Abstract
Aberrant smooth muscle cell (SMC) plasticity has been implicated in a variety of vascular disorders including atherosclerosis, restenosis, and abdominal aortic aneurysm (AAA) formation. While the pathways governing this process remain unclear, epigenetic regulation by specific microRNAs (miRNAs) has been demonstrated in SMCs. We hypothesized that additional miRNAs might play an important role in determining vascular SMC phenotype. Microarray analysis of miRNAs was performed on human aortic SMCs undergoing phenotypic switching in response to serum withdrawal, and identified 31 significantly regulated entities. We chose the highly conserved candidate miRNA-26a for additional studies. Inhibition of miRNA-26a accelerated SMC differentiation, and also promoted apoptosis, while inhibiting proliferation and migration. Overexpression of miRNA-26a blunted differentiation. As a potential mechanism, we investigated whether miRNA-26a influences TGF-β-pathway signaling. Dual-luciferase reporter assays demonstrated enhanced SMAD signaling with miRNA-26a inhibition, and the opposite effect with miRNA-26a overexpression in transfected human cells. Furthermore, inhibition of miRNA-26a increased gene expression of SMAD-1 and SMAD-4, while overexpression inhibited SMAD-1. MicroRNA-26a was also found to be downregulated in two mouse models of AAA formation (2.5- to 3.8-fold decrease, P < 0.02) in which enhanced switching from contractile to synthetic phenotype occurs. In summary, miRNA-26a promotes vascular SMC proliferation while inhibiting cellular differentiation and apoptosis, and alters TGF-β pathway signaling. MicroRNA-26a represents an important new regulator of SMC biology and a potential therapeutic target in AAA disease.
Smooth muscle cell (SMC) pathology is crucial to the progression of almost all vascular disorders (Thompson et al., 1997; Doran et al., 2008; Inoue and Node, 2009). The vascular SMC is unusual among muscle cells in its lack of terminal differentiation, possessing the ability to assume a proliferative, migratory phenotype capable of synthesizing extracellular matrix (ECM) (Owens, 1995). In conditions such as atherosclerosis or post-angioplasty restenosis, SMC proliferation and increased cell cycling are viewed as deleterious and maladaptive (Cai, 2006). In contrast, SMC re-growth is considered desirable during aneurysmal dilation, which is typified by medial thinning (Hoshina et al., 2004).
SMC phenotypic modulation occurs in response to a variety of stimuli, including growth factors, reactive oxygen species, mechanical stress, and changes in extracellular structural elements (Owens, 1995; McDonald and Owens, 2007). Despite considerable progress, no definitive master regulators of SMC plasticity have yet been identified.
In the last decade, it has become clear that endogenous methods of post-transcriptional genetic regulation exist, and that these mechanisms play a significant role in determining cell fate and behavior (Fazi and Nervi, 2008). MicroRNAs (miRNAs) are single-stranded, non-coding RNAs ~ 22 nucleotides in length, that typically function to induce degradation or translational repression of specific target mRNAs (Li et al., 2008; Boettger et al., 2009). Despite the existence of fewer than 1,000 known human miRNAs (Kong et al., 2009), it is now apparent that more than 30% of the genome is regulated in this way (Gartel and Kandel, 2008). Functionally, miRNAs participate in every major cellular process, and have been implicated in conditions such as cancer, heart disease, and developmental abnormalities (Asli et al., 2008; Gartel and Kandel, 2008). In the vasculature, miRNAs regulate angiogenesis, endothelial cell function, and vascular inflammation (Urbich et al., 2008). However, little is known about the specific effects of miRNAs in SMCs. The majority of studies to date have focused on a handful of largely pro-differentiation miRNAs and their role in neointimal formation (Li et al., 2008; Boettger et al., 2009; Liu et al., 2009; Zhang, 2009).
To increase our understanding of vascular SMC pathobiology, we have performed the first microarray study of miRNA expression in differentiating, growth-arrested human aortic SMCs. As detailed below, we identified a group of miRNAs that are differentially regulated during this process. A promising, largely un-studied candidate—miRNA-26a—was found to inhibit SMC differentiation and apoptosis while promoting proliferation and migration, possibly through a mechanism that targets TGF-β/BMP pathway signaling. MicroRNA-26a was also significantly downregulated in developing murine abdominal aortic aneurysms (AAAs) in vivo, and may represent a novel therapeutic target in conditions of pathological aneurysmal dilation.
Materials and Methods
Cell culture
Human aortic SMCs (Lonza, Walkersville, MD, passage #3) were propagated in growth media SmGM-2 (Lonza) in 5% fetal bovine serum (FBS) as per manufacturer’s instructions. Control plates were harvested at 80% confluence. Serum-starved plates were incubated for 48 h (SS48) or 72 h (SS72) in serum-free basal media (SmBM) to induce growth arrest and expression of SMC differentiation genes, according to conventional protocols (Owens et al., 1986; Fager et al., 1989). Experimental plates were harvested for RNA or protein analysis at ~90% confluence. Samples included cells from two separate lots, as well as two technical replicates per vial per time point.
Human embryonic kidney (HEK)-293 cells (ATCC, Manassas, VA) were propagated in DMEM supplemented with 10% FBS, l-glutamine, sodium pyruvate, and antibiotics (Invitrogen, Carlsbad, CA) and used in transfection protocols described below.
MicroRNA and total RNA preparation, quantitative reverse-transcription PCR
Total RNA was isolated using the miRNeasy Mini Kit (Qiagen, Valencia, CA) as per standard methods. RNA was quantitated by Nanodrop, and RNA and miRNA quality were verified using the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA—all sample RNA integrity numbers > 9). For quantitation of gene transcription, cDNA was generated with M-MuLV reverse transcriptase, and then amplified on the ABI PRISM 7900HT with Taqman primers/probes (Applied Biosystems, Foster City, CA). Probes included smooth muscle α-actin (ACTA2), smooth muscle myosin heavy chain (MYH11), transgelin (TAGLN), SMAD family members 1 and 4 (SMAD1 and SMAD4), epidermal growth factor receptor (EGFR), and high mobility group AT-hook 2 (HMGA2) all normalized to 18S internal controls.
MicroRNAs were quantitated using Taqman miRNA Reverse Transcription Kits and Taqman MicroRNA Assays (Applied Biosystems) or the GenoExplorer miRNA qRT-PCR Kit (GenoSensor Corp., Tempe, AZ). Samples were normalized to endogenous controls rnu48 or 5S RNA (human) and sno202 (mouse). All fold changes were calculated by the method of ΔΔCt.
MicroRNA array hybridization and analysis
RNA samples were pooled by treatment group and hybridized to the Agilent Human miRNA 8×15k Array (v2) G4470B as per manufacturer’s protocol. This array contains probes for 723 human microRNAs from the Sanger database v.10.1. Arrays were scanned using an Agilent Microarray Scanner and Feature Extractor (software v 9.5.1). Array results were confirmed for miRNA-26a and miRNA-663 by qRT-PCR.
Array analysis
QC reports were generated by the Feature Extraction software, and the array data were analyzed with Genespring GX 10.0.2 (Agilent). Arrays were examined with QC metrics, principal components analysis (PCA), and hierarchical agglomerative clustering (HAC). One chip (six arrays) clustered separately and was therefore excluded. Two additional arrays were excluded for QC reasons. The remaining 10 arrays were subjected to one-way ANOVA (cutoff of P < 0.05), with Benjamini–Hochberg correction for multiple testing.
Significant miRNAs were required to show >2.0-fold expression change from control at one of the time points. Confirmatory pairwise analysis was performed between the control and 72 h time points using statistical analysis of microarrays (SAMs) with false detection ratio (FDR) < 1 (Tusher et al., 2001). Targetscan 5.1 (Friedman et al., 2009) and Pictar (Krek et al., 2005) were used to identify putative miRNA gene targets. Enrichment for KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway categories and Gene Ontology (GO) biological process categories were determined using DAVID (NCBI), with P < 0.05 cutoff. All microarray data were submitted to the National Center for Biotechnology Information’s Gene Expression Omnibus (GSE19544; http://www.ncbi.nlm.nih.gov/geo/).
