Abstract
Type I strains of Helicobacter pylori (Hp) possess a pathogenicity island, cag, that encodes the effector protein cytotoxin-associated gene A (CagA) and a type four secretion system. After translocation into the host cell, CagA affects cell shape, increases cell motility, abrogates junctional activity, and promotes an epithelial to mesenchymal transition-like phenotype. Transgenic expression of CagA enhances gastrointestinal and intestinal carcinomas as well as myeloid and B-cell lymphomas in mice, but the mechanism of the induced cancer formation is not fully understood. Here, we show that CagA subverts the tumor suppressor function of apoptosis-stimulating protein of p53 (ASPP2). Delivery of CagA inside the host results in its association with ASPP2. After this interaction, ASPP2 recruits its natural target p53 and inhibits its apoptotic function. CagA leads to enhanced degradation of p53 and thereby, down-regulates its activity in an ASPP2-dependent manner. Finally, Hp-infected cells treated with the p53-activating drug Doxorubicin are more resistant to apoptosis than uninfected cells, an effect that requires ASPP2. The interaction between CagA and ASPP2 and the consequent degradation of p53 are examples of a bacterial protein that subverts the p53 tumor suppressor pathway in a manner similar to DNA tumor viruses. This finding may contribute to the understanding of the increased risk of gastric cancer in patients infected with Hp CagA+ strains.
Keywords: microbial pathogenesis, type IV secretion
Host infection with Helicobacter pylori (Hp) is associated with pathology, including peptic ulcer diseases, gastric adenocarcinoma, and mucosal-associated lymphoid tissue (MALT) lymphoma. Hp type I strains encode a type four secretion system (1) that allows the bacterium to deliver the effector protein cytotoxin-associated gene A (CagA) directly inside the host cells (2–4). CagA is one of the most important factors that link infection with Hp to the development of gastric cancer. Mongolian gerbils challenged with type I Hp strains develop gastric dysplasia and adenocarcinoma 12 wk postinfection in a CagA-dependent manner (5).
On delivery, CagA localizes to the plasma membrane where the full-length protein interacts with components of the apical junctional complex of the cell, including ZO-1, Jam, and E-Cadherin (6–8). In addition, the tyrosines contained in the EPIYA (Glu-Pro-Ile-Tyr-Ala) motif of CagA are phosphorylated by c-Src/Lyn and Abl kinases (9, 10). This event activates a receptor tyrosine kinase (RTK) signaling cascade, which promotes the loss of cell polarity and enhances the invasiveness of the cell, both of which are reminiscent of an epithelial to mesenchymal transition (EMT) phenotype. The C-terminal and N-terminal domains of CagA are both required to exploit the full activity of the protein, although they have distinct functions (7).
Several proteins bind to the C terminus of CagA either in a phosphorylation-dependent (i.e., SHP-2, Grb-2, Csk, and Crk/L) (11–14) or -independent manner (i.e., Par-1 and c-Met) (15, 16). Expression of the C-terminal domain of CagA alone initiates RTK signaling and induces scattering of the cell. However, the junctional complexes of the cell remain intact, and the cells do not become migratory or invasive.
In contrast to the C terminus of CagA, few proteins were found to interact with its N terminus. In the absence of the N-terminal domain, the C terminus of CagA is primarily localized to the cytoplasm. Coexpression of the C- and N-terminal domains induces a strong accumulation of both domains near the plasma membrane. Thus, the main role ascribed to the N terminus is to target CagA to the plasma membrane (7).
Earlier reports have linked injection or exogenous expression of CagA to the activation of several oncogenic pathways. For example, destabilization of the E-Cadherin/β-catenin complex by CagA induces abnormal activation of the wingless/int (WNT)/β-catenin pathway (17, 18). However, the effects of CagA on tumor suppressor pathways have remained obscure. Using a proteomic approach, we here show that the N terminus of CagA interacts with the tumor suppressor apoptosis-stimulating protein of p53 (ASPP2). ASPP2 is a proapoptotic protein that associates with and activates the tumor suppressor p53 on DNA damage or oncogenic stimuli, thereby inducing apoptosis. We show that, after binding to CagA, ASPP2 binds its natural ligand p53. However, the interaction between ASPP2 and p53 results in proteasomal degradation of p53 and consequently, inhibition of the apoptotic response of the host cell. CagA, thus, hijacks ASPP2 and alters its activity in a manner that promotes cell survival and favors transformation.
Results
CagA Associates with the Tumor Suppressor ASPP2.
