Abstract
Background
Although the therapeutic outcome of acquired aplastic anemia has improved markedly with the introduction of immunosuppressive therapy using antithymocyte globulin and cyclosporine, a significant proportion of patients subsequently relapse and require second-line therapy. However, detailed analyses of relapses in aplastic anemia children are limited.
Design and Methods
We previously conducted two prospective multicenter trials of immunosuppressive therapy for children with aplastic anemia: AA-92 and AA-97, which began in 1992 and 1997, respectively. In this study, we assessed the relapse rate, risk factors for relapse, and the response to second-line treatment in children with aplastic anemia treated with antithymocyte globulin and cyclosporine.
Results
From 1992 to 2007, we treated 441 children with aplastic anemia with standard immunosuppressive therapy. Among the 264 patients who responded to immunosuppressive therapy, 42 (15.9%) relapsed. The cumulative incidence of relapse was 11.9% at 10 years. Multivariate analysis revealed that relapse risk was significantly associated with an immunosuppressive therapy regimen using danazol (relative risk, 3.15; P=0.001) and non-severe aplastic anemia (relative risk, 2.51; P=0.02). Seventeen relapsed patients received additional immunosuppressive therapy with antithymocyte globulin and cyclosporine. Eight patients responded within 6 months. Seven of nine non-responders to second immunosuppressive therapy received hematopoietic stem cell transplantation and five are alive. Eleven patients underwent hematopoietic stem cell transplantation directly and seven are alive.
Conclusions
In the present study, the cumulative incidence of relapse at 10 years was relatively low compared to that in other studies mainly involving adult patients. A multicenter prospective study is warranted to establish optimal therapy for children with aplastic anemia.
Keywords: children, aplastic anemia, relapse, risk factors, immunosuppressive therapy
Introduction
Aplastic anemia (AA) is thought to be an immune-mediated bone marrow disease, characterized by bone marrow aplasia and peripheral blood pancytopenia. Currently, two effective treatments are available for this disorder: allogeneic bone marrow transplantation and immunosuppressive therapy. Bone marrow transplantation from a human leukocyte antigen (HLA)-matched sibling donor can cure the majority of transplanted patients with severe AA.1 The outcome after bone marrow transplantation has been markedly better in children than in adults, with less frequent and severe graft-versus-host disease and better overall survival.2,3 However, most children with severe AA have no matched sibling donor and rely on immunosuppressive therapy as first-line treatment.
The combination of antithymocyte globulin and cyclosporine is now considered the standard immunosuppressive regimen for children with severe AA who lack a matched sibling donor.4 Recent large trials of combined immunosuppressive therapy for severe AA in children demonstrated that the response rate is greater than 60% and the 3- to 5-year survival rate is approximately 90%.5–7 However, relapse and clonal evolution with transformation to myelodysplasia or acute myeloid leukemia remain significant problems after immunosuppressive therapy, and long-term, event-free survival is less impressive than after bone marrow transplantation.4,8 We previously reported the results of a multicenter trial of immunosuppressive therapy for children with AA (AA-92 study).5 In the AA-92 study, the response rate at 6 months was 71%, with the probability of survival at 4 years being greater than 90%. However, a significant proportion of patients subsequently relapsed and required second-line therapy. To select the optimal therapy for such patients, a detailed analysis concerning relapse after response to immunosuppressive therapy is very important; however, analyses of relapse of AA in children after the standard combined immunosuppressive regimen are very limited.9–11 Although the European Group for Blood and Marrow Transplantation (EMBT) reported an analysis of relapse of AA after immunosuppressive therapy in a large number of patients, the study populations were primarily adults treated in the 1970s and 1980s with antithymocyte globulin monotherapy.9 A report from the Italian Association of Pediatric Hematology and Oncology focused mainly on the response to cyclosporine and dependence after immunosuppressive therapy.10 A single-center retrospective analysis from the National Institutes of Health showed excellent long-term survival with a 33% cumulative incidence of relapse at 10 years in children with severe AA who responded to the standard immunosuppressive therapy; however, a detailed analysis of relapse that included risk factors was not provided.11
We previously conducted two prospective multicenter studies: the AA-92 and AA-97, which began in November 1992 and October 1997, respectively.5,12 From 1992 to 2007, 473 children with AA were treated with immunosuppressive therapy in these studies, and 441 of the children were treated with antithymocyte globulin plus cyclosporine. In the present study, we assessed the relapse rate, risk factors for relapse, response to second-line treatment, and prognosis after relapse in AA children treated with an antithymocyte globulin/cyclosporine-based regimen.
