Abstract
Gamma activity has been linked to a variety of different cognitive processes and exists in both transient and persistent forms. Across studies, different brain regions have been suggested to contribute to gamma activity. Multiple studies have shown that the function of gamma oscillations may be related to temporal binding of early sensory information to relevant top-down processes. Given this hypothesis, we expected gamma oscillations to subserve general brain mechanisms that contribute to the development of cognitive and linguistic systems. The present study aims to examine the predictive relations between resting-state cortical gamma power density at a critical point in language and cognitive acquisition (i.e. 16, 24 and 36 months), and cognitive and language output at ages 4 and 5 years.
Our findings show that both 24- and 36-month gamma power are significantly correlated with later language scores, notably Non-Word Repetition. Further, 16-, 24- and 36-month gamma were all significantly correlated with 4-year PLS-3 and CELF-P sentence structure scores.
Although associations reported here do not reflect a direct cause and effect of early resting gamma power on later language outcomes, capacity to generate higher power in the gamma range at crucial developmental periods may index better modulation of attention and allow easier access to working memory, thus providing an advantage for overall development, particularly in the linguistic domain. Moreover, measuring abilities at times when these abilities are still emergent may allow better prediction of later outcomes.
Keywords: Resting EEG, Gamma power, Working memory, Language, Cognitive development
1. Introduction and literature review
Previous studies have examined activity in the gamma band using many types of techniques including electrophysiological data recorded from single and multiple neurons in animal models up through the collection of dense array electroencephalograms (EEG) or magnetoencephalography (MEG) recordings in humans. Gamma activity has been reported to exist in local as well as distributed cortical networks and in both transient and persistent forms [72, 22, 9]. Different brains regions such as thalamus and Medial Temporal Lobe (MTL), and in particular, hippocampus have been suggested to contribute prominently to gamma activity [59, 72]. Activity in the gamma band has been linked to a variety of different cognitive processes including feature binding [64], attention [26, 69, 7, 42], working memory [68, 52, 55] and associative learning [23, 62, 76]. Further, it has been suggested that gamma oscillations provide an effective mechanism for the synchronization [53] of neuronal activity at both global and local scales [10] and that local and long-range synchronization changes across development [74]. While gamma oscillations occur within diverse contexts, with differing temporal dynamics, and with varied relations to sensory and cognitive events, there is evidence that spontaneous, steady-state, evoked and induced gamma oscillations might be generated by the same neural circuits and have a similar physiology [2, 34, 35, 50]. In general, those studies report that higher gamma activity is associated with better task performance. Nevertheless, Herrmann et al.’s [35] review of gamma oscillations in neuropsychiatric disorders also reports increased gamma activity observed in some situations, specifically for patients with attention deficit hyperactivity disorder (ADHD).
There are strikingly few studies that track maturational patterns of oscillations in normally developing children and fewer still that include frequencies in the gamma range. However, the characteristic EEG patterns observed across age and across frequencies suggest a more general developmental pattern across frequency bands. Specifically, it has been observed that activity in the lower frequency bands decreases and activity in higher frequencies increases as children mature (e.g. [16]). For example, John et al. [38] fitted linear age regression functions on four broad-band EEG indices (delta, theta, alpha and beta) at different locations on the scalp in normally developing children aged 6 to 16 years. The resultant 32 parameters of the EEG were then examined in different groups of children. A high incidence of significant deviation from these parameters was found in children with learning disabilities and those at risk for various neurological disorders [1]. This seems to suggest that children whose indices significantly deviate from the normative regression functions may exhibit different maturational trajectories of early brain development [1, 12]. The studies just cited did not examine EEG patterns within the gamma range, but examination of oscillations in the 30Hz to 80Hz gamma range in human electroencephalograms (EEG) suggests that such high frequency activity is also developmentally regulated and has been shown to increase as a function of age in children between 3 and 12 years of age, most strikingly over frontal regions [66]. A similar pattern has been described for evoked gamma in children from 1 month to 5 years, 6 months (e.g. [43]). On the cellular level, fast oscillations play an essential role in coincidental neural activity important for perceptual binding, synaptic plasticity and in modulating the precise temporal coordination that is being fine-tuned across early development [63, 5, 41, 74, 73]. Those few studies that do examine either resting-state or evoked gamma across childhood suggest that high frequency oscillations may well support general mechanisms that underlie maturation of cortical regions, facilitate cross-cortical longer range synchrony and promote development of efficient neural networks [6, 74, 73].
Support for this statement can be inferred from studies that examine the putative role of neural synchrony in the development of cortical networks. These studies have been reviewed by Uhlhaas et al. [73] including studies of normal brain development as well as for neurodevelopmental disorders, such as autism spectrum disorders (ASDs) and schizophrenia. Across labs and studies, developmental changes in the frequency, amplitude and synchronization of both resting-state and task-related neural synchrony were closely related to brain maturation and to maturational patterns of cognitive function [73]. Gamma oscillations were shown to emerge during early childhood and precise temporal coordination through neural synchrony continued to show maturational change through early adulthood. The slow maturation of neural synchrony is compatible with changes in the myelination of cortico-cortical connections and with late development of GABAergic neurotransmission [18, 17, 74]. One quite recent study [74] examined the development of functional networks in older children through adulthood by recording EEGs to a Gestalt perception task. Pronounced increases in gamma-band power and in both theta and beta phase synchrony were seen across age groups (6–21 years) in conjunction with improvements in reaction times and detection rates, thus providing support for the premise that increases in neural synchrony and brain maturation trajectories are related.
Recently, alterations in resting brain power in clinical populations has also been demonstrated, including attenuation in the delta range in small-for-gestational-age infants [49] and lower power in the very low frequency bands (0.02–0.20 Hz) for children diagnosed with attention deficit/hyperactivity disorder [33]. There are also a few studies looking at shifts in the proportion of “evoked” gamma across age that suggest that the biggest changes in the proportion of the gamma band occurs after 5 years of age (e.g. [43]). Whether this is the same for resting gamma is not known. The development of neural synchrony seems to reflect ongoing maturation and restructuring of functional networks [73, 74]. Fries [24] conceptualizes gamma band synchronization as a fundamental mechanistic process that plays a role for all cortical computation. Taken together, these findings suggest that gamma synchronization is important for the development of cortical networks (e.g. motor, cognitive and perceptual processes that include language).
