Abstract
Low bone mineral density (BMD) is prevalent in human immunodeficiency virus (HIV)–infected subjects. Initiation of antiretroviral therapy is associated with a 2%–6% decrease in BMD over the first 2 years, a decrease that is similar in magnitude to that sustained during the first 2 years of menopause. Recent studies have also described increased fracture rates in the HIV-infected population. The causes of low BMD in individuals with HIV infection appear to be multifactorial and likely represent a complex interaction between HIV infection, traditional osteoporosis risk factors, and antiretroviral-related factors. In this review, we make the point that HIV infection should be considered as a risk factor for bone disease. We recommend screening patients with fragility fractures, all HIV-infected post-menopausal women, and all HIV-infected men ≥50 years of age. We also discuss the importance of considering secondary causes of osteoporosis. Finally, we discuss treatment of the more severe cases of bone disease, while outlining the caveats and gaps in our knowledge.
INTRODUCTION
As the number of older human immunodeficiency virus (HIV)–infected persons expands, the importance of aging-related co-morbidities, such as osteoporosis and fractures, has increased. This review describes the current knowledge of the epidemiology and pathogenesis of reduced bone mineral density (BMD) in HIV-infected patients, discusses the authors’ recommendations regarding screening and treatment considerations, and highlights areas of controversy and uncertainty.
DEFINITIONS OF OSTEOPENIA, OSTEOPOROSIS, AND OSTEOMALACIA
Osteoporosis is a skeletal disorder characterized by compromised bone strength predisposing to an increased risk of fracture [1]. The diagnosis of osteoporosis can be based on a history of fragility fracture (a fracture resulting from trauma equivalent or less than a fall from a standing position). Osteoporosis may also be diagnosed before a fracture occurs by measuring BMD by dual-energy x-ray absorptiometry (DXA). The World Health Organization (WHO) classifies BMD as Normal, Osteopenia, or Osteoporosis according to the number of standard deviations (SDs) below the mean BMD for a healthy, young (25–35 years of age), sex- and ethnicity-matched reference population (T-score) [2]. In post-menopausal women and men 50 years of age and older, a T-score less than or equal to −2.5 at the hip or spine is defined as osteoporosis. Osteopenia is defined as a T-score between −1 and −2.49. In older populations, the risk of fracture approximately doubles for each SD decrease below the young normal mean [3]. For patients younger than 50 years of age, the Z-score (SD below a sex- and ethnicity-matched population of the same age) is preferred; a value less than or equal to −2.0 considered to be abnormal [4]. The diagnosis of osteoporosis in this younger population should not be made on the basis of BMD testing alone [4].
Osteomalacia refers to impaired mineralization of the bone matrix, most often caused by severe vitamin D deficiency. Although osteoporosis and osteomalacia are separate conditions with distinct etiologies and treatments, both may be associated with low BMD by DXA and fractures.
LOW BMD IN HIV INFECTION
Low BMD has been reported in many cross-sectional studies involving younger [5–10] and older [11–14] HIV-infected individuals. In one meta-analysis, the prevalence of osteoporosis was 3 times higher among HIV-infected patients than among HIV-negative control subjects, especially among those receiving antiretroviral therapy (ART) [15]. Several studies have shown that BMD decreases by 2%–6% within the first 2 years after initiation of various ART regimens [16–18], a decrease in BMD similar to that sustained during the first 2 years of menopause [19]. BMD appears to be relatively stable in patients who receive established ART [20–22]. Importantly, studies reporting increased fracture rates in the HIV-infected population are starting to emerge, with rates 30%–70% higher than those among matched uninfected control subjects [23–26].
POTENTIAL ETIOLOGIES OF LOW BMD IN HIV INFECTION
The causes of low BMD in HIV appear to be multifactorial and likely represent a complex interaction between HIV infection, traditional osteoporosis risk factors exacerbated by consequences of chronic HIV infection (eg, poor nutrition and low weight), high rates of tobacco and alcohol use, low vitamin D levels, and ART-related factors [9, 22, 27–29]. ART-naive subjects [5, 15] have a high prevalence of osteopenia, which suggests that uncontrolled viremia can impact BMD, likely mediated by effects of systemic inflammation on bone remodeling. Specifically, HIV proteins increase osteoclastic activity [30] and decrease bone formation by promoting osteoblast apoptosis [31, 32]. Furthermore, elevated tumor necrosis factor (TNF) α increases osteoclast-mediated bone resorption without concomitant increases in bone formation [33].
Other comorbidities that are common in individuals with HIV infection also impact BMD. The prevalence of low vitamin D levels is 60%–75% in different HIV-infected cohorts [34–36], and hypogonadism also likely contributes [37]. Lipoatrophy may mediate bone loss through the complicated relationships between central signaling of adipocyte hormones [38, 39]. As in the general population [39], relative central fat accumulation has been associated with lower BMD in some HIV-infected populations [40, 41]. The mechanisms underlying this association deserve further attention.
Initiation of ART induces a marked and clinically significant loss of BMD (2%–6%), regardless of the initial choice of ART [16, 18, 27, 29, 42]. Although early studies suggested that bone loss was attributable to protease inhibitor (PI)–based ART [9, 43, 44], others have failed to confirm the association [45–47]. Of greatest interest for most clinicians, the nucleoside reverse-transcriptase inhibitor tenofovir (TDF) has been strongly associated with an acute decrease in BMD; in a recently presented study, there was more bone loss in patients with HIV suppression who were switched to TDF than in those who were switched to abacavir (ABC) [27, 48]. Two prospective studies involving subjects who were initiating their first ART regimen showed that TDF-containing regimens led to a significantly larger decrease in spine and hip BMD than did ABC-containing regimens [42, 49]. In addition, in ACTG5224, use of regimens containing the PI atazanavir-ritonavir led to a greater decrease in lumbar spine—but not hip—BMD, when compared with use of efavirenz (EFV) containing regimens. The mechanisms involved in bone loss associated with particular ART regimens are not well understood. TDF may affect bone indirectly through proximal tubule toxicity, resulting in phosphate wasting and increased bone turnover [50], whereas EFV and PIs may affect BMD indirectly through vitamin D metabolism [51–55].
BONE DISEASE IN SELECTED POPULATIONS
Children and adolescents
Peak bone mass is achieved during adolescence and young adulthood and is a key determinant of bone mass in later life [56]. Thus, the effect of HIV infection and/or ART on this process is a critical area of research. Variation in the pattern and dynamics of skeletal growth complicate bone mass measurements in children and youth, and normative databases are insufficient [57, 58]. Techniques that are influenced by body size (eg, DXA) can be confounded by HIV-associated delays in growth [59–61]. Despite these caveats, almost all studies involving perinatally infected children have reported lower-than-expected bone mass [62–74], as well as hormonal and calcium deficiencies [66, 68, 75, 76]. Bone abnormalities may be worse in subjects who are receiving ART [65, 71], particularly TDF [70, 77]; in those with advanced HIV disease [64, 70, 75, 77]; and in pubertal males [78]. Optimal nutrition, exercise, and lifestyle changes should be emphasized in this population.
Resource-limited settings (RLS)
More than 90% of HIV-infected people live in RLS, where nutritional deficiencies are also highly prevalent [79]. Access to effective ART has improved life expectancy for HIV-infected patients in RLS. Although the burden of metabolic bone disease has not been clarified in these populations, osteoporosis would be expected in an aging population. In addition, vitamin D deficiency is very prevalent across the globe [80–83] and may contribute to bone complications in RLS. Defining the prevalence of osteoporosis, fracture risk, and consequences of vitamin D deficiency in RLS is an important topic for investigation.