Targeted miRNA inhibition and overexpression
MicroRNA-26a was inhibited with an antisense antagomiR (Applied Biosystems). SMCs were transfected with high efficiency using the Amaxa Nucleofector system (Lonza) employing protocol U-025. Successful transfection (>85% of all cells) was confirmed by visual fluorescent microscopic analysis and fluorescence-activated cell sorting (FACS) sorting for fluorescently labeled control anti-miR. The in vitro experiments detailed below included an untransfected cohort of cells, and cohorts transfected with 100 nM of a probe directed against miRNA-26a (AM10249) or a validated Cy3-labeled control probe known to have no effect on any human miRNA (AM17011), as well as a cohort transfected with miRNA-let7c as a positive control. Overexpression studies utilized 100nM of hsa-miRNA-26a Pre-miR miRNA Precursor (Ambion—AM17100), in parallel with positive (pre-miR hsa-miRNA-1) and negative controls from the Pre-miR miRNA Precursor Starter Kit (Ambion—AM1540).
Boyden chamber chemotaxis assays
To analyze SMC migration, a modified Boyden chamber assay was performed, as previously described (Ishida et al., 2006). Briefly, 6-well trans-well migration chambers with 8 µm pores (Becton Dickinson (BD), Franklin Lakes, NJ) were employed. AntagomiR-transfected human aortic SMCs (5 × 104 cells/well) were serum starved in SmBM for 24 h and then plated in the upper chamber in 1ml of SmBM. Two milliliters of SmGM-2 media were added to the lower chamber, and the cells were allowed to migrate for 24 h at 37°C. Cells that migrated to the lower chamber were fixed in methanol, stained with 0.1% crystal violet and manually counted in a blinded fashion (eight high-power fields/well).
Proliferation and cell survival assays
A modified MTT (3-[4,5-dimethyl-thiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay was performed to analyze SMC viability, as previously described (Erl et al., 2003). AntagomiR-transfected SMCs were grown in 96-well plates for 24–36 h and then incubated for 4 h in the presence of 10µl of MTT AB solution (Millipore, Billerica, MA). The formazan product was dissolved by addition of 100 µl acidic isopropanol (0.04N HCl) and absorbance was measured at 570 nm (reference wavelength 630 nm) on an ELISA plate reader.
Apoptosis assays
Programmed cell death rates were assessed with a commercially available apoptosis assay (BD). AntagomiR-transfected human aortic SMCs were treated with 10% H2O2 in serum free media for 6 h. Then, 1 × 105 cells were harvested and stained with 10 µl FITC Annexin V and 10µl propidium iodide and FACS sorted within 1 h (BD FACSCaliber, 530 nm [FL1] and >575 nm [FL3]).
Western blots
Transfected human aortic SMCs were serum starved in SmBM for 48 h and then treated with transforming growth factor-β (5 ng/ml, hTGF-β1, R&D Systems, Minneapolis, MN) for an additional 24 h. Total cellular protein was isolated and analyzed as previously described (Yue et al., 2010). Cell lysates were subjected to Western blotting with primary antibodies directed against human SMAD-1 (1:500), and SMAD-4 (1:1,000) (Abcam, Cambridge, MA) and an HRP-conjugated goat–anti-rabbit secondary antibody (1:2,000) (Cell Signaling, Danvers, MA). Exposed films were scanned, and integrated band densities were obtained after background subtraction using ImageJ software in a blinded fashion.
SMAD reporter assays
TGF-β-induced signal transduction was probed using a SMAD reporter construct Cignal Reporter Assay Kit (SABiosciences, Frederick, MD—CCS-017L), which encodes the firefly luciferase reporter gene under the control of a minimal (m)CMV promoter and tandem repeats of the SMAD transcriptional response element (TRE). Firefly reporters came premixed with separate plasmids constitutively expressing Renilla luciferase. Negative and positive reporter controls and SMAD reporter with and without pre-miR precursors (pre-miRNA-26a or negative control), and antagomiRs (anti-miRNA-26a or negative control) were transfected in triplicate into both human aortic SMCs and HEK-293 cells with Lipofectamine 2000 as per manufacturer’s protocol (Invitrogen 11668-019). After 6 h of transfection, the media were changed to Opti-MEM I + 1% NEAA (Invitrogen), with or without hTGF-β1 at 2.5 ng/ml. Cells were then harvested after 18 h and analyzed by Dual-Luciferase Reporter Assay (Promega, Madison, WI) per kit protocol. Results were normalized to basal activity of untreated SMAD reporter.
Murine AAA models
All protocols were approved by the Administrative Panel on Laboratory Animal Care at Stanford University (http://labanimals.stanford.edu/). Both the porcine pancreatic elastase (PPE) and the ApoE−/−/Angiotensin II (AngII) models were employed in this study to evaluate two relevant models of AAA disease.
First, male ApoE−/− mice (C57BL/6J background) (n = 4/group), aged 10 weeks old (25–30 g, Jackson Laboratories, Bar Harbor, ME) underwent AAA creation via elastase infusion, as previously described (Azuma et al., 2009). Briefly, the infra-renal abdominal aortas were isolated and temporary ligatures were applied. Saline solution containing 4.5U/ml type 1 PPE (E-1250; Sigma, St. Louis, MO) was instilled for 5 min at 100 mmHg. The aortotomy was closed with 10–0 suture, and aortic flow restored. Mice were euthanized at time 0, 3 days, and 7 days, and harvested aortas were immediately flash-frozen in liquid nitrogen. Standard immunohistochemistry analysis employing a monoclonal SMC α-actin antibody (Clone A14; Sigma) was performed (Chun et al., 2008).
A second cohort of male ApoE−/− (C57BL/6J background) mice were exposed to continuous AngII infusions (1.4 mg/kg/day, Sigma) via subcutaneous osmotic minipumps (model 2004, Alzet, Cupertino, CA) (Chun et al., 2008). In a separate study, whole genome transcriptional profiling of supra-renal abdominal aortic tissue was performed to identify expression changes versus saline control at the 1-week time point (Spin et al., 2009). Genes upregulated with AngII at day 7 were cross-referenced against the putative target list for miRNA-26a. Using DAVID, we identified overlapping GO biological process categories within the common gene lists.
Statistical analysis
Data are presented as mean ± SEM. All in vitro experiments included at least four replicates per group. Data were subjected to the Kolmogorov–Smirnov test to determine distribution. Groups were compared using the two-tailed Student’s t-test for parametric data. A value of P < 0.05 was considered statistically significant. Data analysis was performed on GraphPad Prism 3.02 software.
Results
SMC differentiation, gene expression, and miRNA modulation with serum starvation
As expected, serum-starved human aortic smooth muscle cells (AoSMC) showed significant and generally progressive increases in the expression of differentiation marker genes by qRT-PCR at 48 and 72 h, including smooth muscle myosin heavy chain (9.2- and 37.8-fold), SM α-actin (15.6- and 20.8-fold), and SM22α/transgelin (2.3- and 2.0-fold) (P < 0.001 for all comparisons vs. control).
