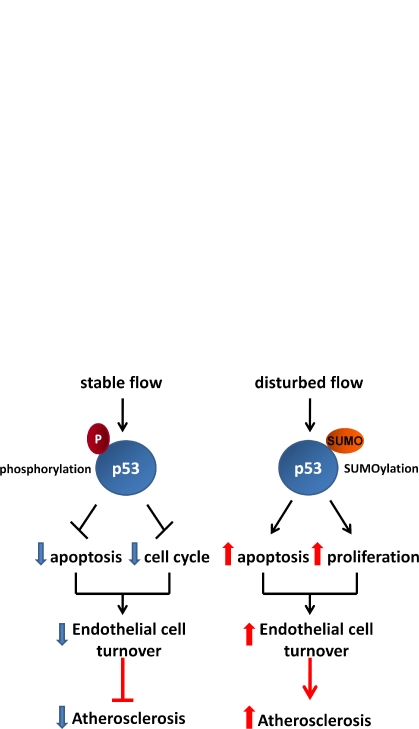
p53 coordinates the opposing effects of stable and disturbed blood flow on endothelial cell turnover. (A) A proposed pathway including a timeline by which disturbed flow is sensed by mechanosensors, which induces peroxynitrite (ONOO−) production, PKCζ phosphorylation, activation of E3 SUMO ligase PIASy, SUMOylation of p53, and its translocation to the cytosol, where it binds Bcl-2. Upon binding SUMOylated p53, Bcl-2 likely releases bax, which stimulates cytochrome c release from mitochondria, leading to apoptosome formation, caspase activation, and subsequent apoptosis. (B) Posttranslational modification of p53 (phosphorylation and SUMOylation under stable and disturbed flow, respectively) determines cell turnover and atherosclerosis. P, phosphorylation of p53.
In contrast, disturbed flow increases endothelial apoptosis and proliferation, resulting in a high turnover rate that corresponds to hot spots of increased endothelial permeability, inflammation, and atherosclerosis (Chiu and Chien, 2011). The mechanism by which disturbed flow regulates these endothelial responses involves the production of reactive oxygen species (ROS), which react with NO to form peroxynitrite and regulation of proatherogenic genes (Sorescu et al., 2004; Ni et al., 2010). Despite intense study, however, the mechanisms by which flow regulates endothelial turnover are unclear.
The tumor suppressor p53 plays a crucial role in determining the fate of apoptosis or cell cycle arrest in response to various stresses. In response to DNA damage or stress, cells increase p53 levels in the nucleus to up-regulate the expression of proapoptotic genes or cell cycle–regulating genes (Lee and Bernstein, 1995; Mihara et al., 2003; Teodoro et al., 2006). The p53 protein is also known to induce apoptosis by a nonnuclear, mitochondrial-dependent mechanism. p53 binds to and inhibits antiapoptotic members of the Bcl-2 family that reside in the mitochondrial surface, such as Bcl-2 or Bcl-xL, resulting in increased mitochondrial membrane permeability, the release of mitochondrial cytochrome c into the cytosol, and the initiation of the apoptotic caspase cascade (Fig. 1 A; Mihara et al., 2003).
The roles of p53 in atherosclerosis and flow-sensitive endothelial biology are confusing. In human atherosclerotic plaques, p53 expression is increased in endothelial cells, implying a role for p53 as an atherosclerosis-promoting factor (Ihling et al., 1998). Also, overexpression of p53 in a transgenic mouse line has been shown to induce endothelial dysfunction and inflammation by down-regulating transcription of klf2, an important antiatherogenic gene (Kumar et al., 2011). Surprisingly, however, p53 overexpression did not exacerbate atherosclerosis in transgenic mice (Sanz-González et al., 2007). To further confuse matters, p53 knockout enhanced atherosclerosis in mice, despite reducing vascular cell turnover (Guevara et al., 1999). These conflicting results may be caused by differential roles of p53 in different cell types involved in atherosclerosis, such as endothelial cells, smooth muscle cells, and macrophages, or even in the same cell under different stress conditions (Mercer et al., 2007).
In endothelial cells, the role of p53 in flow-dependent regulation of endothelial apoptosis and cell cycle arrest has only been partially described. Stable flow causes cell cycle arrest in a p53-dependent manner by stimulating JNK-mediated phosphorylation of p53, which in turn up-regulates expression of the cell cycle–inhibitory proteins GADD45 and p21cip1, resulting in cell cycle arrest (Fig. 1 B; Lin et al., 2000). However, the role of p53 in shear-induced apoptosis has not been determined. Now, Heo et al. (in this issue) reports a novel mechanism by which disturbed flow induces apoptosis of endothelial cells via SUMOylation of p53 in a PKCζ-dependent manner.
