Abstract
Liver transplantation in patients with active hepatitis C virus (HCV) infection is followed by almost universal recurrence of viral infection. The control of HCV infection has been characterized largely in terms of the HCV-specific function of T-lymphocytes and the adaptive immune response. Emerging data suggest that components of the innate immune system, including natural killer cells, have a central role in determining the nature of posttransplant HCV infection and the likelihood of response to antiviral therapy. This review examines the emerging evidence implicating innate immunity in the pathogenesis of posttransplant HCV infections and the potential therapeutic implications of these observations.
Hepatitis C virus (HCV) –induced liver injury is the primary indication for orthotopic liver transplantation (OLT) in the United States [1]. Recurrent HCV infection occurs in at least 75%–80% of recipients, of whom 10%–21% go on to develop fibrosis and cirrhosis [2, 3]. OLT recipients with HCV infection have higher rates of allograft failure and death relative to virus-free recipients [4–8]. The difference in outcomes may reflect the effect of HCV infection on the graft and on graft rejection, as well as the role of HCV in promoting other opportunistic infections and posttransplant malignancies [5, 6, 9]. None of the standard antiviral regimens used to treat posttransplant HCV have been consistently well tolerated or efficacious [10]. Thus, the pathophysiology of recurrent infection and the factors contributing to adverse outcomes are important topics for investigation. In general, the control of posttransplant HCV infection has been studied in the context of adaptive, cellular or humoral immunity. This review explores emerging evidence that implicates effectors of innate immunity, notably natural killer (NK) cells, in the pathogenesis of recurrent HCV and may suggest novel approaches to therapeutic interventions for viral infection in transplant recipients.
HCV AND THE ADAPTIVE IMMUNE RESPONSE
All components of innate and adaptive immunity are involved in the pathogenesis of posttransplant HCV infection. Containment of HCV infection requires a coordinated, vigorous, and sustained multispecific CD4+ and CD8+ T cell response to the virus. HCV epitopes include both core and nonstructural proteins (NS3, NS4, and NS5) [11]. Clearance of acute HCV infection has been correlated with the rapid expansion of CD4+ and CD8+ T cells [12–16]. Maintenance of viral clearance is associated with persistence of HCV-specific CD4+ T cells, with the production of memory CD8+ T cells, and with the elaboration of interferon-γ [16–19]. Progression to chronic HCV in patients who have not undergone transplantation seems to be related to exhaustion of adaptive immune function [20]. The role of the humoral immune response to the containment of HCV infection is controversial. Neutralizing antibodies to surface viral glycoproteins E1 and E2 occurs during the course of infection, regardless of HCV genotype [21]. However, although antibodies interfere with receptor binding, antibody presence does not correlate well with viral clearance in patients with acute HCV infection [22].
Reconstitution of the adaptive immune system after transplantation is associated with an improved antiviral response and attenuation of the severity of recurrent HCV infection [23–27]. Because hepatic allografts are HLA-mismatched between donor and recipient, immune responses to HCV within the liver occur largely in the context of indirect pathways for antigen presentation. The role of the indirect pathway in HCV infection after transplantation is incompletely characterized. The existence of donor major histocompatibility complex (MHC)–restricted, HCV-specific CD8+ T cells has been revealed [28]. Unfortunately, the development of such donor-HLA–restricted CD8+ T cells generally occurs after the reestablishment of allograft HCV infection. Indirect evidence for the role of the indirect pathway in immunity to HCV infection comes from studies suggesting that cyclosporine does not increase HCV viremia; the indirect pathway is reported to be less sensitive to cyclosporine than the direct pathway [29, 30]. The indirect pathway may also contribute to the generation of regulatory T cells that may suppress HCV-specific immune responses [30]. The net clinical impact of the indirect pathway in the pathogenesis of posttransplant HCV infection and allograft injury remains to be elucidated.
THE KINETICS OF POSTTRANSPLANT HCV INFECTION
The kinetics of HCV infection in the early posttransplant period underscores the possible role of the innate immune system in antiviral defenses. During the transplantation procedure, the HCV viral load decreases precipitously with removal of the diseased organ [31]. However, virus is detectable in the blood within 12 h of reperfusion of the allograft and generally returns to pretransplant baseline levels within days of transplantation [31]. This relapse reflects the presence of extrahepatic viral stores and recurrent infection and may not correlate well in the early posttransplant period with intrahepatic or membrane-associated virus that would be expected to be associated with liver injury. Viral loads will often exceed pretransplant levels, likely as a result of the healthier cellular environment for viral replication rather than the effects of immunosuppression [32]. Not all viral species carry equal avidity for hepatic cell surface receptors; virus internalization is a complex process involving virus species, receptor, the low-density lipoprotein receptor, other receptors, and cell-specific factors.
After infection, the individual carries multiple circulating HCV quasispecies differing in viral envelope proteins; viral variants are produced by means of immune selection pressure and the error-prone HCV RNA polymerase at genomic hotspots, such as the highly variable regions 1 and 2 of the E2 protein. Viral quasispecies act as a collective unit with high virologic diversity associated with susceptibility to immune modulation or antiviral therapies prior to the emergence of more virulent strains. In individuals with greater diversity of HCV quasispecies and who demonstrate early but nonsustained virologic responses to antiviral therapy, the time course suggests a role for the innate immune system in the early phase response [33]. Some, but not all, studies have correlated increased homogeneity of quasispecies with greater severity of HCV recurrence after transplantation [34–39].
When HCV-positive donor allografts are transplanted into HCV-positive recipients, either the recipient or donor strain prevails; expulsion of the competing strain occurs as early as 1 day after transplantation [40]. The timing of this process suggests, rather than an immune process or selection based on relative replicative efficiency, that the nature of posttransplant infection is determined by competition of quasispecies for viral entry. Studies of quasispecies pretransplant, postperfusion, and postallograft infection suggest that the allograft selects a fraction of the quasispecies variants found in serum and that posttransplant evolution of this fraction retains conservation of the E2 residues [41–46]. This conservation suggests a role for the interaction between E2 and specific allograft receptors in the development of posttransplant HCV infection. A candidate receptor may be the CD81 molecule of hepatic NK cells.
NK CELLS AND VIRAL INFECTION
NK cells function at the interface of the adaptive and innate immune systems. NK cells have been implicated in host defenses against a variety of human viruses, including the herpes viruses (Epstein-Barr virus and cytomegalovirus) and HCV (Figure 1) [47–51]. Antiviral effects are mediated via cytokines (interferon-γ, interleukin-12, and tumor necrosis factor) and chemokines (CCL3) and/or cytolysis of abnormal or infected cells through secretion of granzyme or perforin. [52, 53, 54] Cytolytic activities of NK cells for virally infected cells may be restricted to anatomic compartments, such as the spleen (for cytomegalovirus [CMV]) or liver (for HCV with interferon-γ) [53]. NK cells recognize target cells via FCIII receptor (CD16) binding of IgG complexes on opsonized targets or specific NK receptor binding of target ligands [52]. These ligands are typically MHC class I molecules and may be stress inducible [54]. Depending on the specific ligand-receptor pair, NK receptors contribute to either inhibition or activation of NK cells, in part related to the presence or absence of cytoplasmic immunoreceptor tyrosine-based inhibitory motifs [53, 54]. The magnitude of NK cell activation is tightly regulated by the cumulative input of multiple activating and inhibitory receptors [54]. The latter are thought to serve an important role in preserving self-tolerance.