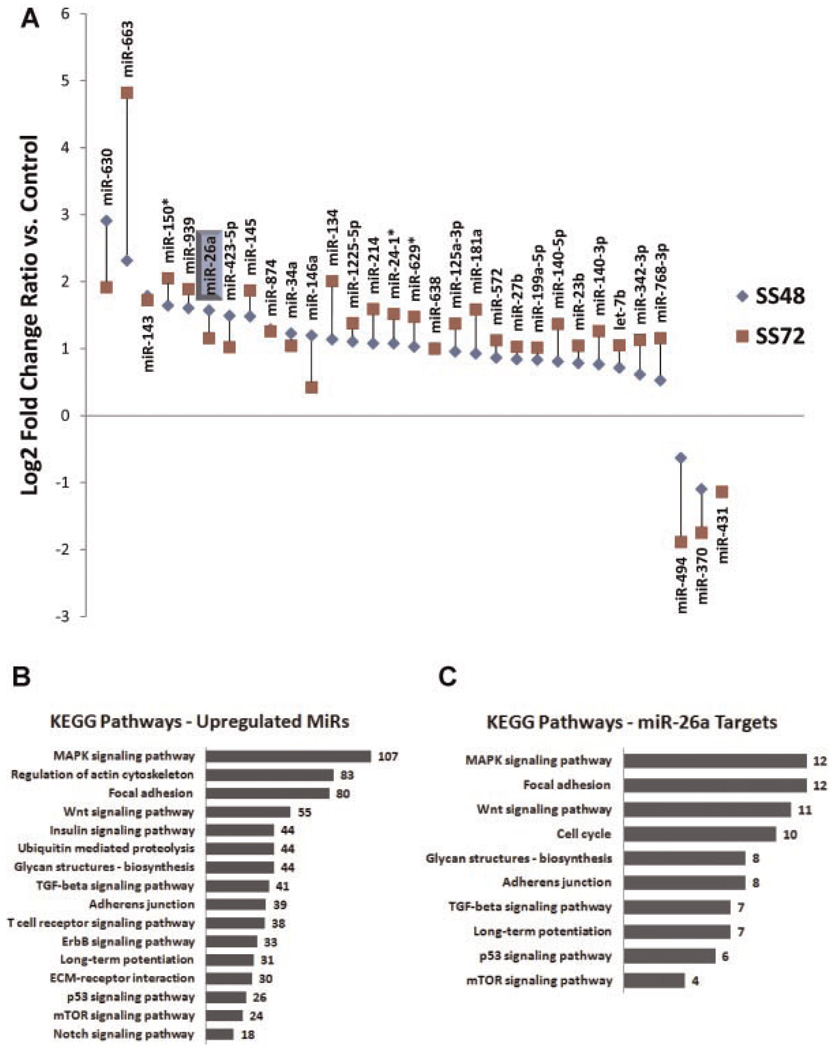
MicroRNA array analysis comparing control (serum-fed) and serum-starved samples (SS48 and SS72) yielded 135 significantly regulated miRNAs. Thirty-one of these miRNAs had fold changes >2.0 (28 upregulated and 3 downregulated), and each of these was also significant at FDR < 1 by SAM (Fig. 1A and Supplementary Table 1A). Most changes occurred in a progressive fashion over time (71% showed higher fold changes vs. control at 72 h than at 48 h).
Fig. 1.
MicroRNAs are differentially regulated during SMC differentiation and are predicted to target genes relevant to vascular biology. A: After 48 h (SS48) and 72 h (SS72) of serum starvation, 31 miRNAs were significantly modulated. The expression change (log 2) for each regulated miRNA is detailed in part A, ordered by fold change. See text for statistics. MicroRNA-26a is highlighted. B: Analysis of the conserved putative target lists for all 28 upregulated miRNAs (using Targetscan, Pictar, and DAVID) revealed several significantly enriched KEGG pathways (P < 0.05). Number of target genes/category shown. C: Analysis of the conserved putative targets of miRNA-26a reveals several enriched KEGG pathways (P < 0.05). Number of target genes/category shown.
Treatment groups showed clear separation with PCA and HAC clustering algorithms (Supplementary Fig. 1A). A few miRNA known to be associated with vascular gene regulation and with SMCs in particular were differentially regulated by both methods used, but only passed a 1.5-fold change cutoff, including miRNA-21 (1.6-fold increased at 72 h), and miRNA-221 (1.5-fold decreased at 72 h). Another established SMC differentiation-related miRNA, miRNA-145, was up 3.6-fold at 72 h, and was the highest-ranked miRNA by SAM.
Targetscan and Pictar were used to identify putative conserved gene targets of the differentially upregulated miRNAs. Resultant gene lists were probed for significantly overabundant canonical pathways (KEGG) and GO biological process groups using DAVID (Fig. 1B) (Huang da et al., 2007, 2009). Apart from miRNA-145, miRNA-26a was the highest-ranked (by SAM) feature for both time-point comparisons that was enriched for putative targets likely related to SMC biology, atherosclerosis, and aortic aneurysm formation, including genes from the cell cycling, TGF-β, cell adhesion, p53, and Wnt pathways among others (Fig. 1C). In particular, miRNA-26a is believed to regulate SMAD-1 (Luzi et al., 2008), and also has SMAD-4 as a putative target. We confirmed the expression levels for miRNA-26a (1.54-fold increase vs. serum fed [range 1.2–2.1]) with 72 h of serum starvation by Taqman/qRT-PCR in aortic SMC from three separate individuals.
MicroRNA-26a regulates several aspects of SMC biology including proliferation, differentiation, migration, and apoptosis
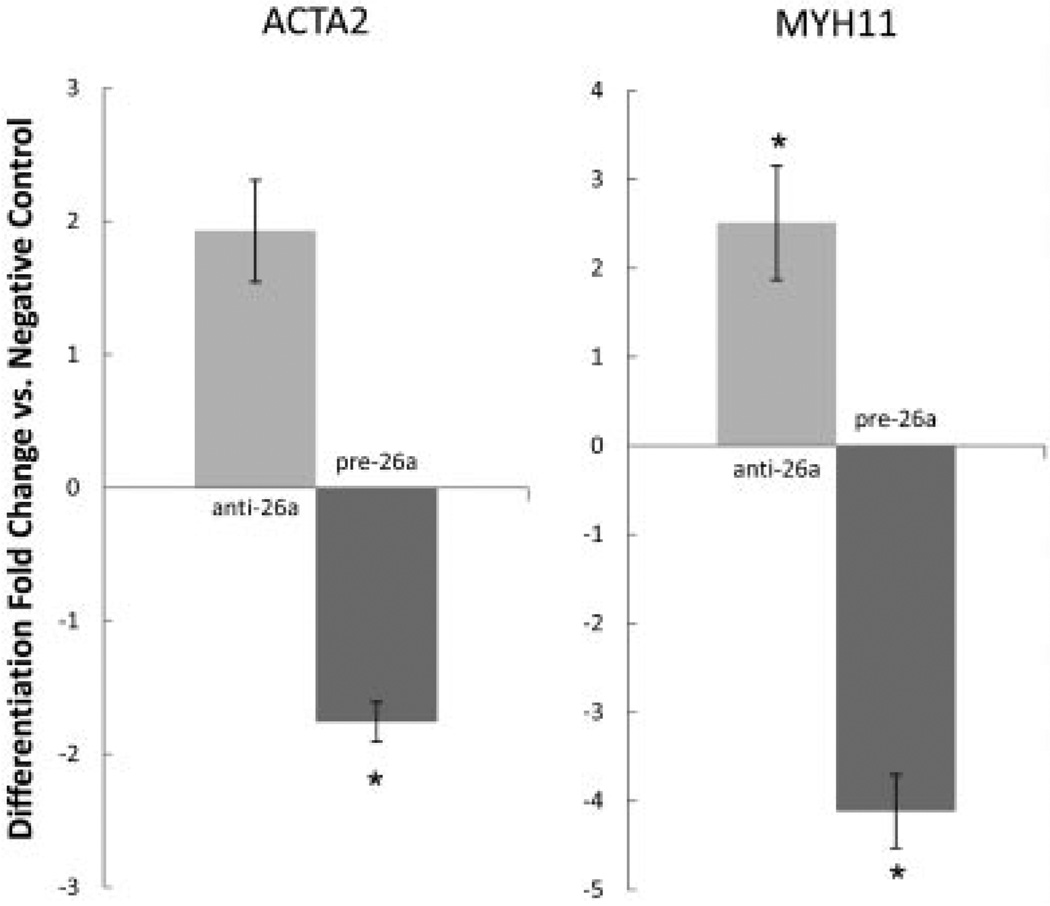
Because miRNA-26a was significantly upregulated during SMC differentiation and was predicted to regulate critical cell-fate pathways, we next investigated the physiological properties of this miRNA in a series of loss-of-function experiments. Interestingly, human aortic SMCs deficient in miRNA-26a actually demonstrated a modest acceleration in appearance of differentiation markers relative to control cells (Fig. 2). Cells transfected with the anti-miRNA-26a antagomiR revealed a more pronounced rise in both smooth muscle myosin heavy chain (MYH11) expression (25.7 ± 8.2 vs. 9.4 ± 2.7-fold increase, P < 0.05) and smooth muscle α-actin (ACTA2) (3.3 ± 0.6 vs. 2.7 ± 0.9-fold increase) after 72 h of serum starvation, compared to cells transfected with a control antagomiR. Further, aortic SMCs transfected with miRNA-26a pre-miR precursor and serum starved for 72 h displayed significant blunting of the usual increase in differentiation marker expression when compared with transfected negative control pre-miR (MYH11: 4.2 ± 0.4 vs. 16.8 ± 2.6-fold increase, P < 0.001; ACTA2: 9.2 ± 0.7 vs. 16.0 ± 0.5-fold increase, P < 0.05).
