Abstract
We have physically mapped and cloned a 2.5-Mb chromosomal segment flanking the centromeric end of the major histocompatibility complex (MHC). We characterized in detail 27 YACs, 144 cosmids, 51 PACs, and 5 BACs, which will facilitate the complete genomic sequencing of this region of chromosome 6. The contig contains the genes encoding CSBP, p21, HSU09564 serine kinase, ZNF76, TCP-11, RPS10, HMGI(Y), BAK, and the human homolog of Tctex-7 (HSET). The GLO1 gene was mapped further centromeric in the 6p21.2–6p21.1 region toward TCTE-1. The gene order of the GLO1–HMGI(Y) segment in respect to the centromere is similar to the gene order in the mouse t-chromosome distal inversion, indicating that there is conservation in gene content but not gene order between humans and mice in this region. The close linkage of the BAK and CSBP genes to the MHC is of interest because of their possible involvement in autoimmune disease.
The 2.5-Mb chromosomal segment flanking the centromeric end of the major histocompatibility complex (MHC) spans the boundary of the 6p21.2 (Giemsa dark) and 6p21.3 (Giemsa light) bands. This segment is of interest because of the association of the MHC with diseases, particularly of autoimmune etiology and the synteny to the mouse t-complex distal inversion on chromosome 17 (Hamvas et al. 1996). The conservation between the 4-Mb human MHC and the mouse H2 complex is well documented (Hanson and Trowsdale 1991; Amadou et al. 1995), but for the region centromeric to the MHC, it is not well established.
The mouse t-complex is an intensively studied region containing genes, which, when mutated or rearranged, cause a number of effects including embryonic lethality, male sterility, recombination suppression, and transmission ratio distortion (Silver 1993b; Foreijt et al. 1994). A part of this complex, within the distal inversion extending from the mouse Hmgi(y) gene to the H2-K region, contains a number of testis-expressed genes (Abe et al. 1988; Yeom et al. 1992). Testis-expressed sequences have been previously identified centromeric to the MHC (Ragoussis et al. 1992).
In humans, this region harbors a gene involved in retinitis pigmentosa (ArP14) (Shugart et al. 1995) whereas chromosomal rearrangements with breakpoints within the region are associated with neoplasia (Johansson et al. 1993; Nilbert et al. 1995; Xiao et al. 1997).
To investigate the relationship of this human segment to the mouse t-complex distal inversion, we have assembled YAC and bacterial clone contigs and mapped sequences with known homology between mice and humans. We screened a variety of genomic libraries in different cloning vectors to obtain clones, and we used both fingerprinting- and hybridization-based approaches to construct contigs. The resulting contigs have enabled the construction of transcript maps and are a first step toward construction of sequence-ready bacterial clone contigs.
RESULTS
Construction of a YAC Contig
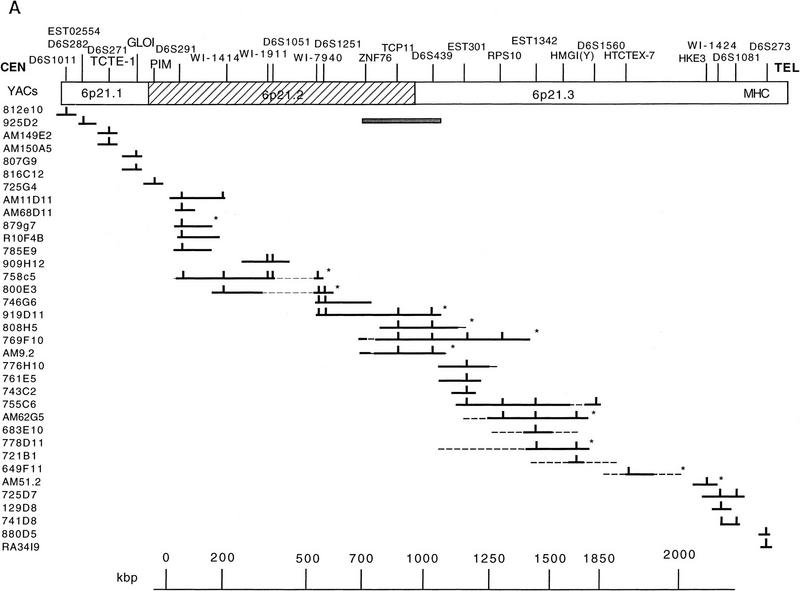
As a first step toward cloning the region, we constructed a YAC contig. This was completed by screening the CEPH, ICRF, and ICI libraries with the markers D6S273, D6S291, D6S439, D6S29, HMGI(Y), TCTE-1, Motilin, and GLO1 by PCR and with PIM1 and HKE3 probes by hybridization. In parallel, the Genèthon Quickmap programs were used to identify 175 clones from the CEPH YAC library that potentially linked the D6S291 marker to D6S273. Additional markers were used as released by the Whitehead database and by Sheffield et al. (1996). The results of these PCR- and hybridization-based screenings are shown in Figure 1A.
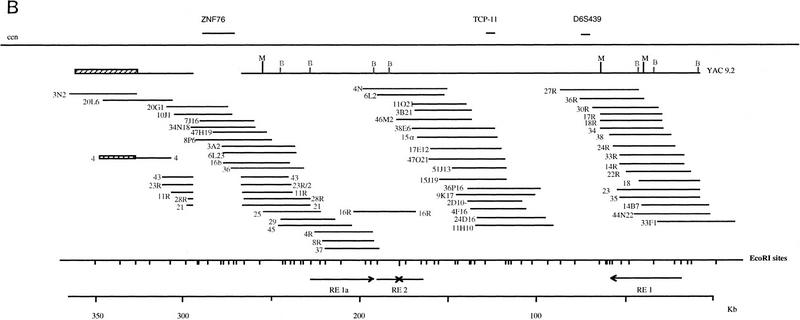
Figure 1.
(A) YAC contig covering the 6p21.2–21.3 region. YACs from three libraries are listed to the left and markers at the top. The YACs and markers in 6p21.1 are shown ordered toward the centromere. Vertical bars indicate the presence of markers in the particular clone and thin interrupted lines indicate chimeric parts. An asterisk indicates that the clone has been used to isolate the cosmids shown in B and Fig. 4. The shaded bar under the ZNF76–D6S439 region indicates the finely mapped segment shown in B. (B) Restriction map of the 350-kb ZNF76–TCP-11 region. The positions of the two genes and D6S439 are shown above, and the position of MluI and BssHII sites as determined by PFGE analysis of YAC 9.2 and digestion of cosmids are below. Cosmids are derived from the chromosome 6 library or by subcloning the YAC 9.2. The left end of the YAC is chimeric to 6p12 (hatched bar) and the same was found for the subcloned cosmid 4 that hybridizes by FISH to 6p21 and 6p21.2. The region deleted in YAC 9.2 is indicated by a gap in cosmids 43,23R/2,11R, 28R, and 21 (see also Fig. 3). The EcoRI map derived from the cosmids and the position of the repeated elements with relative orientation are presented at the bottom.
Additional clones were isolated with probes mapping further centromeric. These clones were with TCTE-1, AM150a5, AM149e2, AM149e3 (ICRF library); with GLO1, 807g9 and 816c12 (CEPH library); with PIM1, 756g4 (CEPH library) in addition to 4D3 and 5.2 containing PIM1 (described in Ragoussis et al. 1992). A clone positive for the Motilin PCR product, 648c8 (CEPH library) was isolated; however, subsequent mapping by FISH did not allow a localization on 6p.
No positives were identified in any of the three YAC libraries with the probe for the BAK gene. When high-density filters containing all isolated clones were screened, the ICI clone RA15B12d (isolated with the D6S29 marker) was positive and was positioned on the map retrospectively (see Fig. 4, below). The same YAC was positive with cosmids containing the BAK gene, but it does not contain the entire coding sequence, as shown by PCR analysis (J.A. Herberg, unpubl.). It is possible that even very low expression of this gene is lethal to yeast (Ink et al. 1997).
