Abstract
Neurodegenerative diseases, such as multiple sclerosis represent global health issues. Accordingly, there is an urgent need to understand the pathogenesis of this and other central nervous system disorders, so that more effective therapeutics can be developed. Cerebrospinal fluid is a potential source of important reporter molecules released from various cell types as a result of central nervous system pathology. Here, we report the development of an unbiased approach for the detection of reactive cerebrospinal fluid molecules and target brain proteins from patients with multiple sclerosis. To help identify molecules that may serve as clinical biomarkers for multiple sclerosis, we have biotinylated proteins present in the cerebrospinal fluid and tested their reactivity against brain homogenate as well as myelin and myelin-axolemmal complexes. Proteins were separated by two-dimensional gel electrophoresis, blotted onto membranes and probed separately with biotinylated unprocessed cerebrospinal fluid samples. Protein spots that reacted to two or more multiple sclerosis-cerebrospinal fluids were further analyzed by matrix assisted laser desorption ionization-time-of-flight time-of-flight mass spectrometry. In addition to previously reported proteins found in multiple sclerosis cerebrospinal fluid, such as αβ crystallin, enolase, and 14–3-3-protein, we have identified several additional molecules involved in mitochondrial and energy metabolism, myelin gene expression and/or cytoskeletal organization. These include aspartate aminotransferase, cyclophilin-A, quaking protein, collapsin response mediator protein-2, ubiquitin carboxy-terminal hydrolase L1, and cofilin. To further validate these findings, the cellular expression pattern of collapsin response mediator protein-2 and ubiquitin carboxy-terminal hydrolase L1 were investigated in human chronic-active MS lesions by immunohistochemistry. The observation that in multiple sclerosis lesions phosphorylated collapsin response mediator protein-2 was increased, whereas Ubiquitin carboxy-terminal hydrolase L1 was down-regulated, not only highlights the importance of these molecules in the pathology of this disease, but also illustrates the use of our approach in attempting to decipher the complex pathological processes leading to multiple sclerosis and other neurodegenerative diseases.
Multiple sclerosis (MS)1 is an inflammatory disorder of the central nervous system (CNS), characterized by focal demyelinating lesions and axonal degeneration and loss (1–3). Although the etiology of this disease remains largely unknown, it is generally recognized that the immune system contributes to the pathogenesis of MS and that a complex interplay between environmental and genetic factors are involved. One of the biochemical markers of MS is an increased level of immunoglobulins (IgG) in the cerebrospinal fluid (CSF), particularly during exacerbation (4). It is now recognized that at the site of active demyelination, the perivascular cells consist predominantly of CD4+ activated T lymphocytes secreting various cytokines, clonally restricted B cells and antigen presenting cells that express class II antigen (5–7). Immunological responses to various known antigens, including viruses have been related to an increased IgG in the CSF in MS (8, 9), but such responses account for only a small proportion of all oligoclonal IgG. Moreover, no unique pattern of reactivity has as yet been described across ethnic and geographic boundaries and the full array of effectors and/or regulators resulting in myelin damage and axonal pathology remain uncertain.
Given that the CSF compartment is in close anatomical contact with the brain interstitial fluid, attempts have been made in recent years to identify molecules that are generated during the pathogenesis of CNS disorders (10–15). These approaches include immunoblotting (16), antigen microarrays (17, 18) and proteomic profiling of the CSF (19, 20). Although these different experimental paradigms have led to the identification of several molecules including immunoglobulins (11–13, 15), their exact pathophysiological role(s) remain to be determined. Moreover, most of these studies have analyzed the reactivity of CSF to defined brain antigens or have used as detection reagents, secondary antibodies specific for a defined class of immunoglobin, thus precluding an unbiased analysis of CSF reactivity to unselected CNS components. This is an important issue because MS-CSF can induce several pathological effects such as axonal damage in culture and clonal expansions of plasma cells in the brain that have been shown to produce myelin-specific antibodies (21–22). Thus, deciphering the reactivity of immune and nonimmune molecules present in diseased-CSF may lead to the discovery of disease-specific molecules. This in turn could help understand the pathogenesis of MS as well as the identification of novel therapeutic targets.
