Abstract
Background
Alterations of the short arm of chromosome 12 (12p) occur in various hematologic malignancies and ETV6 and CDKN1B, which are located on 12p, have been implicated as leukemogenic genes of interest.
Design and Methods
We selected seven patients with myeloid malignancies and small 12p deletions detected by fluorescence in situ hybridization encompassing only the region centromeric of ETV6 and further evaluated them by single nucleotide polymorphism microarrays.
Results
The minimally deleted region contained only nine genes. These genes were subsequently analyzed by microarray expression profiling in an independent cohort of 781 patients, most, but not all, of whom had different hematologic malignancies CREBL2, MANSC1, and CDKN1B were expressed in more than 25% of cases, while the other six genes were expressed in only a minority of cases. As CDKN1B is a cell cycle regulator and functions as a tumor suppressor gene, this gene was selected for further expression studies in 286 patients with acute myeloid leukemia. When comparing patients with low CDKN1B expression (expression level <1,160; 1st quartile) with those with intermediate or high expression (2nd–4th quartiles), certain mutations were observed more frequently in the former: RUNX1-RUNX1T1 (11/83, 13.3% versus 5/203; 2.5%; P=0.001), PML-RARA rearrangements (11/83, 13.3% versus 4/203, 2.0%; P<0.001), 11q23/MLL rearrangements (6/83, 7.2% versus 4/203, 2.0%; P=0.038), and FLT3-TKD mutations (7/63, 11.1% versus 6/167, 3.6%; P=0.047). The median overall survival of patients with low CDKN1B expression was longer than that of patients with intermediate/high expression (not reached versus 14.9 months; P=0.005). Likewise, patients with low CDKN1B expression had a longer event-free survival than those with intermediate/high expression (31.0 versus 9.7 months; P=0.013).
Conclusions
CDKN1B is an interesting candidate gene as a potential biomarker for prognostication in acute myeloid leukemia.
Keywords: 12p deletion, ETV6, CDKN1B, acute myeloid leukemia (AML), prognosis
Introduction
Cytogenetic alterations of chromosome 12p have been described in various hematologic malignancies, such as acute myeloid leukemia (AML), myelodysplastic syndromes (MDS), myeloproliferative neoplasms, and lymphomas.1 Two genes located on 12p are considered to be relevant for leukemogenesis: ETV6 and CDKN1B.1 ETV6, belonging to the ETS gene family, can be fused to various other genes, e.g. t(5;12)(q33;p13)/ETV6-PDGFRB in MDS, or the t(12;22)(p13;q11)/ETV6-MN1 in AML and MDS. Molecular studies have indicated that the smallest region of overlapping deletions may include both CDKN1B and ETV6 or might be localized between these two genes.2,3 In contrast, Takeuchi et al. reported two loss of heterozygosity segments, one including CDKN1B and one region possibly containing ETV6.4 The product of the CDKN1B gene, p27CDKN1B, acts as negative regulator of the cell cycle.5 Therefore, and based on the fact that cytogenetically detectable 12p deletions are frequent in acute leukemias,6 it may be hypothesized that the 12p region contains a tumor suppressor gene.1
To further evaluate the genes of relevance, we first selected seven patients with myeloid malignancies in whom small interstitial 12p deletions (encompassing the region centromeric to ETV6 only) had been identified in routine diagnostic work-up. These seven cases were studied by single nucleotide polymorphism microarray analysis to identify and characterize the minimally deleted region. As CDKN1B (cyclin-dependent kinase inhibitor 1B) was a gene of interest within the minimally deleted region, we evaluated its expression in an independent large cohort of 781 patients, most of whom had different hematologic malignancies, and investigated its impact on clinical outcomes and the association with cytogenetic and molecular subgroups in a subset of 286 AML patients from this cohort.
Design and Methods
Patients
Patients with interstitial 12p deletions
We first queried our database for cases with AML or chronic myeloid malignancies and small 12p deletions following diagnostic processing. Cases qualified for this study if fluorescence in situ hybridization (FISH) with probes flanking the ETV6 locus on 12p13.1 (ABBOTT, Wiesbaden, Germany) revealed an interstitial 12p deletion at the centromeric side of the ETV6 gene in order to verify small interstitial 12p deletions. Seven patients (4 with AML, 1 with chronic myelomonocytic leukemia, 1 with MDS; and 1 with primary myelofibrosis) fulfilled these criteria and were further investigated by single nucleotide polymorphism microarray analysis to identify the minimally deleted region (Genome-Wide Human SNP Array 6.0; Affymetrix, Santa Clara, CA USA).
Bone marrow samples had been sent for diagnostic processing from different hematologic centers to the MLL Munich Leukemia Laboratory in the period between August 2005 and June 2010. Patients gave informed consent to laboratory analyses and to the use of their data for research. The study was conducted in accordance with the rules of the local Internal Review Board and the tenets of the revised Helsinki protocol.
