Abstract
The diversity of highly active bacterial communities in cryoconite holes on three Arctic glaciers in Svalbard was investigated using terminal restriction fragment length polymorphism (T-RFLP) of the 16S rRNA locus. Construction and sequencing of clone libraries allowed several members of these communities to be identified, with Proteobacteria being the dominant one, followed by Cyanobacteria and Bacteroidetes. T-RFLP data revealed significantly different communities in holes on the (cold) valley glacier Austre Brøggerbreen relative to two adjacent (polythermal) valley glaciers, Midtre Lovénbreen and Vestre Brøggerbreen. These population compositions correlate with differences in organic matter content, temperature and the metabolic activity of microbial communities concerned. No within-glacier spatial patterns were observed in the communities identified over the 2-year period and with the 1 km-spaced sampling. We infer that surface hydrology is an important factor in the development of cryoconite bacterial communities.
Keywords: svalbard, bacterial diversity, biogeography, T-RFLP, evenness, spatial scaling
Introduction
Glaciers cover ∼10% of the earth's land area, and host diverse viral, prokaryotic and eukaryotic life-forms (Gunde-Cimerman et al., 2003; Christner et al., 2003a; Anesio et al., 2007). Ironically, it is only as scientific interest has focused more closely on glaciers as a consequence of their vulnerability to climate change (Lemke et al., 2007) that the perception of glaciers as inhospitable masses of ice is being displaced by the recognition of their biological diversity and an appreciation of the biogeochemical consequences of such activity (Hodson et al., 2008).
Cryoconite holes are the most biologically active habitats within glacial ecosystems (Säwström et al., 2002). High Arctic glaciers often contain significant amounts, up to 36 g m−2 of cryoconite sediment (Hodson et al., 2007), with cryoconite holes covering up to 6% of the glacier surface (Fountain et al., 2004). These cylindrical holes are formed on the ice surface by localized melting that is caused by the reduced albedo associated with the progressive accumulation of dark-coloured supraglacial material (Gerdel and Drouet, 1960; Takeuchi et al., 2001; Takeuchi, 2002).
Arctic and Alpine holes are generally open to the atmosphere during each summer, permitting the exchange of gases, nutrients and biological propagules (Hodson et al., 2007). In contrast, Antarctic cryoconite holes, most extensively studied on glaciers in the McMurdo dry valleys, commonly become lidded over by ice, insulating the contents from the influence of atmosphere and meltwater for as long as 10 years (Tranter et al., 2004; Hodson et al., 2008). In both cases, these cylindrical melt holes comprise pelagic (meltwater) and benthic (granular organic/mineral sediment) zones (Gerdel and Drouet, 1960).
On the basis of measurement of photosynthesis and respiration rates of cryoconite holes on glaciers in Svalbard, Greenland and the Alps, Anesio et al. (2009) have estimated that the net primary production of cryoconite habitats may be as much as 64 000 T yr−1 of carbon, challenging previous assumptions that glacial ecosystems are heterotrophic and dependent on an Aeolian flux of allochthonous organic matter (Stibal et al., 2008). Most of this biological activity and biomass can be found in the cryoconite sediment. Photosynthesis rates of nearly 160 μg C l−1 h−1 in the sediment (>10-fold greater than the meltwater above it) have been documented in High Arctic cryoconite holes (Säwström et al., 2002). Similarly, concentrations of bacteria, virus-like particles and nanoflagellates are higher in cryoconite sediment. Examinations of cryoconite organisms by Säwström et al. (2002) and Porazinska et al. (2004) found that several bacterial taxa, in addition to ciliate, metazoan and algal species were present.
There is currently only one published culture-independent study of bacterial diversity in cryoconite sediment, in which Christner et al. (2003b) sequenced 16S rRNA genes taken from an Antarctic cryoconite hole. Despite the importance of this study, it raises further questions relating to the means whereby bacteria colonize cryoconite holes and the dynamics of bacterial communities, both temporally, between different seasons or years, and spatially, between different holes (within and between glaciers). During the ablation season, cryoconite material in open holes can be redistributed over the glacier surface by various hydrological processes, and also transported off the glacier to proglacial areas (Hodson et al., 2008).
In this study, we have combined genetic fingerprinting by terminal restriction fragment length polymorphism (T-RFLP) (Liu et al., 1997) with sequencing of 16S rRNA gene clone libraries to examine the distributions of bacterial communities in cryoconite holes from three adjacent glaciers in the high Arctic. We were particularly interested in detecting changes in community structure as a result of spatial and temporal variation, as well as in relation to the levels of microbial activity.
Methods
Field sites and sampling conditions
Sampling was conducted during July 2006 and 2007 on two polythermal valley glaciers (Midtre Lovénbreen (ML) and Vestre Brøggerbreen (VB)), and one cold valley glacier (Austre Brøggerbreen (AB)) located near the Ny Ålesund research station at Spitsbergen in the high Arctic archipelago of Svalbard (78°55′N 11°56′E). In all, 36 samples were collected from these three glaciers during the sample-collection periods (six cryoconites were sampled at each glacier each year). Cryoconite samples were harvested by aspirating debris with a sterile 50 ml syringe into sterile tubes. The location of sampling sites (Figure 1) was recorded in relation to ablation stakes planted by the Norsk Polar Institutt's annual mass-balance monitoring campaigns on AB and ML, and by the global positioning system.
Figure 1.
Ortho-rectified aerial photograph from 1977 (S77 0731, 0732, Norwegian Polar Institute) showing glaciated areas in the area of Ny Ålesund (courtesy of Tim James, Swansea University, Wales, UK) with the location of the study area on Svalbard indicated (inset). The positions of the cryoconite holes sampled in 2007 are indicated by crosses (top and bottom holes are labeled). The positions of ablation stake lines on glaciers AB and ML are indicated by diamonds. The upper and lower extents of sampling in 2006 are indicated by stake labels (AB: S3–S6; ML: S2–S5). Scale bar indicates 1 km.
Sample handling and DNA extraction
Samples were transferred to the NERC Arctic Research Station at Ny Ålesund before being frozen until transported by air, and frozen in an insulated container with freeze blocks, until archived at −80 °C in Aberystwyth. DNA extractions were conducted aseptically using 250 mg (fresh weight) sub-samples of cryoconite sediment using the MoBio Laboratories (Solana, CA, USA) PowerSoil DNA extraction kit, as directed by the manufacturer, with DNA being eluted from the spin columns in a final volume of 100 μl elution buffer.