Although the initial events that lead to myelin and axonal damage in MS are still unknown, there is now the realization that axonal damage is a major determinant for the clinical deficits which characterize this disease (3, 23, 24). Importantly, the functional co-existence of myelin and the underlying axon is crucial to the meticulous organization and integrity of both structures (25, 26). For example, axon diameter, which is critical for the conduction of impulses, is regulated by myelin and in turn many myelin genes are regulated by axons (27, 28). Therefore, in MS, molecules involved in the organization, maintenance and functionality of both myelin and axons could serve as potential targets for either autoimmune attack or other damaging insults resulting in the impairment of myelin-axonal communication.
Given that CSF can serve as a valuable reporter for the ensuing pathogenesis in MS and/or other neurological conditions in which axonal degeneration and demyelination occur, we devised an unbiased methodology, whereby the proteins of unprocessed CSF samples were biotinylated and the pattern of binding to brain proteins, enriched fractions of myelin, and myelin-axolemmal complexes were analyzed, using two-dimensional-immunoblotting technique. Protein spots that reacted to biotinylated MS-CSF were analyzed and identified by matrix-assisted laser desorption ionization (MALDI)-time-of-flight (TOF)-TOF mass spectrometry. By comparing the reactivity of CSF from MS patients with that of controls, we have identified novel reactive CSF proteins and target proteins in the brain. As a proof of principle we have validated two of these targets in chronic-active MS lesions.
EXPERIMENTAL PROCEDURES
CSF Samples and Isolation of Myelin and Myelin-Axolemmal Complexes
Both control brain and MS-CSF samples were obtained from the Human Brain and Spinal Fluid Resource Center, CA, USA (Table I). These unprocessed samples were kept frozen at −80 °C until used. A rapid separation of myelin and myelin-axolemmal junctions was carried out according to the method described in Menon et al., (29). Briefly, 1 g of white matter was homogenized in ice-cold 0.32 m sucrose containing 2 mm EGTA and protease mixture inhibitors (Sigma Chemicals) in a dounce homogenizer until a homogeneous suspension was visually recognized (≈15 strokes). The brain homogenate was layered on top of ice-cold 0.85 m sucrose/2 mm EGTA solution. The material was spun at 140,000 × g for 1 h at 4 °C. The crude fractions of myelin at the 0.32/0.85 M sucrose interface and the 0.85 m sucrose suspension up to the pellet (myelin-axolemmal junctions) were collected separately. In order to minimize cytosolic contaminants, these fractions were subjected to osmotic shock and centrifuged at 35,000 × g for 15 min, as previously described (29). The pellets were suspended in 2 mm EGTA solution containing 1× protease inhibitors (Sigma Chemicals). The protein content was determined using the Bio-Rad DC protein assay kit (Bio-Rad, Hercules, CA).
Table I. Laboratory features of MS and control CSF samples. M: Male; F: Female.
| Pair and sample numbera | CSF from | Age | IgG synthesis (g/L) | IgG CSF (g/L) | Albumin CSF (g/L) | |
|---|---|---|---|---|---|---|
| Pair 1 | 5922 | Spondylosis, Cervical | 42 m | 3.7 | 3.7 | 22.0 |
| 5282 | MS | 49 m | 40.8 | 20.0 | 38.0 | |
| Pair 2 | 6828 | Myocardial Infarction | 44 m | 27.5 | 15.0 | 42.0 |
| 7046 | MS | 27 m | 35.4 | 12.5 | 30.5 | |
| Pair 3 | 5191 | Peripheral Neuropathy | 66 m | 3.3 | 11.5 | 31.0 |
| 8582 | MS | 54 m | 10.7 | 4.1 | 18.7 | |
| Pair 4 | 5475 | Osteo-Arthritis, Cervical | 52 m | 0.1 | 3.8 | 21.7 |
| 5273 | MS | 57 m | 16.6 | 7.3 | 13.0 | |
| Pair 5 | 5658 | Diabetic Demyelinating Neuropathy | 59 m | 3.1 | 10.4 | 74.0 |
| 5536 | MS | 64 F | 1.8 | 3.3 | 18.5 | |
a Sample numbers are from the Human Brain and Spinal Fluid Resource Center., CA.
