Abstract
The ability to respond to perturbations in endoplasmic reticulum (ER) function is a fundamentally important property of all cells, but ER stress can also lead to apoptosis. In settings of chronic ER stress, the associated apoptosis may contribute to pathophysiological processes involved in a number of prevalent diseases, including neurodegenerative diseases, diabetes, atherosclerosis and renal disease. The molecular mechanisms linking ER stress to apoptosis are the topic of this review, with emphases on relevance to pathophysiology and integration and complementation among the various apoptotic pathways induced by ER stress.
The ability of cells to respond to perturbations in ER function, or ‘ER stress’, is critical for cell survival, but chronic or unresolved ER stress can lead to apoptosis. Factors that perturb ER function and contribute to the development of ER stress include increases in protein synthesis or protein misfolding rates that exceed the capacity of protein chaperones (‘client load’), alterations in calcium stores in the ER lumen, oxidative stress and disturbances to the redox balance in the ER lumen1. In multicellular eukaryotes, ER stress is sensed by three upstream signalling proteins that, when activated, begin a cascade of corrective actions1. The activity of these three pathways collectively constitutes an ER-specific unfolded protein response (UPR).
The evolutionarily oldest branch of the UPR is triggered by the activation of a combined nuclease and kinase called IRE1 (inositol-requiring protein-1), which exists in mammals as two isoforms: IRE1α, which is expressed in all cells, and IRE1β, which is restricted to the gastrointestinal and respiratory tracts. All of the ER-stress transducers, including IRE1, become activated when there is an imbalance of unfolded proteins and chaperones. Activation is probably initiated by both ER-stress-driven changes in heterologous protein interactions, such as dissociation of the protein chaperone BiP (immunoglobulin-binding protein; Grp78), and by direct binding to unfolded proteins1. These lumenal events control the linked processes of transautophosphorylation, nucleotide binding and oligomerization2. Together they promote the cytosolic effector function of IRE1: highly sequence-specific endoribonucleolytic cleavage and subsequent splicing of an mRNA encoding a critical transcription factor called XBP-1. The spliced form of XBP-1 induces the expression of a large number of genes involved in almost all aspects of the UPR (except the suppression of translation initiation), whereas the unspliced form represses such gene expression1. Under certain conditions, the IRE1 nuclease can also degrade and thus block the translation of a wide variety of mRNA species, although the possible physiologic and pathologic functions of this action are still being investigated3.
A second branch of the UPR is initiated by activation of the kinase PERK (protein kinase RNA (PKR)-like ER kinase), which similarly to IRE1, responds to ER stress by autophosphorylation and homomultimerization1. PERK phosphorylates the α-subunit of the translation initiation factor eIF2 (eukaryotic translation initiation factor-2), which results in the attenuation of global translation initiation. However, translation of the gene encoding the transcription factor ATF4 (activating transcription factor-4) is favoured by limiting amounts of active eIF2, and thus the expression of ATF4 and its key downstream target, CHOP (C/EBP-homologous protein; also known as GADD153; gene name Ddit3), are increased when eIF2α is phosphorylated by PERK1. CHOP, usually through interactions with other transcriptional regulators, participates in ER-stress-corrective actions through induction or suppression of a number of genes. A particularly important transcriptional target of CHOP and ATF4 is GADD34 (growth arrest and DNA damage-inducible protein-34), a substrate-specific regulatory subunit of a holo-phosphatase complex that dephosphorylates phosphorylated eIF2α and thus restores global protein translation and suppresses ATF4 translation to basal levels1,4.
A third branch of the UPR involves the protease-mediated activation of a transcriptional factor called ATF6 (activating transcription factor-6; ref. 1). ATF6 has a major role in chaperone induction and can also transcriptionally induce CHOP. Interestingly, ATF6 is also capable of binding and sequestering the transcription factor CREB, which has the net effect of suppressing hepatic gluconeogenesis5.
Although all three branches are usually activated by any given ER stress event, the timing of activation can differ6. In particular, prolonged ER stress leads to the sequential activation then deactivation of the IRE1α, ATF6 and PERK pathways, respectively7. This timing sequence probably has implications for ER-stress-induced apoptosis. It should also be noted that in addition to the suppressive action of CHOP-induced GADD34 on phosphorylated eIF2α, one or more branches of the UPR can be downregulated by other mechanisms specific to certain settings8–12. These mechanisms may help cells adapt to physiologic but prolonged ER stress.
Overview of ER-stress-induced apoptosis
Prolonged activation of IRE1 and CHOP can trigger apoptosis in cells under certain physiologic and pathophysiologic conditions13. In normal physiology, UPR-induced apoptosis may be a means to eliminate the few cells in an ER-stressed environment that remain uncorrected despite the actions of the UPR. Damaged but non-apoptotic cells can elicit inflammation, whereas apoptosis is usually accompanied by efficient phagocytic clearance that both prevents post-apoptotic necrosis and induces an anti-inflammatory response14. Another possible role for physiologic ER-stress-induced apoptosis may be as a host defence mechanism against the intracellular organism Mycobacterium tuberculosis15.
In contrast to these scenarios of limited and selective apoptosis, chronic ER stress can induce widespread pathologic apoptosis16. Non-resolving ER-stress-induced apoptosis is becoming increasingly recognized as an important pathogenic factor in a number of widespread and devastating diseases, including neurodegenerative diseases, diabetes, atherosclerosis and renal disease16.
In the sections below, we will discuss molecular mechanisms of ER-stress-induced apoptosis, meaning cell death mediated directly by signalling pathways responsive to ER stress.
Molecular mechanisms of apoptosis triggered by IRE1α signalling
In organisms such as yeast and worms, the IRE1 branch of the UPR has an essential role in defending cells and tissues against the lethal consequences of ER stress17,18. Thus, when the IRE1 branch is silenced in these organisms, they become more sensitive to agents that perturb protein folding in the ER. In contrast, mammalian cells lacking the widely expressed IRE1α and its major downstream effector XBP-1 are not conspicuously hypersensitive to ER stressors that perturb protein folding in the ER. Rather, these cells seem to alter their development in a manner that places them at less risk for ER stress, that is by failing to acquire the ability to synthesize and fold large amounts of secreted proteins19–21.