T-RFLP and clone libraries
For T-RFLP, PCR amplification of bacterial DNA extracted from all 36 of the sampled cryoconites was conducted with bacterial primers 27F (5′-tagged with fluorophore Cy5) and 1389R and analyzed by capillary electrophoresis, after restriction digestion with HaeIII. Primers specific for archaea (Arch021F/Arch958R) and methanogenic archaea (mcrAF/mcrAR) were also tested. Detailed methodology for T-RFLP and PCR with archaea-specific primers is given in Supplementary Data S1.
For clone libraries, DNA extracted from the sediment of 12 cryoconites (a subset of two per glacier per sampling year, as indicated in Table 1) was used as template in real-time PCR reactions with the primers 27F and 1389R to crudely estimate differences in bacterial abundance (as described in Supplementary Data S1) prior to use as template DNA for clone libraries. For each glacier, one clone library was constructed after pooling of 16 PCR products (four replicates for four samples; two holes per year). For each clone library, 32 clones were sequenced. Further details of the clone library methodologies are given in Supplementary Data S1.
Table 1. Cryoconite holes sampled during this study during 2006 and 2007 on glaciers AB, ML and VB.
| Sample code | Physico-chemical data | Clone Library | T-RFLP No. of peaks | T-RFLP Shannon diversity index (H′) | T-RFLP Gini coefficient (Gcorr) |
|---|---|---|---|---|---|
| AB601 | + | + | ND | ND | ND |
| AB602 | + | 42 | 4.12 | 0.340 | |
| AB603 | + | 16 | 2.657 | 0.389 | |
| AB604 | + | 32 | 3.53 | 0.430 | |
| AB605 | + | 9 | 2.129 | 0.309 | |
| AB606 | + | + | 17 | 2.845 | 0.322 |
| AB701 | + | 35 | 3.926 | 0.301 | |
| AB702 | 17 | 2.751 | 0.369 | ||
| AB703 | 20 | 2.878 | 0.422 | ||
| AB704 | 7 | 1.767 | 0.416 | ||
| AB705 | 16 | 2.709 | 0.358 | ||
| AB706 | + | 12 | 2.364 | 0.362 | |
| AB mean | 20.3 | 2.88 | 0.365 | ||
| ML601 | + | + | 33 | 3.53 | 0.426 |
| ML602 | + | 47 | 4.261 | 0.469 | |
| ML603 | + | 37 | 3.635 | 0.500 | |
| ML604 | 12 | 2.283 | 0.448 | ||
| ML605 | 37 | 3.679 | 0.484 | ||
| ML606 | + | 39 | 3.721 | 0.488 | |
| ML701 | + | 24 | 3.15 | 0.406 | |
| ML702 | 15 | 2.55 | 0.439 | ||
| ML703 | 14 | 2.537 | 0.391 | ||
| ML704 | 8 | 1.886 | 0.419 | ||
| ML705 | 13 | 2.458 | 0.375 | ||
| ML706 | + | 12 | 2.307 | 0.407 | |
| ML mean | 24.3 | 3.00 | 0.438 | ||
| VB601 | + | + | 43 | 4.115 | 0.366 |
| VB602 | + | 7 | 1.763 | 0.421 | |
| VB603 | + | 34 | 3.767 | 0.388 | |
| VB604 | 35 | 3.613 | 0.445 | ||
| VB605 | 21 | 3.005 | 0.386 | ||
| VB606 | + | 41 | 3.87 | 0.440 | |
| VB701 | + | 39 | 3.892 | 0.368 | |
| VB702 | 30 | 3.555 | 0.376 | ||
| VB703 | 24 | 3.227 | 0.387 | ||
| VB704 | 6 | 1.532 | 0.469 | ||
| VB705 | 25 | 3.237 | 0.366 | ||
| VB706 | + | 7 | 1.775 | 0.380 | |
| VB mean | 26.0 | 3.11 | 0.399 | ||
| By glacier | |||||
| ANOVA (P) | 0.558 | 0.787 | 0.001 | ||
| ANOVA (F) | 0.59 | 0.24 | 9.24 | ||
| By year | |||||
| ANOVA (P) | 0.005 | 0.015 | 0.134 | ||
| ANOVA (F) | 8.91 | 6.56 | 2.35 | ||
ND indicates low quality T-RFLP data.
Measurement of microbial activity
Primary production (14C incorporation) and community respiration (oxygen consumption in dark incubations) were measured for several of the cryoconite holes sampled in 2006 for analyses of bacterial diversity. Six cryoconites from AB and three each from ML and VB were sampled. Depth and area measurements using photographs processed by Cell^Five software (Olympus Software Imaging Solutions) were also made.
For measurement of net primary production, 2 ml of cryoconite sediment was collected with a syringe into 80-ml Whirlpak bags (Nasco, Fort Atkinson, WI, USA). In total, 10 μl of NaH214CO3 (370 KBq activity) was added to three 100 ml replicates, which were incubated in situ (unshaded on the glacier surface) for 24 h, with two parallel samples wrapped with aluminium foil serving as dark controls. Immediately after incubation, bags were transported to the laboratory and their contents filtered onto pre-weighed Whatman glass fiber filters (GF1; 47 mm diameter), acidified with HCl and dried overnight. Samples were counted with an LS6000IC (Beckman Coulter Inc., Brea, CA, USA) scintillation counter with internal quench curve. Dissolved inorganic carbon at the beginning of the incubations was calculated according to titration of alkalinity. Both net primary production and dissolved inorganic carbon for sediments were calculated on a dry-weight basis. pCO2 was calculated from dissolved inorganic carbon and pH was measured by using the method of Sturmm and Morgan (1970).
Community respiration was monitored by measurements of dissolved oxygen consumption using a dissolved oxygen meter (550A; YSI Hydrodata Ltd, Letchworth, Herts, UK) with a 0.01 mg l−1 resolution. Cryoconite sediment (∼10 g) was collected from the bottom of each cryoconite hole and distributed into triplicate 100-ml Pyrex glass bottles with ground glass stoppers. Initial dissolved oxygen concentration was measured immediately and the bottles were incubated in the dark at in situ temperature. Oxygen consumption was converted to carbon, using a respiratory quotient of 1. The debris used in the incubations was also dried overnight at 70 °C and weighed for calculation of community respiration on dry-weight basis.
Inorganic matter content and mineral analyses
The inorganic matter content was analyzed by drying 1 g of cryoconite sediment in pre-dried and weighed crucibles at 105 °C for 48 h. Following cooling in a desiccator, samples were re-weighed (0.1 mg accuracy) and heated to 450 °C in a furnace for 24 h. The ashed material was cooled in a desiccator and re-weighed to determine the organic content.