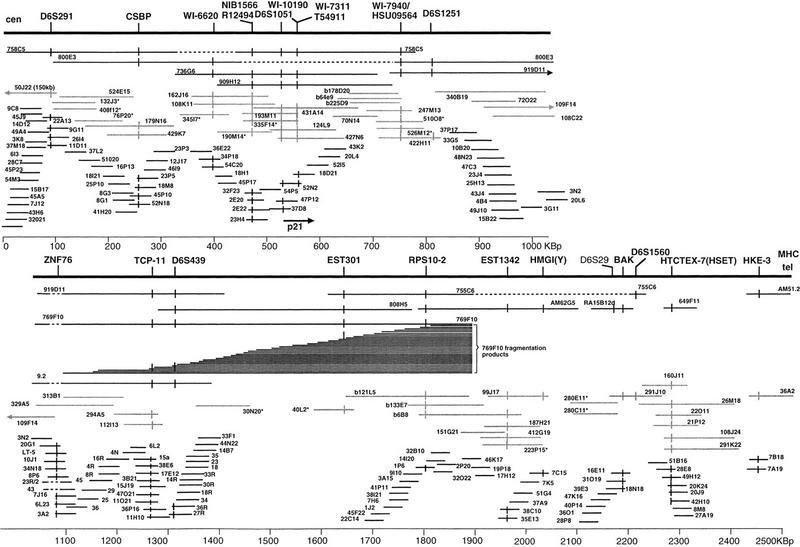
Figure 4.
Composite map of 2.5 Mb centromeric to the MHC. The backbone of YAC clones is shown at the top, underneath PAC and BAC clones (shaded) and cosmid clones (black). The extent of the bars indicates the length of the clones as established by PFGE and restriction enzyme digestion. Asterisks beside the clone address indicates that the size of the clone has not been determined. Markers are at the top and vertical bars on clones indicate positive screening results. The position of the D6S29 marker has not been confirmed in other clones except RA15B12d.
To establish that the YAC clones were derived from chromosome 6p, several overlapping clones were localized on chromosome 6 by FISH. All YACs that gave a signal on 6p mapped in the 6p21.2–6p21.3 interval (see FISH mapping, below), except YAC 816c12, containing GLO1, which was localized in the telomeric part of the 6p21.1 band. To establish the position of GLO1 in relationship to the TCTE-1 gene, another human homolog of a mouse t-complex gene, which is reported to map within the 6p21.1 chromosomal band at the marker D6S271, we mapped two YAC clones containing TCTE-1 (AM149e2 and AM150a5) within 6p21.1, proximal to 816c12. This indicates that GLO1 maps distal to TCTE-1 in humans. These data agree with data produced by the Whitehead institute, where YAC 816c12 is positioned in a contig distal to YACs containing D6S271. The YACs 812e10 and 923g9 containing D6S1011 were mapped at the centromeric end of the 6p21.1 band, close to 6p12. These YACs are reported as containing the marker D6S438 by the Whitehead database, the position of which agrees with this localization.
The YACs 683e10 and 649f11 failed to produce a signal on 6p but they were positioned on the map (Fig. 1) according to marker content and subsequent cosmid hybridization results. Finally, two YACs, 880d5 and RA34I9, containing D6S273 were mapped within the class III region because they hybridized to probes for HSP70.
Construction of Cosmid Pockets
A backbone of YAC clones was used to screen the ICRF chromosome 6-specific cosmid library. YACs 879g7, 758c5, 800e3, 808h5, 919d11, 769f10, 9.2, 62g5, 778d11, and 649f11 (Fig. 1A) were used to select 802 cosmids in total. The cosmids were picked into 96-well plates and spotted at high density onto filters. All of the YACs in Figure 1A were used to rescreen the cosmids and construct pockets. In addition, the YAC clone 9.2 was subcloned into the Supercos cosmid vector to enrich for clones mapping to the TCP-11 region. Sixty-five clones were picked and used along with the cosmids resulting from the hybridization screen.
Construction of Cosmid Contigs
The construction of cosmid contigs was performed by use of hybridization-based approaches: First, selected cosmids from individual pockets were used to hybridize the YAC filters and confirm the position of the particular cosmid and pocket. In all, 105 cosmids were used as hybridization probes on the YAC and cosmid filters. In addition, 59 probes derived from cosmid internal or end fragments and YAC end fragments were used. As a result, 346 cosmids were isolated from the chromsome 6-specific cosmid library, of which 190 were confidently assigned to contigs and mapped to defined positions.
Individual probes corresponding to known genes were also used to identify positive clones. Thus cosmids hybridizing to human KE3, mouse Tctex-7, BAK, TCP-11, ZNF76, and p21 (WAF-1) were initially identified within the isolated cosmids. The PIM-1 probe failed to identify any cosmids from the chromosome 6 library. Therefore the YAC 725g4 was used to isolate a number clones, two of which, 40D7 and 49O12 hybridized back to the YAC and were used for FISH mapping as representatives of this locus.
To aid the contig construction in the ZNF76–RSP10 region, the YAC 769f10 was fragmented by use of the vector pBCL8.1. Ninety-six products with a range of 100–800 kb were sized by PFGE and used to position probes from the region covered by YAC 9.2. Cosmids/probes were hybridized to gridded YAC fragmentation products, and the results were used to orient the cosmid contig encompassing YAC 9.2 relative to the other contigs (not shown and Fig. 4, below). The results also established the order of markers in the region (Fig. 4, below).
The presence of the CSBP gene was detected by PCR. Probes for the mouse Tctex-11 and Tctex-3 genes were also used, but these failed to detect any positives in both our selection of cosmids and the entire chromosome 6 library filters. The cosmid contigs were verified subsequently by double-digest fingerprinting after micropreparation in a 96-well format. By combining of the hybridization and fingerprinting data, nine contigs were constructed. The order of these contigs was determined not only according to their hybridization pattern on gridded YACs but also by elongated chromatin fiber (ECF) and interphase FISH techniques (see below). Further evidence of location and order was obtained by PFGE on genomic DNA.
BAC/PAC Screening and Contig Construction
To extend the contigs and to close gaps between the cosmids, the human BAC library was screened with end probes from cosmid 32022, resulting in the isolation of three BAC clones. One PAC clone was isolated by PCR with the marker WI-7940 and end probes from this PAC identified an additional three BAC clones. Another PAC clone was isolated by PCR with marker D6S1560 and in parallel by hybridization with the coding region of BAK. Whole cosmids and end probes were used for isolating PAC clones by hybridization to increase the depth of the contigs. Twenty cosmids were hybridized to the PAC library in two sets of ten. Ninety-one PACs were identified as positive. PAC/BAC walking with the use of end probes produced fifteen additional PAC clones.
The positioning of the BAC and PAC clones was verified by PCR with the relevant markers at each position and secondary screenings when appropriate. The overlaps of PAC and BAC clones were verified by hybridizations and fingerprinting, and the size of all key clones was determined by PFGE following NotI digestion. The fingerprinting patterns of the PACs and BACs were compared to the patterns obtained from the cosmids, leading to the construction of contigs that incorporated all types of clones.
In total, 82 clones were positioned, forming contigs that overlap with and extend the cosmid contigs, 50 of which are presented on the map in Figure 4 (below). Examples of these contigs can be seen at the Sanger Centre’s chromosome 6 database (http://www.sanger.ac.uk/HGP/Chr6/) as contig 2 (around D6S291), contig 8 (containing p21), contig 3 (WI-7940), and contig 6 (D6S439).
