Abstract
The 5-hydroxytryptamine 2A (5-HT2A) receptor is a member of the G protein-coupled receptor superfamily (GPCR) and plays a key role in transducing a variety of cellular signals elicited by serotonin (5-HT; 5-hydroxytryptamine) in both peripheral and central tissues. Recently, we discovered that the ERK/MAPK effector p90 ribosomal S6 kinase 2 (RSK2) phosphorylates the 5-HT2A receptor and attenuates 5-HT2A receptor signaling. This raised the intriguing possibility of a regulatory paradigm whereby receptor tyrosine kinases (RTKs) attenuate GPCR signaling (i.e., ‘inhibitory cross-talk’) by activating RSK2 [Strachan et al. (2009) J. Biol. Chem. 284, 5557-5573]. We report here that activation of multiple endogenous RTKs such as the epidermal growth factor receptor (EGFR), the platelet-derived growth factor receptor (PDGFR), and ErbB4 significantly attenuates 5-HT2A receptor signaling in a variety of cell types including mouse embryonic fibroblasts (MEFs), mouse vascular smooth muscle cells (mVSMCs), and primary cortical neurons. Importantly, genetic deletion of RSK2 completely prevented signal attenuation, thereby suggesting that RSK2 is a critical mediator of inhibitory cross-talk between RTKs and 5-HT2A receptors. We also discovered that P2Y purinergic receptor signaling was similarly attenuated following EGFR activation. By directly testing multiple endogenous growth factors/RTK pathways and multiple Gq-coupled GPCRs, we have now established a cellular mechanism whereby RTK signaling cascades act via RSK2 to attenuate GPCR signaling. Given the pervasiveness of growth factor signaling, this novel regulatory mechanism has the potential to explain how 5-HT2A receptors are regulated in vivo, with potential implications for human diseases in which 5-HT2A or RTK activity is altered (e.g. neuropsychiatric and neurodevelopmental disorders).
The GPCR superfamily mediates essential functions in organisms as diverse as unicellular choanoflagellates and humans (1, 2). In humans, GPCRs comprise approximately 2% of the genome to transduce signals elicited by both endogenous and exogenous ligands (3-5). Not surprisingly, GPCR dysregulation is associated with many human diseases (6), thus explaining why GPCRs are successful therapeutic targets and remain the focus of intense drug discovery efforts (7).
The Gq-coupled 5-HT2A receptor, in particular, is one of 14 GPCRs that mediates the pleiotropic actions of 5-HT in both peripheral and central tissues (8, 9). The 5-HT2A receptor is an important therapeutic target for a large number of psychiatric and medical diseases (9), and is also the site of action of most, but not all hallucinogens which function as 5-HT2A receptor agonists (10, 11)(Keiser et al., 2009, in press). Additionally, atypical antipsychotics (e.g., clozapine) are thought to mediate their therapeutic actions, at least in part, by antagonizing 5-HT2A receptors (12).
We recently discovered a novel regulatory mechanism whereby RSK2 interacts with 5-HT2A serotonin receptors and attenuates receptor signaling via direct receptor phosphorylation (13, 14). RSK2 is a multifunctional ERK/MAPK effector activated downstream of growth factor signal cascades involving RTKs (15). This raised the intriguing possibility of a new regulatory mechanism whereby RTKs attenuate GPCR signaling (referred to here as ‘inhibitory cross-talk‘) by activating RSK2. These studies led to the initial discovery that activation of the EGFR attenuates 5-HT2A receptor signaling, presumably via RSK2 activation (14). These preliminary data were intriguing for several reasons including: (1) they suggested for the first time that the 5-HT2A receptor is part of an emerging regulatory paradigm whereby activated RTKs attenuate GPCR signaling (16-22), and (2) they were the first to identify RSK2 as a novel mediator of inhibitory cross-talk between growth factor-activated RTKs and a GPCR.
In this paper, we show that activation of various endogenous RTKs (i.e. EGFR, PDGFR, and ErbB4) significantly attenuates 5-HT2A receptor signaling in multiple cell types (i.e., in MEFs, mVSMCs, and primary cortical neurons). In contrast, insulin-like growth factor 1 (IGF-1), which only weakly activates RSK2, fails to attenuate 5-HT2A receptor signaling. Together with evidence that genetic deletion of RSK2 is sufficient to prevent RTK-mediated signal attenuation in all tested cellular backgrounds, these findings support a novel role for RSK2 in inhibitory cross-talk between RTKs and the 5-HT2A receptor. Significantly, we also discovered that P2Y purinergic receptor signaling, which is regulated by RSK2, was similarly attenuated following EGF receptor activation in wild-type (RSK2+/+) MEFs. By testing several endogenous growth factors/RTK pathways and multiple Gq-coupled GPCRs, we have now established a cellular mechanism whereby RTK signaling cascades attenuate GPCR signaling through RSK2. These findings provide an initial framework for a conserved regulatory mechanism whereby RTKs act via RSK2 to attenuate GPCR signaling, and given the complexity of cellular signaling, have the potential to explain how these receptors are regulated in vivo.
Moreover, because null mutations of RSK2 lead to Coffin-Lowry Syndrome which exhibits behaviors characteristic of 5-HT2A dysregulation including a schizophrenia-like psychosis and cognitive impairment (23), these findings may explain, in part, some of the clinical manifestations of this neurodevelopmental disease..
Experimental Procedures
Materials
Cell culture reagents including fetal bovine serum (FBS), Dulbecco’s modified essential medium (DMEM), Trypsin-EDTA, F-12 nutrient mixture, OptiMEM, neurobasal medium, B27 supplement, Hank’s balanced salt solution (HBSS), sodium pyruvate, penicillin, and streptomycin were supplied by Gibco (Invitrogen, Carlsbad, CA). Serotonin, 5-methoxy-N,N-dimethyltryptamine (5-methoxyDMT), human EGF, human PDGF-AB and PDGF-BB, human TGF-α, IGF-1, papain, probenecid, bovine serum albumin (BSA), low molecular weight poly-L-lysine, sodium tetraborate, L-glutamine, and all other standard reagents were supplied by Sigma-Aldrich Corp. (St. Louis, MO). Boric acid was supplied by EMD Chemicals (Gibbstown, NJ). MDL100907 and lisuride were acquired as previously detailed (12). Collagenase II was obtained from Worthington Biochemical Corp. (Lakewood, NJ) and elastase (grade II) was supplied by Roche Applied Science (Indianapolis, IN). Restriction endonucleases were supplied by New England Biolabs (Ipswich, MA). [3H]-Ketanserin was obtained from PerkinElmer Life and Analytical Sciences (Waltham, MA). Protein A/G agarose was supplied by Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Serum Dialysis
We extensively dialyzed FBS to remove the 5-HT present in serum. Briefly, 500 mL FBS was placed into dialysis tubing (Spectra/Por 3500 MWCO, Spectrum Laboratories, Rancho Dominguez, CA) and equilibrated with 4 L of cold dialysis buffer (120 mM NaCl, 10 mM Tris-HCl, pH 7.5 at RT) for 24 hr at 4°C with stirring. The buffer was changed five times totaling 120 hr of dialysis. The dialyzed FBS was then sterile-filtered (0.22 μm, Millipore) and aliquots were stored at −20°C until further use. HPLC-electrochemical detection analysis of the dialyzed serum determined that, when used at a concentration of 5%, our dialyzed culture medium contained 33 times less 5-HT than commercially dialyzed FBS (i.e., 0.039 nM vs. 1.3nM 5-HT).
Cell Culture and Transfection
The RSK2+/+ and RSK2 knockout (RSK2−/−) MEFs stably expressing similar levels of 5-HT2A receptors were generated previously by Sheffler et al. (13) using MEFs originally isolated from RSK2+/+ and RSK2−/− mice (24). Mouse VSMCs and cortical neurons were isolated as detailed below. HEK293T cells were obtained from the American Type Culture Collection (Manassas, VA). All cell lines were cultured at 37°C in a humidified environment in the presence of 5% CO2. Specifically, HEK293T and mVSMC cell lines were maintained in standard medium (DMEM supplemented with 10% FBS, 1 mM sodium pyruvate, 100 units/mL penicillin, and 100 μg/mL streptomycin). Polyclonal populations of MEFs stably expressing FLAG-tagged rat 5-HT2A receptors were cultured in standard medium supplemented with 4 μg/mL puromycin to maintain selection pressure. Primary cortical neurons were maintained in complete neurobasal medium (neurobasal medium, 1× B27 supplement, 0.5 mM L-glutamine, 25 μM glutamate, 100 units/mL penicillin, and 100 μg/mL streptomycin). Fugene6 (Roche) was used exactly as described by the manufacturer to transiently transfect sub-confluent HEK 293T cells.
cDNA Constructs
For generation of stable cell lines, the rat 5-HT2A receptor containing a cleavable N-terminal H. influenza hemagglutinin membrane insertion signal sequence (25) and N-terminal FLAG (DYKDDDDK) affinity tag (FLAG-5-HT2A) (26) was subcloned into the pBABE retroviral vector containing a puromycin resistance gene (FLAG-5-HT2A-pBABEpuro) (27). Briefly, 5′ EcoRI and 3′ SalI restriction sites were introduced into the FLAG-5-HT2A sequence via the following PCR primers: 5′AAAGAATTCGCCACCATGAAGACGATCAT3′ (EcoRI highlighted) and 5′AAAGTCGACTCACACACAGCTAACCTTTTC3′ (SalI highlighted). The FLAG-5-HT2A-pBABEpuro construct was sequence-verified (Case Western Reserve University Genomics Core Facility, Cleveland, OH) and determined via competition radioligand binding assays to bind 5-HT with characteristic affinity (http://pdsp.med.unc.edu/pdsp.php)(28).
For infection of primary cortical neurons, the rat 5-HT2A receptor containing the green fluorescent protein (GFP) inserted between amino acids 452 and 453 within the C-terminus (5-HT2A-GFP-CT) (29) was subcloned into the FUGW lentiviral construct (5-HT2A-GFP-CT-FUGW) (30). Briefly, 5′ XbaI and 3′ EcoRI restriction sites were introduced into the 5-HT2A-GFP-CT sequence via the following primers: 5′AAAATCTAGAGCCACCATGGAAATTCTTTGTGAAG3′ (XbaI highlighted) and 5′TTTTGAATTCTCACACACAGCTAACCTTTTCATTC3′ (EcoRI highlighted). The resulting 5-HT2A-GFP-CT-FUGW construct was sequence-verified by automated sequencing (UNC-Chapel Hill DNA sequencing facility, Chapel Hill, NC).
Microarray Analysis and Pathway Generation
Microarray studies were performed previously by Sheffler et al. (13) to compare gene expression profiles in RSK2+/+ and RSK2−/− MEFs. For pathway analysis of RSK2−/− and RSK2+/+ fibroblast gene expression patterns, GenMAPP and MAPPFinder software packages were used as previously detailed (13, 31, 32).
Isolation of Mouse Aortic Vascular Smooth Muscle Cells
Mouse aortic VSMCs were isolated from 12-week old mice (three mice per genotype), as previously detailed (33). Briefly, mice were sacrificed by cervical dislocation and immediately perfused with 25 mL of 1× HBSS (without Ca2+ and Mg2+). Under sterile conditions, the abdominal/thoracic aorta extending from the ilial bifurcation to aortic arch was carefully microdissected and rinsed with HBSS. The pooled aorta were then incubated with collagenase buffer (175 U/mL in HBSS, filtered through 0.2 μm polyethersulfone membrane) for 15 min at 37°C in the presence of 5% CO2. After the adventitial layer was removed, the aorta were incubated with DMEM supplemented with 10% FBS overnight at 37°C in the presence of 5% CO2. The next day the aorta were cut into 2 mm segments and incubated with digestion buffer (175 U/mL of collagenase and 0.125 mg/mL of elastase in HBSS, filtered through 0.2 μm polyethersulfone membrane) for 1 hr at 37°C in the presence of 5% CO2. Following digestion, the tissue was dissociated with a glass Pasteur pipette, DMEM supplemented with 10% FBS was added, and the cells were collected via centrifugation (200 × g for 8 min). The cells were re-suspended in DMEM supplemented with 20% FBS, transferred to a T-25 cm flask, and the cells were incubated overnight at 37°C in the presence of 5% CO2. The next day the cells were carefully washed with DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin and cultured normally with medium changes every three days. Cells were determined to be SMCs via immunofluorescence assays using the rabbit polyclonal smooth muscle α-actin antibody exactly as described by the manufacturer (1:1000, Abcam Inc., Cambridge, MA). For all experiments, mVSMCs were used between passages three and seven.