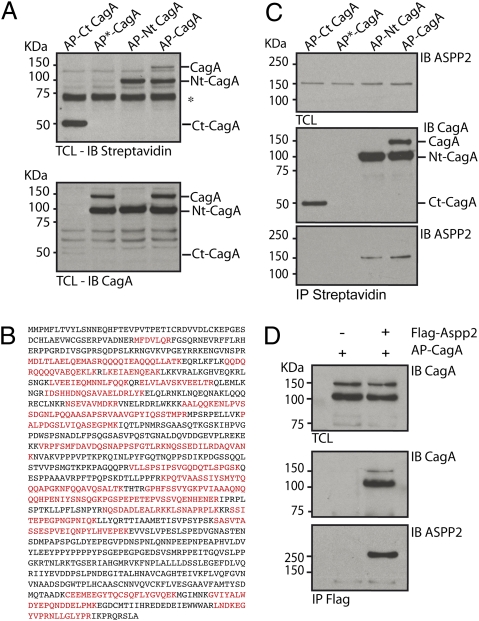
To investigate the role of the individual domains of CagA, we used an in vivo biotinylation approach combined with affinity purification to identify interacting proteins that selectively bind one of two domains in addition to the full-length protein (Fig. S1A). A biotin acceptor peptide (AP) followed by a tobacco etch virus (TEV) cleavage site was placed N-terminally of full-length (AP-CagA), N terminus (AP-Nt CagA), or C terminus (AP-Ct CagA) of CagA. We included a biotin-resistant version of the acceptor peptide fused to full-length CagA (AP*-CagA) (Fig. S1A) as a negative control. The fusion constructs were coexpressed in a doxycycline-inducible manner in T-REx293 cells together with the biotin ligase BirA, which biotinylates the AP tag as revealed by streptavidin HRP immunoblotting (Fig. 1A). We chose this strategy to provide an affinity handle at the extremity of the constructs, while allowing for proper integration into possible complexes with host proteins with otherwise minimal perturbations. After overnight induction with doxycycline, 35S methionine/cysteine-labeled cells were lysed, and biotinylated proteins were recovered by absorption onto streptavidin-conjugated beads. The material was treated with TEV protease to release CagA and any proteins associated with it, which were then analyzed by SDS/PAGE and autoradiography. We observed a prominent band of 150 kDa unique to samples from cells expressing full-length CagA or its N-terminal domain (Fig. S1B). On a preparative scale, we identified, by liquid chromatography LC coupled with tandem MS (MS/MS), several CagA-interacting proteins previously linked to its localization or activity, including Cadherin and a member of the partitioning defective protein complex (Par-3) (Fig. S1 B and D). In addition, LC-MS/MS analysis of the 150 kDa polypeptide unambiguously identified, with excellent sequence coverage, ASPP2, which interacted with full-length CagA and its N terminus (Fig. 1 B and C and Fig. S1C) but not with its C terminus (Fig. 1C). Immunoprecipitation of FLAG-ASPP2 from cells coexpressing AP-CagA confirmed the interaction between ASPP2 and CagA (Fig. 1D).
Fig. 1.
CagA interacts with the tumor suppressor ASPP2. (A) Exogenous expression of BirA induces biotinylation of the AP-tagged CagA constructs. T-REx 293 stable transfectants were treated overnight with Dox to induce the expression of the indicated constructs together with the biotin ligase BirA. Total cell lysates were immunoblotted (IB) with streptavidin-HRP (Upper) and anti-CagA antibody (Lower). Full-length AP-CagA is partially processed into two fragments. Both full-length and N-terminal fragments are recognized by streptavidin-HRP. *Nonspecific band recognized by streptavidin-HRP. (B) AP-Nt CagA associates with endogenous ASPP2. Amino acid sequence of ASPP2. Peptides highlighted in bold were identified by LC-MS/MS in T-REx 293 expressing AP-Nt CagA and BirA. (C) Exogenously expressed CagA binds ASPP2 through its N-terminal domain. T-REx 293 stable transfectants were treated overnight with Dox to induce the expression of the indicated constructs together with BirA. Biotinylated proteins were retrieved by immunoprecipitation (IP) with streptavidin-conjugated beads. Total cell lysate (TCL) and IP were IB with the indicated antibodies. (D) HEK293T cells were cotransfected with FLAG-ASPP2 and AP-CagA. CagA was retrieved by IP with anti-CagA antibody, and immunoprecipitates were IB with the indicated antibodies.
CagA Relocalizes ASPP2 on Hp Infection.
ASPP2 is part of the ASPP family, which includes the proapoptotic ASPP1 and the antiapoptotic iASPP. Both ASPP1 and ASPP2 are known activators of the tumor suppressor p53 in response to DNA damage or oncogenic stimuli (19). The C terminus of ASPP2 transiently interacts with the DNA binding domain of p53, and after this interaction, p53 induces the expression of genes involved in apoptosis, although the molecular mechanism used by ASPP2 to prime p53 to the apoptotic response is still unclear (20, 21). ASPP2 also binds other factors with roles in apoptosis (Bcl-2 and Yes-associated protein) (22–24) and control of cell growth (p65/RelA subunit of NF-κB, adenomatous polyposis coli tumor suppressor, and protein phosphatase-1) (24–26).
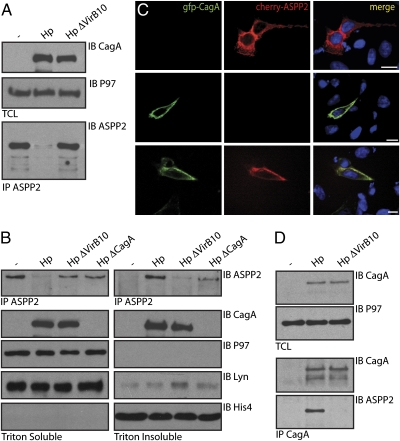
To place this newly identified interaction in the context of infection with Hp, we infected the gastric adenocarcinoma cell line (AGS), bearing WT p53, with WT Hp (G27) or the isogenic HpΔVirb10, which lacks the secretion machinery required for CagA injection. Surprisingly, we were unable to detect ASPP2 in cells infected with WT Hp when lysis was performed using a mild, Brij-containing buffer (Fig. 2A). However, Hp-infected cells lysed in an SDS-based buffer showed a slight increase in total ASPP2 levels compared with cells infected with either ΔVirb10 or ΔCagA Hp mutants (Fig. S2A). These results suggest that Hp induces relocalization rather than degradation of ASPP2.