Detailed EcoRI, BssHII, and MluI Map of the YAC 9.2 Region
The region contained within YAC 9.2 was analyzed further by detailed hybridization screening with a series of cosmid fragments resulting from EcoRI digestion of the subcloned cosmids and end fragments of the chromosome 6 cosmids. Partial digestion with EcoRI of two subcloned cosmids was also performed, and the EcoRI digestion pattern of all the cosmids coming from the region was analyzed manually. All cosmids were investigated further for the presence of BssHII and MluI sites, which were assigned according to the partial digestion patterns of YAC 9.2. All the restriction sites were ordered (Fig. 1B), revealing the existence of two locations with multiple BssHII restriction sites, suggesting possible CpG islands. The detailed analysis also revealed an internal deletion of YAC 9.2, which was carried over to the subcloned cosmids. The 26-kb deletion was bridged by use of the chromosome 6 cosmids. Within this deleted segment lies the coding region of ZNF76. The deletion is also present in all the overlapping YACs that were screened from the region.
Another feature of this region is the existence of a specific repetitive element that extends over 33 kb. The element contains multiple EcoRI and BamHI fragments that cross-hybridize to one another. The repetitive element could not be suppressed by competition with total human DNA. The repeats contained BssHII and MluI restriction sites at varying locations, facilitating the separation and ordering of the EcoRI fragments. The repetitive element is present three times in the YAC 9.2 region, twice in reverse orientation and once in a combined form (Fig. 1B.).
The detailed analysis of the region allowed the positioning of TCP-11, ZNF76, and D6S439 within specific EcoRI fragments and the generation of a 350-kb restriction map of the region.
FISH Analysis and Long-Range Analysis by PFGE
The YAC clones that were used to assemble the contigs were mapped by FISH on metaphase chromosomes to 6p21.2–6p21.3. YAC 880d5 gave signals on 6p21.3, 3p21.3, and 14q22; RA34I9 localized on 6p21.31; 725d7 localized on 6p21.3, but also on 4q32, and 14q12–q13; and YAC 769f10 localized on 6p21.3. AM62g5, 879g7, and 756g4 were mapped to 6p21.2–p21.3 and 778d11 and RA10F4B to 6p21.2. 778d11 gave an additional signal on 11q24 and 879G7 on 2q32. YAC 816c12 was mapped to the distal part of 6p21.1, while AM149e2, AM150a5, and 925d2 mapped to 6p21.1 and 812e10 at the centromeric end of 6p21.1. The YACs 649f11 and 683e10 gave signals only on 4q25 and 4q27, respectively, suggesting that most of their insert is not derived from chromosome 6.
The BAC clones 6B8, 64E9, and 121L5 were localized on 6p21.2 whereas BAC 133E7 localized on 6p21.2–p21.3. The PACs 225D9 and 247M13 were localized on 6p21.2 and 6p21.2–p21.3, respectively. The following cosmids were localized on 6p21.3: 7A19, 7B18, 49H12, 39E3. The cosmids 7C15, 1J2, 33F1, 4R, 30R, 9G11 and 20L4 were localized on 6p21.2–p21.3. Cosmid 4, a subclone of YAC9.2 containing one of the YAC ends, was localized on 6p21.2 and 6p12.2–p12.3, confirming the chimeric nature of the YAC, but no other subclone hybridized to 6p12.2–p12.3. The cosmids 3N2, 33G5, 48N23, 36E22, 37P17, 23P5, 46I9, 16P13, and 11D11 were localized on 6p21.2. The cosmids 40D7 and 49O12, isolated by use of the YAC 725G4, were localized on 6p21.2.
The order of cosmid, PAC, BAC, or YAC clone trios was determined in interphase nuclei. Orders established were: 45P17–PAC 225D9–48N23 (ratio 36:6); 33F1–7K5–12B11 (ratio 30:14); 33F1–40E15–39E3 (ratio 21:5); and 40E15–7K5–39E3 (ratio 21:8). The cosmid 1J20, found to map within the MHC class III region, was used to establish the order of clones in respect to the MHC. This was as follows: 1J2–39E3–1J20 (ratio 10:3); and 51B16–7B18–1J20 (ratio 12:2).
The distance between individual clones within a range of 100–1000 kb was established by FISH on calibrated interphase nuclei. These were as follows: 46K17–87 ± 125 kb–1J2–390 ± 22 kb–7C15; 7B18–185 ± 35 kb–51B16–250 ± 35 kb–7K5; 20G1–323 ± 35 kb–33F1–321 ± 46 kb–14I20; 18H1–580 ± 65 kb–48N23 ≤ 50 kb–3N2; and 46I9–100 ± 35 kb–45P17–320 ± 40 kb–3N2 (see Fig. 2).
Figure 2.
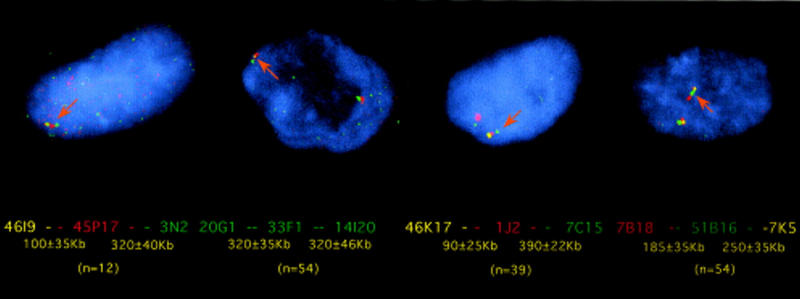
Determination of cosmid clone order and distances by interphase FISH across the 6p21.2–6p21.3 region. From left to right (combinations of three clones): 20G1 (digoxigenin labeled), 33F1 (double labeled), and 14I20 (biotin labeled); 46K17 (double labeled), 1J2 (digoxigenin labeled), and 7C15 (biotin labeled); 7B18 (digoxigenin labeled), 51B16 (biotin labeled), and 7K5 (double labeled); and 46I9 (double labeled), 45P17 (digoxigenin labeled), and 3N2 (biotin labeled). In brackets below is the number of nuclei measured.
For higher resolution (10- to 400-kb range), chromatin fibers were used, which also confirmed the overlap between clones, as well as the distances established by analysis of the EcoRI restriction pattern of cosmids. The distances were defined as cosmids, reflecting the average length of signal on the ECF when a single cosmid was used as a probe. The results were 9G11–2/3cos–16P13; 20L4–2/3cos–52N2 ⩾ 2cos–9G11; 20L4–5.8cos–9G11; 23P5–2cos–36E22–45P17; 23P3–1.3cos–36E22–45P17; 18H1 ⩾ 2.2cos–33G5–48N23; 6L23–2cos–11H10–1.5cos–33F1; and 7B18–7A19–1.6cos–49H12 (see Fig. 3). The same technique allowed the demonstration of the internal deletion of YAC 9.2. In this case, we compared the subcloned cosmid 23R/2 and cosmid LT-5, which contains the coding region of ZNF76. This comparison revealed that ∼30 kb around the ZNF76 region was deleted in YAC 9.2 (see Fig. 3).
Figure 3.