In this work, the authors tested whether disturbed flow induces apoptosis by p53- and PKCζ-dependent mechanisms in endothelial cells. They further hypothesized that peroxynitrite produced in response to disturbed flow mediates the apoptotic pathway in these cells. These hypotheses were based on the following previous observations: (a) flow-disturbed regions show enhanced endothelial cell apoptosis (Zeng et al., 2009); (b) PKCζ is activated in flow-disturbed endothelium in the porcine aorta (Magid and Davies, 2005); (c) human atherosclerotic endothelium shows increased levels of p53 expression (Ihling et al., 1998); and (d) both atherosclerotic lesions and flow-disturbed regions show evidence of increased levels of peroxynitrite (Patel et al., 2000; Hsiai et al., 2007).
To test these hypotheses, the authors developed a cone and plate shear device that enables the exposure of endothelial cells to laminar or disturbed shear stress. Using this system, they showed that disturbed flow phosphorylates PKCζ on T410 and T560 residues in a time-dependent manner in human umbilical vein endothelial cells (HUVECs). Then, they showed that disturbed flow induces HUVEC apoptosis by a PKCζ-dependent mechanism by using PKCζ siRNA or dominant-negative PKCζ. Next, they tested whether peroxynitrite produced in endothelial cells by disturbed flow was responsible for PKCζ activation and apoptosis. Using both chemical peroxynitrite and reagents affecting peroxynitrite levels (ebselen, N-nitro-l-arginine methyl ester, or Mn (III)tetrakis(4-benzoic acid)porphyrin chloride), they showed that this reactive nitrogen species mediated the activation of PKCζ and apoptosis. Unexpectedly, however, they found that neither disturbed flow nor peroxynitrite up-regulated p53 level and that peroxynitrite actually inhibited p53 transcription. By using a series of elegant molecular biological approaches and immunoprecipitation experiments, they determined that disturbed flow induced p53 SUMOylation, which led to the translocation of nuclear p53 into the cytoplasm where it bound Bcl-2. p53 SUMOylation was mediated by PKCζ binding to the E3 SUMO ligase PIASy. The binding between PKCζ and PIASy was then mapped to the C-terminal segment of the PKCζ kinase domain and the RING domain of PIASy. Surprisingly, although PIASy-mediated SUMOylation of p53 required PKCζ binding, PIASy was not phosphorylated by PKCζ, indicating a phosphorylation-independent activation of PIASy by PKCζ. Importantly, point mutations of p53 SUMOylation sites or a truncation mutant lacking the p53 nuclear export sequence abolished translocation of p53 to the cytosol and apoptosis induced by disturbed flow, indicating the critical importance of SUMOylation and nuclear export into the cytosol for p53’s apoptotic action.
These findings were validated in vivo by staining experiments with flow-disturbed lesser curvature and stable greater curvature regions of the mouse aortic arch. Endothelial cells in the lesser curvature regions show higher levels of apoptosis, total PKCζ, phosphorylated PKCζ, and nitrotyrosine staining, a marker of peroxynitrite. They also found increased perinuclear localization of p53 in lesser curvature regions compared with greater curvature regions, which is consistent with their in vitro findings.
These novel insights raise several questions: First, it is not clear how and where SUMOylated p53 binds to Bcl-2. The current immunostaining study in HUVECs suggests that disturbed flow and peroxynitrite induce translocation of p53 into the cytosol, but its specific subcellular location is not clear. However, p53 is known to bind Bcl-2 or Bcl-xL on the mitochondrial surface, leading to mitochondrial pore formation and cytochrome c release (Mihara et al., 2003). Whether disturbed flow works by this same mechanism needs to be clarified. Second, the authors show that p53 is only transiently SUMOylated in response to disturbed flow (at ∼3 h). By 6 h, p53 is no longer SUMOylated, suggesting an active de-SUMOylation event. What is the underlying mechanism? Third, the question still remains how p53 determines the balance between apoptosis, cell survival, and cell proliferation and how this ultimately controls endothelial cell turnover under various flow environments. Lastly, although the current study provides correlative in vivo evidence, cell type–specific knockout, knockin, or overexpression models of p53 in mice will be required to fully understand its role in flow-depensecadent regulation of cell turnover and vascular disease.
Acknowledgments
H. Jo’s work was supported by funding from National Institutes of Health grants HL87012, HL75209, and HHSN268201000043C and a World Class University Project from the Ministry of Education, Science and Technology of South Korea.