Isolation of Primary Cortical Neurons
Cortical neurons were prepared as previously detailed (34-36). Briefly, pups (postnatal day<2) were genotyped and euthanized by decapitation. The entire frontal cortex of RSK2+/+ and RSK2−/− animals was microdissected from whole brain, followed by digestion in neurobasal medium containing 0.1% papain and 0.2% BSA at 37°C for 20 min. The medium was then replaced with complete neurobasal medium and the digested tissue was mechanically dissociated via trituration with a glass Pasteur pipette. The supernatant was transferred to a new sterile 1.5 mL tube and cells were collected via centrifugation (200 × g for 5 min). The cell pellet was then resuspended in conditioned complete neurobasal medium. Cells were counted and seeded at a density of 50,000 cells/well onto poly-L-lysine-coated 96 well plates (0.1 mg/mL low molecular mass poly-L-lysine, 0.625% boric acid, 0.955% sodium tetraborate) and cultured normally.
Lentivirus Production and Infection
Lentiviral infection of primary cortical neurons was performed essentially as previously described (35). In brief, a pre-determined mixture of 5-HT2A-GFP-CT-FUGW and the viral packaging constructs VSVG and Delta 8.9 (ratio= 3.3 FUGW: 2.5 Delta 8.9 : 1 VSVG) were co-transfected into HEK293T cells using Fugene6. Forty-eight hours after transfection the medium containing virus was removed, pooled, and a virus pellet was obtained via centrifugation (26,000 × g for 5 hr). The virus pellet was re-suspended in PBS, concentrated approximately 40-fold using Amicon UltraCel 100K filters (Millipore), and then tested for infection and expression of 5-HT2A-GFP in HEK293T cells. A pre-determined amount of concentrated lentivirus was then applied to primary cortical neurons cultured for 7-10 days in vitro.
Immunoprecipitation and Western Blotting
Immunoprecipitation of RSK2 and detection of Ser(P)-386 following growth factor treatment was performed as previously described (37). Briefly, RSK2+/+ and RSK2−/− cells were treated with EGF (100ng/mL) or IGF-1 (10nM) for various times and solubilized with cold lysis buffer (50 mM Tris-HCl, 150mM NaCl, 1% tergitol, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, EDTA-free protease inhibitors, 50mM NaF, 50mM β-glycerolphosphate, 5mM sodium pyrophosphate, and 0.1mM sodium orthovanadate, pH 8.0) for 20 min at 4°C. Supernatants were collected via centrifugation (15,000 × g, 30 min) and equal amounts of Protein A/G-cleared lysate were incubated with mouse monoclonal anti-RSK2 (2μg) for 2 hr at 4°C, followed by incubation with Protein A/G agarose for 2 hr at 4°C. Immunopurified complexes were extensively washed with lysis buffer, eluted with 2× SDS sample buffer (125 mM Tris-HCl, 4% sodium dodecyl sulfate, 20% glycerol, 200 mM dithiothreitol, 0.2% Bromphenol Blue, pH 6.8), and stored at −80°C until further use.
Proteins were immunoblotted using standard procedures (28). Specifically, proteins were resolved on 10% SDS-PAGE gels, electroblotted onto nitrocellulose membranes (BioRad Laboratories, Hercules, CA), and blocked with standard blocking buffer (Tris-buffered saline, 0.1% Tween-20, and 5% nonfat dehydrated milk) for 1 hr at RT. Membranes were then incubated with primary antibodies diluted in standard blocking buffer or phospho-specific blocking buffer (5% BSA and TBST). Specifically, RSK2 was detected using the goat polyclonal RSK2 antibody (1:1000, Santa Cruz Biotechnology, Inc.) and the rabbit polyclonal Ser(P)-386 antibody (1:1000, Cell Signaling Technology, Inc., Danvers, MA). The EGFR was detected using the rabbit polyclonal EGFR antibody (1:500, Santa Cruz Biotechnology, Inc.) and the mouse monoclonal Tyr(P)-1068 antibody (1:500, Cell Signaling Technology, Inc.). Phosphorylated IGF-1 R was detected using the rabbit polyclonal Tyr(P)-1158/1162/1163 antibody (1:1000, Upstate Millipore).Membranes were washed extensively with Tris-buffered saline + 0.1% Tween-20 (TBST) and subsequently incubated for 1 hr at RT with secondary horseradish peroxidase-conjugated antibodies raised against mouse, rabbit and goat IgG (1:1000, Vector Laboratories, Burlingame, CA). Membranes were washed extensively and proteins were detected using SuperSignal West Pico chemiluminescent substrate (Thermo Fisher Scientific, Inc., Rockford, IL). Immunoreactive bands were imaged and quantified using Kodak Imaging software (Eastman Kodak, New Haven, CT). Sum pixel intensity values were analyzed via the one-tailed, paired t test (significance defined as p<0.05)(Graphpad Software, Inc., La Jolla, CA).
Fluorometric Imaging Plate Reader (FLIPRTetra) Analysis of Intracellular Ca2+ Release
Intracellular Ca2+ release was measured in MEFs and mVSMCs via FLIPRTetra assays using a Ca2+ assay kit (Molecular Devices, Sunnyvale, CA) as previously detailed (13, 14). Briefly, MEFs were plated at a density of 25,000 cells/well into black-wall, clear-bottom 96-well tissue culture plates (Greiner Bio-One, Monroe, NC), whereas mVSMCs were plated at a density of 10,000 cells/well into black-wall, clear-bottom 384-well tissue culture plates (Greiner Bio-One). The cells were cultured in dialyzed medium (DMEM, 5% FBS dialyzed to <0.05 nM 5-HT, 1 mM sodium pyruvate, 100 units/mL penicillin, and 100 μg/mL streptomycin) and serum-free medium (DMEM, 0.1% BSA, 100 units/mL penicillin, and 100 μg/mL streptomycin) for 24-40 hr before the assay for normal and growth factor desensitization experiments, respectively. For both experiments, the cells were incubated with Ca2+ assay buffer (20 mM HEPES, 1× HBSS, 2.5 mM probenecid, and Ca2+ assay reagent, pH 7.4) for 60 min at 37°C prior to initiating the FLIPR program. However, for growth factor desensitization experiments the cells were incubated with growth factors diluted in Ca2+ assay buffer (for 30 min and 60 mintime points). After dye loading, the FLIPRTetra was programmed to add agonist approximately 10 seconds after establishing baseline relative fluorescence unit (RFU) values (excitation 470-495, emission 515-575 nm). RFU values were collected every second for 5 min and the average baseline values were subtracted from maximum RFU values. Values were expressed relative to the maximal untreated response in each cell line and analyzed by nonlinear regression to generate fit parameters of potency (EC50) and maximal signaling (Emax) (Graphpad software). The F test was used to determine the statistical significance (defined as p<0.05) of the fit parameters in growth factor-treated vs. untreated cells.
Analysis of Intracellular Ca2+ Release in Primary Cortical Neurons
Cortical neurons were isolated, cultured and imaged as described previously (35, 36). In brief, 48 hr after lentivirus infection each well was imaged for total GFP fluorescence using the BD Pathway 855 high content imaging microscope equipped with environmental control. Ca2+ flux was then determined using the FLIPR Ca2+ assay kit (Molecular Probes) as detailed by the manufacturer. In brief, prior to live cell imaging, cells were washed 1× with phosphate buffered saline followed by 60 min incubation with Ca2+ assay buffer (20 mM HEPES, 1× HBSS, 2.5 mM probenecid, 0.57 mM ascorbic acid, and Ca2+ assay reagent, pH 7.4). Assays using growth factors were performed similarly except that growth factor was added during the dye loading step. Cells were maintained at 37°C during the entire period of observation and were imaged for 20 sec prior to drug addition to obtain baseline dye fluorescence. The liquid handling capability of the BD Pathway 855 was used to add 10× drug and then fluorescence images were obtained for 120 sec. To control for subtle differences in receptor expression, Ca2+ responses were normalized to GFP intensity/well using custom written macros for Excel (Microsoft, Redmond, WA) and Image J (U. S. National Institutes of Health, Bethesda, Maryland). Values were expressed as fold over baseline and a two-tailed, paired t test was used to determine the statistical significance (defined as p<0.05) of responses in growth factor-treated vs. untreated cells.
Results
RSK2 is required for EGF-induced attenuation of 5-HT2A receptor signaling
We recently determined that RSK2 interacts with the 5-HT2A serotonin receptor and attenuates signaling via direct receptor phosphorylation (13, 14). Moreover, preliminary data suggested that EGFR activation attenuates 5-HT2A receptor signaling, presumably by activating RSK2. Considering the potential for describing how 5-HT2A receptors are regulated in cells, and perhaps in vivo, we applied pharmacological and genetic approaches to determine if various RTKs, including members of the EGFR family, require RSK2 for attenuating 5-HT2A receptor signaling.
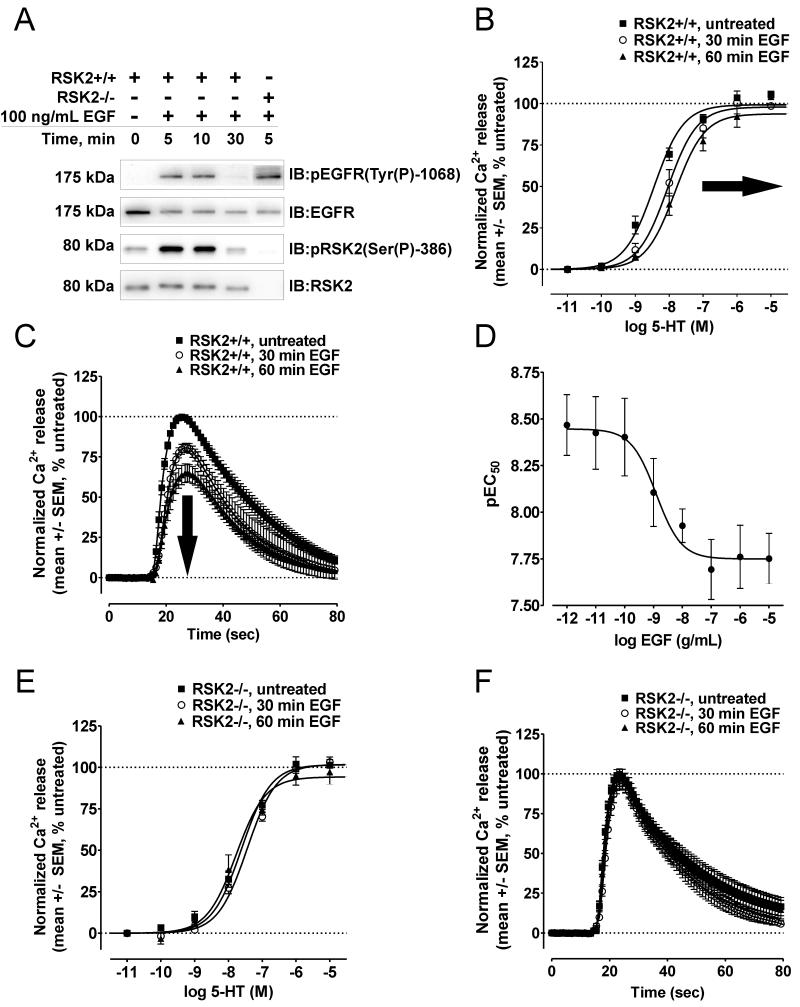
First, we activated the EGFR (also known as ErbB1) in RSK2+/+ and RSK2−/− MEFs with two canonical agonists (i.e., EGF and TGF-α) and monitored 5-HT2A receptor responsiveness using 5-HT2A agonists of varying intrinsic efficacies. Consistent with our initial EGFR findings (14), EGF significantly attenuated 5-HT2A receptor signaling in RSK2+/+ MEFs. As shown in Figure 1A, 100ng/mL EGF maximally activated the EGFR and RSK2 in these cells. When receptor signaling was assayed, we found that EGF pretreatment resulted in significant rightward shifts in 5-HT concentration-response curves (CRCs) as early as 30min, with maximal effects reached within 60 min (Figure 1B, Table 1). According to classical concepts of receptor pharmacology, these rightward shifts in 5-HT CRCs denoted decreases in agonist potency, most likely resulting from attenuation of receptor signaling given the short time scale of the experiment (38). To best illustrate this decrease in 5-HT potency, we compared the 5-HT2A-mediated Ca2+ responses elicited by a sub-maximal, EC50 concentration of 5-HT (i.e., 10nM). As shown in Figure 1C, 10nM 5-HT elicited significantly lower Ca2+ responses after treating with EGF for 30 min (peak Ca2+ release= 99.7 +/− 0.1% vs. 79.7 +/− 3.1% in untreated and EGF-treated RSK2+/+ MEFs, respectively; N=3 to 9, p<0.05) and 60 min (peak Ca2+ release= 99.7 +/− 0.08% vs. 63.7 +/− 6.0% in untreated and EGF-treated RSK2+/+ MEFs, respectively; N=7 to 9, p<0.05). Although it was clear that EGF decreased 5-HT signaling, these results could be explained by non-specific effects associated with a single, supramaximal concentration of EGF. To address this concern we determined that EGF attenuated 5-HT2A receptor signaling with an IC50 of 1.3 ng/mL (Figure 1D), a value that is within the concentration range typically observed for EGFR-mediated signaling events (39).