In the only study to date investigating resting gamma-band power in a prospective fashion across early development, Benasich and colleagues examined gamma activity in a group of children at 16, 24 and 36 months of age [6]. The authors reported individual differences in the distribution of frontal gamma power (31–50Hz) that was associated with emerging linguistic and cognitive skills as well as attentional measures assessed at the same ages. This was the case for typically developing children as well as children at higher risk for language disorders as a function of a family history of language-based learning disorders (LLD). The 16 through 36 month age range examined in the Benasich et al. study [6] is a particularly significant time developmentally. A dramatic burst in linguistic acquisition and cognitive growth is occurring over this time period with large amounts of individual variation in language development also seen at these ages [21]. At this point in development, children are achieving important cognitive concepts like object permanence, are beginning to engage in symbolic reasoning, and are beginning to think in increasingly complex ways. These behavioral results suggest that the emergence of high frequency neural synchrony may be critical for normative cognitive and linguistic development, and that those children at higher risk for language impairments (e.g. born into a family with a history of LLD) may lag in this process [6]. Importantly, these findings demonstrate that infant cognitive ability correlates with power density functions even in an “idling” brain, that is a brain not engaged in an active perceptual or cognitive task.
Given these findings that resting EEG gamma power is strongly associated with concurrent language and cognitive skills for normally-developing children as well as children at higher risk for language disorders, the question remains as to whether resting gamma power density at a particular age would be an ongoing predictor of later linguistic and cognitive abilities. Increased power and synchronization in the high-frequency ranges (e.g. gamma and beta) is known to be mediated mainly by cortico-cortical connections and is thought to serve as a mechanism for the integration of distributed signals over different temporal and spatial scales [18, 44, 74]. These networks may then sub-serve the development of cognition and language. Thus the present study examines the question of whether frontal gamma power measured via resting EEG at an early age (16, 24 and 36 months of age) is predictive of later cognitive and linguistic performance. We also consider the role of emergent abilities and of developmental time windows in the particular skills that might be associated with earlier EEG power. In particular, we ask what later abilities might reflect optimal establishment of high frequency (31–50Hz) cortical oscillatory activity, that in turn supports the establishment of efficient information (e.g. linguistic) processing networks. Here we examine the ongoing longitudinal data collected from a subset of the sample described in [6] and explore the associations between resting EEG gamma power during the language burst (16, 24 and 36 months of age) and cognitive and linguistic performance at ages 4 and 5 years of ages.
2. Materials and Methods
2.1. EEG Assessment at 16, 24 and 36 months of age
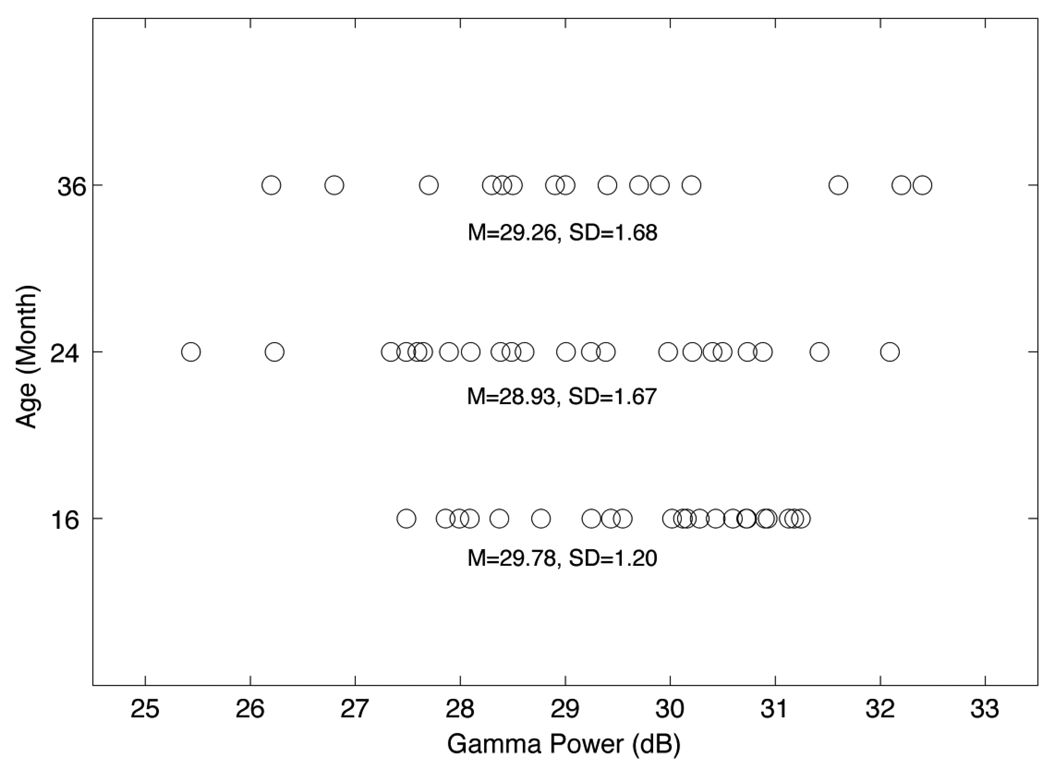
The Geodesic Sensor Net (Electrical Geodesics Inc., Eugene, OR) with 62 scalp sites was used to collected EEG data. At each visit, following the placement of EEG electrodes on the scalp, the children participated in a passive auditory oddball procedure containing two blocked stimulus conditions separated by a three-minute break with no stimuli being presented during the break. A three-minute block of EEG was collected while the child quietly played on the parent’s lap. This 3-minute “resting” EEG was analyzed and provided the gamma power data for the present study. The raw EEG data were sampled at 250 Hz and filtered offline at 1.5–57 Hz. Artifacts including eye blinks and muscle movements were identified by visual inspection and Independent Component Analysis (ICA) [39] and removed from the EEG data. The data were re-referenced by whole-head average algorithm. The artifact-free epochs were submitted to power spectral analysis using Fast Fourier Transform (FFT). The log transform for absolute power was: 10 log 10(×). A continuous power density function (averaged across all the prefrontal and frontal electrodes) was generated for each child at each age. The 31–50 Hz band was considered representative of the gamma range. The mean of the gamma range power function was taken as the average gamma power density for each child. As noted, the gamma data used in the predictive analyses described here was collected at 16, 24 and 36 months of age with group means of 29.78dB, 28.93dB and 29.26dB, and standard deviations of 1.20dB, 1.67dB and 1.68dB respectively (see Figure 1). The concurrent results and EEG data processing details for these ages were reported in Benasich et al., 2008 [6].
Figure 1.
Mean gamma power data plotted by age at 16-, 24- and 36-months-of-age
2.2. Behavioral Assessment at 4 and 5 years
The Stanford-Binet Intelligence Scale-4th ed
[SB-4] [70] was administered at 4 and 5 years of age to assess overall cognitive ability. The following cognitive clusters were administered and are reported on here: Verbal Reasoning, Abstract/Visual Reasoning, Quantitative Reasoning, and Short-Term Memory as well as individual subset score. Subtest scores are reported as standard age scores (SAS: mean = 50, SD = 8) and cluster scores were reported as mean = 100, SD = 16.