Fig. 2.
CagA targets and relocalizes ASPP2 on Hp infection. Hp infection targets ASPP2. (A) AGS cells were infected for 6 h with the indicated Hp strains [multiplicity of infection (moi) = 1:100] or left uninfected and lysed in 1% Brij-containing buffer. TCLs were immunoblotted with the indicated antibodies. The ASPP2 input material is not shown because of the low expression level of the endogenous protein. ASPP2 was retrieved by IP with an anti-ASPP2 antibody, and the immunoprecipitates were IB with the indicated antibodies. (B) Hp infection induces relocalization of ASPP2 to a triton-resistant fraction. AGS cells were infected for 7 h with the indicated Hp strains (moi = 1:100) or left uninfected and lysed in Triton-X100 (T-X100) -containing buffer. The T-X100 insoluble fraction was then extracted in SDS buffer. Fractions were IB with the indicated antibodies or IP with anti-ASPP2 and IB with anti-ASPP2. P97 and Lyn serve as cytoplasmic markers, and HistoneH4 was a marker for nuclei. (C) Exogenously expressed CagA and ASPP2 colocalize near the plasma membrane. AGS cells cotransfected with FLAG-ASPP2 and Cherry-CagA are stained with anti-FLAG (green) and Hoechst (blue) (SI Materials and Methods and Fig. S2B). (Scale bar: 10 μm.) (D) Endogenous ASPP2 interacts with CagA during the course of in vivo Hp infection. AGS cells were infected for 7 h with the indicated Hp strains at an moi = 1:100 or left uninfected and lysed by sonication in Nonidet P-40–containing buffer. TCLs were immunoblotted with the indicated antibodies. The ASPP2 input material is not shown because of the low expression level of the endogenous protein. CagA was retrieved by IP with an anti-CagA antibody, and the immunoprecipitates were IB with the indicated antibodies.
To further explore this possibility, AGS cells were infected with Hp or the isogenic mutants ΔVirB10 or ΔCagA and lysed in 0.5% Triton X-100. As observed before, the amount of endogenous ASPP2 recovered was reduced in samples exposed to WT Hp (Fig. 2B). However, resolubilization of the Triton-resistant pellet in SDS lysis buffer confirmed that ASPP2 was relocalized to a Triton-resistant fraction, which contains cell remnants, nuclei, Triton-resistant membranes, and bacteria (Fig. 2B).
To determine whether CagA affects the localization of ASPP2, we transfected AGS cells with Cherry-ASPP2 alone or in combination with GFP-CagA. Cherry-ASPP2 is present in the cytoplasm and is excluded from the nucleus. Coexpression of Cherry-ASPP2 with GFP-CagA shows strong colocalization, and CagA recruits ASPP2 to a region close to the plasma membrane (Fig. 2C and Fig. S2B). Although there is no clear consensus as to the exact localization of ASPP2 (27, 28), a recent study described the targeting of ASPP2 to tight junctions (29). Because full-length CagA is also found close to the plasma membrane and junctional complexes, CagA likely recruits ASPP2 to form a complex near the plasma membrane.
To confirm the interaction between CagA and ASPP2 on infection, we immunoprecipitated CagA from Hp-infected AGS cells that were lysed by sonication. ASPP2 immunoblotting confirmed the CagA-ASPP2 interaction in the course of infection with WT but not ΔVirB10 Hp (Fig. 2D). Thus, we confirmed that CagA equally targets ASPP2 in transfectants and on in vivo infection, and it relocalizes ASPP2 to a region near the plasma membrane.
ASPP2 Forms a Complex with p53 on Delivery of CagA.
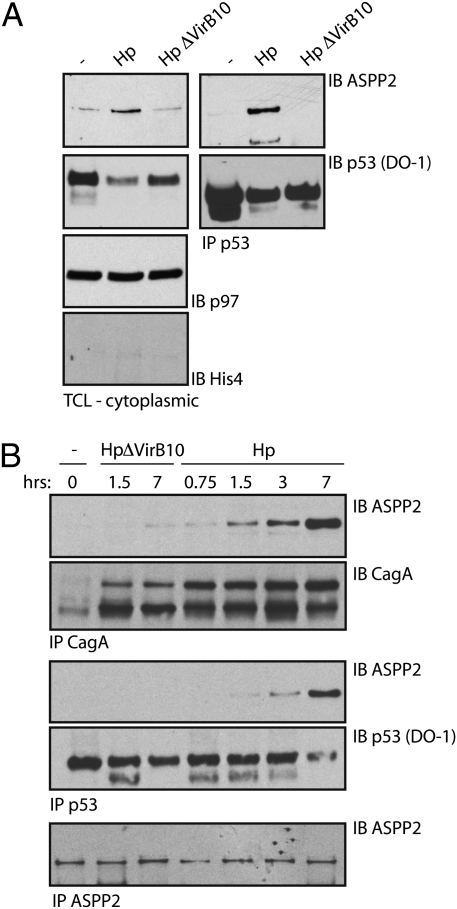
To determine whether the binding of CagA to ASPP2 and the subsequent redistribution of ASPP2 affects the interaction between ASPP2 and p53, AGS cells were infected with WT or ΔVirb10 mutant Hp, and the cytoplasmic fraction of endogenous p53 was immunoprecipitated. In the absence of infection, ASPP2 does not bind p53 (Fig. 3A). However, in cells exposed to WT Hp, we observed an association between endogenous p53 and ASPP2. This interaction requires CagA, because it was not observed in cells infected with the ΔVirb10 mutant Hp (Fig. 3A). Thus, injection of CagA facilitates the interaction between p53 and ASPP2. Of note, treatment of cells with Doxorubicin (Dox), a DNA damaging agent that activates p53, induces the association of ASPP2 with p53 as well as apoptosis in a concentration-dependent manner (Fig. S3 A and B) without affecting the cellular distribution of ASPP2 (compared with cells infected with WT Hp) (Fig. S3C).
