Abstract
Adults who have recovered from an episode of invasive pneumococcal disease demonstrate defective B cell activation in response to αδ-dex, a polyclonal polysaccharide mimic, compared with matched control subjects. The defect is not overcome by CD4+ T cell assistance and may explain the relatively poor response to pneumococcal vaccination in survivors of invasive pneumococcal disease.
Invasive pneumococcal disease (IPD) is characterized by isolation of Streptococcus pneumoniae from normally sterile sites, including the bloodstream, and generally occurs in individuals at extremes of age and those with relatively specific immunological impairment, including inherited or acquired defects of complement pathways [1], splenic function [2], or inter- or intracellular signaling [3]. However, a significant proportion of adults who develop invasive infection have no apparent pre-existing risk factor [4].
Patients who have recovered from IPD have relatively impaired antibody responses to S. pneumoniae polysaccharide vaccination (PPV) [5, 6]. Capsular polysaccharide is a thymus-independent type 2 (TI-2) antigen; thus, PPV-induced antibody and a major component of natural immunity to IPD is provided by a TI-2 response. We therefore postulated that in vitro challenge with the TI-2 antigenic mimic αδ-dex might unmask a subtle underlying defect in this pathway in patients who have experienced IPD. αδ-dex is composed of anti-IgD monoclonal antibodies conjugated to a dextran backbone and, used experimentally ex vivo, results in polyclonal stimulation of B cells. This technique overcame the need to assess responses of extremely rare serotype-specific cells, which may have been altered by IPD [5, 7, 8].
PARTICIPANTS AND METHODS
Adult volunteers (aged 18–65 years) who had successfully recuperated from pneumococcal bacteraemia and age-, sex-, and ethnicity-matched control subjects were recruited to a case-control study. This study was approved by the United Kingdom National Research Ethics Service (2008, 08/H1302/7). All identifiable data were anonymized before inclusion in a study database. Case patients were identified by documented episodes of pneumococcal bacteremia with use of microbiology records held at Sheffield Teaching Hospitals NHS Foundation Trust. The sample size required to detect a significant difference in response (α = 0.05; 1-β=80%) was determined a priori to be 10. Case patients were included irrespective of infecting S. pneumoniae serotype or prior and/or subsequent pneumococcal vaccination and were in good physical health at the time of venepuncture. Exclusion criteria included known and/or probable immunosuppression resulting from disease (including HIV infection, current or previous cancer, or conditions causing hyposplenism) or treatment (including steroids, chemotherapy, or immunomodulatory agents).
Control subjects were selected from healthy hospital and university employees who were unrelated to but age, sex, and ethnicity matched to each case patient. After detailed explanation of the study and provision of informed consent, a medical history was obtained and venepuncture was performed. Blood samples from each case patient and control subject were processed in parallel at arrival in the laboratory.
Peripheral blood mononuclear cells (PBMCs) were isolated from 50 mL of heparinized fresh blood by centrifugation with lymphocyte separation media (BioWhittaker). Polyclonal stimulation of B and T cells was subsequently performed using αδ-dex, plate-bound anti-CD3 or plate-bound anti-CD3, and anti-CD28 in combination, as described elsewhere [8]. Cells were incubated at 37°C for 96 h before harvesting. Activation and proliferation of B and T cells was measured by flow cytometry (LSRII; BD) using carboxyfluoroscein succinimidyl ester (CFSE; Invitrogen) and the following fluorochrome conjugated antibodies: CD14 pacific blue, CD25 phycoerythrin (PE), CD86 PE-alexafluor700, CD19 PE-Cy7, and CD4 allophycocyanin (all Caltag-Medsystems). Nonviable cells and monocytes were excluded on the basis of UV Live/Dead staining (Invitrogen) or CD14 expression, respectively.
Activation marker levels were assessed using median fluorescence intensity of the relevant fluorochrome. CFSE proliferation was measured using a proliferation analysis platform (FlowJo; Treestar) to calculate the divisional index (mean number of divisions undertaken by each cell).