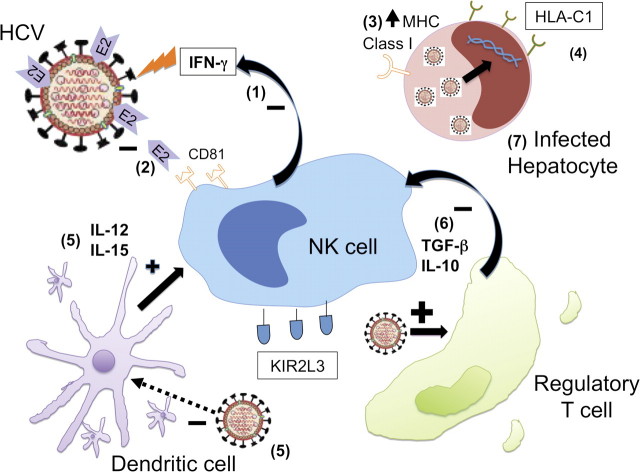
Figure 1.
Modulation of innate immunity during hepatitis C virus (HCV) infection. HCV alters the innate immune response at multiple sites. In response to HCV infection, (1) natural killer (NK) cells elaborate interferon-γ to mediate antiviral effects. However, (2) HCV E2 protein binds the NK CD81 receptor, decreasing release of interferon-γ and cytotoxic granules by NK cells. (3) HCV core protein increases major histocompatibility complex class I expression on infected hepatocytes, decreasing NK cell activity against infected cells. (4) Protection from, and clearance of, HCV infection have been associated with a KIR2L3 and HLA-C1 receptor-ligand pairing. (5) Dendritic cells release cytokines IL-12 and IL-15, which augment NK cell function and survival. However, HCV depresses dendritic cell function and number. (6) HCV increases the regulatory T cell population in the liver. Regulatory T cells secrete transforming growth factor–β and IL-10 to decrease NK cell function. (7) HCV proteins ablate signaling pathways in the infected cell to block intracellular pathways associated with innate immunity. In the infected cell, the nonstructural proteins of HCV, notably NS3/4a protein, interact with various host adaptor molecules to block type I interferon induction pathways and the antiviral effects of interferon. Plus sign, activating; minus sign, inhibiting.
The relationship of NK cells to HCV infection is under investigation. NK cells are activated in patients acutely infected with HCV, notably in virologic responders to interferon-α and ribavirin therapy [55, 56]. Chronic HCV infection is associated with reduced NK cell frequency and function (perforin and interferon-γ secretion) in the peripheral blood and in the liver [57–59]. Achievement of undetectable HCV viral load after pegylated interferon-α therapy was associated with increased NK cell numbers, as well as expression of the activating NK cell receptor (NKG2D) and of perforin [59]. NK functions were not reconstituted in patients for whom therapy did not achieve viral clearance [59].
Viral infections elicit the synthesis of interferon-α/β and interleukin-12, which stimulate NK cells to secrete interferon-γ, and TNF-α [53]. These cytokines activate a series of antiviral pathways, chemokines (eg, monokine induced by interferon-γ or Mig, CX3CL1), production of nitric oxide, and immunoregulatory effects, including the stimulation of adaptive immune responses [53]. Interferon-γ inhibits HCV replication and may contribute to activation of the virus-specific immune response [60]. The therapeutic effects of ribavirin have been correlated with an ability to enhance cellular responses to inteferon-γ [61]. Single nucleotide polymorphisms near the gene encoding type III interferon, IL-28B, significantly affect the HCV treatment response [62–64]. HCV may evade the innate immune response of the infected hepatocyte by attenuating the activation of intracellular signaling pathways responsible for expression of interferon-β and interferon-stimulated genes, and the antiviral effects of interferon on HCV [60].
Multiple viruses have mechanisms that enable them to evade NK cell defenses. Murine CMV encodes a protein, m144, which mimics MHC class 1 and engages NK cell inhibitory receptors [65]. Human CMV proteins downregulate cellular ligands for the activating NK receptor, NKG2D [66–68]. Influenza virions infect NK cells causing apoptosis; the hemagglutinin protein depresses NK cell cytotoxicity upon internalization [69]. Similarly, HCV seems to downregulate NK natural cytotoxicity receptors [70]. It may also depress NK cell cytotoxicity by means of the HCV core protein, which has been shown to upregulate the expression of MHC class I in infected hepatocytes [71, 72]. Furthermore, the HCV-E2 protein, an envelope protein of HCV, may cross-link the tetraspanin CD81 surface receptor, decreasing NK cell function. Two groups have reported in vitro systems in which recombinant HCV-E2 protein was used to tether the CD81 receptor on NK cells, resulting in decreased NK cell function [73, 74]. Inhibition of NK cell function occurred with immobilized HCV virions; the physiologic relevance of such exposures is uncertain [75, 76].
MODULATORS OF NK CELL FUNCTION IN RECURRENCE OF HCV INFECTION
NK Cell Receptors, Dendritic Cells, and Regulatory T Cells
Two main families of NK receptors exist that tightly regulate NK activity: the Ly49 family (killer immunoglobulin receptor [KIR]) and CD94/NKG2 [77].In individuals, one receptor type may be relatively overexpressed [78]. The Ly49 family in mice comprises more than 10 Ly49 genes, including both activating and inhibitory receptors [79]. In humans, the functions of the Ly49 family are subserved by a distinct family, the KIR genes. Inhibitory Ly49 molecules bind primarily MHC class I ligands, whereas the ligands for activating Ly49 molecules may include MHC class I and MHC class I–like molecules expressed by viruses. The murine CMV m157 gene product decreases NK function by means of Ly49H binding [79]. In steady state, the Ly49 receptors allow murine NK cells to discriminate “self” cells expressing MHC class I molecules from foreign or infected cells that are “missing self” MHC class I molecules. The absence of self MHC class I molecules relieves NK cell inhibition and activates NK cell cytotoxicity against infected or foreign cells.
The CD94/NKG2 family also comprises inhibitory (eg, CD94/NKG2A) and activating (eg, CD94/NKG2C) receptors [54]. During stress, a signal peptide derived from heat shock protein 60 binds to the ligand for these receptors, HLA-E, decreasing affinity for the inhibitory receptor, and upregulates NK activity [80]. Potential target cells express MHC class I chain-related genes (MICA/B) under stress that bind the NK activating receptor, NKG2D [81–83].