Fig. 3.
ASPP2 associates with p53 on intracellular delivery of CagA. (A) Hp infection induces the association between endogenous p53 and ASPP2. AGS cells were infected with the indicated Hp strains (1:100 moi) for 7 h or left uninfected; then, they were sonicated in Nonidet P-40–containing buffer, and cytoplasmic p53 was IP. TCLs and immunoprecipitates were IB with the indicated antibodies, and p97 served as loading control as well as a cytoplasmic marker. His4 serves as a nuclear marker. (B) The CagA-ASPP2 interaction precedes the association between ASPP2 and p53. AGS cells were infected with the indicated Hp strains (moi = 1:100) or not infected, harvested at different time points, and processed as described in A. p53, CagA, and ASPP2 IP were IB with the indicated antibodies (SI Materials and Methods and Fig. S5A).
To exclude possible competition between CagA and p53 for binding to ASPP2, we mapped the region of ASPP2 required for binding to CagA. We cotransfected FLAG-tagged fragments of ASPP2 with AP-CagA in HEK293T cells (Fig. S4A). Only full-length ASPP2 and its 861aa N-terminal region, but not its predicted N-terminal coiled coil domain or C terminus, were recovered together with AP-CagA (Fig. S4B). Thus, although p53 binds ASPP2 at its C terminus, CagA binds at its N terminus and thus, is unlikely to compete with p53.
To understand the sequence of events trigged by the translocation of CagA, we determined the kinetics between the interaction of CagA-ASPP2 and ASPP2-p53. AGS cells were infected with WT Hp or ΔVirb10 mutant and harvested at different time points. We detected an association between CagA and ASPP2 already 90 min postinfection (p.i.), with a additional increase as time progressed (Fig. 3B and Fig. S5A). In contrast, the association between ASPP2 and cytoplasmic p53 was observed not earlier than 3 h p.i. and reached a peak 7 h p.i. This suggests that ASPP2 recruits the cytoplasmic pool of p53 after ASPP2 has been engaged by CagA. We did not detect a ternary complex composed of CagA, ASPP2, and p53. Thus, CagA modulates both the localization of ASPP2 as well as its interaction with the tumor suppressor p53.
To determine whether the interaction between ASPP2 and p53 involves phosphorylation of CagA and initiation of RTK signaling, we infected AGS cells with the mutant Hp EPISA, which translocates a phosphorylation-resistant version of CagA. In cells infected with Hp-EPISA, p53 binds ASPP2 to the same extent as seen for WT Hp (Fig. S5B). Thus, CagA stimulates the association between ASPP2 and p53 in a phosphorylation-independent manner.
CagA Stimulates Proteasomal Degradation of p53 in an ASPP2-Dependent Manner.
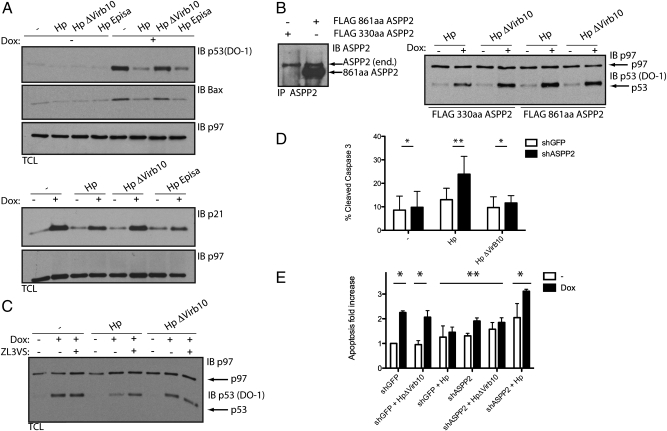
p53 is a transcription factor that regulates a variety of target genes. It is usually rapidly degraded by the proteasome but stabilized on DNA damage or cellular stress. Because ASPP2 recruits cytoplasmic p53 on Hp infection, we hypothesized that the transcriptional activity of p53 might be altered on translocation of CagA. We examined the levels and activity of p53 in response to DNA damage (Dox) in cells previously infected with Hp. Treatment of cells with Dox elicited a drastic increase in p53 levels, and as a consequence, its downstream effectors p21 and Bax were up-regulated (Fig. 4A). However, Dox treatment of cells infected with WT or the EPISA Hp mutant inhibited accumulation of p53 (Fig. 4A). Accordingly, a decrease in p53 also inhibited its transcriptional activity; Bax and p21 are not up-regulated in Dox-treated and Hp-infected cells. The failure to up-regulate p21 and Bax is a direct consequence of reduced levels of p53, because infection of the p53-deficient isogenic cell line with Hp or Hp ΔVirb10 mutant followed by Dox treatment did not affect Bax or p21 levels (Fig. S6B). WT Hp and the EPISA mutant affect p53 levels equally. Phosphorylation of CagA is, therefore, not required for inhibition of the p53 response and suggests a role for the phosphorylation-independent interaction between CagA and ASPP2.
Fig. 4.