Figure 1.
RSK2 is required for inhibitory cross-talk between the EGFR and the 5-HT2A receptor. The EGFR and RSK2 were activated with 100ng/mL EGF (A) and then 5-HT2A-mediated Ca2+ responses were measured via FLIPRtetra assays in RSK2+/+ (B-D) and RSK2−/− (E-F) MEFs. A, Immunoblots showing that EGF treatment (100ng/mL) activated the EGFR (Tyr(P)-1068, top panels) and RSK2 (Ser(P)-386, bottom panels) in RSK2+/+ MEFs. The EGFR was similarly activated in RSK2−/− MEFs; however, RSK2 was not detected in RSK2−/− MEFs (bottom panel). Shown are representative immunoblots of three independent experiments. B, In RSK2+/+ MEFs, CRCs for 5-HT were significantly shifted rightward (i.e., decreased 5-HT potency, bold arrow) following 30 min (o), and 60 min (▲) EGF treatments relative to untreated cells (■). Shown are the results (mean +/− SEM) of three to ten independent experiments performed in duplicate (p<0.05). C, In RSK2+/+ MEFs, 5-HT2A-mediated Ca2+ responses elicited by an EC50 concentration of 5-HT (10 nM) were significantly attenuated following 30 min (o) and 60 min (▲) EGF treatments relative to untreated cells (■). Shown are normalized Ca2+ traces (untreated set to 100%, mean +/− SEM) of three to nine independent experiments (p<0.05). D, In RSK2+/+ MEFs, EGF attenuated 5-HT2A-mediated Ca2+ responses with an IC50 of 1.3ng/mL. Shown are the results (mean +/− SEM) of four independent experiments performed in duplicate. E, In RSK2−/− MEFs, CRCs for 5-HT were not significantly shifted following EGF treatments ((30 min (o) and 60 min (▲)) relative to untreated cells (■). Shown are the results (mean +/− SEM) of six independent experiments performed in duplicate (p>0.05). F, In RSK2−/− MEFs, 5-HT2A-mediated Ca2+ responses elicited by an EC50 concentration of 5-HT (10 nM) were not decreased after 30 min (o) and 60 min (▲) EGF treatments relative to untreated cells (■). Shown are the normalized Ca2+ traces (untreated set to 100%, mean +/− SEM) of three to six independent experiments (p>0.05).
Table 1.
Effects of various growth factors on the signaling of GPCR ligands in MEFs.
| Cell type | RSK2+/+ MEFs | RSK2−/− MEFs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time treated with growth factor |
0 min | 30 min | 60 min | 0 min | 30 min | 60 min | |||||||
| RTK ligand |
GPCR ligand |
pEC50 ± SEMa,b |
Emax± SEM, %c |
pEC50 ± SEMa,b |
Emax± SEM, %c |
pEC50 ± SEMa,b |
Emax± SEM, %c |
pEC50 ± SEMa,b |
Emax± SEM, %c |
pEC50 ± SEMa,b |
Emax± SEM, %c |
pEC50 ± SEMa,b |
Emax± SEM, %c |
| EGF | 5-HT | 8.45 +/− 0.06 |
99.3 +/− 2.1 |
8.05 +/− 0.09d |
97.9 +/− 3.5 |
7.83 +/− 0.09d |
93.8 +/− 3.5 |
7.61 +/− 0.07 |
102 +/− 3.0 |
7.45 +/− 0.05 |
102 +/− 2.0 |
7.78 +/− 0.10 |
94.3 +/− 3.4 |
| EGF | 5- methoxy DMT |
7.59 +/− 0.10 |
86.7 +/− 3.3 |
NDe | ND | 7.17 +/− 0.07d |
79.6 +/− 2.2 |
7.05 +/− 0.06 |
80.4 +/− 2.0 |
NDe | ND | 7.05 +/− 0.07 |
79.1 +/− 2.3 |
| EGF | Lisuride | 6.57 +/− 0.09 |
54.8 +/− 2.1 |
ND | ND | 6.49 +/− 0.11 |
40.5 +/− 1.8d |
5.63 +/− 0.14 |
42.4 +/− 2.7 |
ND | ND | 5.90 +/− 0.20 |
37.2 +/− 3.4 |
| TGF-α | 5-HT | 8.65 +/− 0.05 |
99.9 +/− 1.8 |
8.40 +/− 0.07d |
94.4 +/− 2.6 |
8.23 +/− 0.04d |
91.8 +/− 1.6d |
8.07 +/− 0.06 |
100 +/− 2.0 |
8.08 +/− 0.05 |
99.2 +/− 1.8 |
8.01 +/− 0.05 |
100 +/− 1.8 |
| IGF-1 | 5-HT | 8.53 +/− 0.09 |
100 +/− 3 |
8.46 +/− 0.09 |
96.8 +/− 3.4 |
8.54 +/− 0.07 |
93.7 +/− 2.4 |
8.26 +/− 0.05 |
100 +/− 2 |
8.54 +/− 0.08d |
93.4 +/− 2.2d |
8.53 +/− 0.08d |
91.7 +/− 2.2d |
| EGF | ATP | 5.16 +/− 0.05 |
99.7 +/− 2.0 |
4.95 +/− 0.06d |
88.4 +/− 2.5d |
4.88 +/− 0.09d |
82.5 +/− 3.2d |
5.37 +/− 0.09 |
98.6 +/− 3.3 |
5.34 +/− 0.09 |
93.4 +/− 3.2 |
5.30 +/− 0.09 |
100 +/− 3.5 |
Agonist-induced Ca2+ responses were quantified via FLIPR assays in untreated and growth factor–treated RSK2+/+ and RSK2−/− MEFs. The fit parameters of potency (EC50) and maximal signaling (Emax) were obtained from nonlinear regression (GraphPad software) and represent the mean +/− SEM of at least three independent experiments performed in duplicate.
pEC50 values are represented as −log of EC50 in M.
The maximum response of agonist (Emax) in untreated cells was set equal to 100%.
The F test was used to determine the statistical significance (defined as p<0.05) of the fit parameter compared to untreated cells.
ND, not determined.
In contrast to these results in RSK2+/+ MEFs, EGF cannot attenuate 5-HT2A receptor signaling in RSK2−/− MEFs. Specifically, EGF did not significantly alter 5-HT potency in RSK2−/− MEFs as evidenced by superimposed 5-HT CRCs (Figure 1E and Table 1). Moreover, 5-HT2A-mediated Ca2+ responses elicited by an EC50 concentration of 5-HT were not significantly decreased after treating with EGF for 30 min (peak Ca2+ release= 99.7 +/− 0.2% vs. 93.9 +/− 4.2% in untreated and EGF-treated RSK2−/− MEFs, respectively; N=3 to 6, p>0.05) and 60 min (peak Ca2+ release= 99.7 +/− 0.2% vs. 96.8 +/− 6.3% in untreated and EGF-treated RSK2−/− MEFs, respectively; N=6, p>0.05) (Figure 1F).
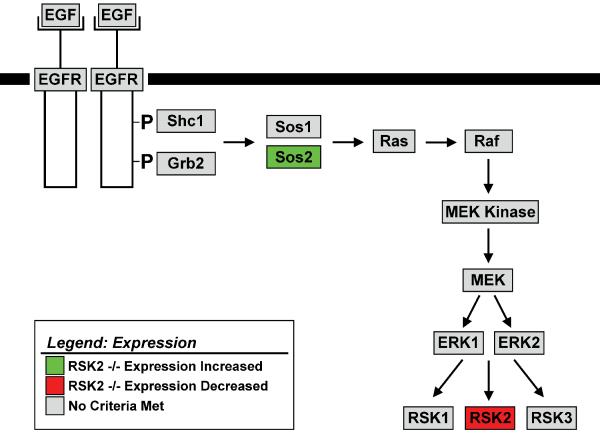
It was conceivable that differences in EGFR signal transduction between RSK2+/+ and RSK2−/− MEFs could account for the differential effects of EGF. To evaluate this possibility, we compared the mRNA expression profiles of genes constituting the EGFR signal transduction pathway in RSK2+/+ and RSK2−/− MEFs. As shown in Figure 2, analysis of microarray studies revealed no substantial differences in gene expression profiles between RSK2+/+ and RSK2−/− MEFs that could account for lack of attenuation in RSK2−/− MEFs. Only two differences were apparent in RSK2−/− cells: (1) a decrease in RSK2 mRNA (as predicted in knock-out cells) and (2) an increase in Sos2 mRNA. Additionally, at the protein level we found that EGFR activation was similar between RSK2+/+ and RSK2−/− MEFs after 5 min of EGF treatment (472 +/− 131% vs. 491 +/− 96% for EGFR phosphorylation in RSK2+/+ and RSK2−/− MEFs, respectively; N=3, p>0.05) (Figure 1A). Taken together, these findings indicate (1) that RSK2 is a critical mediator of inhibitory cross-talk between EGF and 5-HT2A receptors in MEFs and (2) that the effects are not due to compensatory changes in expression of EGFR signaling partners.
Figure 2.
Genes involved in EGFR signal transduction are expressed at similarly in RSK2+/+ and RSK2−/− MEFs. The microarray data quantifying gene expression in RSK2+/+ and RSK2−/− MEFs was published previously by Sheffler et al. (13). Here we overlaid the mRNA expression levels of EGFR signal transduction geness in RSK2+/+ and RSK2−/− MEFs with gene-expression color criterion and fold-changes from the programs GenMAPP and MAPPFinder. Gray colored genes are equally expressed in RSK2+/+ and RSK2−/− MEFs. Green colored genes show greater than a 2-fold increase in expression in RSK2−/− MEFs compared to RSK2+/+ fibroblasts. Red colored genes show greater than a 2-fold decrease in expression in RSK2−/− MEFs compared to RSK2+/+ fibroblasts.
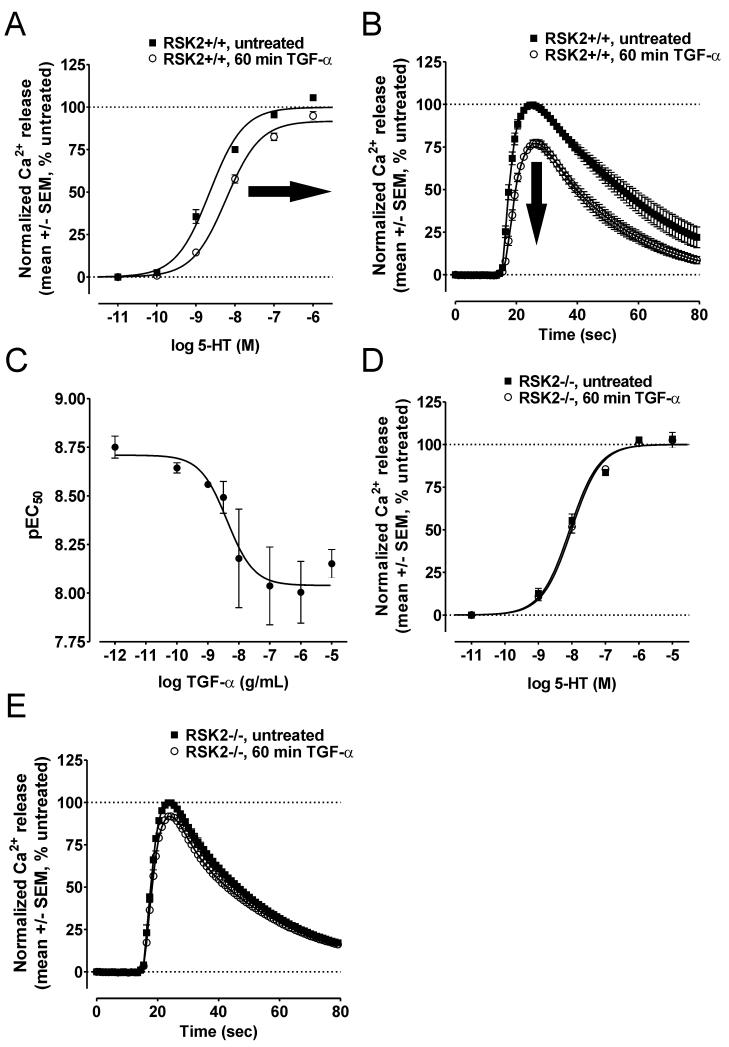
We further confirmed that RSK2 was required for inhibitory cross-talk by activating the EGFR in RSK2+/+ and RSK2−/− MEFs with another selective and potent EGFR agonist, TGF-α. Similar to our results with EGF, TGF-α attenuated 5-HT2A receptor signaling in RSK2+/+ MEFs. Specifically, 1hr treatment with TGF-α decreased 5-HT potency, as illustrated by significant rightward shifts in 5-HT CRCs (Figure 3A and Table 1) and significant decreases in 5-HT2A-mediated Ca2+ release elicited by an EC50 concentration of 5-HT (peak Ca2+ release= 99.7 +/− 0.2% vs. 76.9 +/− 2.2% in untreated and TGF-α-treated RSK2+/+ MEFs, respectively; N=5, p<0.05) (Figure 3B). Moreover, TGF-α attenuated 5-HT2A receptor signaling in RSK2+/+ MEFs with an IC50 of 4.1 ng/mL (Figure 3C), again consistent with EGFR-mediated signaling events. In agreement with our previous experiments using EGF, TGF-α treatment did not attenuate 5-HT2A receptor signaling in RSK2−/− MEFs (Figure 3D and Table 1). Moreover, we did not detect large decreases in 5-HT2A-mediated Ca2+ release elicited by an EC50 concentration of 5-HT (peak Ca2+ release= 99.8 +/− 0.2% vs. 91.7 +/− 1.2% in untreated and TGF-α-treated RSK2−/− MEFs, respectively; p<0.05) (Figure 3E).