Fig. 2.
MicroRNA-26a regulates the rise of SMC markers during serum starvation induced differentiation over 72 h. Left: Smooth muscle α-actin (ACTA2) expression is enhanced by anti-miRNA-26a, and blunted by miRNA-26a pre-miR precursor when compared to negative control antagomiR and pre-miR. Right: Similar findings are seen for smooth muscle myosin heavy chain (MYH11) expression. *P < 0.05.
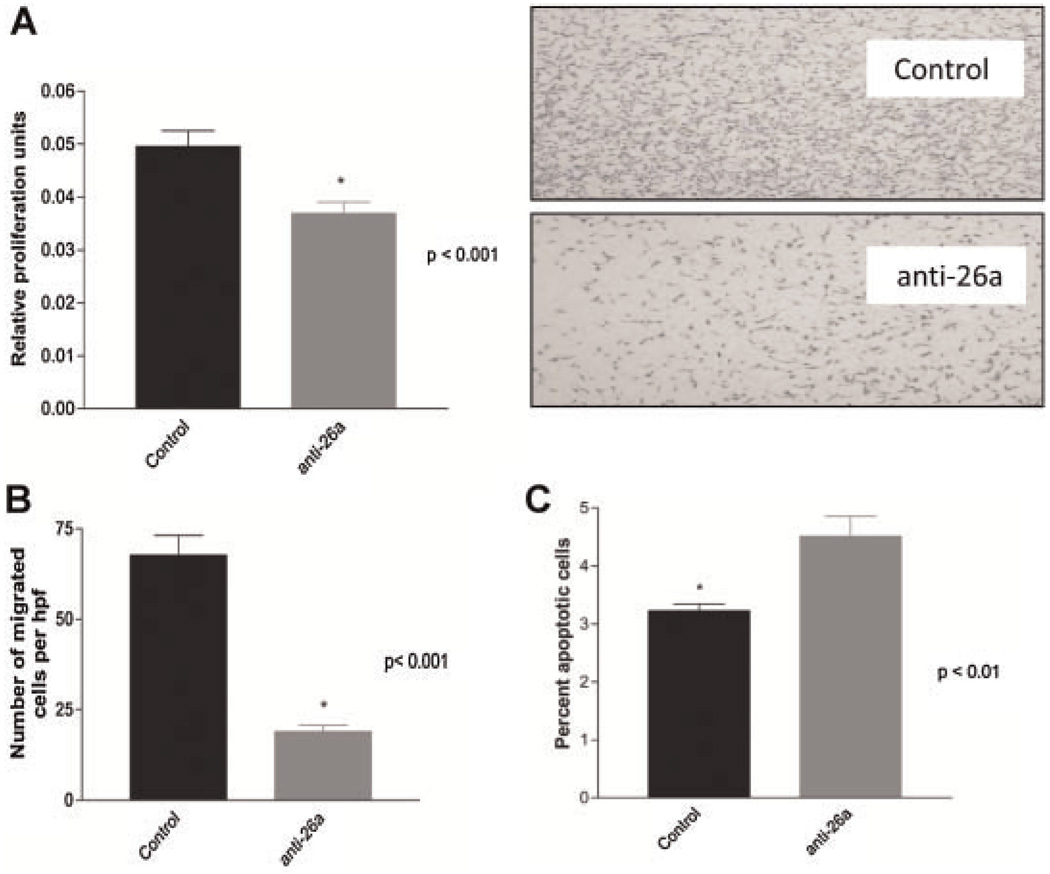
In keeping with this anti-differentiation effect, aortic SMCs deficient in miRNA-26a also revealed significant reductions in proliferation (0.037 ± 0.002 vs. 0.050 ± 0.002 relative proliferation units, P < 0.001) and migration (18.9 ± 1.8 vs. 67.6 ± 5.5 cells/hpf, P < 0.001), and a significant increase in the rate of H2O2-induced apoptosis (4.5 ± 0.3% vs. 3.2 ± 0.1%, P < 0.01), relative to control transfected cells (Fig. 3A–C).
Fig. 3.
MicroRNA-26a regulates multiple aspects of SMC phenotype. Relative to control-transfected cells, human aortic SMC deficient in miRNA-26a display. A: Reduced rates of cellular proliferation. B: Reduced rates of cellular migration (representative Boyden chambers shown top right, 6 × magnification). C: Enhanced rates of apoptosis.
MicroRNA-26a regulates the TGF-β signaling cascade
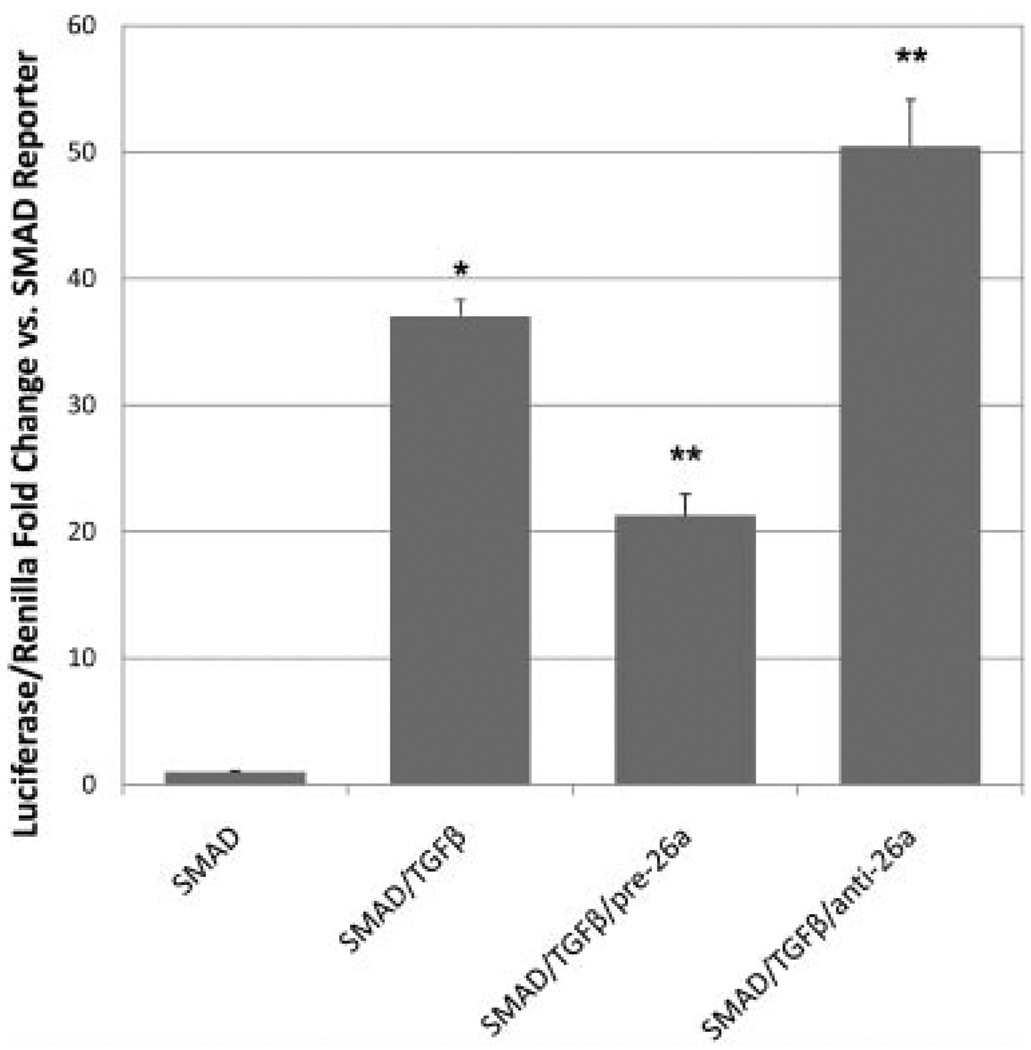
In order to elucidate the mechanism by which miRNA-26a modulates SMC processes, we assessed the effect of miRNA-26a on the pro-differentiation TGF-β cascade (Fig. 4). Cells were co-transfected with a SMAD firefly luciferase reporter, a constitutively expressing Renilla luciferase reporter, and either miRNA-26a antagomiR or pre-miR precursor. Success of the transfection protocol was confirmed using green fluorescent protein-expressing positive controls.