The NonWord Repetition Test
[NWR] [27] was also administered at 4 and 5 years of age. NWR measures phonological working memory skills thought to be related to the acquisition of vocabulary and reading skills. In this test, the child hears a single unfamiliar phonological item, such as “barrazon”, and attempts to immediately repeat it. The task consists of 45 nonsense words divided into 5 subsets each increasing in syllable length (1- through 5-syllables). The 1-syllable subset is composed of 5 non-words while the rest contain 10 each. Test items are presented in random order so that syllable length varies throughout the test. Children receive scores for each of the 5 subtests. Since the 1-syllable test has low test-retest reliability 28], that subscale has been dropped from the Total test score. Total raw scores ranged from 0–40. Age-appropriate norms are not as yet available for this measure.
The Preschool Language Scale-3rd ed
[PLS-3] [80] assesses receptive (Auditory Comprehension) and expressive (Expressive Communication) language skills in children from birth to 6 years and 11 months and for this study was administered at 4 years of age. The test yields standard scores (mean = 100, SD = 15), percentile ranks, and age scores for the subscales as well as a total language score. For the purpose of this study, standard scores for the Auditory Comprehension and Expressive Communication subscales were used.
The Clinical Evaluation of Language Fundamentals - Preschool
[CELF-P] [78] was administered at 4 years of age. Children were evaluated with two subtests of the CELF-P (mean = 10 SD = 3): Sentence Structure (SS) and Word Structure (WS). The Sentence Structure subtest evaluates comprehension of sentence formation rules, while the Word Structure subtest evaluates the child’s use of morphological rules and forms.
Test of Language Development
[TOLD: P3] [47] was administered at 5 years of age. Five subtests (grammatic completion GC, grammatic understanding GU, sentence imitation SI; mean 10, SD = 3) were used to assess the child’s understanding and use of grammatical structures. A composite score for Syntax SYQ (mean = 100, SD = 15) was derived by combining scores from the grammatic completion, grammatic understanding, and sentence imitation subtests.
Woodcock Reading Mastery Tests-Revised
[WRMT-R] [79]; Letter- Identification subtest (mean = 100, SD= 15) was administered at 5 years of age. This subtest measures the subject’s ability to identify upper and lower case letters.
2.3. Participants
The participants in this study were a subset of those participating in a larger longitudinal study examining the association between early processing of auditory information and language development. Families were recruited from urban and suburban communities in New Jersey and were assigned to family history positive (FH+) or family history negative (FH−) groups based on parental report of family history of language-based learning disorder (LLD); for additional information see [14]. The FH+ children are considered to be at significantly higher risk for language disorders by virtue of their family history of LLD [13].
The sample consisted of 40 children with gamma data and 45 children with complete behavioral data. Table 1 shows the number of participants that had gamma data at any one age point and corresponding behavioral data at outcome ages. This table also presents the sample mean and standard deviation for the behavioral assessments.
Table 1.
Means of the behavioral measures for 4- and 5–year-olds
| At 4 Years: | At 5 Years: | ||||||
|---|---|---|---|---|---|---|---|
| N | M | SD | N | M | SD | ||
| Stanford-Binet | Stanford-Binet | ||||||
| Composite Score | 27(9+) | 113 | 9 | Composite Score | 23(8+) | 114* | 10 |
| Quantitative Reasoning | 27(9+) | 111 | 13 | Quantitative Reasoning | 23(8+) | 114 | 11 |
| Verbal Reasoning Scale | 27(9+) | 116 | 8 | Verbal Reasoning Scale | 23(8+) | 115 | 8 |
| Short-term Memory | 27(9+) | 108 | 10 | Short-term Memory | 23(8+) | 107 | 12 |
| Abstract/Visual Reasoning | 27(9+) | 110 | 12 | Abstract/Visual Reasoning | 23(8+) | 112 | 13 |
| Absurdities | 27(9+) | 59 | 5 | Absurdities | 23(8+) | 58 | 4 |
| Vocabulary | 27(9+) | 56 | 4 | Vocabulary | 23(8+) | 55 | 6 |
| Comprehension | 27(9+) | 56 | 5 | Comprehension | 23(8+) | 57 | 7 |
| Bead Memory | 27(9+) | 51 | 6 | Bead Memory | 23(8+) | 54 | 6 |
| Sentence Memory | 27(9+) | 56 | 7 | Sentence Memory | 23(8+) | 53 | 6 |
| Copying | 27(9+) | 52 | 6 | Copying | 23(8+) | 52 | 5 |
| Pattern Analysis | 27(9+) | 57 | 7 | Pattern Analysis | 23(8+) | 59* | 7 |
| Non-Word Repetition Test | Non-Word Repetition Test | ||||||
| 2 Syllables | 27(9+) | 9 | 1 | 2 Syllables | 24(8+) | 9 | 2 |
| 3 Syllables | 27(9+) | 8 | 3 | 3 Syllables | 24(8+) | 8 | 2 |
| 4 Syllables | 27(9+) | 5* | 2 | 4 Syllables | 24(8+) | 6 | 2 |
| 5 Syllables | 27(9+) | 5 | 3 | 5 Syllables | 24(8+) | 7 | 2 |
| Total | 27(9+) | 27 | 7 | Total | 24(8+) | 29 | 6 |
| Preschool Language Scale | Test of Language development | ||||||
| Auditory Comprehension | 27(9+) | 116 | 14 | Grammatic Completion | 25(8+) | 12* | 2 |
| Expressive | 27(9+) | 113 | 14 | Grammatic Understanding | 25(8+) | 12 | 2 |
| Clinical Evaluation of Language Fundamental | Sentence Imitation | 25(8+) | 12 | 2 | |||
| Syntax Quotient | 25(8+) | 112* | 11 | ||||
| Word Structure | 26(8+) | 11 | 2 | Woodcock Reading Mastery | |||
| Sentence Structure | 26(8+) | 12* | 4 | Letter Identification subtest | 25(8+) | 109 | 11 |
Number of FH+ children.
t-test of FH+ and FH− groups different, p<=0.05.
As noted, this study is part of a larger ongoing longitudinal study on cognitive and language development of infants with (FH+) and without a family history (FH−) of language based learning disorder (LLD). While infants recruited into the family history positive (FH+) group were at higher risk for developing LLD, none of the children in the present study were themselves diagnosed with any language or learning disabilities. T-tests confirmed that there were no group differences in this sample between children from FH+ and FH− families on 40 of the 46 measures and thus for all further analyses FH+ and FH− children were treated as one continuous group.
3 Results
To examine whether EEG gamma power at 16-, 24-, and 36-months was associated with later cognitive and language abilities, Pearson Product Moment Correlations were computed between mean gamma power at each age and performance on a series of language and cognitive tasks at both 4 and 5 years of age.