Design and Methods
Patients
Two consecutive prospective studies were designed by the Japan Childhood Aplastic Anemia Study Group and involved 79 hospitals in Japan. The eligibility criteria have been described previously.5 The severity of disease was determined according to currently used criteria.13,14 Disease was considered severe if at least two of the following were present: (i) neutrophil count less than 0.5×109/L; (ii) platelet count less than 20×109/L; and (iii) reticulocyte count less than 20×109/L with a hypocellular bone marrow. AA was considered very severe if the above criteria for severe disease were fulfilled and the neutrophil count was less than 20×109/L. Non-severe disease was defined by at least two of the following: (i) neutrophil count less than 1.0×109/L, (ii) platelet count less than 50×109/L; and (iii) reticulocyte count less than 60×109/L with a hypocellular bone marrow. Allogeneic bone marrow transplantation was recommended for those patients with severe or very severe disease who had a matched sibling donor. This study was approved by the Ethic Committee of Hyogo Children Hospital.
Treatment
The details of the immunosuppressive therapy administered were described in previous reports.5,12 Immunosuppressive therapy consisted of horse antithymocyte globulin (Lymphoglobulin; Genzyme Corp., Cambridge, MA, USA) (15 mg/kg per day, days 1 to 5), cyclosporine (6 mg/kg per day, days 1 to 180, with subsequent adjustments to maintain the whole blood cyclosporine concentration between 100 to 200 ng/mL), and methylprednisolone for prophylaxis against allergic reactions (2 mg/kg per day for 5 days, with subsequent halving of the dose every week until discontinuation on day 28). Patients with very severe AA were treated with immunosuppressive therapy plus granulocyte-colony stimulating factor (G-CSF) (Filgrastim; Kirin, Tokyo, Japan) [400 μg/m2 on day 1, with responding patients (neutrophil count > 1.0×109/mL) receiving the same dose three times a week for 3 months in the AA-92 study and for 60 days in the AA-97 study]. In the AA-92 study, the addition of G-CSF to immunosuppressive therapy for patients with severe AA and non-severe AA was randomized, while in the AA-97 study, G-CSF was not given to patients with severe AA or non-severe AA except to those with documented severe infection. All patients in the AA-92 study received danazol at a dose of 5 mg/kg/day for 6 months, and danazol was discontinued without tapering.
Assessments
A complete response was defined for all patients as a neutrophil count greater than 1.5×109/L, a platelet count greater than 100×109/L, and a hemoglobin level greater than 11.0 g/dL. For patients with severe AA and very severe AA, a partial response was defined as a neutrophil count greater than 0.5×109/L, a platelet count greater than 20×109/L, a hemoglobin level greater than 8.0 g/dL, and no requirement for blood transfusions. For patients with non-severe AA, a partial response was defined as a neutrophil count greater than 1.0×109/L, a platelet count greater than 30×109/L, a hemoglobin level greater than 8.0 g/dL, and no requirement for blood transfusions.5 In patients with a complete response on day 180, the cyclosporine dose was tapered down slowly (10% of adjusted dose per month). In those with a partial response, cyclosporine was continued for another 6 months to allow further improvement of blood counts. Tapering of cyclosporine was started on day 360 (10% every 2 weeks) regardless of response.
The hematologic response was evaluated 6 months after the initiation of therapy. Relapse was defined by conversion to no response from a partial or complete response and/or the requirement for blood transfusions.5
Statistical analysis
Failure-free survival curves were calculated by the Kaplan-Meier method, and evaluated by the log-rank test. The Cox proportional hazards model was used to assess the risk factors for relapse after immunosuppressive therapy using both univariate and multivariate analyses. The estimated magnitude of the relative risk (RR) is shown along with the 97.5% confidence interval (CI). Cumulative incidence using the competing risk method, as described by Fine and Gray,15 was used for the assessment of factors predicting relapse. The competing events of relapse were death and transplantation.