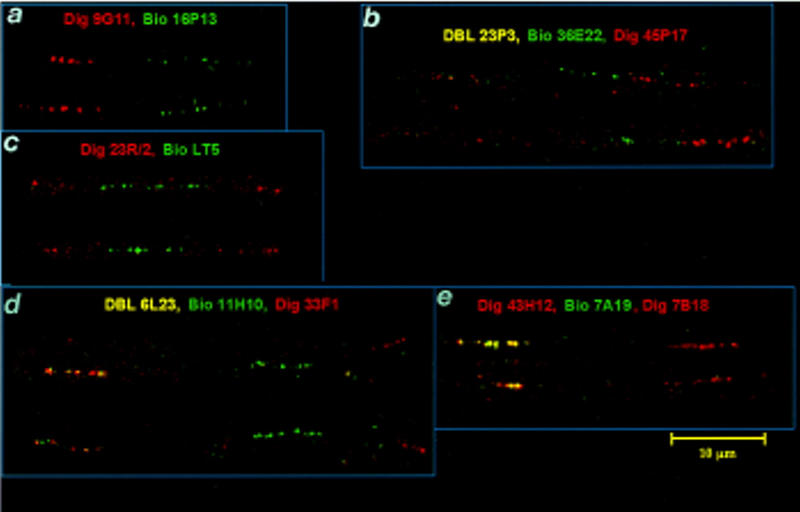
ECF–FISH analysis of cosmids across the 6p21.2–6p21.3 region. From centromere to telomere: (a) The distance between cosmids 9G11 and 16P13 in the D6S291 region is about one-half cosmid length. (b) Ordering the cosmids 23P3–36E22–45P17. (c) Visualization of the deletion in YAC 9.2 that encompasses ZNF76. The cosmid LT-5 containing ZNF76 is flanked by the subcloned cosmid 23R/2. (d) Ordering of cosmids in the region ZNF76–TCP-11. (e) The cosmid 49H12 (right) containing HTCTEX-7 is two cosmid lengths apart from the overlapping cosmids 7A19 and 7B18, which contain HKE3.
Further confirmation of distances and order was derived from PFGE analysis with probes derived from the HKE3, HTCTEX-7, TCP-11, ZNF76, CSBP, HMGI(Y), BAK, and p21 regions (data not shown). In particular, the position of the cosmid contig containing HTCTEX-7 was established by the detection of a 440-kb NotI and a 230-kb MluI fragment that also hybridized with the probe for HKE3. This result is consistent with NotI and MluI fragments that extend toward the centromere encompassing RING1, RING2, and HKE3 (Hanson and Trowsdale 1991). The distance between HKE3 and Col11α2 is 100 kb. In line with the distances obtained by FISH, the detection of these fragments positioned HTCTEX-7 proximal to HKE3 and within 130 kb of it. The human homolog of Tctex-7 (HTCTEX-7) was identified as HSET by (Ando et al. 1994), and our data are in full agreement with the position given in their paper.
Mapping of Genes and ESTs within the Contigs
The cDNAs corresponding to the BAK gene, the HMGI(Y) gene, HTCTEX-7, EST 1342, RPS10, the serine kinase HSU09564(WI-7940), TCP-11, ZNF76, p21, EST T54911, and EST R12494 were all mapped within cosmid, PAC, or BAC clones (see Fig. 4). EST 301 was present on YAC 769f10, but no cosmids were identified by PCR. The EST is highly similar to RPS10, (in EST 301 the ORF for the RPS10 protein is disrupted) and indeed hybridizes to cosmids containing RPS10. Because RPS10 is detected by PCR with primers derived from intronic sequences, in contrast to EST 301, where the primers are derived from cDNA sequences, it is likely that this EST corresponds to a RPS10 pseudogene. The position for this pseudogene, was established by PCR screening of the YAC fragmentation products of 769f10. All products with a size of 250 kb and above contained EST 301, whereas products with a size of 100 kb and above contained RPS10. The D6S439 marker was present in products of 550 kb and longer as well as within the contig (see Fig. 4). These data establish that EST 301 maps between RPS10 and D6S439, 150 kb centromeric to the RPS10 gene.
The marker WI-7940, corresponding to the mRNA for serine kinase HSU09564, was positioned centromeric to ZNF76 on PACs 247M13, 510O8, and 526M12. The p21 (WAF-1) gene was mapped to cosmids 52N2 (5′ end) and 18D21 (3′ end) by PCR and hybridizes together with the markers WI-10190, WI-7311, and the EST T54911, all of which correspond to the p21 gene.
These results, combined with the location of markers (Fig. 4) establish the following order: Cen–D6S291–CSBP–WI-6620–NIB1566 and R12494–D6S1051/p21–5′–WI-10190/WI-71311/p21–3′/T54911–WI-7940/HSU09564–ZNF76–TCP-11–D6S439–EST 301–RPS10–EST 1342–HMGI(Y)–D6S29–BAK–D6S1560–HTCTEX-7(HSET)–HKE-3–MHC. The distances between markers and genes were also determined (Fig. 4). Other ESTs tested but not found in the YAC/bacterial clone contigs were D6S1535E, D6F304S2E, D6F310S2E (reported to map on chromosome 6), EST 02289, EST 02554 as well as the markers FB2A D6S394–D6S389 and D6S329. Of these, EST 02554 was detected on YAC 925d2 reported to be part of a contig around the D6S271 marker at 6p21.1.
We were not able to locate the PIM1 gene accurately. The CEPH YAC 725g4 isolated with the PIM1 cDNA has been mapped to 6p21.2–21.3 on metaphase chromosomes. By comparison, the ICRF YACs that were isolated previously were mapped to 6p21.2 by the same technique. There were no Whitehead data about this YAC in the database release of 6/9/96. Cosmids isolated from the chromosome 6 library with YAC 725g4 were mapped back to the YAC and to chromosome 6p21.2 (clone 40D7, 49O12). Interphase FISH mapping of 40D7 in combination with cosmids from the D6S291–D6S439 interval did not yield any consistent order or distances. We have observed inconsistent distance and orders in cases where the distances between the probes was >600 kb. This observation would indicate that PIM1 is not within the 2.5-Mb region presented here. This hypothesis was supported by PFGE results, where the bands hybridizing to PIM1 did not correspond. The EST map data position PIM1 in the D6S291–D6S1641bin (the next marker toward the centromere), and, therefore, in combination with our data, the most likely location for PIM1 would be centromeric of D6S291 within 6p21.2.
DISCUSSION
We have constructed a physical map by generating YAC, PAC, BAC, and cosmid contigs covering the region between the D6S291 marker and the MHC, crossing the 6p21.2 and 6p21.3 chromosomal bands. The contigs have been generated by use of a combination of techniques. Initial assembly was performed by hybridization of YAC-derived probes to organized library filters followed by hybridization of whole cosmids or probes derived from them to cosmid and YAC filters. After contig assembly, the position of the clones within the contigs was confirmed by fingerprinting. In general there was overall agreement between the data obtained with both techniques.
The 2500 kb are covered in three bacterial clone contigs. One contig, extending from PAC 50J22 to 30N20 and covering 1500 kb from D6S291 to D6S439 inclusive, a second covering 400 kb from PAC40L2 to cosmid 7C15 containing the markers EST 301 to HMGI(Y) and one covering 350–400 kb from PAC280E11 to PAC26M18, containing BAK and HTCTEX-7. The gaps between these contigs reflect mainly, on the one hand, regions not represented in the cosmid library, as the cosmid contigs are the result of exhaustive walking steps, and, on the other hand, the paucity of additional markers available at the time, that could enable a direct screen of genomic libraries. Closing the gaps through multiple walking steps in the PAC library has not been completed because of time constraints. However, PFGE and high resolution FISH mapping data confirm that the gap between the cosmids 33F1 and 22C14 in the D6S439–EST 301 region is 200 kb, part of which is covered with the PACs 30N20 and 40L2. The same techniques suggest that the gap between cosmid 7C15 and the PACs 280E11/280C11 is either not existing or below 10 kb, and the gap between PAC 291K22 and the cosmids 7B18/7A19 is on the order of 5–20 kb (see Fig. 4).