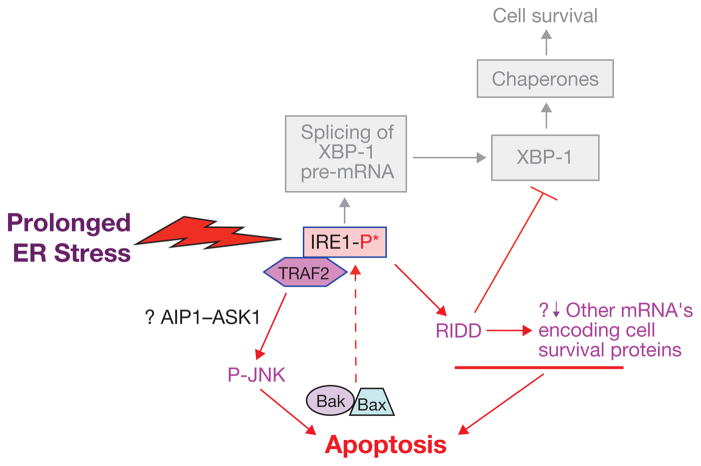
Thus, IRE1-deficient mammalian cells not only survive, but they may actually survive better than wild-type cells under prolonged ER stress. In vitro, the RNase activity of IRE1 is highly sequence specific22,23, and at physiologically low levels of signalling, the endoribonuclease of IRE1 is exquisitely sequence specific in vivo as well24. At higher levels of stress signalling, however, there is evidence in cultured cells that IRE1 may contribute to rather promiscuous degradation of membrane-associated mRNAs through a process referred to as regulated IRE1 dependent decay or RIDD3,25. Although RIDD may help to defend cells against ER stress by degrading ER-associated mRNAs and thus limiting new protein translation, it may also be a mechanism of apoptosis in the setting of severe ER stress. In experiments in which IRE1 activity was manipulated towards RIDD in an insulin-producing pancreatic beta cell line (INS-1), ER-stress-induced apoptosis was enhanced26 (Fig. 1). Moreover, similar findings were observed with induced overexpression of IRE1α, which is a way to activate IRE1 RNase activity through transphosphorylation in the absence of ER stress. However, when IRE1 was allosterically activated through a pseudokinase mechanism, generalized mRNA degradation and apoptosis were not observed, despite intact XBP1 splicing26. Whether these findings are applicable to pancreatic beta cell death in the setting of diabetes or in other cases of pathologic ER-stress-induced cell death remains to be explored in vivo.
Figure 1.
Prolonged activation of IRE1 may promote apoptosis. Studies with cultured cells have identified a pro-apoptotic IRE1–TRAF2–JNK pathway that can be activated by prolonged ER stress. Signal transduction between IRE1–TRAF2 and phosphorylation of JNK may be mediated in certain settings by the MAP kinase kinase kinase (MAPKKK) ASK1 and its activator, AIP1. JNK-induced apoptosis may involve the pro-apoptotic Bcl-2 family members, Bax and Bak, which in turn, can amplify the IRE1 signal. Prolonged IRE1-mediated activation of the RIDD pathway may promote apoptosis by degrading mRNAs encoding essential cell-survival proteins, including XPB1 itself.
Other possible links between IRE1 and ER-stress-induced apoptosis may entail interaction of IRE1 with proteins involved in apoptosis signalling. For example, co-immunoprecipitation experiments suggest that mammalian IRE1α binds Bak and Bax, proteins involved in the mitochondrial pathway of apoptosis (Fig. 1). This interaction seems to be important for IRE1α activation27.
Phosphorylated, activated mammalian IRE1 also interacts with the adaptor protein TRAF2 (tumour necrosis factor receptor (TNFR)-associated factor-2) and promotes a cascade of phosphorylation events that ultimately activates Jun amino-terminal kinase (JNK, ref. 28; Fig. 1). Given the links between sustained JNK activity and cell death29,30, JNK activity may link IRE1-mediated ER stress signalling to cell death in certain settings31. Genetic tools to address the physiological significance of this hypothesized link are not available, as there are no known mutations in IRE1 or TRAF2 that selectivity disrupt this signalling node. However, an intriguing study suggests that ER-localized Bim and Puma selectively activate the TRAF2–JNK arm of IRE1 signalling, biasing the response away from XBP-1 activation in favour of JNK32. The experimental system described relies on overexpression of modified versions of these BH3-only apoptosis effectors, with no contribution of luminal protein misfolding stress, and thus it is unclear how these events might tie in with cell death mediated by ER stress.
A key goal for future studies of the role of IRE1 in apoptosis is to define mechanisms and show relevance in vivo. This goal may be achievable through mutations in IRE1 that could drive a wedge between potential cell death pathways (that is, RIDD and apoptotic protein interaction) and cell-survival pathways (that is, XBP-1 splicing), but such mutations have not yet been reported. However, the use of chemical tools may prove helpful in achieving this goal. In vitro studies with the yeast IRE1 enzyme have revealed that certain flavenols can stabilize the IRE1 dimer, promoting XBP-1 splicing in the absence of IRE1 phosphorylation33. Although flavenols are too pleiotropic to be of use in vivo, it is intriguing to consider that more selective compounds acting on the mammalian enzyme may favour XBP-1 splicing, which enhances the ability of cells to cope with ER stress, over JNK activation, which may be linked to cell death. Given the powerful negative feedback that exists between XBP-1 and IRE1 (ref. 1), even partial biasing of the outputs of IRE1 could have significant effects on physiology.
Molecular mechanisms of CHOP-induced apoptosis
Studies using Chop-null mice have established the role of CHOP in ER-stress-induced apoptosis in a number of disease models, including renal dysfunction34, diabetes35–37, ethanol-induced hepatocyte injury38, Parkinson’s disease39, experimental colitis40, advanced atherosclerosis41,42 and cardiac-pressure overload43. However, the molecular mechanisms remain incompletely understood.
