Abstract
We characterized the Schizosaccharomyces pombe arc3 gene, whose product shares sequence homology with that of the budding yeast ARC18 and human ARPC3/p21 subunits of the Arp2/3 complex. Our data showed that Arc3p co-localizes with F-actin patches at the cell ends, but not with F-actin cables or the equatorial actin ring, and binds other subunits of the Arp2/3 complex. Gene deletion analysis showed that arc3 is essential for viability. When arc3 expression was repressed, F-actin patches became dispersed throughout the cell with greatly reduced mobility. Furthermore in arc3-repressed cells, endocytosis was also inhibited. Human ARPC3 rescued the viability of the S. pombe arc3 null mutant; in addition, ARPC3 also localizes to F-actin patches in human cells. These data suggest that Arc3p is an evolutionarily conserved subunit of the Arp2/3 complex required for proper F-actin organization and efficient endocytosis.
INTRODUCTION
The Arp2/3 complex is a well-established nucleator of actin polymerization and responsible for the formation and dynamics of several kinds of actin structures in a number of model organisms (Goley and Welch, 2006). The Arp2/3 complex is composed of at least seven highly conserved subunits, two of which, Arp2 and Arp3 are structurally related to actin and proposed to act as nuclei to promote actin polymerization (Machesky and Gould, 1999). Since the Arp2/3 complex frequently associates with the sides of preexisting actin filaments and initiates polymerization at an angle, its activity often leads to the formation of highly branched F-actin sturctures (Wear, et al., 2000). The remaining subunits have been hypothesized to play regulatory roles (Welch, et al., 1997) as well as maintain the structural integrity of the complex (Zhao, et al., 2001). Reconstitution experiments suggest that the p41, p21 and p16 subunits are located in the periphery of the complex and seem to influence actin polymerization efficiency and activation by WASP (Gournier, et al., 2001). p41 can be phosphorylated by PAK1 to influence cell migration (Vadlamudi, et al., 2004). The p20 and p34 subunits seem to be confined to the complex's core and to be required for the structural integrity of the complex and its ability to bind existing actin filaments (Gournier, et al., 2001). Arc18, the predicted S.cerevisiae homolog of the arc3/p21 subunit is recruited to the mitochondria and the arc18 deletion mutant shows impaired mitochondrial transport (Fehrenbacher, et al., 2005).
The Arp2/3 complex is known to associate with and participate in actin polymerization in both Schizosaccharomyces pombe (Sirotkin, et al., 2005) and Saccharomyces cerevisiae (Winter, et al., 1997). In S. pombe, three F-actin structures are readily identifiable. During interphase, F-actin patches concentrate at the growing ends of the cell (Marks, et al., 1986; Verde, et al., 1995), and during early mitosis, these patches relocate to the cell equator (Wu, et al., 2006). F-actin also foms cable-like structures that extend along the long axis of the cell throughout the cell cycle. Just before anaphase-B , F-actin filaments also form a ring encircling the cell equator, which plays a key role in providing the constrictive force needed for cytokinesis (Noguchi, et al., 2001; Wu, et al., 2006). The Arp2/3 complex associates with F-actin patches, but not with F-actin cables or the equatorial ring, and is required for the proper organization and mobility of these patches (McCollum, et al., 1996; Pelham and Chang, 2001). They are spatially associated with endocytic vesicles (Gachet and Hyams, 2005) and have been proposed to play a role in their formation and internalization (Girao, et al., 2008). This is supported by the observations that the S. cerevisiae WASP (Wiskott-Aldrich Syndrome Protein) ortholog, LAS17, and S. pombe Cdc42, activators of the Arp2/3 complex, are requried for clathrin-mediated endocytosis (Murray and Johnson, 2001; Naqvi, et al., 1998). However, whether all Arp2/3 subunits are required for efficient endocytosis has not been determined. Furthermore, although Arp2/3 complex subunit orthologs from different species are highly conserved at the protein sequence level, it is not know whether the function of different subunits is also conserved across evolution.
In a recent study of a mutant (carrying deletion in the yin6 gene) with defective proteasomes, we isolated the S. pombe arc3 gene, which is highly homologus to human ARPC3 and S. cerevisiae ARC18 (Welch, et al., 1997), and showed that it is needed for proteasomes to maintain high mobility (Cabrera, et al., 2010). In this study, we further characterize the role of arc3 in regulating F-actin organization and endocytosis. Our results showed that unlike its S. cerevisiae ortholog ARC18, arc3 is essential for viability. We further showed that Arc3 is required for proper organization and high mobility of F-actin patches and efficient endocytosis. The essential function of the arc3 gene can be fully rescued by the human ARPC3, which also localizes to F-actin patches in human cells, suggesting that their functions are evolutionarily conserved.
MATERIALS AND METHODS
Growth conditions and reagents
Cells were grown in either yeast extract (rich) medium (YEAU) or synthetic minimal medium (MM) with appropriate supplements (Chen, et al., 1999). To depolymerize F-actin, Lat A stock solution (1 mM, Sigma) was prepared in DMSO. To repress the nmt promoter, thiamine was added from a 20 µM stock (Sigma) after autoclaving. We carried out all the experiments with cells pregrown to early logarithmic phase (2–5×106 cells/ml). For growth experiments on plates, cells were serially diluted 1:5. HeLa cells were obtained from the American Type Culture Collection and cultured in Dulbecco's Modified Eagle's Medium (DMEM) (Gibco) supplemented with 10% FBS, 100 IU/ml penicillin, 0.1 mg/ml streptomycin, and 2 mM glutamine (Gibco). Subconfluent cells were transfected with 200 ng of plasmid using Lipofectamine 2000 (Invitrogen).