Results
Patients’ characteristics
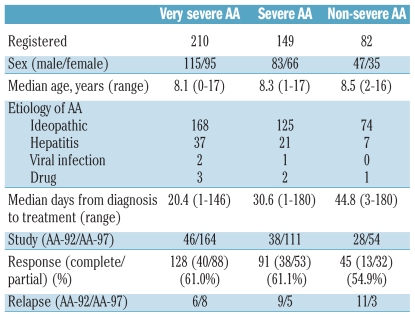
In the AA-92 and AA-97 studies, 441 AA children were treated with antithymocyte globulin plus cyclosporine between 1992 and 2007. The characteristics of all the patients studied are summarized in Table 1. There were 112 and 329 patients in the AA-92 and AA-97 studies, respectively. The median age of all these patients was 8.3 years (range, 0 to 17 years). Patients with very severe (n=210), severe (n=149) and non-severe disease (n=82) received initial immunosuppressive therapy consisting of antithymocyte globulin and cyclosporine. Six months after the initial immunosuppressive therapy, 264 patients (59.9%) had achieved a complete response (n = 91) or partial response (n=173). Among the 264 patients who responded to immunosuppressive therapy, 42 (15.9%) subsequently relapsed. The cumulative incidence of relapse was 11.9% at 10 years and the median time from diagnosis to relapse was 21 months (range, 6 to 138 months). The median time from response to antithymocyte globulin therapy to relapse was 22 months (range, 2 to 135 months).
Table 1.
Patients’ pretreatment characteristics.
Risk factors for relapse
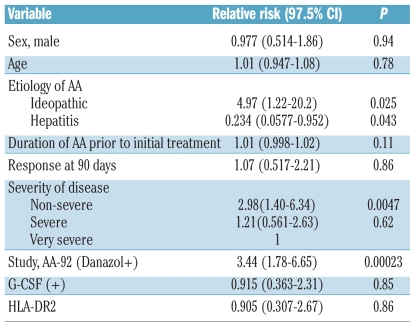
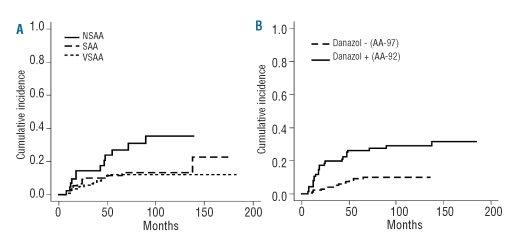
Two hundred and sixty-four patients with a total of 42 events were eligible for analyses of risk factors for relapse. In univariate analysis, two parameters, non-severe disease (RR=2.98, 97.5% CI 1.40 – 6.34, P=0.0047) and use of danazol (RR=3.44, 97.5% CI 1.78 – 6.65, P=0.00023), were statistically significant risk factors (Table 2). In contrast, the relative risk of relapse for patients with post-hepatitis AA was significantly lower than the relative risk for patients with idiopathic AA (RR=0.234, P=0.043). Gender, age, duration of AA prior to initial treatment, early response (within 90 days after immunosuppressive therapy), use of G-CSF, and HLA-DR2 could not be identified as risk factors. In multivariate analysis, two factors, non-severe AA (RR=2.51, 97.5% CI 1.15 – 5.46, P=0.02) and use of danazol (RR=3.15, 97.5% CI 1.62 – 6.12, P=0.001) remained statistically significant. Figure 1A shows the cumulative incidence of relapse of patients with non-severe AA (35.3%), severe AA (12.9%), and very severe AA (12.0%) 10 years after the first immunosuppressive therapy. The cumulative relapse rate of patients with non-severe AA was significantly higher than that of patients with severe AA (P=0.025) or very severe AA (P=0.005). Figure 1B shows the actuarial risk of relapse at 10 years among patients treated with danazol (29.0%) and in the group not treated with danazol (9.8%) (P<0.001).
Table 2.
Risk factors for relapse in patients with aplastic anemia by univariate analysis.
Figure 1.
Cumulative incidence of relapse after immunosuppressive therapy in children with aplastic anemia. (A) The cumulative relapse rate of patients with non-severe aplastic anemia (NSAA) was significantly higher than that of patients with severe aplastic anemia (SAA) (P=0.025) and very severe aplastic anemia (VSAA) (P=0.005) 10 years after the first immunosuppressive therapy. (B) The actuarial risk of relapse at 10 years was significantly higher in the group treated with danazol (29.0%) than in the group not treated with danazol (9.8%) (P<0.001).