Figure 3.
RSK2 is required for TGF-α-induced attenuation of 5-HT2A receptor signaling. The EGFR was activated with 100ng/mL TGF-α and then 5-HT2A-mediated Ca2+ responses were measured via FLIPRtetra assays in RSK2+/+ (A-C) and RSK2−/− (D-E) MEFs. A, In RSK2+/+ MEFs, the CRC for 5-HT was significantly shifted rightward (i.e., decreased potency, bold arrow) and downward (i.e., decreased maximal signaling, bold arrow) following 60 min (o) TGF-α treatment relative to untreated cells (■). Shown are the results (mean +/− SEM) of five independent experiments performed in duplicate (p<0.05). B, In RSK2+/+ MEFs, activation of 5-HT2A receptors with an EC50 concentration of 5-HT (10 nM) was significantly attenuated following 60 min (o) TGF-α treatment relative to untreated cells (■). Shown are the normalized Ca2+ traces (untreated set to 100%, mean +/− SEM) of five independent experiments (p<0.05). C, In RSK2+/+ MEFs, TGF-α attenuated 5-HT2A-mediated Ca2+ responses with an IC50 of 4.1ng/mL. Shown are the results (mean +/− SEM) of three independent experiments performed in duplicate. D, In RSK2−/− MEFs, the CRC for 5-HT was not significantly shifted following 60 min (o) TGF-α treatment relative to untreated cells (■). Shown are the results (mean +/− SEM) of three independent experiments performed in duplicate (p>0.05). E, In RSK2−/− MEFs, activation of 5-HT2A receptors with an EC50 concentration of 5-HT (10 nM) was not significantly decreased following 60 min (o) TGF-α treatment relative to untreated cells (■). Shown are the normalized Ca2+ traces (untreated set to 100%, mean +/− SEM) of three independent experiments (p>0.05).
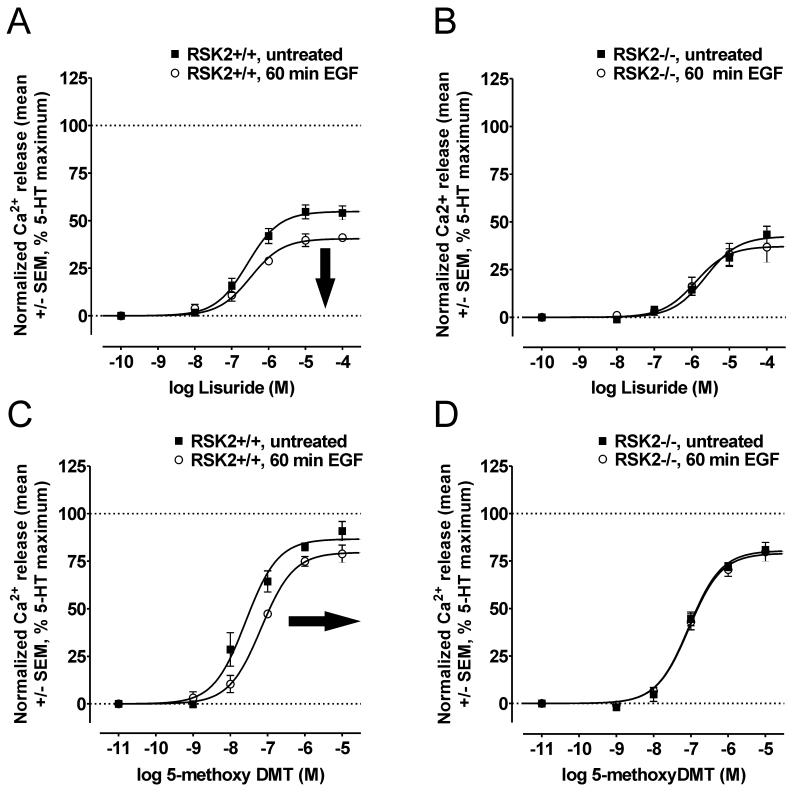
In addition to full agonists, partial agonists display characteristic and predictable signaling behaviors under conditions of decreased receptor responsiveness. Notably, in this context, full agonists commonly exhibit effects on potency but lesser effects on maximal signaling, while partial agonists commonly display decreases in maximal signaling (i.e., large downward shifts in CRCs) (38). Thus, we predicted that EGF treatment would decrease the maximal signaling of the weak partial agonist lisuride with minimal effects on potency; whereas the potency of the strong partial agonist 5-methoxyDMT would be significantly decreased. Consistent with these predictions, EGF treatment significantly decreased the maximal signaling of lisuride in RSK2+/+ MEFs (Figure 4A, Table 1). Furthermore, 5-methoxyDMT displayed behaviors intermediate between the full agonist 5-HT and the weak partial agonist lisuride, exhibiting a minor decrease in maximal signaling and a significant decrease in potency (Figure 4C, Table 1). In agreement with a requirement for RSK2, we did not observe significant shifts in the CRCs of either partial agonist in RSK2−/− MEFs (Figures 4B and 4D, Table 1). Taken together, these pharmacological and genetic approaches strongly support the hypothesis that EGFRs act via RSK2 to attenuate 5-HT2A receptor signaling in MEFs.
Figure 4.
The unique pharmacology of partial agonists shows that RSK2 is required for EGFR-mediated attenuation of 5-HT2A receptor signaling. The EGFR was activated with 100ng/mL EGF and then 5-HT2A-mediated Ca2+ responses elicited by the weak partial agonist lisuride (A-B) and the strong partial agonist 5-methoxyDMT (C-D) were measured via FLIPRtetra assays in RSK2+/+ and RSK2−/− MEFs. A, In RSK2+/+ MEFs, the CRC for lisuride was significantly shifted downward (i.e., decreased maximal signaling, bold arrow) following 60 min (o) EGF treatment relative to untreated cells (■). Shown are the results (mean +/− SEM) of three independent experiments performed in duplicate (p<0.05). B, In RSK2−/− MEFs, the CRC for lisuride was not significantly decreased following EGF treatment relative to untreated cells (■). Shown are the results (mean +/− SEM) of three independent experiments performed in duplicate (p>0.05). C, In RSK2+/+ MEFs, the CRC for 5-methoxyDMT was significantly shifted rightward (i.e., decreased potency, bold arrow) following 60 min (o) EGF treatment relative to untreated cells (■). Shown are the results (mean +/− SEM) of three independent experiments performed in duplicate (p<0.05). D, In RSK2−/− MEFs, the CRC for 5-methoxyDMT was not significantly shifted following EGF treatment relative to untreated cells (■). Shown are the results (mean +/− SEM) of three independent experiments performed in duplicate (p>0.05).
RSK2 is required for PDGFR-mediated attenuation of endogenous 5-HT2A receptor signaling in primary mVSMCs
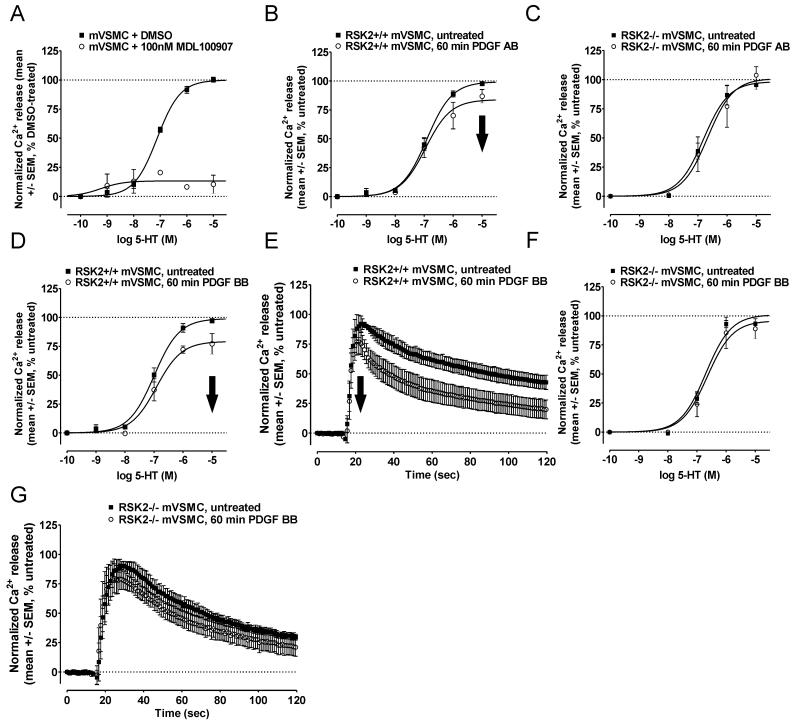
The growth factor PDGF is a potent mitogen, chemoattractant, and survival factor that activates RSKs downstream of PDGFR activation in VSMCs (40, 41). VSMCs also endogenously express 5-HT2A receptors which produce measurable Ca2+ responses in FLIPR assays (Figure 5A) (42). Therefore, VSMCs isolated from RSK2+/+ and RSK2−/− mice represented an intact model system whereby we could test: (1) whether inhibitory cross-talk occurs between additional RTKs and endogenously expressed 5-HT2A receptors, and (2) to what extent this requires RSK2.
Figure 5.
RSK2 is required for inhibitory cross-talk between the PDGFR and the 5-HT2A receptor. 5-HT2A-mediated Ca2+ responses were measured via FLIPRtetra assays (A) in RSK2+/+ and RSK2−/− mVSMCs following treatment with the PDGF ligands PDGF AB (B-C) and PDGF BB (D-G). A, 5-HT elicits robust Ca2+ responses in RSK2+/+ mVSMCs which can be blocked with the 5-HT2A-specific antagonist MDL100907 (100nM). Shown are the results (mean +/− SEM) of two independent experiments performed in duplicate. B, In RSK2+/+ mVSMCs, the CRC for 5-HT was significantly shifted downward (i.e., decreased maximal signaling, bold arrow) following 60 min (o) PDGF AB treatment relative to untreated cells (■). Shown are the results (mean +/− SEM) of four to six independent experiments performed in duplicate (p<0.05). C, In RSK2−/− mVSMCs, the CRC for 5-HT was not significantly shifted following PDGF AB treatment relative to untreated cells (■). Shown are the results (mean +/− SEM) of four independent experiments performed in duplicate (p>0.05). D, In RSK2+/+ mVSMCs, the CRC for 5-HT was significantly shifted downward (i.e., decreased maximal signaling, bold arrow) following 60 min (o) PDGF BB treatment relative to untreated cells (■). Shown are the results (mean +/− SEM) of four to six independent experiments performed in duplicate (p<0.05). E, In RSK2+/+ mVSMCs, 5-HT2A-mediated Ca2+ responses elicited by a maximal concentration of 5-HT (10 μM) were significantly attenuated following 60 min (o) PDGF BB treatment relative to untreated cells (■). Shown are the normalized Ca2+ traces (untreated set to 100%, mean +/− SEM) of four independent experiments (p<0.05). F, In RSK2−/− mVSMCs, the CRC for 5-HT was not significantly shifted following PDGF BB treatment relative to untreated cells (■). Shown are the results (mean +/− SEM) of four independent experiments performed in duplicate (p>0.05). G, In RSK2−/− mVSMCs, 5-HT2A-mediated Ca2+ responses elicited by a maximal concentration of 5-HT (10 μM) were not significantly decreased following PDGF BB treatment relative to untreated cells (■). Shown are the normalized Ca2+ traces (untreated set to 100%, mean +/− SEM) of four independent experiments (p>0.05).