Plasmid constructions
The pREP41ARC3 expression vector was described elsewhere (Cabrera, et al., 2010). Full length arc3 was amplified from an S. pombe cDNA library (Norbury and Moreno, 1997) and cloned into the BamHI site of pVJL11 to generate pVJLARC3. Full length arc4 was amplified from the S. pombe cDNA library described above and cloned into the BamHI site of pGADgh to generate pGADARC4. ARPC3 was amplified from cDNA was obtained from Open Biosystems and cloned into the SalI sites of pEGFP-N1 or pREP1 to generate pARPC3EGFP and pREP1ARPC3.
Strain constructions
The parental wild-type S. pombe strain used in this study was SP870 (h90, ade6-M210, leu1-32, ura4-D18) unless indicated otherwise. Cells expressing Arc3-MYC and GFP were generated by tagging Arc3 with 13× MYC and GFP respectively at the C-terminus using homologous recombination via a PCR-based method (Bähler, et al., 1998). The creation of an arc3::ura4/+ strain was described elsewhere (Cabrera, et al., 2010). Crn1-GFP was kindly provided by Dr. Fred Chang. To determine if arc3 is an essential gene, arc3::ura4/+ cells were induced to sporulate, and the viability of individual spores was assayed by tetrad analysis. To study the function of arc3, its expression was repressed in the ARC3NMT strain (Cabrera, et al., 2010), which carries an arc3 deletion (arc3::ura4) and the pREP41ARC3 plasmid to express arc3 from a thiamine-represible nmt promoter. Repression of arc3 for microscopy experiments was achieved by incubating cells in MM with 200 nM thiamine for 16 to 20 hours. Repression of arc3 to assay growth was achieved by growing ARC3NMT cells on MM plates containing 1 nM thiamine.
Immunoprecipitation
Approximately 50 OD units of cells expressing Arc3-Myc were lysed using glass beads in PBS (pH 7.4) containg 0.1% Triton X-100. Lysate was centrifuged for 5 minutes at 16000×g and the supernatant was added to 40 µl of Protein A beads (Roche) and incubated in the presence of either anti-Myc antibody (9E10), anti-Arp2 (Morrell, et al., 1999) or mouse IgG (Roche) as a control. The beads were washed three times with PBS plus 0.1% Triton X-100 and solubilized in 1× SDS loading buffer. The resulting samples were analyzed by western blot with anti-Myc (9E10, 1:100), anti-Arp2 ((Morrell, et al., 1999), 1:1000) and anti-tubulin (TAT1, 1:1000) antibodies.
Fluorescence Microscopy
The general procedures for staining F-actin in fixed yeast cells were as described (Sawin and Nurse, 1998) except staining was done with both Rhodamine-Phalloidin and Alexa Fluor 488-Phalloidin as required. To stain F-actin in HeLa cells, the cells were washed with PBS, fixed in PBS plus 4% paraformaldehyde, washed and permeabilized with PBS plus 0.5% Triton X-100. These cells were then incubated with PBS containing 0.2 units/ml Rhodamine-Phalloidin, washed, and mounted for observation. Representative images were collected using Olympus IX70 and BX61 fluorescent microscopes. To depolymerize actin, yeast cells were grown in YEAU, treated with 10 µM Latrunculin A for 30 mins and then washed in YEAU and grown for 1 hour. Images were collected at 30 min after Latrunculin A and 1 hour after washing. Identical treatment was given to HeLa cells. To determine actin patch localization, projection images of deconvolved stacks of Z-sections of cells stained with Alexa-488 Phalloidin were used. To measure Crn1-GFP velocity, time lapse microscopy was performed. A given Crn1-GFP dot was tracked frame by frame (0.5 sec/frame) for a total of 20 sec by the MetaMorph software (Molecular Devices), and the total distance it traveled was measured. To assay endocytosis, arc3nmt cells were pregrown to early log phase at 30 °C in minimal medium, concentrated 10-fold before FM 4–64 (8.15 µM) was added, and observed by time lapse microscopy (Gachet and Hyams, 2005).