Grain-size distributions of inorganic debris samples were determined, to a bin resolution of 1 ϕ, by dry sieving fractions coarser than 125 μm and by settling (Micromeritics SediGraph 5100, Norcross, GA, USA) for fractions finer than 125 μm. The finest bin analyzed was ‘finer than 1 μm' (>10 ϕ). Thin sections were prepared of cryoconite sediment, which were embedded in Araldite DBF resin (Huntsman Advanced Materials, Salt Lake City, UT, USA) and mounted on glass slides for examination using a Photomicroscope III polarizing microscope (Zeiss, Oberkochen, Germany) under illumination with both plane and cross-polarized light.
Statistical analyses
Data were initially organized in MS Excel. Analysis of variance was conducted using Minitab 1.13. PRIMER6 and PERMANOVA+ (version 6.1.12 and version 1.0.2; Primer-E, Ivybridge, UK) respectively were used to conduct permutation multivariate analysis of variance (PERMANOVA) (Anderson, 2001) and canonical analysis of principal coordinates (CAP) (Anderson and Willis, 2003) on Bray–Curtis distances of fourth-root log or log(X+1) transforms of T-RFLP abundance data. PERMANOVA was conducted using default settings with 9999 unrestricted permutations, while CAP was conducted using default settings.
Pareto–Lorenz distribution curves were plotted from the cumulative relative abundance of each T-RF and its cumulative proportion of the number of T-RFs for each cryoconite hole. Gini coefficients (Gcorr) were calculated geometrically (Dorfman, 1979) by estimating the area under the Lorenz curves by trapezoid approximation using the equation:
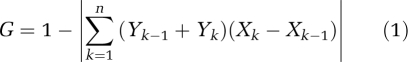 |
where Y is the cumulative relative abundance and X is the cumulative proportion of T-RFs. Gcorr was corrected to minimize the bias in estimating the underlying population, as it may result in skewed estimations of Gcorr (Weiner and Solbrig, 1984; Deltas, 2003)):
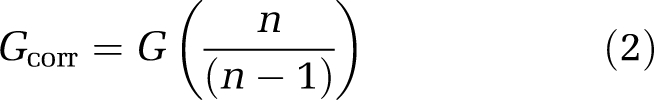 |
In order to explore spatial relationships between cryoconite holes on the same glacier, matrices of measures of similarity, richness and evenness determined from pair-wise comparison of T-RFLP profiles were generated. For distance–decay relationships of similarity, Bray–Curtis distances were generated using MVSP 3.1, (Kovach Computing Services, Anglesey, Wales, UK). For richness (S, number of T-RFs) and evenness, corrected Gcorr was calculated for pair-wise means of relative abundances from T-RFLP profiles. These measurements of community structure were tested for correlation against a matrix of geographical distances between cryoconite holes obtained from geographic information system mapping (MapInfo Professional 7.8; http://www.mapinfo.com) by conducting Mantel tests using PAST 1.80 (Hammer et al., 2001).
Canonical correspondence analysis (CCA; a constrained ordination method combining multiple regression with the underlying ordination) was conducted using MVSP 3.1, following the guidance of ter Braak (1986). Environmental variables were first standardized against the mean and s.d. of each variable in MS Excel and imported into MVSP for CCA by the Hill algorithm.
Results
T-RFLP analyses
T-RFLP analysis was conducted on 36 samples (six samples in each of the 2 years from each of the three glaciers) but one sample failed to produce an acceptable profile. The number of peaks for bacterial T-RFs (broadly equivalent to ‘species richness') within the range of the size markers (60–600 bp) ranged from 6 to 47 per sample and Shannon Diversity Index ranged from 1.53 to 4.26 (Table 1). Both species richness and diversity were higher in 2006 than in 2007 (ANOVA: P=0.005, F=8.91; P=0.015, F=6.56, respectively), but there was no significant difference between the three glaciers.
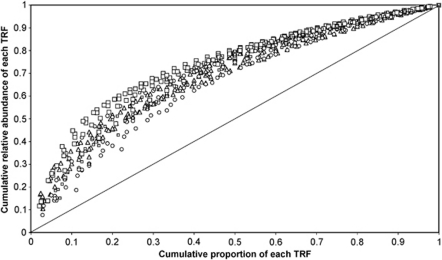
The evenness of the bacterial communities in the holes was described using Pareto–Lorenz distribution curves (De Schryver et al., 2008; Marzorati et al., 2008) and the corrected Gini coefficients (Gcorr) (Vainio et al., 2005; Halet et al., 2006; Wittebolle et al., 2008) derived for each hole. The Pareto–Lorenz distribution curves obtained by plotting the cumulative relative abundance of each T-RF against its cumulative proportion of the number of T-RFs for each cryoconite hole are shown in Figure 2. Gcorr derived from these Pareto–Lorenz distribution curves (Table 1) showed that the communities on ML were significantly more uneven than those on AB (ANOVA P=0.001, F=9.24). No significant difference between years was observed but comparison of each glacier–year combination showed that the communities sampled from ML in 2006 were significantly more uneven than those on AB (both years) and VB (2007 only).
Figure 2.
Pareto–Lorenz distribution curves for bacterial T-RF abundance patterns from the three glaciers. Samples from ML cryoconites (squares) show the most uneven distribution of taxa, with AB (circles) showing the most even distribution, and samples from VB (triangles) being intermediate. The diagonal gray line indicates a theoretical even distribution. Gini coefficients derived from Pareto–Lorenz curves for AB samples were significantly different (Tukey's honest significant difference) from ML and VB samples.
To test the hypotheses that: (i) cryoconites on different glaciers harbor distinct bacterial communities and (ii) these communities vary from year to year, we used PERMANOVA, a powerful statistical tool for the hypothesis testing of multivariate datasets (McArdle and Anderson, 2001; Anderson, 2005), along with CAP. CAP, widely used for analysis of T-RFLP data (Nikolcheva and Barlocher, 2005; Gleeson et al., 2008), is a constrained ordination method, which also cross-validates the model it derives.
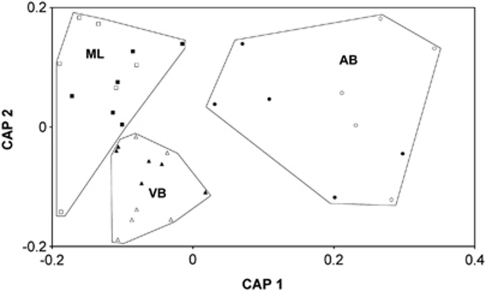
PERMANOVA analysis of T-RFLP data showed a significant difference in cryoconite bacterial community structures between glaciers (P=0.0001; Pseudo-F=5.34) and also between years (P=0.016; Pseudo-F=2.74) but no significant interaction between these factors. Pair-wise testing using PERMANOVA on the basis of T-RFLP data revealed highly significant differences in bacterial community between glaciers, with communities on AB differing from both ML and VB (P=0.0005 and P=0.0001, respectively) but there being no significant difference between communities on ML and VB (P=0.0795). This is consistent with both the clustering patterns observed following CAP ordination (Figure 3) and also detrended correspondence analysis (Supplementary Data S2). The derived model correctly assigns 83.3% (VB), 72.7% (AB) and 83.3% (ML) of samples to the correct glacier (overall 80% correct).