One of the more widely cited mechanisms of CHOP-induced apoptosis is suppression of the pro-survival protein Bcl-2 (Fig. 2), which was based initially on a study showing correlations among CHOP expression, oxidative stress, apoptosis and downregulation of Bcl-2 in a CHOP-transfected rat fibroblast cell line44. Most importantly, genetic restoration of Bcl-2 rescued the CHOP-transfected cells from both oxidative stress and apoptosis. The mechanism may involve the ability of CHOP to interact with one or more transcriptional repressors to decrease Bcl2 transcription44. In thapsigargin-treated MEFs, CHOP nuclear translocation, Bcl2 transcriptional suppression and apoptosis were shown to require CHOP interaction with an isoform of C/EBPβ called liver inhibitory protein (LIP)45.
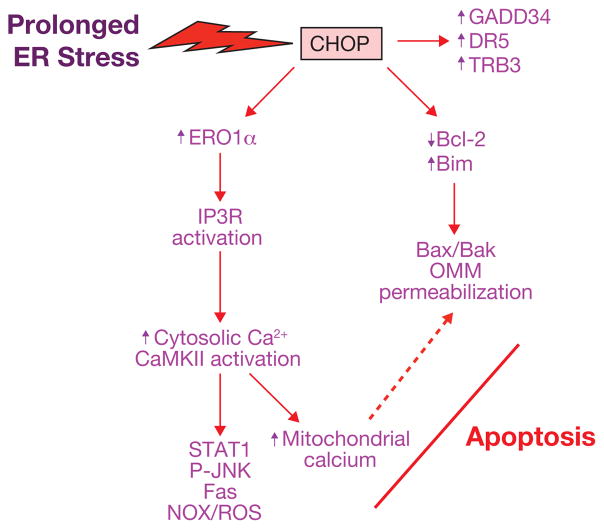
Figure 2.
Pathways through which prolonged activation of CHOP may promote apoptosis. Studies with both cultured cells and gene-targeted mouse models have identified a number of possible pro-apoptotic actions of prolonged CHOP expression, as depicted here and explained in detail in the text. Note that two of the major cell death pathways — the ERO1α–IP3R–Ca2+–CaMKII pathway and the Bcl-2 family member pathway — may lead to a common pro-apoptotic endpoint of outer mitochondrial membrane (OMM) permeabilization (dotted arrow; see text for details).
A correlation between CHOP-mediated apoptosis and downregulation of Bcl-2 in vivo was shown in a mouse model of cardiomyocyte apoptosis, where there was a small but statistically significant decrease in cardiomyocyte Bcl-2 in ER-stressed wild-type mice but not in Chop−/− mice43. However, a direct molecular link between CHOP and Bcl-2 in this setting remains to be explored, and in general there has been no documentation that Bcl-2 restoration in vivo rescues mice from CHOP-induced apoptosis and tissue dysfunction.
Bcl-2 mediates cell survival through sequestration of BH3-only proteins, such as Bad, Bim, Noxa and Puma, which are necessary for Bax–Bak-mediated mitochondrial permeabilization and apoptosis46. With regard to BH3-only proteins, a study using multiple ER stressors demonstrated the importance of Bim in ER-stress-induced apoptosis47 (Fig. 2). Moreover, Bim−/− mice were protected from tunicamycin-induced renal epithelial cell apoptosis. ER stress increased Bim levels through both decreased proteasomal degradation and CHOP–C/EBPα-mediated gene induction. In another study, palmitate-induced ER stress was shown to be associated with CHOP–AP-1-dependent upregulation of Puma, Bax activation and apoptosis48. The role of Bax in CHOP-induced apoptosis was suggested initially from studies using cultured macrophages42,49,50 and then later shown in the aforementioned model of ER-stress-induced cardiomyocyte apoptosis, where Bax levels increased with ER stress in a CHOP-dependent manner43 (Fig. 2).
Another mechanism implicated in CHOP-induced apoptosis is oxidative stress. Prolonged ER stress can both hyperoxidize the ER lumen, which may result in H2O2 leakage into the cytoplasm, and directly induce cytotoxic reactive oxygen species (ROS) in the cytoplasm. Oxidation of the ER lumen is induced by the CHOP transcriptional target ER oxidase 1α (ERO1α)51. In normal physiology, this promotes disulfide bond formation in newly translated proteins, but partial silencing of ero-1 in Caenorhabditis elegans protected the organism from tunicamycin-induced death51. This has led to the speculation that with prolonged ER stress, ERO1 may promote a hyperoxidizing environment that leads to cell death. In the setting of diabetes, CHOP deficiency suppresses pancreatic beta cell apoptosis, and this protection was associated with decreased ERO1α, suppression of oxidative-stress markers and induction of anti-oxidant genes36.
Recent work has suggested a specific molecular mechanism that might link ERO1α to CHOP-induced apoptosis (Fig. 2). Recent in vitro and in vivo data has shown that CHOP-induced apoptosis involves activation of pro-apoptotic cytoplasmic calcium signalling pathways52–54. In particular, UPR-CHOP-induced apoptosis can be blocked by buffering cytoplasmic calcium52. Cytoplasmic calcium triggers apoptosis by activating the calcium-sensing kinase CaMKII, which in turn triggers a number of downstream apoptosis pathways53,54. A causative role for CaMKII in ER-stress-induced apoptosis was observed in a number of different cell types exposed to various ER stressors and in tunicamycin-treated mice53,55. The role of ERO1α is suggested by the observation that CHOP-induced ERO1α activates the ER calcium-release channel IP3R1, which is crucial for the signalling events triggered by cytoplasmic calcium55. ERO1α-induced IP3R1 activation may involve disulfide bond formation in a lumenal loop of IP3R1 (refs 51, 56).