Repeated immunosuppressive therapy versus hematopoietic stem cell transplantation as second-line therapy
Among 42 relapsed patients, 17 received a second course of immunosuppressive therapy with antithymocyte globulin and cyclosporine. Eight of these 17 patients responded within 6 months and are alive. Seven of nine non-responders to second immunosuppressive therapy received hematopoietic stem cell transplantation (HSCT) as salvage therapy. The hematopoietic stem cell donors were HLA-matched unrelated bone marrow donors (n=4), unrelated cord blood donors (n=2) and one matched sibling donor. Five of seven patients are alive following HSCT. Eleven patients underwent HSCT directly from an alternative donor (unrelated bone marrow donor, n=7; unrelated cord blood donor, n=1, HLA-mismatched family donor, n=3) and seven are alive. The estimated failure-free survival from the beginning of second-line therapy was 63.6% in the HSCT group compared with 47.1% in the groups treatment with repeated immunosuppressive therapy (P=0.96). The overall survival rate did not differ between the immunosuppressive therapy group (84.7%) and the HSCT group (63.6%) after second-line treatment (P=0.07). Other patients were treated with cyclosporine alone (n=6) or bone marrow transplantation from a matched sibling donor (n=6). Two patients did not receive second-line treatments. One patient developed clonal evolution to myelodysplastic syndrome after 65 months, and the second developed acute myeloid leukemia after 37 months. Two patients showed clonal evolution to paroxysmal nocturnal hemoglobinuria after 138 months and 55 months. There were seven deaths among the 42 patients who initially relapsed. The causes of death were HSCT-related complications (n=5), acute myeloid leukemia (n=1) and bacteremia (n=1). The overall 10-year survival rates for patients with very severe AA, severe AA, and non-severe AA were 82.2±3.3%, 82.1±4.7% and 98.2±1.8%, respectively.
Discussion
Analysis of relapse in children with AA responding to immunosuppressive therapy will provide valuable information for the management of childhood AA. Here, we present the results of a comprehensive analysis of the largest consecutive series of AA children treated with standard immunosuppressive therapy. Relapse of AA after immunosuppressive therapy is relatively common, with actuarial risks of 30 – 40% having been reported.16–18 In the present study, the cumulative incidence of relapse at 10 years was 11.9%, which is relatively low compared with that found in other studies that primarily involved adult patients.16–18 Differences in the study populations may explain the discrepancy between the results of our current study and those of the other studies. A recent Italian study of childhood AA showed a 16% cumulative incidence of relapse, which is comparable with that found in our study.10
Multivariate analysis of the data from this retrospective multicenter study shows that the use of danazol was the most statistically significant risk factor for relapse. From 1992 to 2007, 441 children with newly diagnosed AA were treated with immunosuppressive therapy consisting of antithymocyte globulin and cyclosporine with (the AA-92 study) or without danazol (the AA-97 study). There are several reports of the efficacy of anabolic steroids in the treatment of AA. A randomized trial from the EBMT SAA working party demonstrated that the addition of an anabolic steroid (oxymetholone) to antithymocyte globulin treatment improved the response rate of patients with treated AA.14 In our study, consistent with that report, the response rate at 6 months was higher in the patients who received immunosuppressive therapy with danazol (67.9%) than in the group of patients who received immunosuppressive therapy without danazol (57.1%). Furthermore, our results also showed that the cumulative relapse rate was significantly higher in the patients treated with immunosuppressive therapy plus danazol (Figure 1B). The reason danazol has an impact on relapse is unknown. However, it is possible that a number of cases with an androgen-responsive congenital bone marrow failure syndrome such as dyskeratosis congenita were hidden in our series of AA patients, and discontinuation of danazol was responsible for relapse. Recent reports have shown that a bone marrow failure syndrome of variable severity due to dyskeratosis congenita may be present in otherwise phenotypically normal individuals, and can masquerade as acquired AA.19–22 We found mutations in the telomerase reverse transcriptase (TERT) gene, which is one of the genes causing dyskeratosis congenita, in two of 96 Japanese children with acquired AA.23 Recently, more dyskeratosis congenita genes have been discovered. It is possible that more cases with an androgen-responsive dyskeratosis congenita were hidden in our series of AA patients. Alternatively, danazol may inhibit complete eradication of pathological T-cell clones by antithymocyte globulin through an unknown mechanism. Understanding the effects of androgens and developing androgen-mimetic drugs could be of significant benefit.