Fig. 4.
MicroRNA-26a controls the activity of a TGF-β-activated SMAD reporter element. HEK-293 cells were dual-transfected with firefly luciferase SMAD reporter/constitutively expressing Renilla luciferase and either miRNA-26a antagomiR or pre-miR precursor, and treated with TGF-β. As expected, TGF-β treatment increased transcription of the SMAD-responsive TREs (SMAD/TGFβ), *P < 0.05 versus untreated reporter (SMAD). Addition of pre-miRNA-26a (SMAD/TGFβ/pre-26a) caused a significant drop in signaling compared with TGF-β-treated cells, while miRNA-26a antagomiR (SMAD/TGFβ/anti-26a) led to a significant activity increase, **P < 0.05.
As expected, basal TGF-β pathway activity in growing AoSMC and HEK-293 cells was quite low, and comparable to negative control reporter. Addition of hTGF-β1 at 2.5 ng/ml to HEK-293 cells led to a substantial (~37-fold) increase in SMAD reporter activation. AntagomiR inhibition of miRNA-26a combined with TGF-β in HEK-293 cells caused a significant increase in SMAD activity beyond that seen with TGF-β alone (1.36-fold, P < 0.05), while pre-miR-induced overexpression of miRNA-26a significantly decreased SMAD activity levels (−1.74-fold, P < 0.05). MicroRNA-26a antagomiR and pre-miR precursor transfection had no impact on basal SMAD activity in the absence of TGF-β, implying that this mechanism requires pathway activation by TGF-β in order to exert an effect. Negative control pre- and antagomiRs did not significantly alter reporter activity (Supplementary Fig. 2). Despite lower SMAD-reporter luciferase signal levels in AoSMCs, the reporter activity was similar to the pattern observed for HEK-293 cells (data not shown).
MicroRNA-26a targets the expression of SMAD-1 and SMAD-4, members of the TGF-β superfamily signaling cascade
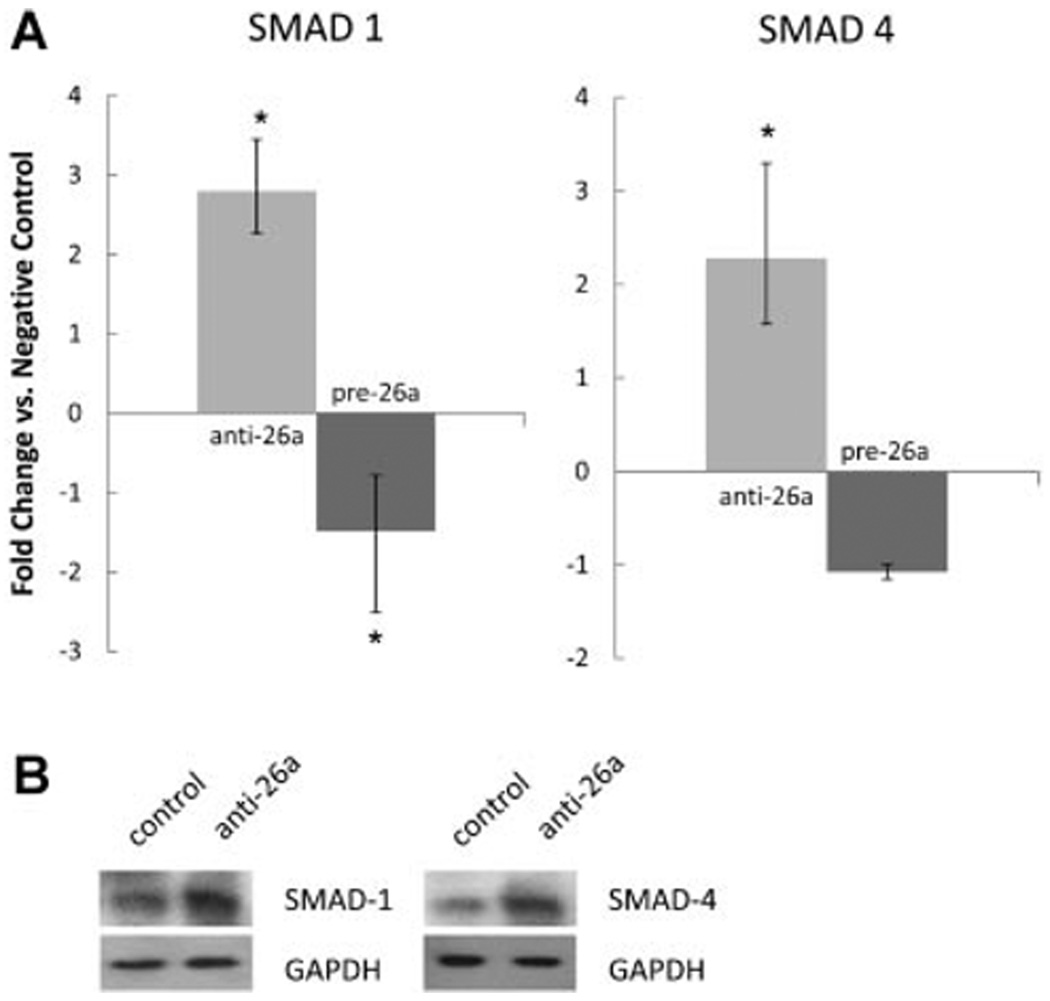
Because miRNA-26a has been predicted to target SMAD-1 and SMAD-4, we next assessed the direct effects of miRNA-26a on the expression of these pro-differentiation TGF-β/BMP cascade molecules (Fig. 5A). Compared to negative control transfected cells, SMC transfected with anti-miRNA-26a antagomiR displayed increases in the expression of both SMAD1 (2.3- to 3.5-fold-change range, P < 0.05) and SMAD4 (1.6- to 3.3-fold-change range, P < 0.05) after 72 h of serum starvation. Negative control antagomiR caused no change in SMAD-1/4 expression, while transfection with anti-miRNA-let7c as a positive control caused a 2.0- to 5.1-fold-change range increase of HMGA2 (P < 0.05).
Fig. 5.
A: SMAD-1 and SMAD-4 are putative targets of miRNA-26a. Human aortic SMCs deficient in miRNA-26a display enhanced expression ofSMAD-1 and SMAD-4, while overexpression of miRNA-26a lowers expression ofSMAD-1 (*P < 0.05). Error bars indicate fold-change range derived per protocol (Applied Biosystems). B: Representative Western blots confirm increased SMAD-1/4 levels with inhibition of miRNA-26a in cells treated with 5 ng/ml of TGF-β. Left lanes: negative control antagomiR. Right lanes: anti-miRNA-26a.
In keeping with the results of these knockdown experiments, overexpression of miR-26a decreased SMAD-1 expression compared with negative control pre-miR precursor transfected cells an average of 1.5-fold (P < 0.05), although SMAD-4 expression did not change significantly. No change was seen between negative control pre-miR precursor and mock-transfected cells for SMAD-1/4. For comparison, transfected positive control pre-miR-1 decreased its known target EGFR 1.7-fold versus negative control pre-miR precursor (P < 0.05).
To confirm this effect at the protein level, transfected AoSMCs were subsequently treated with TGF-β and then harvested for Western blot analysis. One day after treatment, cells deficient in miRNA-26a displayed a qualitative increase in the rate of SMAD-1 and SMAD-4 expression after TGF-β exposure (Fig. 5B). Densitometric analysis of these blots confirmed a 1.59-fold greater expression of SMAD-1 protein and a 1.16-fold greater expression of SMAD-4 protein in cells treated with 5 ng/ml of TGF-β.
