Abstract
Ni-phosphine complexes were used as catalysts for the cycloaddition of various ketenes and diynes. In general, 2,4-cyclohexadieonones were formed instead of products arising from decarbonylation of the ketenes.
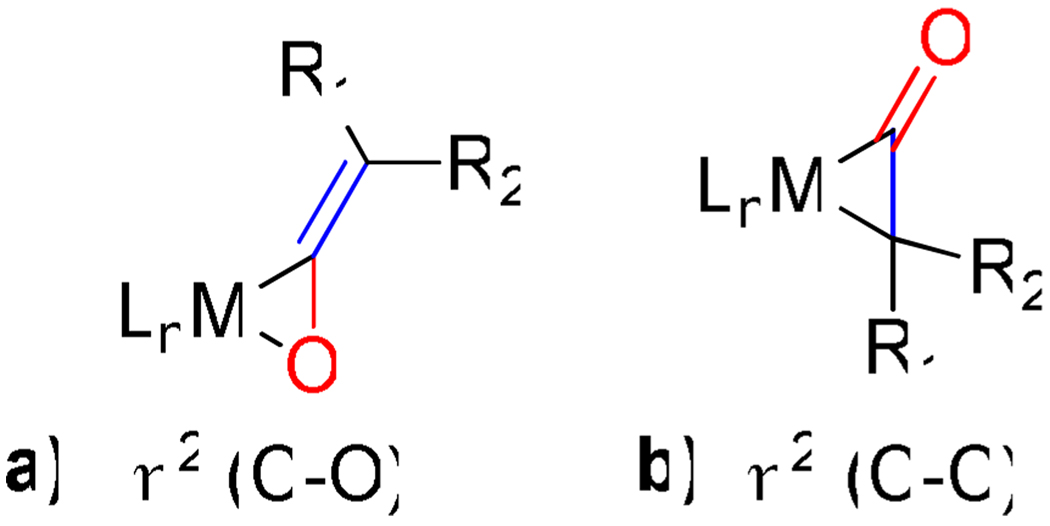
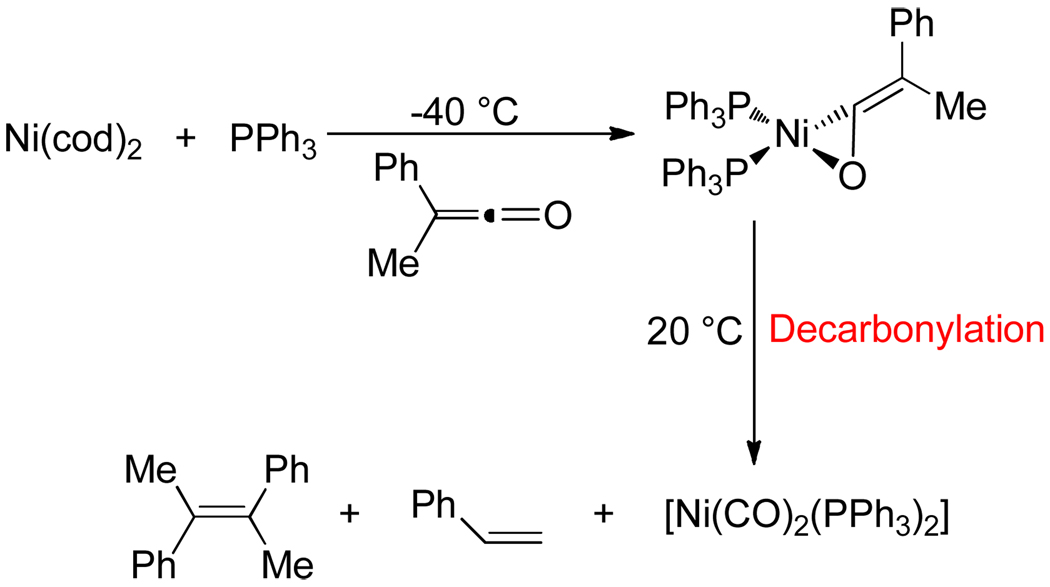
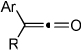
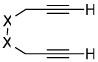
Almost every possible unsaturated starting material (alkynes, alkenes, dienes, CO2, nitriles, isocyanates, carbonyls etc.) has been employed as a substrate in transition metal catalyzed cycloadditions.1,2 Despite this rich history of cycloaddition chemistry, ketene substrates are notoriously absent.3 An insufficient reactivity between potential transition metal catalysts and ketenes is not the problem. Ketenes easily form η2-complexes with various metals (Ni, Pd, Pt, Co, Rh, Ir etc.).4,5 Furthermore, two modes of coordination, C-O or C-C binding, are available to ketenes (Figure 1). The inability of these η2-complexes to undergo further reactions with other unsaturated coupling partners lies in their propensity to decarbonylate and form stable, unreactive M-CO complexes (Scheme 1).4,5,6 In addition, ketenes often undergo homo-dimerization under thermal conditions.7 Given these pitfalls, we were surprised and delighted to discover that Ni-phosphine catalysts mediate the cycloaddition of ketenes and diynes to afford cyclohexadienones in good yields.8,9 Herein, we report these results.