Figure 3.
Canonical analysis of principal coordinates (CAP) ordination on the basis of Bray–Curtis similarity indices of fourth-root transformed T-RFLP profiles of bacterial communities from cryoconites on the three glaciers at Svalbard (AB, circles, ML, squares and VB, triangles; filled markers, 2006, empty markers, 2007.) Polygons have been added to define cryoconites from the same glacier.
The changes in bacterial communities between 2006 and 2007 were further explored using PERMANOVA to examine each glacier separately. There was a clear difference between years for ML (P=0.004, Pseudo-F=6.24), but not for AB and VB (P=0.233 and 0.380, respectively). The mean linear correlation coefficient of log-transformed T-RFLP abundance data for all pair-wise comparisons of all the holes on each glacier was lowest for AB (Supplementary Data S3), suggesting, as indicated by the spread of sites in Figure 3, that the bacterial communities on AB were the most heterogeneous, whereas those on ML and VB were more homogeneous in terms of community structure. Only one cryoconite was unequivocally sampled in both years (the uppermost hole on VB –VB601/VB701). Pair-wise comparisons of T-RF abundance data using Pearson's product–moment correlation showed that the bacterial communities in these holes were more similar to each other (r2=0.834) than to any other holes sampled.
Spatial variation of cryoconite communities
Global positioning system location of cryoconite holes in 2007 permitted the creation of distance matrices for the holes sampled on each glacier using the geographic information system (Supplementary Data S4). As it was clear that bacterial communities on AB were distinct, spatial relationships between the bacterial communities in the cryoconite holes were investigated separately for each glacier. The mean relative abundance of T-RFLP peaks was calculated pair-wise for all the holes on each glacier. Matrices of mean species richness (number of T-RFs) and Gcorr were then derived, and Mantel tests were conducted in PAST to assess the correlation between the spatial matrix and the other matrices. A taxon–distance relationship was found for holes on ML and VB but not AB at α=0.05 (Table 2). Bray–Curtis distance relationships were also tested between pairs of holes, but no meaningful spatial correlation was found.
Table 2. Mantel test correlation between physical distance and community structure.
|
ML |
AB |
VB |
||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Bray–Curtis distance | 0.001 | 0.007 | 0.000 | 0.392 | 0.002 | 0.169 |
| Taxa (S) | 0.838 | 0.041 | 0.044 | 0.491 | 0.407 | 0.047 |
| Gini coefficient (Gcorr) | 0.534 | 0.067 | −0.497 | 0.944 | 0.233 | 0.178 |
r indicates the coefficient of correlation and P the significance of this correlation.
Correlation of bacterial diversity with cryoconite hole biogeochemistry
In 2006, measurements were conducted in situ and contemporaneously on 31 cryoconite holes, out of which 11 were also subjected to T-RFLP analysis (Supplementary Data S5). Significant differences between glaciers were observed in:
Temperature (P=0.004), with AB cryoconites showing a higher mean temperature (0.6 °C versus 0.1 °C and 0.2 °C for ML and VB, respectively);
percentage of organic content (P<0.001; AB significantly lower than ML and VB);
sediment primary production (P<0.001, AB significantly lower than ML and VB);
respiration rate (P<0.001; AB significantly lower than ML and VB).
Thus, cryoconite holes on different glaciers also show significant differences in their biogeochemical and metabolic activity, with AB differing from both ML and VB in a manner similar to the variation observed in T-RFLP profiles, and consistent with the findings of Anesio et al. (2009) regarding primary production and respiration.
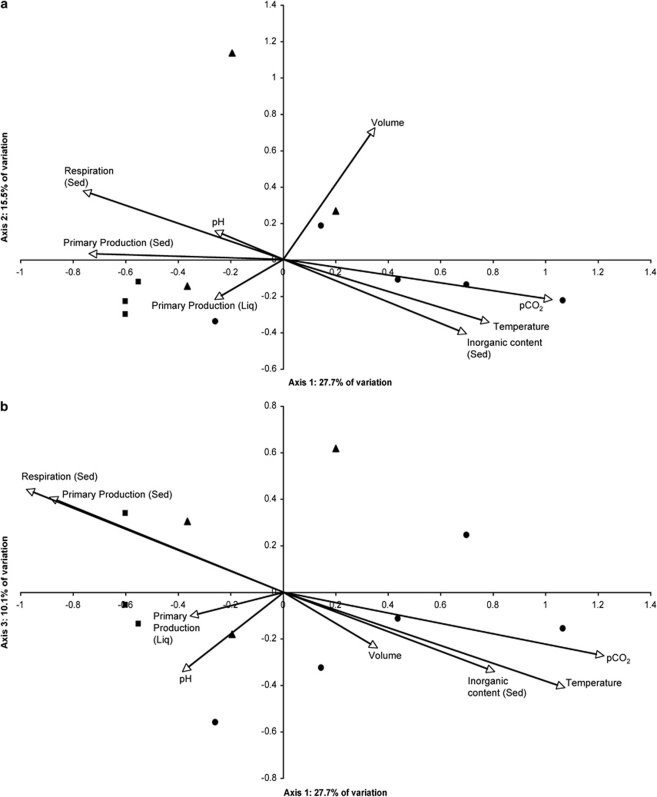
The CCA was used to show whether any of these environmental variables accounted for changes in bacterial cryoconite community structure between holes. All environmental variables measured were initially included in the model but several were removed in a step-wise manner in order to develop a robust model, which explained as much variance as possible while avoiding multi-collinearity and high variance inflation. This model (Figure 4) explained 80.1% of the total variation within the T-RFLP profiles in relation to 9 of 11 environmental variables measured, with 66.5% of the explainable variance being explained within the first three axes (eigenvalues>0.1: Supplementary Data S6).
Figure 4.
Canonical correspondence analysis of the effect of environmental variables on bacterial TRFs from cryoconite holes sampled in 2006 on AB (circles), ML (squares) and VB (triangles), using a model described by (a) axes 1 and 2 and (b) axes 1 and 3. Axes 1,2 and 3 explain 27.7, 15.5 and 10.1% of the total variance, respectively.