Modulation of NK cell activity is also regulated by cytokines produced by other immune and inflammatory cells. Dendritic cells (DCs) and NK cells participate in a complex crosstalk that results in reciprocal activation (Figure 1) [84]. The control mechanisms include DC elaboration of soluble IL-12 to stimulate NK cell cytotoxicity and IL-15 to enhance NK cell survival [85].
Recent studies have shown that regulatory T cells (Tregs and Foxp3+CD4+CD25+) inhibit NK cell function. Data from murine studies suggest that membrane-bound transforming growth factor (TGF)–β on Tregs mediates NK cell inhibition by downregulating the NKGD2 receptor [86].In mice, Tregs may interfere with DC-NK cell crosstalk by limiting DC interaction with self-reactive CD4+ T cells in the lymph node [87]. This interaction seems to be critical to DC expression of IL-15.
Local Microenvironment: Hepatic NK Cells
The hepatic NK cell compartment is increasingly recognized as unique. Although NK cells form only 5%–15% of peripheral blood mononuclear cells, they comprise 30%–50% of intrahepatic lymphocytes [88]. The results of a recent study in mice suggested that hepatic NK cells could be distinguished from splenic NK cells by the presence of decreased levels of activating receptor (Ly49) expression, increased inhibitory receptor (NKG2A) expression, and decreased interferon- γ secretion. Splenic NK cells adoptively transferred into the murine liver assumed the liver NK cell phenotype, suggesting that the hepatic environment influences NK cell function [89]. Such changes in the cellular phenotype are likely to have functional consequences. In a C57BL/6 mouse model, NK cell control of murine CMV infection required perforin in the spleen but not in the liver [90]. This distinction may be specific to the mouse strain studied and was not replicated in a subsequent study [91].
NKT cells are a T cell subset that express NK cell markers (NK1.1 or Ly-49), an activated phenotype, and a restricted T cell receptor repertoire. Although some of the functions ascribed to NKT cells have been attributed more recently to NK cells, NKT cells are substantial producers of interferon-γ and might participate in the antiviral immune response. However, the role of NKT cells in hepatic antiviral defenses is uncertain, because NKT cells represent only ∼4% of resident human intrahepatic lymphocytes [92].
NK Receptor Genotypes and Recurrent HCV Infection after Liver Transplantation
The relationship of inhibitory KIR genotypes to clinical outcomes after HCV infection has been studied. Ligands for the inhibitory KIRs chiefly include HLA-C and HLA-Bw4. The former ligand exists as 2 allotypes, HLA-C1 and C2. Each KIR haplotype differs in the ligand affinity. For example, the inhibitory KIR2L3 haplotype has a lower affinity for HLA-C1 antigen, rendering NK cells more easily activated for cytolytic attack [93]. Among individuals at risk, protection from, and clearance of, HCV infection have been associated with a KIR2L3 and HLA-C1 receptor-ligand pairing [93–95]. The KIR2L3–HLA-C1 haplotype-ligand pair has also been associated with sustained virologic response to antiviral therapy [95, 96]. By contrast, KIR-ligand mismatch and recipient KIR2L3 haplotype have been correlated with recurrent allograft hepatitis, perhaps because both are associated with a reduction in KIR inhibition of NK cells (P = .04) [97]. This correlation has been found to be independent of antiviral therapy and immunosuppressive regimen. Neither a KIR ligand mismatch nor recipient KIR2L3 haplotype was associated with acute allograft rejection. A study of 44 OLT patients receiving therapy for posttransplant HCV revealed that the lack of antiviral response to therapy was associated with the absence of the activating NK receptor haplotype, KIR2DS2 (P = .008) [98]. It is difficult to ascribe this finding to an isolated impairment of innate immunity, because KIR2DS2 is also expressed on T cells. The study also could not distinguish a KIR2DS2 correlation from one with the inhibitory counterpart, KIR2DL2, because both haplotypes are found in the same populations.
Another NK receptor-ligand pair, the inhibitory CD94/NKG2A receptor and HLA-E, may also modulate host response to HCV infection. HLA-E has 2 alleles, HLA-ER and HLA-EG. The former has less cell surface expression with resultant reduction in the probability of inhibitory receptor binding to inhibit NK cell activity [99]. Thus, individuals with HLA-E R/R would be expected to exhibit more rapid NK cell activation and increased viral clearance. Consistent with this hypothesis, 308 patients who were susceptible to HCV genotypes 2 and 3 were generally found to lack the HLA-ER allele (P < .001) [100]. Correlation between HLA-E allele match and the risk for recurrent posttransplant HCV infection has not been explored.
Treg Enrichment in the Hepatic and HCV Environment
Peripheral regulatory T cells (Tregs) include IL-10–secreting Tr1 cells, TGF-β–secreting Tr3 cells, and Fox3p+CD4+CD25+ cells [101]. Coculture studies show that the hepatic microenvironment may influence DC and NK crosstalk, favoring enrichment of the hepatic CD4+CD25+ Treg population [102]. HCV infection may also enrich the hepatic Treg cell population; this enrichment may persist after liver transplantation. Immune profiles of liver biopsies show a CD4+:Foxp3+ratio of 10:1 in patients with primary biliary cirrhosis, compared with a ratio of 2:1 in patients with chronic HCV [103]. Compared with uninfected recipients, OLT recipients with posttransplant HCV may have an enhanced peripheral Foxp3+CD4+CD25+ T cell population [104, 105]. The mechanisms underlying these observations are uncertain. The loss of Treg function has been associated with more effective clearance of acute HCV infection (P = .02) [106]. Longitudinal examination of the transcriptome and proteome in sera and livers of transplant recipients has demonstrated an association between development of post-transplant hepatitis C at five years with Treg cell markers and Treg-associated cytokines, TGF-beta and IL-10 [105].
Dendritic Cell Impairment by the HCV-Infected Liver
Although direct HCV infection of DCs is rare, HCV is associated with decreased numbers of peripheral DCs in patients with chronic and posttransplant disease [107, 108, 109]. In vitro attenuation of DC function by HCV has been reported but not reliably validated [110, 111]. In vivo, DCs from patients with chronic HCV infection show decreased release of inflammatory cytokines [112, 113]. HCV may directly interfere with DC activation of NK cells by downregulating DC expression of the MICA/B ligands that activate NK cells [114]. Although the liver allograft brings a population of uninfected DCs to the recipient, these hepatic DCs are distinguished from other subsets by their tolerigenic ability. Products from enteric bacteria may maintain hepatic DCs in an immature, hyporesponsive state [115]. Although these immature hepatic DCs may reduce the immunogenicity of liver allografts, they may also contribute to the development of posttransplant HCV infection [116, 117].