Analysis of Biotinylated Human Cerebrospinal Fluid Proteins
Five control and six MS-CSF samples were chosen for the biotinylation analysis (Table I). In order to have more relevant and informative CSF controls than that of healthy individuals, which contain very low amount of proteins, including immunoglobulins (8), samples from four different neurological and nonneurological diseases with nonimmune etiologies plus one sample from a diabetic patient with demyelinating neuropathy were selected (Table I). This later sample was chosen as this disease affects distinct regions of the CNS and is associated with antibody and cell-mediated immune responses to a variety of neuronal structures (30–32). Contents of a single tube from No-Weigh phospho-biotin (Thermo Fisher) was mixed with 170 μl of MilliQ water and pipetted up and down five times. One hundred microliters biotin for 1000 μl of CSF was used for biotinylation. After addition of biotin to CSF, the tubes were covered with aluminum foil and incubated at room temperature on a rotator for 3 h. For each analysis, three different gels were electrophoresed: two gels having 1 μg each and one gel with 3 μg of total biotinylated CSF proteins. The gel with 3 μg was silver stained to visualize the proteins and the other two (1 μg of sample) were subjected to western/far Western blotting (29, 33). Total biotinylated proteins on one of the blots were visualized using streptavidin-horseradish peroxidase (HRP). Presence of immunoglobulins were detected using HRP conjugated anti-human Ig G, A, and M (Sigma Chemicals) on the second blot. Reactivity of biotinylated CSF proteins and CSF immunoglobulins were visualized on Kodak films using ECL supersignal solution (Pierce).
Two-dimensional Gel Electrophoresis
One milligram of myelin, myelin-axolemmal complexes or human brain homogenate was solubilized in 300 μl of lysis buffer [1% ASB-14 (Calbiochem, San Diego, CA)/25 mm Tris/5 mm EDTA/2x protease inhibitor (Sigma Chemicals) mixture] at 37 °C for 30 min. Proteins were precipitated by adding two volumes of 100% ethanol at −20 °C overnight. The precipitated material was spun at 18,000 × g (sigma centrifuge) for 60 min and resuspended in 20 μl of 20% ASB-14 prior to sonication. These samples were then mixed with rehydration buffer (200 μl) [7 M urea (MP Biomedicals, Solon, OH)/2 M thiourea (Thermo Fisher)/4% CHAPS]. 200 μg of total protein were loaded per 7-cm strip (GE healthcare, Immobiline dry strip, pI 3–10) in a total of 125 μl of rehydration buffer containing a final concentration of focusing components 2% ASB-14, 2% IPG buffer pI 3–10 and 100 mm dithiotreitol (DTT). After loading the samples onto 7-cm IPGphor strip, they were overlaid with 3.5–5 ml of mineral oil and rehydrated actively at 30 V for 18 h. The steps involved in isoelectric focusing were as follows; (a) at the end of 18 h run, filter wicks were saturated with 200 mm DTT or water was placed at the cathode (DTT wick) and anode (water wick) ends respectively. (b) 300 V for 30 min, (c) 1000 V for 30 min (d) 5000 V for 1.5 h (e) 5000 V for 30 min and finally on hold at 50 V for 12 h. After focusing of the proteins, the IPG strips were incubated in equilibration buffer (50 mm Tris, pH 8.8/6 M urea/30% glycerol/2% SDS) with 1% DTT for 15 min, then in equilibration buffer with 2% iodoacetamide for 15 min. On occasion, the strips were kept at −20 °C until further use.
SDS-PAGE and Western/Far Western blotting
Proto-Gel (National Diagnostics, Atlanta, GA) was used for making 12% acrylamide gel. Western/far Western blotting was performed as previously described (29, 33). IPG strips were placed on SDS/PAGE gels and sealed in place with 0.5% agarose. two-dimensional SDS/PAGE was performed using a Bio-Rad mini protean system. Gels were transferred onto polyvinylidene fluoride (Immobilon-P, Millipore, Billerica, MA) for immunoblotting or stained with colloidal Coomassie brilliant blue (Sigma).