WHOM TO SCREEN FOR BONE DISEASE IN HIV?
Recently published guidelines for the management of low BMD in the general US population recommend a DXA for persons of any age with a fragility fracture, women ≥65 years of age, and men ≥70 years of age (Figure 1) [4]. For those with an additional risk factor, the recommendation is to perform a DXA in younger post-menopausal women and men ≥50 years of age [4]. Although HIV infection is not listed as a condition that is associated with low BMD, we believe that current evidence supports the inclusion of HIV-infection among other risk factors. Thus, we recommend a DXA scan for all HIV-infected post-menopausal women and men ≥50 years. Our position is more aggressive than the recommendation from the Infectious Diseases Society of America that suggests a DXA for HIV-infected subjects ≥50 years of age with additional risk factors for osteopenia and/or osteoporosis, although those factors are so prevalent that most HIV-positive patients would qualify [84]. If the results of the test do not warrant medical treatment, the test should be repeated every 2–5 years, depending on the proximity to thresholds for therapy. DXA scans in younger HIV-infected persons are probably not indicated, because the risk for fracture is low. One of the most powerful predictors of future fragility fractures in the general population is a history of fragility fracture [85]. Patients who have a history of fragility fracture should be evaluated by DXA regardless of age or sex, which is a diagnostic modality clearly underutilized in this population [86].
Figure 1.
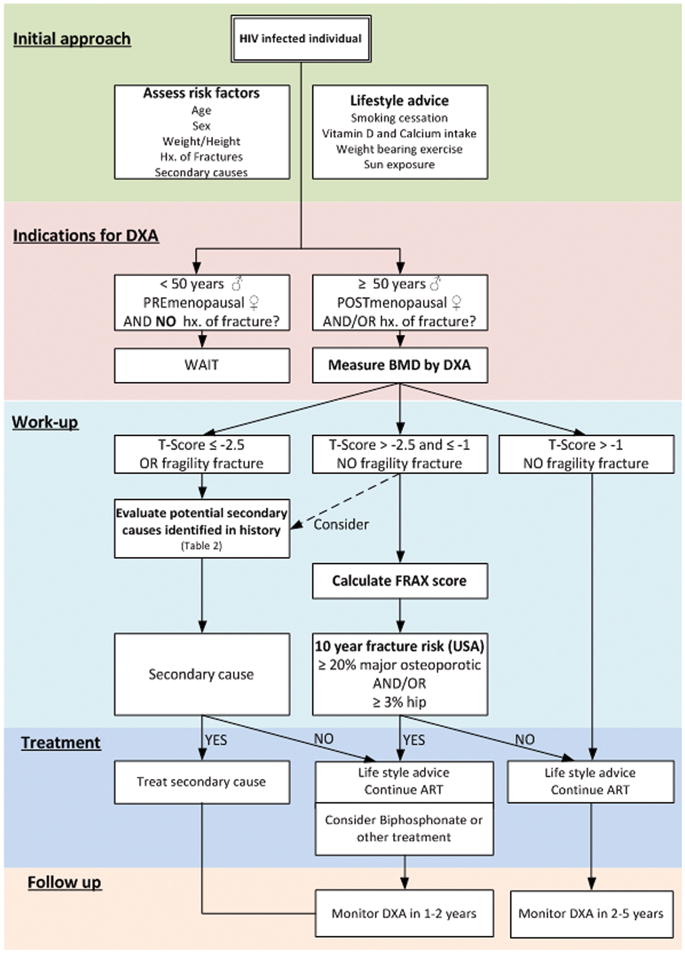
Approach to bone problems in patients with human immunodeficiency virus (HIV) infection (adapated from Dolin et al [126]). ART, antiretroviral therapy; BMD, bone mineral density; DXA, dual-energy x-ray absorptiometry; FRAX, Fracture Risk Assessment Tool; Hx, history.
WORK-UP TO RULE OUT SECONDARY CAUSES OF OSTEOPOROSIS
Secondary osteoporosis is osteoporosis that is caused or exacerbated by a specific disease process or medication. Table 1 includes a list of diseases, medications, and behaviors associated with low BMD and fractures in the general population. In post-menopausal women [87], pre-menopausal women [88, 89], and men <50 years of age [90] with low BMD in the general population, the prevalence of secondary causes is 44%–90%.
Table 1.
Conditions Associated With Osteoporosis and Fractures
| Category | Condition(s) |
|---|---|
| Genetic disorders | Cystic fibrosis, hemochromatosis, idiopathic hypercalciuria |
| Hypogonadal states | Anorexia nervosa, early menopause, low testosterone (men), premenopausal oligomenorrhea, prolactinoma, Turner’s and Kleinfelter’s syndromes |
| Other endocrine disorders | Acromegaly, adrenal insufficiency, Cushing’s syndrome, diabetes mellitus (1 and 2), primary hyperparathyroidism, thyrotoxicosis |
| Gastrointestinal diseases | Bariatric surgery, celiac disease, gastrectomy, inflammatory bowel disease, malabsorption, primary biliary cirrhosis |
| Hematologic disorders | Hemophilia, leukemias and lymphomas, multiple myeloma, sickle cell disease, thalassemia, systemic mastocytosis |
| Pulmonary diseases | Emphysema, sarcoidosis |
| Infectious and inflammatory diseases | Rheumatoid arthritis, lupus |
| Lifestyle choices and/or habits | Alcohol (>3 drinks/day), dietary calcium deficiency, methadone/opiates, physical inactivity, tobacco use |
| Miscellaneous | Chronic metabolic acidosis, congestive heart failure, chronic infection, chronic kidney disease, depression, idiopathic scoliosis, immobilization, multiple sclerosis, organ transplantation, vitamin D deficiency |
| Medications | Anticoagulants, anticonvulsants, glitazones, antipsychotics, antiretrovirals, cyclosporines, tacrolimus, cytotoxic drugs, glucocorticoids, gonadotropin-releasing hormone agonists, lithium, excess thyroxine, methotrexate, proton pump inhibitors |
NOTE. Conditions that are particularly relevant to patients with human immunodeficiency virus infection or AIDS are shown in bold.
The most common secondary causes in men are hypogonadism, alcoholism, and glucocorticoid exposure, which together account for 40%–60% of cases [90], whereas premenopausal estrogen deficiency and glucocorticoid exposure are the most common secondary causes in women, accounting for 35%–40% of cases [88]. In HIV-infected individuals, low BMD has been linked most frequently to low body weight [91] but has also been linked to testosterone or estrogen deficiency, glucocorticoids, malabsorption, tobacco use, alcohol and opiate abuse, nadir CD4+ cell count, duration of HIV infection, lipodystrophy, insulin resistance, and hyperlactatemia [92, 93]. There are significant clinical consequences of secondary osteoporosis, because older women and men with metabolic disorders associated with secondary osteoporosis have a 2–3-fold higher risk of hip and vertebral fractures [94–96]. Fortunately, the majority of causes of secondary osteoporosis can be suspected or diagnosed on the basis of a thorough history and physical examination. However, if no cause is apparent, the tests summarized in Table 2 were shown to have 92% sensitivity for detecting secondary causes of osteoporosis [87].
Table 2.