In these studies we activated PDGFRs with PDGF-AB and PDGF-BB, the principal PDGF ligands in serum (43). As evidenced by significant downward shifts in 5-HT CRCs following 60 min treatments with PDGF-AB (Figure 5B) and PDGF-BB (Figure 5D), activation of the PDGFR resulted in attenuation of 5-HT2A receptor signaling (Table 2). To best illustrate this we showed that PDGF-BB treatment significantly decreased 5-HT2A-mediated Ca2+ responses in RSK2+/+ MEFs elicited by a saturating concentration of 5-HT (i.e., 10μM) (peak Ca2+ release= 90.2 +/− 0.7% vs. 67.0 +/− 8.5% in untreated and PDGF-BB-treated RSK2+/+ MEFs, respectively; N=4, p<0.05) (Figure 5E). As expected, PDGF treatments did not significantly reduce the maximal signaling of 5-HT in RSK2−/− mVSMCs (Figures 5C and 5F). Moreover, PDGF-BB treatment failed to significantly decrease 5-HT2A-mediated Ca2+ responses in RSK2−/− MEFs elicited by a saturating concentration of 5-HT (peak Ca2+ release= 90.3 +/− 3.8% vs. 77.9 +/− 7.6% in untreated and PDGF-BB-treated RSK2−/− MEFs, respectively; N=4, p>0.05)(Figure 5G). Together with our results using two different EGFR agonists, these results strongly suggest that RSK2 is required for inhibitory cross-talk between multiple growth factor signaling pathways and the 5-HT2A receptor.
Table 2.
Effects of PDGFR agonists on 5-HT2A receptor signaling in mVSMCs.
| Cell type | RSK2+/+ mVSMCs | RSK2−/− mVSMCs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time treated with growth factor |
0 min | 30 min | 60 min | 0 min | 30 min | 60 min | |||||||
| RTK ligand |
GPCR ligand |
pEC50 ± SEMa,b |
Emax± SEM, %c |
pEC50 ± SEMa,b |
Emax± SEM, %c |
pEC50 ± SEMa,b |
Emax± SEM, %c |
pEC50 ± SEMa,b |
Emax± SEM, %c |
pEC50 ± SEMa,b |
Emax± SEM, %c |
pEC50 ± SEMa,b |
Emax± SEM, %c |
| PDGF AB |
5-HT | 6.91 +/− 0.05 |
99.4 +/− 2.2 |
6.80 +/− 0.13 |
90.5 +/− 5.4 |
6.95 +/− 0.15 |
83.9 +/− 5.6d |
6.80 +/− 0.10 |
98.3 +/− 4.4 |
6.38 +/− 0.09 |
102 +/− 4.2 |
6.66 +/− 0.22 |
101 +/− 10.8 |
| PDGF BB |
5-HT | 7.00 +/− 0.06 |
99.0 +/− 2.7 |
6.88 +/− 0.17 |
94.5 +/− 7.3 |
6.92 +/− 0.14 |
79.2 +/− 5.1d |
6.70 +/− 0.08 |
101 +/− 3.5 |
6.57 +/− 0.14 |
108 +/− 7.8 |
6.64 +/− 0.19 |
95.8 +/− 7.7 |
Agonist-induced Ca2+ responses were quantified via FLIPR assays in untreated and growth factor–treated RSK2+/+ and RSK2−/− mVSMCs. The fit parameters of potency (EC50) and maximal signaling (Emax) were obtained from nonlinear regression (GraphPad software) and represent the mean +/− SEM of at least three independent experiments performed in duplicate.
pEC50 values are represented as −log of EC50 in M.
The maximum response of agonist (Emax) in untreated cells was set equal to 100%.
The F test was used to determine the statistical significance (defined as p<0.05) of the fit parameters in growth factor-treated and untreated cells.
IGF-1 weakly activates RSK2 in MEFs and does not attenuate 5-HT2A receptor signaling
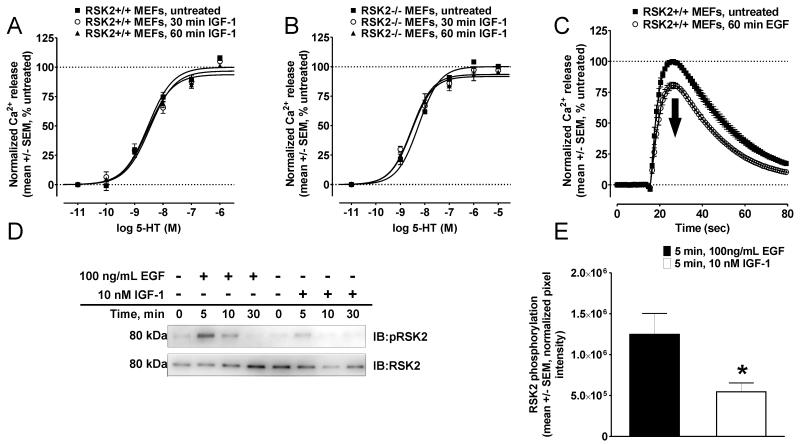
We have demonstrated using various RTK agonists (i.e. EGF, TGF-α, and PDGF), cell lines (MEFs and VSMCs), and GPCR ligands (i.e. 5-HT, 5-methoxy-DMT, and lisuride) that RTKs require RSK2 to attenuate 5-HT2A receptor signaling. However, it was unknown whether insulin or IGF-1, which have been shown to attenuate the signaling of GPCRs including the closely related 5-HT2C receptor (22), also attenuate 5-HT2A receptor signaling.
In initial experiments testing insulin, we determined that insulin showed no effect on 5-HT2A receptor signaling, despite modest activation of RSK2 (data not shown). However, upon closer examination we discovered that insulin receptors are not expressed at detectable levels in RSK2+/+ and RSK2−/− MEFs (Figure S1), suggesting that RSK2 activation is mediated via the IGF-1 R which has low affinity for insulin (44). Since both RSK2+/+ and RSK2−/− MEFs express equal amounts of IGF-1 R (Figure S1), we next determined if IGF-1 could attenuate 5-HT2A receptor signaling. As shown in Figure 6A and 6B, IGF-1 treatment did not result in large shifts in 5-HT CRCs in RSK2+/+ or RSK2−/− MEFs (Table 1), identical to our results with insulin. Importantly, these results could not be explained by a general deficiency in RTK signaling since a 1hr treatment with EGF attenuated 5-HT2A signaling in parallel control experiments (peak Ca2+ release= 99.9 +/− 0.1% vs. 80.7 +/− 2.3% in untreated and EGF-treated RSK2+/+ MEFs, respectively; N=3) (Figure 6C). Additional experimentation showed that the IGF-1R is activated by IGF-1 (Figure S2). Together, these findings suggested that the mechanism(s) underlying inhibitory cross-talk between an RTK and a GPCR (e.g., RSK2 activation) engenders some level of specificity. One such possibility was that IGF-1 treatment only modestly activated RSK2 (24). Indeed, as shown in Figure 6D and quantified in Figure 6E, maximal activation of RSK2 by IGF-1 was significantly less when compared to EGF (0.546 × 106 +/− 0.107 × 106 vs. 1.25 × 106 +/− 0.25 × 106 for IGF-1 and EGF, respectively; N=3, p<0.05). Thus, robust activation of RSK2 by RTKs seems to be required for inhibitory cross-talk with the 5-HT2A receptor. Regardless of the mechanism, it is likely that some degree of specificity exists given the potential physiological importance of RTK-GPCR crosstalk.
Figure 6.
IGF-1 weakly activates RSK2 and does not attenuate 5-HT2A receptor signaling. 5-HT2A-mediated Ca2+ responses were measured via FLIPRtetra assays in RSK2+/+ and RSK2−/− MEFs following treatment with 10 nM IGF-1 (A and B) or 100ng/mL EGF (C). For Western blotting, a phospho-specific antibody was used to detect activation of RSK2 (D and E). A and B, In RSK2+/+ and RSK2−/− MEFs, CRCs for 5-HT were not significantly shifted following 30 min (o) and 60 min (▲) IGF-1 treatments relative to untreated cells (■). Shown are the results (mean +/− SEM) of three independent experiments performed in duplicate (p>0.05). C, Control experiments in RSK2+/+ MEFs showed that 5-HT2A-mediated Ca2+ responses elicited by an EC50 concentration of 5-HT (10 nM) were significantly attenuated following 60 min (o) 100ng/mL EGF treatment relative to untreated cells (■). Shown are the normalized Ca2+ traces (untreated set to 100%, mean +/− SEM) of three independent experiments performed in duplicate (p<0.05). D, Immunoblot showing that EGF robustly activated RSK2 (Ser(P)-386) in RSK2+/+ MEFs; whereas IGF-1 weakly activated RSK2 in RSK2+/+ MEFs. Shown are representative data from three independent experiments. E, Quantification of immunoblots in (D) showing that maximal activation of RSK2 at 5 min was significantly greater after EGF treatment than after IGF-1 treatment (*, p<0.05). Shown are the results (sum pixel intensity normalized to total RSK2, mean +/− SEM) of three independent experiments.
RSK2 is required for EGF-mediated attenuation of endogenous P2Y purinergic receptor signaling
RSK2 attenuates the signaling of additional GPCRs endogenously expressed in MEFs, including P2Y purinergic receptors (13). Therefore, we hypothesized that EGFR activation, in addition to regulating 5-HT2A receptors, could also attenuate P2Y receptor signaling in a RSK2-dependent manner. By testing this hypothesis we could begin to address whether this novel regulatory mechanism is conserved across multiple GPCRs that show sensitivity to RSK2 regulation.
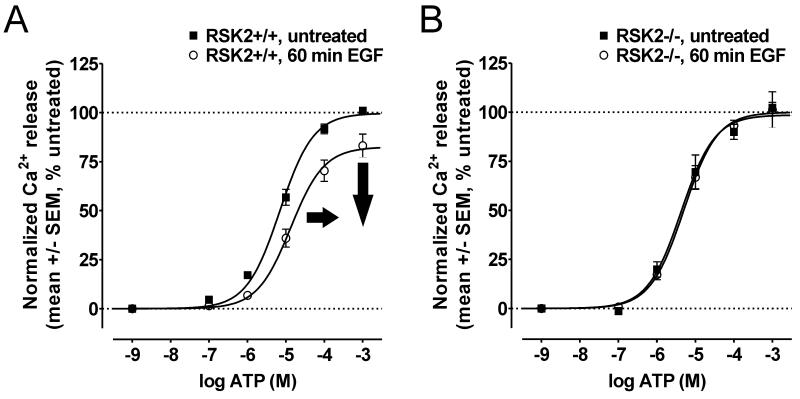
As shown in Figure 7A, EGF treatment significantly reduced ATP signaling in RSK2+/+ MEFs (Table 1). Specifically, we observed significant decreases in ATP maximal signaling (Emax=99.7 +/− 2.0% vs. 82.5 +/− 3.2% in untreated and EGF-treated RSK2+/+ MEFs, respectively; N=5, p<0.05) and potency (7.0 μM vs. 13 μM for untreated and hEGF-treated, respectively; N=5, p<0.05) following 60 min EGF treatment. Similar to our observations in mVSMCs (Figure 5), decreased maximal signaling of the full agonist ATP was consistent with desensitization of endogenously expressed P2Y receptors. However, treating RSK2−/− MEFs with EGF failed to significantly decrease ATP maximal signaling or potency (Figure 7B, Table 1). These data are important because they provide the first evidence for a common regulatory mechanism whereby RTKs act via RSK2 to regulate the signaling of multiple GPCRs.
Figure 7.