Independent cohort of patients with different hematologic malignancies analyzed for CDKN1B expression
Following the characterization of the minimally deleted region in the above seven cases with different myeloid malignancies, we selected an independent cohort of 781 cases consisting of 666 cases with different hematologic malignancies and 115 samples of reactive non-malignant bone marrow from our gene expression database in order to analyze the expression of genes located in the minimally deleted region. All cases were investigated by oligonu-cleotide microarrays (HG-U133 Plus 2.0; Affymetrix). Expression levels of the nine genes (including CDKN1B) from the minimally deleted region on 12p13 were evaluated. With the aim of correlating CDKN1B expression levels with clinical outcomes and with genetic subgroups, we finally selected from this cohort a subset of 286 AML patients with available clinical follow-up data: 135 females and 151 males with a median age of 65.7 years (range, 19.7 – 88.1 years). Patients with AML were treated with intensive chemotherapy protocols and some were included in controlled trials by German study groups.
Cytogenetics and fluorescence in situ hybridization
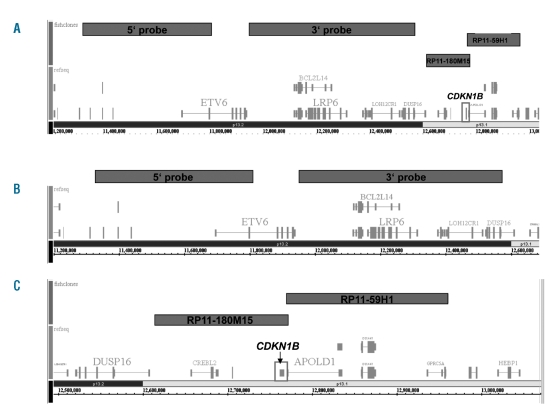
Chromosome banding was carried out according to previously described standard methods.7 In cases with small 12p deletions interphase FISH was performed using probes flanking the ETV6 region on chromosome 12p13.1 (ABBOTT). The 5′ probe covers the telomeric end of ETV6 and the 3′ probe covers a region centromeric to ETV6 (Figure 1A, B). FISH was also performed using two bacterial artificial chromosome (BAC) clones localized centromeric to ETV6 and covering the CDKN1B gene (RP11-180M15 physical map position 12,614,233 to 12,772,124 and RP11-59H1 physical map position 12,759,639 to 12,961,302; BlueGnome, Cambridge, UK) (Figure 1A, C).
Figure 1.
(A) Localization of the FISH probes flanking the ETV6 gene on chromosome 12p13 being used for the investigation of 12p deletions in various myeloid malignancies and localization of the FISH probes covering the CDKN1B gene (BAC RP11-180M15 and RP11-59H1). (B) Magnification of the ETV6 region. (C) Magnification of the CDKN1B region.
Molecular mutation screening
Molecular analyses for FLT3-ITD/TKD8,9 and MLL-PTD aberrations,10 as well as mutations of NPM1,11 CEPBA,12 NRAS,13 RUNX1,14 and IDH115 genes were performed as described previously.
Single nucleotide polymorphism array analysis
Seven patients with small interstitial 12p deletions, in whom the centromeric ETV6′ probe was deleted while the telomeric probe was retained, were investigated with the Genome-Wide Human SNP Array 6.0 (Affymetrix) featuring more than 1.8 million markers of genetic variation, including single nucleotide polymorphisms as well as probes for the detection of copy number variations. Briefly, total genomic DNA (500 ng) was digested with NspI and StyI restriction enzymes and ligated to adaptors that recognize the cohesive 4 bp overhangs. All fragments resulting from restriction enzyme digestion, regardless of size, were substrates for adaptor ligation. A generic primer that recognizes the adaptor sequence was used to amplify adaptor-ligated DNA fragments using the TITANIUM DNA Amplification kit (Clontech, Mountain View, CA, USA). The polymerase chain reaction conditions described by the manufacturer of the kit were applied to preferentially amplify fragments in the 200 to 1,100 bp size range. Polymerase chain reaction amplification products for each restriction enzyme digest were subsequently combined and purified using polystyrene beads (Agencourt, Beverly, MA, USA). The DNA was then fragmented, labeled and hybridized to the microarray. All microarray raw data were generated using Affymetrix GeneChip Command Console software version 1.1 following the manufacturer’s recommendations. Further analysis was performed using Genotyping Console version 3.0.1 and the included genome browser (Affymetrix). Copy number alterations that overlapped with regions of reported copy number variations, i.e. polymorphic regions that exist as genomic variants in the population, were filtered and removed from further analysis. Single nucleotide polymorphism microarray data were deposited online in the Gene Expression Omnibus (GEO) database under the accession number GSE27832.
Gene expression profiling
The sample preparation assay for gene expression profiling using Affymetrix HG-U133 Plus 2.0 microarrays and subsequent data analysis were performed as previously reported.16,17
Statistics
Survival curves were calculated for overall survival and event-free survival according to the method of Kaplan-Meier and compared using the two-sided log rank test. Overall survival was defined as the time from diagnosis of AML to death or last follow-up. Event-free survival was defined as the time from diagnosis of AML to treatment failure, relapse, death, or last follow-up. Results with a two-sided P value of less than 0.05 were considered statistically significant. SPSS software version 14.0.1 software (SPSS, Chicago, IL, USA) was used for the statistical analyses.
Results
Fluorescence in situ hybridization analysis of ETV6
Small interstitial 12 deletions identified by a signal constellation of two signals for the telomeric probe and only one signal for the centromeric probe were detected in seven cases with myeloid malignancies: four AML (3 de novo, 1 secondary to MDS), one MDS, one primary myelofibrosis, one chronic myelomonocytic leukemia. These seven cases were, therefore, chosen for subsequent investigation by single nucleotide polymorphism microarray analysis to characterize the minimally deleted region better.
