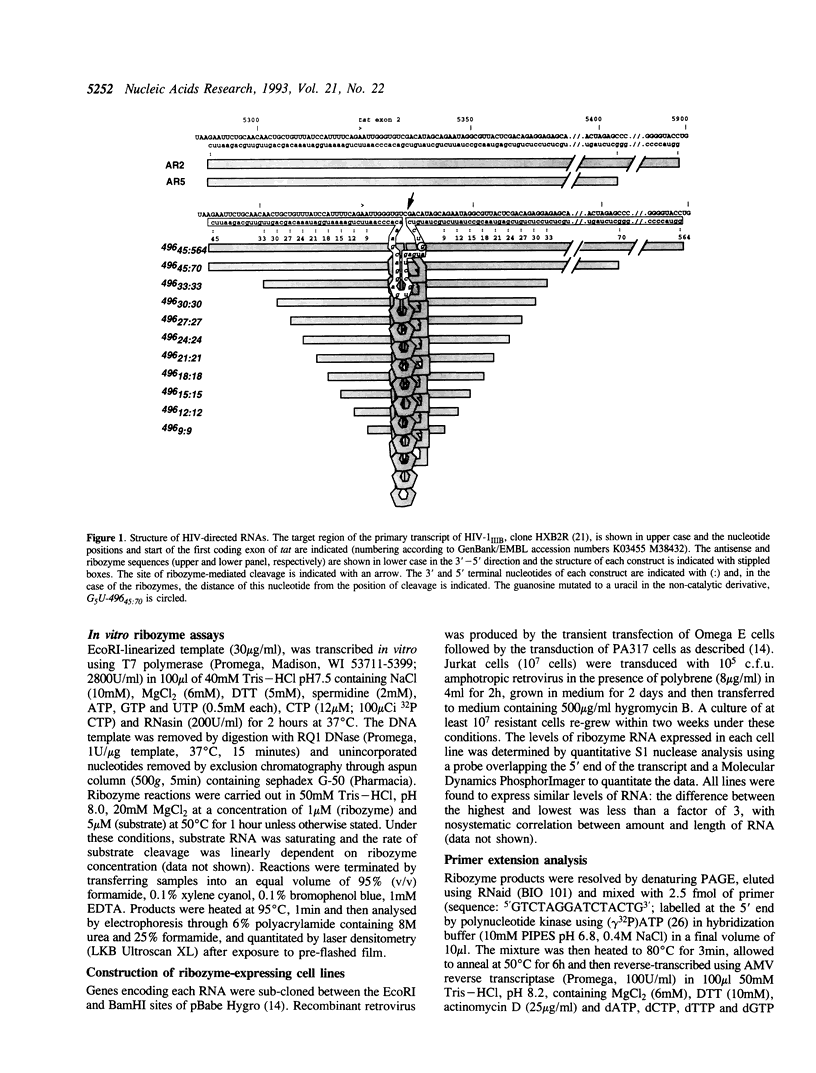
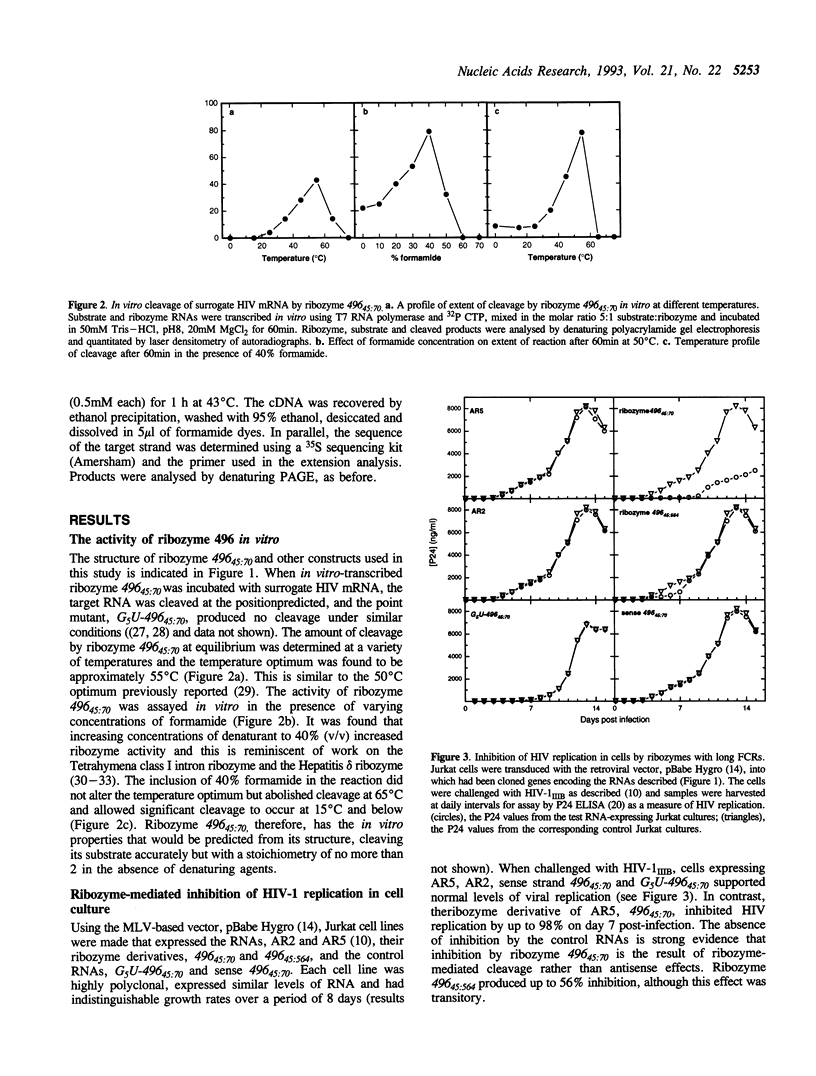
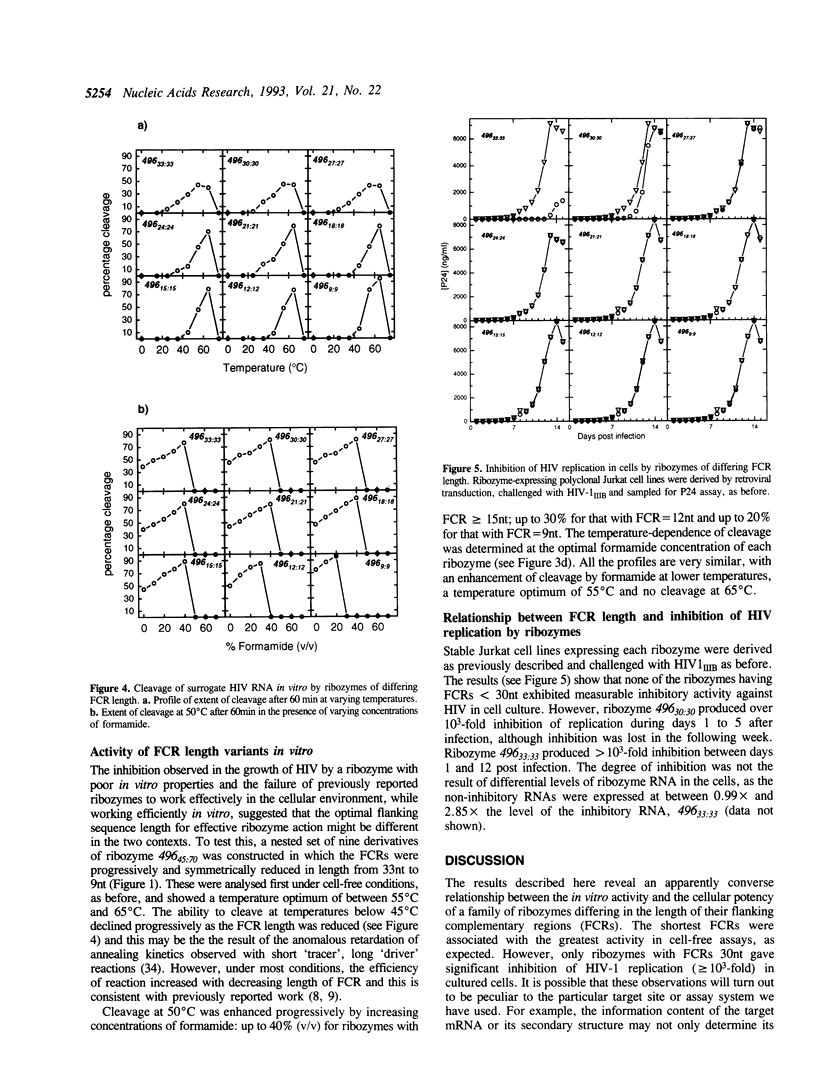
Abstract
Self-cleaving RNAs (ribozymes) can be engineered to cleave target RNAs of choice in a sequence-specific manner (1). Consequently, they could be used to inhibit virus replication or to analyse host gene function in vivo. However, ribozymes that are catalytic in vitro are generally disappointing when analysed in cells unless expressed at high levels relative to their target RNAs (2, 3). Here we provide evidence that this can be overcome by optimizing ribozyme structure using cellular rather than cell-free assays. We show that ribozymes of relatively long flanking complementary regions (FCRs), while poor catalysts in vitro, can produce profound inhibition of HIV replication in cells. By examining a series of ribozymes in which the FCRs vary from 9 to 564 nucleotides, we establish that the optimum length for activity in the cell is > or = 33 nucleotides.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buzayan J. M., Gerlach W. L., Bruening G. Satellite tobacco ringspot virus RNA: A subset of the RNA sequence is sufficient for autolytic processing. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8859–8862. doi: 10.1073/pnas.83.23.8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron F. H., Jennings P. A. Specific gene suppression by engineered ribozymes in monkey cells. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9139–9143. doi: 10.1073/pnas.86.23.9139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlin M. E., Galau G. A., Britten R. J., Davidson E. H. Studies on nucleic acid reassociation kinetics: V. Effects of disparity in tracer and driver fragment lengths. Nucleic Acids Res. 1978 Jun;5(6):2073–2094. doi: 10.1093/nar/5.6.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claude A., Arenas J., Hurwitz J. The isolation and characterization of an RNA helicase from nuclear extracts of HeLa cells. J Biol Chem. 1991 Jun 5;266(16):10358–10367. [PubMed] [Google Scholar]
- Collin M., James W., Gordon S. Development of techniques to analyse the formation of HIV provirus in primary human macrophages. Res Virol. 1991 Mar-Jun;142(2-3):105–112. doi: 10.1016/0923-2516(91)90045-5. [DOI] [PubMed] [Google Scholar]