FUTURE DIRECTIONS
Observations on the role of the innate immune system in recurrent HCV infection after transplantation are preliminary but beginning to find clinical application. Pretransplant peripheral NK cell levels may predict the severity of HCV recurrence in OLT recipients [118]. In a recent clinical trial, 14 HCV-positive recipients elected to receive a donor lymphocyte infusion, rich in NK and NKT cells, on day 3 after transplantation. All had undetectable HCV RNA levels by 1 week after transplantation, and 1 participant has maintained a durable response 20 months after infusion. Eight control recipients refused the infusion and never achieved virologic control during the study period [119].
Experience with NK cell immunotherapy for hematologic malignancies may suggest approaches relevant to HCV infection in liver transplant recipients. In haploidentical stem cell transplant recipients with donor-recipient mismatches at the HLA-C or HLA-Bw4 loci, a population of donor NK cells with KIR ligands may recognize recipient cells as non-self and mediate attack [120]. Recipients of haploidentical stem cell transplants and recipients of cord blood for acute myelogenous leukeumia (AML) with this HLA mismatch have been shown to have improved disease-free survival and time to relapse. This has been attributed to NK cell–mediated cytotoxicity of tumor cells [121–124]. The potential role for NK cell therapies in HCV infection remains undefined. As for any hematopoietic cellular transplant, the potential risks of neutropenia-associated infections and transplant-associated malignancies may temper enthusiasm for NK cell therapies [125].
Anti-KIR monoclonal antibodies may circumvent some of the logistical hurdles associated with NK cell infusions and, in a phase I trial, have been shown to augment endogenous NK cell activity against AML [126]. Blockade of other inhibiting NK receptors has increased NK activity against AML cells in vitro [127]. Similar monoclonal antibodies might be considered as a part of anti-HCV therapy for transplant recipients with the risk that enhanced NK cell activity may exacerbate allograft rejection.
In the process of evasion of the innate immune response, HCV also adversely affects the development of antigen-specific adaptive immunity and contributes to viral persistence and resistance to therapy. As increasing numbers of patients become eligible for OLT, therapeutic interventions aimed at the innate immune response and mechanisms that enhance viral persistence may provide novel means by which to control posttransplant HCV infections and to extend allograft survival.
Acknowledgments
Financial support. This work was supported by the National Institutes of Health (training grant 5T32AI007529) to A.N.
Potential conflicts of interest. J.A.F. is a consultant and holds stock options for Primera Dx and is also a consultant for Elan Pharmaceuticals.
References
- 1.McCashland TM. Management of liver transplant recipients with recurrent hepatitis C. Curr Opin Organ Transplant. 2009;14:221–4. doi: 10.1097/MOT.0b013e32832ade76. [DOI] [PubMed] [Google Scholar]
- 2.Gane EJ, Portmann BC, Naoumov NV, et al. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996;334:815–20. doi: 10.1056/NEJM199603283341302. [DOI] [PubMed] [Google Scholar]
- 3.Feray C, Caccamo L, Alexander GJ, et al. European collaborative study on factors influencing outcome after liver transplantation for hepatitis C. Gastroenterology. 1999;117:619–25. doi: 10.1016/s0016-5085(99)70454-3. [DOI] [PubMed] [Google Scholar]
- 4.Forman LM, Lewis JD, Berlin JA, Feldman HI, Lucey MR. The association between hepatitis C infection and survival after orthotopic liver transplantation. Gastroenterology. 2002;122:889–96. doi: 10.1053/gast.2002.32418. [DOI] [PubMed] [Google Scholar]
- 5.Thuluvath PJ, Krok KL, Segev DL, Yoo HY. Trends in post-liver transplant survival in patients with hepatitis C between 1991 and 2001 in the United States. Liver Transpl. 2007;13:719–24. doi: 10.1002/lt.21123. [DOI] [PubMed] [Google Scholar]
- 6.McCaughan GW, Shackel NA, Strasser SI, Dilworth P, Tang P. Minimal but significant improvement in survival for non-hepatitis C-related adult liver transplant patients beyond the one-year posttransplant mark. Liver Transpl. 2010;16:130–7. doi: 10.1002/lt.21978. [DOI] [PubMed] [Google Scholar]
- 7.Neumann UP, Berg T, Bahra M, et al. Long-term outcome of liver transplants for chronic hepatitis C: a 10-year follow-up. Transplantation. 2004;77:226–31. doi: 10.1097/01.TP.0000101738.27552.9D. [DOI] [PubMed] [Google Scholar]
- 8.Velidedeoglu E, Mange KC, Frank A, et al. Factors differentially correlated with the outcome of liver transplantation in HCV+ and HCV– recipients. Transplantation. 2004;77:1834–42. doi: 10.1097/01.tp.0000130468.36131.0d. [DOI] [PubMed] [Google Scholar]
- 9.McTaggart RA, Terrault NA, Vardanian AJ, Bostrom A, Feng S. Hepatitis C etiology of liver disease is strongly associated with early acute rejection following liver transplantation. Liver Transpl. 2004;10:975–85. doi: 10.1002/lt.20213. [DOI] [PubMed] [Google Scholar]
- 10.Gurusamy KS, Tsochatzis E, Xirouchakis E, Burroughs AK, Davidson BR. Antiviral therapy for recurrent liver graft infection with hepatitis C virus. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD006803.pub3. CD006803. [DOI] [PubMed] [Google Scholar]
- 11.Schirren CA, Jung MC, Gerlach JT, et al. Liver-derived hepatitis C virus (HCV)-specific CD4+ T cells recognize multiple HCV epitopes and produce interferon gamma. Hepatology. 2000;32:597–603. doi: 10.1053/jhep.2000.9635. [DOI] [PubMed] [Google Scholar]
- 12.Missale G, Bertoni R, Lamonaca V, et al. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J Clin Invest. 1996;98:706–14. doi: 10.1172/JCI118842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diepolder HM, Zachoval R, Hoffmann RM, et al. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet. 1995;346:1006–7. doi: 10.1016/s0140-6736(95)91691-1. [DOI] [PubMed] [Google Scholar]
- 14.Aberle JH, Formann E, Steindl-Munda P, et al. Prospective study of viral clearance and CD4+ T-cell response in acute hepatitis C primary infection and reinfection. J Clin Virol. 2006;36:24–31. doi: 10.1016/j.jcv.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 15.Lechner F, Wong DK, Dunbar PR, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerlach JT, Diepolder HM, Jung MC, et al. Recurrence of hepatitis C virus after loss of virus-specific CD4+ T-cell response in acute hepatitis C. Gastroenterology. 1999;117:933–41. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- 17.Takaki A, Wiese M, Maertens G, et al. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578–82. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- 18.Chang KM, Thimme R, Melpolder JJ, et al. Differential CD4+ and CD8+ T-cell responsiveness in hepatitis C virus infection. Hepatology. 2001;33:267–76. doi: 10.1053/jhep.2001.21162. [DOI] [PubMed] [Google Scholar]