Work-Up for Secondary Causes of Osteopenia and/or Osteoporosis
| History and physical examination with focus on secondary causes of osteoporosis |
| Perform complete blood count |
| Perform routine blood chemistry tests |
| Determine creatinine, blood urea nitrogen, total calcium, phosphate, albumin, and alkaline phosphatase levels |
| Determine serum 25-hydroxyvitamin D level |
| Perform parathyroid hormone and thyroid-stimulating hormone tests |
| Perform 24-hour urine test for calcium and creatinine |
| Determine total and free testosterone (for men) |
| Determine estradiol, follicle-stimulating hormone, luteinizing hormone, and prolactin levels in young amenorrheic women |
| Simultaneous serum phosphate and creatinine, spot urine phosphate, and creatinine to calculate the fractional excretion of phosphate (for patients receiving tenofovir) |
NOTE. Adapted from Dolin et al [126].
When vitamin D deficiency is pronounced, patients may experience osteomalacia, the diagnosis of which relies on a combination of clinical symptoms and biochemical abnormalities. Osteoporosis is asymptomatic until the development of a bone fracture, whereas severe osteomalacia can lead to bone pain, muscle weakness, and stiffness. Laboratory abnormalities include low calcium and phosphorus levels, low 25 hydroxyvitamin D (25[OH]D) levels, and elevated alkaline phosphatase and parathyroid hormone (PTH) levels.
WHO SHOULD BE TREATED FOR OSTEOPOROSIS?
After evaluation and treatment of secondary causes of reduced BMD, we recommend pharmacologic treatment of osteoporosis for post-menopausal women and men ≥50 years with a T-score of the total hip, femoral neck, or lumbar spine less than or equal to −2.5 or in those with a history of fragility fracture, in accordance with the most recent guidelines from the National Osteoporosis Foundation [4]. For those with osteopenia, the 10-year risk for both major osteoporotic fracture (hip, shoulder, wrist, and clinical vertebral combined) and hip fracture alone [98] should be calculated, using the WHO Fracture Risk Assessment Tool (FRAX) [97]. If the 10-year risk of all osteoporotic fracture is ≥20% or risk of hip fracture is ≥3% (the cost-effective threshold set in the United States), consideration should be given to starting pharmacologic therapy. In patients with osteopenia who have a history of height loss, plain radiographs of the thoracic and lumbar spine or use of a DXA with vertebral fracture assessment software may be useful to further risk-stratify patients, because clinically silent vertebral fractures are common and would trigger the use of pharmacologic therapy, regardless of the FRAX score or BMD. In addition, all subjects with osteopenia, regardless of FRAX score, deserve a work-up for secondary causes of bone loss and non-pharmacologic interventions. It should be noted that the FRAX has not been validated in HIV-infected persons, and there is concern that the FRAX-derived 10-year risk of fracture may under-estimate risk in HIV-infected patients [98].
GENERAL GUIDELINES FOR GOOD BONE HEALTH
In practice, it is important to focus on factors important to bone health, including adequate nutrition, particularly calcium and vitamin D intake. Because of the high prevalence of low BMD in HIV infection, we recommend that HIV-infected subjects receive 1000–1500 mg of calcium and 800–1000 IU of vitamin D daily. The amount of daily sun exposure sufficient for maintaining vitamin D levels without increasing the risk of skin cancer is unknown. Muscle strengthening and balance exercises to prevent falls should also be recommended. In post-menopausal women, exercise that puts a physical load on the bone was shown to improve BMD and reduce fracture [99]. Thirty minutes of weight-bearing exercise (including jogging or walking) at least 3 days a week has been recommended [100]. Smoking cessation and limitation of alcohol intake are strongly recommended.
Treatment of secondary causes
If a secondary cause of low BMD is identified, specific treatment addressing the underlying problem should be instituted. Of the conditions listed in Table 1, vitamin D deficiency and phosphate wasting deserve special consideration.
Vitamin D can be replaced by vitamin D2 (ergocalciferol) or the more bioavailable vitamin D3 (cholecalciferol) [101, 102]. A standard repletion regimen does not exist for healthy adults. Examples of replacement regimens [103, 104] include D2 50,000 IU weekly for 8–12 weeks and then monthly thereafter or D3 2000 IU daily for 12 weeks then D3 1000–2000 IU daily. In general, we prefer daily dosing of vitamin D for 25(OH)D concentrations >15 ng/mL. For those with lower 25(OH)D concentrations, particularly those with secondary hyperparathyroidism or evidence of osteomalacia, high dose loading can be considered. However, there are limited data evaluating the effect of vitamin D loading strategies (eg, D2 50,000 IU weekly for 8–12 weeks) on fracture risk and that very high yearly loading doses (500,000 U vitamin D3 each year) may be associated with an increased risk of fractures and falls [105]. Measurements of 25(OH)D at the end of replacement intervals should be considered to ensure adequate levels. The goal should be to achieve 25(OH)D level of >32 ng/mL, although some experts recommend levels in the 40–50 ng/mL range [104]. An incremental dose of D3 of 40 IU will increase 25(OH)D by 0.4 ng/mL [106]. Vitamin D deficiency may attenuate the efficacy of bisphosphonates and increase the risk of bisphosphonate-related hypocalcemia [107, 108]. Vitamin D deficiency should therefore be corrected prior to initiation of bisphosphonates therapy, particularly intravenous therapy.
Phosphate wasting, if severe, may cause osteomalacia. Effective treatment of osteomalacia may rapidly reverse low BMD. In a TDF-treated patient with fragility fracture or Z-score less than or equal to −2.0, discontinuation of TDF should be considered in the presence of urinary phosphate wasting and hypophosphatemia. For patients with significant hypophosphatemia, calcium (1–2 g/day) and phosphorous (1–2 g/day) should be given to remineralize the bone, and any concomitant vitamin D deficiency should be corrected.
SPECIFIC PHARMACOLOGIC INTERVENTIONS
Bisphosphonates
Bisphosphonates are considered to be first-line therapy. These medications bind to the bone matrix and inhibit osteoclast-mediated bone resorption. In numerous randomized, placebo-controlled trials, they have been shown to reduce the risk of fracture in patients without HIV infection [109]. Oral bisphosphonates are given either weekly (alendronate) or monthly (risedronate and ibandronate). Intravenous bisphosphonates include ibandronate (given every 3 months) and zoledronic acid (given yearly). All reduce the risk of vertebral fractures, and all but ibandronate lower the risk of non-spine (hip) fractures [109]. Intravenous formulations should be considered for those who do not tolerate or are noncompliant with oral bisphosphonates. Both alendronate and zoledronic acid has been evaluated in HIV-infected patients in 48-week randomized controlled trials and have shown effects on BMD and tolerability that were similar to those found in the general population [110–113].
Adverse effects of bisphosphonates
Oral bisphosphonates may be associated with esophageal irritation and dyspepsia, are poorly absorbed, and must be taken on an empty stomach. Patients should be instructed to administer with a glass of water, to remain upright, and not to eat or drink anything for at least 30 min [114]. Osteonecrosis of the jaw has been reported in patients who received bisphosphonates, although the incidence is very rare (<1 case per 100,000 person-years) [115]. The most consistent risk factor is recent prior dental surgery or extraction [116]. Therefore, patients who require dental work should have this work completed and be given time to heal before bisphosphonate initiation [116]. Atrial fibrillation and esophageal cancers have been associated with bisphosphonate use in some trials [117]; however, there is insufficient evidence to establish a causal relationship [118]. In addition, there is concern about the potential adverse effects of chronic suppression of bone turnover with bisphosphonates, which may prevent the repair of microdamage to the bone architecture and compromise bone strength and, paradoxically, predispose to fracture in some patients [119]. There have been recent reports of unusual femur fractures (subtrochanteric) in patients who received long-term bisphosphonates [120]. Nevertheless, 10-year data with alendronate in post-menopausal women showed continued increases in BMD and no increase in the risk of fracture over time [121, 122]. Given the prolonged effects of bisphosphonates and the uncertainty of their long-term safety, the optimal duration of treatment with bisphosphonates is unclear, but some experts recommend discontinuation after 5 years with careful observation [123]. Because HIV-infected subjects are now expected to live for many decades, the decision at to when to start or stop therapy is difficult and deserves to be investigated in long-term clinical trials.