In our cohort of patients with non-severe AA, most patients were transfusion-dependent. In the AA-92 and AA-97 studies, 82 patients with non-severe AA were treated with the standard immunosuppressive regimen consisting of antithymocyte globulin and cyclosporine. Six months after the initial immunosuppressive therapy, 13 patients had achieved a complete response and 32 patients achieved a partial response. Among the 32 patients who achieved a partial response, 14 patients later relapsed. However, 18 patients with non-severe AA patients who achieved a partial response maintained their hematologic response, and 12 of them subsequently achieved a complete response. When childhood non-severe AA is treated with supportive care, 67% of patients progress to develop severe AA, suggesting that it is important to consider early immunosuppressive therapy.24 Our data indicate that immunosuppressive therapy is beneficial for some patients with non-severe AA.
A previous Japanese study showed that the addition of G-CSF to immunosuppressive therapy increased the hematologic response rate after 6 months and reduced the relapse rate in adult patients with severe AA.25 Recently, Gurion et al. conducted a systematic review and meta-analysis of randomized controlled trials comparing treatments with immunosuppressive therapy with or without hematopoietic growth factors in patients with AA. The addition of hematopoietic growth factors did not affect mortality, response rate, or occurrence of infections, but did significantly decrease the risk of relapse.26 The data from our AA-92 trial were included in this meta-analysis. In contrast to the other five studies in the meta-analysis, only our study included patients with non-severe AA, who had a significantly higher relapse rate than that of patients with either severe AA or very severe AA. Differences in the study populations may explain the discrepancy between the results of our current study and those of the other studies in the meta-analysis. To compare our results with the other studies, we excluded patients with non-severe AA from the statistical analysis, and compared the risk of relapse between patients who did or did not receive G-CSF. The results again showed no significant differences in the relative risk between them (RR=2.71, 97.5% CI 0.614 – 12.0, P=0.19).
The majority of patients who experienced relapse responded to reintroduction of immunosuppressive agents.27 Our present study also demonstrates that a second course of immunosuppressive therapy was a safe and effective treatment for the patients who relapsed after the first immunosuppressive therapy. However, an optimal second immunosuppressive therapy regimen has not yet been established. Furthermore, about half of the relapsing patients eventually received HSCT in our study. The treatment choice was based on center-related preferences or on anecdotal evidence. A multicenter prospective study is warranted to establish optimal therapy for these patients.
Supplementary Material
Appendix
The following centers and persons participated in the Japan Childhood Aplastic Anemia Study Group: Japanese Red Cross Nagoya First Hospital (K. Kato); Kyoto Prefectural University of Medicine (S. Morimoto); Kobe University School of Medicine (Y. Takeshima); Hyogo College of Medicine (Y. Ohtsuka); Tokai University (H. Yabe); Shizuoka Children’s Hospital (J. Mimaya); Fukushima Medical University (A. Kikuta); Tokyo Metropolitan Children’s Medical Center, Tokyo (T. Kaneko); Osaka City General Hospital (J. Hara); Nagoya University (S. Kojima) ; Jichi Medical School (T. Yamauchi); Kagoshima University (Y. Kawano); Okayama University (M. Oda); Hokkaido University (R. Kobayashi); Hiroshima University (S. Nishimura); Kanazawa University (S. Koizumi); Keio University (T. Mori); Hiroshima Red Cross Atomic Bomb Hospital (K. Hamamoto); Chiba University (T. Sato); Hirosaki University (E. Ito); Teikyo University School of Medicine (F. Ohta); Tottori University (T. Kawakami); Dokkyo University School of Medicine (K. Sugita); Kumamoto National Hospital (K. Takagi); Seirei Hamamatsu Hospital (T. Matsubayashi); Hyogo Children’s Hospital (Y. Kosaka); Yokohama City University (K. Ikuta); Yamaguchi University (H. Ayukawa); Kanagawa Children’s Medical Center (T. Kigasawa); Hirakata City Hospital (C. Kawakami); Nakadohri General Hospital (A. Watanabe); Gumma Children’s Hospital (T. Shitara); National Defence Medical College (I. Sekine); Gifu University School of Medicine (K. Isogai); Kumamoto University School of Medicine (S. Morinaga); University of Ryukyu (N. Hyakuna); Narita Red Cross Hospital (K. Sunami); Asahikawa Medical College (M. Yoshida); Nagoya City University (Y. Ito).