- 19.Urbani S, Amadei B, Fisicaro P, et al. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology. 2006;44:126–39. doi: 10.1002/hep.21242. [DOI] [PubMed] [Google Scholar]
- 20.Spangenberg HC, Viazov S, Kersting N, et al. Intrahepatic CD8+ T-cell failure during chronic hepatitis C virus infection. Hepatology. 2005;42:828–37. doi: 10.1002/hep.20856. [DOI] [PubMed] [Google Scholar]
- 21.Mancini N, Diotti RA, Perotti M, et al. Hepatitis C virus (HCV) infection may elicit neutralizing antibodies targeting epitopes conserved in all viral genotypes. PLoS One. 2009;4:e8254. doi: 10.1371/journal.pone.0008254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan DE, Sugimoto K, Newton K, et al. Discordant role of CD4 T-cell response relative to neutralizing antibody and CD8 T-cell responses in acute hepatitis C. Gastroenterology. 2007;132:654–66. doi: 10.1053/j.gastro.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 23.Gruener NH, Jung MC, Ulsenheimer A, et al. Analysis of a successful HCV-specific CD8+ T cell response in patients with recurrent HCV-infection after orthotopic liver transplantation. Liver Transpl. 2004;10:1487–96. doi: 10.1002/lt.20300. [DOI] [PubMed] [Google Scholar]
- 24.Weston SJ, Leistikow RL, Reddy KR, et al. Reconstitution of hepatitis C virus–specific T-cell–mediated immunity after liver transplantation. Hepatology. 2005;41:72–81. doi: 10.1002/hep.20507. [DOI] [PubMed] [Google Scholar]
- 25.Schirren CA, Zachoval R, Gerlach JT, et al. Antiviral treatment of recurrent hepatitis C virus (HCV) infection after liver transplantation: association of a strong, multispecific, and long-lasting CD4+ T cell response with HCV-elimination. J Hepatol. 2003;39:397–404. doi: 10.1016/s0168-8278(03)00266-6. [DOI] [PubMed] [Google Scholar]
- 26.Rosen HR, Hinrichs DJ, Gretch DR, et al. Association of multispecific CD4+ response to hepatitis C and severity of recurrence after liver transplantation. Gastroenterology. 1999;117:926–32. doi: 10.1016/s0016-5085(99)70352-5. [DOI] [PubMed] [Google Scholar]
- 27.Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosen HR, Hinrichs DJ, Leistikow RL, et al. Cutting edge: identification of hepatitis C virus-specific CD8+ T cells restricted by donor HLA alleles following liver transplantation. J Immunol. 2004;173:5355–9. doi: 10.4049/jimmunol.173.9.5355. [DOI] [PubMed] [Google Scholar]
- 29.Kakumu S, Takayanagi M, Iwata K, et al. Cyclosporine therapy affects aminotransferase activity but not hepatitis C virus RNA levels in chronic hepatitis C. J Gastroenterol Hepatol. 1997;12:62–6. doi: 10.1111/j.1440-1746.1997.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 30.Gokmen MR, Lombardi G, Lechler RI. The importance of the indirect pathway of allorecognition in clinical transplantation. Curr Opin Immunol. 2008;20:568–74. doi: 10.1016/j.coi.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Retortillo M, Forns X, Feliu A, et al. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology. 2002;35:680–7. doi: 10.1053/jhep.2002.31773. [DOI] [PubMed] [Google Scholar]
- 32.Powers KA, Ribeiro RM, Patel K, et al. Kinetics of hepatitis C virus reinfection after liver transplantation. Liver Transpl. 2006;12:207–16. doi: 10.1002/lt.20572. [DOI] [PubMed] [Google Scholar]
- 33.Abbate I, Lo Iacono O, Di Stefano R, et al. HVR-1 quasispecies modifications occur early and are correlated to initial but not sustained response in HCV-infected patients treated with pegylated- or standard-interferon and ribavirin. J Hepatol. 2004;40:831–6. doi: 10.1016/j.jhep.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 34.Arenas JI, Gallegos-Orozco JF, Laskus T, et al. Hepatitis C virus quasi-species dynamics predict progression of fibrosis after liver transplantation. J Infect Dis. 2004;189:2037–46. doi: 10.1086/386338. [DOI] [PubMed] [Google Scholar]
- 35.Lyra AC, Fan X, Lang DM, et al. Evolution of hepatitis C viral quasispecies after liver transplantation. Gastroenterology. 2002;123:1485–93. doi: 10.1053/gast.2002.36546. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan DG, Wilson JJ, Carithers RL, Jr., Perkins JD, Gretch DR. Multigene tracking of hepatitis C virus quasispecies after liver transplantation: correlation of genetic diversification in the envelope region with asymptomatic or mild disease patterns. J Virol. 1998;72:10036–43. doi: 10.1128/jvi.72.12.10036-10043.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez-Fueyo A, Gimenez-Barcons M, Puig-Basagoiti F, et al. Influence of the dynamics of the hypervariable region 1 of hepatitis C virus (HCV) on the histological severity of HCV recurrence after liver transplantation. J Med Virol. 2001;65:266–75. doi: 10.1002/jmv.2029. [DOI] [PubMed] [Google Scholar]
- 38.Doughty AL, Painter DM, McCaughan GW. Post-transplant quasispecies pattern remains stable over time in patients with recurrent cholestatic hepatitis due to hepatitis C virus. J Hepatol. 2000;32:126–34. doi: 10.1016/s0168-8278(00)80199-3. [DOI] [PubMed] [Google Scholar]
- 39.Pessoa MG, Bzowej N, Berenguer M, et al. Evolution of hepatitis C virus quasispecies in patients with severe cholestatic hepatitis after liver transplantation. Hepatology. 1999;30:1513–20. doi: 10.1002/hep.510300610. [DOI] [PubMed] [Google Scholar]
- 40.Ramirez S, Perez-del-Pulgar S, Carrion JA, et al. Hepatitis C virus superinfection of liver grafts: a detailed analysis of early exclusion of non-dominant virus strains. J Gen Virol. 2010;91:1183–8. doi: 10.1099/vir.0.018929-0. [DOI] [PubMed] [Google Scholar]
- 41.Hughes MG, Jr., Rudy CK, Chong TW, et al. E2 quasispecies specificity of hepatitis C virus association with allografts immediately after liver transplantation. Liver Transpl. 2004;10:208–16. doi: 10.1002/lt.20060. [DOI] [PubMed] [Google Scholar]
- 42.Ramirez S, Perez-Del-Pulgar S, Carrion JA, et al. Hepatitis C virus compartmentalization and infection recurrence after liver transplantation. Am J Transplant. 2009;9:1591–601. doi: 10.1111/j.1600-6143.2009.02666.x. [DOI] [PubMed] [Google Scholar]
- 43.Schramm F, Soulier E, Royer C, et al. Frequent compartmentalization of hepatitis C virus with leukocyte-related amino acids in the setting of liver transplantation. J Infect Dis. 2008;198:1656–66. doi: 10.1086/592986. [DOI] [PubMed] [Google Scholar]