Second-line osteoporosis therapies
In postmenopausal women, osteoporosis can be effectively treated with estrogen-replacement therapy. However, because of the risk of cancer (breast and endometrial), cardiovascular disease, and deep-vein thrombosis, these therapies cannot be recommended as first line treatment. In postmenopausal women, the selective estrogen receptor modulator raloxifene may be a reasonable alternative to bisphosphonates, because it does not increase the risk of breast cancer [85]. Intranasal calcitonin has relatively low efficacy, compared with that of bisphosphonates. An analogue of PTH, teriparatide, stimulates new bone formation by increasing the number and/ or activity of the bone-forming osteoblasts, but it should be reserved for patients with severe osteoporosis and those who have experienced treatment with bisphosphonates. PTH should not be used concomitantly with bisphosphonates [124]. These therapies have not been specifically evaluated in HIV-infected patients.
Antiretroviral changes in patients with low BMD
Although certain ART regimens may be associated with greater decreases in BMD, there is currently no evidence to suggest that switching ART will improve BMD and reduce fracture risk in HIV-infected patients.
Role of tenofovir in osteoporosis
Although TDF exposure has been associated with increased bone loss in HIV-infected patients initiating ART [27, 42, 49] and those receiving suppressive ART regimens [48], there have been no properly powered studies to date that have linked TDF use to fracture. Ongoing studies investigating the use of TDF for HIV prophylaxis and for the treatment hepatitis B infection will be useful in determining the effect of TDF use, independent of HIV infection or the host inflammatory response. At this juncture, there is insufficient evidence to recommend against TDF use in a patient with known low BMD prior to ART initiation. However, alternative ART choice or closer bone monitoring after TDF initiation may be considered for subjects with fragility fractures or known osteoporosis. Additional investigation into the mechanisms and clinical impact of TDF use on bone health is required.
Monitoring osteoporosis treatment
DXA should be monitored 1–2 years after initiation of osteoporosis therapy [57]. If BMD is stable or improved, consideration can be given to less frequent monitoring. If BMD decreases, compliance, procedures for taking the drug, or underlying secondary causes should be re-evaluated. Bone turnover markers decrease by 30%–50% within 6 weeks after initiation of bisphosphonates [125], although a relationship between changes in these markers and changes in BMD has not been demonstrated in individuals with HIV infection [110]. The use of bone markers in this setting has not been addressed in the current guidelines [57].
Referral to a specialist
Consultation with an experienced endocrinologist or rheumatologist should be considered when: (1) the osteoporosis is unexpectedly severe, (2) there are significant secondary causes contributing to low BMD, and (3) in cases of treatment intolerance or failure.
Conclusion
Low BMD is very common among HIV-infected subjects. BMD decreases further after ART initiation. Fracture rates are increased among older HIV-infected patients. The pathogenesis of low BMD in individuals with HIV infection remains only partially elucidated, and studies aimed at clarifying the mechanisms underlying bone loss related to ART initiation are urgently needed. Better understanding of the mechanisms of bone loss would permit targeted interventions to prevent ART-induced bone loss and mitigate fracture risk among aging HIV-infected patients. We recommend aggressive screening and treatment of the more-severe cases, while outlining the caveats and gaps in our knowledge.
Acknowledgments
G.A.M. has received research grant support from Bristol-Myers Squibb, GlaxoSmithKline, Gilead, and Merck; has served as a consultant for GlaxoSmithKline, Gilead, Abbott, and Bristol-Myers Squibb; and is currently the Chair of the Data Safety Monitoring Board for a Pfizer-sponsored study. P.T. has consulted for Tibotec, Bristol-Myers Squibb, and Pfizer. E.S. has received research support from Merck, Novartis, and Eli Lilly. E.T.O. has received research grants from Merck, GlaxoSmithKline, Gilead, Abbott, Tibotec, and Boehinger Ingelheim through Washington University; has served as a consultant for Tibotec and Glaxo-SmithKline; and has served on speakers’ bureau or received honoraria from Merck, Tibotec, GlaxoSmithKline, Bristol-Myers Squibb, GlaxoSmithKline, Monogram Sciences, and Gilead. J.S.H. has received a research grant from Novartis. J.L.S. has received grants from Gilead, Tibotec, and Merck; is on a clinical advisory board for Gilead, Tibotec, Bristol-Myers Squibb, and Merck; and is a speaker for Tibotec, Merck, Gilead, and Bristol-Myers Squibb. T.T.B. has served as a consultant for Bristol-Myers Squibb, GlaxoSmithKline, Gilead, and VIIV Healthcare and has received research funding from Merck and GlaxosmithKline.
Footnotes
Potential conflicts of interest.
All other authors: no conflicts.
References
- 1.Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94(6):646–650. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 2.Assessment of fracture risk and its application to screening for post-menopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 3.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312(7041):1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foundation NO. Clinician’s guide to prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation; 2010. [Google Scholar]
- 5.Bruera D, Luna N, David DO, Bergoglio LM, Zamudio J. Decreased bone mineral density in HIV-infected patients is independent of antiretroviral therapy. AIDS. 2003;17(13):1917–1923. doi: 10.1097/00002030-200309050-00010. [DOI] [PubMed] [Google Scholar]
- 6.Carr A, Miller J, Eisman JA, Cooper DA. Osteopenia in HIV-infected men: association with asymptomatic lactic acidemia and lower weight pre-antiretroviral therapy. AIDS. 2001;15(6):703–709. doi: 10.1097/00002030-200104130-00005. [DOI] [PubMed] [Google Scholar]
- 7.Dolan SE, Huang JS, Killilea KM, Sullivan MP, Aliabadi N, Grinspoon S. Reduced bone density in HIV-infected women. AIDS. 2004;18(3):475–483. doi: 10.1097/00002030-200402200-00014. [DOI] [PubMed] [Google Scholar]
- 8.Knobel H, Guelar A, Vallecillo G, Nogues X, Diez A. Osteopenia in HIV-infected patients: is it the disease or is it the treatment? AIDS. 2001;15(6):807–808. doi: 10.1097/00002030-200104130-00022. [DOI] [PubMed] [Google Scholar]