Fluorescence in situ hybridization analysis of CDKN1B
In two cases with (12p) deletion of the centromeric ETV6 probe and retained telomeric ETV6 signal, which had also been analyzed by single nucleotide polymorphism arrays, material was available for additional FISH analysis with RP11-180M15 and RP11-59H1. In one case both BAC were deleted, while in the other case one signal was present for BAC RP11-180M15, but two signals were detected for BAC RP11-59H1.
Karyotypes of cases with small interstitial 12p deletions
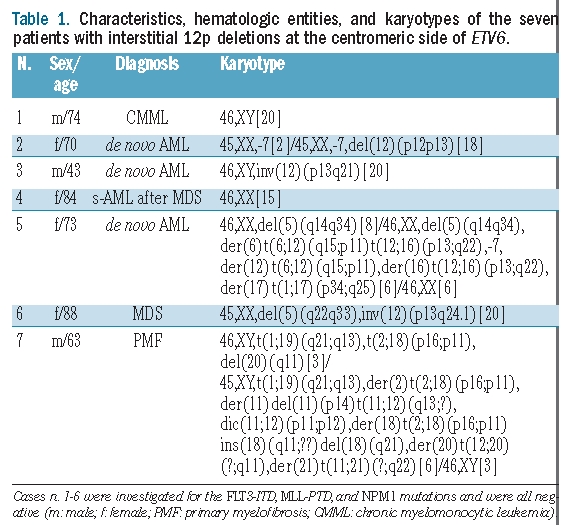
Table 1 shows the results of the cytogenetic analysis of the above seven cases with myeloid malignancies and small interstitial centromeric 12p deletions. Two patients had normal karyotypes (cases #1 and #4). Five patients had aberrant karyotypes: two cases had a pericentric inversion of chromosome 12 (cases #3 and #6; in case #6 combined with a 5q deletion), one case had a 12p deletion combined with monosomy 7 (case #2), and two had complex aberrant karyotypes involving 12p within unbalanced rearrangements (cases #5 and #7).
Table 1.
Characteristics, hematologic entities, and karyotypes of the seven patients with interstitial 12p deletions at the centromeric side of ETV6.
Characterization of the interstitial 12p deletion by single nucleotide polymorphism microarrays
The median size of the 12p deletions was 1.6 Mb (range, 1.2 – 10.6 Mb). The minimally deleted region had a size of 815 kb (starting at 12,005,749 bp from pter and ending at 12,820,773 bp from pter, based on hg18). The single nucleotide polymorphisms located nearest to the breakpoints of the minimally deleted region are shown in Online Supplementary Table S2. Overall, nine genes were localized within the minimally deleted region: BCL2L14, LRP6, MANSC1, DUSP16, CREBL2, GPR19, CDKN1B, APOLD1, and hsa-mir-613 (Figure 2).
Figure 2.
Characterization of the minimally deleted region following single nucleotide polymorphism microarray analysis of seven patients with different myeloid malignancies and interstitial 12p deletions. Single nucleotide polymorphism array karyograms (Affymetrix Genotyping Console V.3.0.1, based on hg18) of region 12p are shown. For each of the seven patients the smooth signals are depicted. The ETV6 gene is highlighted by a box, the borders of the minimal deleted region are marked with black bars.
Expression of genes derived from the minimally deleted region in 781 patients with different hematologic malignancies or non-leukemic bone marrow
We subsequently evaluated the expression of the nine genes from the minimally deleted region in 666 cases with different acute or chronic leukemias and 115 individuals with non-malignant bone marrow samples. Expression of the respective genes was observed in variable proportions of patients: CREBL2 in 100.0% (781/781), CDKN1B in 100.0% (781/781), MANSC1 in 76.1% (594/781), APOLD1 in 24.0% (187/781), LRP6 in 16.0% (125/781), DUSP16 in 15.0% (117/781), LOH12CR1 in 20.0% (156/781), GPR19 in 1.0% (8/781) and BCL2L14 in 7.0% (55/781). Thus, only three of nine genes - CREBL2 (cAMP response element-binding protein-like-2), MANSC1 (MANSC domain-containing protein 1) and CDKN1B (cyclin-dependent kinase inhibitor 1B) - were expressed in more than 25% of all cases, and the range of mRNA expression was higher for CDKN1B than for the remaining eight genes. The gene expression data are shown in detail in Online Supplementary Table S1.
The subset of 399 patients with AML or MDS from this cohort (286 AML, 113 MDS) was subdivided into four quartiles according to CDKN1B expression levels. The range of CDKN1B expression was 83.6 to 4,498.2 (median, 1,582.4). Levels of CDKN1B expression in the patients in the 1st (lowest) quartile (n=99 patients) ranged from 83.6 to 1,160, in the 2nd quartile (n=100) from 1,210.8 – 1,582.2, in the the 3rd (n=100) from 1,582.4 to 2,006.9, and in the 4th (n=100) from 2,007.2 to 4,498.2. According to the upper range of the expression levels encompassing the 1st quartile, an expression level of 1,160 was used as a threshold to separate cases with low CDKN1B expression (expression level <1.160; “low expressers”; 1st quartile) from those with intermediate/high CDKN1B expression (expression level ≥1,160; 2nd – 4th quartiles; “intermediate/high expressers”).