Figure 1.
Modes of ketene coordination.
Scheme 1.
We initially discovered that the combination of 10 mol% Ni(COD)2 and 10 mol% DPPF successfully catalyzed the cycloaddition of diyne 1 and phenyl ethyl ketene a (Equation 1). The cycloaddition afforded a carbocyclic product (1a) that resulted from the coupling of the C=C bond of ketene a rather than a pyran (1a’), which would have resulted from the coupling of the C=O bond.2j,k Other ligands and conditions were evaluated in an effort to optimize reaction conditions (Table 1). In most cases, by-products arising from dimerization of diyne and ketene were observed (entries 1–8). However, we found that high yields were obtained when either DPPF or DPPB was employed as the ligand. Ultimately, the following optimized conditions were employed: 5 mol% catalyst loading (Ni(COD)2 and DPPB in 1:1 ratio) at a 0.1 M reaction concentration in toluene at 60 °C.10
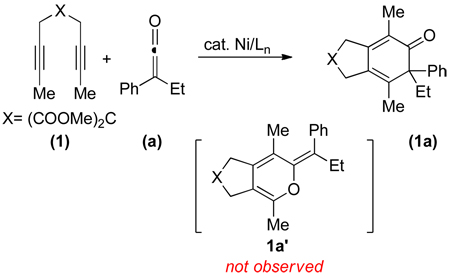 |
(1) |
Table 1.
Ni-Catalyzed Cycloaddition of Diynes and Ketenesa
| 1 | 1a | |||
|---|---|---|---|---|
| Entry | Ligand (Ln) | Ni:Ln | % Conv.b | % Yieldb |
| 1 | IPrc | 1:2 | 100 | 12 |
| 2 | SIPrc | 1:2 | 63 | 3 |
| 3 | PPh3 | 1:2 | 100 | 39 |
| 4 | PCy3 | 1:2 | 100 | 20 |
| 5 | MePPh2 | 1:2 | 100 | 54 |
| 6 | CyPPh2 | 1:2 | 100 | 31 |
| 7 | DPPE | 1:1 | 32 | 2 |
| 8 | DCPE | 1:1 | 22 | - |
| 9 | DPPF | 1:1 | 100 | >99 (86)d |
| 10 | DPPB | 1:1 | 100 | 86 (86)d |
Reaction conditions: 5 mol% Ni catalyst, diyne (1 equiv, 0.05), ketene (1.2 equiv), benzene, 60 °C, 12 h.
GC yield analyzed using decane as an internal standard.
The catalyst solutions were equilibrated f or at least 6 h before use.
Isolated yields.
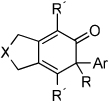
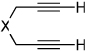
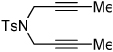
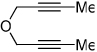
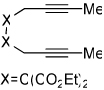
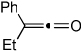
Importantly, we found that ketenes other than a could be used as substrates in the cycloaddition reaction and that a variety of cyclohexadienones could be prepared with these optimized reaction conditions (Table 2). For example, diyne 1 not only reacted with ketene a but also with a diaryl ketene b as well as a ketene with increased steric hinderance c (entries 1–3). Diynes that are prone to cyclotrimerization side reactions,11 such as the phenyl substituted diyne 2 and terminal diynes 3 and 4, were also successfully converted to their respective cyclohexadienone products in moderate yields (entries 4–6). In addition, cycloaddition products could be prepared from sulfonamide diynes and diyne-ethers (entries 7–9).