- 9.Tebas P, Powderly WG, Claxton S, et al. Accelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapy. AIDS. 2000;14(4):F63–F67. doi: 10.1097/00002030-200003100-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teichmann J, Stephan E, Lange U, et al. Osteopenia in HIV-infected women prior to highly active antiretroviral therapy. J Infect. 2003;46(4):221–227. doi: 10.1053/jinf.2002.1109. [DOI] [PubMed] [Google Scholar]
- 11.Arnsten JH, Freeman R, Howard AA, Floris-Moore M, Lo Y, Klein RS. Decreased bone mineral density and increased fracture risk in aging men with or at risk for HIV infection. AIDS. 2007;21(5):617–623. doi: 10.1097/QAD.0b013e3280148c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnsten JH, Freeman R, Howard AA, Floris-Moore M, Santoro N, Schoenbaum EE. HIV infection and bone mineral density in middle-aged women. Clin Infect Dis. 2006;42(7):1014–1020. doi: 10.1086/501015. [DOI] [PubMed] [Google Scholar]
- 13.Jones S, Restrepo D, Kasowitz A, et al. Risk factors for decreased bone density and effects of HIV on bone in the elderly. Osteoporos Int. 2008;19(7):913–918. doi: 10.1007/s00198-007-0524-8. [DOI] [PubMed] [Google Scholar]
- 14.Yin M, Dobkin J, Brudney K, et al. Bone mass and mineral metabolism in HIV+ postmenopausal women. Osteoporos Int. 2005;16(11):1345–1352. doi: 10.1007/s00198-005-1845-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20(17):2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 16.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr. 2009;51(5):554–561. doi: 10.1097/QAI.0b013e3181adce44. [DOI] [PubMed] [Google Scholar]
- 17.Cassetti I, Madruga JV, Suleiman JM, et al. The safety and efficacy of tenofovir DF in combination with lamivudine and efavirenz through 6 years in antiretroviral-naive HIV-1–infected patients. HIV Clin Trials. 2007;8(3):164–172. doi: 10.1310/hct0803-164. [DOI] [PubMed] [Google Scholar]
- 18.Duvivier C, Kolta S, Assoumou L, et al. Greater decrease in bone mineral density with protease inhibitor regimens compared with non-nucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS. 2009;23(7):817–824. doi: 10.1097/QAD.0b013e328328f789. [DOI] [PubMed] [Google Scholar]
- 19.Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008;93(3):861–868. doi: 10.1210/jc.2007-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolland MJ, Grey AB, Horne AM, et al. Bone mineral density is not reduced in HIV-infected caucasian men treated with highly active antiretroviral therapy. Clin Endocrinol (Oxf) 2006;65(2):191–197. doi: 10.1111/j.1365-2265.2006.02572.x. [DOI] [PubMed] [Google Scholar]
- 21.Dolan SE, Kanter JR, Grinspoon S. Longitudinal analysis of bone density in human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 2006;91(8):2938–2945. doi: 10.1210/jc.2006-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mondy K, Yarasheski K, Powderly WG, et al. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus–infected individuals. Clin Infect Dis. 2003;36(4):482–490. doi: 10.1086/367569. [DOI] [PubMed] [Google Scholar]
- 23.Dao C, Young B, Buchacz K, et al. Higher and increasing rates of fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared to the general US population, 1994 to 2008 [abstract 128]. Program and abstracts of the 17th Conference on Retroviruses and Opportunistic Infections; 18 February 2010; San Francisco, CA. [Google Scholar]
- 24.Womack J, Goulet J, Gibert C, et al. HIV-infection and fragility fracture risk among male veterans [abstract 129]. Program and abstracts of the 17th Conference on Retroviruses and Opportunistic Infections; 18 February 2010; San Francisco, CA. [Google Scholar]
- 25.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab. 2008;93(9):3499–3504. doi: 10.1210/jc.2008-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collin F, Duval X, Le Moing V, et al. Ten-year incidence and risk factors of bone fractures in a cohort of treated HIV1-infected adults. AIDS. 2009;23(8):1021–1024. doi: 10.1097/QAD.0b013e3283292195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292(2):191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 28.Overton E, Mondy K, Bush T, et al. Factors associated with low bone mineral density (BMD) in a chort of HIV-Infected U.S. adults—baseline results from the SUN study [abstract 836]. Program and abstracts of the 13th Conference on Retroviruses and Opportunistic Infections; 26 February 2007; Los Angeles, CA. [Google Scholar]
- 29.Tebas P, Umbleja T, Dube M. Initiation of ART is associated with bone loos independent of the specific ART regimen: Results of ACTG A5005s [abstract 837]. Program and abstracts of the 14th Conference on Retroviruses and Opportunistic Infections; 26 February 2007; Los Angeles, CA. [Google Scholar]
- 30.Fakruddin JM, Laurence J. HIV-1 Vpr enhances production of receptor of activated NF-kappaB ligand (RANKL) via potentiation of glucocorticoid receptor activity. Arch Virol. 2005;150(1):67–78. doi: 10.1007/s00705-004-0395-7. [DOI] [PubMed] [Google Scholar]
- 31.Borderi M, Gibellini D, Crignis ED, et al. HIV-1 Induces apoptosis in primary osteoblasts: an alternative mechanism in the development of osteopenia and osteoporosis [abstract 759]. Program and abstracts of the 15th Conference on Retroviruses and Opportunistic Infections; February 2009; Montreal, Canada. [Google Scholar]
- 32.Gibellini D, De Crignis E, Ponti C, et al. HIV-1 triggers apoptosis in primary osteoblasts and HOBIT cells through TNFalpha activation. J Med Virol. 2008;80(9):1507–1514. doi: 10.1002/jmv.21266. [DOI] [PubMed] [Google Scholar]
- 33.Bismar H, Diel I, Ziegler R, Pfeilschifter J. Increased cytokine secretion by human bone marrow cells after menopause or discontinuation of estrogen replacement. J Clin Endocrinol Metab. 1995;80(11):3351–3355. doi: 10.1210/jcem.80.11.7593450. [DOI] [PubMed] [Google Scholar]
- 34.Guaraldi G, Orlando G, Squillace N, et al. Prevalence of secondary causes of osteoporosis among HIV-infected individuals. Program and abstracts of the 8th International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV; San Francisco, CA. 24–26 September 2006. [Google Scholar]
- 35.Rodriguez M, Daniels B, Gunawardene S, Robbins GK. High frequency of vitamin D deficiency in ambulatory HIV-Positive patients. AIDS Res Hum Retroviruses. 2009;25(1):9–14. doi: 10.1089/aid.2008.0183. [DOI] [PubMed] [Google Scholar]
- 36.Stephensen CB, Marquis GS, Kruzich LA, Douglas SD, Aldrovandi GM, Wilson CM. Vitamin D status in adolescents and young adults with HIV infection. Am J Clin Nutr. 2006;83(5):1135–1141. doi: 10.1093/ajcn/83.5.1135. [DOI] [PubMed] [Google Scholar]