3.1 Post hoc Analysis of Electrode-by-Electrode Scalp Correlations
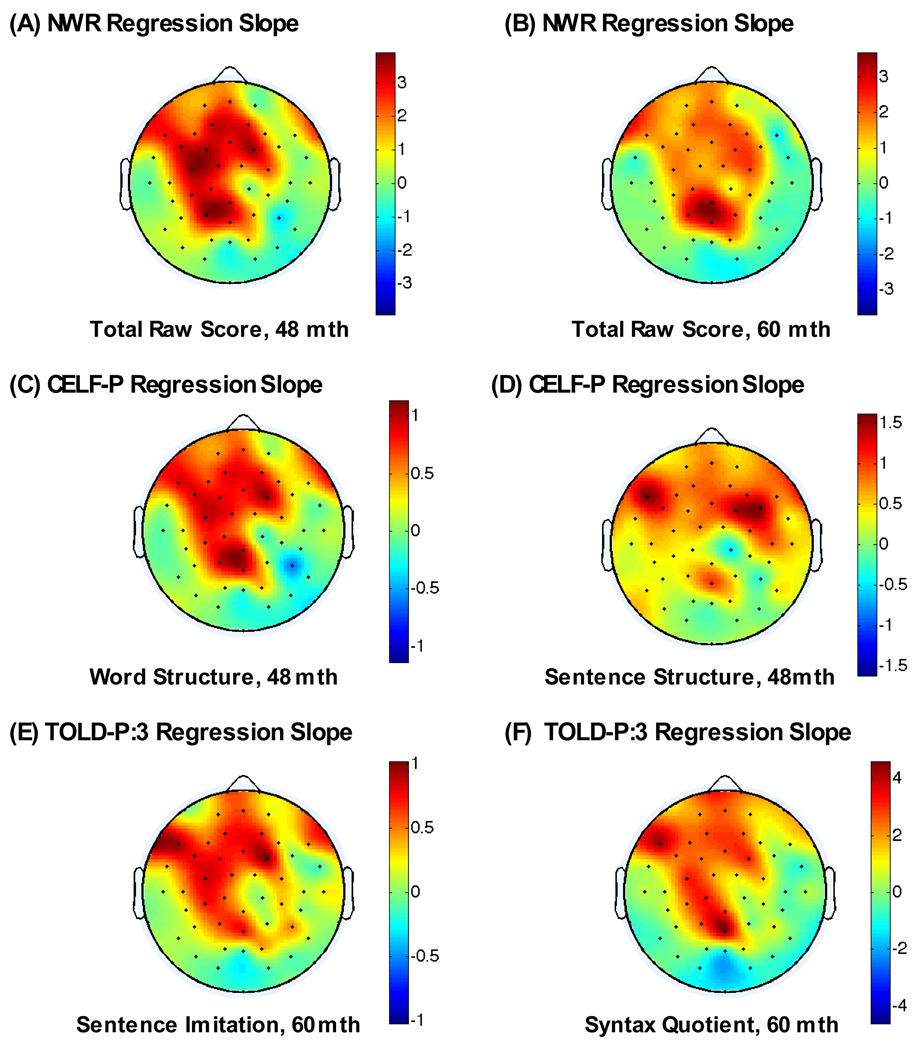
As we had focused primarily on Frontal and Prefrontal regions based on a qualitative measure (i.e. examination of power topograms see [6]), we performed post hoc electrode-by-electrode analyses including all 62 scalp sites for a subset1 of outcomes to examine which areas were most highly correlated with the language outcomes of interest. The patterns of outcomes revealed were similar to the findings described above, when only frontal and pre-frontal regions were examined. To illustrate the scalp regions where mean gamma power correlates with language outcome when all electrodes are included, we show results for 36-month gamma power (see Figure 2A–F). The results indicate that across tests and across age, the correlations are predominately in frontal, and to a lesser extent, temporal regions and tended to be left lateralized.
Figure 2.
Correlation topograms showing maps of the electrode-by-electrode regression slopes for 36-month gamma power with NonWord Repetition (NWR) Total Raw Score, CELF-P Word and Sentence Structure Score, and TOLD-P:3 Sentence Imitation and Syntax Quotient score at 4 and 5 years of age
3.2 Gamma and general cognitive ability
The Stanford-Binet Intelligence Scale was used to assess general cognitive ability. Correlation analyses did not reveal any significant associations between gamma power and IQ at any age, with only one exception; 36-month gamma was associated with scores on the 5-year Verbal Reasoning cluster, r = 0.67, p<0.01
3.3 Early gamma and 4 and 5-year phonological working memory
The Non-Word Repetition Test (NWR) was used to assess phonological working memory at 4 and 5 years of age. There were no significant correlations observed among 16-month gamma and any 4- or 5-year NWR syllable subtests. Table 2 shows the correlations between 24- and 36-month gamma and for NWR 2, 3, 4, and 5 syllables and total score. Results revealed that 24- and 36-month gamma power was significantly associated with both 4- and 5-year NWR test performance.
Table 2.
Correlations of 24- and 36-month gamma power with 4- and 5-year NonWord Repetition test performance
| 24-month mean gamma | 36-month mean gamma | |
|---|---|---|
| 4-Year NWR syllable length | (N=17) | (N=16) |
| 2 syllables | 0.18 | 0.01 |
| 3 syllables | 0.53* | 0.48 |
| 4 syllables | 0.65** | 0.53* |
| 5 syllables | 0.58* | 0.55* |
| Total Score | 0.61* | 0.51* |
| 5-Year NWR syllable length | (N=15) | (N=14) |
| 2 syllables | 0.42 | 0.32 |
| 3 syllables | 0.52* | 0.32 |
| 4 syllables | 0.65** | 0.57* |
| 5 syllables | 0.69** | 0.54* |
| Total Score | 0.72** | 0.52* |
Pearson correlation coefficient:
p<=0.05 and
p<=0.01.
3.4 Early gamma and 4-year expressive and receptive language abilities
Significant associations were found between resting gamma power at all ages and later language ability. Table 3 shows the correlation results between 16-, 24- and 36-month mean gamma density and 4-year PLS-3 and CELF-P scores. Gamma power at 36-months was associated with the PLS-3 comprehension score (r = 0.59, p = 0.02), performance on the CELF-P Sentence Structure (r = 0.56, p = 0.03), and with CELF-P Word Structure (r = 0.67, p = 0.01) standard scores. The 16- and 24-month gamma measures were significantly correlated only with the CELF-P sentence structure score: r = 0.69, p = 0.01; r = 0.65 and p = 0.01 respectively. Mean gamma band density at all three ages, from 16- to 36-months, showed significant correlations with CELF-P syntax comprehension score. The 16- and 24 month power measures have even stronger correlations with the CELF-P sentence structure subtest that measures the ability to understand sentences that incorporate various syntactic structures.
Table 3.