- 44.Hughes MG, Jr., Chong TW, Smith RL, et al. HCV infection of the transplanted liver: changing CD81 and HVR1 variants immediately after liver transplantation. Am J Transplant. 2005;5:2504–13. doi: 10.1111/j.1600-6143.2005.01060.x. [DOI] [PubMed] [Google Scholar]
- 45.Schvoerer E, Soulier E, Royer C, et al. Early evolution of hepatitis C virus (HCV) quasispecies after liver transplant for HCV-related disease. J Infect Dis. 2007;196:528–36. doi: 10.1086/519691. [DOI] [PubMed] [Google Scholar]
- 46.Lyra AC, Fan X, Di Bisceglie AM. CD81 binding regions of hepatitis C virus remain conserved after liver transplantation. Braz J Infect Dis. 2004;8:126–32. [PubMed] [Google Scholar]
- 47.Kuijpers TW, Baars PA, Dantin C, van den Burg M, van Lier RA, Roosnek E. Human NK cells can control CMV infection in the absence of T cells. Blood. 2008;112:914–5. doi: 10.1182/blood-2008-05-157354. [DOI] [PubMed] [Google Scholar]
- 48.Sullivan JL, Byron KS, Brewster FE, Purtilo DT. Deficient natural killer cell activity in X-linked lymphoproliferative syndrome. Science. 1980;210:543–5. doi: 10.1126/science.6158759. [DOI] [PubMed] [Google Scholar]
- 49.Harada S, Bechtold T, Seeley JK, Purtilo DT. Cell-mediated immunity to Epstein-Barr virus (EBV) and natural killer (NK)-cell activity in the X-linked lymphoproliferative syndrome. Int J Cancer. 1982;30:739–44. doi: 10.1002/ijc.2910300610. [DOI] [PubMed] [Google Scholar]
- 50.Fleisher G, Starr S, Koven N, Kamiya H, Douglas SD, Henle W. A non-X-linked syndrome with susceptibility to severe Epstein-Barr virus infections. J Pediatr. 1982;100:727–30. doi: 10.1016/s0022-3476(82)80572-6. [DOI] [PubMed] [Google Scholar]
- 51.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–5. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 52.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 53.Biron CA, Brossay L. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol. 2001;13:458–64. doi: 10.1016/s0952-7915(00)00241-7. [DOI] [PubMed] [Google Scholar]
- 54.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–74. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 55.Amadei B, Urbani S, Cazaly A, et al. Activation of natural killer cells during acute infection with hepatitis C virus. Gastroenterology. 2010;138:1536–45. doi: 10.1053/j.gastro.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee S, Watson MW, Flexman JP, Cheng W, Hammond T, Price P. Increased proportion of the CD56(bright) NK cell subset in patients chronically infected with hepatitis C virus (HCV) receiving interferon-alpha and ribavirin therapy. J Med Virol. 2010;82:568–74. doi: 10.1002/jmv.21742. [DOI] [PubMed] [Google Scholar]
- 57.Meier UC, Owen RE, Taylor E, et al. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol. 2005;79:12365–74. doi: 10.1128/JVI.79.19.12365-12374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawarabayashi N, Seki S, Hatsuse K, et al. Decrease of CD56+ T cells and natural killer cells in cirrhotic livers with hepatitis C may be involved in their susceptibility to hepatocellular carcinoma. Hepatology. 2000;32:962–9. doi: 10.1053/jhep.2000.19362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dessouki O, Kamiya Y, Nagahama H, et al. Chronic hepatitis C viral infection reduces NK cell frequency and suppresses cytokine secretion: reversion by anti-viral treatment. Biochem Biophys Res Commun. 2010;393:331–7. doi: 10.1016/j.bbrc.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 60.Gale M, Jr., Foy EM. Evasion of intracellular host defence by hepatitis C virus. Nature. 2005;436:939–45. doi: 10.1038/nature04078. [DOI] [PubMed] [Google Scholar]
- 61.Feld JJ, Lutchman GA, Heller T, et al. Ribavirin improves early responses to peginterferon through improved interferon signaling. Gastroenterology. 2010;139(1):154–62.e4. doi: 10.1053/j.gastro.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–9. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- 63.Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100–4. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- 64.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 65.Kubota A, Kubota S, Farrell HE, Davis-Poynter N, Takei F. Inhibition of NK cells by murine CMV-encoded class I MHC homologue m144. Cell Immunol. 1999;191:145–51. doi: 10.1006/cimm.1998.1424. [DOI] [PubMed] [Google Scholar]
- 66.Chalupny NJ, Rein-Weston A, Dosch S, Cosman D. Down-regulation of the NKG2D ligand MICA by the human cytomegalovirus glycoprotein UL142. Biochem Biophys Res Commun. 2006;346:175–81. doi: 10.1016/j.bbrc.2006.05.092. [DOI] [PubMed] [Google Scholar]
- 67.Dunn C, Chalupny NJ, Sutherland CL, et al. Human cytomegalovirus glycoprotein UL16 causes intracellular sequestration of NKG2D ligands, protecting against natural killer cell cytotoxicity. J Exp Med. 2003;197:1427–39. doi: 10.1084/jem.20022059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stern-Ginossar N, Elefant N, Zimmermann A, et al. Host immune system gene targeting by a viral miRNA. Science. 2007;317:376–81. doi: 10.1126/science.1140956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mao H, Tu W, Liu Y, et al. Inhibition of human natural killer cell activity by influenza virions and hemagglutinin. J Virol. 2010;84:4148–57. doi: 10.1128/JVI.02340-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wen C, He X, Ma H, et al. Hepatitis C virus infection downregulates the ligands of the activating receptor NKG2D. Cell Mol Immunol. 2008;5:475–8. doi: 10.1038/cmi.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herzer K, Falk CS, Encke J, et al. Upregulation of major histocompatibility complex class I on liver cells by hepatitis C virus core protein via p53 and TAP1 impairs natural killer cell cytotoxicity. J Virol. 2003;77:8299–309. doi: 10.1128/JVI.77.15.8299-8309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Horner SM, Gale M., Jr Intracellular innate immune cascades interferon defenses that control hepatitis C virus. J Interferon Cytokine Res. 2009;29:489–98. doi: 10.1089/jir.2009.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tseng CT, Klimpel GR. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J Exp Med. 2002;195:43–9. doi: 10.1084/jem.20011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Crotta S, Stilla A, Wack A, et al. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J Exp Med. 2002;195:35–41. doi: 10.1084/jem.20011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoon JC, Shiina M, Ahlenstiel G, Rehermann B. Natural killer cell function is intact after direct exposure to infectious hepatitis C virions. Hepatology. 2009;49:12–21. doi: 10.1002/hep.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Crotta S, Brazzoli M, Piccioli D, Valiante NM, Wack A. Hepatitis C virions subvert natural killer cell activation to generate a cytokine environment permissive for infection. J Hepatol. 2010;52:183–90. doi: 10.1016/j.jhep.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 77.Boyton RJ, Altmann DM. Natural killer cells, killer immunoglobulin-like receptors and human leucocyte antigen class I in disease. Clin Exp Immunol. 2007;149:1–8. doi: 10.1111/j.1365-2249.2007.03424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Valiante NM, Uhrberg M, Shilling HG, et al. Functionally and structurally and distinct NK cell receptor repertoires in the peripheral blood of two human donors. Immunity. 1997;7:739–51. doi: 10.1016/s1074-7613(00)80393-3. [DOI] [PubMed] [Google Scholar]