Footnotes
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Storb R. Bone marrow transplantation for aplastic anemia. Cell Transplant. 1993;2(5):365–79. doi: 10.1177/096368979300200503. [DOI] [PubMed] [Google Scholar]
- 2.Horowitz MM. Current status of allogeneic bone marrow transplantation in acquired aplastic anemia. Semin Hematol. 2000;37(1):30–42. doi: 10.1016/s0037-1963(00)90028-3. [DOI] [PubMed] [Google Scholar]
- 3.Ades L, Mary JY, Robin M, Ferry C, Porcher R, Esperou H, et al. Long-term outcome after bone marrow transplantation for severe aplastic anemia. Blood. 2004;103(7):2490–7. doi: 10.1182/blood-2003-07-2546. [DOI] [PubMed] [Google Scholar]
- 4.Davies JK, Guinan EC. An update on the management of severe idiopathic aplastic anaemia in children. Br J Haematol. 2007;136(4):549–64. doi: 10.1111/j.1365-2141.2006.06461.x. [DOI] [PubMed] [Google Scholar]
- 5.Kojima S, Hibi S, Kosaka Y, Yamamoto M, Tsuchida M, Mugishima H, et al. Immunosuppressive therapy using antithymocyte globulin, cyclosporine, and danazol with or without human granulocyte colony-stimulating factor in children with acquired aplastic anemia. Blood. 2000;96(6):2049–54. [PubMed] [Google Scholar]
- 6.Bacigalupo A, Bruno B, Saracco P, Di Bona E, Locasciulli A, Locatelli F, et al. Anti-lymphocyte globulin, cyclosporine, prednisolone, and granulocyte colony-stimulating factor for severe aplastic anemia: an update of the GITMO/EBMT study on 100 patients. European Group for Blood and Marrow Transplantation (EBMT) Working Party on Severe Aplastic Anemia and the Gruppo Italiano Trapianti di Midollo Osseo (GITMO) Blood. 2000;95(6):1931–4. [PubMed] [Google Scholar]
- 7.Führer M, Rampf U, Baumann I, Faldum A, Niemeyer C, Janka-Schaub G, et al. Immunosuppressive therapy for aplastic anemia in children: a more severe disease predicts better survival. Blood. 2005;106(6):2102–4. doi: 10.1182/blood-2005-03-0874. [DOI] [PubMed] [Google Scholar]
- 8.Kojima S, Ohara A, Tsuchida M, Kudoh T, Hanada R, Okimoto Y, et al. Risk factors for evolution of acquired aplastic anemia into myelodysplastic syndrome and acute myeloid leukemia after immunosuppressive therapy in children. Blood. 2002;100(3):786–90. doi: 10.1182/blood.v100.3.786. [DOI] [PubMed] [Google Scholar]
- 9.Schrezenmeier H, Marin P, Raghavachar A, McCann S, Hows J, Gluckman E, et al. Relapse of aplastic anaemia after immunosuppressive treatment: a report from the European Bone Marrow Transplantation Group SAA Working Party. Br J Haematol. 1993;85(2):371–7. doi: 10.1111/j.1365-2141.1993.tb03181.x. [DOI] [PubMed] [Google Scholar]
- 10.Saracco P, Quarello P, Iori AP, Zecca M, Longoni D, Svahn J, et al. Cyclosporin A response and dependence in children with acquired aplastic anaemia: a multicentre retrospective study with long-term observation follow-up. Br J Haematol. 2008;140(2):197–205. doi: 10.1111/j.1365-2141.2007.06903.x. [DOI] [PubMed] [Google Scholar]
- 11.Scheinberg P, Wu CO, Nunez O, Young NS. Long-term outcome of pediatric patients with severe aplastic anemia treated with antithymocyte globulin and cyclosporine. J Pediatr. 2008;153(6):814–9. doi: 10.1016/j.jpeds.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosaka Y, Yagasaki H, Sano K, Kobayashi R, Ayukawa H, Kaneko T, et al. Prospective multicenter trial comparing repeated immunosuppressive therapy with stem-cell transplantation from an alternative donor as second-line treatment for children with severe and very severe aplastic anemia. Blood. 2008;111(3):1054–9. doi: 10.1182/blood-2007-08-099168. [DOI] [PubMed] [Google Scholar]
- 13.Camitta BM, Thomas ED, Nathan DG, Gale RP, Kopecky KJ, Rappeport JM, et al. A prospective study of androgens and bone marrow transplantation for treatment of severe aplastic anemia. Blood. 1979;53(3):504–14. [PubMed] [Google Scholar]