Investigation of 286 patients with acute myeloid leukemia
Considering the multiple functions of CDKN1B in the cell cycle (including cell proliferation, differentiation, and apoptosis),18 this gene is a putative tumor suppressor and might play an important role in the pathogenesis of cases with 12p deletions. We, therefore, analyzed CDKN1B expression in 286 AML cases with gene expression data and available survival data (a subset from the above cohort) in more detail. The median level of CDKN1B expression in the AML cohort was 1,508.6 (range, 83.6–4,498.2). Eighty-three AML patients (29.0% of the cohort) showed an expression level <1,160 and were considered to be “low CDKN1B expressers” according to the above definition.
Correlation of CDKN1B expression with genetic subtypes in acute myeloid leukemia
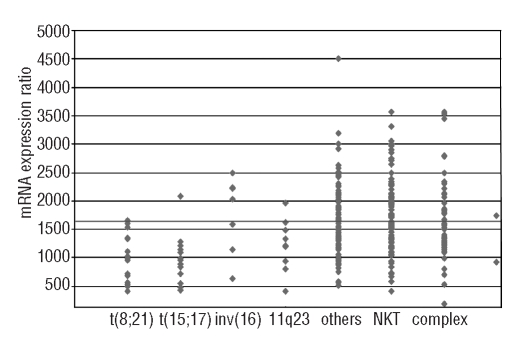
We compared the frequencies of distinct cytogenetic subtypes in the 286 AML patients with low (n=83) and intermediate/high CDKN1B expression (n=203) (Table 2A). Favorable karyotypes were significantly over-represented in the group of CDKN1B low expressers when compared to the group of intermediate/high expressers (24/83, 28.9% versus 14/203, 6.9%; P<0.001), which was due to significantly higher frequencies of t(8;21)/RUNX1-RUNX1T1 (P=0.001) and t(15;17)/PML-RARA (P<0.001) in the patients with low CDKN1B expression. 11q23/MLL rearrangements were more frequent in the CDKN1B low expressers than in the intermediate/high expressers (P=0.038). Normal karyotypes were significantly less frequent in the low expressers (21/83, 25.3% versus 78/203, 38.4%; P=0.04) (Figure 3). FLT3-TKD were found more frequently in the low expressers than in the intermediate/high expressers (7/63, 11.1% versus 6/167, 3.6%; P=0.047). Only one of 13 FLT3-TKD-positive patients had a favorable karyotype (inv(16)/t(16;16)/CBFB-MYH11). There were no significant differences for any of the other molecular markers, i.e. FLT3-ITD, MLL-PTD, and NPM1 mutations, between the low and the intermediate/high expressers (Table 2B).
Table 2A.
Distribution of cytogenetic alterations in 286 patients with AML according to different CDKN1B expression levels. There were 83 “low” and 203 “intermediate/high” expressers.
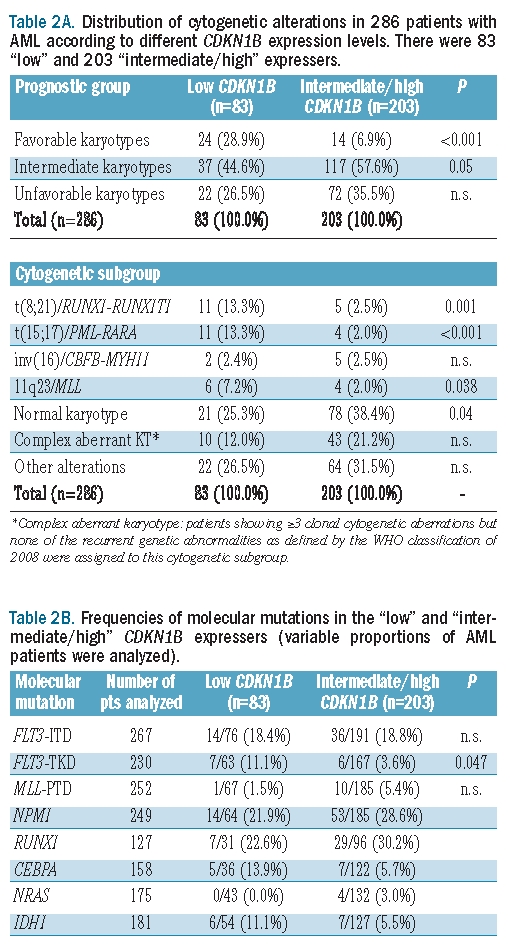
Figure 3.
mRNA expression ratios of CDKN1B in different cytogenetic AML subgroups (n=286). The gray line marks the CDKN1B expression ratio of 1,160 which separated patients from the 1st and 2nd–4th quartiles.
Table 2B.
Frequencies of molecular mutations in the “low” and “intermediate/high” CDKN1B expressers (variable proportions of AML patients were analyzed).