Detection of CSF Reactivity on Western/Far Western Blots
After blotting, membranes were blocked with protein free Tween blocking buffer (Pierce) for 45 min and were then incubated first with control biotinylated CSF sample as follows. Forty microliters (∼80 μg of CSF proteins) of control biotinylated CSF sample was added to 1 ml of 5% gelatin (Sigma) in Tris-buffered saline (TBS) containing 0.1% TW20 and applied onto blot in plastic bags and kept overnight rotating at 4 °C. After 16 h incubation, the blots were taken out and washed with TBS-TW20 (0.2%) three times. These blots were then incubated with 5% milk in TBS-TW-20 (0.1%) containing streptavidin-HRP (Pierce) at 1:4000 dilutions for 40 min, washed as above with TBS-TW20 three times and developed using supersignal ECL solution (Pierce) and exposed to Kodak x-ray films. The spots obtained were considered control CSF reactivity spots. After exposure to obtain the control CSF reactivity, the blots that were probed with control biotinylated CSF were washed immediately for three times 10 mins each with TBS-TW20. These same blots were again incubated overnight with biotinylated CSF from MS patients [40 μl (∼80 μgs of CSF proteins)/ml of 5% gelatin (Sigma) in TBS-TW20] as described above for control. Blots were then washed and developed as described for the control CSF incubations. A schematic representation of our procedure is shown in supplemental Fig. 1.
Qualitative Protein Identification of MS-CSF Reactive Spots
To qualitatively identify the spots that reacted with MS-CSF, the exact same blots of brain homogenate, myelin and myelin-axolemmal complexes were first probed with control biotinylated CSF to identify the control reacting spots and then probed with biotinylated MS-CSF to visualize the MS-CSF reactive spots. This was done by comparing the reactivity of one control CSF, arbitrarily paired with one MS-CSF. Autoradiograms provided confirmation of differential reactivities. Protein spots that were reactive to two or more MS-CSF samples were selected for MALDI-TOF-TOF mass spectrometry analysis. Spot selection was therefore based on comigration from corresponding Coomassie-stained two-dimensional gels. Because the blots used for probing both control and MS CSF were the same, spots identified with control CSF, could be subtracted from the MS CSF spots by superimposing the autoradiograms. The potential of false positive identification through comigrating proteins within a single spot was minimized by probing two-dimensional blots generated from isolated myelin and myelin-axolemmal complexes samples with the same CSF that was used for the two-dimensional blots generated from brain homogenates. Moreover, because identical results were obtained when triplicate myelin two-dimensional blots were probed with the same control CSF sample (data not shown), it is unlikely that the detection of spots occurred by chance. Using this strategy we were able to identify and characterize 91 spots. These spots were then excised from the Coomassie-stained two-dimensional gels and trypsin digested. Digest samples were cospotted onto the MALDI target plate with Matrix solution of 10 mg/ml a-cyano-4-hydroxycinnamic acid (Laser BioLabs, Sophia-Antipolis, France) in 50% acetonitrile 0.1% trifluoroacetic acid. The samples were analyzed on an Applied Biosystems (Foster City, CA) 4700 Proteomics Analyzer MALDI TOF/TOF in reflectron mode with a mass range of 800 to 3500 Da, focus mass of 1400 Da at 1500 shots per spectra. The 4700 Series Explorer software selects the 12 most intense peptides as precursor masses for tandem MS (MS/MS) analysis, and acquiring in the order of decreasing intensity. MS/MS analysis is carried out in reflector mode with spectra summed to 2500 shots/spectrum.
The probability mass function and MS/MS data was compiled by the GPS explorer software Ver. 3 (build 311) (Applied Biosystems, Foster City, CA) and searched against the National Center for Biotechnology Information nonredundant and Swiss-Prot databases using the MASCOT search engine (version 1.9, Matrix Science Inc., London, UK) with Human taxonomy selected. The following search parameters used were: missed cleavages, 1; peptide mass tolerance, ± 50 ppm; peptide fragment tolerance, ± 0.3 Da; peptide charge, 1+; fixed modifications, carbamidomethyl; Variable modification, oxidation (Met). Scores above 53 for each protein was considered significant. Among the 91 spots analyzed by mass spectrometry, 43 spots had significant scores and 15 of those proteins appeared repeatedly. When several members of a protein family were matched, only with the proteins with the top scores and unique matched sequences were reported. These spots showed reactivity against myelin, myelin-axolemmal complex and brain homogenate proteins as compared with the controls tested (Table II). The characterization and specificity of this antibody is shown in supplemental Fig. 2.