Correlations of 16-, 24-and 36-month gamma power with 4-year PLS-3 and CELF-P scores
| 16-month mean gamma |
24-month mean gamma |
36-month mean gamma |
|
|---|---|---|---|
| 4-Year PLS-3 | (N=14) | (N=17) | (N=16) |
| Expressive | 0.15 | 0.15 | 0.40 |
| Receptive | 0.41 | 0.38 | 0.59* |
| 4-Year CELF-P | (N=13) | (N=17) | (N=15) |
| Word | 0.40 | 0.34 | 0.67** |
| Sentence | 0.69** | 0.65** | 0.56* |
Pearson correlation coefficient:
p<=0.05 and
p<=0.01.
3.5 Early gamma and language abilities at 5 years of age
We also examined scores on specific subtests of the TOLD-P3, including grammatic understanding (GU), grammatic completion (GC), sentence imitation (SI) and syntax composite score (SYQ). The abilities measured by the composite score (SYQ) are related to grammar and sentence structure, most notably the ability to sequence words and to organize phrases to form grammatically appropriate sentences [47]. No associations were seen between TOLD-P3 scores and 16-month gamma power. However, at both 24- and 36-months, gamma power was significantly associated with 5-year Sentence Imitation (r = 0.53, p = 0.04; r = 0.53, p = 0.05). In addition, at 36-months, gamma power was significantly associated with the SYQ (r = 0.56, p = 0.04). Table 4 details the associations seen at 24- and 36-months.
Table 4.
Correlations of 24- and 36-month gamma power and 5-Year TOLD-P3 Scores
| 24-month mean gamma | 36-month mean gamma | |
|---|---|---|
| 5-Year TOLD-P3 | (N=17) | (N=16) |
| Grammatic Understanding | 0.10 | 0.39 |
| Sentence imitation | 0.53* | 0.53* |
| Grammatic completion | 0.25 | 0.48 |
| Syntax Composite quotients | 0.33 | 0.56* |
Pearson correlation coefficient:
p<=0.05 and
p<=0.01.
4. Discussion
A series of Pearson’s correlations were performed examining children’s 16-, 24- and 36- month mean resting gamma power (31–50Hz), at frontal and pre-frontal regions, and the cognitive and linguistic outcomes for the same children at 4- and 5-years of age. The results described here strongly suggest that resting gamma power, as measured across 16- through 36-months, a time of dramatic increases in linguistic and cognitive abilities, is a good predictor of specific components of language development at 4- and 5-years of age. We understand that the associations reported here do not reflect a direct cause and effect of early resting gamma power densities on later language outcomes, but rather, index the synchronous activity of large interconnected networks of neurons that underlie cognitive functions, such as memory formation, language development and sensory processing, but are not themselves specific cognitive substrates. However, the findings presented here suggest that variation in the capability of a particular brain to generate activity in the gamma range or to switch into a gamma state on demand, might well index establishment of critical neural synchronies that underlie emergence of high frequency oscillatory activity and in turn the establishment of efficient information (e.g. linguistic) processing.
4.1 Gamma Oscillations
Gamma frequency components have been studied in both visual and language processing. In this context, it is important to understand that gamma oscillation is not proposed to represent information itself, but rather to provide a temporal structure for correlations in the neurons that do encode specific information [19, 30, 65]. Generally, human data are in good agreement with animal studies suggesting the role of gamma synchronization in the binding and selection of distributed information [11, 65, 29, 64]. Both evoked and induced gamma oscillations have been shown to be correlates of numerous cognitive functions. In the visual domain, gamma bursts have been observed as a function of object perception [3]. Faces induce more gamma activity than rotated unrecognizable faces [40] and lead to more synchronization among brain areas within the gamma band [61]. Also gamma activity can be detected when subjects suddenly recognize a meaningful picture within random-dot patterns (autostereoscopic pictures) [58]. Visual feature binding also has been associated with gamma activity [67, 40, 46]. The uniquely human ability of language has also been associated with gamma activity: words evoke higher levels of gamma in human cortex than do pseudo-words [57]. The mechanisms that underlie many of the cognitive functions that induce gamma activity are hypothesized to also support memory and attentional access. This can be gleaned from the few studies that examine memory and attention. For example, when subjects are required to actively maintain visual stimuli in working memory, higher levels of gamma are detected as compared to a condition not requiring memorization [68]. Tones also evoke gamma bursts [50] and attended tones evoke larger auditory gamma peaks than unattended ones [71]. Further, a number of other studies have shown positive correlations between gamma activity and learning and memory in older populations [45, 25, 32, 20]. Overall studies that specifically focus on measuring both gamma oscillations and behavior seem to suggest that gamma activity and its synchronization across brain regions might well serve as a mechanism by which distributed signals can be integrated over different temporal and spatial scales [55].
4.2 Gamma power, phonological working memory and syntax
Gamma power in this study showed significant correlations with both phonological working memory and syntactic skills. This is an intriguing result. Why would we see such a pattern? What do these two linguistic abilities have in common? The connection between phonological working memory and syntactical skill has been reported and then debated for many years and conflicting theoretical interpretations of these results have been offered. For example, van Daal et al. [77] studied 5-year-old Dutch speaking children with severe language problems. Structural equation modeling (SEM) was used to explore the relations between the different factors; phonological memory was shown to predict phonological abilities and phonological abilities also predicted to syntactic abilities. In a similar vein, Ullman and Pierpont [75] reported a link between impairments of non-word repetition and syntax, and suggested a general neural model (the Procedural Deficit Hypothesis) that predicted a deficit in a network of interconnected structures embedded in frontal/basal-ganglia circuits that impacts procedural memory and learning, and that is in turn implicated in both syntax acquisition and phonological working memory. These authors emphasize the importance of taking account of both behavioral and neural correlates.
Similarly, Joanisse and Seidenberg [37], used a connectionist model to study development of phonology and syntax in specific language impairment (SLI) employing a two-model simulation experiment. One network was trained to simulate how normally developing children learn syntax and the second network was trained using subtle distortions in phonology to simulate imperfect phonological representations as in SLI. The authors demonstrated that syntactic impairments could be directly tied to limitations in working memory capacity and phonological knowledge [37]. They further concluded that the pattern of results fit a model that entails a single mechanism, rather than a dual-mechanism model as suggested by Ullman and colleagues [54, 75]. It is not clear which of these models accurately capture the relations among these abilities; however, to date it has not been shown that such precise phonological and syntactic deficits share common underlying neurological substrate. However, Fries [24] observes that gamma synchronization occurs in multiple brain areas, including sensory cortex, hippocampus, motor cortex, spinal cord and subcortical nuclei -- as long as the regions are functionally activated, and hypothesizes that such high frequency oscillations may indeed serve a critical role in cortical computation. Thus, the relations seen here between resting gamma power, phonological working memory and syntactic skills could plausibly be supported by the essential role that coincidental neural activity plays in the general mechanisms that underlie maturation of cortical regions, facilitate cross-cortical longer range synchrony and promote development of efficient neural networks [6, 74, 73].