CagA inhibits p53 accumulation and transcriptional activity in an ASPP2-dependent manner. (A) CagA inhibits the accumulation and transcriptional activity of p53. HCT116p53+/+ cells were infected for 24 h with the indicated Hp strains (moi = 1:50) or left uninfected. Where indicated, cells were treated 5 h postinfection with 1 μg/mL Dox for 1.5 h. SDS-TCLs were IB with the indicated antibodies. p97 serves as loading control. We used HCT116 p53+/+ cells, because these can be infected with Hp (SI Materials and Methods and Fig. S6A) and their p53 pathway is well-characterized (41). (B) CagA inhibits accumulation of p53 in an ASPP2-dependent manner. HCT116 stably transfected with the indicated constructs were treated as indicated in A. To compare the level of endogenous ASPP2 with the overexpressed constructs, SDS lysates were IP and IB with ASPP2 antibody. This experiment is representative of three independent experiments. FLAG 330-aa ASPP2 construct is not shown, because the ASPP2 antibody does not recognize this short fragment (SI Materials and Methods and Fig. S4A). (C) CagA induces proteasomal degradation of p53. HCT116 cells were infected for 10 h with the indicated Hp strains (1:100 moi) or left uninfected. Where indicated, cells were treated 3 h postinfection with 1 μg/mL Dox for 1 h. Where indicated, ZL3VS was added for the remaining hours before harvesting the cells. TCLs were IB with the indicated antibodies. (D) ASPP2 inhibits apoptosis on Hp infection. Levels of cleaved Caspase-3 (CC3) assayed by flow cytometry in shGFP (white bars) or shASPP2 (construct #3; black bars) AGS cells that were infected for 24 h with the indicated Hp strains (1:50 moi) or left uninfected. Error bars ± SEM (n = 5). *P = ns; **P < 0.05. Significance was tested using two-way ANOVA Bonferroni multiple-comparison test. (E) Dox-induced apoptosis is inhibited by Hp in an ASPP2-dependent manner. Relative increase in CC3 in shGFP (white bars) or shASPP2 (construct #3; black bar) cells infected for 24 h with the indicated Hp strains (1:50 moi) or left uninfected and treated with 1 μg/mL Dox for 1 h. Error bars ± SEM (n = 3). *P < 0.05; **P = ns. Significance was tested using two-way ANOVA Bonferroni multiple-comparison test.
To investigate whether the failure to up-regulate p53 indeed requires ASPP2, we expressed the FLAG-tagged N-terminal segment of ASPP2 (FLAG 861-aa ASPP2) followed by Hp infection and Dox treatment. This N-terminal part of ASPP2 binds CagA, but the absence of the ankyrin (ANK), proline-rich, and SH3 domains prevents the binding to p53 (Fig. S4 C and D). Thus, this ASPP2 fragment is expected to compete with endogenous ASPP2 for binding to CagA and therefore, prevent the downstream events. Indeed, p53 accumulated normally on Dox treatment of cells infected with Hp and expressing the N-terminal 861-aa fragment of ASPP2, whereas in Hp-infected control cells (expressing FLAG 330-aa ASPP2), up-regulation of p53 was inhibited (Fig. 4B). Thus, CagA-mediated inhibition of p53 expression is dependent on ASPP2.
To test whether CagA targets p53 for degradation by the proteasome, cells were treated with Dox and subsequently incubated with the proteasome inhibitor ZL3VS. This caused accumulation of p53 in uninfected as well as the ΔVirb10 Hp mutant-infected cells (Fig. 4C). Inhibition of p53 degradation was effective as well in cells infected with WT Hp and treated with Dox, indicating that CagA-induced degradation of p53 is mediated by the proteasome (Fig. 4C).
Down-Regulation of ASPP2 Promotes the Apoptotic Response on Hp Infection.
Under normal conditions, the tumor suppressor function of the ASPP2-p53 pathway is exerted mainly through induction of the apoptotic response. However, in Hp-infected cells, CagA promotes the interaction between p53 and ASPP2, leading to an enhanced degradation of p53 and therefore, inhibition of its transcriptional activity. Hp induces only a slight increase in the apoptotic response of infected cells, and apoptosis is inhibited by the delivery of CagA, which also activates a prosurvival pathway that stabilizes and enhances the antiapoptotic response (30). To determine if ASPP2 is necessary to block apoptosis on Hp challenge, we tested the apoptotic response of Hp-infected cells in which expression of ASPP2 was reduced by means of a specific shRNA (Fig. S7A). After 24 h of infection with WT Hp, the apoptotic response was determined by measuring cleaved caspase-3 levels by cytofluorimetric analysis. Apoptosis was enhanced in shASPP2 Hp-infected cells compared with shGFP Hp-infected cells (Fig. 4D and Fig. S7B). In contrast, no difference was observed between shGFP or shASPP2 cells either infected with the ΔVirb10 Hp mutant or uninfected (Fig. 4D and Fig. S7B). Thus, we observe an increase in apoptosis in ASPP2-depleted and Hp-infected cells, suggesting that CagA hijacks the tumor suppressor function of ASPP2 to target p53.
To test this more directly, we treated cells with Dox. In control cells treated with Dox, we observed a 2.5-fold increase in cleaved caspase-3 (Fig. 4E). A similar increase was observed in cells infected with the ΔVirb10 Hp mutant before Dox treatment. In contrast, we saw a marked resistance to Dox in cells infected with WT Hp. This effect was strictly dependent on ASPP2, because shASPP2 cells infected with WT Hp were no longer resistant to Dox-induced apoptosis (Fig. 4E). Combined, these results suggest that CagA inhibits apoptosis by binding the tumor suppressor ASPP2 and through this interaction p53 is degraded and its apoptotic function inhibited.