Table II. MS-CSF reactive Protein-ID from two-dimensional blots. Spots that reacted with MS-CSF were excised from the corresponding Coomassie stained gels and processed for mass spectrometric identification as described in the methods. Any score greater than 56 is considered significant, proteins reported below this score have been manually verified.
| Spot numbers on gels | Protein name | Accession number | Mascot search score | Sequence coverage % | Total peptides matched | Number confirmed with MSMS | Total masses unmatched |
|---|---|---|---|---|---|---|---|
| 1 | Fructosebisphosphate aldolase A | P04075 | 209 | 47 | 11 | 3 | 42 |
| 2 | Alpha enolase | P06733 | 220 | 34 | 14 | 3 | 41 |
| 3 | Phosphoglycerate kinase 1 | P00558 | 173 | 51 | 15 | 2 | 45 |
| 4 | Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | P04406 | 105 | 24 | 6 | 3 | 57 |
| 5 | Dihydropteridine reductase | P09417 | 80 | 40 | 6 | 1 | 34 |
| 6 | Triosephosphate isomerase | P60174 | 294 | 67 | 15 | 7 | 42 |
| 7 | ATP synthase subunit alpha, mitochondrial precursor | P25705 | 188 | 38 | 20 | 4 | 47 |
| 8 | Pyruvate kinase isozymes M1/M2 | P14618 | 154 | 40 | 18 | 1 | 44 |
| 9 | Gamma-enolase | P09104 | 366 | 55 | 23 | 8 | 45 |
| 10 | Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta 1 | P62873 | 53 | 16 | 5 | 1 | 23 |
| 11 | Collaspsin response mediator protein-2 (CRMP-2) (DRP-2) | Q16555 | 428 | 59 | 25 | 10 | 50 |
| 12 | Ubiquitin carboxyl-terminal hydrolase isozyme L1 (UCH-L1/PGP 9.5) | P09936 | 73 | 40 | 6 | 2 | 29 |
| 13 | Heat shock-related 70 kDa protein 2 | P54652 | 111 | 24 | 13 | 2 | 44 |
| 14 | Creatine kinase B-type | P12277 | 92 | 39 | 10 | 2 | 69 |
| 15 | Cofilin-1 | P23528 | 119 | 30 | 4 | 2 | 39 |
| 16 | Aspartate aminotransferase, cytoplasmic | P17174 | 56 | 19 | 6 | 0 | 22 |
| 17 | 2′,3′-cyclicnucleotide 3′-phosphodiesterase (CNPase) | P09543 | 303 | 34 | 17 | 5 | 43 |
| 18 | Alpha crystallin ß chain | P02511 | 137 | 61 | 12 | 0 | 36 |
| 19 | Quaking protein | Q96PU8 | 25 | 9 | 2 | 0 | 22 |
| 20 | 14-3-3 protein zeta/delta | P63104 | 66 | 24 | 5 | 1 | 69 |
| 14-3-3 protein gamma | P61981 | 64 | 17 | 4 | 2 | 69 | |
| 21 | Peptidylprolyl cis-trans isomerase A (Cyclophilin A) | P62937 | 56 | 48 | 6 | 0 | 34 |
| 22 | Voltage dependent anion-selective channel protein 1(VDAC-1) | P21796 | 53 | 17 | 3 | 1 | 29 |
| 23 | Actin β | P60709 | 289 | 38 | 15 | 6 | 46 |
| Actin γ | P63261 | 350 | 65 | 17 | 4 | 58 | |
| 24 | Tubulin β 4 | P04350 | 532 | 72 | 33 | 9 | 42 |
| Tubulin α-1B chain | P68363 | 481 | 51 | 19 | 7 | 55 |
Production of Phosphorylation Site-specific Antibody for CRMP-2 (p-CRMP-2)
A rabbit polyclonal antibody against CRMP-2, (a microtubule associated protein involved in neuron development and axon pathfinding), phosphorylated at Thr-555 (anti-pT555), was generated using the chemically synthesized phosphopeptide Cys-Ile550-Pro-Arg-Arg-Thr-Thr(P)-Gln-Arg-Ile-Val-Ala560 as an antigen (34). This peptide was used in antibody blocking experiments along with the unphosphorylated peptide as a control. The characterization and specificity of this antibody is shown in supplemental Fig. 2.