- Cotten M., Birnstiel M. L. Ribozyme mediated destruction of RNA in vivo. EMBO J. 1989 Dec 1;8(12):3861–3866. doi: 10.1002/j.1460-2075.1989.tb08564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten M., Schaffner G., Birnstiel M. L. Ribozyme, antisense RNA, and antisense DNA inhibition of U7 small nuclear ribonucleoprotein-mediated histone pre-mRNA processing in vitro. Mol Cell Biol. 1989 Oct;9(10):4479–4487. doi: 10.1128/mcb.9.10.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedor M. J., Uhlenbeck O. C. Substrate sequence effects on "hammerhead" RNA catalytic efficiency. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1668–1672. doi: 10.1073/pnas.87.5.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodchild J., Kohli V. Ribozymes that cleave an RNA sequence from human immunodeficiency virus: the effect of flanking sequence on rate. Arch Biochem Biophys. 1991 Feb 1;284(2):386–391. doi: 10.1016/0003-9861(91)90313-8. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988 Aug 18;334(6183):585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- Hertel K. J., Pardi A., Uhlenbeck O. C., Koizumi M., Ohtsuka E., Uesugi S., Cedergren R., Eckstein F., Gerlach W. L., Hodgson R. Numbering system for the hammerhead. Nucleic Acids Res. 1992 Jun 25;20(12):3252–3252. doi: 10.1093/nar/20.12.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger J. A., Turner D. H., Zuker M. Improved predictions of secondary structures for RNA. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7706–7710. doi: 10.1073/pnas.86.20.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W., Crisell P., Rhodes A. Gene inhibition of HIV-1 replication. A comparative and mechanistic study. Ann N Y Acad Sci. 1992 Oct 28;660:274–275. doi: 10.1111/j.1749-6632.1992.tb21082.x. [DOI] [PubMed] [Google Scholar]
- Lo K. M., Biasolo M. A., Dehni G., Palú G., Haseltine W. A. Inhibition of replication of HIV-1 by retroviral vectors expressing tat-antisense and anti-tat ribozyme RNA. Virology. 1992 Sep;190(1):176–183. doi: 10.1016/0042-6822(92)91203-7. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern J. P., Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990 Jun 25;18(12):3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K., Yoo C., Kim U., Murray J. M., Estes P. A., Cash F. E., Liebhaber S. A. Substrate specificity of the dsRNA unwinding/modifying activity. EMBO J. 1991 Nov;10(11):3523–3532. doi: 10.1002/j.1460-2075.1991.tb04916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñol-Roma S., Choi Y. D., Matunis M. J., Dreyfuss G. Immunopurification of heterogeneous nuclear ribonucleoprotein particles reveals an assortment of RNA-binding proteins. Genes Dev. 1988 Feb;2(2):215–227. doi: 10.1101/gad.2.2.215. [DOI] [PubMed] [Google Scholar]