- 79.Dimasi N, Biassoni R. Structural and functional aspects of the Ly49 natural killer cell receptors. Immunol Cell Biol. 2005;83:1–8. doi: 10.1111/j.1440-1711.2004.01301.x. [DOI] [PubMed] [Google Scholar]
- 80.Michaelsson J, Teixeira de Matos C, Achour A, Lanier LL, Karre K, Soderstrom K. A signal peptide derived from hsp60 binds HLA-E and interferes with CD94/NKG2A recognition. J Exp Med. 2002;196:1403–14. doi: 10.1084/jem.20020797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Watzl C. The NKG2D receptor and its ligands—recognition beyond the "missing self"? Microbes Infect. 2003;5:31–7. doi: 10.1016/s1286-4579(02)00057-6. [DOI] [PubMed] [Google Scholar]
- 82.Tieng V, Le Bouguenec C, du Merle L, et al. Binding of Escherichia coli adhesin AfaE to CD55 triggers cell-surface expression of the MHC class I-related molecule MICA. Proc Natl Acad Sci U S A. 2002;99:2977–82. doi: 10.1073/pnas.032668099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Groh V, Bahram S, Bauer S, Herman A, Beauchamp M, Spies T. Cell stress-regulated human major histocompatibility complex class I gene expressed in gastrointestinal epithelium. Proc Natl Acad Sci U S A. 1996;93:12445–50. doi: 10.1073/pnas.93.22.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gerosa F, Gobbi A, Zorzi P, et al. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol. 2005;174:727–34. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- 85.Ferlazzo G, Pack M, Thomas D, et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci U S A. 2004;101:16606–11. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ralainirina N, Poli A, Michel T, et al. Control of NK cell functions by CD4+ CD25+ regulatory T cells. J Leukoc Biol. 2007;81:144–53. doi: 10.1189/jlb.0606409. [DOI] [PubMed] [Google Scholar]
- 87.Terme M, Chaput N, Combadiere B, Ma A, Ohteki T, Zitvogel L. Regulatory T cells control dendritic cell/NK cell cross-talk in lymph nodes at the steady state by inhibiting CD4+ self-reactive T cells. J Immunol. 2008;180:4679–86. doi: 10.4049/jimmunol.180.7.4679. [DOI] [PubMed] [Google Scholar]
- 88.Stegmann KA, Bjorkstrom NK, Veber H, et al. Interferon-alpha-induced TRAIL on natural killer cells is associated with control of hepatitis C virus infection. Gastroenterology. 2010;138:1885–97. doi: 10.1053/j.gastro.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 89.Lassen MG, Lukens JR, Dolina JS, Brown MG, Hahn YS. Intrahepatic IL-10 maintains NKG2A+Ly49– liver NK cells in a functionally hyporesponsive state. J Immunol. 2010;184:2693–701. doi: 10.4049/jimmunol.0901362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tay CH, Welsh RM. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J Virol. 1997;71:267–75. doi: 10.1128/jvi.71.1.267-275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Loh J, Chu DT, O'Guin AK, Yokoyama WM, Virgin HW., IV Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver. J Virol. 2005;79:661–7. doi: 10.1128/JVI.79.1.661-667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Karadimitris A, Gadola S, Altamirano M, et al. Human CD1d-glycolipid tetramers generated by in vitro oxidative refolding chromatography. Proc Natl Acad Sci U S A. 2001;98:3294–8. doi: 10.1073/pnas.051604498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khakoo SI, Thio CL, Martin MP, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–4. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 94.Romero V, Azocar J, Zuniga J, et al. Interaction of NK inhibitory receptor genes with HLA-C and MHC class II alleles in hepatitis C virus infection outcome. Mol Immunol. 2008;45:2429–36. doi: 10.1016/j.molimm.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Knapp S, Warshow U, Hegazy D, et al. Consistent beneficial effects of killer cell immunoglobulin-like receptor and 2DL3 and group 1 human leukocyte antigen-C following exposure to hepatitis C virus. Hepatology. 2010;51:1168–75. doi: 10.1002/hep.23477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vidal-Castineira JR, Lopez-Vazquez A, Diaz-Pena R, et al. Effect of killer immunoglobulin-like receptors in the response to combined treatment in patients with chronic hepatitis C virus infection. J Virol. 2010;84:475–81. doi: 10.1128/JVI.01285-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Arias AE, Haworth SE, Belli LS, et al. Killer cell immunoglobulin-like receptor genotype and killer cell immunoglobulin-like receptor-human leukocyte antigen C ligand compatibility affect the severity of hepatitis C virus recurrence after liver transplantation. Liver Transpl. 2009;15:390–9. doi: 10.1002/lt.21673. [DOI] [PubMed] [Google Scholar]
- 98.Askar M, Avery R, Corey R, et al. Lack of killer immunoglobulin-like receptor 2DS2 (KIR2DS2) and KIR2DL2 is associated with poor responses to therapy of recurrent hepatitis C virus in liver transplant recipients. Liver Transpl. 2009;15:1557–63. doi: 10.1002/lt.21878. [DOI] [PubMed] [Google Scholar]
- 99.Strong RK, Holmes MA, Li P, Braun L, Lee N, Geraghty DE. HLA-E allelic variants: correlating differential expression, peptide affinities, crystal structures, and thermal stabilities. J Biol Chem. 2003;278:5082–90. doi: 10.1074/jbc.M208268200. [DOI] [PubMed] [Google Scholar]
- 100.Schulte D, Vogel M, Langhans B, et al. The HLA-E(R)/HLA-E(R) genotype affects the natural course of hepatitis C virus (HCV) infection and is associated with HLA-E-restricted recognition of an HCV-derived peptide by interferon-gamma-secreting human CD8+ T cells. J Infect Dis. 2009;200:1397–401. doi: 10.1086/605889. [DOI] [PubMed] [Google Scholar]