Correlation of CDKN1B expression with clinical outcomes in acute myeloid leukemia
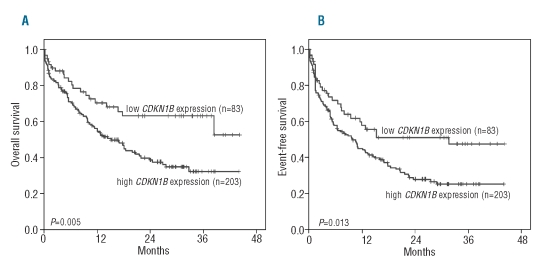
We compared the survival outcomes of the AML patients in relation to their CDKN1B expression. First, thresholds of CDKN1B expression determined in the AML/MDS cohorts as described above were used to separate the AML patients into different categories. Low expressers had a longer median overall survival (not reached versus 14.9 months; P=0.005; Figure 4A) and event-free survival (31.0 versus 9.7 months; P=0.013; Figure 4B) than the intermediate/high expressers. The results and Kaplan Meier plots for overall survival and event-free survival of the cohort separated into quartiles are presented in the Online Supplement Part A and Online Supplementary Figure S1.
Figure 4.
Overall survival (A) and event-free survival (B) depending on low versus intermediate/high CDKN1B expression in 286 patients with AML. The cut-offs of CDKN1B expression were determined in the AML/MDS cohort.
When the cut-off levels for the separation of the different CDKN1B expression levels were those defined separately in the AML cohort, low expressers again had significantly better survival (Online Supplement Part B and Online Supplementary Figures S2 and S3). With regards to overall survival, univariate Cox regression analysis showed that intermediate/high CDKN1B expression was significantly associated with shorter overall survival and a relative risk of 2.0 (P=0.005). However, when the parameters favorable and unfavorable karyotypes were added to the model, CDKN1B expression did not have an independent impact on overall survival (P=0.069). Likewise, univariate Cox regression analysis showed that intermediate/high CDKN1B expression was significantly associated with shorter event-free survival and a relative risk of 1.7 (P=0.014) and when the parameters favorable and unfavorable karyotypes were added to the model, CDKN1B expression did not have an independent impact on event-free survival (P=0.186). When the analysis was restricted to patients with AML and a normal karyotype, no significant differences were seen in overall survival and event-free survival between CDKN1B low and high expressers. Expression of the CREBL2 and MANSC1 genes showed no significant correlations with survival (data not shown).
Discussion
Previous studies suggested a pathological role for the 12p region in subsets of AML patients. Gupta et al. performed single nucleotide polymorphism array studies in 463 AML patients aged 15 – 55 years. With respect to 12p, losses were detected in seven cases (1.5%) while no gains or acquired copy neutral loss of heterozygosity was observed. 12p deletions were associated with adverse risk karyotypes.19 In a study by Andreasson et al., who investigated 79 patients with AML and MDS with interphase FISH, a high frequency of cryptic 12p deletions was detected in cytogenetically normal AML (10%), whereas the rate was only 2% in cytogenetically aberrant AML without 12p aberrations.1 Using genome-wide single nucleotide polymorphism microarray analysis, Bullinger et al. found acquired copy number alterations in 49% of cytogenetically normal AML cases. Two cases showed a 12p13 deletion. The minimally altered region on 12p13 was 1.42 Mb in size and included, among others, the ETV6 gene.20
With the aim of further defining the role of the 12p13 region in myeloid malignancies and characterize the relevant genes, we selected seven cases with myeloid malignancies and small interstitial 12p deletions (localized in the region centromeric to ETV6) for additional in-depth high-resolution single nucleotide polymorphism microarray studies and were able to identify the minimally deleted region encompassing only nine genes. Subsequent analysis of the expression of these genes in an independent cohort of 666 patients with different hematologic malignancies and 115 non-leukemic bone marrow samples demonstrated expression of only three genes in higher proportions of patients: CDKN1B, CREBL2, and MANSC1.
The CDKN1B gene encodes the p27CDKN1B protein which is assumed to act as a negative regulator of the cell cycle.5 This protein belongs to the family of CDK inhibitors that bind to cyclin/CDK complexes, leading to arrest of cell division.18 Experiments by See et al. in a murine model showed that p27 deficiency affected chromosomal stability and resulted in a decrease of mitotic cells. As chromatin breaks increased, Rad51-dependent repair of double-stranded DNA breaks seemed hindered in p27-deficient cells leading to chromosomal instability.21 Using single nucleotide polymorphism microarrays, Akagi et al. demonstrated small copy number changes, including deletions of NF1, ETV6/TEL, CDKN2A, and CDKN2B, in 24% of AML/MDS patients with normal karyotypes. A relationship between the amount of chromosomal changes and mRNA expression levels was verified by mRNA microarray analysis.22 Performing analysis of mRNA expression in patients with AML, we had been able to demonstrate that numerical or structural gains (e.g. trisomy 8) or losses (e.g. 5q-deletions) result in increased or decreased expression of the associated genes, respectively.23 This allowed the conclusion that 12p deletions involving the CDKN1B gene are likely to result in low CDKN1B expression and in a decrease of p27CDKN1B.