Autopsy Specimens
Human cerebral tissue from autopsy specimens was obtained via the Department of Anatomical Pathology, The Royal Melbourne Hospital, in accordance with the ethical guidelines outlined by the National Health and Medical Research Council (NHMRC). Sections used for histology were from a 52-year-old male with a 7-year history of Multiple Sclerosis (MS-1) and a 38-year-old female with an 11-year history of Multiple Sclerosis (MS-2). Both had multiple chronic-active lesions on histopathology (35). These samples were fixed by immersion in 10% buffered formalin and processed for histology. Sections were stained with Luxol fast blue (LFB) to identify the plaques of demyelination as previously reported (23).
Immunohistochemistry
Paraffin embedded sections (10 μm) were dewaxed and washed in phosphate-buffered saline (PBS; pH7.4). Heat-induced epitope retrieval was performed by the standard microwave oven method using 0.1 m citrate buffer (pH6.0) for 10 min, cooled and washed in PBS (5 min x2). Sections were incubated with 6% hydrogen peroxide (15 min at room temperature) to quench endogenous peroxidase followed by 0.5% Triton X 100 for 5 min. The sections were incubated with 5% goat serum (Sigma) in PBS for 60 min followed by primary antibody for 60 min both at room temperature in a humidified chamber. The after primary antibodies were used: polyclonal rabbit anti-phospho-Thr555-CRMP-2 at 1:50 dilution, mouse neurofilament SMI 31 monoclonal IgG (Covance, Princeton, NJ) at 1:2000 dilution and mouse Class III, β-Tubulin (TUJ1) monoclonal IgG (Covance) at 1:250 dilution and polyclonal rabbit IgG anti UCH-L1/PGP9.5 (Thermo Scientific) at 1:200 dilution. Sections were washed in PBS-0.05% Tween-20 (pH7.4) for 5 min 3× before being incubated with appropriate HRP conjugated secondary antibody; goat anti-mouse (1:200 dilution, Calbiochem, San Diego, CA) or goat anti-rabbit (1:300 dilution, Calbiochem). The Avidin-Biotin peroxidase (ABC) method was used for the sections of MS-2 incubated with SMI 31 and β-Tubulin antibodies. In brief, sections were blocked with avidin and biotin prior to incubation with the primary antibody followed by goat anti-mouse IgG-biotinylated (Vector, Burlingame, CA) (1:250) and the ABC complex (Vector). All sections were finally washed in PBS-0.05% Tween-20 (5 min, 3×) followed by incubation with ImmPACT™ diaminobenzidine peroxidase substrate (Vector) for 6 min and counterstained with Harris hematoxylin (Amber Scientific Midvale, Australia) for 20 s. Sections were dehydrated through absolute ethanol and xylene and mounted using DPX. Isotype-matched immunoglobulins (rabbit IgG, mouse IgG; Zymed Laboratories Inc.) were used as negative controls.
Immunofluorescence
Immunofluorescence was performed on the same tissue blocks used for immunohistochemistry. Dewax, epitope retrieval, serum blocking and primary antibody incubation was performed as described above for immunohistochemistry. After thorough washing with PBS-0.05% Tween-20 (5 min, 3×), sections were incubated with the appropriate secondary antibody; anti-rabbit Alexa Fluor 488 conjugate or anti-mouse Alexa Fluor 647 conjugate (both 1:1000 dilution, Invitrogen) for 60 min at room temperature. 4′, 6-Diamidino-2-phenylidole dilactate (DAPI; 1:10000 dilution; Molecular Probes, Carlsbad, CA) was added to sections to stain nuclei. Sections were mounted using fluorescence mounting media (Dako, USA).
Image Acquisition and Processing
Bright field microscopy analysis and images were acquired under the 40× objective lens of an Olympus Provis Ax70 microscope and an Olympus DP70 color digital camera. Immunofluorescence was analyzed with an Olympus Fluoview FV1000 confocal microscope (Olympus, GmbH, Germany) and FV10-ASW software (version 1.7.2.2; Olympus) and the images taken using PLAPOx100 OI NA: 1.4 objective lens. Images were acquired in the XY scan mode at 100 μs/pixel sequentially using Kalman line integration. Immunohistochemistry images were saved as RGB TIFF files. Immunofluorescence images were saved as gray scale TIFF files. Images were imported into Adobe Photoshop v9.0.2 (Adobe Systems Incorporated, USA) and minor brightness and contrast adjustments performed in a linear manner. Immunofluorescence images were colored and combined to produce pseudo-colored merged images.