- 14.Bacigalupo A, Chaple M, Hows J, Van Lint MT, McCann S, Milligan D, et al. Treatment of aplastic anaemia (AA) with antilymphocyte globulin (ALG) and methylprednisolone (MPred) with or without androgens: a randomized trial from the EBMT SAA working party. Br J Haematol. 1993;83(1):145–51. doi: 10.1111/j.1365-2141.1993.tb04645.x. [DOI] [PubMed] [Google Scholar]
- 15.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 16.Schrezenmeier H, Marin P, Raghavachar A, McCann S, Hows J, Gluckman E, et al. Relapse of aplastic anaemia after immunosuppressive treatment: a report from the European Bone Marrow Transplantation Group SAA Working Party. Br J Haematol. 1993;85(2):371–7. doi: 10.1111/j.1365-2141.1993.tb03181.x. [DOI] [PubMed] [Google Scholar]
- 17.Frickhofen N, Heimpel H, Kaltwasser JP, Schrezenmeier H. Antithymocyte globulin with or without cyclosporin A: 11-year follow-up of a randomized trial comparing treatments of aplastic anemia. Blood. 2003;101(4):1236–42. doi: 10.1182/blood-2002-04-1134. [DOI] [PubMed] [Google Scholar]
- 18.Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia; association between hematologic response and long-term outcome. JAMA. 2003;289 (3):1130–5. doi: 10.1001/jama.289.9.1130. [DOI] [PubMed] [Google Scholar]
- 19.Vulliamy T, Marrone A, Dokal I, Mason PJ. Association between aplastic anaemia and mutations in telomerase RNA. Lancet. 2002;359(9324):2168–70. doi: 10.1016/S0140-6736(02)09087-6. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi H, Baerlocher GM, Lansdorp PM, Chanock SJ, Nunez O, Sloand E, et al. Mutations of the human telomerase RNA gene (TERC) in aplastic anemia and myelodysplastic syndrome. Blood. 2003;102 (3):916–8. doi: 10.1182/blood-2003-01-0335. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi H, Calado RT, Ly H, Kajigaya S, Baerlocher GM, Chanock SJ, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352(14):1413–24. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 22.Fogarty PF, Yamaguchi H, Wiestner A, Baerlocher GM, Sloand E, Zeng WS, et al. Late presentation of dyskeratosis congenita as apparently acquired aplastic anaemia due to mutations in telomerase RNA. Lancet. 2003;362(9396):1628–30. doi: 10.1016/S0140-6736(03)14797-6. [DOI] [PubMed] [Google Scholar]
- 23.Liang J, Yagasaki H, Kamachi Y, Hama A, Matsumoto K, Kato K, et al. Mutations in telomerase catalytic protein in Japanese children with aplastic anemia. Haematologica. 2006;91(5):656–8. [PubMed] [Google Scholar]
- 24.Howard SC, Naidu PE, Hu XJ, Jeng MR, Rodriguez-Galindo C, Rieman MD, et al. Natural history of moderate aplastic anemia in children. Pediatr Blood Cancer. 2004;43(5):545–51. doi: 10.1002/pbc.20131. [DOI] [PubMed] [Google Scholar]
- 25.Teramura M, Kimura A, Iwase S, Yonemura Y, Nakao S, Urabe A, et al. Treatment of severe aplastic anemia with antithymocyte globulin and cyclosporin A with or without G-CSF in adults: a multicenter randomized study in Japan. Blood. 2007;110(6):1756–61. doi: 10.1182/blood-2006-11-050526. [DOI] [PubMed] [Google Scholar]
- 26.Gurion R, Gafter-Gvili A, Paul M, Vidal L, Ben-Bassat I, Yeshurun M, et al. Hematopoietic growth factors in aplastic anemia patients treated with immunosuppressive therapy-systematic review and meta-analysis. Haematologica. 2009;94(5):712–9. doi: 10.3324/haematol.2008.002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheinberg P, Nunez O, Young NS. Retreatment with rabbit anti-thymocyte globulin and cyclosporin for patients with relapsed or refractory severe aplastic anaemia. Br J Haematol. 2006;133(6):622–7. doi: 10.1111/j.1365-2141.2006.06098.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.