Table 2.
Ni-Catalyzed Cycloaddition of Diynes and Ketenesa
| Entry | Diyne | Ketene | Product | Yieldbc |
|---|---|---|---|---|
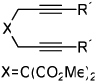 |
 |
 |
||
| 1 | 1 (R′ = Me) | a Ar= Ph R=Et | 1a | 82% |
| 2 | 1 | b Ar= Ph R=Ph | 1b | 46% |
| 3 | 1 | c Ar= Ph R=i-Pr | 1C | 50% |
| 4 | 2 (R′ = Ph) | a Ar= Ph R=Et | 2a | 65% |
 |
||||
| 5 | 3 (X=C(CO2Et)2) | a Ar= Ph R=Et | 3a | 54% |
| 6 | 4 (X=CH2) | a Ar= Ph R=Et | 4a | 35% |
 |
||||
| 7 | 5 | a Ar= Ph R=Et | 5a | 50% |
| 8 | 5 | d Ar= Ph R=n-Pr | 5d | 55% |
 |
||||
| 9 | 6 | a Ar= Ph R=Et | 6a | 33% |
 |
 |
 |
||
| 10 | 7 | a Ar= Ph R=Et | 7a | 91% |
| 11 | 7 | e Ar= p-OMe-Ph R=Et | 7e | 81% |
| 12 | 7 | f Ar= p-Me-Ph R=Et | 7f | 80% |
| 13 | 7 | g Ar= p-F-Ph R=Et | 7g | >99%c |
| 14 | 7 | h Ar= Ph R=Me | 7h | 65% |
| 15 | 7 | d Ar= Ph R=n-Pr | 7d | 76% |
 |
 |
|||
| 16 | 7 | i | 7i-i' | 82% |
 |
 |
|||
| 17 | 7 | j | 7j | 76% |
 |
 |
 |
||
| 18 | 8 (X=C(CO2Et)2) | a | 8a | 78% |
| 19 | 9 (X= CH2) | a | 9a | 33% |
Reaction conditions: 5 mol% Ni(COD)2, 5 mol% DPPB, diyne (1 equiv, 0.1 M), ketene (1.2 equiv) in toluene at 60 °C, 5 h.
Isolated yields.
Average of at least two runs.
Crude ketene was used.
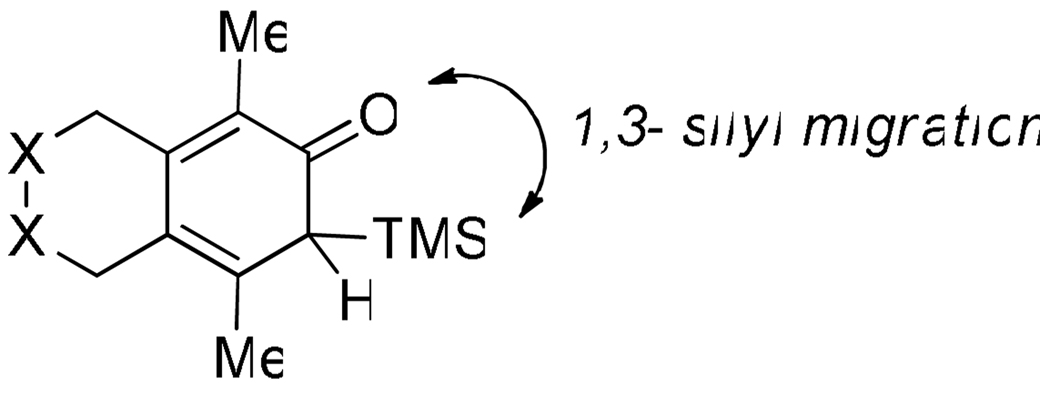
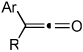
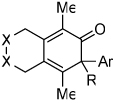
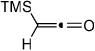
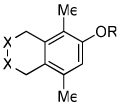
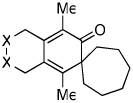
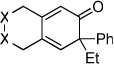
Diynes separated by a four atom linker instead of a three atom linker afforded cyclohexadienones in higher yields (entries 10–18). For example, the reaction between diyne 7 and ketene a afforded the product in 91% (vs. 82% with diyne 1, entries 10 vs. 1, respectively). We found that ketenes bearing an electron withdrawing groups in the para-position (-F, entry 13) enhanced the formation of the carbocyclic product whereas ketenes bearing an electron-donating group in the para-position (-OMe, -Me, entries 11–12) had the opposite effect. Interestingly, the cycloaddition between diyne 7 and trimethylsilyl ketene i gave a phenolic product resulting from a facile 1,3-silyl migration (Figure 2).12 The reaction of ketene j afforded a spiro-bicyclic product in good yield (entry 17). Again, terminal diynes 8 and 9 were also found to afford carbocyclic products as evidenced by the formation of 8a and 9a (entries 18 and 19 respectively).13
Figure 2.