RSK2 is required for inhibitory cross-talk between the EGFR and the P2Y purinergic receptor. The EGFR was activated with 100ng/mL EGF and then P2Y-mediated Ca2+ responses were measured via FLIPRtetra assays in RSK2+/+ (A) and RSK2−/− (B) MEFs. A, In RSK2+/+ MEFs, the CRC for ATP was significantly shifted rightward (i.e., decreased ATP potency, bold arrow) and downward (ie., decreased maximal signaling, bold arrow) following 60 min (o) EGF treatment relative to untreated cells (■). Shown are the results (mean +/− SEM) of five independent experiments performed in duplicate (p<0.05). B, In RSK2−/− MEFs, the CRC for ATP was not significantly shifted following EGF treatment relative to untreated cells (■). Shown are the results (mean +/− SEM) of five independent experiments performed in duplicate (p>0.05).
Growth factors essential for normal brain function attenuate 5-HT2A receptor signaling in cortical neurons
We have presented multiple lines of evidence to show that 5-HT2A signaling is indeed attenuated following activation of several endogenous RTKs in multiple cell types. In addition to expression in peripheral tissues, RTKs are widely expressed throughout the brain (e.g., in the cortex) and are activated by endogenous ligands such as EGF and NRG-1 (45). Since 5-HT2A receptors are also highly expressed in the cortex (46), it was tempting to speculate that cross-talk between RTKs and 5-HT2A receptors could explain how 5-HT2A receptors are regulated in cortical neurons.
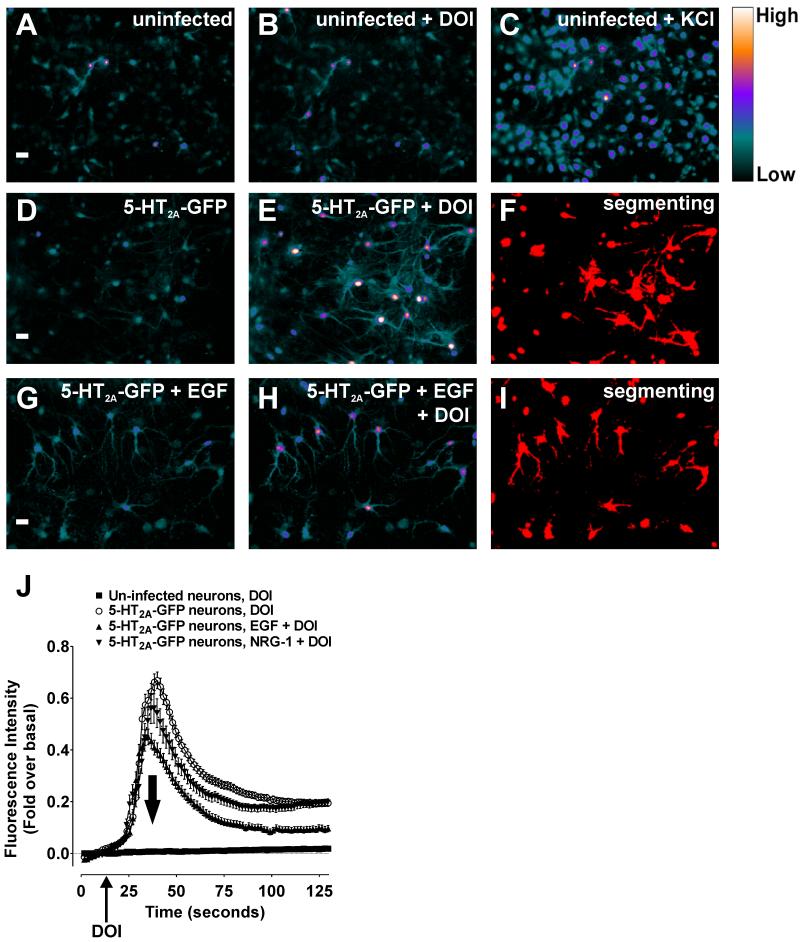
To test this possibility, we developed a live cell imaging technique to measure 5-HT2A receptor signaling in cortical neurons in the presence and absence of growth factors. As shown in Figure 8A and 8B, uninfected neurons were unresponsive to the 5-HT2A/2C selective agonist DOI, despite robust Ca2+ responses following depolarization with 80 mM KCl (Figure 8C). However, DOI elicited measurable Ca2+ responses in neurons only after infection with GFP-tagged 5-HT2A receptors (Figure 8D and 8E), thus ensuring specificity of the DOI response. We then quantified these DOI-induced responses in untreated (Figure 8E) and growth factor-treated (Figure 8H) neurons via manual segmenting (Figure 8F and 8I). As shown in Figure 8J and quantified in Table 3, treatment with either EGF or NRG-1 significantly reduced the Ca2+ response elicited by DOI. In these studies we present the first evidence that inhibitory cross-talk occurs between RTKs and GPCRs in neurons. Most importantly, our data show that RTKs attenuate 5-HT2A signaling in neurons-a finding with enormous potential for explaining how 5-HT2A receptors are regulated in the brain.
Figure 8.
Growth factors essential for normal brain function attenuate 5-HT2A receptor signaling in cortical neurons. Cortical neurons were isolated from RSK2+/+ mice and cultured for ~14 days in vitro (scale bar is 20μm). These neurons were loaded with Ca2+ dye for 60 min prior to stimulation with the 5-HT2A-selective agonist DOI (1μM) and 5-HT2A-mediated Ca2+ responses (see color scale on right) were measured by live cell fluorescence microscopy in uninfected cortical neurons (A-C) and cortical neurons infected with GFP-tagged 5-HT2A receptors (D-I). Growth factor-mediated changes in signaling were detected by comparing Ca2+ responses in untreated neurons (D-E) and neurons treated with 100ng/mL EGF (G-I) and 100ng/mL NRG-1 (images not shown). Ca2+ responses were quantified by segmenting the total area of responding cells and assessing the mean fluorescence intensity/area (F, I, J). A and B, The 5-HT2A-selective agonist DOI did not produce measurable Ca2+ responses in uninfected neurons. C, The uninfected neurons produced measurable Ca2+ responses upon depolarization with 80mM KCl. D, Shown are cortical neurons that were infected with lentivirus encoding GFP-tagged 5-HT2A receptors prior to stimulation with DOI. E and F, DOI produced robust Ca2+ responses in infected neurons which were quantified via manual segmenting. G, Shown are cortical neurons infected with GFP-tagged 5-HT2A receptors loaded with Ca2+ imaging dye containing 100ng/mL EGF for 60 min prior to stimulation with DOI. H and I, The 5-HT2A-selective agonist DOI produced weak Ca2+ responses in infected neurons treated with EGF. J, The DOI-induced 5-HT2A Ca2+ responses in infected neurons (o) was significantly greater than those measured from neurons pretreated with EGF (▲) or NRG-1 (▼) (p<0.05). No response was detected for uninfected neurons (■). Quantified results (mean +/− SEM) are shown from three independent experiments.
Table 3.
Effects of EGFR family agonists on 5-HT2A receptor signaling in cortical neurons.
| RTK ligand | GPCR ligand |
Peak Ca2+ releasea |
Area under curve |
|---|---|---|---|
| None | DOI | 0.67 +/− 0.03 | 33.7 +/− 1.3 |
| EGF | DOI | 0.45 +/−0.03b | 20.9 +/− 1.2b |
| NRG-1 | DOI | 0.56 +/− 0.06 | 28.6 +/− 1.7b |
Agonist-induced Ca2+ responses in untreated and growth factor–treated cortical neurons were quantified using the FLIPR Ca2+ assay kit and BD Pathway 855 high content imaging microscope. To control for subtle differences in 5-HT2A-GFP receptor expression, Ca2+ responses were normalized to GFP intensity/well using custom written macros for Excel (Microsoft) and Image J (U. S. National Institutes of Health). Values were expressed as fold over baseline and represent the mean +/− SEM of three independent experiments.
A two-tailed, paired t test was used to determine the statistical significance (defined as p<0.05) of responses in growth factor-treated vs. untreated cells.
Discussion
The three major findings in this paper are: 1) multiple endogenous RTK receptors and their ligands attenuate 5-HT2A receptor responsiveness in several physiologically relevant cell types; 2) RSK2 is required for RTK-mediated attenuation of 5-HT2A receptor signaling, and 3) RTK activation similarly attenuates P2Y purinergic signaling in a RSK2-dependent manner. By directly testing multiple endogenous growth factors/RTK pathways and multiple Gq-coupled GPCRs, we have now established a cellular mechanism whereby RTK signaling cascades attenuate GPCR signaling through RSK2. Importantly, these findings support a novel paradigm of inhibitory cross-talk between RTKs and GPCRs and extend it to include a larger mechanism whereby RTKs act via RSK2 to regulate the signaling of multiple GPCRs.
RSK2 is required for inhibitory cross-talk between RTKs and the 5-HT2A receptor in a variety of cell types
Consistent with evidence for inhibitory cross-talk between RTKs and select GPCRs (i.e., the β1-, β2-, α1B-, and α1D-adrenergic receptors, and 5-HT2C receptor) (16-22), our data demonstrate that activation of the EGFR attenuates 5-HT2A receptor signaling in MEFs, VSMCs and cortical pyramidal neurons. Moreover, we discovered that this novel regulatory pathway requires RSK2. We verified that the EGFR requires RSK2 to attenuate 5-HT2A receptor signaling by observing the signaling of both full and partial 5-HT2A agonists in RSK2+/+ and RSK2−/− cells. Since changes to GPCR responsiveness affect each agonist class differently, this approach allowed us to unambiguously identify RTK-mediated effects on receptor signaling. Explicitly, full agonists have a large receptor reserve and are resistant to changes in the population of functional receptors (i.e., resulting from receptor desensitization or down-regulation). As a result, full agonists signal maximally but with lower potency under conditions of receptor desensitization in cells over-expressing a GPCR (i.e., CRCs are right-shifted) (38). However, both the maximal signaling and potency of full agonists is decreased under conditions of receptor desensitization in cells with endogenous GPCR expression (i.e., CRCs are predominantly shifted downward with minor rightward shifts). Partial agonists, on the other hand, have low receptor reserve and are more sensitive to changes in the population of functional GPCRs. As a result, partial agonists typically signal with lower efficacy and potency under conditions of receptor desensitization irrespective of receptor expression (38). In line with these predictions, we observed that EGF significantly decreased full agonist (i.e., 5-HT) potency and partial agonist (i.e., lisuride) efficacy in high-expressing RSK2+/+ MEFs. Additionally, growth factor treatment significantly decreased the maximal signaling of full agonists (i.e., 5-HT and ATP) when their cognate receptors were expressed at endogenous levels in RSK2+/+ mVSMCs and MEFs. Taken together, our results in RSK2+/+ cells are consistent with RTK-mediated attenuation of 5-HT2A receptor signaling. Importantly, none of these predicted effects were observed in RSK2−/− MEFs, thus supporting the hypothesis that RTKs act via RSK2 to attenuate 5-HT2A receptor signaling.
Alternatively, these results could be explained by differences in gene expression profiles between RSK2+/+ and RSK2−/− cells. However, microarray data show that the expression of genes required for EGFR signal transduction are not significantly different between RSK2+/+ and RSK2−/− MEFs. Thus, the simplest explanation for our results remains that RSK2 is a critical mediator of cross-talk between the EGFR and 5-HT2A receptor.
In addition to our results in RSK2+/+ MEFs, we observed that 5-HT2A signaling was significantly decreased following activation of endogenous RTKs in mVSMC and cortical neuron primary cell lines. Considering the physiological importance of inhibitory cross-talk, it is attractive to speculate that growth factor signaling may be relevant for regulating the 5-HT2A receptor in the CNS. In support of this, members of the EGFR family are widely expressed throughout the brain and regulate a variety of functions including proliferation, differentiation, maturation, and survival of a variety of neurons (45). Interestingly, the ErbB4 neuregulin receptor, which is a member of the EGFR family, is expressed throughout the mature brain and is known to reside in some of the same cortical layers (47) as the 5-HT2A receptor (48). Moreover, ErbB4 interacts with PSD-95, a post-synaptic density protein that associates with and regulates 5-HT2A receptor signaling and trafficking in vitro and in vivo (26, 29, 35, 49). Thus, considering the pervasiveness of growth factor signaling in the brain, as well as its overlapping expression with 5-HT2A receptors, RTK signaling could modulate 5-HT2A receptor signaling in vivo. Intriguingly, aberrant signaling of both RTKs and 5-HT2A receptors has been associated with neuropsychiatric disorders such as depression and schizophrenia (47, 50-52). Together, these findings suggest that a more complete understanding of the mechanism(s) underlying inhibitory cross-talk between RTKs and 5-HT2A receptors is of considerable therapeutic importance.