RESULTS
Validation of Biotinylation of CSF Proteins
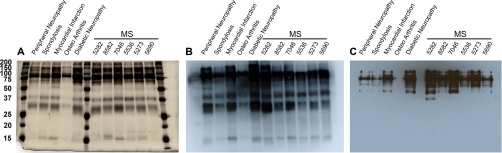
In order to identify unbiased CSF reactivity (by antibodies or any other moieties) toward proteins of myelin, myelin-axolemmal complexes, and total brain homogenate, we first tagged CSF proteins with biotin. Biotinylation was chosen for two main reasons: first, any proteins with a reactive amine group can be biotinylated and second, biotinylation is an event that does not discriminate between proteins, because all proteins essentially have reactive amines. The effectiveness of CSF biotinylation was analyzed, by comparing the 3 μg-silver stained gels from the five control and the six MS-CSF samples, with that of the 1 μg-biotinylated CSF using western/far Western blotting and subsequent staining with avidin-HRP. As shown in Figs. 1A and 1B, the pattern of biotin conjugated proteins in the CSF remained similar to that of the silver stained gel. The presence of immunoglobulins in the CSF, was confirmed by staining the blotted-biotinylated CSF with anti-human Ig G, A, and M. (Fig. 1C). However, additional bands were present on the biotinylated CSF blot, indicating the presence of nonimmunoglobulin reactive proteins in the CSF.
Fig. 1.
Analysis of biotinylated CSF from controls and MS patients. A, Silver stained 12% gel showing the profile of biotinylated CSF proteins (3 μg) from different samples. B, western/far Western blot of the biotinylated CSF proteins (1 μg) probed with streptavidin-HRP to show the extent of biotinylation. Note the extent of similarity in pattern with silver stained gel on the left. C, western/far Western blot of biotinylated CSF proteins (1 μg) probed with anti-human Ig GAM showing Fc chains of immunoglobulins.
Identification of Reactive Brain Proteins Using Biotinylated MS-CSF
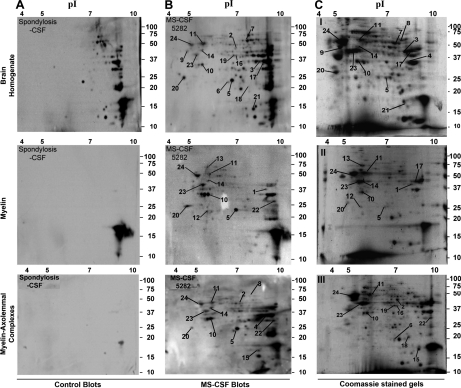
To identify the reactivity of MS-CSF molecules to brain proteins, enriched fractions of compact myelin and the myelin-axolemmal complexes were subjected to two-dimensional gel electrophoresis. Proteins from each gel were transferred onto polyvinylidene fluoride membranes and western/far Western blotting performed using biotinylated CSF from the five control subjects and from five of the six biotinylated MS patients, namely samples 5282, 8582, 7046, 5536, and 5273. MS-CSFs reactive spots (Fig. 2) were excised out from Coomassie-stained gels and after tryptic digestion, subjected to mass spectrometric analysis to identify the proteins. With the exception of the diabetic demyelinating neuropathy, none of the control CSFs reacted to any of the spots observed with MS-CSFs (Fig. 3 and supplemental Table S1). The list of the 24 MS-CSF reactive proteins identified by our approach is shown in Table II. Among those, six were only reactive to the MS-CSFs: these are GAPDH, pyruvate kinase isozymes, UCH-L1/PGP 9.5, heat shock-related 70 kDa protein 2, CNPase and αβ-crystallin. The consitancy of the MS-CSF reactivity to many of these proteins, especially CRMP-2, dihydropteridine reductase, creatine kinase, guanine nucleotide-binding protein and quaking protein between the brain homogenate, myelin, and myelin-axolemmal complexes is shown in Fig. 3 and the supplemental Table S1.
Fig. 2.
Representative two-dimensional blot showing the reactivity of Control and MS-CSF to different brain preparations. Two-dimensional gels of brain homogenate, myelin and myelin-axolemmal complex preparations were subjected to western/far Western blotting, probed first with the biotinylated spondylosis CSF control and developed using streptavidin-HRP (A). The same blots were washed thoroughly and probed again with the paired MS-CSF sample 5282 (B) as described in the methods. (C) Corresponding Coomassie stained two-dimensional gels of brain homogenate, myelin and myelin-axolemmal complexes. Spots that showed reactivity to MS-CSF were numbered. Spots common to both blots were no further assessed. Note the reproducibility of the different spots both on the blots (B) and Coomassie stained gels (C).