Proposed intermediate.
The standard reaction conditions were applied to unsymmetrical diyne 10. We were delighted to selectively obtain one regioisomer 10a in 66% yield (equation 2). The regiochemistry of 10a was determined by NOESY-1D spectroscopy.
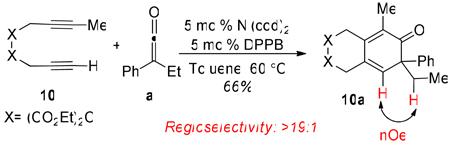 |
(2) |
The asymmetric formation of quaternary stereocenters remains a formidable challenge to organic chemists.14 With this in mind, we also investigated the development of an asymmetric version of the cycloaddition reaction. Initial investigations employing (R)-BINAP as a ligand gave dismal results. That is, no reaction was observed when standard reaction conditions (5 mol% catalyst, 0.1M diyne, 60 °C, and toluene) were employed. However, carbocyclic product was generated when the temperature was elevated to 80 °C. Although a relatively low yield (38%) was obtained, excellent enantioselectivity (99%) was observed (equation 3). A higher yield was obtained when the reaction temperature was increased to 100 °C. Gratifyingly, only a slight decrease in ee was observed at a higher temperature (100 °C).
In conclusion, we have successfully incorporated ketenes in [2+2+2] cycloaddition reactions with diynes. Decarbonylation of the ketene starting materials was not observed. Instead, a variety of 2,4-cyclohexadienones were formed. Enantionpure cyclohexadienone product was obtained when (R)-BINAP was used as the ligand. Efforts to develop a general asymmetric catalyst system and to understand the mechanistic details of this cycloaddition chemistry are underway.
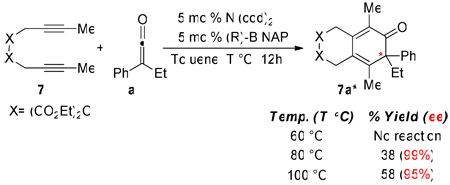 |
(3) |
Supplementary Material
Acknowledgment
We gratefully acknowledge the NSF and the NIGMS (5RO1GM076125) for support of this research. R.C. thanks CNPq-Brasil (#201732/2008-4) for a postdoctoral fellowship. We thank Professor Sigman at the University of Utah for the use of a chiral GC instrument.