Discussion
Intracellular bacterial and viral pathogens that establish persistent infections have evolved multiple strategies to interfere with host signaling pathways and immune responses. They do so to create an intracellular safe haven while avoiding killing the host cell. Indeed, several viral oncogenes (e.g., T-antigen E1B-55Kd and HPV E6) inactivate the tumor suppressor activity of the host cell by targeting and degrading p53 when the infection is established (31–33). Although Hp is not an intracellular pathogen, its interactions with host cells may provide certain survival benefits for the bacterium and contribute to the establishment of a chronic infection. Indeed, Hp uses host plasma membranes as a site for replication and formation of microcolonies (34). In addition, colonization of the stomach by Hp is enhanced when the apoptotic response of gastric epithelial cells is impaired. CagA injection induces the up-regulation of the antiapoptotic protein MCL-1, and consequently, the infected cells become more resistant to normal cell turnover in the stomach (30). Hp, thus, benefits from improved survival of the host cell. We here provide an example of a bacterial virulence factor that directly counteracts a tumor suppressor function of the host in a fashion similar to that used by viral oncogenes. We show that the tumor suppressor ASPP2 associates with CagA. After this interaction, ASPP2 recruits and binds cytosolic p53, which is then degraded by the proteasome. CagA-mediated degradation of p53 results in resistance to the apoptotic response in an ASPP2-dependent manner (Fig. S8). Of note, RUNX3 is also sent to the proteasome after CagA interaction, suggesting a link between CagA and the proteasomal machinery of the infected cell (35).
We did not detect the formation of a ternary complex between CagA, ASPP2, and p53, perhaps owing to a transient nature of any of the interactions. It, therefore, remains unclear whether the associations between CagA-ASPP2 and ASPP2-p53 occur simultaneously or sequentially and in which cytosolic region the ASPP2-p53 complex is formed.
ASPP2 is best known for its role as tumor suppressor, and as such, it enhances the apoptotic response of the cell. On DNA damage, ASPP2 associates with and activates the transcription factor p53, resulting in increased expression of BAX, PUMA, and PIG3, all of which are positive regulators of programmed cell death. The C terminus of ASPP2 interacts with the DNA-binding domain of p53, but it is unclear how this interaction leads to activation of p53. The N terminus of ASPP2 is required to enhance the transcriptional activity of p53, suggesting that this domain may have a regulatory role and determine the outcome of the ASPP2-p53 interaction (19, 21). Posttranslational modification of the N terminus of ASPP2, as well as an alteration in the set of proteins that interact with this domain, may determine the fate of p53 and thereby, affect the apoptotic response. For example, cytosolic DDA3 binds ASPP2 and prevents activation of p53 without affecting the ASPP2-p53 interaction (28). Likewise, CagA may modulate the activity of ASPP2 by simply binding its N terminus, inducing a conformational change, or altering its posttranslational modification. Alternatively, CagA may sequester ASPP2 and consequently, p53 in the cytoplasm, thereby altering its function. Indeed, cytoplasmic ASPP1 functions as oncogene, whereas nuclear ASPP1 acts as tumor suppressor (36). The function of ASPP2 may be similarly dictated by its localization, and CagA may affect this phenomenon. Thus, how CagA hijacks and misregulates the tumor suppressor function of ASPP2 requires further investigation.
The function of ASPP2 is not restricted to activation of p53 but includes regulation of cell–cell adhesion and polarity (Fig. S8). Drosophila dASPP localizes at adherens junctions and regulates the activity of C-terminal kinase (dCsk). Loss of function of dASPP increases cell spreading and apoptosis. In mammalian cells, ASPP2 associates with Par-3, a complex crucial for the formation and localization of the apical–junctional complex (AJC). ASPP2-depleted cells are defective in the formation of tight junctions and acquire a migratory phenotype (29, 37–39). Thus, ASPP2 may have a dual role. First, it functions as a tumor suppressor by enhancing the transcriptional activity of p53. Second, it may act as regulator of cell–cell adhesion in a p53-independent manner. Does CagA affect both of these seemingly unrelated functions of ASPP2? Here, we have shown that the interaction between CagA and ASPP2 results in misregulation of its function as tumor suppressor. The notion that the presence of CagA affects the function of ASPP2 as a regulator of cell adhesion and polarity is an attractive hypothesis. Similar to ASPP2, CagA disrupts cell polarity and enhances the invasive ability of its target cells. Thus, it is plausible that the CagA association with and misregulation of ASPP2 contributes to this phenotype as well. Interactors of CagA identified by LC-MS/MS not only reveal the presence of ASPP2 but also Par-3, possibly as a consequence of its interaction with ASPP2 (Fig. S1D). Thus, Hp possibly hijacks both functions of ASPP2 to simultaneously cause an increased resistance to apoptotic stimuli and impose the aggressive and invasive phenotype associated with gastric cancer (Fig. S8).