Case patients recruited who had not received 23-valent pneumococcal polysaccharide (23-PPV) vaccination after their bacteremic episode were advised to consult their primary care physician in accordance with current UK recommendations.
Differences between paired case patients and control subjects were assessed by analysis of variance with use of a posttest Bonferroni correction for multiple analyses.
RESULTS
Ten healthy adults who had recovered from an episode of IPD were initially recruited to the study; one was excluded from the final analysis, however, because of concurrent medical problems. The mean age of the remaining 9 case patients (5 women and 4 men) was 40.2 years (range, 18–63 years), and they were recruited a median of 56.5 weeks after the documented bacteremia episode (range, 17–106 weeks). The implicated S. pneumoniae serotypes were 1 (2 cases), 4 (1 case), 5 (3 cases), and 7F (2 cases); one was unknown. Two case patients had received 23-PPV subsequent to the IPD episode. With the exception of their admission for IPD, all case patients recruited were otherwise healthy; most had never smoked (n = 6) or had smoking history of <2 pack-years, seldom drank alcohol (median 1 iu/week), and had no previous medical or surgical history of note. None of the case patients had risk factors for or blood result anomalies suggestive of underlying immunodeficiency. Of interest, 4 of 9 case patients recalled a family history of severe pneumonia and/or chest infection in a first-degree relative; one mother (the case patient) was admitted to hospital contemporaneously with her daughter, who was also subsequently given a diagnosis of IPD, presumably as a result of parallel nasopharyngeal colonization events. One case patient reported a confirmed episode of meningococcal meningitis in an infant second-degree relative.
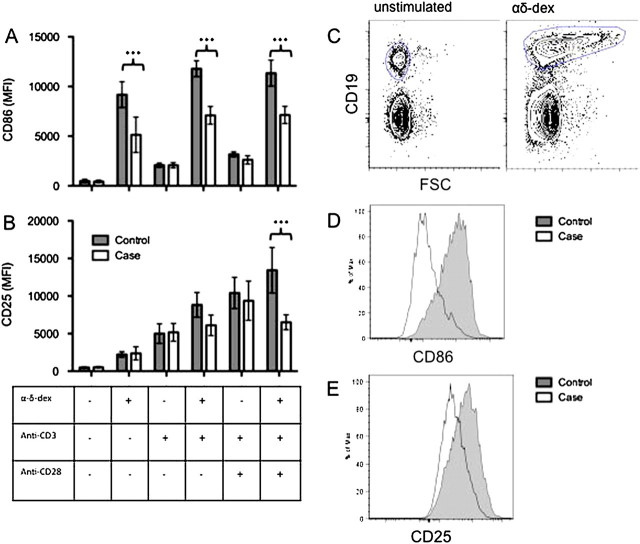
Case patients demonstrated a significantly reduced level of B cell activation in response to αδ-dex, in comparison with matched control subjetcs. This was manifest as a reduction in the expression of the level of the B cell activation marker CD86 (Figure 1a) and a parallel reduction in B cell CD25 expression (Figure 1b). The addition of plate-bound anti-CD3 and plate-bound anti-CD3/anti-CD28 did not overcome the defect seen in response to αδ-dex.
Figure 1.
PBMCs were extracted from cases patients and matched control subjects and stimulated for 96 h with platebound anti-CD3±anti-CD28 and/or the TI-2 antigen mimic, αδ-dex. Activation of CD19-expressing B-cells was measured by CD86 MFI of CD19+ cells (A) and CD25 MFI of CD19+ cells (B). C, Example gating strategy for B cells. D, Example histogram of CD86 expression. E, Example histogram of CD25 expression (n = 9). mean±SEM, ANOVA+ Bonferroni selected pairs posttest.*** P < .001.
There was no detected difference in B cell proliferation. No difference was detected in levels of T cell activation (CD25 expression) or proliferation (data not shown).