- 37.Teichmann J, Lange U, Discher T, Lohmeyer J, Stracke H, Bretzel RG. Bone mineral density in human immunodeficiency virus–1 infected men with hypogonadism prior to highly-active-antiretroviral-therapy (HAART) Eur J Med Res. 2009;14(2):59–64. doi: 10.1186/2047-783X-14-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosen CJ, Klibanski A. Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med. 2009;122(5):409–414. doi: 10.1016/j.amjmed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 39.Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab. 2009;94(9):3387–3393. doi: 10.1210/jc.2008-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown TT, Ruppe MD, Kassner R, et al. Reduced bone mineral density in human immunodeficiency virus-infected patients and its association with increased central adiposity and postload hyperglycemia. J Clin Endocrinol Metab. 2004;89(3):1200–1206. doi: 10.1210/jc.2003-031506. [DOI] [PubMed] [Google Scholar]
- 41.Huang JS, Wilkie SJ, Sullivan MP, Grinspoon S. Reduced bone density in androgen-deficient women with acquired immune deficiency syndrome wasting. J Clin Endocrinol Metab. 2001;86(8):3533–3539. doi: 10.1210/jcem.86.8.7728. [DOI] [PubMed] [Google Scholar]
- 42.McComsey GA, Kitch D, Daar E, et al. Bone and limb fat outcomes of ACTG A5224s, a substudy of ACTG A5202: a prospective, randomized, partially blinded phase III trial of ABC/3TC or TDF/FTC with EFV or ATV/r for initial treatment of HIV-1 infection [abstract 106LB]. Program and abstracts of the 17th Conference on Retroviruses and Opportunistic Infections; 16–19 February 2010; San Francisco, CA. [Google Scholar]
- 43.Madeddu G, Spanu A, Solinas P, et al. Bone mass loss and vitamin D metabolism impairment in HIV patients receiving highly active antiretroviral therapy. Q J Nucl Med Mol Imaging. 2004;48(1):39–48. [PubMed] [Google Scholar]
- 44.Fernandez-Rivera J, Garcia R, Lozano F, et al. Relationship between low bone mineral density and highly active antiretroviral therapy including protease inhibitors in HIV-infected patients. HIV Clin Trials. 2003;4(5):337–346. doi: 10.1310/4X0H-UVMJ-BHYW-CPFB. [DOI] [PubMed] [Google Scholar]
- 45.Amiel C, Ostertag A, Slama L, et al. BMD is reduced in HIV-infected men irrespective of treatment. J Bone Miner Res. 2004;19(3):402–409. doi: 10.1359/JBMR.0301246. [DOI] [PubMed] [Google Scholar]
- 46.Cozzolino M, Vidal M, Arcidiacono MV, Tebas P, Yarasheski KE, Dusso AS. HIV-protease inhibitors impair vitamin D bioactivation to 1,25-dihydroxyvitamin D. AIDS. 2003;17(4):513–520. doi: 10.1097/00002030-200303070-00006. [DOI] [PubMed] [Google Scholar]
- 47.Dusso A, Vidal M, Powderly W, Yarashesky K, Tebas P. Protease inhibitors inhibit in vitro conversion of 25[OH]-vitamin D to 1,25[OH]2-vitamin D. Antivir Ther. 2000;5:19. [Google Scholar]
- 48.Cooper DA, Bloch M, Humphries A, et al. Simplification with fixed-dose tenofovir-emtricitaine or abacavir-lamivudine in adults with suppressed HIV repliation (The Steal Study): A randomized, open-label, 96-week, non-inferiority trial [abstract 576]. Program and abstracts of the 16th Conference on Retroviruses and Opportunistic Infections; 8–11 February 2009; Montreal, Canada. [Google Scholar]
- 49.Stellbrink H, Moyle G, Orkin C, et al. Assessment of safety and efficacy of abacavir/lamivudine and tenofovir/emtricitabine in treatment-naive HIV-1 infected subjects. ASSERT: 48-Week Result. Program and abstracts of the 12th European AIDS Conference; 11–14 November 2009; Cologne, Germany. [Google Scholar]
- 50.Fux CA, Rauch A, Simcock M, et al. Tenofovir use is associated with an increase in serum alkaline phosphatase in the Swiss HIV Cohort Study. Antivir Ther. 2008;13(8):1077–1082. [PubMed] [Google Scholar]
- 51.Ellfolk M, Norlin M, Gyllensten K, Wikvall K. Regulation of human vitamin D(3) 25-hydroxylases in dermal fibroblasts and prostate cancer LNCaP cells. Mol Pharmacol. 2009;75(6):1392–1399. doi: 10.1124/mol.108.053660. [DOI] [PubMed] [Google Scholar]
- 52.Fabbriciani G, De Socio GV. Efavirenz and bone health. AIDS. 2009;23(9):1181. doi: 10.1097/QAD.0b013e32832bab0f. [DOI] [PubMed] [Google Scholar]
- 53.Herzmann C, Arasteh K. Efavirenz-induced osteomalacia. AIDS. 2009;23(2):274–275. doi: 10.1097/QAD.0b013e32831f4685. [DOI] [PubMed] [Google Scholar]
- 54.Landriscina M, Altamura SA, Roca L, et al. Reverse transcriptase inhibitors induce cell differentiation and enhance the immunogenic phenotype in human renal clear-cell carcinoma. Int J Cancer. 2008;122(12):2842–2850. doi: 10.1002/ijc.23197. [DOI] [PubMed] [Google Scholar]
- 55.Mouly S, Lown KS, Kornhauser D, et al. Hepatic but not intestinal CYP3A4 displays dose-dependent induction by efavirenz in humans. Clin Pharmacol Ther. 2002;72(1):1–9. doi: 10.1067/mcp.2002.124519. [DOI] [PubMed] [Google Scholar]
- 56.Mora S, Gilsanz V. Establishment of peak bone mass. Endocrinol Metab Clin North Am. 2003;32(1):39–63. doi: 10.1016/s0889-8529(02)00058-0. [DOI] [PubMed] [Google Scholar]
- 57.Lewiecki EM, Gordon CM, Baim S, et al. International Society for Clinical Densitometry 2007 adult and pediatric official positions. Bone. 2008;43(6):1115–1121. doi: 10.1016/j.bone.2008.08.106. [DOI] [PubMed] [Google Scholar]
- 58.Webber CE, Sala A, Barr RD. Accounting for body size deviations when reporting bone mineral density variables in children. Osteoporos Int. 2009;20(1):113–121. doi: 10.1007/s00198-008-0642-y. [DOI] [PubMed] [Google Scholar]
- 59.Gertner JM, Kaufman FR, Donfield SM, et al. Delayed somatic growth and pubertal development in human immunodeficiency virus-infected hemophiliac boys: Hemophilia Growth and Development Study. J Pediatr. 1994;124(6):896–902. doi: 10.1016/s0022-3476(05)83177-4. [DOI] [PubMed] [Google Scholar]
- 60.Moye J, Jr, Rich KC, Kalish LA, et al. Natural history of somatic growth in infants born to women infected by human immunodeficiency virus. Women and Infants Transmission Study Group. J Pediatr. 1996;128(1):58–69. doi: 10.1016/s0022-3476(96)70428-6. [DOI] [PubMed] [Google Scholar]
- 61.Wren TA, Kim PS, Janicka A, Sanchez M, Gilsanz V. Timing of peak bone mass: discrepancies between CT and DXA. J Clin Endocrinol Metab. 2007;92(3):938–941. doi: 10.1210/jc.2006-1570. [DOI] [PubMed] [Google Scholar]
- 62.Arpadi S, Horlick M, Shane E. Metabolic bone disease in human immunodeficiency virus-infected children. J Clin Endocrinol Metab. 2004;89(1):21–23. doi: 10.1210/jc.2003-031942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arpadi SM, Horlick M, Thornton J, Cuff PA, Wang J, Kotler DP. Bone mineral content is lower in prepubertal HIV-infected children. J Acquir Immune Defic Syndr. 2002;29(5):450–454. doi: 10.1097/00126334-200204150-00004. [DOI] [PubMed] [Google Scholar]
- 64.Jacobson DL, Spiegelman D, Duggan C, et al. Predictors of bone mineral density in human immunodeficiency virus-1 infected children. J Pediatr Gastroenterol Nutr. 2005;41(3):339–346. doi: 10.1097/01.mpg.0000174468.75219.30. [DOI] [PubMed] [Google Scholar]
- 65.Mora S, Sala N, Bricalli D, Zuin G, Chiumello G, Vigano A. Bone mineral loss through increased bone turnover in HIV-infected children treated with highly active antiretroviral therapy. AIDS. 2001;15(14):1823–1829. doi: 10.1097/00002030-200109280-00011. [DOI] [PubMed] [Google Scholar]