- Pontius B. W., Berg P. Renaturation of complementary DNA strands mediated by purified mammalian heterogeneous nuclear ribonucleoprotein A1 protein: implications for a mechanism for rapid molecular assembly. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8403–8407. doi: 10.1073/pnas.87.21.8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Rhodes A., James W. Inhibition of heterologous strains of HIV by antisense RNA. AIDS. 1991 Feb;5(2):145–151. doi: 10.1097/00002030-199102000-00003. [DOI] [PubMed] [Google Scholar]
- Rhodes A., James W. Inhibition of human immunodeficiency virus replication in cell culture by endogenously synthesized antisense RNA. J Gen Virol. 1990 Sep;71(Pt 9):1965–1974. doi: 10.1099/0022-1317-71-9-1965. [DOI] [PubMed] [Google Scholar]
- Rittner K., Sczakiel G. Identification and analysis of antisense RNA target regions of the human immunodeficiency virus type 1. Nucleic Acids Res. 1991 Apr 11;19(7):1421–1426. doi: 10.1093/nar/19.7.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein S. P., Been M. D. Evidence that genomic and antigenomic RNA self-cleaving elements from hepatitis delta virus have similar secondary structures. Nucleic Acids Res. 1991 Oct 11;19(19):5409–5416. doi: 10.1093/nar/19.19.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin S. Z., Markham P. D., Wong-Staal F., Franchini G., Kalyanaraman V. S., Gallo R. C. Restricted expression of human T-cell leukemia--lymphoma virus (HTLV) in transformed human umbilical cord blood lymphocytes. Virology. 1983 Aug;129(1):51–64. doi: 10.1016/0042-6822(83)90395-1. [DOI] [PubMed] [Google Scholar]
- Saxena S. K., Ackerman E. J. Ribozymes correctly cleave a model substrate and endogenous RNA in vivo. J Biol Chem. 1990 Oct 5;265(28):17106–17109. [PubMed] [Google Scholar]