We now provide the mechanism responsible for the reduction of p53 on delivery of CagA. The link to the p53 pathway has been suggested in the context of B-cell lymphoma and epithelial cells, although a direct connection between CagA and p53 has not been shown (14, 40). We show that the degradation of p53 is a consequence of the recruitment and misregulation of ASPP2 by CagA. We speculate that altered localization and/or posttranslational modification of ASPP2 might be responsible for its subverted tumor suppressor function and drive ASPP2 in an aberrant complex with p53. Hp and DNA tumor viruses both target tumor suppressor functions. Although viruses have an obligatory intracellular lifestyle, CagA is delivered to the host transiently. The association between Hp and disease might hinge on sustained injection of CagA and the consequent deregulation of p53 during chronic Helicobacter infection. Not only does CagA promote an epithelial to mesenchymal transition, the deregulation of the ASPP2/p53 tumor suppressor pathway in CagA-recipient cells may predispose the target cells to a cancerous transformation.
Materials and Methods
Bacterial Strains and Infections.
Hp strain G27 and the isogenic mutants ΔVirb10, ΔCagA, and EPISA were described previously (9). Strains were cultured as described in SI Materials and Methods.
Protein Interaction Studies.
To detect cytoplasmic endogenous p53/ASPP2 and CagA/ASPP2 complex during the course of Hp infection, cells were lysed by sonication in Nonidet P-40–containing buffer (0.05% Nonidet P-40, 20 mM Hepes, pH 7.8, 50 mM KCl, 5 mM EDTA, 5% Glycerol) supplemented with complete protease inhibitors (Roche) and cleared by centrifugation. To detect endogenous p53/ASPP2 interaction during the course of Dox treatment, cells were lysed by sonication in 20 mM Hepes (pH 7.8), 400 mM KCl, 5% Glycerol, 5 mM EDTA, and 0.4% Np40 supplemented with complete protease inhibitors (Roche) and cleared by centrifugation. Detailed description of the immoprecipitation is in SI Materials and Methods and Table S1.
Flow Cytometry.
Cells were stained with Cleaved Caspase 3 antibody according to the manufacturer's instructions. Cells were analyzed using a Becton Dickinson FACS-Calibur, and apoptotic profiles were generated using FlowJo 8.5.3 software.
Image Acquisition.
Cells were processed as described in ref. 6. Images were acquired using a spinning disk confocal microscope and a 3-W water-cooled laser with an acoustic-optic tunable filter (Prairie Technologies). The system incorporated a Nikon TE2000-U inverted microscope using a Nikon_60 magnification, 1.4 numerical aperture, and differential interference contrast oil lens. Nikon type A immersion oil was used (Nikon). The Hamamatsu Orca ER camera (C4742-95–12ERG), Metamorph software (Molecular Devices), and Volocity 5.4 (PerkinElmer) were used for acquisition.
Supplementary Material
Acknowledgments
We thank B. Park, M. Pacold, S. Censini, T. Brummelkamp, and R.A. Weinberg for critical review of the article; X. Lu for the ASPP2 cDNA construct; and B. Vogelstein for the HCT116 p53+/+ and p53−/− cell lines. A.G.V.d.V was supported by the Boehringer Ingelheim Fonds.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106200108/-/DCSupplemental.
References
- 1.Censini S, et al. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asahi M, et al. Helicobacter pylori CagA protein can be tyrosine phosphorylated in gastric epithelial cells. J Exp Med. 2000;191:593–602. doi: 10.1084/jem.191.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odenbreit S, et al. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 4.Stein M, Rappuoli R, Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci USA. 2000;97:1263–1268. doi: 10.1073/pnas.97.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franco AT, et al. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 2008;68:379–387. doi: 10.1158/0008-5472.CAN-07-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amieva MR, et al. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430–1434. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagnoli F, Buti L, Tompkins L, Covacci A, Amieva MR. Helicobacter pylori CagA induces a transition from polarized to invasive phenotypes in MDCK cells. Proc Natl Acad Sci USA. 2005;102:16339–16344. doi: 10.1073/pnas.0502598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murata-Kamiya N, et al. Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene. 2007;26:4617–4626. doi: 10.1038/sj.onc.1210251. [DOI] [PubMed] [Google Scholar]
- 9.Stein M, et al. c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Mol Microbiol. 2002;43:971–980. doi: 10.1046/j.1365-2958.2002.02781.x. [DOI] [PubMed] [Google Scholar]
- 10.Poppe M, Feller SM, Römer G, Wessler S. Phosphorylation of Helicobacter pylori CagA by c-Abl leads to cell motility. Oncogene. 2007;26:3462–3472. doi: 10.1038/sj.onc.1210139. [DOI] [PubMed] [Google Scholar]
- 11.Higashi H, et al. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002;295:683–686. doi: 10.1126/science.1067147. [DOI] [PubMed] [Google Scholar]
- 12.Mimuro H, et al. Grb2 is a key mediator of helicobacter pylori CagA protein activities. Mol Cell. 2002;10:745–755. doi: 10.1016/s1097-2765(02)00681-0. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki M, et al. Interaction of CagA with Crk plays an important role in Helicobacter pylori-induced loss of gastric epithelial cell adhesion. J Exp Med. 2005;202:1235–1247. doi: 10.1084/jem.20051027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsutsumi R, Higashi H, Higuchi M, Okada M, Hatakeyama M. Attenuation of Helicobacter pylori CagA × SHP-2 signaling by interaction between CagA and C-terminal Src kinase. J Biol Chem. 2003;278:3664–3670. doi: 10.1074/jbc.M208155200. [DOI] [PubMed] [Google Scholar]