The contigs consist of 27 YACs, 144 cosmids, 51 PAC, and 5 BAC clones covering 2.5 Mb centromeric to the MHC. This region contains genes that have been implicated in the development of neoplasia, such as p21 and HMGI(Y) (Fedele et al. 1996). Of particular interest is the finding that 6p21 rearrangements involve the region around the HMGI(Y) gene (Kazmierczak et al. 1996). Apart from affecting the expression of the HMGI(Y) gene, these rearrangements may also influence the expression of other genes in the vicinity, such as BAK. The BAK gene product is a potent inducer of apoptosis and shows widespread expression in human tissues. It is a homolog of the Bcl2 gene, and transgenic mice expressing the human Bcl2 gene constitute a model for systemic autoimmune disease resembling the human disorder systemic lupus erythematosus (Strasser et al. 1991).
In humans, the genotype of the MHC is the strongest genetic determinant for many autoimmune diseases, and BAK maps just 300 kb centromeric to the MHC class II region. Another gene of immunological interest in the region is the gene encoding CSBP, a protein kinase activated by mitogens and stress stimuli, which plays a critical role in cytokine production (IL-1, TNF) (Lee et al. 1994). TNF maps further toward the telomere within the MHC class III region. The mapping of CSBP close to the MHC increases the number of functionally related genes within this chromosomal segment. A number of genes that are possibly involved in immune processes have also been identified telomeric of the class I region (Gruen and Weissman 1997), and it is possible that genes functionally linked to genes within the MHC are located within several megabases either side of the complex. Therefore, it would be interesting to investigate any involvement of these genes in autoimmunity or other diseases associated with the MHC. The function of the HSU09564 serine kinase is not established. It is located at the 3′ end of p21, which encodes a mediator of p53 function that plays a role in cell cycle regulation.
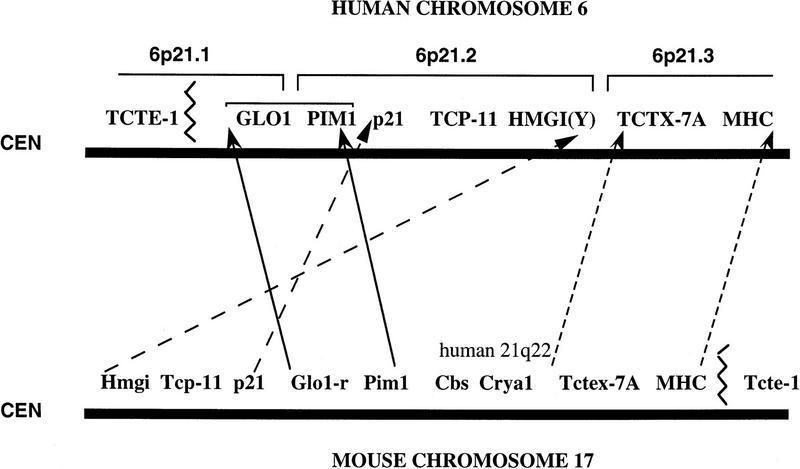
Of further particular interest is the comparison of this map to the map of mouse chromosome 17. There, the wild-type gene order is cen–Hmgi(y)–Tcp-11–Tctex-7–Cdkn1(p21)–Glo1–Pim1–Crya-1–Tctex-7A–H2ke3–Mhc–Tcte1–Tel (Lai et al. 1994; Hamvas et al. 1996). Apart from the segment around Crya1 that maps to human chromosome 21, the other genes have been mapped to chromosome 6p. Our data indicate that, in humans, the segment HMGI(Y)–GLO1 is inverted, thus resembling the mouse t-complex distal inversion in which the gene order is Cen–Pim1–Glo1–Hmgi(y) rather than the wild type (Fig. 5). Also, this segment is considerably closer to the MHC in humans than in mice. Sporadic reports of HLA associations with neural tube defects, spontaneous abortion, and infertility, and observations of transmission distortion and deficits of homozygotes have been reported. Although the findings remain controversial (Kostyu 1994; Jin et al. 1995), this close linkage may explain some of the data. In addition, the region between Hmgi(y) and Tcp-11 contains the tf locus in mouse and the region between Tcp-11 and Tctex-7B the Hst4 locus and loci for t-complex lethal mutations (Silver 1993a; Foreijt et al. 1994). Human homologs of these loci would then map within 6p21.2–6p21.3.
Figure 5.
Human–mouse comparative map between the 6p21.1–6p21.3 segment of chromosome 6 and mouse chromosome 17. Blocks of genes are conserved but the relative position and orientations of these blocks are different between the two species.
The gene order in relationship to the centromere for the segment HTCTEX-7–HKE3–MHC resembles the mouse wild type (Fig. 5). The breakpoint of continuous synteny between mice and humans centromeric to the MHC is between the HMGI(Y) and HTCTEX-7 genes (the position of Bak in mouse is not accurately established) and coincides with chromosomal breakpoints observed in neoplasias. In mouse, the segment Tctex-7A–Crya1 contains the Tctex-8–11 genes, the Eke1-5 genes, and Rab11b (Lai et al. 1994). Although we did not detect any chromosome 6 cosmid clone for Tctex-11 gene, some of the human homologs of these genes may still map on this chromosome.
When the physical map constructed here is compared to the genetic map, there is good agreement between the genetic distance of D6S291–D6S273 reported to be 5 cM and the physical distance, which is 4.2 Mb (as D6S273 maps about 1.7 Mb away from the end of this contig). The physical mapping of D6S1081 and D6S1051 centromeric of the MHC and of D6S1011 at 6p21.1 allows also the anchoring of the genetic map generated by Sheffield et al. (1996) to this chromosomal segment.
The PAC clones presented in this study serve as templates for genomic sequencing at the Sanger Centre as part of the effort to completely sequence human chromosome 6. This effort combined with cDNA selection and exon trapping experiments (N. Tripodis and J. Ragoussis, in prep.) will lead to the construction of a complete transcript map of the 6p21.2–6p21.3 region.
All data presented in this paper have been submitted to GDB (accession no. GDB:6279934).
METHODS
PCR Primers and Conditions
PCR primers and conditions were: for D6S291, D6S273, D6S439 as described by Gyapay et al. (1994); for markers D6S1011, D6S1051, and D6S1081 as described by Sheffield et al. (1995); and for the ESTs FB2A, 02554, 301, and 1324 as described by Pappas et al. (1995). Primers and conditions for markers WI-6620, NIB1566, WI-10190, WI-7311, WI-7940, RPS10-2, and D6S1560 were as described in the Whitehead Institute database, release 11/10/1996 (http://www-genome.wi.mit.edu/cgi-bin/contig/phys_map). Primers and conditions for the ESTs T54911 and R12494 were as described by Schuler et al. (1996) and for ESTs D6S1535E, D6F304S2E, and D6F310S2E as described by Evans et al. (1996).
Additional primers and conditions are as follows: For HMGI(Y) (CCTTTGCTTCACTTGGTTTACCC/GGTAGTCAGGGACAGTCATCAC) Tann = 68°C, 1.5 mm MgCl2, 2100-bp product (Friedman et al. (1993); for p21(WAF1) (CTTTCTAGGAGGGAGACAC/GTTCCGCTGCTAATCAAAG) Tann = 57.5°C, 1.5 mm, 99-bp product (el-Deiry et al. 1993); for TCTE-1 (B10C:TCTGACAGTTCCGGAGTGC/B10D:AGAGCCTGGTCTCACAAGAG) Tann = 62°C, 1.5 mm MgCl2, 332-bp product (Kwiatkowski et al. 1991); for Motilin (Mota:CCCTGACTGTCGCTGTTCC/Motb:GTGCACGTGGTCTGAGTGG) Tann = 62°C, 248 bp (Gasparini et al. 1994); for glyoxylase (Glo1-L:GGACTGATGGATCACTGTCCC/Glo1-R:GCAGCCTCTGAAGGGAACTG) Tann = 62°C, 3 mm MgCl2, 192-bp product; for CSBP (AAAAGGAGAGAAAGTGTA/TCCACCTGTTCCTCTTTTCAT) 48°C, 1.5 mm MgCl2, 547-bp product (McDonnell et al. 1995).