Cryoconite temperature, pCO2 and percentage inorganic content exert the strongest positive influences on environmental axis 1, with respiration and primary production acting most negatively. The impact of pCO2 can be interpreted in the context of the highly autotrophic nature of cryoconite communities (Anesio et al., 2009). Thus, low pCO2 would correlate with active cryoconite phototrophs, and, as observed, high primary production. The relatively small temperature differences (<1 °C) are unlikely to exert a strong direct influence on community structure but may reflect differences in rates of meltwater flow into cryoconites, as discussed below.
Respiration rate, temperature and inorganic content also exert contrasting influences on environmental axis 2, but, interestingly, cryoconite volume has a positive influence on axis 2. Volume is a composite of depth and surface area, and is itself largely filled with meltwater, so this could reflect inputs into the cryoconites, in terms of either solar radiation or nutrients within meltwater. In all CCA plots, AB holes are resolved from both ML and VB, reflecting the differences shown earlier for bacterial communities. The dominant environmental influences on AB holes are also different (Supplementary Data S6), with the vectors of temperature, pCO2 and inorganic matter being localized to the AB sites, while markers of microbial activity are strong influences on ML and VB sites.
As the high inorganic content (84–99% of dry-weight) of cryoconites sampled suggests the dominance of inorganic minerals in the structure of cryoconite sediment, representative samples from each glacier were analyzed to determine the mineral composition and size. Thin sections of cryoconite sediment viewed using polarized light microscopy consisted of a broad range of mineral types (Supplementary Data S7). Cryoconite sediment from hole ML701 contained quartz, white and biotite mica, feldspar and appeared consistent with metamorphic origins, as did a representative sample from hole AB703. Cryoconite sediment from VB701 contained clasts of sedimentary rocks, as well as carbonate, magnetite and biotite mica. In contrast to other samples, the biotite mica appeared to be more degraded in VB701, perhaps as a result of weathering; carbonate grains in the sample also appeared to contain structures resembling biological material. However, in general, the mineral particles in the cryoconite sediments examined had angular edges, suggesting local origin and recent deposition.
Particle size analysis conducted on pooled cryoconite ashes from holes sampled in 2006 showed that the particles from AB were finer than those taken from the other glaciers (Supplementary data S7). Calculation of overall surface area:volume ratio for the particle size profile found that this was 944 mm2 mg−1 for AB, 715 mm2 mg−1 for VB and 563 mm2 mg−1 for ML. Thus, the expected correlation between finer particles and increased biological activity (that is, greater surface area for biofilm formation and weathering; see above and Supplementary Data S5) was not found.
Clone library diversity
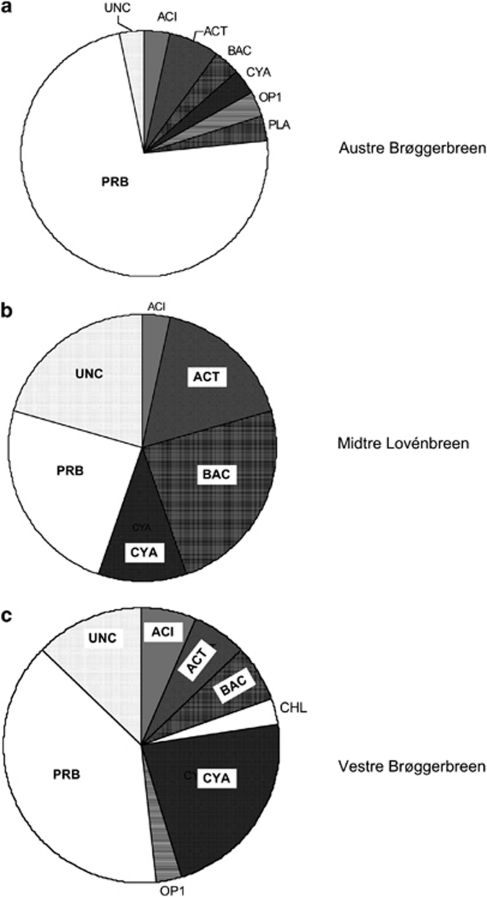
Bacterial 16S sequence data were obtained for 90/96 clones, after removal of potential chimeric sequences using Bellerophon (Huber et al., 2004) and Pintail (Ashelford et al., 2005). The sequences have been deposited at the Genbank under accession numbers FN824532–FN824621. Sequence similarity (% identity) was determined using SeqMatch and alignment of the clones identified 54 OTUs at the 97% identity cutoff threshold (Supplementary Data S8). Nearly all OTUs (47/54) were matched to existing RDP-II sequences in the RDP taxonomic hierarchy (using the RDP Bayesian hierarchy classifier) and included 22 groups, which were previously found in Antarctic and alpine environments. Of the seven major bacterial lineages present, Proteobacteria were dominant (46% of clones), followed by Cyanobacteria (12%), Bacteroidetes (11%) and Actinobacteria (10%), with members of the phyla Acidobacteria, Chloroflexus, Planctomycetes and candidate phylum OP10 also being present (Figure 5).
Figure 5.
Phylum level classification of clones by glacier (n=32 per glacier; pooled from two holes per glacier per year); (UNC: Unclassified bacteria; ACI: Acidobacteria; ACT: Actinobacteria; BAC: Bacteroidetes; CHL: Chloroflexus; CYA; Cyanobacteria; OP1: OP10; PLA: Planctomycetes; PRB: Proteobacteria).
As these clone libraries were generated from four pooled samples for each glacier, it was possible to compare the abundance of OTUs and phyla between glaciers by χ2-analysis (Supplementary Data S9). Five OTUs showed significantly different distributions between glaciers, with, for instance, the OTU52 (Sphingobacteriales) being found only on ML (P=0.003). As can be seen in Figure 5, proteobacteria were more dominant on AB (P=0.014), mainly because of the greater abundance of alphaproteobacteria (P=0.035) and the absence of betaproteobacteria (mostly Burkholderiales) on ML. Among the alphaproteobacteria, members of the order Sphingomonadaceae were dominant (18/29). Sphingomonas species comprised about 33% of the culturable bacteria isolated by Margesin et al. (2002) from Alpine cryoconites, but here they were mostly found on AB (8/9 clones; P=0.002). In contrast, Sphingobacteriales (Bacteroidetes) were mostly found on ML (7/9 clones; P=0.018).
In silico digestion of trimmed clone sequences with HaeIII identified 42 distinct T-RFs among the 94 clones (Supplementary Data S8). Only 1 OTU was predicted to have a T-RF outside the 60–600 bp range of the analysis, and of the 15 OTUs represented by multiple (⩽7) clones, 7 were predicted to give multiple T-RF peaks (±2 bp); with only one of these having more than two T-RFs. The three most abundant T-RFs across all T-RFLP samples at 294, 227 and 187 bp (±2 bp), respectively occurred seven, four and three times among the 90 clones.