- 15.Saadat I, et al. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature. 2007;447:330–333. doi: 10.1038/nature05765. [DOI] [PubMed] [Google Scholar]
- 16.Churin Y, et al. Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J Cell Biol. 2003;161:249–255. doi: 10.1083/jcb.200208039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Botham CM, Wandler AM, Guillemin K. A transgenic Drosophila model demonstrates that the Helicobacter pylori CagA protein functions as a eukaryotic Gab adaptor. PLoS Pathog. 2008;4:e1000064. doi: 10.1371/journal.ppat.1000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franco AT, et al. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci USA. 2005;102:10646–10651. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samuels-Lev Y, et al. ASPP proteins specifically stimulate the apoptotic function of p53. Mol Cell. 2001;8:781–794. doi: 10.1016/s1097-2765(01)00367-7. [DOI] [PubMed] [Google Scholar]
- 20.Bergamaschi D, et al. iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human. Nat Genet. 2003;33:162–167. doi: 10.1038/ng1070. [DOI] [PubMed] [Google Scholar]
- 21.Gorina S, Pavletich NP. Structure of the p53 tumor suppressor bound to the ankyrin and SH3 domains of 53BP2. Science. 1996;274:1001–1005. doi: 10.1126/science.274.5289.1001. [DOI] [PubMed] [Google Scholar]
- 22.Naumovski L, Cleary ML. Bcl2 inhibits apoptosis associated with terminal differentiation of HL-60 myeloid leukemia cells. Blood. 1994;83:2261–2267. [PubMed] [Google Scholar]
- 23.Espanel X, Sudol M. Yes-associated protein and p53-binding protein-2 interact through their WW and SH3 domains. J Biol Chem. 2001;276:14514–14523. doi: 10.1074/jbc.M008568200. [DOI] [PubMed] [Google Scholar]
- 24.Yang JP, et al. NF-kappaB subunit p65 binds to 53BP2 and inhibits cell death induced by 53BP2. Oncogene. 1999;18:5177–5186. doi: 10.1038/sj.onc.1202904. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa H, et al. APCL, a central nervous system-specific homologue of adenomatous polyposis coli tumor suppressor, binds to p53-binding protein 2 and translocates it to the perinucleus. Cancer Res. 2000;60:101–105. [PubMed] [Google Scholar]
- 26.Helps NR, Barker HM, Elledge SJ, Cohen PT. Protein phosphatase 1 interacts with p53BP2, a protein which binds to the tumour suppressor p53. FEBS Lett. 1995;377:295–300. doi: 10.1016/0014-5793(95)01347-4. [DOI] [PubMed] [Google Scholar]
- 27.Uhlmann-Schiffler H, Kiermayer S, Stahl H. The DEAD box protein Ddx42p modulates the function of ASPP2, a stimulator of apoptosis. Oncogene. 2009;28:2065–2073. doi: 10.1038/onc.2009.75. [DOI] [PubMed] [Google Scholar]
- 28.Sun WT, Hsieh PC, Chiang ML, Wang MC, Wang FF. p53 target DDA3 binds ASPP2 and inhibits its stimulation on p53-mediated BAX activation. Biochem Biophys Res Commun. 2008;376:395–398. doi: 10.1016/j.bbrc.2008.08.168. [DOI] [PubMed] [Google Scholar]
- 29.Sottocornola R, et al. ASPP2 binds Par-3 and controls the polarity and proliferation of neural progenitors during CNS development. Dev Cell. 2010;19:126–137. doi: 10.1016/j.devcel.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Mimuro H, et al. Helicobacter pylori dampens gut epithelial self-renewal by inhibiting apoptosis, a bacterial strategy to enhance colonization of the stomach. Cell Host Microbe. 2007;2:250–263. doi: 10.1016/j.chom.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Sarnow P, Ho YS, Williams J, Levine AJ. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982;28:387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- 32.Schutzbank T, Robinson R, Oren M, Levine AJ. SV40 large tumor antigen can regulate some cellular transcripts in a positive fashion. Cell. 1982;30:481–490. doi: 10.1016/0092-8674(82)90245-8. [DOI] [PubMed] [Google Scholar]
- 33.Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 34.Tan S, Tompkins LS, Amieva MR. Helicobacter pylori usurps cell polarity to turn the cell surface into a replicative niche. PLoS Pathog. 2009;5:e1000407. doi: 10.1371/journal.ppat.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsang YH, et al. Helicobacter pylori CagA targets gastric tumor suppressor RUNX3 for proteasome-mediated degradation. Oncogene. 2010;29:5643–5650. doi: 10.1038/onc.2010.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vigneron AM, Ludwig RL, Vousden KH. Cytoplasmic ASPP1 inhibits apoptosis through the control of YAP. Genes Dev. 2010;24:2430–2439. doi: 10.1101/gad.1954310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Langton PF, Colombani J, Aerne BL, Tapon N. Drosophila ASPP regulates C-terminal Src kinase activity. Dev Cell. 2007;13:773–782. doi: 10.1016/j.devcel.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Langton PF, et al. The dASPP-dRASSF8 complex regulates cell-cell adhesion during Drosophila retinal morphogenesis. Curr Biol. 2009;19:1969–1978. doi: 10.1016/j.cub.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 39.Cong W, et al. ASPP2 regulates epithelial cell polarity through the PAR complex. Curr Biol. 2010;20:1408–1414. doi: 10.1016/j.cub.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 40.Wei J, et al. Regulation of p53 tumor suppressor by Helicobacter pylori in gastric epithelial cells. Gastroenterology. 2010;139:1333–1343. doi: 10.1053/j.gastro.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bunz F, et al. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.