We, therefore, analyzed CDKN1B expression in 286 AML patients with clinical follow-up data available using whole-genome expression microarrays and performed correlations with the karyotypes and clinical outcomes. Based on their levels of CDKN1B expression patients were separated into two subgroups: 83 cases with low CDKN1B expression (1st quartile) and 203 cases with intermediate/high CDKN1B expression (2nd –4th quartiles). Low CDKN1B expression was significantly over-represented in AML with the reciprocal t(8;21)/RUNX1-RUNX1T1 (P=0.001), t(15;17)/PML-RARA (P<0.001), and 11q23/MLL-rearrangements (P=0.038), whereas normal karyotypes were significantly over-represented in the CDKN1B intermediate/high expressers (P=0.04). Thus, distinct genetic alterations might be able to modify CDKN1B expression, for example by the stimulation of signaling pathways. The interaction between these reciprocal rearrangements and CDKN1B expression levels does, however, remain unclear. Interestingly, FLT3-TKD were more frequent in the low CDKN1B expressers than in the intermediate/high expressers (P=0.047). Future studies should clarify whether such an association might contribute to explain the lesser adverse impact of FLT3-TKD than of FLT3-ITD on clinical outcomes. Other molecular mutations had no significant impact on CDKN1B expression in our study while a significant association of low CDKN1B expression with reciprocal gene rearrangements was observed. Analysis of signaling pathways might contribute to a better understanding of this observation.
The WHO classification in 2008 listed for the first time aberrant expression of genes as molecular genetic alterations affecting outcome in AML.24 So far, high expression of BAALC,25 ERG,26 or MN127 has been shown to be associated with unfavorable outcome in normal karyotype AML. In addition, high EVI1 expression was suggested to predict poor outcome, particularly in patients with intermediate cytogenetic risk AML.28 We, therefore, analyzed the prognostic impact of CDKN1B expression in a cohort of 286 cases of AML. Patients with low CDKN1B expression had significantly better overall survival (P=0.005) and event-free survival (P=0.013) compared to patients with higher expression. The better prognosis of AML patients with low CDKN1B expression (probably related to low p27CDKN1B expression) might be due to enhanced cell cycle function and increased susceptibility to cytotoxic agents. In contrast to our results, Yokozawa et al. suggested that high p27 expression has a favorable prognostic impact in patients with AML: the authors performed correlations of p27 expression levels, as determined by immunoblot analysis, with the clinical courses of 72 patients with AML. Patients with high p27 expression had a significantly better disease-free survival than that of patients with low or moderate p27 levels (78% versus 19%; P=0.004).29 It was suggested that the p27 protein might have a positive as well as a negative impact on cell cycle function.30
The interactions between CDKN1B expression, p27 protein levels and clinical outcomes in patients with AML do, therefore, deserve further investigation. A relationship between p27 expression levels and proliferation rates in hematologic malignancies has already been suggested.31 Performing immunohistochemistry in lymphomas, Erlanson et al. demonstrated high p27 expression in small lymphocytes while centroblasts and immunoblasts showed weak or no p27 staining. This was suggestive of an inverse correlation between proliferation rates and p27 expression,32 which would support our hypothesis that low CDKN1B expression is associated with high proliferation and, thereby, with a favorable response to chemotherapy. This is in line with our data regarding low CDKN1B expression in AML subtypes with high response rates to chemotherapy.
In conclusion, deletions of the 12p13 region are a recurrent phenomenon in patients with AML and other myeloid disorders, confirming previous data, e.g. from Silva et al., who recently reported on three patients with 12p deletions in a cohort of 52 patients with FAB M0 AML.33 However, the minimally deleted region in the 12p-deleted cases reported by Silva et al. comprised the ETV6 gene but excluded the CDKN1B gene. Others, such as Andreasson et al., described CDKN1B within the deleted region of 12p in AML and MDS,1 and Sato et al. were able to localize the smallest region of overlap of 12p deletions in hematologic malignancies to a small genomic region that was bordered by the ETV6/TEL and CDKN1B/KIP genes.2 In our study, CDKN1B was the most interesting candidate with respect to the nine genes located within the minimally deleted region of 12p. CDKN1B expression levels were shown to be significantly correlated with distinct cytogenetic subgroups. Our study suggests a close link of the t(15;17)/PML-RARA, the t(8;21)/RUNX1-RUNX1T1, and the 11q23/MLL rearrangements with low CDKN1B expression. Similar to other data on the expression of BAALC, MN1, or ERG,25,27,34 we here demonstrate for the first time, in a cohort of 286 patients with AML, that the expression of CDKN1B is associated with clinical outcome. However, CDKN1B expression had a significant impact on survival outcomes only in univariate analysis, and it could not be shown that CDKN1B expression had an impact independently of karyotype. Further studies should confirm the prognostic value of CDKN1B expression in myeloid malignancies and evaluate whether this parameter could contribute to therapeutic decisions in AML.
Supplementary Material
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
The information provided by the authors about contributions from persons listed as authors and in acknowledgments is available with the full text of this paper at www.haematologica.org.
Financial and other disclosures provided by the authors using the ICMJE (www.icmje.org) Uniform Format for Disclosure of Competing Interests are also available at www.haematologica.org.