- 66.Mora S, Zamproni I, Beccio S, Bianchi R, Giacomet V, Vigano A. Longitudinal changes of bone mineral density and metabolism in antiretroviral-treated human immunodeficiency virus-infected children. J Clin Endocrinol Metab. 2004;89(1):24–28. doi: 10.1210/jc.2003-030767. [DOI] [PubMed] [Google Scholar]
- 67.Mora S, Zamproni I, Giacomet V, Cafarelli L, Figini C, Vigano A. Analysis of bone mineral content in horizontally HIV-infected children naive to antiretroviral treatment. Calcif Tissue Int. 2005;76(5):336–340. doi: 10.1007/pl00020973. [DOI] [PubMed] [Google Scholar]
- 68.O’Brien KO, Razavi M, Henderson RA, Caballero B, Ellis KJ. Bone mineral content in girls perinatally infected with HIV. Am J Clin Nutr. 2001;73(4):821–826. doi: 10.1093/ajcn/73.4.821. [DOI] [PubMed] [Google Scholar]
- 69.Pitukcheewanont P, Safani D, Church J, Gilsanz V. Bone measures in HIV-1 infected children and adolescents: disparity between quantitative computed tomography and dual-energy X-ray absorptiometry measurements. Osteoporos Int. 2005;16(11):1393–1396. doi: 10.1007/s00198-005-1849-9. [DOI] [PubMed] [Google Scholar]
- 70.Purdy JB, Gafni RI, Reynolds JC, Zeichner S, Hazra R. Decreased bone mineral density with off-label use of tenofovir in children and adolescents infected with human immunodeficiency virus. J Pediatr. 2008;152(4):582–584. doi: 10.1016/j.jpeds.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosso R, Vignolo M, Parodi A, et al. Bone quality in perinatally HIV-infected children: role of age, sex, growth, HIV infection, and anti-retroviral therapy. AIDS Res Hum Retroviruses. 2005;21(11):927–932. doi: 10.1089/aid.2005.21.927. [DOI] [PubMed] [Google Scholar]
- 72.Soler Palacin P, Torrent A, Rossich R, et al. Osteoporosis and multiple fractures in an antiretroviral-naive, HIV-positive child. J Pediatr Endocrinol Metab. 2007;20(8):933–938. doi: 10.1515/jpem.2007.20.8.933. [DOI] [PubMed] [Google Scholar]
- 73.Tan BM, Nelson RP, Jr, James-Yarish M, Emmanuel PJ, Schurman SJ. Bone metabolism in children with human immunodeficiency virus infection receiving highly active antiretroviral therapy including a protease inhibitor. J Pediatr. 2001;139(3):447–451. doi: 10.1067/mpd.2001.117005. [DOI] [PubMed] [Google Scholar]
- 74.Vignolo M, Rosso R, Parodi A, Viscoli C. Quantitative ultrasound and bone density in vertically HIV-infected children. Clin Infect Dis. 2006;43(1):112–113. doi: 10.1086/504950. author reply, 3–4. [DOI] [PubMed] [Google Scholar]
- 75.Stagi S, Bindi G, Galluzzi F, Galli L, Salti R, de Martino M. Changed bone status in human immunodeficiency virus type 1 (HIV-1) perinatally infected children is related to low serum free IGF-I. Clin Endocrinol Oxf. 2004;61(6):692–699. doi: 10.1111/j.1365-2265.2004.02150.x. [DOI] [PubMed] [Google Scholar]
- 76.Matarazzo P, Palomba E, Lala R, et al. Growth impairment, IGF I hyposecretion and thyroid dysfunction in children with perinatal HIV-1 infection. Acta Paediatr. 1994;83(10):1029–1034. doi: 10.1111/j.1651-2227.1994.tb12977.x. [DOI] [PubMed] [Google Scholar]
- 77.Hazra R, Gafni RI, Maldarelli F, et al. Tenofovir disoproxil fumarate and an optimized background regimen of antiretroviral agents as salvage therapy for pediatric HIV infection. Pediatrics. 2005;116(6):e846–e854. doi: 10.1542/peds.2005-0975. [DOI] [PubMed] [Google Scholar]
- 78.Jacobson DL, Lindsey JC, Gordon CM, et al. Total body and spinal bone mineral density across Tanner stage in perinatally HIV-infected and uninfected children and youth in PACTG 1045. AIDS. 2010;24(5):687–696. doi: 10.1097/QAD.0b013e328336095d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Villamor E. A potential role for vitamin D on HIV infection? Nutr Rev. 2006;64(5 Pt 1):226–233. doi: 10.1301/nr.2006.may.226-233. [DOI] [PubMed] [Google Scholar]
- 80.Pettifor JM. Nutritional rickets: deficiency of vitamin D, calcium, or both? Am J Clin Nutr. 2004;80(6 Suppl):1725S–1729S. doi: 10.1093/ajcn/80.6.1725S. [DOI] [PubMed] [Google Scholar]
- 81.Puri S, Marwaha RK, Agarwal N, et al. Vitamin D status of apparently healthy schoolgirls from two different socioeconomic strata in Delhi: relation to nutrition and lifestyle. Br J Nutr. 2008;99(4):876–882. doi: 10.1017/S0007114507831758. [DOI] [PubMed] [Google Scholar]
- 82.Dannhauser A, van Staden AM, van der Ryst E, et al. Nutritional status of HIV-1 seropositive patients in the Free State Province of South Africa: anthropometric and dietary profile. Eur J Clin Nutr. 1999;53(3):165–173. doi: 10.1038/sj.ejcn.1600691. [DOI] [PubMed] [Google Scholar]
- 83.Sachan A, Gupta R, Das V, Agarwal A, Awasthi PK, Bhatia V. High prevalence of vitamin D deficiency among pregnant women and their newborns in northern India. Am J Clin Nutr. 2005;81(5):1060–1064. doi: 10.1093/ajcn/81.5.1060. [DOI] [PubMed] [Google Scholar]
- 84.Aberg JA, Kaplan JE, Libman H, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(5):651–681. doi: 10.1086/605292. [DOI] [PubMed] [Google Scholar]
- 85.Johnell O, Kanis JA, Black DM, et al. Associations between baseline risk factors and vertebral fracture risk in the Multiple Outcomes of Raloxifene Evaluation (MORE) Study. J Bone Miner Res. 2004;19(5):764–772. doi: 10.1359/JBMR.040211. [DOI] [PubMed] [Google Scholar]
- 86.McComsey GA, Huang JS, Woolley IJ, et al. Fragility fractures in HIV-infected patients: need for better understanding of diagnosis and management. J Int Assoc Physicians AIDS Care Chic Ill. 2004;3(3):86–91. doi: 10.1177/154510970400300303. [DOI] [PubMed] [Google Scholar]
- 87.Tannenbaum C, Clark J, Schwartzman K, et al. Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab. 2002;87(10):4431–4437. doi: 10.1210/jc.2002-020275. [DOI] [PubMed] [Google Scholar]
- 88.Cohen A, Fleischer J, Freeby MJ, McMahon DJ, Irani D, Shane E. Clinical characteristics and medication use among premenopausal women with osteoporosis and low BMD: the experience of an osteoporosis referral center. J Womens Health Larchmt. 2009;18(1):79–84. doi: 10.1089/jwh.2008.0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cohen A, Shane E. In: Primer on the metabolic bone diseases and other disorders of bone and mineral metbolism: premenopausal osteoporosis. 7. Rosen C, editor. New York: JW Wiley; 2008. [Google Scholar]
- 90.Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29(4):441–464. doi: 10.1210/er.2008-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bolland MJ, Grey AB, Gamble GD, Reid IR. CLINICAL review: low body weight mediates the relationship between HIV infection and low bone mineral density: a meta-analysis. J Clin Endocrinol Metab. 2007;92(12):4522–4528. doi: 10.1210/jc.2007-1660. [DOI] [PubMed] [Google Scholar]
- 92.Chew NS, Doran PP, Powderly WG. Osteopenia and osteoporosis in HIV: pathogenesis and treatment. Curr Opin HIV AIDS. 2007;2(4):318–323. doi: 10.1097/COH.0b013e3281a3c092. [DOI] [PubMed] [Google Scholar]