On the other hand, the link in this study could, more simply, be one of “emergent function”, in essence what skills are children acquiring at these particular ages. As children add new language skills to their repertoire across development, they are frequently on a steep learning curve. Moreover, as children vary in their rate of acquisition, some children are further advanced than others, thus the outcome measure contains a greater degree of variability. These characteristics become more important as the sample size decreases. It is also clear from previous work [6, 38] that lower levels of gamma in resting EEG, may well be a marker for slower brain maturation.
4.3 Importance of the Developmental Time Window
In the initial paper exploring resting gamma [6], we chose to look at a developmental period (16 through 36 months of age) characterized by a dramatic burst in cognitive and linguistic abilities [4, 21], that is hypothesized to correlate with a steep increase in synaptic density in relevant cortical regions [15, 36, 56]. Major changes in forebrain organization including continuing maturation and myelination of temporal and frontal areas, and elaboration of the extensive cortical and subcortical circuits that are thought to subserve coordinated high-frequency activity are also highlights of this developmental period [51, 56]. What is the importance of this dynamic period of growth and reorganization? It illustrates our contention that across age, the periods when new abilities are being acquired and consolidated provide a unique window of opportunity in which to observe the underlying neural dynamics that accompany behavioral change. For example, the data presented here show that 4-year-old syntax comprehension strongly correlates with mean gamma power at the ages of 16-, 24- and 36-months. This may be attributed to the fact that at 4 years of age, rapid progress is being made in the acquisition of grammar, for example, in the use of verb inflections [60], irregular words and in sentence embedding and conjoining [48]. 24-month gamma power also showed strong associations with both phonological working memory and with syntax at 4 years. Areas of language that are still being mastered (are “emergent”) require more focused attention and broader cortical input. Thus significant individual variability is seen in acquisition of grammatical and syntactical skills at these ages [8] as well as during the 16 to 36 month language burst (although different skills are being acquired). And, as noted there is significant variability in resting gamma during the 16–36 month age range. Gamma power has been shown to increase in tandem with maturation of information processing and cognitive functions in both younger [38] as well as in older children (3–12 years) with a peak at about 4–5 years of age [66]. These findings suggest that the developmental time window when EEG power and language is being measured is very important. Thus choosing time periods for measurement when there is maximal variability, basically when abilities are emerging, enhances the ability to track developmental outcomes.
4.4 Conclusions
Our results clearly show robust associations between gamma power in resting EEG at 16, 24 and 36 months of age and later language, specifically phonological memory (NWR) and syntactical skill. We suggest that the ability of the child’s brain to activate in the gamma range during information processing or perhaps differences in the underlying neural capabilities to generate these high frequency oscillations, may index better modulation of attention and allow easier access to working memory thus providing an advantage for overall development, particularly in the linguistic domain. Thus, the capability to generate higher power in certain frequency ranges (30~50Hz gamma) at certain crucial developmental periods, for instance during the 16–36 month language and cognitive burst may well confer an advantage in setting up efficient and automatized language systems. In addition, measuring abilities at times when they are still emergent appears to allow better prediction of later outcomes.
Research Highlights.
16-, 24-, & 36-month resting gamma predicted to later phonological working memory.
Early gamma power predicted to 4–5 year language including syntactical skills.
Resting gamma may tap neural synchronies critical to efficient language processing.
Measuring emergent abilities may allow better prediction of later outcomes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Computing analyses for all regions for all electrodes severely reduces power, thus we only examined those variables significantly correlated in our first set of analyses.
References
- 1.Ahn H, Prichep L, John ER, Baird H, Trepetin M, Kaye H. Developmental equations reflect brain dysfunctions. Science. 1980;210(4475):1259–1262. doi: 10.1126/science.7434027. [DOI] [PubMed] [Google Scholar]
- 2.Basar E. EEG-Brain dynamics relation between EEG and brain evoked potentials. Amsterdam: Elsevier; 1980. [Google Scholar]
- 3.Basar E, Basar-Eroglu C, Karakas S, Schürmann M. Brain oscillations in perception and memory. International Journal of Psychophysiology. 2000;35(2–3):95–124. doi: 10.1016/s0167-8760(99)00047-1. [DOI] [PubMed] [Google Scholar]
- 4.Bates EA, Thal D, Janowsky JS. Handbook of Neuropsychology. Amsterdam: Elsevier; 1992. Early language development and its neural correlates. [Google Scholar]
- 5.Ben-Ari Y. Developing networks play a similar melody. Trends in neurosciences. 2001;24(6):353–360. doi: 10.1016/s0166-2236(00)01813-0. [DOI] [PubMed] [Google Scholar]
- 6.Benasich AA, Gou Z, Choudhury N, Harris KD. Early cognitive and language skills are linked to resting frontal gamma power across the first 3 years. Behavioural Brain Research. 2008;195(2):215–222. doi: 10.1016/j.bbr.2008.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bichot NP, Rossi AF, Desimone R. Parallel and serial neural mechanisms for visual search in macaque area V4. Science. 2005;308(5721):529–534. doi: 10.1126/science.1109676. [DOI] [PubMed] [Google Scholar]
- 8.Brown R. A first language: The early stages. Cambridge, MA: Harvard University Press; 1973. [Google Scholar]
- 9.Buhl EH, Tamas G, Fisahn A. Cholinergic activation and tonic excitation induce persistent gamma oscillations in mouse somatosensory cortex in vitro. Journal of Physiology. 1998;513:117–126. doi: 10.1111/j.1469-7793.1998.117by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 11.Castelo-Branco M, Goebel R, Neuenschwander S, Singer W. Neural synchrony correlates with surface segregation rules. Nature. 2000;405:685–689. doi: 10.1038/35015079. [DOI] [PubMed] [Google Scholar]
- 12.Chabot RJ, di Michele F, Prichep L, John ER. The clinical role of computerized EEG in the evaluation and treatment of learning and attention disorders in children and adolescents. The Journal of Neuropsychiatry & Clinical Neurosciences. 2001;13:171–186. doi: 10.1176/jnp.13.2.171. [DOI] [PubMed] [Google Scholar]