Footnotes
Supporting Information Available: Detailed experimental procedures and compound characterization (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Leboeuf D, Gandon V, Malacria M. Transition Metal-Mediated [2+2+2] Cycloadditions. In: Ma S, editor. Handbook of Cyclization Reactions. Vol. 1. Weinheim: Wiley-VCH; 2009. pp. 367–406. [Google Scholar]; (b) Lautens M, Klute W, Tam W. Chem. Rev. 1996;96:49. doi: 10.1021/cr950016l. [DOI] [PubMed] [Google Scholar]; (b) Chopade P, Louie J. Adv. Synth. Catal. 2006;348:2307. [Google Scholar]; (c) Louie J. Curr. Org. Chem. 2005;9:605. [Google Scholar]; (d) Dominguez G, Pérez-Castells J. Chem. Soc. Rev. 2011 doi: 10.1039/c1cs15029d. ASAP. [DOI] [PubMed] [Google Scholar]
- 2.(a) Sato Y, Nishimata T, Mori M. J. Org. Chem. 1994;59:6133. doi: 10.1021/jo030309b. [DOI] [PubMed] [Google Scholar]; (b) Ikeda S, Watanabe H, Sato Y. J. Org. Chem. 1998;63:702. doi: 10.1021/jo980987b. [DOI] [PubMed] [Google Scholar]; (c) Wender PA, Jenkins TE. J. Am. Chem. Soc. 1989;111:6432. [Google Scholar]; (d) Ni Y, Montgomery J. J. Am. Chem. Soc. 2004;126:11162. doi: 10.1021/ja046147y. [DOI] [PubMed] [Google Scholar]; (e) Louie J, Gibby JE, Farnworth MV, Tekavec TN. J. Am. Chem. Soc. 2002;124:15188. doi: 10.1021/ja027438e. [DOI] [PubMed] [Google Scholar]; (f) McCormick MM, Duong HA, Zuo G, Louie J. J. Am. Chem. Soc. 2005;127:5030. doi: 10.1021/ja0508931. [DOI] [PubMed] [Google Scholar]; (g) Duong HA, Cross MJ, Louie J. J. Am. Chem. Soc. 2004;126:11438. doi: 10.1021/ja046477i. [DOI] [PubMed] [Google Scholar]; (h) Tanaka K, Wada A, Noguchii K. Org. Lett. 2005;7:4737. doi: 10.1021/ol052041b. [DOI] [PubMed] [Google Scholar]; (i) Oberg KM, Lee EE, Rovis T. Tetrahedron. 2009;65:5056. doi: 10.1016/j.tet.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Tekavec TN, Louie J. Org. Lett. 2005;7:4037. doi: 10.1021/ol0515558. [DOI] [PubMed] [Google Scholar]; (k) Tekavec TN, Louie J. J. Org. Chem. 2008;73:2641. doi: 10.1021/jo702508w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ketenes were coupled with alkynes using Rh- catalyst. However, products arising from a β-hydride elimination step are obtained. Kondo T, Niimi M, Yoshida Y, Wada K, Mitsudo T, Kimura Y, Toshimitsu A. Molecules. 2010;15:4189. doi: 10.3390/molecules15064189.
- 4.For excellent review on interaction of ketenes with various metals, See: Geoffery GL, Bassner SL. Adv. Organomet. Chem. 1988;28:1.
- 5.For various metal- ketene complexes, See: [Ni] Hoberg H, Korff J. J. Organomet. Chem. 1978;152:255. Sugai R, Miyashita A, Nohira H. Chem. Lett. 1988;17:1403. Miyashita A, Grubbs RH. Tetrahedron Lett. 1981;22:1255. Miyashita A, Shitara H, Nohira H. J. Chem. Soc. Chem. Commun. 1985:850. [Pt] Schorpp K, Beck W. Z. Naturforsch. 1973;28B:738. Miyashita A, Shitara H, Nohira H. Organometallics. 1985;4:1463. [Ti,Zr] Straus DA, Grubbs RH. J. Am. Chem. Soc. 1982;104:5499. [Ir] Lo HC, Grotjahn DB. J. Am. Chem. Soc. 1997;119:2958. Grotjahn DB, Bikzhanova GA, Collins LSB, Concolino T, Lam K-C, Rheingold AL. J. Am. Chem. Soc. 2000;122:5222. Grotjahn DB, Collins LSB, Wolpert M, Bikzhanova GA, Lo HC, Combs D, Hubbard JL. J. Am. Chem. Soc. 2001;123:8260. doi: 10.1021/ja004324z. Grotjahn DB, Hoerter JM, Hubbard JL. J. Am. Chem. Soc. 2004;126:8866. doi: 10.1021/ja048489+. [Rh] Grotjahn DB, Hoerter JM, Hubbard JL. Organometallics. 1999;18:5614. Werner H, Bleuel E. Angew. Chem., Int. Ed. 2001;40:145. doi: 10.1002/1521-3773(20010105)40:1<145::aid-anie145>3.0.co;2-g.