DISCUSSION
These data suggest that the peripheral blood B cells of otherwise healthy adults who have experienced an episode of IPD have a subtle, measurable defect in response to a TI-2 antigen mimic. The reduction in B cell activation was consistent across the 9 patients analyzed.
The primary role of CD86 expression by B cells is to provide a costimulatory signal to T cells via CD28 cross-linking. The reduced up-regulation of CD86 in response to anti-CD28 suggests that B cells from this patient group may be unable to cooperate effectively with T cells. Because T cell responses to anti-CD3 and anti-CD28 remained unchanged, it may be expected that, although responses to polysaccharide are inadequate in these individuals at high risk, they are not totally defective; thus, clinical protection from a conjugated-polysaccharide vaccine may be more effective. Impaired B cell expression of CD25 (the α chain of the IL-2 receptor) indicates a relative failure of B cells to respond to combined stimulation from αδ-dex and T cell help. Impaired B cell expression of CD86 and CD25 has also been observed in patients with type-1 common variable immunodeficiency [9]; none of our case patients were hypogammaglobulinaemic, had a history of known vaccine failure, or had any of the clinical features commonly associated with this condition. We previously demonstrated a similar finding in adults experiencing serogroup C meningococcal disease [8]; a deficiency in B cell response to αδ-dex, both without and with anti-CD3/anti-CD28, was seen, which was detected as reduced B cell proliferation rather than a reduction in B cell activation marker expression. However, the assay sensitivities would likely vary between the 2 studies with different operators and even different flow cytometers being used; thus, we cannot conclude that there is not a common defect among individuals who have experienced these 2 conditions, both characterized by bacteremia with encapsulated pathogens.
We cannot conclude definitively whether the defect observed is one that predates the IPD episode or is either a permanent or temporary legacy of it. Cumulative exposure to polysaccharide antigens has been postulated to result in eventual hyporesponsiveness to polysaccharide vaccination [10]; however, our stimulus mimics that of TI-2 antigens, such as S. pneumoniae polysaccharide, but is nonantigen specific, activating B cells polyclonally. The possibility described by O'Brien et al [10] is therefore not likely to be the cause of this finding; it appears to be more likely that this assay has identified a subtle polyclonal intrinsic defect that is a contributing factor in susceptibility to infection rather than the result of infection with S. pneumoniae. Both Neisseria meningitidis and S. pneumoniae invade the bloodstream after a period of asymptomatic colonization, during which a humoral immune response is normally initiated. Cross-sectional community carriage rates of the 2 organisms among adults is high (up to 35% for N. meningitidis [11] and 14% for S. pneumoniae [12]); however, the incidence of both invasive meningococcal disease and IPD are much lower than this. By the time an individual reaches adulthood, it is likely there has been cumulative immunizing exposure to both organisms; in the case of S. pneumoniae, nasopharyngeal colonization induces serotype-independent antibody responses [13]. By deduction, the occurrence of IPD in an adult can be a consequence of relatively poor humoral response to prior natural exposure. Therefore, it is possible that subtle B cell defects might underlie invasive disease caused by encapsulated bacteria, being permissive to translocation from the colonizing niche (nasopharynx) to the bloodstream.
The evidence presented here suggests that otherwise healthy adults [4] with a history of IPD have an underlying defect that manifests as relative failure of peripheral B cells to activate in response to TI-2 antigens. This may have implications for local and national vaccination guidelines [14]. Further work is needed to delineate the precise cause of this defective response.
Acknowledgments
We thank Marc Daigneault and David Dockrell for invaluable assistance and advice on measurement of apoptosis and Tim Mitchell for kindly providing pneumolysin.
Financial support. This work was supported by Colin Beattie Memorial Trust, National Institute for Health Research (T.D. Academic Clinical Fellowship), and the Meningitis Research Foundation (grant 0604).