- 13.Choudhury N, Benasich AA. A Family Aggregation Study: The Influence of Family History and Other Risk Factors on Language Development. Behavioural Brain Research. 2003;46:261–272. doi: 10.1044/1092-4388(2003/021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choudhury N, Leppanen PHT, Leevers HJ, Benasich AA. Infant information processing and family history of specific language impairment: converging evidence for RAP deficits from two paradigms. Developmental science. 2007;10(2):213–236. doi: 10.1111/j.1467-7687.2007.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Annals of Neurology. 1987;22(4):487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- 16.Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Age and sex effects in the EEG: development of the normal child. Clinical neurophysiology. 2001;112(5):806–814. doi: 10.1016/s1388-2457(01)00488-6. [DOI] [PubMed] [Google Scholar]
- 17.Cobb R, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- 18.Engel AK, Konig P, Kreiter AK, Singer W. Interhemispheric synchronization of oscillatory neuronal responses in cat visual cortex. Science. 1991;252:1177–1179. doi: 10.1126/science.252.5009.1177. [DOI] [PubMed] [Google Scholar]
- 19.Engel AK, König P, Schillen TB. Why does the cortex oscillate? Current biology. 1992;2(6):332–334. doi: 10.1016/0960-9822(92)90898-k. [DOI] [PubMed] [Google Scholar]
- 20.Fell J, Klaver P, Lehnertz K, Grunwald T, Schaller C, Elger C, Fernandez G. Human memory formation is accompanied by rhinal-hippocampal coupling and decoupling. Nature neuroscience. 2001;4(12):1259–1264. doi: 10.1038/nn759. [DOI] [PubMed] [Google Scholar]
- 21.Fenson LS, Dale PS, Reznick JS, et al. Mac Arthur Communicative Development Inventories: User's Guide and Technical Manual. San Diego, CA: Singular; 1993. [Google Scholar]
- 22.Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- 23.Fitzgibbon SP, Pope KJ, Mackenzie L, Clark CR, Willoughby JO. Cognitive tasks augment gamma EEG power. Clinical neurophysiology. 2004;115(8):1802–1809. doi: 10.1016/j.clinph.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Fries P. Neuronal Gamma-Band Synchronization as a Fundamental Process in Cortical Computation. Annual Review of Neuroscience. 2009;32:209–224. doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- 25.Fries P, Fernández G, Jensen O. When neurons form memories. Trends in Neurosciences. 2003;26(3):123–124. doi: 10.1016/S0166-2236(03)00023-7. [DOI] [PubMed] [Google Scholar]
- 26.Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of Oscillatory Neuronal Synchronization by Selective Visual Attention. Science. 2001;291(5508):1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 27.Gathercole SE, Baddeley AD. Evaluation of the role of phonological STM in the development of vocabulary in children:Alongitudinal study. Journal of Memory and Language. 1989;28:200–213. [Google Scholar]
- 28.Gathercole SE, Willis CS, Baddeley AD, Emslie H. The Children's Test of Nonword Repetition: a test of phonological working memory. Memory. 1994;2(2):103–127. doi: 10.1080/09658219408258940. [DOI] [PubMed] [Google Scholar]
- 29.Gray CM. The temporal correlation hypothesis of visual feature integration: still alive and well. Neuron. 1999;24(1):31–47. doi: 10.1016/s0896-6273(00)80820-x. [DOI] [PubMed] [Google Scholar]
- 30.Gray CM, Engel AK, König P, Singer W. Synchronization of oscillatory neuronal responses in cat striate cortex: temporal properties. Visual neuroscience. 1992;8(4):337–347. doi: 10.1017/s0952523800005071. [DOI] [PubMed] [Google Scholar]
- 31.Gray CM, König P, Engel AK, Singer W. Oscillatory response in the cat visual cortex exhibit intercolumnar synchronization which reflects global stimulus properties. Nature. 1989;338:334–337. doi: 10.1038/338334a0. [DOI] [PubMed] [Google Scholar]
- 32.Gruber T, Müller MM, Keil A. Modulation of induced gamma band responses in a perceptual learning task in the human EEG. Journal of cognitive neuroscience. 2002;14(5):732–744. doi: 10.1162/08989290260138636. [DOI] [PubMed] [Google Scholar]
- 33.Helps SK, Broyd SJ, James CJ, Karl A, Chen W, Sonuga-Barke EJ. Altered spontaneous low frequency brain activity in attention deficit/hyperactivity disorder. Brain Research. 2010 Mar 31;1322:134–143. doi: 10.1016/j.brainres.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 34.Herrmann CS. Human EEG responses to 1–100 Hz flicker: resonance phenomena in visual cortex and their potential correlation to cognitive phenomena. Experimental brain research. 2001;137(3–4):346–353. doi: 10.1007/s002210100682. [DOI] [PubMed] [Google Scholar]
- 35.Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clinical neurophysiology. 2005;116(12):2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Huttenlocher PR. Morphometric study of human cerebral cortex development. Neuropsychologia. 1990;28:517–527. doi: 10.1016/0028-3932(90)90031-i. [DOI] [PubMed] [Google Scholar]
- 37.Joanisse MF, Seidenberg MS. Phonology and syntax in specific language impairment: evidence from a connectionist model. Brain and language. 2003;86(1):40–56. doi: 10.1016/s0093-934x(02)00533-3. [DOI] [PubMed] [Google Scholar]
- 38.John ER, Ahn H, Pricep L, Trepetin M, Brown D, Kaye H. Developmental equations for the electroencephalogram. Science. 1980;210:1255–1258. doi: 10.1126/science.7434026. [DOI] [PubMed] [Google Scholar]
- 39.Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clinical Neurophysiology. 2000;111(10):1745–1758. doi: 10.1016/s1388-2457(00)00386-2. [DOI] [PubMed] [Google Scholar]
- 40.Keil A, Müller MM, Ray WJ, Gruber T, Elbert T. Human gamma band activity and perception of a gestalt. The Journal of Neuroscience. 1999;19(12):7152–7161. doi: 10.1523/JNEUROSCI.19-16-07152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khazipov R, Luhmann HJ. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends in Neurosciences. 2006;29(7):414–418. doi: 10.1016/j.tins.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- 43.Lippé S, Martinez-Montes E, Arcand C, Lassonde M. Electrophysiological study of auditory development. Neuroscience. 2009;164(3):1108–1118. doi: 10.1016/j.neuroscience.2009.07.066. [DOI] [PubMed] [Google Scholar]
- 44.Löwel S, Singer W. Selection of intrinsic horizontal connections in the visual cortex by correlated neuronal activity. Science. 1992;255:209–212. doi: 10.1126/science.1372754. [DOI] [PubMed] [Google Scholar]
- 45.Miltner WH, Braun C, Arnold M, Witte H, Taub E. Coherence of gamma-band EEG activity as a basis for associative learning. Nature. 1999;397:434–436. doi: 10.1038/17126. [DOI] [PubMed] [Google Scholar]
- 46.Müller MM, Bosch J, Elbert T, Kreiter A, Sosa MV, Sosa PV, Rockstroh B. Visually induced gamma-based responses in human electroencephalographic activity – a link to animal studies. Experimental brain research. 1996;12(1):96–102. doi: 10.1007/BF00227182. [DOI] [PubMed] [Google Scholar]