DNA Probes
The probes and hybridization conditions for tctex 7, tctex 3, and tctex 11 were described previously (Ha et al. 1991). The probes for PIM1, TCP-11, and ZNF76 were described by Ragoussis et al. (1992), for HKE3 (RPS18) by Hanson and Trowsdale (1991), and for BAK by Kiefer et al. (1995). The probe for D6S29 was described by Hoff et al. (1988).
Genomic Libraries
YAC libraries The ICI (Anand et al. 1990), CEPH (Albertsen et al. 1990), and ICRF (Larin et al. 1991; clone prefix AM) were screened courtesy of Dr. D. Bentley (Sanger Centre) at Guy’s Hospital. Hybridization filters and clones of the human PAC library (Ioannou et al. 1994) were obtained from the MRC–Human Genome Mapping Project-Resource Centre and the Sanger Centre (Hinxton, Cambridge, UK). The BAC clones were isolated by hybridization of filters obtained from Genome Systems (Kim et al. 1996). BAC 6B8, reported to map on 6p21 (Genome Mapping and Sequencing, Cold Spring Harbor, NY 1994 and Gene Mappers, American Society of Human Genetics Meeting 95) was obtained from Dr. J. Korenberg (UCLA).
YAC 9.2 was subcloned into the cosmid vector Supercos1 after partial MboI digestion as described previously (Ragoussis and Monaco 1996).
PFGE
YACs, PACs, and BACs were sized by PFGE. YACs were analyzed as described previously (Ragoussis 1996). The following conditions were used for PACs: 6 V/cm, pulse time 1–20 sec for 16 hr at 12°C in a Bio-Rad DRII apparatus. PFGE of genomic DNA was performed as described previously (Ragoussis et al. 1992).
YAC and Bacterial Clone Screening and Characterization
The chromosome 6 library filters (Nizetic et al. 1994) were screened with Alu PCR probes and whole YAC DNA as described previously (Ragoussis and Monaco 1996). Generation of high-density YAC and cosmid filters and preparation of DNA pools for PCR-based screening was as described previously (Bentley et al. 1992; Cole et al. 1992). YAC end probes were generated by vectorette PCR, and the same technique was applied to PACs, BACs, and cosmids (Ragoussis and Olavesen 1997). Cosmid, PAC, and BAC clone DNA was prepared by use of standard techniques.
YAC Fragmentation
YACs were fragmented by use of the vector pBCL8.1 (Lewis et al. 1992), and the products were analyzed by the arrayed preparation method (Markie 1995).
Cosmid to PAC Hybridization
Miniprepped cosmid DNA was digested with HindIII. Then, 10–15 ng of DNA was random oligolabeled with [α-32P]dCTP and incubated for 3 hr at room temperature. With sonicated cosmid vector and sonicated human DNA as competitors, the labeled probe was hybridized to the PAC library for 18 hr at 65°C (Gregory 1997).
Fingerprinting and Contig Construction
Micropreps of clones were performed as described previously (Birnboim and Doly 1979; Gibson and Sulston 1987). Double restriction digest fingerprinting was as first described by Coulson et al. (1986), with the modification of a one-step reaction (Gregory 1997). Briefly, two restriction enzymes (HindIII and Sau3A) and a DNA polymerase (AMV-reverse transcriptase) were employed. The HindIII sites alone were labeled in the first position with [α-32P]dATP before closure with a ddGTP terminator. The bands were separated on a 4% denaturing polyacrylamide gel. Then, gels were dried to the glass plate and exposed to sensitized autoradiographic film overnight at −70°C in a cassette with intensifying screens and for 3 days at room temperature without screens. Gels were digitized with an Amersham Film Reader. Then, the program Image (Sulston et al. 1989) was used to process and enter the gel. The program FPC (Soderlund and Longden 1996; Gregory 1997) was used to analyze clones. This program has an ACeDB (a Caenorhabditis elegans database) format and uses the probability of coincidence equation adopted from contig 9. Each comparison produces a negative log score. The lower this number, the greater the likelihood that the overlap is genuine.
Chromosome Walking
End probes from cosmids were generated by digestion of the clones with EcoRI, blotting, and hybridization with Sp6 (for lawrist vector cosmids) or T3 (Supercos vector cosmids) and T7 (lawrist and Supercos) primers (Ragoussis and Olavesen 1997). The bands corresponding to the particular cosmid end were cut out of an LMP gel and used as hybridization probes. Alternatively, end probes were generated by vectorette PCR. The cosmids were digested with EcoRV, PvuII, or RsaI. End primers, specific for the particular vector end were used in combination with the vectorette primers. The same procedure was used for PAC and BAC clones.
FISH
FISH on metaphase spreads and interphase nuclei was performed as described previously (Olavesen et al. 1995). ECFs were prepared from actively dividing normal male lymphoblasts by use of the technique of Parra and Windle (1993) on slides that had been previously coated with gelatin and poly-l–lysine as described by Heiskanen et al. (1994, 1995). Briefly, glass slides were treated for 30 sec in each of 0.2 n HCl, distilled H2O, and acetone, and allowed to air dry. Then, they were soaked for 5 min in 0.15% gelatin and left to dry overnight at room temperature. The following day, they were soaked twice successively in 0.01% polyl–lysine and again allowed to dry overnight at room temperature. Then, ECFs were prepared on the coated slides as follows: Actively dividing lymphoblasts were washed and resuspended in PBS at 1.5 × 105 cells/ml. Two 2-ml spots were placed ∼1 cm apart at one end of a horizontally positioned slide and allowed to air dry. Each spot was overlaid with 5 ml of 0.5% SDS/50 mm EDTA/200 mm Tris (pH 7.4) and incubated at room temperature for 5 min. The slide was then tilted to an angle of ∼60° to cause the DNA to stream down its length. After air drying at room temperature, the DNA fibers were fixed by treatment in fixative (3:1 methanol/acetic acid) for 3 min. ECF slides were generally prepared 1–3 days before being used for FISH and in the interim were stored at room temperature. FISH on ECFs was performed as for metaphase and interphase nuclei, but with the modification that prehybridization with 50× (wt/wt) Cot-1 DNA was for only 8 min.
Acknowledgments
This work was supported by grants from the Medical Research Council (G9215293), EEC (GENE-CT93-0075), Wellcome (049873) and the Guy’s Hospital Special Trustees. We thank Dr. Karen Artzt and Dr. Young Yeom for the Tctex-7 and Tctex-11 cDNAs; Professor Julie Korenberg for BAC 6B8; Dr. Nigel K. Spurr for the donation of primers for D6S1535E, D6F304S2E, D6F310S2E; Dr. Stepan Beck (Sanger Centre) for his interest in the work; Dr. Mark Olavesen for discussions; and Elisabeth Bentley for technical assistance.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL i.ragoussis@umds.ac.uk; FAX 44-171-955-4444.