In addition to directly promoting hyperoxidizing conditions in the lumen of the ER, the CHOP–ERO1α pathway can induce pro-apoptotic oxidative stress in the cytoplasm. Indeed, one of the consequences of the CHOP–ERO1α–IP3R1–CaMKII pathway is induction of the NADPH (nicotinamide adenine dinucleotide phosphate) oxidase subunit Nox2 and generation of ROS, which is not only essential for apoptosis but which may amplify CaMKII activation as part of a positive feedback loop54,57,58. Interestingly, ROS induced by NADPH oxidase is also part of a positive feedback cycle that activates PKR and thus amplifies CHOP expression, and ER-stress-induced apoptosis is suppressed by Nox2 or PKR deficiency in cultured cells and in vivo54. ROS induced by NADPH oxidase can also be triggered by chronic ER stress secondary to protein misfolding, and oxidative stress in this setting may be exacerbated by high levels of mitochondrial electron transport and by consumption of glutathione48. For example, CHOP-induced death of hepatocytes in a mouse model of protein misfolding was associated with increased oxidative stress and was relieved by the anti-oxidant butylated hydroxyanisole (BHA)59.
The ability of cells to slow protein translation through eIF2α phosphorylation is a key mechanism to prevent oxidative stress and apoptosis in certain settings of physiologic prolonged ER stress60. In those settings, the CHOP transcriptional target GADD34 (Fig. 2), which promotes the dephosphorylation of phosphorylated eIF2α and thus restores protein translation, represents another pro-apoptotic mechanism of prolonged CHOP expression. In vivo support for this concept came from a study showing that tunicamycin-treated mice homozygous for a disabling Gadd34 mutation are protected against renal epithelial cell apoptosis51. In addition, elevated levels of GADD34 may mediate CHOP-induced apoptosis by other mechanisms61,62. However, the role of phosphorylated eIF2α in cell viability may be more complex, as illustrated by a study showing that translational suppression by phosphorylated eIF2α in ER-stressed cultured insulinoma cells blocks the expression of the cell survival protein Mcl-1 and promotes cell death63.
Other CHOP-induced molecules that have been implicated in apoptosis include death receptor-5 (DR5; TRAIL-R2) and Tribbles-related protein 3 (TRB3; Fig. 2). DR5 has been shown to be a mediator of ER-stress-induced death in a number of cultured cancer cell lines64, and TRB3 is necessary for the full apoptotic response in cultured 293 and HeLa cells exposed to tunicamycin65. Data evaluating the importance of DR5 and TRB3 in CHOP-induced apoptosis and tissue dysfunction in vivo are lacking. However, a recent study showed an increase in pancreatic beta cell apoptosis in mice overexpressing a hyper-stable form of TRB3 associated with decreased beta cell function in humans66.
It is intriguing to consider how pathologic CHOP-induced cell death is avoided when prolonged ER stress is an appropriate adaptive response to increased client load, for example in macrophages and other cell types exposed to LPS (lipopolysaccharide) and in B-cell maturation11,67. In both of these examples, a prolonged ER stress response through the XBP-1 and ATF6 chaperone branches is necessary, and yet CHOP-induced apoptosis is avoided11,68–70. Indeed, forced expression of CHOP in an in vivo sepsis model leads to inappropriate cell death and tissue dysfunction11. With LPS, in vitro and in vivo data support a mechanism in which Toll-like receptor signalling leads to ‘resistance’ to the translational effects of phosphorylated eIF2α, resulting in selective suppression of the ATF4–CHOP axis. Thus, CHOP-induced apoptosis is avoided while the protective arms of the UPR remain engaged11. This mechanism appears to be engaged when fitness requires sustained protein synthesis, despite the physiological ER stress imposed, and contrasts markedly with the circumstances reviewed above51,60. In other settings, the eIF2α kinases may be checked by a UPR-induced inhibitor P58IPK (refs 8, 9). However, the relevance of P58IPK to eIF2α activity is called into question by recent evidence that it resides in the ER lumen71,72. Finally, CHOP- and IRE1α-induced apoptosis may be avoided by a phenomenon called pre-conditioning, in which all three branches of the UPR are partially suppressed in cells subjected to low-level ER stress before being exposed to a robust UPR activator10. In this setting, the downregulation of pro-apoptotic proteins is greater than that of pro-survival proteins, such as chaperones, owing to differential mRNA stability10. Pre-conditioning is likely to be highly context-dependent and, in certain pathological settings, cells subjected to even low-level ER stress may undergo CHOP-induced apoptosis if exposed to other factors that suppress compensatory cell-survival pathways in ER-stressed cells73.
Integration among UPR branch apoptosis pathways
Most of the studies investigating ER-stress-induced apoptosis have tended to neglect molecular and/or functional links among the different UPR branch apoptosis pathways. It is possible that two or more UPR branches may induce different steps of a single linear apoptosis pathway (epistasis). In another setting, different UPR branches may separately induce the same pro-apoptotic effector or response, which would amplify that effector or response. Finally, different, complementary UPR branches may promote entirely different apoptosis pathways in a manner that could increase the intensity or chronicity of the cell death response7 (Fig. 3a). Indeed, in most studies where one UPR branch or effector is experimentally silenced, ER-stress-induced apoptosis is not suppressed completely.
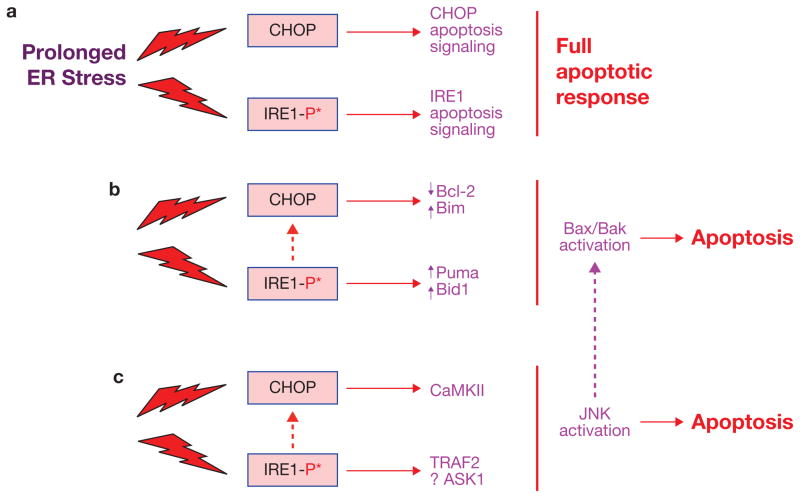
Figure 3.