RESULTS AND DISCUSSION
Arc3 is a subunit of the Arp2/3 complex and associates with F-actin patches
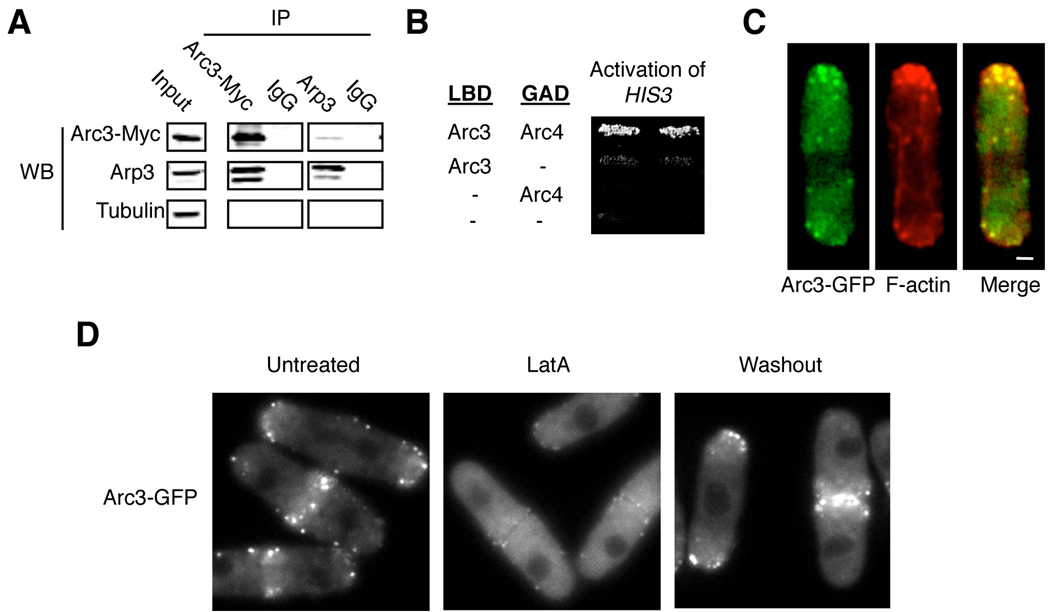
The predicted Arc3 protein is 51 and 54 % identical to the human ARPC3 and S. cerevisiae Arc18p protein, respectively. Because both ARPC3 and Arc18p represent the p21 subunits of the Arp2/3 complex, we named this molecue Arc3 with the assumption that it is an Arp2/3 complex subunit. To test whether Arc3 associates with the Arp2/3 complex, we constructed a strain in which the endogenous Arc3 protein was C-terminally tagged with 13 copies of the c-Myc epitope by homologous recombination. We performed immunoprecipitation experiments and found that Arc3 immunoprecipitated Arp3 and vice versa (Figure 1A). We also found that Arc3 can bind the predicted Arp2/3 subunit Arc4 in a yeast two-hybrid assay (Figure 1B), an interaction also observed between the S. Cervisiae Arc18p and Arc4p orthologs (Zhao, et al., 2001). These results confirm that Arc3 binds components of the Arp2/3 complex.
Figure 1.
Arc3 associates with the Arp2/3 complex in a F-actin dependent manner. (A) Lysates from cells expressing Arc3-MYC were prepared for immunoprecipitation (IP) — antibodies used are as indicated (top) and purified mouse IgG was used as a control. Immunoprecipitated proteins were analyzed by Western blots using antibodies for the indicated proteins. (B) The binding between Arc3 and Arc4 was determined by the yeast two-hybrid system by measuring the activation of the HIS3 reporter gene as described (Chang, et al., 1994). pVJLARC3 and pGADARC4 express Arc3 and Arc4 fused to the LexA DNA binding domain (LBD) and the Gal4 activation domain (GAD), respectively. (C) Cells expressing Arc3-GFP were fixed and stained with rhodamine-conjugated phalloidin to visualize F-actin. We found that nearly every GFP dot overlaps with an F-actin dot/patch, but not with F-actin cables, in all the cells that we examined. A representative cell is shown. (D) Arc3-GFP expressing cells were treated with 10 µM Latrunculin A (LatA) for 30 mins to depolymerize F-actin followed by washing and incubation in regular media (Wash out). Images of Arc3-GFP distribution were collected from untreated cells, from cells treated with LatA for 30 mins and from cells 1 hour after washing out. Bar, 1 µm.
We then tested whether Arc3 would localize to F-actin patches. We generated a strain that expresses the Arc3 fused at its C-terminus to GFP from its endogenous promoter by homologous recombination. Expression of this fusion protein as the sole source of Arc3 does not result in growth abnormalities, suggesting that it is functional. As shown in Figure 1C, Arc3-GFP localizes to cortical dots at the end of the cell that overlap with F-actin patches, as visualized by rhodamine-phalloidin. In dividing cells, both Arc3 and the F-actin patches are visible at the division plane (Figure 1D and data not shown). We note that Arc3-GFP dots do not associate with F-actin cables or F-actin ring at the cell equator. To determine whether this association with F-actin patches is dependent on the integrity of F-actin, we treated cells with the F-actin polymerization inhibitor Latrunculin A, which readily caused the majority of Arc3-GFP to be dispersed throughout the cell, a defect that was efficiently rescued when Latrunculin A was washed out (Figure 1D). We conclude from these results that Arc3 associates with Arp2/3 complex and F-actin patches in a F-actin-dependent manner.