- Sayers J. R., Schmidt W., Wendler A., Eckstein F. Strand specific cleavage of phosphorothioate-containing DNA by reaction with restriction endonucleases in the presence of ethidium bromide. Nucleic Acids Res. 1988 Feb 11;16(3):803–814. doi: 10.1093/nar/16.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J. H., Somoza C., Schockmel G. A., Collin M., Davis S. J., Williams A. F., James W. A rat CD4 mutant containing the gp120-binding site mediates human immunodeficiency virus type 1 infection. J Exp Med. 1993 Apr 1;177(4):949–954. doi: 10.1084/jem.177.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioud M., Natvig J. B., Førre O. Preformed ribozyme destroys tumour necrosis factor mRNA in human cells. J Mol Biol. 1992 Feb 20;223(4):831–835. doi: 10.1016/0022-2836(92)90244-e. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Dinter-Gottlieb G. Antigenomic Hepatitis delta virus ribozymes self-cleave in 18 M formamide. Nucleic Acids Res. 1991 Mar 25;19(6):1285–1289. doi: 10.1093/nar/19.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]
- Wagner R. W., Yoo C., Wrabetz L., Kamholz J., Buchhalter J., Hassan N. F., Khalili K., Kim S. U., Perussia B., McMorris F. A. Double-stranded RNA unwinding and modifying activity is detected ubiquitously in primary tissues and cell lines. Mol Cell Biol. 1990 Oct;10(10):5586–5590. doi: 10.1128/mcb.10.10.5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Shoback D., Stobo J. Role of T3 surface molecules in human T-cell activation: T3-dependent activation results in an increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4169–4173. doi: 10.1073/pnas.81.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. N., Lin Y. J., Lin F. P., Makino S., Chang M. F., Lai M. M. Human hepatitis delta virus RNA subfragments contain an autocleavage activity. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1831–1835. doi: 10.1073/pnas.86.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z., Whitton J. L. An anti-lymphocytic choriomeningitis virus ribozyme expressed in tissue culture cells diminishes viral RNA levels and leads to a reduction in infectious virus yield. J Virol. 1993 Apr;67(4):1840–1847. doi: 10.1128/jvi.67.4.1840-1847.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaug A. J., Grosshans C. A., Cech T. R. Sequence-specific endoribonuclease activity of the Tetrahymena ribozyme: enhanced cleavage of certain oligonucleotide substrates that form mismatched ribozyme-substrate complexes. Biochemistry. 1988 Dec 13;27(25):8924–8931. doi: 10.1021/bi00425a008. [DOI] [PubMed] [Google Scholar]
- Zuker M. On finding all suboptimal foldings of an RNA molecule. Science. 1989 Apr 7;244(4900):48–52. doi: 10.1126/science.2468181. [DOI] [PubMed] [Google Scholar]