Examples of integration among the UPR apoptosis pathways. (a) In the simplest scenario, differential apoptotic signalling downstream of CHOP and IRE1 may lead to only partial cell death responses, and so the full apoptotic response may require activation of both pathways. (b) Both pathways can promote changes in Bcl-2 family protein expression or activity that favour cell death. (c) Both pathways can promote JNK activation, which when prolonged, can trigger cell death. Note that one of the mechanisms by which activated JNK leads to apoptosis is through Bax–Bak activation, possibly leading to further integration (purple dotted arrow). See text for details and for other possible areas of integration.
The fact that CHOP is a transcriptional target of not only ATF4 but also XBP-1 and ATF6 (ref. 1) provides an obvious possible link among all three branches. A caveat here is that the IRE1α–XBP-1 and ATF6 branches may be at relatively low levels of activation compared with the PERK–CHOP branch during the later, apoptotic phase of prolonged ER stress7. Indeed, as noted above, prolonged, pathologic activation of IRE1 may favour RIDD over XBP-1 splicing. Thus, experiments in which XBP-1 and/or ATF6 are silenced in various settings of CHOP-mediated cell death are needed to fully explore this issue.
Alterations in the expression and/or localization of Bcl-2 family proteins represent another possible area of integration (Fig. 3b). The IRE1α branch can affect BH3-only proteins like Puma and Bid74,75, and CHOP can induce another member of this family, Bim47. Thus, the two branches might have additive effects on activation of Bax and Bak, the functional targets of the BH3-only proteins. Moreover, other processes associated with CHOP-induced apoptosis — notably downregulation of Bcl-2 and Mcl-1, which are de-activators of BH3-only proteins, and Bax translocation to the mitochondria — represent additional opportunities for synergy in this pathway, particularly because they are likely to involve different mechanisms from Bim induction47. Pro-apoptotic ER stress is also associated with sustained JNK activation (see above), and a recent in vitro study linked apoptosis under these conditions with activation of Bad31. Integration at the functional level is also likely, because the major pro-apoptotic action of Bax–Bak is through mitochondrial permeabilization46, which is also a key pro-apoptotic end effect of the CHOP–ERO1α–IP3R–calcium–CaMKII pathway42,49,53. Whether Bax and Bak are actually components of the calcium pathway or part of a separate, complementary ER stress–Bax–Bak–mitochondrial pathway is an important question for the future.
In certain cell types, species, and conditions, ER-stress-mediated apoptosis involves caspase-12 activation76. Studies to date suggest that the mechanism involves the interaction of pro-caspase-12 with the IRE1–TRAF2 complex77, but the significance of this interaction remains to be determined. Caspase-12 can also be activated by the calcium-activated protease calpain in settings of ER-stress-induced apoptosis78; therefore it is possible that a CHOP–ERO1α–IP3R–calcium–calpain pathway might also contribute to caspase-12 activation. To date, there is little information on how the silencing of various branches of the UPR affects caspase-12 activation and apoptosis in those settings in which this caspase has a role.
Sustained activation of JNK has been implicated in a number of UPR apoptosis pathways13. IRE1α has been linked to JNK activation in apoptosis settings through TRAF2 and ASK1 (ref. 79). In addition, one of the major downstream effectors of the CHOP–CaMKII pathway is JNK activation, which is essential for ER-stress-induced apoptosis through at least two mechanisms: induction of Fas and induction of Nox2 and subsequent oxidative stress53,54. Therefore, in settings in which both pro-apoptotic IRE1α activation and CHOP expression are prolonged, additive or synergistic activation of JNK may play a crucial role in apoptosis (Fig. 3c). More specifically, this crosstalk may favour prolonged JNK activation, favouring its pro-apoptotic effector function30,31.
The functional importance of crosstalk among various UPR branch-apoptosis pathways needs to be assessed in one and the same in vitro or in vivo model. Moreover, in those cases in which different branches contribute different components in the same pathway, lack of temporal coordination may preclude functional significance7. Careful studies using disease-relevant in vitro and in vivo models in which several branches and sub-branches of the UPR are manipulated simultaneously will be needed to define those situations in which functionally significant integration occurs.
Therapeutic implications
Evidence for a role of ER-stress-induced apoptosis in a variety of prevalent chronic diseases make this process a tempting target for therapeutic intervention. A conceptually straightforward strategy would be to globally relieve ER stress itself through the use of so-called proteostasis regulators, including ‘chemical chaperones’80,81, such as 4-phenyl butyric acid (PBA) and tauroursodeoxycholic acid (TUDCA; Fig. 4). Both have been used in humans for a variety of disorders and have beneficial effects in various mouse models of pathologic ER stress82–84, but definitive proof that these beneficial effects are mechanistically linked to suppression of ER stress and ER-stress-induced apoptosis is lacking. For example, PBA possesses histone deacetylase inhibitor activity.85
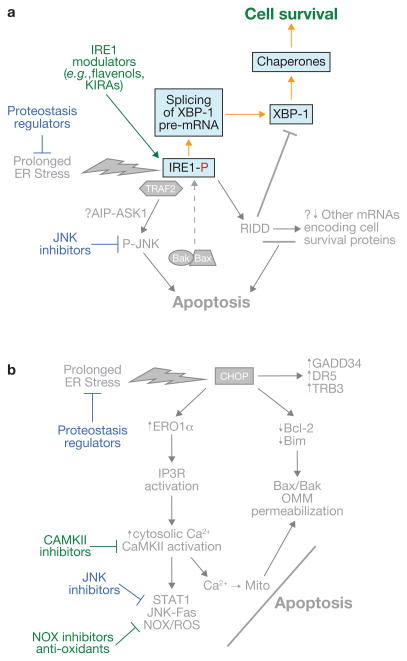
Figure 4.
Examples of therapeutic strategies to prevent cell death in the setting of pathologic, prolonged ER stress. (a, b) Strategies directed towards IRE1- and CHOP-mediated apoptotic pathways, respectively. Note that several of the approaches are applicable to both (blue font). The common approaches include mitigation of ER stress itself by so-called proteostasis regulators and inhibition of JNK. See text for details.