Creation of an arc3 mutant reveals that arc3 is essential for viability
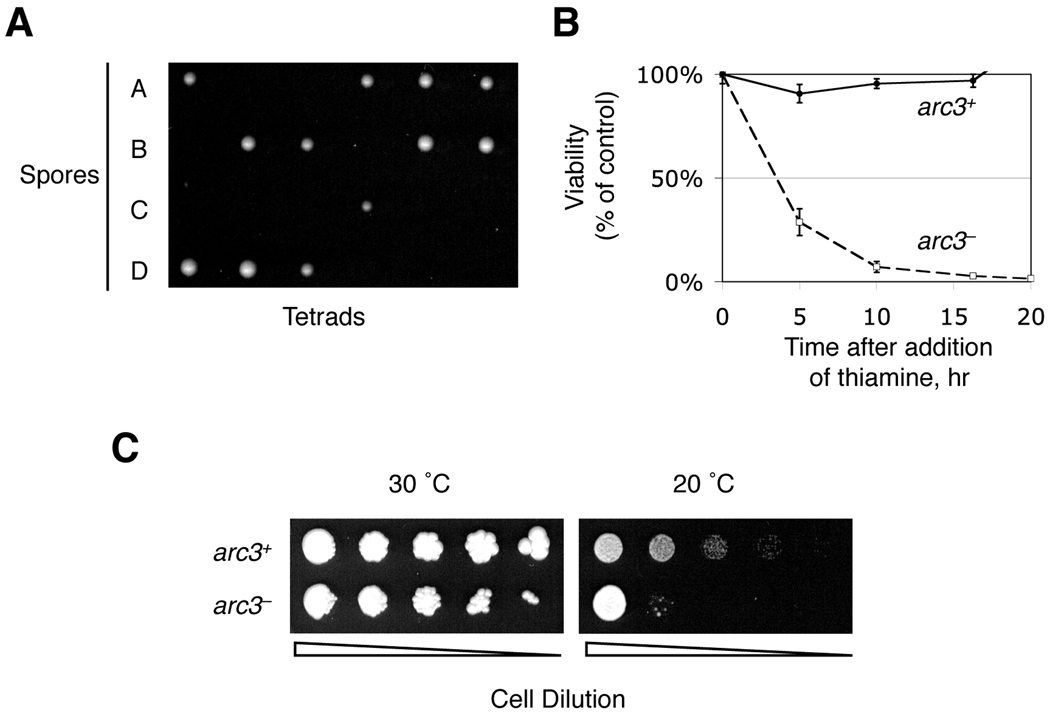
In order to study the function of the arc3 gene, we generated a diploid strain in which one copy of the arc3 was deleted and replaced by the ura4 selectable marker (arc3Δ or arc3::ura4) by homologous recombination. Tetrad analysis revealed that arc3 is essential for viability, since only 2 spores of every tetrad were able to form colonies, both of which are arc3+ (Figure 2A and data not shown).
Figure 2.
arc3 is essential for viability. (A) arc3 null heterozygous diploid cells, arc3Δ/+ (arc3::ura4/+), were induced to sporulate. After tetrad dissection, the spores were allowed to grow at 30°C on YEAU plates. Emerged colonies were further examined for autotrophy markers and none were Ura+, indicating that these were arc3+ cells. (B) The arc3nmt mutant cells (strain ARC3NMT), whose arc3 is expressed under the control of the thiamine-repressible nmt promoter, were first treated with (arc3−) or without (arc3+) 200 nM thiamine to regulate arc3 expression. Time points were taken and identical numbers of cells were spread on MM plates lacking thiamine to measure viability by colony formation (left). (C) WT or arc3nmt mutant cells were serially diluted and spotted on MM plates contatining 1 nM thiamine and incubated at the indicated temperatures.
To facilitate the study of Arc3 functions, we generated a conditional arc3 mutant by transforming arc3+/arc3Δ cells with a plasmid expressing Arc3 from a thiamine-repressible promoter. Upon meiosis and sporulation, we selected the thiamine repressible haploid arc3 mutant cells (arc3nmt). Upon addition of high concentrations of thiamine (200 µM), the expression of Arc3 was repressed with a concurrent increase of lethality which eventually affected nearly 100% of cells (Figure 2B and data not shown). We also observed that under semi-permissive conditions (low thiamine, 1 nM), these cells show impaired growth in the cold, similar to that observed in many mutants with defective actin cytoskeleton (e.g., (Balasubramanian, et al., 1996; McCollum, et al., 1999; McCollum, et al., 1996)) (Figure 2C).