IGF-1 fails to robustly activate RSK2 and does not attenuate 5-HT2A receptor signaling
In stark contrast to our results using EGF, PDGF, and ErbB4 receptor agonists, we discovered that IGF-1 did not attenuate 5-HT2A receptor signaling in either RSK2+/+ or RSK2−/− MEFs. In order to interpret these negative results, we showed that EGF treatment retained the ability to attenuate 5-HT2A-mediated Ca2+ release in parallel control experiments. These data suggest that, unlike EGF receptor activation, IGF-1 signaling does not desensitize 5-HT2A receptors. The reasons for this are unknown, although our data showing that IGF-1 weakly activates RSK2 when compared to EGF suggest that a threshold level of RSK2 activation must be reached in order to elicit 5-HT2A receptor desensitization.
Other mechanisms have been proposed to explain IGF-1-induced GPCR desensitization including phosphorylation of tyrosine residues in the second intracellular loop of the β2-adrenergic receptor and Akt-mediated phosphorylation of the β1-adrenergic receptor (16, 19). However, 5-HT2A receptors are not known to be phosphorylated on tyrosine residues and are not substrates for Akt, perhaps explaining why IGF-1 has no effect on 5-HT2A receptor signaling.
RSK2 is required for growth factor-mediated regulation of multiple GPCRs-evidence from P2Y purinergic receptors
In addition to the 5-HT2A receptor, endogenous P2Y purinergic receptor signaling is regulated by RSK2 (13). Here we provide the first evidence showing that, like 5-HT2A receptors, EGFR activation attenuates P2Y-purinergic receptor signaling in a RSK2-dependent manner. Thus, it appears that RSK2 is a critical mediator of inhibitory crosstalk between multiple RTKs and GPCRs. Interestingly, the β1-adrenergic and PAR-1 thrombinergic receptors are also regulated by RSK2 (13), and it remains to be determined if these receptors are regulated by RTKs in a RSK2-dependent manner. This is an especially intriguing question for the β1AR since it is already known that activation of the IGF-1R regulates β1-adrenergic receptor signaling through activation of PI3 kinase and Akt (16).
A question of important physiological relevance is whether specific RTK signaling pathways influence the signaling of all or only select groups of GPCRs. Our results, along with those of others, indicate that signaling from some Gq-coupled receptors (i.e., 5HT2A, P2Y, α1b-adrenergic, and α1d-adrenergic) are similarly attenuated by one RTK, the EGFR (20, 21). Other RTKs, such as insulin and IGF-1 receptors, are well-known to decrease the signaling of some Gs-coupled GPCRs such as β1- and β2-adrenergic receptors (16, 53). However, insulin and IGF-1 receptors attenuate signaling from only some (i.e., 5-HT2C), but not all (i.e., M1 muscarinic or 5-HT2A) Gq-coupled GPCRs ((22), Figure 6). Therefore, a robustness of this signaling crosstalk is evident and RTK inhibitory crosstalk to GPCRs will likely emerge as a receptor-specific phenomenon. Ultimately, further studies testing many RTKs and GPCRs will help elucidate if this crosstalk is a conserved phenomenon.
In summary, multiple lines of evidence suggest that RSK2 is a critical mediator of inhibitory cross-talk between RTKs and the 5-HT2A receptor. Specifically, this study presents the first evidence that 5-HT2A receptor signaling is attenuated by the growth-factor-mediated activation of RTKs endogenously expressed in multiple cell types including physiologically relevant mVSMCs and cortical neurons. Moreover, genetic deletion of RSK2 was sufficient to block these effects, thus demonstrating that RSK2 is required for the inhibitory cross-talk between RTKs and 5-HT2A receptors in all relevantcell types examined. Intriguingly, we discovered that the P2Y purinergic receptor, whose signaling is also regulated by RSK2, is similarly attenuated following EGFR activation in RSK2+/+ MEFs. Taken together, these findings provide the initial framework for a conserved regulatory mechanism whereby multiple RTKs act via the ERK/MAPK effector RSK2 to attenuate GPCR signaling. Most importantly, inhibitory cross-talk between RTKs and 5-HT2A receptors could provide insight into how these receptors are regulated in vivo.
Supplementary Material
Figure S1. Genes involved in IGF-1 R signal transduction are expressed at similar levels in RSK2+/+ and RSK2−/− MEFs. The microarray data quantifying gene expression in RSK2+/+ and RSK2−/− MEFs was produced previously by Sheffler et al. (13). Here we overlaid the mRNA expression levels of IR and IGF-1 R signal transduction genes in RSK2+/+ and RSK2−/− MEFs with gene-expression color criterion and fold-changes from the programs GenMAPP and MAPPFinder. Gray colored genes are equally expressed in RSK2+/+ and RSK2−/− MEFs. Green colored genes show greater than a 2-fold increase in expression in RSK2−/− MEFs compared to RSK2+/+ fibroblasts. Red colored genes show greater than a 2-fold decrease in expression in RSK2−/− MEFs compared to RSK2+/+ fibroblasts. As shown, the gene encoding the IR was not expressed in RSK2+/+ and RSK2−/− MEFs. However, the genes encoding the IGF-1 R and downstream effectors were expressed at comparable levels in RSK2+/+ and RSK2−/− MEFs. Raw microarray data and gene information can be found in the Supporting Information zip file.
Figure S2. IGF-1 activates the IGF-1 R in RSK2+/+ MEFs. Here we determined using cell lysates that 10 nM IGF-1 activated the IGF-1 R (Tyr(P)1158/1162/1163) in RSK2+/+ MEFs. Shown is an immunoblot representative of three independent experiments.
Acknowledgment
We thank Kimberly Molnar for her expert assistance with mVSMC isolation and Dr. Betsy Pehek for HPLC-electrochemical detection analysis of dialyzed serum.
Abbreviations
- 5-HT
serotonin
- 5-HT2A
serotonin 2A receptor
- GPCR
G protein-coupled receptor
- RSK2
p90 ribosomal S6 kinase 2
- ERK/MAPK
extracellular signal regulated kinase/mitogen activated protein kinase
- CRC
concentration-response curve
- RTK
receptor tyrosine kinase
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- IGF-1
insulin-like growth factor 1
- IGF-1 R
insulin-like growth factor 1 receptor
- TGF-α
transforming growth factor-alpha
- PDGF
platelet-derived growth factor
- PDGFR
platelet-derived growth factor receptor
- NRG-1
neuregulin-1
- GFP
green fluorescent protein
- 5-methoxyDMT
5-methoxy-N,N-dimethyltryptamine
- DOI
(±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride
- FBS
fetal bovine serum
- DMEM
Dulbecco’s modified Eagle’s medium
- HBSS
Hank’s buffered salt solution
- MEF
mouse embryonic fibroblast
- mVSMC
mouse vascular smooth muscle cell
- RFU
relative fluorescent unit
Footnotes
Supporting Information. Supporting information is available showing (1) microarray analysis of genes involved in IR and IGF-1 R signaling pathways in RSK2+/+ and RSK2−/− MEFs and (2) IGF-1 activation of the IGF-1 R. This material is available free of charge via the internet at http://pubs.acs.org.
This work was supported by RO1MH61887, NO1MH32004, U19MH82441, T32HD040127, and UNC Neurodevelopmental Disorders Research Center.
References
- 1.King N, Hittinger CT, Carroll SB. Evolution of key cell signaling and adhesion protein families predates animal origins. Science. 2003;301:361–363. doi: 10.1126/science.1083853. [DOI] [PubMed] [Google Scholar]
- 2.Schioth HB, Nordstrom KJ, Fredriksson R. Mining the gene repertoire and ESTs for G protein-coupled receptors with evolutionary perspective. Acta Physiol (Oxf) 2007;190:21–31. doi: 10.1111/j.1365-201X.2007.01694.x. [DOI] [PubMed] [Google Scholar]
- 3.Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, Amanatides P, Ballew RM, Huson DH, Wortman JR, Zhang Q, Kodira CD, Zheng XH, Chen L, Skupski M, Subramanian G, Thomas PD, Zhang J, Miklos G. L. Gabor, Nelson C, Broder S, Clark AG, Nadeau J, McKusick VA, Zinder N, Levine AJ, Roberts RJ, Simon M, Slayman C, Hunkapiller M, Bolanos R, Delcher A, Dew I, Fasulo D, Flanigan M, Florea L, Halpern A, Hannenhalli S, Kravitz S, Levy S, Mobarry C, Reinert K, Remington K, Abu-Threideh J, Beasley E, Biddick K, Bonazzi V, Brandon R, Cargill M, Chandramouliswaran I, Charlab R, Chaturvedi K, Deng Z, Di Francesco V, Dunn P, Eilbeck K, Evangelista C, Gabrielian AE, Gan W, Ge W, Gong F, Gu Z, Guan P, Heiman TJ, Higgins ME, Ji RR, Ke Z, Ketchum KA, Lai Z, Lei Y, Li Z, Li J, Liang Y, Lin X, Lu F, Merkulov GV, Milshina N, Moore HM, Naik AK, Narayan VA, Neelam B, Nusskern D, Rusch DB, Salzberg S, Shao W, Shue B, Sun J, Wang Z, Wang A, Wang X, Wang J, Wei M, Wides R, Xiao C, Yan C, Yao A, Ye J, Zhan M, Zhang W, Zhang H, Zhao Q, Zheng L, Zhong F, Zhong W, Zhu S, Zhao S, Gilbert D, Baumhueter S, Spier G, Carter C, Cravchik A, Woodage T, Ali F, An H, Awe A, Baldwin D, Baden H, Barnstead M, Barrow I, Beeson K, Busam D, Carver A, Center A, Cheng ML, Curry L, Danaher S, Davenport L, Desilets R, Dietz S, Dodson K, Doup L, Ferriera S, Garg N, Gluecksmann A, Hart B, Haynes J, Haynes C, Heiner C, Hladun S, Hostin D, Houck J, Howland T, Ibegwam C, Johnson J, Kalush F, Kline L, Koduru S, Love A, Mann F, May D, McCawley S, McIntosh T, McMullen I, Moy M, Moy L, Murphy B, Nelson K, Pfannkoch C, Pratts E, Puri V, Qureshi H, Reardon M, Rodriguez R, Rogers YH, Romblad D, Ruhfel B, Scott R, Sitter C, Smallwood M, Stewart E, Strong R, Suh E, Thomas R, Tint NN, Tse S, Vech C, Wang G, Wetter J, Williams S, Williams M, Windsor S, Winn-Deen E, Wolfe K, Zaveri J, Zaveri K, Abril JF, Guigo R, Campbell MJ, Sjolander KV, Karlak B, Kejariwal A, Mi H, Lazareva B, Hatton T, Narechania A, Diemer K, Muruganujan A, Guo N, Sato S, Bafna V, Istrail S, Lippert R, Schwartz R, Walenz B, Yooseph S, Allen D, Basu A, Baxendale J, Blick L, Caminha M, Carnes-Stine J, Caulk P, Chiang YH, Coyne M, Dahlke C, Mays A, Dombroski M, Donnelly M, Ely D, Esparham S, Fosler C, Gire H, Glanowski S, Glasser K, Glodek A, Gorokhov M, Graham K, Gropman B, Harris M, Heil J, Henderson S, Hoover J, Jennings D, Jordan C, Jordan J, Kasha J, Kagan L, Kraft C, Levitsky A, Lewis M, Liu X, Lopez J, Ma D, Majoros W, McDaniel J, Murphy S, Newman M, Nguyen T, Nguyen N, Nodell M, Pan S, Peck J, Peterson M, Rowe W, Sanders R, Scott J, Simpson M, Smith T, Sprague A, Stockwell T, Turner R, Venter E, Wang M, Wen M, Wu D, Wu M, Xia A, Zandieh A, Zhu X. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 4.Kristiansen K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol Ther. 2004;103:21–80. doi: 10.1016/j.pharmthera.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Kroeze WK, Sheffler DJ, Roth BL. G-protein-coupled receptors at a glance. J Cell Sci. 2003;116:4867–4869. doi: 10.1242/jcs.00902. [DOI] [PubMed] [Google Scholar]
- 6.Thompson MD, Burnham WM, Cole DE. The G protein-coupled receptors: pharmacogenetics and disease. Crit Rev Clin Lab Sci. 2005;42:311–392. doi: 10.1080/10408360591001895. [DOI] [PubMed] [Google Scholar]
- 7.Lagerstrom MC, Schioth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- 8.Kroeze WK, Kristiansen K, Roth BL. Molecular biology of serotonin receptors structure and function at the molecular level. Curr Top Med Chem. 2002;2:507–528. doi: 10.2174/1568026023393796. [DOI] [PubMed] [Google Scholar]