To circumvent these potential problems, researchers have turned their attentions to more focused manipulations of the pro-apoptotic UPR. As covered in this review, cell culture studies have suggested strategies in which modulatory ligands would be able to promote pro-survival over pro-apoptotic functions of IRE1 (refs 26, 33; Fig. 4a). Several drug strategies targeting the PERK–CHOP pathways of apoptosis have also been tried in mouse models of chronic ER stress (Fig. 4b). Salubrinal is a compound that blocks the dephosphorylation of eIF2α86 and at certain doses can promote survival in certain cell culture and mouse disease models associated with prolonged ER stress87–89, although the mechanism is unknown. With regard to the CHOP-oxidative stress-calcium pathway of apoptosis, compounds that inhibit ERO1 have also been shown to protect MEFs from tunicamycin-induced apoptosis in culture90, and genetic or pharmacologic CaMKII inhibition blocks ER-stress-induced apoptosis in a number of disease models53,91,92.
One of the major concepts developed in this review, namely integration and complementation among various UPR branch-apoptosis pathways, suggests that targeting only one branch pathway may not be as efficient as targeting two branches. Combining two or more drugs might accomplish this objective, but a number of potential targets — including the pro-apoptotic Bcl-2 family members and JNK — are common to both the pro-apoptotic IRE1α and CHOP pathways and thus may require only one drug. In particular, JNK inhibitors represent an area of high interest; they show promise in a number of pre-clinical models, including those associated with prolonged ER stress, and are currently being investigated in phase I clinical trials for inflammatory disorders93 (http://www.celgene.com/pdfs/product_pipeline.pdf). Continued progress in pre-clinical models and a favourable safety profile in humans may pave the way for early phase trials in humans with diseases driven by pathologic ER-stress-induced apoptosis.
Conclusion and perspectives
There is increasing evidence that apoptosis triggered by ER stress is involved in the pathogenesis or exacerbation of a number of widespread disease processes. Research in this area has provided rich mechanistic insight into how the IRE1 and PERK–CHOP branches can lead to apoptosis and has suggested promising and innovative areas of potential translational application. However, as reviewed herein, many of the studies use one or just a few ER stressors and cell types, and thus rapid progression to more pathophysiologically relevant in vitro and animal models is essential. This gap is particularly apparent for studies on IRE1-mediated pathways of apoptosis — a challenging area given the dichotomy between the effects of XBP-1 versus JNK and RIDD on cell viability. In addition, the possibility of molecular crosstalk between the various pathways of the UPR or consideration of how their separate but complementary actions may have critical roles in apoptosis has not been adequately addressed by most of the published studies in this area. Progress in these areas is essential to gain a more global understanding of this topic and to optimize the design of new therapeutic strategies. Given the prevalence and devastating nature of the diseases in which ER-stress-induced apoptosis is involved, and the fact that many of these diseases are favoured by the increasingly prevalent trends of increased longevity, decreased physical activity and over-nutrition, the impetus for achieving these goals is clear.
Acknowledgments
I.T. and D.R. gratefully acknowledge current and past members of their laboratories who contributed to the studies described herein. This work was supported by National Institutes of Health Grants HL087123, HL075662, and HL054591 to I.T. and by a Wellcome Trust Principal Research Fellowship to D.R.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Contributor Information
Ira Tabas, Email: iat1@columbia.edu, Departments of Medicine, Anatomy & Cell Biology, and Physiology & Cellular Biophysics, Columbia University, New York, NY 10032, USA.
David Ron, Institute of Metabolic Sciences, University of Cambridge, Cambridge, CB2 0QQ, UK.
References
- 1.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 2.Ron D, Hubbard SR. How IRE1 reacts to ER stress. Cell. 2008;132:24–26. doi: 10.1016/j.cell.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 4.Ma Y, Hendershot LM. Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. J Biol Chem. 2003;278:34864–34873. doi: 10.1074/jbc.M301107200. [DOI] [PubMed] [Google Scholar]
- 5.Seo HY, et al. Endoplasmic reticulum stress-induced activation of activating transcription factor 6 decreases cAMP-stimulated hepatic gluconeogenesis via inhibition of CREB. Endocrinology. 2010;151:561–568. doi: 10.1210/en.2009-0641. [DOI] [PubMed] [Google Scholar]
- 6.DuRose JB, Tam AB, Niwa M. Intrinsic capacities of molecular sensors of the unfolded protein response to sense alternate forms of endoplasmic reticulum stress. Mol Biol Cell. 2006;17:3095–3107. doi: 10.1091/mbc.E06-01-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin JH, et al. IRE1 signaling affects cell fate during the unfolded protein response. Science. 2007;318:944–949. doi: 10.1126/science.1146361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan W, et al. Control of PERK eIF2α kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc Natl Acad Sci USA. 2002;99:15920–15925. doi: 10.1073/pnas.252341799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Huizen R, Martindale JL, Gorospe M, Holbrook NJ. P58IPK, a novel endoplasmic reticulum stress-inducible protein and potential negative regulator of eIF2α signaling. J Biol Chem. 2003;278:15558–15564. doi: 10.1074/jbc.M212074200. [DOI] [PubMed] [Google Scholar]
- 10.Rutkowski DT, et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woo CW, et al. Adaptive suppression of the ATF4–CHOP branch of the unfolded protein response by toll-like receptor signaling. Nat Cell Biol. 2009;11:1473–1480. doi: 10.1038/ncb1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshida H, Oku M, Suzuki M, Mori K. pXBP1(U) encoded in XBP1 pre-mRNA negatively regulates unfolded protein response activator pXBP1(S) in mammalian ER stress response. J Cell Biol. 2006;172:565–575. doi: 10.1083/jcb.200508145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fadok VA, Bratton DL, Henson PM. Phagocyte receptors for apoptotic cells: recognition, uptake and consequences. J Clin Invest. 2001;108:957–962. doi: 10.1172/JCI14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seimon TA, et al. Induction of ER stress in macrophages of tuberculosis granulomas. PLoS ONE. 2010;5:e12772. doi: 10.1371/journal.pone.0012772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen X, et al. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell. 2001;107:893–903. doi: 10.1016/s0092-8674(01)00612-2. [DOI] [PubMed] [Google Scholar]