REFERENCES
- Abe K, Wei JF, Wei FS, Hsu YC, Uehara H, Artzt K, Bennett D. Searching for coding sequences in the mammalian genome: The H-2K region of the mouse MHC is replete with genes expressed in embryos. EMBO J. 1988;7:3441–3449. doi: 10.1002/j.1460-2075.1988.tb03218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albertsen HM, Abderrahim H, Cann HM, Dausset J, Le Paslier D, Cohen D. Construction and characterization of a yeast artificial chromosome library containing seven haploid human genome equivalents. Proc Natl Acad Sci. 1990;87:4256–4260. doi: 10.1073/pnas.87.11.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadou C, Ribouchon MT, Mattei MG, Jenkins NA, Gilvert DJ, Copeland N, Avoustin P, Pontarotti P. Localization of new genes and markers to the distal part of the human major histocompatibility complex (MHC) region and comparison with the mouse: New insights on the evolution of mammalian genomes. Genomics. 1995;26:9–20. doi: 10.1016/0888-7543(95)80077-y. [DOI] [PubMed] [Google Scholar]
- Anand R, Riley JH, Butler R, Smith JC, Markham AF. A 3.5 genome equivalent multi access YAC library: Construction, characterization, screening and storage. Nucleic Acids Res. 1990;18:1951–1956. doi: 10.1093/nar/18.8.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando A, Kikuti YY, Kawata H, Okamoto N, Imai T, Eki T, Yokoyama K, Soeda E, Ikemura T, Abe K, Inoko H. Cloning of a new kinesin-related gene located at the centromeric end of the human MHC region. Immunogenetics. 1994;39:194–200. doi: 10.1007/BF00241260. [DOI] [PubMed] [Google Scholar]
- Bentley DR, Todd C, Collins J, Holland J, Dunham I, Hassock S, Bankier A, Giannelli F. The development and application of automated gridding for efficient screening of yeast and bacterial ordered libraries. Genomics. 1992;12:534–541. doi: 10.1016/0888-7543(92)90445-x. [DOI] [PubMed] [Google Scholar]
- Birnboim HC, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole CG, Patel K, Shipley J, Sheer D, Bobrow M, Bentley DR, Dunham I. Identification of region-specific yeast artificial chromosomes using pools of Alu element-mediated polymerase chain reaction probes labeled via linear amplification. Genomics. 1992;14:931–938. doi: 10.1016/s0888-7543(05)80114-4. [DOI] [PubMed] [Google Scholar]
- Coulson A, Sulston J, Brenner S, Karn J. Toward a physical map of the genome of the nematode Caenorhabditis elegans. Proc Natl Acad Sci. 1986;83:7821–7825. doi: 10.1073/pnas.83.20.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C, Bouzyk M, Cox S, Warne D, Bryant SP, Spurr NK. Chromosomal assignment of 79 cDNAs from a range of human tissues. Genomics. 1996;31:130–134. doi: 10.1006/geno.1996.0021. [DOI] [PubMed] [Google Scholar]
- Fedele M, Bandiera A, Chiappetta G, Battista S, Viglietto G, Manfioletti G, Casamassimi A, Santoro M, Giancotti V, Fusco A. Human colorectal carcinomas express high levels of high mobility group HMGI(Y) proteins. Cancer Res. 1996;56:1896–1901. [PubMed] [Google Scholar]
- Foreijt J, Artzt K, Barlow D, Hamvas R, Lindahl KF, Lyon M, Klein J, Silver LM. Mouse chromosome 17. Mamm Genome. 1994;5:238–258. [PubMed] [Google Scholar]
- Friedman M, Holth L, Zoghbi H, Reeves R. Organization, inducible-expression and chromosome localization of the human HMG-I(Y) nonhistone protein gene. Nucleic Acids Res. 1993;21:4259–4267. doi: 10.1093/nar/21.18.4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini P, Grifa A, Savasta S, Merlo I, Bisceglia L, Totaro A, Zelante L. The motilin gene: Subregional localization, tissue expression, DNA polymorphisms and exclusion as a candidate gene for the HLA-associated immotile cilia syndrome. Hum Genet. 1994;94:671–674. doi: 10.1007/BF00206962. [DOI] [PubMed] [Google Scholar]
- Gibson TJ, Sulston JE. Preparation of large numbers of plasmid DNA samples in microtiter plates by the alkaline lysis method. Gene Anal Techniques. 1987;4:41–44. doi: 10.1016/0735-0651(87)90016-1. [DOI] [PubMed] [Google Scholar]
- Gregory S. Contig assembly by fingerprinting. In: Dear PH, editor. Genome mapping: A practical approach. Oxford, UK: Oxford University Press; 1997. . (In press.) [Google Scholar]
- Gruen, J.R. and S.M. Weissman. 1997. Evolving views of the MHC. Blood (in press). [PubMed]
- Gyapay G, Morissette J, Vignal A, Dib C, Fizames C, Millasseau P, Marc S, Bernardi G, Lathrop M, Weissenbach J. The 1993-94 Genethon human genetic linkage map. Nature Genet. 1994;7:246–249. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Ha H, Howard CA, Yeom YI, Abe K, Uehara H, Artzt K, Bennett D, Cuenda A, Cohen P, Buee-Scherrer V, Goedert M. Several testis-expressed genes in the mouse t-complex have expression differences between wild-type and t-mutant mice. Dev Genet. 1991;12:318–332. doi: 10.1002/dvg.1020120409. [DOI] [PubMed] [Google Scholar]
- Hamvas R, Trachtulec Z, Forejt J, Williams RW, Arzt K, Fischer-Lindahl K, Silver LM. Encyclopedia of the mouse genome V. Mouse chromosome 17. Mamm Genome. 1996;6:S281–S299. [PubMed] [Google Scholar]
- Hanson IM, Trowsdale J. Colinearity of novel genes in the class II regions of the MHC in mouse and human. Immunogenetics. 1991;34:5–11. doi: 10.1007/BF00212306. [DOI] [PubMed] [Google Scholar]
- Heiskanen M, Karhu R, Hellsten E, Peltonen L, Kallioniemi OP, Palotie A. High resolution mapping using fluorescence in situ hybridization to extended DNA fibers prepared from agarose-embedded cells. Biotechniques. 1994;17:928–933. [PubMed] [Google Scholar]
- Heiskanen M, Hellsten E, Kallioniemi OP, Makela TP, Alitalo K, Peltonen L, Palotie A. Visual mapping by fiber-FISH. Genomics. 1995;30:31–36. doi: 10.1006/geno.1995.0005. [DOI] [PubMed] [Google Scholar]
- Hoff M, Nakamura Y, Holm T, Gillilan S, O’Connell PO, Leppert M, Lathrop GM, Lalouel JM, White R. Isolation and mapping of a polymorphic DNA sequence (pHHH157) on chromosome 6p [D6S29] Nucleic Acids Res. 1988;16:5217. doi: 10.1093/nar/16.11.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ink B, Zornig M, Baum B, Hajibagher N, James C, Chittenden T, Evan G. Human Bak induces cell death in Schizosaccharomyces pombe with morphological changes similar to those with apoptosis in mammalian cells. Mol Cell Biol. 1997;17:2468–2474. doi: 10.1128/mcb.17.5.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou PA, Amemiya CT, Garnes J, Kroisel PM, Shizuya H, Chen C, Batzer MA, de Jong PJ. A new bacteriophage P1-derived vector for the propagation of large human DNA fragments. Nature Genet. 1994;6:84–89. doi: 10.1038/ng0194-84. [DOI] [PubMed] [Google Scholar]
- Jin K, Ho HN, Speed TP, Gill TJR. Reproductive failure and the major histocompatibility complex. Am J Hum Genet. 1995;56:1456–1467. [PMC free article] [PubMed] [Google Scholar]