- 9.Berger M, Gray JA, Roth BL. The Expanded Biology of Serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB. Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci U S A. 2002;99:11934–11939. doi: 10.1073/pnas.182234399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willins DL, Berry SA, Alsayegh L, Backstrom JR, Sanders-Bush E, Friedman L, Roth BL. Clozapine and other 5-hydroxytryptamine-2A receptor antagonists alter the subcellular distribution of 5-hydroxytryptamine-2A receptors in vitro and in vivo. Neuroscience. 1999;91:599–606. doi: 10.1016/s0306-4522(98)00653-8. [DOI] [PubMed] [Google Scholar]
- 13.Sheffler DJ, Kroeze WK, Garcia BG, Deutch AY, Hufeisen SJ, Leahy P, Bruning JC, Roth BL. p90 ribosomal S6 kinase 2 exerts a tonic brake on G protein-coupled receptor signaling. Proc Natl Acad Sci U S A. 2006;103:4717–4722. doi: 10.1073/pnas.0600585103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strachan RT, Sheffler DJ, Willard B, Kinter M, Kiselar JG, Roth BL. Ribosomal S6 kinase 2 directly phosphorylates the 5-hydroxytryptamine 2A (5-HT2A) serotonin receptor, thereby modulating 5-HT2A signaling. J Biol Chem. 2009;284:5557–5573. doi: 10.1074/jbc.M805705200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frodin M, Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol. 1999;151:65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- 16.Gavi S, Yin D, Shumay E, Wang HY, Malbon CC. Insulin-like growth factor-I provokes functional antagonism and internalization of beta1-adrenergic receptors. Endocrinology. 2007;148:2653–2662. doi: 10.1210/en.2006-1569. [DOI] [PubMed] [Google Scholar]
- 17.Hadcock JR, Port JD, Gelman MS, Malbon CC. Cross-talk between tyrosine kinase and G-protein-linked receptors. Phosphorylation of beta 2-adrenergic receptors in response to insulin. J Biol Chem. 1992;267:26017–26022. [PubMed] [Google Scholar]
- 18.Karoor V, Baltensperger K, Paul H, Czech MP, Malbon CC. Phosphorylation of tyrosyl residues 350/354 of the beta-adrenergic receptor is obligatory for counterregulatory effects of insulin. J Biol Chem. 1995;270:25305–25308. doi: 10.1074/jbc.270.43.25305. [DOI] [PubMed] [Google Scholar]
- 19.Karoor V, Malbon CC. Insulin-like growth factor receptor-1 stimulates phosphorylation of the beta2-adrenergic receptor in vivo on sites distinct from those phosphorylated in response to insulin. J Biol Chem. 1996;271:29347–29352. doi: 10.1074/jbc.271.46.29347. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Sainz JA, Romero-Avila MT, Molina-Munoz T, Ldel C. Medina. Insulin induces alpha1B-adrenergic receptor phosphorylation and desensitization. Life Sci. 2004;75:1937–1947. doi: 10.1016/j.lfs.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Perez CE, Calvo-Ochoa E, Kalashnikova EV, Reyes-Cruz G, Romero-Avila MT, Garcia-Sainz JA. Receptor tyrosine kinases regulate alpha1D-adrenoceptor signaling properties: phosphorylation and desensitization. Int J Biochem Cell Biol. 2009;41:1276–1283. doi: 10.1016/j.biocel.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 22.Hurley JH, Zhang S, Bye LS, Marshall MS, DePaoli-Roach AA, Guan K, Fox AP, Yu L. Insulin signaling inhibits the 5-HT2C receptor in choroid plexus via MAP kinase. BMC Neurosci. 2003;4:10. doi: 10.1186/1471-2202-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanauer A, Young ID. Coffin-Lowry syndrome: clinical and molecular features. J Med Genet. 2002;39:705–713. doi: 10.1136/jmg.39.10.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruning JC, Gillette JA, Zhao Y, Bjorbaeck C, Kotzka J, Knebel B, Avci H, Hanstein B, Lingohr P, Moller DE, Krone W, Kahn CR, Muller-Wieland D. Ribosomal subunit kinase-2 is required for growth factor-stimulated transcription of the c-Fos gene. Proc Natl Acad Sci U S A. 2000;97:2462–2467. doi: 10.1073/pnas.97.6.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan XM, Kobilka TS, Kobilka BK. Enhancement of membrane insertion and function in a type IIIb membrane protein following introduction of a cleavable signal peptide. J Biol Chem. 1992;267:21995–21998. [PubMed] [Google Scholar]
- 26.Xia Z, Gray JA, Compton-Toth BA, Roth BL. A direct interaction of PSD-95 with 5-HT2A serotonin receptors regulates receptor trafficking and signal transduction. J Biol Chem. 2003;278:21901–21908. doi: 10.1074/jbc.M301905200. [DOI] [PubMed] [Google Scholar]
- 27.Morgenstern JP, Land H. A series of mammalian expression vectors and characterisation of their expression of a reporter gene in stably and transiently transfected cells. Nucleic Acids Res. 1990;18:1068. doi: 10.1093/nar/18.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray JA, Sheffler DJ, Bhatnagar A, Woods JA, Hufeisen SJ, Benovic JL, Roth BL. Cell-type specific effects of endocytosis inhibitors on 5-hydroxytryptamine(2A) receptor desensitization and resensitization reveal an arrestin-, GRK2-, and GRK5-independent mode of regulation in human embryonic kidney 293 cells. Mol Pharmacol. 2001;60:1020–1030. doi: 10.1124/mol.60.5.1020. [DOI] [PubMed] [Google Scholar]
- 29.Xia Z, Hufeisen SJ, Gray JA, Roth BL. The PDZ-binding domain is essential for the dendritic targeting of 5-HT2A serotonin receptors in cortical pyramidal neurons in vitro. Neuroscience. 2003;122:907–920. doi: 10.1016/s0306-4522(03)00589-x. [DOI] [PubMed] [Google Scholar]
- 30.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 31.Dahlquist KD, Salomonis N, Vranizan K, Lawlor SC, Conklin BR. GenMAPP, a new tool for viewing and analyzing microarray data on biological pathways. Nat Genet. 2002;31:19–20. doi: 10.1038/ng0502-19. [DOI] [PubMed] [Google Scholar]
- 32.Doniger SW, Salomonis N, Dahlquist KD, Vranizan K, Lawlor SC, Conklin BR. MAPPFinder: using Gene Ontology and GenMAPP to create a global gene-expression profile from microarray data. Genome Biol. 2003;4:R7. doi: 10.1186/gb-2003-4-1-r7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moon SK, Thompson LJ, Madamanchi N, Ballinger S, Papaconstantinou J, Horaist C, Runge MS, Patterson C. Aging, oxidative responses, and proliferative capacity in cultured mouse aortic smooth muscle cells. Am J Physiol Heart Circ Physiol. 2001;280:H2779–2788. doi: 10.1152/ajpheart.2001.280.6.H2779. [DOI] [PubMed] [Google Scholar]
- 34.Ahlemeyer B, Baumgart-Vogt E. Optimized protocols for the simultaneous preparation of primary neuronal cultures of the neocortex, hippocampus and cerebellum from individual newborn (P0.5) C57Bl/6J mice. J Neurosci Methods. 2005;149:110–120. doi: 10.1016/j.jneumeth.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 35.Abbas AI, Yadav PN, Yao WD, Arbuckle MI, Grant SG, Caron MG, Roth BL. PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. J Neurosci. 2009;29:7124–7136. doi: 10.1523/JNEUROSCI.1090-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen MS, Zhang C, Shokat KM, Taunton J. Structural bioinformatics-based design of selective, irreversible kinase inhibitors. Science. 2005;308:1318–1321. doi: 10.1126/science1108367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kenakin T. A Pharmacology Primer: Theory, Application, and Methods. Third ed Elsevier Science & Technology Books; 2009. [Google Scholar]
- 39.Schiffer HH, Reding EC, Fuhs SR, Lu Q, Piu F, Wong S, Littler PL, Weiner DM, Keefe W, Tan PK, Nash NR, Knapp AE, Olsson R, Brann MR. Pharmacology and signaling properties of epidermal growth factor receptor isoforms studied by bioluminescence resonance energy transfer. Mol Pharmacol. 2007;71:508–518. doi: 10.1124/mol.106.027656. [DOI] [PubMed] [Google Scholar]
- 40.Tallquist M, Kazlauskas A. PDGF signaling in cells and mice. Cytokine Growth Factor Rev. 2004;15:205–213. doi: 10.1016/j.cytogfr.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Mackeigan JP, Murphy LO, Dimitri CA, Blenis J. Graded mitogen-activated protein kinase activity precedes switch-like c-Fos induction in mammalian cells. Mol Cell Biol. 2005;25:4676–4682. doi: 10.1128/MCB.25.11.4676-4682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garnovskaya MN, Nebigil CG, Arthur JM, Spurney RF, Raymond JR. 5-Hydroxytryptamine2A receptors expressed in rat renal mesangial cells inhibit cyclic AMP accumulation. Mol Pharmacol. 1995;48:230–237. [PubMed] [Google Scholar]
- 43.Ross R. Platelet-derived growth factor. Lancet. 1989;1:1179–1182. doi: 10.1016/s0140-6736(89)92760-8. [DOI] [PubMed] [Google Scholar]
- 44.De Meyts P, Whittaker J. Structural biology of insulin and IGF1 receptors: implications for drug design. Nat Rev Drug Discov. 2002;1:769–783. doi: 10.1038/nrd917. [DOI] [PubMed] [Google Scholar]
- 45.Wong RW, Guillaud L. The role of epidermal growth factor and its receptors in mammalian CNS. Cytokine Growth Factor Rev. 2004;15:147–156. doi: 10.1016/j.cytogfr.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. J Comp Neurol. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 47.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Willins DL, Deutch AY, Roth BL. Serotonin 5-HT2A receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse. 1997;27:79–82. doi: 10.1002/(SICI)1098-2396(199709)27:1<79::AID-SYN8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 49.Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, Pelkey KA, Yang DJ, Xiong WC, Salter MW, Mei L. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- 50.Buckley PF, Mahadik S, Pillai A, Terry A., Jr. Neurotrophins and schizophrenia. Schizophr Res. 2007;94:1–11. doi: 10.1016/j.schres.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 51.Kalkman HO. Altered growth factor signaling pathways as the basis of aberrant stem cell maturation in schizophrenia. Pharmacol Ther. 2009;121:115–122. doi: 10.1016/j.pharmthera.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Roth BL, Willins DL, Kroeze WK. G protein-coupled receptor (GPCR) trafficking in the central nervous system: relevance for drugs of abuse. Drug Alcohol Depend. 1998;51:73–85. doi: 10.1016/s0376-8716(98)00067-2. [DOI] [PubMed] [Google Scholar]
- 53.Gavi S, Shumay E, Wang HY, Malbon CC. G-protein-coupled receptors and tyrosine kinases: crossroads in cell signaling and regulation. Trends Endocrinol Metab. 2006;17:48–54. doi: 10.1016/j.tem.2006.01.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Genes involved in IGF-1 R signal transduction are expressed at similar levels in RSK2+/+ and RSK2−/− MEFs. The microarray data quantifying gene expression in RSK2+/+ and RSK2−/− MEFs was produced previously by Sheffler et al. (13). Here we overlaid the mRNA expression levels of IR and IGF-1 R signal transduction genes in RSK2+/+ and RSK2−/− MEFs with gene-expression color criterion and fold-changes from the programs GenMAPP and MAPPFinder. Gray colored genes are equally expressed in RSK2+/+ and RSK2−/− MEFs. Green colored genes show greater than a 2-fold increase in expression in RSK2−/− MEFs compared to RSK2+/+ fibroblasts. Red colored genes show greater than a 2-fold decrease in expression in RSK2−/− MEFs compared to RSK2+/+ fibroblasts. As shown, the gene encoding the IR was not expressed in RSK2+/+ and RSK2−/− MEFs. However, the genes encoding the IGF-1 R and downstream effectors were expressed at comparable levels in RSK2+/+ and RSK2−/− MEFs. Raw microarray data and gene information can be found in the Supporting Information zip file.
Figure S2. IGF-1 activates the IGF-1 R in RSK2+/+ MEFs. Here we determined using cell lysates that 10 nM IGF-1 activated the IGF-1 R (Tyr(P)1158/1162/1163) in RSK2+/+ MEFs. Shown is an immunoblot representative of three independent experiments.