- 101.Dolganiuc A, Szabo G. T cells with regulatory activity in hepatitis C virus infection: what we know and what we don't. J Leukoc Biol. 2008;84:614–22. doi: 10.1189/jlb.1107770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jinushi M, Takehara T, Tatsumi T, et al. Natural killer cell and hepatic cell interaction via NKG2A leads to dendritic cell-mediated induction of CD4 CD25 T cells with PD-1-dependent regulatory activities. Immunology. 2007;120:73–82. doi: 10.1111/j.1365-2567.2006.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ward SM, Fox BC, Brown PJ, et al. Quantification and localisation of FOXP3+ T lymphocytes and relation to hepatic inflammation during chronic HCV infection. J Hepatol. 2007;47:316–24. doi: 10.1016/j.jhep.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 104.Ciuffreda D, Codarri L, Buhler L, et al. Hepatitis C virus infection after liver transplantation is associated with lower levels of activated CD4+CD25+CD45RO+IL-7ralphahigh T cells. Liver Transpl. 2010;16:49–55. doi: 10.1002/lt.21959. [DOI] [PubMed] [Google Scholar]
- 105.Carpentier A, Conti F, Stenard F, et al. Increased expression of regulatory Tr1 cells in recurrent hepatitis C after liver transplantation. Am J Transplant. 2009;9:2102–12. doi: 10.1111/j.1600-6143.2009.02743.x. [DOI] [PubMed] [Google Scholar]
- 106.Smyk-Pearson S, Golden-Mason L, Klarquist J, et al. Functional suppression by FoxP3+CD4+CD25high regulatory T cells during acute hepatitis C virus infection. J Infect Dis. 2008;197:46–57. doi: 10.1086/523651. [DOI] [PubMed] [Google Scholar]
- 107.Bain C, Fatmi A, Zoulim F, Zarski JP, Trepo C, Inchauspe G. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512–24. doi: 10.1053/gast.2001.21212. [DOI] [PubMed] [Google Scholar]
- 108.Navas MC, Fuchs A, Schvoerer E, Bohbot A, Aubertin AM, Stoll-Keller F. Dendritic cell susceptibility to hepatitis C virus genotype 1 infection. J Med Virol. 2002;67:152–61. doi: 10.1002/jmv.2204. [DOI] [PubMed] [Google Scholar]
- 109.Kanto T, Inoue M, Miyatake H, et al. Reduced numbers and impaired ability of myeloid and plasmacytoid dendritic cells to polarize T helper cells in chronic hepatitis C virus infection. J Infect Dis. 2004;190:1919–26. doi: 10.1086/425425. [DOI] [PubMed] [Google Scholar]
- 110.Saito K, Ait-Goughoulte M, Truscott SM, et al. Hepatitis C virus inhibits cell surface expression of HLA-DR, prevents dendritic cell maturation, and induces interleukin-10 production. J Virol. 2008;82:3320–8. doi: 10.1128/JVI.02547-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shiina M, Rehermann B. Cell culture-produced hepatitis C virus impairs plasmacytoid dendritic cell function. Hepatology. 2008;47:385–95. doi: 10.1002/hep.21996. [DOI] [PubMed] [Google Scholar]
- 112.Anthony DD, Yonkers NL, Post AB, et al. Selective impairments in dendritic cell-associated function distinguish hepatitis C virus and HIV infection. J Immunol. 2004;172:4907–16. doi: 10.4049/jimmunol.172.8.4907. [DOI] [PubMed] [Google Scholar]
- 113.Ulsenheimer A, Gerlach JT, Jung MC, et al. Plasmacytoid dendritic cells in acute and chronic and hepatitis C virus infection. Hepatology. 2005;41:643–51. doi: 10.1002/hep.20592. [DOI] [PubMed] [Google Scholar]
- 114.Jinushi M, Takehara T, Kanto T, et al. Critical role of MHC class I-related chain A and B expression on IFN-alpha-stimulated dendritic cells in NK cell activation: impairment in chronic hepatitis C virus infection. J Immunol. 2003;170:1249–56. doi: 10.4049/jimmunol.170.3.1249. [DOI] [PubMed] [Google Scholar]
- 115.Lunz JG, 3rd, Specht SM, Murase N, Isse K, Demetris AJ. Gut-derived commensal bacterial products inhibit liver dendritic cell maturation by stimulating hepatic interleukin-6/signal transducer and activator of transcription 3 activity. Hepatology. 2007;46:1946–59. doi: 10.1002/hep.21906. [DOI] [PubMed] [Google Scholar]
- 116.Bertolino P, McCaughan GW, Bowen DG. Role of primary intrahepatic T-cell activation in the 'liver tolerance effect'. Immunol Cell Biol. 2002;80:84–92. doi: 10.1046/j.0818-9641.2001.01048.x. [DOI] [PubMed] [Google Scholar]
- 117.Benseler V, McCaughan GW, Schlitt HJ, Bishop GA, Bowen DG, Bertolino P. The liver: a special case in transplantation tolerance. Semin Liver Dis. 2007;27:194–213. doi: 10.1055/s-2007-979471. [DOI] [PubMed] [Google Scholar]
- 118.Golden-Mason L, Rosen HR. Natural killer cells: primary target for hepatitis C virus immune evasion strategies? Liver Transpl. 2006;12:363–72. doi: 10.1002/lt.20708. [DOI] [PubMed] [Google Scholar]
- 119.Ohira M, Ishiyama K, Tanaka Y, et al. Adoptive immunotherapy with liver allograft-derived lymphocytes induces anti-HCV activity after liver transplantation in humans and humanized mice. J Clin Invest. 2009;119:3226–35. doi: 10.1172/JCI38374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ruggeri L, Capanni M, Casucci M, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94:333–9. [PubMed] [Google Scholar]
- 121.Ruggeri L, Mancusi A, Capanni M, et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: challenging its predictive value. Blood. 2007;110:433–40. doi: 10.1182/blood-2006-07-038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Willemze R, Rodrigues CA, Labopin M, et al. KIR-ligand incompatibility in the graft-versus-host direction improves outcomes after umbilical cord blood transplantation for acute leukemia. Leukemia. 2009;23:492–500. doi: 10.1038/leu.2008.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pende D, Marcenaro S, Falco M, et al. Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood. 2009;113:3119–29. doi: 10.1182/blood-2008-06-164103. [DOI] [PubMed] [Google Scholar]
- 124.Vago L, Forno B, Sormani MP, et al. Temporal, quantitative, and functional characteristics of single-KIR-positive alloreactive natural killer cell recovery account for impaired graft-versus-leukemia activity after haploidentical hematopoietic stem cell transplantation. Blood. 2008;112:3488–99. doi: 10.1182/blood-2007-07-103325. [DOI] [PubMed] [Google Scholar]
- 125.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 126.Romagne F, Andre P, Spee P, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009;114:2667–77. doi: 10.1182/blood-2009-02-206532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Godal R, Bachanova V, Gleason M, et al. Natural killer cell killing of acute myelogenous leukemia and acute lymphoblastic leukemia blasts by killer cell immunoglobulin-like receptor-negative natural killer cells after NKG2A and LIR-1 blockade. Biol Blood Marrow Transplant. 2010;16:612–21. doi: 10.1016/j.bbmt.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]