References
- Chiu J.J., Chien S. 2011. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 91:327–387 10.1152/physrev.00047.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara N.V., Kim H.S., Antonova E.I., Chan L. 1999. The absence of p53 accelerates atherosclerosis by increasing cell proliferation in vivo. Nat. Med. 5:335–339 10.1038/6585 [DOI] [PubMed] [Google Scholar]
- Heo K.-S., Lee H., Nigro P., Thomas T., Le N.-T., Chang E., McClain C., Reinhart-King C.A., King M.R., Berk B.C., Fujiwara K., Woo C.-H., Abe J.-i. 2011. PKCζ mediates disturbed flow-induced endothelial apoptosis via p53 SUMOylation. J. Cell Biol. 193:867–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiai T.K., Hwang J., Barr M.L., Correa A., Hamilton R., Alavi M., Rouhanizadeh M., Cadenas E., Hazen S.L. 2007. Hemodynamics influences vascular peroxynitrite formation: Implication for low-density lipoprotein apo-B-100 nitration. Free Radic. Biol. Med. 42:519–529 10.1016/j.freeradbiomed.2006.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihling C., Haendeler J., Menzel G., Hess R.D., Fraedrich G., Schaefer H.E., Zeiher A.M. 1998. Co-expression of p53 and MDM2 in human atherosclerosis: implications for the regulation of cellularity of atherosclerotic lesions. J. Pathol. 185:303–312 [DOI] [PubMed] [Google Scholar]
- Kumar A., Kim C.S., Hoffman T.A., Naqvi A., Dericco J., Jung S.B., Lin Z., Jain M.K., Irani K. 2011. p53 impairs endothelial function by transcriptionally repressing Kruppel-Like Factor 2. Arterioscler. Thromb. Vasc. Biol. 31:133–141 10.1161/ATVBAHA.110.215061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.M., Bernstein A. 1995. Apoptosis, cancer and the p53 tumour suppressor gene. Cancer Metastasis Rev. 14:149–161 10.1007/BF00665797 [DOI] [PubMed] [Google Scholar]
- Lin K., Hsu P.P., Chen B.P., Yuan S., Usami S., Shyy J.Y., Li Y.S., Chien S. 2000. Molecular mechanism of endothelial growth arrest by laminar shear stress. Proc. Natl. Acad. Sci. USA. 97:9385–9389 10.1073/pnas.170282597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magid R., Davies P.F. 2005. Endothelial protein kinase C isoform identity and differential activity of PKCzeta in an athero-susceptible region of porcine aorta. Circ. Res. 97:443–449 10.1161/01.RES.0000179767.37838.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J., Mahmoudi M., Bennett M. 2007. DNA damage, p53, apoptosis and vascular disease. Mutat. Res. 621:75–86 [DOI] [PubMed] [Google Scholar]
- Mihara M., Erster S., Zaika A., Petrenko O., Chittenden T., Pancoska P., Moll U.M. 2003. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell. 11:577–590 10.1016/S1097-2765(03)00050-9 [DOI] [PubMed] [Google Scholar]
- Nam D., Ni C.W., Rezvan A., Suo J., Budzyn K., Llanos A., Harrison D., Giddens D., Jo H. 2009. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 297:H1535–H1543 10.1152/ajpheart.00510.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni C.W., Qiu H., Rezvan A., Kwon K., Nam D., Son D.J., Visvader J.E., Jo H. 2010. Discovery of novel mechanosensitive genes in vivo using mouse carotid artery endothelium exposed to disturbed flow. Blood. 116:e66–e73 10.1182/blood-2010-04-278192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R.P., Moellering D., Murphy-Ullrich J., Jo H., Beckman J.S., Darley-Usmar V.M. 2000. Cell signaling by reactive nitrogen and oxygen species in atherosclerosis. Free Radic. Biol. Med. 28:1780–1794 10.1016/S0891-5849(00)00235-5 [DOI] [PubMed] [Google Scholar]
- Sanz-González S.M., Barquín L., García-Cao I., Roque M., González J.M., Fuster J.J., Castells M.T., Flores J.M., Serrano M., Andrés V. 2007. Increased p53 gene dosage reduces neointimal thickening induced by mechanical injury but has no effect on native atherosclerosis. Cardiovasc. Res. 75:803–812 10.1016/j.cardiores.2007.05.002 [DOI] [PubMed] [Google Scholar]
- Sorescu G.P., Song H., Tressel S.L., Hwang J., Dikalov S., Smith D.A., Boyd N.L., Platt M.O., Lassègue B., Griendling K.K., Jo H. 2004. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ. Res. 95:773–779 10.1161/01.RES.0000145728.22878.45 [DOI] [PubMed] [Google Scholar]
- Teodoro J.G., Parker A.E., Zhu X., Green M.R. 2006. p53-mediated inhibition of angiogenesis through up-regulation of a collagen prolyl hydroxylase. Science. 313:968–971 10.1126/science.1126391 [DOI] [PubMed] [Google Scholar]
- Zeng L., Zampetaki A., Margariti A., Pepe A.E., Alam S., Martin D., Xiao Q., Wang W., Jin Z.G., Cockerill G., et al. 2009. Sustained activation of XBP1 splicing leads to endothelial apoptosis and atherosclerosis development in response to disturbed flow. Proc. Natl. Acad. Sci. USA. 106:8326–8331 10.1073/pnas.0903197106 [DOI] [PMC free article] [PubMed] [Google Scholar]