Discussion
To our knowledge, this is the first detailed comparison of bacterial diversity in cryoconite holes on neighboring glaciers. Previous microbiological analyses have predominantly focused on culture- and microscopy-based approaches (Margesin et al., 2002; Mueller and Pollard, 2004). Morphological examination of phototrophic microbes has been useful in comparison of Arctic and Antarctic cryoconite holes (Porazinska et al., 2004; Stibal et al., 2006), but the limited range of observable morphology hinders the application of this approach to most non-photosynthetic bacteria. Although archaea have been reported from several peri-glacial habitats (Sjöling and Cowan, 2003; Nicol et al., 2006), other PCR-based investigations have also failed to detect their presence (Pointing et al., 2009); (Supplementary Data S1). Further study using PCR-independent approaches (for example, phospholipid ether lipid analysis; Gattinger et al., 2003) could usefully be applied to provide a definitive determination of the occurrence of archaea within cryoconites.
Our 16S rRNA gene clone library analysis confirms the presence of at least seven bacterial phyla within cryoconite sediments. The dominance of the alpha- and betaproteobacteria is consistent with the earlier culture-based studies of Margesin et al. (2002) of Alpine cryoconite bacteria and the 16S rRNA clones sequenced by Christner et al. (2003a) from an Antarctic cryoconite. The paucity of cyanobacterial sequences (12% of clones) implies that cyanobacteria are not the sole contributors to the high rates of primary production within cryoconite holes. Although non-cyanobacterial photosynthetic bacteria such as Rhodobacter, Rhodopila and Chloroflexi are present in the clone library, it is likely that eukaryotic photosynthetic organisms such as algae and diatoms are significant contributors to primary productivity in cryoconite holes (M Yallop and A Anesio, unpublished data). SeqMatch classification of bacterial clones also indicates that several of the taxa identified in this study are closely related to sequences identified at other cold, but mostly freshwater, environments. For instance, Phormidium pristleyi (OTU8) and other mat-forming Oscillatoriales have been found to be the most abundant cyanobacteria in cryoconite holes globally (Wharton et al., 1981; Takeuchi et al., 2000; Mueller et al., 2001). However, the majority of OTUs identified in this study have similar sequences to those identified in temperate habitats, indicating that the cryoconite bacterial community also contains OTUs from broadly distributed taxa.
Several bacterial groups are characterized by an uneven spatial distribution between the three glaciers sampled, consistent with the differences observed in T-RFLP profiles between glaciers. The highly significant difference resolved by PERMANOVA and other multivariate analyses of T-RFLP profiles between AB and ML/VB is intriguing.
The annual mass-balance monitoring conducted by the Norsk Polar Institutt (Dowdeswell et al., 1997) includes two of the glaciers studied here, with both AB and ML experiencing similar surface melt-rates of up to 1–2 m per year (Hagen et al., 2003), and it is likely that surface melt rates would be similar on VB. The upper surfaces of all three glaciers therefore experience ubiquitous melting in their lower reaches during the months of summer.
Although there is no reason to believe that the surface melt rate varies significantly between these three glaciers, supraglacial meltwater drainage may well differ at the sites investigated for at least two reasons. First, each of the glaciers included in the study has a unique shape and basal boundary. These differences manifest themselves as correspondingly unique flow fields and structural configurations (Hambrey et al., 2005). As detailed patterns of surface meltwater drainage are to some degree controlled by glacier structure, it follows that at least the detail of each glacier's supraglacial meltwater network will be different. Second, supraglacial drainage networks generally evolve downglacier, with the proportion of smaller feeder channels gradually reducing relative to that of larger trunk channels, as the former merge into the latter. Thus, other things being equal, the greater the upglacier drainage ‘fetch' above a sample site, the more discrete and less widespread the local drainage will be (Hodgkins, 1997; Nienow and Hubbard, 2005). As AB has a considerably longer fetch than ML or VB (Figure 1), it follows that the supraglacial drainage system is likely to be more discretely organized into fewer, larger channels in the area concerned. Although field observations on the three glaciers involved tentatively support this inference, it remains to be tested rigorously and is considered a research priority.
The low degree of spatial scaling that we have found on individual glaciers (Mantel test) and the greater variability between glaciers may be because of the downhill redistribution of both microbial propagules and larger particles by supraglacial melt-water over the glacier surface. Most of the open cryoconite holes are to some extent bathed with meltwater, and may be connected by supraglacial melt streams. Mueller and Pollard (2004) suggested that physico-chemical conditions and taxa in cryoconites on White Glacier in the Canadian High Arctic can be ‘reset' by these interconnections.
However, such connections within glaciers do not exist between glaciers. In this study, the three glaciers were separated by de-glaciated ridgelines and 240 m or more apart at the closest. However, the sampled holes on ML and VB were 2–7 km apart, yet showed no significant difference in community structure. Thus, considerations of spatial proximity do not adequately explain the different T-RFLP profiles of bacterial communities on different glaciers. Equally, the CCA resolves different clusters of bacterial community structures in relation to the environmental variables included in the model. Cryoconites from ML and VB cluster closely suggesting similar environmental pressures on bacterial community structures, whereas AB cryoconites are well resolved during ordination, even from each other, and show differing influences from environmental variables on AB cryoconite holes. Therefore, these variations in the route and rate of surface meltwater drainage potentially influence the availability of nutrients, debris and propagules to cryoconite holes on the glacier surface. In particular, this would restrict their availability to the cryoconite holes on the surfaces of longer and predominantly cold glaciers resulting in more heterogenous environmental conditions than on glaciers with less well-developed surface drainage.
This is consistent with the observations that AB cryoconite sediments have considerably lower primary production and respiration rates and occupy a greater environmental gradient, as resolved by CCA, than both ML and VB. The relative abundance of T-RFs was more even on AB than on ML/VB, possibly reflecting a lower degree of functional organization, with unstable conditions constraining the emergence of dominant taxa (Marzorati et al., 2008). This may be because of a lower and more sporadic influx of nutrients as a result of the more isolated nature of the cryoconite holes on AB. Correspondingly, the ordination space occupied by the bacterial T-RFLP profiles of cryoconite sites on AB is considerably greater than that of ML and VB using both detrended correspondence analysis and CAP.
Though dust particles on AB were finer, we did not identify any clear effect of inorganic particle size or composition from representative samples of cryoconite sediment that could correlate with the observed patterns of microbial diversity and activity. Although a complex and subtle set of influences and interactions between the biotic constituents of cryoconite and the inorganic substrates on which they reside no doubt exist, at the scale of this study, none could be identified as having a clear role in shaping microbial community structure.