MicroRNA-26a is downregulated during AAA formation
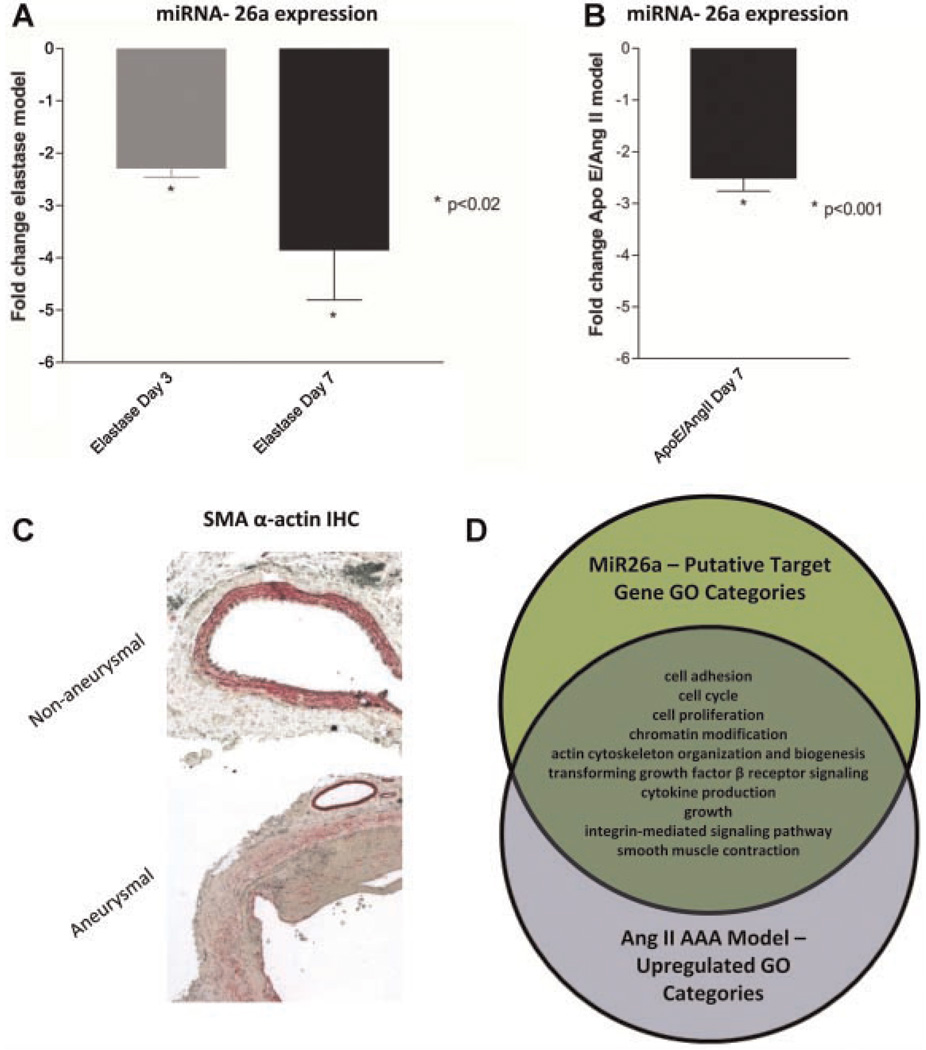
We next evaluated the expression of miRNA-26a in two murine AAA development models to extend our in vitro findings in vivo. Compared to baseline, miRNA-26a expression in the aneurysm wall was reduced significantly 3 days after infusion in the elastase model (2.3-fold by qRT-PCR, P < 0.001), and this reduction was accentuated further at 7 days (3.8-fold, P < 0.02) (Fig. 6A). Similar results were observed in the AngII–ApoE−/− AAA model, where miRNA-26a was also significantly downregulated within the suprarenal aortic wall 1 week after beginning AngII infusion (vs. saline infusion, 2.5-fold, P < 0.001, Fig. 6B).
Fig. 6.
MicroRNA-26a is downregulated during AAA formation. A, B: Compared to baseline, miRNA-26a expression is significantly decreased in both the murineelastase aneurysm model (A) and the ApoE−/−/Ang II aneurysm model (B) during lesion progression. C: Loss of miRNA-26a occurs at a time point when SMC de-differentiation is pronounced. Representative micrographs of non-dilated vessels (top) and day 7 elastase-treated aneurysmal aortas (bottom) reveal arelative deficiency of SM-α actin-expressing medial SMCs as AAAs develop (20 × magnification, IHC for SM-α actin in red). Note the preserved staining in the unaffected vasavasorum of the aneurysmal vessel. D: Predicted miRNA-26a targets are differentially regulated during AAA progression. The Venn diagram demonstrates overlap of Gene Ontology categories between miRNA-26a target genes and genes significantly upregulated during AAA development in the ApoE−/−/AngII model, minimum 2 genes/category.
Histopathological analysis revealed that the observed decrease in miRNA-26a expression coincided temporally with decreased SM α-actin staining, and vessel dilation (Fig. 6C) (Van Vickle-Chavez et al., 2006). In addition, the expression of potentially related microRNA miRNA-21 was increased in the elastase model of AAA (2.5-fold increase at day 3, P < 0.001; 3.9-fold increase at day 7).
The list of conserved putative miRNA-26a target genes was cross-referenced against a list of aortic genes, we found to be upregulated in the AngII–ApoE−/− model AAA wall at 7 days, in which classic as well as novel genes were identified (e.g., Spp1, Ctgf, Mmp2, Mmp3, and thousands more—full article under review) (Spin et al., 2009). Of 535 predicted conserved miRNA-26a target genes, 124 were increased during AAA formation (including such genes as Smad1 and Smad4, Loxl2, and Inhbb). As shown in Figure 6D, many of these genes were members of the cell cycling, apoptosis, proliferation/migration, cytokine production, and TGF-β receptor pathway signaling GO categories, linking miRNA-26a more closely to disease-relevant vascular SMC processes.
Discussion
Smooth muscle cell plasticity is a critical determinant of vessel wall disease. The ability of these cells to modulate their phenotype from a quiescent, contractile state to a proliferative, synthetic profile has been implicated in conditions such as atherosclerosis, post-angioplasty restenosis, and aortic aneurysm formation (Thompson et al., 1997; Hoshina et al., 2004; Cai, 2006; Doran et al., 2008; Inoue and Node, 2009). While some of the basic aspects underlying regulation of SMC phenotypic change have been elucidated, the ways in which miRNAs might initiate or modify the process are not well understood.
We sought to clarify this question by performing the first in vitro miRNA microarray analysis of human vascular smooth muscle cells induced to differentiate via serum starvation/growth arrest. Employing an agnostic whole-genome approach, we identified 28-upregulated and 3-downregulated miRNAs that were significantly and sustainably altered during the differentiation process.
As expected, several miRNAs known to be involved in vascular physiology were found to be differentially regulated with serum starvation, including miRNA-145, miRNA-21, and miRNA-221 (although of these only miRNA-145 showed > 2.0-fold change vs. control). However, the majority of the significantly regulated miRNAs, we identified have never previously been implicated in SMC biology. Bioinformatic analysis revealed that the putative targets of the upregulated miRNAs were enriched for several ontologic pathways relevant to SMC phenotypic change and disease, including cytoskeletal regulation, Wnt and TGF-β pathways, p53 signaling, and cell–matrix interactions (Fig. 1).
Among the highest-ranked significant genes was miRNA-26a. Ontologic analysis singled out this miRNA as a particularly interesting candidate. Given its highly conserved status (in contrast to the most highly regulated gene, miRNA-663, which does not exist in the mouse or rat) and large number of predicted targets within SMC-related pathways (Fig. 1), we surmised that miRNA-26a could be a key regulator of SMC physiology.
Despite being significantly upregulated during SMC differentiation, we found that knockdown of miRNA-26a actually accelerated the appearance of SMC differentiation marker genes with serum starvation, while overexpression of miRNA-26a blunted marker appearance. We postulate that the differentiation-associated upregulation of miRNA-26a might be a compensatory negative-feedback mechanism, and that miRNA-26a actually serves as an inhibitor of SMC differentiation. In keeping with this hypothesis, we found that cells deficient in miRNA-26a displayed significantly decreased rates of cell cycling, and were less able to effectively migrate toward a growth factor/serum gradient relative to control cells. Furthermore, cells deficient in miRNA-26a displayed significantly enhanced rates of programmed cell death.