- 6.For decarbonylation of ketene complexes, See: Hofman P, Perez-Moya LA, Steigelman O, Riede J. Organometallics. 1992;11:1167. [Co] Young DA. Inorg. Chem. 1973;12:482. Hong P, Sonogashira K, Hagihara N. Tetrahedron Lett. 1971:1105. [Pd] Mitsudo T, Kadokura M, Watanabe Y. J. Org. Chem. 1987;52:1695. Mitsudo T, Kadokura M, Watanabe Y. J. Org. Chem. 1987;52:3186. Mitsudo T, Kadokura M, Watanabe Y. Tetrahedron Lett. 1985;26:3697. Goll JM, Fillion E. Organometallics. 2008;27:3622. [Fe] Mills OS, Redhouse AD. J. Chem. Soc. Chem. Commun. 1966:444. [Os] Arce AJ, Deeming AJ. J. Chem. Soc. Chem. Commun. 1982:364. [Pt] Miyashita A, Shitara H, Nohira H. Organometallics. 1985;4:1463. [Rh] Hong P, Nishii N, Sonogashira K, Hagihara N. J. Chem. Soc. Chem. Commun. 1972:993. Kondo T, Tokoro Y, Ura Y, Wada K, Mitsudo T. ChemCatChem. 2009;1:82. [Ir] Grotjahn DB, Bikzhanova GA, Collins LSB, Concolino T, Lam K-C, Rheingold AL. J. Am. Chem. Soc. 2000;122:5222. Urtel H, Bikzhanova GA, Grotjahn DB, Hofmann P. Organometallics. 2001;20:3938.
- 7.Tidwell TT. Ketenes. Wiley-Interscience: New York; 1995. [Google Scholar]
- 8.For complimentary approach to cyclohexadienones and phenols, See: Tang PC, Wulff WD. J. Am. Chem. Soc. 1984;106:1132. Ming-Yuan L, Madhushaw RJ, Liu RS. J. Org. Chem. 2004;69:7700. doi: 10.1021/jo048983w.
- 9.For natural products containing 2,4-cyclohexadienone core, See: Kaouadji M. J. Nat. Prod. 1986;49:500. Kuo Y, Li S, Huang R, Wu M, Huang H, Lee K. J. Nat. Prod. 2001;64:487. doi: 10.1021/np000261m. Shen Y, Cheng Y, Liaw C, Liou S, Khalil A. J. Nat. Prod. 2007;70:1139. doi: 10.1021/np078006q. Quideau S, Pouysegu L, Deffieux D. Synlett. 2008:467.
- 10.When DPPF is employed as the ligand in the cycloaddition of other substrates, such as diynes 5 and 7 with a, a complex mixture of products that included diyne dimer, ketene, dimer, and cycloadduct was formed which hampered purification. In general, reactions run with DPPB were cleaner. As such, our optimized conditions focused on using DPPB as the ligand of choice.
- 11.Wender PA, Christy JP. J. Am. Chem. Soc. 2007;129:13402. doi: 10.1021/ja0763044. [DOI] [PubMed] [Google Scholar]
- 12.(a) Larson GL, Hernandez D, Lopez-Cepreo I, M. D, Torres LE. J. Org. Chem. 1985;50:5267. [Google Scholar]; (b) Matsuda I, Sato S, Hattori M, Izumi Y. Tetrahedron Lett. 1985;26:3215. [Google Scholar]
- 13.When 3-hexyne and phenyl ethyl ketene (a) were subjected to optimized conditions, the cycloaddition product was not obtained. Instead, only ketene dimerization was observed by GC.
- 14.(a) Corey EJ, Guzman-Perez A. Angew. Chem., Int. Ed. 1998;37:391. doi: 10.1002/(SICI)1521-3773(19980302)37:4<388::AID-ANIE388>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]; (b) Christoffers J, editor. Challenges and Solutions For Organic Synthesis. Weinhein: Wiley-VCH; 2005. [Google Scholar]; (c) Douglas CJ, Overman LE. Proc. Natl. Acad. Sci. USA. 2004;101:5363. doi: 10.1073/pnas.0307113101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.