In contrast, factors that have a clear biotic feedback (for example, humic and fulvic acid content and organic matter) correlate closely with levels of microbial activity and the patterns of bacterial community structure observed, both corroborating an earlier study showing the autochthonous origins of cryoconite carbon (Anesio et al., 2009) and highlighting the importance of biotic processes to the structure and function of cryoconite holes.
Acknowledgments
We wish to express our gratitude for the support provided by the Ny Ålesund community, especially Nick Cox, Rick Atkinson and Rob Smith at the NERC Arctic Research Station. This study was supported by Grants from NERC (NERC NE/D007321/1; NERC NE/G00496X/1) and the Royal Society (RS 2006/R2) to the authors, a PhD scholarship to AE from the Higher Education Funding Council for Wales (Mantais) and funding from the Austrian Academy of Sciences (OEAW) to BS. We thank Dr Tim James and the SLICES project (NERC NE/B505203/1), as well as Dr David Rippin and Dr Jack Kohler for providing GIS mapping data; Dr David Ratkowsky and Dr Gustaf Hendeby for discussions regarding multivariate data analysis; Tommy Ridgeway of IGES for preparing thin sections of cryoconite; and Hilary Worgan and Beatriz Macias of IBERS for assistance with T-RFLP.
Footnotes
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26:32–46. [Google Scholar]
- Anderson MJ. PERMANOVA: A FORTRAN Computer Program for Permutational Multivariate Analysis of Variance. Department of Statistics, University of Auckland: New Zealand; 2005. [Google Scholar]
- Anderson MJ, Willis TJ. Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology. 2003;84:511–525. [Google Scholar]
- Anesio AM, Hodson AJ, Fritz A, Psenner R, Sattler B. High microbial activity on glaciers: importance to the global carbon cycle. Global Change Biol. 2009;15:955–960. [Google Scholar]
- Anesio AM, Mindl B, Laybourn-Parry J, Hodson AJ, Sattler B.2007Viral dynamics in cryoconite holes on a high Arctic glacier (Svalbard) J Geophys Res (G Biogeosci 112G04S3110pp. [Google Scholar]
- Ashelford KE, Chuzhanova NA, Fry JC, Jones AJ, Weightman AJ. At least 1 in 20 16S rRNA sequence records currently held in public repositories is estimated to contain substantial anomalies. Appl Environ Microbiol. 2005;71:7724–7736. doi: 10.1128/AEM.71.12.7724-7736.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christner BC, Kvitko BH, Reeve JN. Molecular identification of Bacteria and Eukarya inhabiting an Antarctic cryoconite hole. Extremophiles. 2003a;7:177–183. doi: 10.1007/s00792-002-0309-0. [DOI] [PubMed] [Google Scholar]
- Christner BC, Mosley-Thompson E, Thompson LG, Reeve JN. Bacterial recovery from ancient glacial ice. Environ Microbiol. 2003b;5:433–436. doi: 10.1046/j.1462-2920.2003.00422.x. [DOI] [PubMed] [Google Scholar]
- De Schryver P, Crab R, Defoirdt T, Boon N, Verstraete W. The basics of bio-flocs technology: the added value for aquaculture. Aquaculture. 2008;277:125–137. [Google Scholar]
- Deltas G. The small-sample bias of the Gini coefficient: Results and implications for empirical research. Rev Econ Stat. 2003;85:226–234. [Google Scholar]
- Dorfman R. Formula for the Gini coefficient. Rev Econ Stat. 1979;61:146–149. [Google Scholar]
- Dowdeswell JA, Hagen JO, Bjornsson H, Glazovsky AF, Harrison WD, Holmlund P, et al. The mass balance of circum-Arctic glaciers and recent climate change. Quatern Res. 1997;48:1–14. [Google Scholar]
- Fountain AG, Tranter M, Nylen TH, Lewis KJ, Mueller DR. Evolution of cryoconite holes and their contribution to meltwater runoff from glaciers in the McMurdo Dry Valleys, Antarctica. J Glaciol. 2004;50:35–45. [Google Scholar]
- Gattinger A, Gunther A, Schloter M, Munich JC. Characterisation of Archaea in soils by polar lipid analysis. Acta Biotechnol. 2003;23:21–28. [Google Scholar]
- Gerdel RW, Drouet F. The cryoconite of the Thule area, Greenland. Trans Am Microsc Soc. 1960;79:256–272. [Google Scholar]
- Gleeson DB, Herrmann AM, Livesley SJ, Murphy DV. Influence of water potential on nitrification and structure of nitrifying bacterial communities in semiarid soils. Appl Soil Ecol. 2008;40:189–194. [Google Scholar]
- Gunde-Cimerman N, Sonjak S, Zalar P, Frisvad JC, Diderichsen B, Plemenitas A. Extremophilic fungi in arctic ice: a relationship between adaptation to low temperature and water activity. Phys Chem Earth. 2003;28:1273–1278. [Google Scholar]
- Hagen JO, Kohler J, Melvold K, Winther JG. Glaciers in Svalbard: mass balance, runoff and freshwater flux. Polar Res. 2003;22:145–159. [Google Scholar]
- Halet D, Boon N, Verstraete W. Community dynamics of methanotrophic bacteria during composting of organic matter. J Biosci Bioeng. 2006;101:297–302. doi: 10.1263/jbb.101.297. [DOI] [PubMed] [Google Scholar]
- Hambrey MJ, Murray T, Glasser NF, Hubbard A, Hubbard B, Stuart G, et al. Structure and changing dynamics of a polythermal valley glacier on a centennial timescale: Midre Lovenbreen, Svalbard. J Geophys Res (Earth Surface) 2005;110:F1. [Google Scholar]
- Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol Electronica. 2001;4:9. [Google Scholar]
- Hodgkins R. Glacier hydrology in Svalbard, Norwegian high Arctic. Quaternary Sci Rev. 1997;16:957–973. [Google Scholar]
- Hodson A, Anesio AM, Ng F, Watson R, Quirk J, Irvine-Fynn T, et al. 2007A glacier respires: Quantifying the distribution and respiration CO2 flux of cryoconite across an entire Arctic supraglacial ecosystem J Geophys Res (G Biogeosci) 112G04S369pp. [Google Scholar]
- Hodson A, Anesio AM, Tranter M, Fountain A, Osborn M, Priscu J, et al. Glacial ecosystems. Ecol Monogr. 2008;78:41–67. [Google Scholar]
- Huber T, Faulkner G, Hugenholtz P. Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics. 2004;20:2317–2319. doi: 10.1093/bioinformatics/bth226. [DOI] [PubMed] [Google Scholar]