Given its predicted gene targets, miRNA-26a may alter vascular SMC biology in part via an inhibitory effect on the signaling pathways downstream of the TGF-β/BMP superfamily of growth factors. Although complementary base pair modeling with thermodynamic stability calculations predicts hundreds of potential targets for miRNA-26a-induced translational repression, two of the most strongly predicted targets for degradation include the TGF-β and bone morphogenetic pathway (BMP)-related pro-differentiation factors, SMAD-1 and SMAD-4. Prior evidence suggested that miRNA-26a inhibits SMAD-1 (Luzi et al., 2008). We confirmed this interaction in SMCs, observing that cells deficient in miRNA-26a expressed more SMAD-1 and SMAD-4 than cells with intact miRNA-26a both at the RNA and protein level. Similarly, overexpression of miRNA-26a suppressed the expression of SMAD-1, although not SMAD-4. Solidifying this link, we found that miRNA-26a inhibited the activity of a SMAD2/3/4-reporter, confirming that miRNA-26a directly modulates the TGF-β signaling cascade.
These effects on the TGF-β pathway, and on SMC proliferation, migration, and apoptosis in particular, suggested that miRNA-26a could be important in AAA development. SMCs display profound de-differentiation early in the formation of experimental animal AAAs (Ailawadi et al., 2009). Some SMC apoptosis also occurs in both human AAA and murine AAA models (Thompson et al., 1997; Henderson et al., 1999; Sho et al., 2005). It was therefore interesting to find that miRNA-26a is significantly and progressively downregulated in two independent mouse models of AAA disease, and that loss of miRNA-26a expression coincides temporally with loss of medial SM α-actin staining and radial aortic dilation, consistent with our in vitro results. It should be noted that other cell types within the aortic wall may have contributed to the observed gene signal, and that some SMC cell loss does occur during AAA formation. However, medial SMC still represent the majority of the cells in the post-treatment aortic wall. MicroRNA-26a has several putative gene targets tied to apoptosis. Notably three of these, BAK1 (BCL2-antagonist/killer 1), PAK2 (p21 protein (Cdc42/Rac)-activated kinase 2), and SULF1 (sulfatase 1) are pro-apoptotic genes also found to be upregulated in the wall of developing AngII–ApoE−/−I murine aortic aneurysms (Spin et al., 2009). More detailed studies of miRNA-26a function in AAA models are needed to clarify its role.
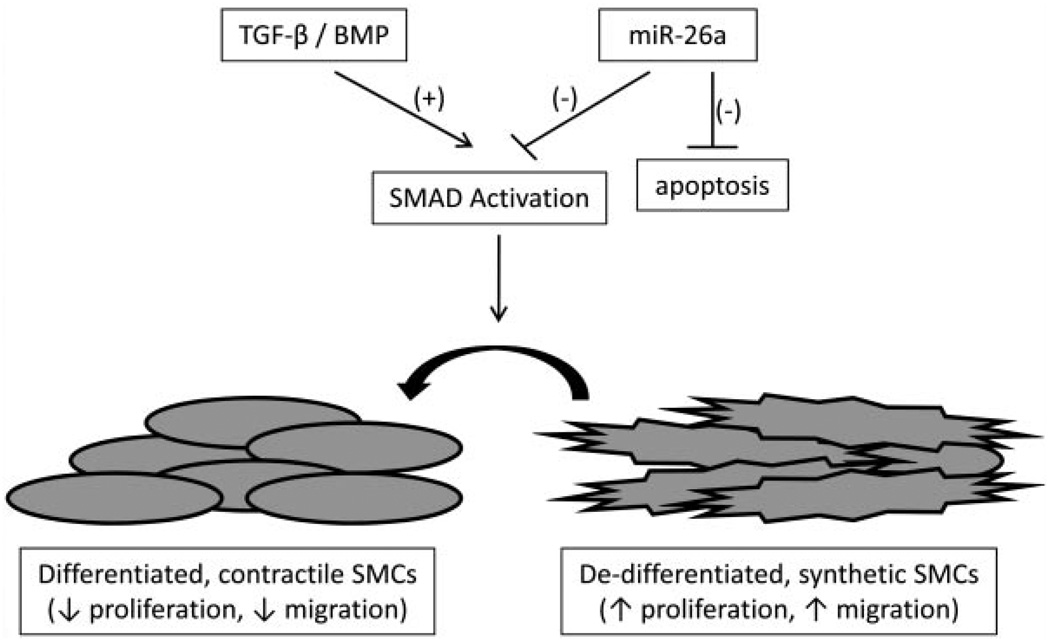
It appears, therefore, that miRNA-26a may function as a ‘biological brake’ that dampens cellular differentiation in culture. By inhibiting critical SMAD signaling proteins downstream of TGF-β/BMP-family receptors, as well as other possible pathway-related targets, miRNA-26a helps maintain the balance of cells in the synthetic and contractile states and governs phenotypic shifting (Fig. 7). A caveat to this hypothesis is that SMAD-1 is primarily an element of BMP-responsive pathways, while SMAD-4 is in the common pathway for BMP and TGF-β. Future experiments will specifically evaluate the effects of miRNA-26a on BMP-triggered signaling. Also, SMAD protein activity is thought to be primarily dependent on posttranslational phosphorylation, suggesting that non-SMAD targets of miRNA-26a may also play a significant role in its modulation of SMC phenotype.
Fig. 7.
Putative mechanism by which miRNA-26a may target TGF-β/BMP signaling to alter SMC biology.
Previously, miRNA-26a had been reported to induce conflicting effects on cellular fate and survival in cell types of differing origins (Luzi et al., 2008; Wong and Tellam, 2008; Ciarapica et al., 2009; Huse et al., 2009; Kota et al., 2009; Sander et al., 2009). In contrast to the findings reported here, miRNA-26a has been implicated as a potent inducer of skeletal myogenesis (Wong and Tellam, 2008; Ciarapica et al., 2009) and a promoter of cell cycling arrest in proliferating hepatocellular carcinoma and lymphomatous cells (Kota et al., 2009; Sander et al., 2009). Conversely, other studies have shown that miRNA-26a can inhibit differentiation as adipose-derived stem cells evolve towards lineage-committed osteoblasts, and in malignant glioblastoma tissue (Luzi et al., 2008; Huse et al., 2009). Such remarkable cell-specific biological heterogeneity has been described previously for other microRNAs (Ji et al., 2007). In the case of miRNA-26a, these observed differences are likely due to varying ratios of antagonistic molecular targets in tissue from differing lineages (Huse et al., 2009). In fact, it is conceivable that the seemingly opposite effects of TGF-β on endothelial and smooth muscle constituents of the vessel wall may be due, in part, to the unique properties of miRNA-26a in different cell types (Pollman et al., 1999).
In conclusion, this microarray-based study found numerous significantly regulated miRNAs with SMC differentiation in culture. In particular, we identified miRNA-26a as a potentially critical regulator of SMC biology, a promoter of cell cycling and migration, and an inhibitor of apoptosis. MicroRNA-26a joins miRNA-21a and miRNA-221 as the only microRNAs known to inhibit SMC differentiation (Ji et al., 2007; Davis et al., 2009; Liu et al., 2009). We hypothesize that miRNA-26a may serve an evolutionary role in the repair of damaged blood vessels, inducing SMC proliferation and medial repopulation in response to pathological conditions such as aneurysm dilation. Future studies should evaluate the effect of miRNA-26a replacement or inhibition on the progression ofAAAdisease—a leading cause of morbidity and mortality.
Supplementary Material
Acknowledgments
This work was supported by NIH 5K08 HL080567-03 (to J.M.S.), K12 HL087746 (to N.J.L.), and 1P50HL083800-01 (to P.S.T.).
Footnotes
Additional supporting information may be found in the online version of this article.