References
- 1.Andreasson P, Johansson B, Arheden K, Billstrom R, Mitelman F, Hoglund M. Deletions of CDKN1B and ETV6 in acute myeloid leukemia and myelodysplastic syndromes without cytogenetic evidence of 12p abnormalities. Genes Chromosomes Cancer. 1997;19(2):77–83. doi: 10.1002/(sici)1098-2264(199706)19:2<77::aid-gcc2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 2.Sato Y, Suto Y, Pietenpol J, Golub TR, Gilliland DG, Davis EM, et al. TEL and KIP1 define the smallest region of deletions on 12p13 in hematopoietic malignancies. Blood. 1995;86(4):1525–33. [PubMed] [Google Scholar]
- 3.Stegmaier K, Takeuchi S, Golub TR, Bohlander SK, Bartram CR, Koeffler HP. Mutational analysis of the candidate tumor suppressor genes TEL and KIP1 in childhood acute lymphoblastic leukemia. Cancer Res. 1996;56(6):1413–7. [PubMed] [Google Scholar]
- 4.Takeuchi S, Bartram CR, Miller CW, Reiter A, Seriu T, Zimmerann M, et al. Acute lymphoblastic leukemia of childhood: identification of two distinct regions of deletion on the short arm of chromosome 12 in the region of TEL and KIP1. Blood. 1996;87(8):3368–74. [PubMed] [Google Scholar]
- 5.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78(1):67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 6.Mitelman F, Johansson B, Mertens F. Mitelman Database of Chromosome Aberrations in Cancer. http://cgap.nci.nih.gov/Chromosomes/Mitelman.
- 7.Schoch C, Schnittger S, Bursch S, Gerstner D, Hochhaus A, Berger U, et al. Comparison of chromosome banding analysis, interphase- and hypermetaphase-FISH, qualitative and quantitative PCR for diagnosis and for follow-up in chronic myeloid leukemia: a study on 350 cases. Leukemia. 2002;16(1):53–9. doi: 10.1038/sj.leu.2402329. [DOI] [PubMed] [Google Scholar]
- 8.Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100(1):59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 9.Bacher U, Haferlach C, Kern W, Haferlach T, Schnittger S. Prognostic relevance of FLT3-TKD mutations in AML: the combination matters - an analysis of 3082 patients. Blood. 2008;111(5):2527–37. doi: 10.1182/blood-2007-05-091215. [DOI] [PubMed] [Google Scholar]
- 10.Schnittger S, Kinkelin U, Schoch C, Heinecke A, Haase D, Haferlach T, et al. Screening for MLL tandem duplication in 387 unselected patients with AML identify a prognostically unfavorable subset of AML. Leukemia. 2000;14(5):796–804. doi: 10.1038/sj.leu.2401773. [DOI] [PubMed] [Google Scholar]
- 11.Schnittger S, Kern W, Tschulik C, Weiss T, Dicker F, Falini B, et al. Minimal residual disease levels assessed by NPM1 mutation specific RQ-PCR provide important prognostic information in AML. Blood. 2009;114(11):2220–31. doi: 10.1182/blood-2009-03-213389. [DOI] [PubMed] [Google Scholar]
- 12.Snaddon J, Smith ML, Neat M, Cambal-Parrales M, Dixon-McIver A, Arch R, et al. Mutations of CEBPA in acute myeloid leukemia FAB types M1 and M2. Genes Chromosomes Cancer. 2003;37(1):72–8. doi: 10.1002/gcc.10185. [DOI] [PubMed] [Google Scholar]
- 13.Bacher U, Haferlach T, Schoch C, Kern W, Schnittger S. Implications of NRAS mutations in AML: a study of 2502 patients. Blood. 2006;107(10):3847–53. doi: 10.1182/blood-2005-08-3522. [DOI] [PubMed] [Google Scholar]
- 14.Schnittger S, Dicker F, Kern W, Wendland N, Sundermann J, Haferlach T, et al. RUNX1 mutations are frequent in de novo AML with noncomplex karyotype and confer an unfavorable prognosis. Blood. 2011;117(8):2348–57. doi: 10.1182/blood-2009-11-255976. [DOI] [PubMed] [Google Scholar]
- 15.Schnittger S, Haferlach C, Ulke M, Alpermann T, Kern W, Haferlach T. IDH1 mutations are detected in 6.6% of 1414 AML patients and are associated with intermediate risk karyotype and unfavorable prognosis in adults younger than 60 years and unmutated NPM1 status. Blood. 2010;116(25):5486–96. doi: 10.1182/blood-2010-02-267955. [DOI] [PubMed] [Google Scholar]
- 16.Kohlmann A, Kipps TJ, Rassenti LZ, Downing JR, Shurtleff SA, Mills KI, et al. An international standardization programme towards the application of gene expression profiling in routine leukaemia diagnostics: the Microarray Innovations in LEukemia study prephase. Br J Haematol. 2008;142(5):802–7. doi: 10.1111/j.1365-2141.2008.07261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohlmann A, Haschke-Becher E, Wimmer B, Huber-Wechselberger A, Meyer-Monard S, Huxol H, et al. Intraplatform reproducibility and technical precision of gene expression profiling in 4 laboratories investigating 160 leukemia samples: the DACH study. Clin Chem. 2008;54(10):1705–15. doi: 10.1373/clinchem.2008.108506. [DOI] [PubMed] [Google Scholar]