- 18.Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- 19.Reimold AM, et al. Plasma cell differentiation requires the transcription factor XBP-1. Nature. 2001;412:300–307. doi: 10.1038/35085509. [DOI] [PubMed] [Google Scholar]
- 20.Zhang K, et al. The unfolded protein response sensor IRE1α is required at 2 distinct steps in B cell lymphopoiesis. J Clin Invest. 2005;115:268–281. doi: 10.1172/JCI21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee AH, Chu GC, Iwakoshi NN, Glimcher LH. XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 2005;24:4368–4380. doi: 10.1038/sj.emboj.7600903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KP, et al. Structure of the dual enzyme Ire1 reveals the basis for catalysis and regulation in nonconventional RNA splicing. Cell. 2008;132:89–100. doi: 10.1016/j.cell.2007.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sidrauski C, Walter P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell. 1997;90:1031–1039. doi: 10.1016/s0092-8674(00)80369-4. [DOI] [PubMed] [Google Scholar]
- 24.Nekrutenko A, He J. Functionality of unspliced XBP1 is required to explain evolution of overlapping reading frames. Trends Genet. 2006;22:645–648. doi: 10.1016/j.tig.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Hollien J, et al. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han D, et al. IRE1α kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138:562–575. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hetz C, et al. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1α. Science. 2006;312:572–576. doi: 10.1126/science.1123480. [DOI] [PubMed] [Google Scholar]
- 28.Urano F, et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 29.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 30.Ventura JJ, et al. Chemical genetic analysis of the time course of signal transduction by JNK. Mol Cell. 2006;21:701–710. doi: 10.1016/j.molcel.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 31.Li B, et al. Differences in endoplasmic reticulum stress signalling kinetics determine cell survival outcome through activation of MKP-1. Cell Signal. 2010;23:35–45. doi: 10.1016/j.cellsig.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 32.Klee M, Pallauf K, Alcala S, Fleischer A, Pimentel-Muinos FX. Mitochondrial apoptosis induced by BH3-only molecules in the exclusive presence of endoplasmic reticular Bak. EMBO J. 2009;28:1757–1768. doi: 10.1038/emboj.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiseman RL, et al. Flavonol activation defines an unanticipated ligand-binding site in the kinase-RNase domain of IRE1. Mol Cell. 2010;38:291–304. doi: 10.1016/j.molcel.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zinszner H, et al. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oyadomari S, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525–532. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oyadomari S, et al. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci USA. 2001;98:10845–10850. doi: 10.1073/pnas.191207498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ji C, Mehrian-Shai R, Chan C, Hsu YH, Kaplowitz N. Role of CHOP in hepatic apoptosis in the murine model of intragastric ethanol feeding. Alcohol Clin Exp Res. 2005;29:1496–1503. doi: 10.1097/01.alc.0000174691.03751.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silva RM, et al. CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J Neurochem. 2005;95:974–986. doi: 10.1111/j.1471-4159.2005.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Namba T, et al. Positive role of CCAAT/enhancer-binding protein homologous protein, a transcription factor involved in the endoplasmic reticulum stress response in the development of colitis. Am J Pathol. 2009;174:1786–1798. doi: 10.2353/ajpath.2009.080864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thorp E, et al. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 2009;9:474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsukano H, et al. The endoplasmic reticulum stress-C/EBP homologous protein pathway-mediated apoptosis in macrophages contributes to the instability of atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2010;30:1925–1932. doi: 10.1161/ATVBAHA.110.206094. [DOI] [PubMed] [Google Scholar]
- 43.Fu HY, et al. Ablation of C/EBP homologous protein attenuates endoplasmic reticulum-mediated apoptosis and cardiac dysfunction induced by pressure overload. Circulation. 2010;122:361–369. doi: 10.1161/CIRCULATIONAHA.109.917914. [DOI] [PubMed] [Google Scholar]
- 44.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiribau CB, Gaccioli F, Huang CC, Yuan CL, Hatzoglou M. Molecular symbiosis of CHOP and C/EBPβ isoform LIP contributes to endoplasmic reticulum stress-induced apoptosis. Mol Cell Biol. 2010;30:3722–3731. doi: 10.1128/MCB.01507-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng EH, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 47.Puthalakath H, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 48.Santos CX, Tanaka LY, Wosniak J, Laurindo FR. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport and NADPH oxidase. Antioxid Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 49.Yao PM, Tabas I. Free cholesterol loading of macrophages is associated with widespread mitochondrial dysfunction and activation of the mitochondrial apoptosis pathway. J Biol Chem. 2001;276:42468–42476. doi: 10.1074/jbc.M101419200. [DOI] [PubMed] [Google Scholar]
- 50.Gotoh T, Terada K, Oyadomari S, Mori M. hsp70–DnaJ chaperone pair prevents nitric oxide- and CHOP-induced apoptosis by inhibiting translocation of Bax to mitochondria. Cell Death Differ. 2004;11:390–402. doi: 10.1038/sj.cdd.4401369. [DOI] [PubMed] [Google Scholar]
- 51.Marciniak SJ, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seimon TA, Obstfeld A, Moore KJ, Golenbock DT, Tabas I. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proc Natl Acad Sci USA. 2006;103:19794–19799. doi: 10.1073/pnas.0609671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Timmins JM, et al. Calcium/calmodulin-dependent protein kinase II links endoplasmic reticulum stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009;119:2925–2941. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li G, Scull C, Ozcan L, Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J Cell Biol. 2010;191:1113–1125. doi: 10.1083/jcb.201006121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li G, et al. Role of ERO1α-mediated stimulation of inositol 1, 4, 5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol. 2009;186:783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Higo T, et al. Subtype-specific and ER lumenal environment-dependent regulation of inositol 1, 4, 5-trisphosphate receptor type 1 by ERp44. Cell. 2005;120:85–98. doi: 10.1016/j.cell.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 57.Palomeque J, et al. Angiotensin II-induced oxidative stress resets the Ca2+ dependence of Ca2+-calmodulin protein kinase II and promotes a death pathway conserved across different species. Circ Res. 2009;105:1204–1212. doi: 10.1161/CIRCRESAHA.109.204172. [DOI] [PubMed] [Google Scholar]