- Johansson M, Dietrich C, Mandahl N, Hambraeus G, Johansson L, Clausen P, Mitelman F, Heim S. Recombinations of chromosomal bands 6p21 and 14q24 characterize pulmonary hamartomas. Br J Cancer. 1993;67:1236–1241. doi: 10.1038/bjc.1993.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak B, Bol S, Wanschura S, Bartnitzke S, Bullerdiek J. PAC clone containing the HMGI(Y) gene spans the breakpoint of a 6p21 translocation in a uterine leiomyoma cell line. Genes Chromosomes Cancer. 1996;17:191–193. doi: 10.1002/(SICI)1098-2264(199611)17:3<191::AID-GCC8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Kiefer MC, Brauer MJ, Wu VC, Powers JJ, Umansky SR, Tomei LD, Barr PJ. Modulation of apoptosis by the widely distributed Bcl-2 homolog Bak. Nature. 1995;374:736–739. doi: 10.1038/374736a0. [DOI] [PubMed] [Google Scholar]
- Kim UJ, Birren BW, Slepak T, Mancino V, Boysen C, Kang HL, Simon MI, Shizuya H. Construction and characterization of a human bacterial artificial chromosome library. Genomics. 1996;34:213–218. doi: 10.1006/geno.1996.0268. [DOI] [PubMed] [Google Scholar]
- Kostyu DD. HLA: Fertile territory for developmental genes? Crit Rev Immunol. 1994;14:29–59. [PubMed] [Google Scholar]
- Kwiatkowski TJ, Jr, Beaudet AL, Trask BJ, Zoghbi HY. Linkage mapping and fluorescence in situ hybridization of TCTE-1 on human chromosome 6p: Analysis of dinucleotide polymorphisms on native gels. Genomics. 1991;10:921–926. doi: 10.1016/0888-7543(91)90180-m. [DOI] [PubMed] [Google Scholar]
- Lai F, Stubbs L, Lehrach H, Huang Y, Yeom Y, Artzt K. Genomic organization and expressed sequences of the mouse extended H-2K region. Genomics. 1994;23:338–343. doi: 10.1006/geno.1994.1509. [DOI] [PubMed] [Google Scholar]
- Larin Z, Monaco A, Lehrach H. Yeast artificial chromosome libraries containing large inserts from mouse and human DNA. Proc Natl Acad Sci. 1991;88:4123–4127. doi: 10.1073/pnas.88.10.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- Lewis BC, Shah NP, Braun BS, Denny CT. Creation of a yeast artificial chromosome fragmentation vector based on lysine-2. Genome Anal Techniques Applic. 1992;9:86–90. doi: 10.1016/1050-3862(92)90003-n. [DOI] [PubMed] [Google Scholar]
- Markie D. Arrayed preparation of YAC DNA for pulsed field gel analysis. Nucleic Acids Res. 1995;23:4526–4527. doi: 10.1093/nar/23.21.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell PC, DiLella AG, Lee JC, Young PR. Localization of the human stress responsive MAP kinase-like CSAIDs binding protein (CSBP) gene to chromosome 6p21.3/21.2. Genomics. 1995;29:301–302. doi: 10.1006/geno.1995.1252. [DOI] [PubMed] [Google Scholar]
- Nilbert M, Heim S, Mandahl N, Floderus U-M, Willen H, Mitelman F. Characteristic chromosome abnormalities, including rearrangements of 6p, del (7q), +12 and t (12,14) in 44 uterine leiomyomas. Hum Genet. 1995;85:605–611. doi: 10.1007/BF00193583. [DOI] [PubMed] [Google Scholar]
- Nizetic D, Monard S, Young B, Cotter F, Zehetner G, Lehrach H. Construction of cosmid libraries from flow-sorted human chromosomes 1, 6, 7, 11, 13, and 18 for reference library resources. Mamm Genome. 1994;5:801–802. doi: 10.1007/BF00292017. [DOI] [PubMed] [Google Scholar]
- Olavesen MG, Davies AF, Broxholme SJ, Wixon J, Senger G, Nizetic D, Campbell RD, Ragoussis J. An integrated map of human chromosome 6p23. Genome Res. 1995;5:342–358. doi: 10.1101/gr.5.4.342. [DOI] [PubMed] [Google Scholar]
- Pappas GJ, Polymeropoulos MH, Boyle JM, Trent JM. Regional assignment by hybrid mapping of 36 expressed sequence tags (ESTs) on human chromosome 6. Genomics. 1995;25:124–129. doi: 10.1016/0888-7543(95)80117-5. [DOI] [PubMed] [Google Scholar]
- Parra I, Windle B. High resolution visual mapping of stretched DNA by fluorescent hybridization. Nature Genet. 1993;5:17–21. doi: 10.1038/ng0993-17. [DOI] [PubMed] [Google Scholar]
- Ragoussis J. Restriction analysis of YACs. Methods Mol Biol. 1996;54:69–74. doi: 10.1385/0-89603-313-9:69. [DOI] [PubMed] [Google Scholar]
- Ragoussis J, Monaco AP. Covering YAC-cloned DNA with phages and cosmids. Methods Mol Biol. 1996;54:157–166. doi: 10.1385/0-89603-313-9:157. [DOI] [PubMed] [Google Scholar]
- Ragoussis J, Olavesen M. Generation of probes for chromosome walking. In: Dear PH, editor. Genome Mapping: A practical approach. Oxford, UK: Oxford University Press; 1997. pp. 255–280. [Google Scholar]
- Ragoussis J, Senger G, Mockridge I, Sanseau P, Ruddy S, Dudley K, Sheer D, Trowsdale J. A testis-expressed Zn finger gene (ZNF76) in human 6p21.3 centromeric to the MHC is closely linked to the human homolog of the t-complex gene tcp-11. Genomics. 1992;14:673–679. doi: 10.1016/s0888-7543(05)80167-3. [DOI] [PubMed] [Google Scholar]
- Schuler GD, Boguski MS, Stewart EA, Stein LD, Gyapay G, Rice K, White RE, Rodriguez-Tome P, Aggarwal A, Bajorek E, et al. A gene map of the human genome. Science. 1996;274:540–546. [PubMed] [Google Scholar]
- Sheffield VC, Weber JL, Buetow KH, Murray JC, Even DA, Wiles K, Gastier JM, Pulido JC, Yandava C, Sunden SL, Mattes G, Businga T, McClain A, Beck J, Scherpier T, Gilliam J, Zhong J, Duyk GM. A collection of tri- and tetranucleotide repeat markers used to generate high quality, high resolution human genome-wide linkage maps. Hum Mol Genet. 1995;4:1837–1844. doi: 10.1093/hmg/4.10.1837. [DOI] [PubMed] [Google Scholar]
- Shugart YY, Banerjee P, Knowles JA, Lewis CA, Jacobson SG, Matise TC, Penchaszadeh G, Gilliam TC, Ott J. Fine genetic mapping of a gene for autosomal recessive retinitis pigmentosa on chromosome 6p21. Am J Hum Genet. 1995;57:499–502. [PMC free article] [PubMed] [Google Scholar]
- Silver LM. Encyclopedia of the mouse genome III. October 1993. Master locus list. Mamm Genome. 1993a;4:2–9. [PubMed] [Google Scholar]
- Silver LM. The peculiar journey of a selfish chromosome: Mouse t haplotypes and meiotic drive. Trends Genet. 1993b;9:250–254. doi: 10.1016/0168-9525(93)90090-5. [DOI] [PubMed] [Google Scholar]
- Soderlund CA, Longden I. FPC. Technical Report SC-01-96. Hinxton, Cambridge, UK: The Sanger Centre; 1996. [Google Scholar]
- Strasser A, Whittingham S, Vaux DL, Bath ML, Adams JM, Cory S, Harris AW. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci. 1991;88:8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J, Mallett F, Durbin R, Horsnell T. Image analysis of restriction enzyme fingerprint autoradiograms. Comput Appl Biosci. 1989;5:101–106. doi: 10.1093/bioinformatics/5.2.101. [DOI] [PubMed] [Google Scholar]
- Xiao S, Lux ML, Reeves R, Hudson TJ, Fletcher JA. HMGI(Y) activation by chromosome 6p21 rearrangements in multilineage mesenchymal cells from pulmonary hamartoma. Am J Pathol. 1997;150:901–910. [PMC free article] [PubMed] [Google Scholar]
- Yeom YI, Abe K, Artzt K. Evolution of the mouse H-2K region: A hot spot of mutation associated with genes transcribed in embryos and/or germ cells. Genetics. 1992;130:629–638. doi: 10.1093/genetics/130.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]