- Lemke P, Ren J, Alley RB, Allison I, Carrasco J, Flato G, et al. 2007Observations: Changes in Snow, Ice and Frozen GroundIn: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB et al (eds).Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press: Cambridge, UK and New York, NY, USA [Google Scholar]
- Liu WT, Marsh LT, Cheng H, Forney LJ. Characterization of microbial diversity by determining terminal restriction fragment length polymorphisms of genes encoding 16S rRNA. Appl Environ Microbiol. 1997;63:4516–4522. doi: 10.1128/aem.63.11.4516-4522.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margesin R, Zacke G, Schinner F. Characterization of heterotrophic microorganisms in alpine glacier cryoconite. Arct Antarct Alp Res. 2002;34:88–93. [Google Scholar]
- Marzorati M, Wittebolle L, Boon N, Daffonchio D, Verstraete W. How to get more out of molecular fingerprints: practical tools for microbial ecology. Environ Microbiol. 2008;10:1571–1581. doi: 10.1111/j.1462-2920.2008.01572.x. [DOI] [PubMed] [Google Scholar]
- McArdle BH, Anderson MJ. Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology. 2001;82:290–297. [Google Scholar]
- Mueller DR, Pollard WH. Gradient analysis of cryoconite ecosystems from two polar glaciers. Polar Biol. 2004;27:66–74. [Google Scholar]
- Mueller DR, Vincent WF, Pollard WH, Fritsen CH. Glacial cryoconite ecosystems: a bipolar comparison of algal communities and habitats. Nova Hedwigia. 2001;123:173–197. [Google Scholar]
- Nicol GW, Tscherko D, Chang L, Hammesfahr U, Prosser JI. Crenarchaeal community assembly and microdiversity in developing soils at two sites associated with deglaciation. Environ Microbiol. 2006;8:1382–1393. doi: 10.1111/j.1462-2920.2006.01031.x. [DOI] [PubMed] [Google Scholar]
- Nienow PW, Hubbard B.2005Surface and englacial drainage of glaciers and ice sheetsIn: Anderson M (ed).Encyclopedia of Hydrological Sciences John Wiley & Sons: London; 2575–2586. [Google Scholar]
- Nikolcheva LG, Barlocher F. Seasonal and substrate preferences of fungi colonizing leaves in streams: traditional versus molecular evidence. Environ Microbiol. 2005;7:270–280. doi: 10.1111/j.1462-2920.2004.00709.x. [DOI] [PubMed] [Google Scholar]
- Pointing SB, Chan Y, Lacap DC, Lau MCY, Jurgens JA, Farrell RL. Highly specialized microbial diversity in hyper-arid polar desert. Proc Natl Acad Sci USA. 2009;106:19964–19969. doi: 10.1073/pnas.0908274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porazinska DL, Fountain AG, Nylen TH, Tranter M, Virginia RA, Wall DH. The biodiversity and biogeochemistry of cryoconite holes from McMurdo Dry Valley glaciers, Antarctica. Arct Antarct Alp Res. 2004;36:84–91. [Google Scholar]
- Säwström C, Mumford P, Marshall W, Hodson A, Laybourn-Parry J. The microbial communities and primary productivity of cryoconite holes in an Arctic glacier (Svalbard 79 degrees N) Polar Biol. 2002;25:591–596. [Google Scholar]
- Sjöling S, Cowan DA. High 16S rDNA bacterial diversity in glacial meltwater lake sediment, Bratina Island, Antarctica. Extremophiles. 2003;7:275–282. doi: 10.1007/s00792-003-0321-z. [DOI] [PubMed] [Google Scholar]
- Stibal M, Sabacka M, Kastovska K. Microbial communities on glacier surfaces in Svalbard: impact of physical and chemical properties on abundance and structure of cyanobacteria and algae. Microb Ecol. 2006;52:644–654. doi: 10.1007/s00248-006-9083-3. [DOI] [PubMed] [Google Scholar]
- Stibal M, Tranter M, Benning LG, Rehak J. Microbial primary production on an Arctic glacier is insignificant in comparison with allochthonous organic carbon input. Environ Microbiol. 2008;10:2172–2178. doi: 10.1111/j.1462-2920.2008.01620.x. [DOI] [PubMed] [Google Scholar]
- Sturmm W, Morgan JJ. Aquatic chemistry: An Introduction Emphasizing Chemical Equilibria in Natural Waters. Wiley Interscience: New York; 1970. [Google Scholar]
- Takeuchi N. Optical characteristics of cryoconite (surface dust) on glaciers: the relationship between light absorbency and the property of organic matter contained in the cryoconite. Ann Glaciol. 2002;34:409–414. [Google Scholar]
- Takeuchi N, Kohshima S, Seko K. Structure, formation, and darkening process of albedo-reducing material (cryoconite) on a Himalayan glacier: a granular algal mat growing on the glacier. Arct Antarct Alp Res. 2001;33:115–122. [Google Scholar]
- Takeuchi N, Kohshima S, Yoshimura Y, Seko K, Fujita K. Characteristics of cryoconite holes on a Himalayan glacier, Yala glacier, Central Nepal. Bull Glaciol Res. 2000;17:51–59. [Google Scholar]
- ter Braak CJF. Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology. 1986;67:1167–1179. [Google Scholar]
- Tranter M, Fountain AG, Fritsen CH, Lyons WB, Priscu JC, Statham PJ, et al. Extreme hydrochemical conditions in natural microcosms entombed within Antarctic ice. Hydrological Processes. 2004;18:379–387. [Google Scholar]
- Vainio EJ, Hallaksela AM, Lipponen K, Hantula J. Direct analysis of ribosomal DNA in denaturing gradients: application on the effects of Phlebiopsis gigantea treatment on fungal communities of conifer stumps. Mycol Res. 2005;109:103–114. doi: 10.1017/s0953756204001406. [DOI] [PubMed] [Google Scholar]
- Weiner J, Solbrig OT. The meaning and measurement of size hierarchies in plant populations. Oecologia. 1984;61:334–336. doi: 10.1007/BF00379630. [DOI] [PubMed] [Google Scholar]
- Wharton RA, Vinyard WC, Parker BC, Simmons GM, Seaburg KG. Algae in cryoconite holes on Canada Glacier in Southern Victorialand, Antarctica. Phycologia. 1981;20:208–211. [Google Scholar]
- Wittebolle L, Vervaeren H, Verstraete W, Boon N. Quantifying community dynamics of nitrifiers in functionally stable reactors. Appl Environ Microbiol. 2008;74:286–293. doi: 10.1128/AEM.01006-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.