Arc3 is required for actin organization and endocytosis
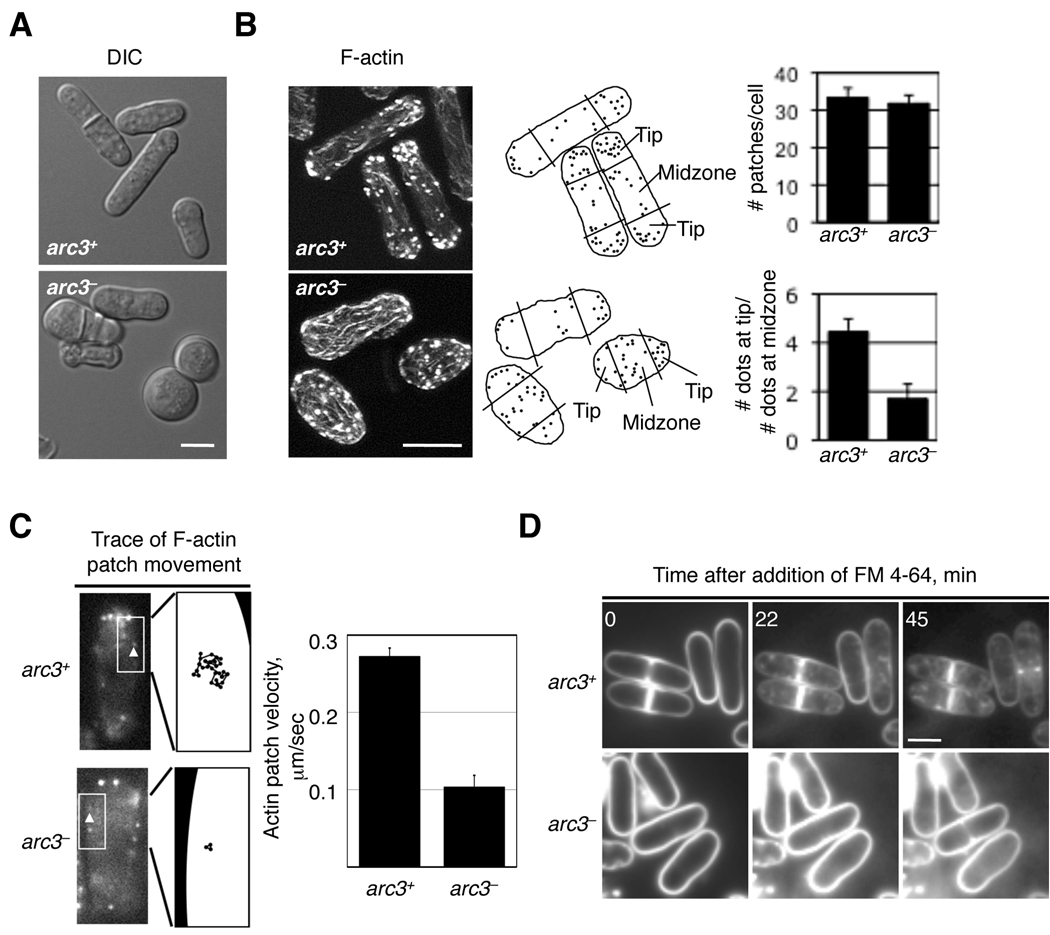
The actin cytoskeleton has been implicated in the regulation of cell polarity (Marks, et al., 1986). In fission yeast, the cell maintains an elongated morphology by growing from two ends of the cell, which are marked by F-actin patches. We examined the effect of reducing arc3 expression on cell shape and F-actin organization. Our data show that while normal cells maintain the typical rod-like shape, arc3-repressed cells became round or ellipsoid in shape (Figure 3A). Staining cells to visualize F-actin structures with Alexa Fluor 488 Phalloidin revealed a defect in actin organization. Whereas F-actin patches are mostly restricted to the cell ends in interphase cells expressing arc3 (arc3+), in arc3-repressed cells (arc3−), F-actin patches were more scattered and could be readily seen in the middle of the cell (Figure 3B). However, arc3 deficiency does not seem to affect other F-actin structures such as F-actin cables, which still extend along the cell body with no significant reduction in length or number, and rings that encircle the cell equator (data not shown). These observations agree with those of the study of Arp2 (Morrell, et al., 1999). These results suggest that Arc3 is required for bi-polar distribution of F-actin patches in fission yeast cells.
Figure 3.
arc3 is required for organization and mobility of F-actin patches and endocytosis. (A) arc3 expression was repressed as in Figure 2B for 20 hours, and both arc3− and arc3+cells were visualized by DIC microscopy. (B) arc3 expression was repressed as in A, the resulting cells were fixed and stained with the F-actin dye Alexa 488-Phalloidin. Shown here are projection images after deconvolution. The length of the cell was measured and dots located in the 25% of the length nearest to the end were counted as localized to that cell end. While F-actin patches are concentrated at the cell ends in arc3+ interphase cells, in arc3-repressed (arc3−) cells, these patches are more diffused whether the cell is round or not (right). The number and the size of F-actin patches are similar in these two types of cells. (C) arc3nmt cells expressing Crn1-GFP were treated with (arc3−) or without 200 nM thiamine (arc3+) for 16 hours and observed by time lapse microscopy. Crn1-GFP dots (left, arrowheads) were individually tracked and the velocity calculated (right). A total of 15 dots in wild type cells and 30 dots in the arc3 mutants were analyzed. We note that the measured Crn1-GFP velocity in wild type cells matches that reported previously (Pelham and Chang, 2001). (D) The arc3 expression was repressed as in (C) before the fluorescent dye FM 4–64 (8.15 mM) was added, and the cells were then observed by time lapse microscopy. This dye first bound the plasma membrane and then readily entered arc3+ cells and ultimately accumulated in the vacuoles. In contrast, in arc3− cells, this dye could only be detected at the plasma membrane. Bars, 5 µm.
F-actin patches are highly mobile and their mobility requires F-actin polymerization and functional Arp2 (Pelham and Chang, 2001). F-actin patches can be tracked by following the localization of GFP-tagged coronin (Crn1), which interacts with Arp2/3 (Pelham and Chang, 2001). We therefore tested whether Arc3 is also required for F-actin patch mobility by following the trayectory of Crn1-GFP containing patches through time. We determined that the average velocity of Crn1-GFP containing patches is reduced nearly three-fold in arc3− cells, and the distance travelled by individual patches is also much shorter (Figure 3C). The formation of endocytic vesicles is also believed to be driven by the Arp2/3 complex, and S. cerevisiae mutants defective in the Arp2/3 complex are deficient in endocytosis (Daugherty and Goode, 2008). We thus tested whether Arc3 is required for endocytosis in S. pombe by measuring the rate of internalization of the membrane dye FM 4–64. Our data showed that while FM 4–64 internalized readily in arc3+ cells, in arc3− cells, FM 4–64 mainly associated with the plasma membrane and did not internalize efficiently (Figure 3D). These results suggest that Arc3 is involved in the movement of F-actin patch function and endocytosis. Collectively, since Arc3 associates with Arp2/3 and controls the same set of functions known to be controlled by Arp2, we conclude that Arc3 is an authentic Arp2/3 subunit in S. pombe.