Literature Cited
- Ailawadi G, Moehle CW, Pei H, Walton SP, Yang Z, Kron IL, Lau CL, Owens GK. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J Thorac Cardiovasc Surg. 2009;138:1392–1399. doi: 10.1016/j.jtcvs.2009.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asli NS, Pitulescu ME, Kessel M. MicroRNAs in organogenesis and disease. Curr Mol Med. 2008;8:698–710. doi: 10.2174/156652408786733739. [DOI] [PubMed] [Google Scholar]
- Azuma J, Asagami T, Dalman R, Tsao PS. Creation of murine experimental abdominal aortic aneurysms with elastase. J Vis Exp. 2009 doi: 10.3791/1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, Braun T. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119:2634–2647. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X. Regulation of smooth muscle cells in development and vascular disease: Current therapeutic strategies. Expert Rev Cardiovasc Ther. 2006;4:789–800. doi: 10.1586/14779072.4.6.789. [DOI] [PubMed] [Google Scholar]
- Chun HJ, Ali ZA, Kojima Y, Kundu RK, Sheikh AY, Agrawal R, Zheng L, Leeper NJ, Pearl NE, Patterson AJ, Anderson JP, Tsao PS, Lenardo MJ, Ashley EA, Quertermous T. Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis. J Clin Invest. 2008;118:3343–3354. doi: 10.1172/JCI34871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarapica R, Russo G, Verginelli F, Raimondi L, Donfrancesco A, Rota R, Giordano A. Deregulated expression of miR-26a and Ezh2 in rhabdomyosarcoma. Cell Cycle. 2009;8:172–175. doi: 10.4161/cc.8.1.7292. [DOI] [PubMed] [Google Scholar]
- Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem. 2009;284:3728–3738. doi: 10.1074/jbc.M808788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812–819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erl W, Hristov M, Neureuter M, Yan ZQ, Hansson GK, Weber PC. HMG-CoA reductase inhibitors induce apoptosis in neointima-derived vascular smooth muscle cells. Atherosclerosis. 2003;169:251–258. doi: 10.1016/s0021-9150(03)00201-6. [DOI] [PubMed] [Google Scholar]
- Fager G, Hansson GK, Gown AM, Larson DM, Skalli O, Bondjers G. Human arterial smooth muscle cells in culture: Inverse relationship between proliferation and expression of contractile proteins. In Vitro Cell Dev Biol. 1989;25:511–520. doi: 10.1007/BF02623563. [DOI] [PubMed] [Google Scholar]
- Fazi F, Nervi C. MicroRNA: Basic mechanisms and transcriptional regulatory networks for cell fate determination. Cardiovasc Res. 2008;79:553–561. doi: 10.1093/cvr/cvn151. [DOI] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartel AL, Kandel ES. miRNAs: Little known mediators of oncogenesis. Semin Cancer Biol. 2008;18:103–110. doi: 10.1016/j.semcancer.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Henderson EL, Geng YJ, Sukhova GK, Whittemore AD, Knox J, Libby P. Death of smooth muscle cells and expression of mediators of apoptosis by T lymphocytes in human abdominal aortic aneurysms. Circulation. 1999;99:96–104. doi: 10.1161/01.cir.99.1.96. [DOI] [PubMed] [Google Scholar]
- Hoshina K, Koyama H, Miyata T, Shigematsu H, Takato T, Dalman RL, Nagawa H. Aortic wall cell proliferation via basic fibroblast growth factor gene transfer limits progression of experimental abdominal aortic aneurysm. J Vasc Surg. 2004;40:512–518. doi: 10.1016/j.jvs.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Huang daW, Sherman BT, Tan Q, Collins JR, Alvord WG, Roayaei J, Stephens R, Baseler MW, Lane HC, Lempicki RA. The DAVID Gene Functional Classification Tool: A novel biological module-centric algorithm to functionally analyze large gene lists. Genome Biol. 2007;8:R183. doi: 10.1186/gb-2007-8-9-r183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Huse JT, Brennan C, Hambardzumyan D, Wee B, Pena J, Rouhanifard SH, Sohn-Lee C, le Sage C, Agami R, Tuschl T, Holland EC. The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev. 2009;23:1327–1337. doi: 10.1101/gad.1777409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Node K. Molecular basis of restenosis and novel issues of drug-eluting stents. Circ J. 2009;73:615–621. doi: 10.1253/circj.cj-09-0059. [DOI] [PubMed] [Google Scholar]
- Ishida N, Hayashi K, Hattori A, Yogo K, Kimura T, Takeya T. CCR1 acts downstream of NFAT2 in osteoclastogenesis and enhances cell migration. J Bone Miner Res. 2006;21:48–57. doi: 10.1359/JBMR.051001. [DOI] [PubMed] [Google Scholar]
- Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res. 2007;100:1579–1588. doi: 10.1161/CIRCRESAHA.106.141986. [DOI] [PubMed] [Google Scholar]
- Kong W, Zhao JJ, He L, Cheng JQ. Strategies for profiling microRNA expression. J Cell Physiol. 2009;218:22–25. doi: 10.1002/jcp.21577. [DOI] [PubMed] [Google Scholar]
- Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, Mendell JR, Mendell JT. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek A, Grun D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M, Rajewsky N. Combinatorial microRNA target predictions. Nat Genet. 2005;37:495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- Li Z, Hassan MQ, Volinia S, van Wijnen AJ, Stein JL, Croce CM, Lian JB, Stein GS. AmicroRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc Natl Acad Sci USA. 2008;105:13906–13911. doi: 10.1073/pnas.0804438105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2009;104:476–487. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzi E, Marini F, Sala SC, Tognarini I, Galli G, Brandi ML. Osteogenic differentiation of human adipose tissue-derived stem cells is modulated by the miR-26a targeting of the SMAD1 transcription factor. J Bone Miner Res. 2008;23:287–295. doi: 10.1359/jbmr.071011. [DOI] [PubMed] [Google Scholar]
- McDonald OG, Owens GK. Programming smooth muscle plasticity with chromatin dynamics. Circ Res. 2007;100:1428–1441. doi: 10.1161/01.RES.0000266448.30370.a0. [DOI] [PubMed] [Google Scholar]
- Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487–517. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- Owens GK, Loeb A, Gordon D, Thompson MM. Expression of smooth muscle-specific alpha-isoactin in cultured vascular smooth muscle cells: Relationship between growth and cytodifferentiation. J Cell Biol. 1986;102:343–352. doi: 10.1083/jcb.102.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollman MJ, Naumovski L, Gibbons GH. Vascular cell apoptosis: Cell type-specific modulation by transforming growth factor-beta1 in endothelial cells versus smooth muscle cells. Circulation. 1999;99:2019–2026. doi: 10.1161/01.cir.99.15.2019. [DOI] [PubMed] [Google Scholar]
- Sander S, Bullinger L, Wirth T. Repressing the repressor: A new mode of MYC action in lymphomagenesis. Cell Cycle. 2009;8:556–559. doi: 10.4161/cc.8.4.7599. [DOI] [PubMed] [Google Scholar]
- Sho E, Sho M, Nanjo H, Kawamura K, Masuda H, Dalman RL. Comparison of cell-type-specific vs. transmural aortic gene expression in experimental aneurysms. J Vasc Surg. 2005;41:844–852. doi: 10.1016/j.jvs.2005.02.027. [DOI] [PubMed] [Google Scholar]
- Spin JM, Hsu M, Azuma J, Tsao PS. Transcriptional profiling and network analysis of the murine angiotensin II-induced abdominal aortic aneurysm model; 2009. Washington, DC: Omni Shoreham Hotel; 2009. p. e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RW, Liao S, Curci JA. Vascular smooth muscle cell apoptosis in abdominal aortic aneurysms. Coron Artery Dis. 1997;8:623–631. doi: 10.1097/00019501-199710000-00005. [DOI] [PubMed] [Google Scholar]
- Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79:581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- Van Vickle-Chavez SJ, Tung WS, Absi TS, Ennis TL, Mao D, Cobb JP, Thompson RW. Temporal changes in mouse aortic wall gene expression during the development of elastase-induced abdominal aortic aneurysms. J Vasc Surg. 2006;43:1010–1020. doi: 10.1016/j.jvs.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Wong CF, Tellam RL. MicroRNA-26a targets the histone methyltransferase Enhancer of Zeste homolog 2 during myogenesis. J Biol Chem. 2008;283:9836–9843. doi: 10.1074/jbc.M709614200. [DOI] [PubMed] [Google Scholar]
- Yue P, Jin H, Aillaud-Manzanera M, Deng AC, Azuma J, Asagami T, Kundu RK, Reaven GM, Quertermous T, Tsao PS. Apelin is necessary for the maintenance of insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;298:E59–E67. doi: 10.1152/ajpendo.00385.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. MicroRNA and vascular smooth muscle cell phenotype: New therapy for atherosclerosis? Genome Med. 2009;1:85. doi: 10.1186/gm85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.