- 47.Newcomer PL, Hammill DD. Test of Language Development-Primary. 3rd ed. Austin: Tex: Pro-Ed; 1997. [Google Scholar]
- 48.Owens R. Language development: An introduction. 5th ed. Boston, Mass: Allyn and Bacon; 2001. [Google Scholar]
- 49.Ozdemir OM, Ergin H, Sahiner T. Electrophysiological assessment of the brain function in term SGA infants. Brain Research. 2009 May 13;1270:33–38. doi: 10.1016/j.brainres.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Pantev C, Makeig S, Hoke M, Galambos R, Hampson S, Gallen C. Human auditory evoked gamma-band magnetic fields. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(20):8996–9000. doi: 10.1073/pnas.88.20.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: A review of magnetic resonance studies. Brain research bulletin. 2001;54(3):255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- 52.Pesaran B, Pezaris JS, Sahani M, Mitra P, Andersen RA. Temporal structure in neuronal activity during working memory in macaque parietal cortex. Nature Neuroscience. 2002;5:805–811. doi: 10.1038/nn890. [DOI] [PubMed] [Google Scholar]
- 53.Pikovsky A, Rosenblum M, Kurths J. Synchronization: A Universal Concept in Nonlinear Sciences. Cambridge, UK: Cambridge University Press; 2003. [Google Scholar]
- 54.Pinker S, Ullman MT. The past and future of the past tense. Trends in cognitive sciences. 2002;6(11):456–463. doi: 10.1016/s1364-6613(02)01990-3. [DOI] [PubMed] [Google Scholar]
- 55.Pipa G, Städtler ES, Rodriguez EF, et al. Performance- and stimulus-dependent oscillations in monkey prefrontal cortex during short-term memory. Frontiers in Integrative Neuroscience. 2009:3–25. doi: 10.3389/neuro.07.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pujol J, Soriano-Mas C, Ortiz H, Sebastián-Gallés N, Losilla JM, Deus J. Myelination of language-related areas in the developing brain. Neurology. 2006;66:304–305. doi: 10.1212/01.wnl.0000201049.66073.8d. [DOI] [PubMed] [Google Scholar]
- 57.Pulvermüller F, Lutzenberger W, Preissl H, Birbaumer N. Spectral responses in the gamma-band – physiological signs of higher cognitive processes. Neuroreport. 1995;6:2059–2064. doi: 10.1097/00001756-199510010-00025. [DOI] [PubMed] [Google Scholar]
- 58.Revonsuo A, Wilenius-Emet M, Kuusela J, Lehto M. The neural generation of a unified illusion in human vision. NeuroReport. 1997;8:3867–3870. doi: 10.1097/00001756-199712220-00006. [DOI] [PubMed] [Google Scholar]
- 59.Ribary U, Ioannides AA, Singh KD, et al. Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. The Proceedings of the National Academy of Sciences of the United States of America. 1991;88:11037–11041. doi: 10.1073/pnas.88.24.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rice ML, Wexler K, Cleave PL. Specific language impairment as a period of extended optional infinitive. Journal of Speech and Hearing Research. 1995;38:850–863. doi: 10.1044/jshr.3804.850. [DOI] [PubMed] [Google Scholar]
- 61.Rodriguez E, George N, Lachaux JP, Martinerie J, Renault B, Varela FJ. Perception's shadow: long-distance synchronization of human brain activity. Nature. 1999;397:430–433. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- 62.Simos PG, Papanikolaou E, Sakkalis E, Micheloyannis S. Modulation of gamma-band spectral power by cognitive task complexity. Brain topography. 2002;14(3):191–196. doi: 10.1023/a:1014550808164. [DOI] [PubMed] [Google Scholar]
- 63.Singer W. Development and plasticity of cortical processing architectures. Science. 1995;270(5237):758–764. doi: 10.1126/science.270.5237.758. [DOI] [PubMed] [Google Scholar]
- 64.Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- 65.Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annual review of neuroscience. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- 66.Takano T, Ogawa T. Characterization of developmental changes in EEG gamma-band activity during childhood using the autoregressive model. Acta paediatrica Japonica. 1998;40(5):446–452. doi: 10.1111/j.1442-200x.1998.tb01966.x. [DOI] [PubMed] [Google Scholar]
- 67.Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. Journal of Neuroscience. 1996;16:4240–4249. doi: 10.1523/JNEUROSCI.16-13-04240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-band activity during the delay of a visual short-term memory task in humans. The Journal of neuroscience. 1998;18(11):4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taylor K, Mandon S, Freiwald WA, Kreiter AK. Coherent oscillatory activity in monkey area V4 predicts successful allocation of attention. Cerebral Cortex. 2005;15(9):1424–1437. doi: 10.1093/cercor/bhi023. [DOI] [PubMed] [Google Scholar]
- 70.Thorndike RL, Hagen EP, Sattler JM. The Stanford-Binet Intelligence Scale: Fourth edition. Chicago: Riverside; 1986. [Google Scholar]
- 71.Tiitinen H, Sinkkonen J, Reinikainen K, Alho K, Lavikainen J, Näätänen R. Selective attention enhances the auditory 40-Hz transient response in humans. Nature. 1993;364:59–60. doi: 10.1038/364059a0. [DOI] [PubMed] [Google Scholar]
- 72.Traub RD, Whittington MA, Stanford IM, fefferys JG. A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature. 1996;383:621–624. doi: 10.1038/383621a0. [DOI] [PubMed] [Google Scholar]
- 73.Uhlhaas PJ, Roux F, Rodriguez E, Rotarska-Jagiela A, Singer W. Neural synchrony and the development of cortical networks. Trends in cognitive sciences. 2010;14(2):72–80. doi: 10.1016/j.tics.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 74.Uhlhaas PJ, Roux F, Singer W, Haenschel C, Sireteanu R, Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. The Proceedings of the National Academy of Sciences of the United States of America. 2009;106(24):9866–9871. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ullman MT, Pierpont EI. Specific language impairments is not specific to language: the procedural deficit hypothesis. Cortex. 2005;41:399–433. doi: 10.1016/s0010-9452(08)70276-4. [DOI] [PubMed] [Google Scholar]
- 76.Ursino M, Magosso E, Cuppini C. Recognition of abstract objects via neural oscillators: interaction among topological organization, associative memory and gamma band synchronization. IEEE transactions on neural networks. 2009;20(2):316–335. doi: 10.1109/TNN.2008.2006326. [DOI] [PubMed] [Google Scholar]
- 77.van Daal J, Verhoeven L, van Leeuwe J, van Balkom H. Working memory limitations in children with severe language impairment. Journal of communication disorders. 2008;41(2):85–107. doi: 10.1016/j.jcomdis.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 78.Wiig EH, Secord WA, Semel E. Clinical Evaluation of Language Fundamentals-Preschool. San Antonio: Tex: The Psychological Corporation; 1992. [Google Scholar]
- 79.Woodcock RW. Woodcock Reading Mastery Tests-Revised examiners manual. Circle Pines, MN: American Guidance Service; 1987. [Google Scholar]
- 80.Zimmerman I, Steiner V, Pond R. Preschool Language Scale. 3rd ed. San Antonio: Tex: The Psychological Corporation; 1992. [Google Scholar]