Yeast Arc3 and human ARPC3 have an evolutionarily conserved function
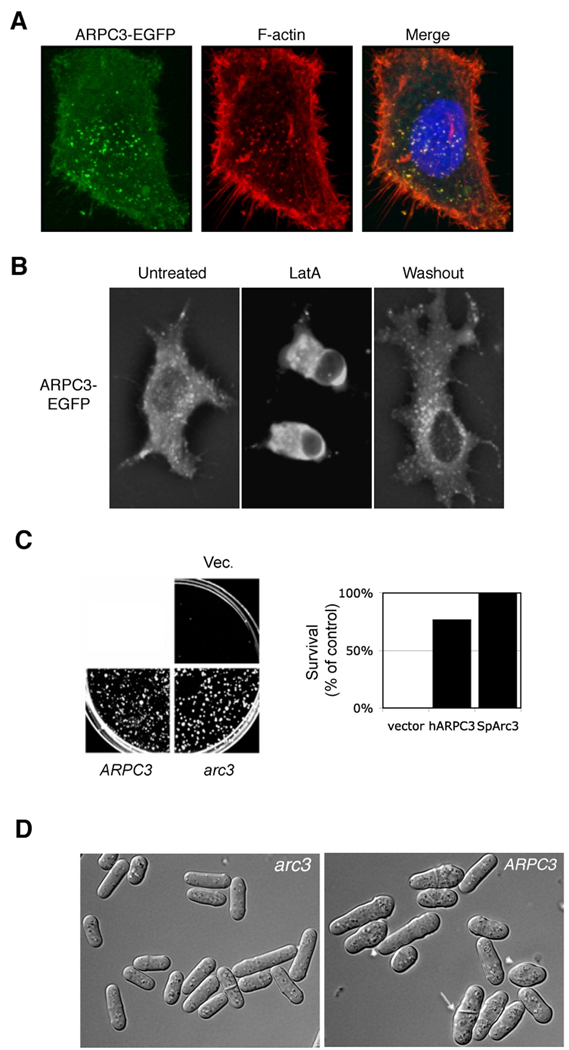
Based on sequence analysis, S. pombe Arc3 is most likely the ortholog of human ARPC3, whose functions have not been reported. We first examined whether human APRC3, like S. pombe Arc3, can associate with F-actin patches in human cells. We took HeLa cells ectopically expressing GFP-tagged ARPC3 and found that it colocalized with F-actin-rich patches visualized by phalloidin staining (Figure 4A). Treatment with Latrunculin A seemed to cause disappearance of Arc3-GFP structures in the cell with a concurrent increase of diffuse signal in cytoplasm (Figure 4B). To determine if the function of the two orthologs is conserved, we transformed arc3+/arc3::ura4 (arc3+/arc3Δ) cells with plasmids expressing either human ARPC3 or S. pombe arc3 or with the vector control. After sporulation, haploid spores were plated on media without uracil to select arc3Δ cells. Our data show that we could only recover viable arc3Δ cells when they carried either human ARPC3 or S. pombe arc3, indicating that human ARPC3 can efficiently rescue colony formation of S. pombe arc3Δ cells (Figure 4C). These cells were further examined by microscopy, and the data show that while most of the cells are elongated in cell-shape, they appeared to be more round, and wider and shorther than normal, and the septum in some mitotic cells was not properly positioned (Figure 4D). While human ARPC3 can replace S. pombe arc3Δ when the cell is grown in solid medium, when arc3Δ cells expressing ARPC3 were transferred to liquid medium, the resulting cells could barely grow. We conclude that S. pombe arc3 is an ortholog of human ARPC3 and that they control a similar set of functions, although human ARPC3 does not fully rescue the phenotype of S. pombe arc3Δ cells.
Figure 4.
arc3 function has been conserved throughout evolution. (A) HeLa cells expressing human ARPC3-EGFP were fixed and stained with rhodamine-phalloidin to visualize F-actin. We found that nearly every GFP dot overlaps with an F-actin dot. (B) HeLa cells expressing ARPC3-EGFP were treated with 10 µM Latrunculin A for 30 min to depolymerize F-actin. These cells were then washed and incubated in regular media for 1 hr before being photographed. (C) Diploid cells heterozygous for arc3 deletion (+/arc3::ura4) were transformed with plasmids expressing S. pombe arc3 (pREP41ARC3), human ARPC3 (pREP1ARPC3) or the empty vector as indicated. Cells were then sporulated, and plated on media with selection for the arc3 deletion. Relative survival compared to control was quantified (right). (D) Colonies from cells transformed with S. pombe arc3 (pREP41ARC3) or human ARPC3 (pREP1ARPC3) obtained as in (C) were grown on MM to log phase and observed under the microscope. The arrowheads show cells with abnormal morphology and the arrow indicates a cell with an improperly positioned septum.