- 18.Sgambato A, Cittadini A, Faraglia B, Weinstein IB. Multiple functions of p27(Kip1) and its alterations in tumor cells: a review. J Cell Physiol. 2000;183(1):18–27. doi: 10.1002/(SICI)1097-4652(200004)183:1<18::AID-JCP3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 19.Gupta M, Raghavan M, Gale RE, Chelala C, Allen C, Molloy G, et al. Novel regions of acquired uniparental disomy discovered in acute myeloid leukemia. Genes Chromosomes Cancer. 2008;47(9):729–39. doi: 10.1002/gcc.20573. [DOI] [PubMed] [Google Scholar]
- 20.Bullinger L, Kronke J, Schon C, Radtke I, Urlbauer K, Botzenhardt U, et al. Identification of acquired copy number alterations and uniparental disomies in cytogenetically normal acute myeloid leukemia using high-resolution single-nucleotide polymorphism analysis. Leukemia. 2010;24 (2):438–49. doi: 10.1038/leu.2009.263. [DOI] [PubMed] [Google Scholar]
- 21.See WL, Miller JP, Squatrito M, Holland E, Resh MD, Koff A. Defective DNA double-strand break repair underlies enhanced tumorigenesis and chromosomal instability in p27-deficient mice with growth factor-induced oligodendrogliomas. Oncogene. 2010;29(12):1720–31. doi: 10.1038/onc.2009.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akagi T, Ogawa S, Dugas M, Kawamata N, Yamamoto G, Nannya Y, et al. Frequent genomic abnormalities in acute myeloid leukemia/myelodysplastic syndrome with normal karyotype. Haematologica. 2009;94(2):213–23. doi: 10.3324/haematol.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schoch C, Kohlmann A, Dugas M, Kern W, Hiddemann W, Schnittger S, et al. Genomic gains and losses influence expression levels of genes located within the affected regions: a study on acute myeloid leukemias with trisomy 8, 11, or 13, monosomy 7, or deletion 5q. Leukemia. 2005;9(7):1224–8. doi: 10.1038/sj.leu.2403810. [DOI] [PubMed] [Google Scholar]
- 24.Swerdlow S, Campo E, Lee Harris N, Jaffe E, Pileri S, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. IARC press; Lyon: 2008. [Google Scholar]
- 25.Baldus CD, Thiede C, Soucek S, Bloomfield CD, Thiel E, Ehninger G. BAALC expression and FLT3 internal tandem duplication mutations in acute myeloid leukemia patients with normal cytogenetics: prognostic implications. J Clin Oncol. 2006;24 (5):790–7. doi: 10.1200/JCO.2005.01.6253. [DOI] [PubMed] [Google Scholar]
- 26.Marcucci G, Baldus CD, Ruppert AS, Radmacher MD, Mrozek K, Whitman SP, et al. Overexpression of the ETS-related gene, ERG, predicts a worse outcome in acute myeloid leukemia with normal karyotype: a Cancer and Leukemia Group B study. J Clin Oncol. 2005;23(36):9234–42. doi: 10.1200/JCO.2005.03.6137. [DOI] [PubMed] [Google Scholar]
- 27.Heuser M, Beutel G, Krauter J, Dohner K, von Neuhoff N, Schlegelberger B, et al. High meningioma 1 (MN1) expression as a predictor for poor outcome in acute myeloid leukemia with normal cytogenetics. Blood. 2006;108(12):3898–905. doi: 10.1182/blood-2006-04-014845. [DOI] [PubMed] [Google Scholar]
- 28.Gröschel S, Lugthart S, Schlenk RF, Valk PJ, Eiwen K, Goudswaard C, et al. High EVI1 expression predicts outcome in younger adult patients with acute myeloid leukemia and is associated with distinct cytogenetic abnormalities. J Clin Oncol. 2010;28(12):2101–7. doi: 10.1200/JCO.2009.26.0646. [DOI] [PubMed] [Google Scholar]
- 29.Yokozawa T, Towatari M, Iida H, Takeyama K, Tanimoto M, Kiyoi H, et al. Prognostic significance of the cell cycle inhibitor p27Kip1 in acute myeloid leukemia. Leukemia. 2000;14(1):28–33. doi: 10.1038/sj.leu.2401640. [DOI] [PubMed] [Google Scholar]
- 30.Chu IM, Hengst L, Slingerland JM. The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer. 2008;8(4):253–67. doi: 10.1038/nrc2347. [DOI] [PubMed] [Google Scholar]
- 31.Belletti B, Nicoloso MS, Schiappacassi M, Chimienti E, Berton S, Lovat F, et al. p27(kip1) functional regulation in human cancer: a potential target for therapeutic designs. Curr Med Chem. 2005;12(14):1589–605. doi: 10.2174/0929867054367149. [DOI] [PubMed] [Google Scholar]
- 32.Erlanson M, Portin C, Linderholm B, Lindh J, Roos G, Landberg G. Expression of cyclin E and the cyclin-dependent kinase inhibitor p27 in malignant lymphomas-prognostic implications. Blood. 1998;92(3):770–7. [PubMed] [Google Scholar]
- 33.Silva FPG, Morolli B, Storlazzi CT, Zagaria A, Impera L, Klein B, Vrieling H, Kluin-Nelemans HC. ETV mutations and loss in AML-M0. Leukemia. 2008;22(8):1639–43. doi: 10.1038/leu.2008.34. [DOI] [PubMed] [Google Scholar]
- 34.Mrozek K, Döhner H, Bloomfield CD. Influence of new molecular prognostic markers in patients with karyotypically normal acute myeloid leukemia: recent advances. Curr Opin Hematol. 2007;14(2):106–14. doi: 10.1097/MOH.0b013e32801684c7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.