- 93.Pollock E, Klotsas AE, Compston J, Gkrania-Klotsas E. Bone health in HIV infection. Br Med Bull. 2009;92:123–133. doi: 10.1093/bmb/ldp037. [DOI] [PubMed] [Google Scholar]
- 94.Kanis J, Johnell O, Gullberg B, et al. Risk factors for hip fracture in men from southern Europe: the MEDOS study. Mediterranean Osteoporosis Study. Osteoporos Int. 1999;9(1):45–54. doi: 10.1007/s001980050115. [DOI] [PubMed] [Google Scholar]
- 95.Melton LJ, 3rd, Atkinson EJ, Khosla S, O’Fallon WM, Riggs BL. Secondary osteoporosis and the risk of vertebral deformities in women. Bone. 1999;24(1):49–55. doi: 10.1016/s8756-3282(98)00150-1. [DOI] [PubMed] [Google Scholar]
- 96.Poor G, Atkinson EJ, O’Fallon WM, Melton LJ., 3rd Predictors of hip fractures in elderly men. J Bone Miner Res. 1995;10(12):1900–1907. doi: 10.1002/jbmr.5650101209. [DOI] [PubMed] [Google Scholar]
- 97.FRAX WHO Fracture Risk Assessment Tool. University of Sheffield; UK: [Accessed 7 September 2010]. http://www.shef.ac.uk/FRAX/ [Google Scholar]
- 98.Lundgren JD, Battegay M, Behrens G, et al. European AIDS Clinical Society (EACS) guidelines on the prevention and management of metabolic diseases in HIV. HIV Med. 2008;9(2):72–81. doi: 10.1111/j.1468-1293.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- 99.Gregg EW, Cauley JA, Seeley DG, Ensrud KE, Bauer DC. Physical activity and osteoporotic fracture risk in older women. Study of Osteoporotic Fractures Research Group. Ann Intern Med. 1998;129(2):81–88. doi: 10.7326/0003-4819-129-2-199807150-00002. [DOI] [PubMed] [Google Scholar]
- 100.Rosen H, Drezner M. In: Overview of the management of osteoporosis in women. Rose B, editor. Wellsley, MA: UpToDate Online 18.2, 2010. http://www.uptodateonline.com. [Google Scholar]
- 101.Armas LA, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89(11):5387–5391. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]
- 102.Romagnoli E, Mascia ML, Cipriani C, et al. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J Clin Endocrinol Metab. 2008;93(8):3015–3020. doi: 10.1210/jc.2008-0350. [DOI] [PubMed] [Google Scholar]
- 103.Cannell JJ, Hollis BW, Zasloff M, Heaney RP. Diagnosis and treatment of vitamin D deficiency. Expert Opin Pharmacother. 2008;9(1):107–118. doi: 10.1517/14656566.9.1.107. [DOI] [PubMed] [Google Scholar]
- 104.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 105.Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303(18):1815–1822. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 106.Vieth R. Critique of the considerations for establishing the tolerable upper intake level for vitamin D: critical need for revision upwards. J Nutr. 2006;136(4):1117–1122. doi: 10.1093/jn/136.4.1117. [DOI] [PubMed] [Google Scholar]
- 107.Maalouf NM, Heller HJ, Odvina CV, Kim PJ, Sakhaee K. Bisphosphonate-induced hypocalcemia: report of 3 cases and review of literature. Endocr Pract. 2006;12(1):48–53. doi: 10.4158/EP.12.1.48. [DOI] [PubMed] [Google Scholar]
- 108.Adami S, Giannini S, Bianchi G, et al. Vitamin D status and response to treatment in post-menopausal osteoporosis. Osteoporos Int. 2009;20(2):239–244. doi: 10.1007/s00198-008-0650-y. [DOI] [PubMed] [Google Scholar]
- 109.Bilezikian JP. Efficacy of bisphosphonates in reducing fracture risk in postmenopausal osteoporosis. Am J Med. 2009;122(2 Suppl):S14–S21. doi: 10.1016/j.amjmed.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 110.McComsey GA, Kendall MA, Tebas P, et al. Alendronate with calcium and vitamin D supplementation is safe and effective for the treatment of decreased bone mineral density in HIV. AIDS. 2007;21(18):2473–2482. doi: 10.1097/QAD.0b013e3282ef961d. [DOI] [PubMed] [Google Scholar]
- 111.Mondy K, Powderly WG, Claxton SA, et al. Alendronate, vitamin D, and calcium for the treatment of osteopenia/osteoporosis associated with HIV infection. J Acquir Immune Defic Syndr. 2005;38(4):426–431. doi: 10.1097/01.qai.0000145352.04440.1e. [DOI] [PubMed] [Google Scholar]
- 112.Bolland MJ, Grey AB, Horne AM, et al. Annual zoledronate increases bone density in highly active antiretroviral therapy-treated human immunodeficiency virus-infected men: a randomized controlled trial. J Clin Endocrinol Metab. 2007;92(4):1283–1288. doi: 10.1210/jc.2006-2216. [DOI] [PubMed] [Google Scholar]
- 113.Huang J, Meixner L, Fernandez S, McCutchan JA. A double-blinded, randomized controlled trial of zoledronate therapy for HIV-associated osteopenia and osteoporosis. AIDS. 2009;23(1):51–57. doi: 10.1097/QAD.0b013e32831c8adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Miller PD. Is there a role for bisphosphonates in chronic kidney disease? Semin Dial. 2007;20(3):186–190. doi: 10.1111/j.1525-139X.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 115.Khan AA, Sandor GK, Dore E, et al. Bisphosphonate associated osteonecrosis of the jaw. J Rheumatol. 2009;36(3):478–490. doi: 10.3899/jrheum.080759. [DOI] [PubMed] [Google Scholar]
- 116.Khosla S, Burr D, Cauley J, et al. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22(10):1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 117.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 118.Wysowski DK. Reports of esophageal cancer with oral bisphosphonate use. N Engl J Med. 2009;360(1):89–90. doi: 10.1056/NEJMc0808738. [DOI] [PubMed] [Google Scholar]
- 119.Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005;90(3):1294–1301. doi: 10.1210/jc.2004-0952. [DOI] [PubMed] [Google Scholar]
- 120.Lenart BA, Neviaser AS, Lyman S, et al. Association of low-energy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporos Int. 2009;20(8):1353–1362. doi: 10.1007/s00198-008-0805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bone HG, Hosking D, Devogelaer JP, et al. Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 2004;350(12):1189–1199. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]
- 122.Black DM, Kelly MP, Genant HK, et al. Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med. 2010;362(19):1761–1771. doi: 10.1056/NEJMoa1001086. [DOI] [PubMed] [Google Scholar]
- 123.Ott SM. Long-term safety of bisphosphonates. J Clin Endocrinol Metab. 2005;90(3):1897–1899. doi: 10.1210/jc.2005-0057. [DOI] [PubMed] [Google Scholar]
- 124.Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med. 2003;349(13):1216–1226. doi: 10.1056/NEJMoa035725. [DOI] [PubMed] [Google Scholar]
- 125.Rosen HN, Moses AC, Garber J, Ross DS, Lee SL, Greenspan SL. Utility of biochemical markers of bone turnover in the follow-up of patients treated with bisphosphonates. Calcif Tissue Int. 1998;63(5):363–368. doi: 10.1007/s002239900541. [DOI] [PubMed] [Google Scholar]
- 126.Dolin R, Masur H, Saag M. In: Bone disorders. 3. Livingstone C, editor. Philadelphia, PA: Elsevier; 2008. [Google Scholar]