CONCLUDING REMARKS
In this study, we identified the S. pombe Arc3 subunit of the Arp2/3 complex. As expected, Arc3 binds other subunits of the Arp2/3 complex, and localizes to F-actin patches. Cells deficient in Arc3, as with the arp2 mutant, contain an F-actin network that is disorganized. In particular, in these arc3-repressed cells, F-actin patches are dispersed throughout the cells with greatly reduced mobility. S. pombe arc3 mutant is also deficient in endocytosis, which is consistent with the observation that proper formation of F-actin patches correlates with efficient endocytosis. Our data also strongly suggest that Arc3 is the ortholog of human ARPC3 and that its function is conserved because (1) ARPC3 expression in S. pombe efficiently rescues lethality of arc3Δ cells in solid medium and (2) ARPC3 also localizes to patches and in HeLa cells the Arp2/3 complex has been shown to be recruited to sites of clathrin-mediated endocytosis (Carreno, et al., 2004). While in S. cerevisiae, deleting many genes encoding Arp2/3 subunits severely impairs cell growth (Winter, et al., 1999), arc18Δ cells are viable. By contrast, in S. pombe, deleting arp2, as well as arc3, induced lethality. Thus while the Arp2/3 complexes in both yeasts control F-actin patch organization and endocytosis, the essential function of Arc3 in S. pombe may be absent or performed by another protein in S. cerevisiae.
Acknowledgements
We thank Kathy Gould, Dan McCollum and Fred Chang for providing materials critical for this study. ECC is supported by grants from the NIH (CA90464 and CA107187), JS by fellowships from the Susan G. Komen Foundation (PDF0402733) and Expedition Inspiration Fund for Breast Cancer Research, and EY by a Graduate Research Fellowship from NSF.
REFERENCES
- Bähler J, Wu JQ, Longtine MS, Shah NG, McKenzie A, 3rd, Steever AB, Wach A, Philippsen P, Pringle JR. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14:943–951. doi: 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Balasubramanian MK, Feoktistova A, McCollum D, Gould KL. Fission yeast Sop2p: a novel and evolutionarily conserved protein that interacts with Arp3p and modulates profilin function. Embo J. 1996;15:6426–6437. [PMC free article] [PubMed] [Google Scholar]
- Cabrera R, Sha Z, Vadakkan TJ, Otero J, Kriegenburg F, Hartmann-Petersen R, Dickinson ME, Chang EC. Proteasome nuclear import mediated by Arc3 can influence efficient DNA damage repair and mitosis in Schizosaccharomyces pombe. Mol Biol Cell. 2010;21:3125–3136. doi: 10.1091/mbc.E10-06-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreno S, Engqvist-Goldstein AE, Zhang CX, McDonald KL, Drubin DG. Actin dynamics coupled to clathrin-coated vesicle formation at the trans-Golgi network. Journal of Cell Biology. 2004;165:781–788. doi: 10.1083/jcb.200403120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EC, Barr M, Wang Y, Jung V, Xu H, Wigler HM. Cooperative interaction of S. pombe proteins required for mating and morphogenesis. Cell. 1994;79:131–141. doi: 10.1016/0092-8674(94)90406-5. [DOI] [PubMed] [Google Scholar]
- Chen CR, Li YC, Chen J, Hou MC, Papadaki P, Chang EC. Moe1, a conserved protein in Schizosaccharomyces pombe, interacts with a Ras effector, Scd1, to affect proper spindle formation. Proc. Natl. Acad. Sci. U S A. 1999;96:517–522. doi: 10.1073/pnas.96.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty KM, Goode BL. Functional surfaces on the p35/ARPC2 subunit of Arp2/3 complex required for cell growth, actin nucleation, and endocytosis. J Biol Chem. 2008;283:16950–16959. doi: 10.1074/jbc.M800783200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbacher KL, Boldogh IR, Pon LA. A role for Jsn1p in recruiting the Arp2/3 complex to mitochondria in budding yeast. Molecular Biology of the Cell. 2005;16:5094–5102. doi: 10.1091/mbc.E05-06-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachet Y, Hyams JS. Endocytosis in fission yeast is spatially associated with the actin cytoskeleton during polarised cell growth and cytokinesis. J Cell Sci. 2005;118:4231–4242. doi: 10.1242/jcs.02530. [DOI] [PubMed] [Google Scholar]
- Girao H, Geli MI, Idrissi FZ. Actin in the endocytic pathway: from yeast to mammals. FEBS Lett. 2008;582:2112–2119. doi: 10.1016/j.febslet.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Goley ED, Welch MD. The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol. 2006;7:713–726. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- Gournier H, Goley ED, Niederstrasser H, Trinh T, Welch MD. Reconstitution of human Arp2/3 complex reveals critical roles of individual subunits in complex structure and activity. Mol Cell. 2001;8:1041–1052. doi: 10.1016/s1097-2765(01)00393-8. [DOI] [PubMed] [Google Scholar]