- 58.Seimon TA, et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12:467–482. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malhotra JD, et al. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci USA. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Back SH, et al. Translation attenuation through eIF2α phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 2009;10:13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yagi A, et al. GADD34 induces p53 phosphorylation and p21/WAF1 transcription. J Cell Biochem. 2003;90:1242–1249. doi: 10.1002/jcb.10711. [DOI] [PubMed] [Google Scholar]
- 62.Shi W, et al. GADD34–PP1c recruited by Smad7 dephosphorylates TGFβ type I receptor. J Cell Biol. 2004;164:291–300. doi: 10.1083/jcb.200307151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Allagnat F, et al. Mcl-1 downregulation by pro-inflammatory cytokines and palmitate is an early event contributing to beta-cell apoptosis. Cell Death Differ. 2010;18:328–337. doi: 10.1038/cdd.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem. 2004;279:45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 65.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4–CHOP pathway and is involved in cell death. EMBO J. 2005;24:1243–1255. doi: 10.1038/sj.emboj.7600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liew CW, et al. The pseudokinase tribbles homolog 3 interacts with ATF4 to negatively regulate insulin exocytosis in human and mouse beta cells. J Clin Invest. 2010;120:2876–2888. doi: 10.1172/JCI36849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iwakoshi NN, Lee AH, Glimcher LH. The X-box binding protein-1 transcription factor is required for plasma cell differentiation and the unfolded protein response. Immunol Rev. 2003;194:29–38. doi: 10.1034/j.1600-065x.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 68.Skalet AH, et al. Rapid B Cell Receptor-induced unfolded protein response in nonsecretory B cells correlates with pro- versus antiapoptotic cell fate. J Biol Chem. 2005;280:39762–39771. doi: 10.1074/jbc.M502640200. [DOI] [PubMed] [Google Scholar]
- 69.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakayama Y, et al. Molecular mechanisms of the LPS-induced non-apoptotic ER stress-CHOP pathway. J Biochem. 2010;147:471–483. doi: 10.1093/jb/mvp189. [DOI] [PubMed] [Google Scholar]
- 71.Rutkowski DT, et al. The role of p58IPK in protecting the stressed endoplasmic reticulum. Mol Biol Cell. 2007;18:3681–3691. doi: 10.1091/mbc.E07-03-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petrova K, Oyadomari S, Hendershot LM, Ron D. Regulated association of misfolded endoplasmic reticulum lumenal proteins with P58/DNAJc3. EMBO J. 2008;27:2862–2872. doi: 10.1038/emboj.2008.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seimon T, Tabas I. Mechanisms and consequences of macrophage apoptosis in atherosclerosis. J Lipid Res. 2009;50(Suppl):S382–S387. doi: 10.1194/jlr.R800032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Younce CW, Kolattukudy PE. MCP-1 causes cardiomyoblast death via autophagy resulting from ER stress caused by oxidative stress generated by inducing a novel Zn-finger protein, MCPIP. Biochem J. 2009;27:43–53. doi: 10.1042/BJ20090976. [DOI] [PubMed] [Google Scholar]
- 75.Upton JP, et al. Caspase-2 cleavage of BID is a critical apoptotic signal downstream of endoplasmic reticulum stress. Mol Cell Biol. 2008;28:3943–3951. doi: 10.1128/MCB.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakagawa T, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 77.Yoneda T, et al. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem. 2001;276:13935–13940. doi: 10.1074/jbc.M010677200. [DOI] [PubMed] [Google Scholar]
- 78.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sekine Y, Takeda K, Ichijo H. The ASK1–MAP kinase signaling in ER stress and neurodegenerative diseases. Curr Mol Med. 2006;6:87–97. doi: 10.2174/156652406775574541. [DOI] [PubMed] [Google Scholar]
- 80.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 81.Perlmutter DH. Chemical chaperones: a pharmacological strategy for disorders of protein folding and trafficking. Pediatr Res. 2002;52:832–836. doi: 10.1203/00006450-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 82.Ozcan U, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Erbay E, et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat Med. 2009;15:1383–1391. doi: 10.1038/nm.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ozcan L, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 85.Kang HL, Benzer S, Min KT. Life extension in Drosophila by feeding a drug. Proc Natl Acad Sci USA. 2002;99:838–843. doi: 10.1073/pnas.022631999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boyce M, et al. A selective inhibitor of eIF2α dephosphorylation protects cells from ER stress. Science. 2005;307:935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 87.Zhu Y, et al. Eif-2a protects brainstem motoneurons in a murine model of sleep apnea. J Neurosci. 2008;28:2168–2178. doi: 10.1523/JNEUROSCI.5232-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin W, et al. Enhanced integrated stress response promotes myelinating oligodendrocyte survival in response to interferon-γ. Am J Pathol. 2008;173:1508–1517. doi: 10.2353/ajpath.2008.080449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saxena S, Cabuy E, Caroni P. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat Neurosci. 2009;12:627–636. doi: 10.1038/nn.2297. [DOI] [PubMed] [Google Scholar]
- 90.Blais JD, et al. A small molecule inhibitor of endoplasmic reticulum oxidation 1 (ERO1) with selectively reversible thiol reactivity. J Biol Chem. 2010;285:20993–21003. doi: 10.1074/jbc.M110.126599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang R, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 92.Khoo MS, et al. Death, cardiac dysfunction, and arrhythmias are increased by calmodulin kinase II in calcineurin cardiomyopathy. Circulation. 2006;114:1352–1359. doi: 10.1161/CIRCULATIONAHA.106.644583. [DOI] [PubMed] [Google Scholar]
- 93.Bogoyevitch MA, Ngoei KR, Zhao TT, Yeap YY, Ng D. C c-Jun N-terminal kinase (JNK) signaling: recent advances and challenges. Biochim Biophys Acta. 2010;1804:463–475. doi: 10.1016/j.bbapap.2009.11.002. [DOI] [PubMed] [Google Scholar]