Potential conflicts of interest. A.L. is employed by Fina BioSolutions. A.H. holds stock in and is a consultant for Adjuvantix, and his institution has received grant money on his behalf from Wellcome Trust, National Institutes of Health, Gates Foundation, and Adjuvantix. All other authors: no conflicts.
References
- 1.Jonsson G, Oxelius VA, Truedsson L, Braconier JH, Sturfelt G, Sjoholm AG. Homozygosity for the IgG2 subclass allotype G2M(n) protects against severe infection in hereditary C2 deficiency. J Immunol. 2006;177:722–8. doi: 10.4049/jimmunol.177.1.722. [DOI] [PubMed] [Google Scholar]
- 2.Ramakrishnan M, Moisi JC, Klugman KP, et al. Increased risk of invasive bacterial infections in African people with sickle-cell disease: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:329–37. doi: 10.1016/S1473-3099(10)70055-4. [DOI] [PubMed] [Google Scholar]
- 3.Ku CL, Picard C, Erdos M, et al. IRAK4 and NEMO mutations in otherwise healthy children with recurrent invasive pneumococcal disease. J Med Genet. 2007;44:16–23. doi: 10.1136/jmg.2006.044446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klemets P, Lyytikainen O, Ruutu P, Ollgren J, Pekka Nuorti J. Invasive pneumococcal infections among persons with and without underlying medical conditions: implications for prevention strategies. BMC Infect Dis. 2008;8:96. doi: 10.1186/1471-2334-8-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borrow R, Stanford E, Waight P, et al. Serotype-specific immune unresponsiveness to pneumococcal conjugate vaccine following invasive pneumococcal disease. Infect Immun. 2008;76:5305–9. doi: 10.1128/IAI.00796-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musher DM, Rueda AM, Nahm MH, Graviss EA, Rodriguez-Barradas MC. Initial and subsequent response to pneumococcal polysaccharide and protein-conjugate vaccines administered sequentially to adults who have recovered from pneumococcal pneumonia. J Infect Dis. 2008;198:1019–27. doi: 10.1086/591629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snapper CM, McIntyre TM, Mandler R, et al. Induction of IgG3 secretion by interferon gamma: a model for T cell-independent class switching in response to T cell-independent type 2 antigens. J Exp Med. 1992;175:1367–71. doi: 10.1084/jem.175.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster RA, Carlring J, McKendrick MW, et al. Evidence of a functional B-cell immunodeficiency in adults who experience serogroup C meningococcal disease. Clin Vaccine Immunol. 2009;16:692–8. doi: 10.1128/CVI.00485-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groth C, Drager R, Warnatz K, et al. Impaired up-regulation of CD70 and CD86 in naive (CD27-) B cells from patients with common variable immunodeficiency (CVID) Clin Exp Immunol. 2002;129:133–9. doi: 10.1046/j.1365-2249.2002.01883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Brien KL, Hochman M, Goldblatt D. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? Lancet Infect Dis. 2007;7:597–606. doi: 10.1016/S1473-3099(07)70210-4. [DOI] [PubMed] [Google Scholar]
- 11.Roberts J, Greenwood B, Stuart J. Sampling methods to detect carriage of Neisseria meningitidis; literature review. J Infect. 2009;58:103–7. doi: 10.1016/j.jinf.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Millar EV, Watt JP, Bronsdon MA, et al. Indirect effect of 7-valent pneumococcal conjugate vaccine on pneumococcal colonization among unvaccinated household members. Clin Infect Dis. 2008;47:989–96. doi: 10.1086/591966. [DOI] [PubMed] [Google Scholar]
- 13.Granat SM, Ollgren J, Herva E, Mia Z, Auranen K, Makela PH. Epidemiological evidence for serotype-independent acquired immunity to pneumococcal carriage. J Infect Dis. 2009;200:99–106. doi: 10.1086/599364. [DOI] [PubMed] [Google Scholar]
- 14.Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23) MMWR Morb Mortal Wkly Rep. 2010;59:1102–6. [PubMed] [Google Scholar]