- Machesky LM, Gould KL. The Arp2/3 complex: a multifunctional actin organizer. Curr Opin Cell Biol. 1999;11:117–121. doi: 10.1016/s0955-0674(99)80014-3. [DOI] [PubMed] [Google Scholar]
- Marks J, Hagan IM, Hyams JS. Growth polarity and cytokinesis in fission yeast: the role of the cytoskeleton. J Cell Sci Suppl. 1986;5:229–241. doi: 10.1242/jcs.1986.supplement_5.15. [DOI] [PubMed] [Google Scholar]
- McCollum D, Balasubramanian M, Gould K. Identification of cold-sensitive mutations in the Schizosaccharomyces pombe actin locus. FEBS Lett. 1999;451:321–326. doi: 10.1016/s0014-5793(99)00619-5. [DOI] [PubMed] [Google Scholar]
- McCollum D, Feoktistova A, Morphew M, Balasubramanian M, Gould KL. The Schizosaccharomyces pombe actin-related protein, Arp3, is a component of the cortical actin cytoskeleton and interacts with profilin. Embo J. 1996;15:6438–6446. [PMC free article] [PubMed] [Google Scholar]
- Morrell JL, Morphew M, Gould KL. A mutant of Arp2p causes partial disassembly of the Arp2/3 complex and loss of cortical actin function in fission yeast. Mol Biol Cell. 1999;10:4201–4215. doi: 10.1091/mbc.10.12.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JM, Johnson DI. The Cdc42p GTPase and its regulators Nrf1p and Scd1p are involved in endocytic trafficking in the fission yeast Schizosaccharomyces pombe. J Biol Chem. 2001;276:3004–3009. doi: 10.1074/jbc.M007389200. [DOI] [PubMed] [Google Scholar]
- Naqvi SN, Zahn R, Mitchell DA, Stevenson BJ, Munn AL. The WASp homologue Las17p functions with the WIP homologue End5p/verprolin and is essential for endocytosis in yeast. Curr Biol. 1998;8:959–962. doi: 10.1016/s0960-9822(98)70396-3. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Arai R, Motegi F, Nakano K, Mabuchi I. Contractile ring formation in Xenopus egg and fission yeast. Cell Struct Funct. 2001;26:545–554. doi: 10.1247/csf.26.545. [DOI] [PubMed] [Google Scholar]
- Norbury C, Moreno S. Cloning cell cycle regulatory genes by transcomplementation in yeast. Methods Enzymol. 1997;283:44–59. doi: 10.1016/s0076-6879(97)83006-6. [DOI] [PubMed] [Google Scholar]
- Pelham RJ, Jr, Chang F. Role of actin polymerization and actin cables in actin-patch movement in Schizosaccharomyces pombe. Nat Cell Biol. 2001;3:235–244. doi: 10.1038/35060020. [DOI] [PubMed] [Google Scholar]
- Sawin KE, Nurse P. Regulation of cell polarity by microtubules in fission yeast. J. Cell Biol. 1998;142:457–471. doi: 10.1083/jcb.142.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin V, Beltzner CC, Marchand JB, Pollard TD. Interactions of WASp, myosin-I, and verprolin with Arp2/3 complex during actin patch assembly in fission yeast. J Cell Biol. 2005;170:637–648. doi: 10.1083/jcb.200502053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadlamudi RK, Li F, Barnes CJ, Bagheri-Yarmand R, Kumar R. p41-Arc subunit of human Arp2/3 complex is a p21-activated kinase-1-interacting substrate. EMBO Rep. 2004;5:154–160. doi: 10.1038/sj.embor.7400079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde F, Mata J, Nurse P. Fission yeast cell morphogenesis: identification of new genes and analysis of their role during the cell cycle. J Cell Biol. 1995;131:1529–1538. doi: 10.1083/jcb.131.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wear MA, Schafer DA, Cooper JA. Actin dynamics: assembly and disassembly of actin networks. Curr Biol. 2000;10:R891–R895. doi: 10.1016/s0960-9822(00)00845-9. [DOI] [PubMed] [Google Scholar]
- Welch MD, DePace AH, Verma S, Iwamatsu A, Mitchison TJ. The human Arp2/3 complex is composed of evolutionarily conserved subunits and is localized to cellular regions of dynamic actin filament assembly. J Cell Biol. 1997;138:375–384. doi: 10.1083/jcb.138.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D, Podtelejnikov AV, Mann M, Li R. The complex containing actin-related proteins Arp2 and Arp3 is required for the motility and integrity of yeast actin patches. Curr Biol. 1997;7:519–529. doi: 10.1016/s0960-9822(06)00223-5. [DOI] [PubMed] [Google Scholar]
- Winter DC, Choe EY, Li R. Genetic dissection of the budding yeast Arp2/3 complex: a comparison of the in vivo and structural roles of individual subunits. Proc Natl Acad Sci U S A. 1999;96:7288–7293. doi: 10.1073/pnas.96.13.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JQ, Sirotkin V, Kovar DR, Lord M, Beltzner CC, Kuhn JR, Pollard TD. Assembly of the cytokinetic contractile ring from a broad band of nodes in fission yeast. J Cell Biol. 2006;174:391–402. doi: 10.1083/jcb.200602032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Yang Z, Qian M, Zhu X. Interactions among subunits of human Arp2/3 complex: p20-Arc as the hub. Biochem Biophys Res Commun. 2001;280:513–517. doi: 10.1006/bbrc.2000.4151. [DOI] [PubMed] [Google Scholar]