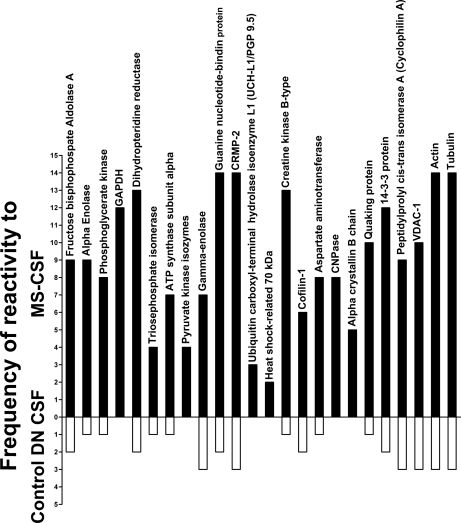
Fig. 3.
Frequency of reactivity of CSF samples to the proteins identified by mass spectrometry. This graph shows the total number of times the five MS-CSF and the control diabetic demyelinating neuropathy (DN) samples reacted to the proteins for each of the gels shown in Fig. 2 and supplemental Fig. 4. None of the other controls showed any reactivity to these proteins. Protein spots that were reactive to two or more MS-CSF samples were selected for identification by mass spectrometry and correspond to the proteins listed in Table II. Note the consistancy of the reactivity to most proteins, especially dihydropteridine reductase, guanine nucleotide-binding protein, CRMP-2, creatine kinase, actin and tubulin.
MS-CSF Reactivity to Molecules Associated with Energy Metabolism
Among the different molecules that showed reactivity to MS-CSF, proteins related to glycolytic pathway were predominant (Table II, Figs. 3 and 4). Six of the ten different enzymes that participate in the breakdown of glucose to pyruvate in the glycolytic pathway reacted with MS-CSF (Table II, Figs. 3 and 4). These are triose phosphate isomerase (TPI), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), α-enolase (AE), fructose bisphosphate aldolase-A, pyruvate kinase (PK), and phosphoglycerate kinase (PGK) (36, 37). Although these enzymes are mainly involved in carrying out their glycolytic functions, they also take part in other cellular responses. For example, GAPDH and AE are involved in transcriptional regulation and apoptosis (36). Similarly, TPI is involved in the regulation of KATP channels and its deficiency leads to neurodegeneration (37, 38). Proteins belonging to mitochondrial complexes also reacted with MS-CSF. These include ATP synthase subunit α and voltage dependent anion-selective channel protein 1 (VDAC-1). We also detected the cytoplasmic form of aspartate aminotransferase, an enzyme that catalyzes the production of N-acetyl-aspartate (NAA) involved in the mitochondrial energy metabolism and is critical for the myelin lipid synthesis (39, 40).
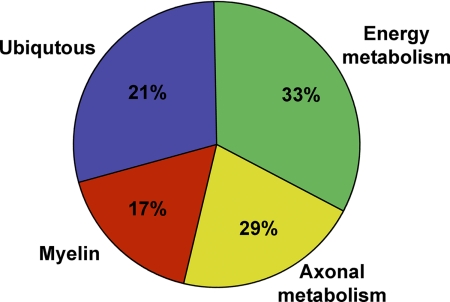
Fig. 4.
Classification of CSF reactivity to various brain proteins. Pie chart showing overall MS-CSF reactivity pattern to various brain proteins. Note the relatively increased levels of CSF reactivity to proteins of energy and axonal metabolism compared with myelin.
MS-CSF Reactivity to Proteins of Myelin and Axonal Metabolism
In comparison to the identified proteins related to energy metabolism, the number of myelin specific proteins identified was relatively low (Table II, Fig. 2, 3, and 4). Those belonging to myelin metabolism include 2′, 3′-cyclicnucleotide 3′-phosphodiesterase (CNPase), a potential autoantigen (15) whose role is not yet defined and the quaking protein, an RNA-binding protein that plays a central role in myelination. MS-CSF reacted to a significant number of proteins belonging to cytoskeletal architecture and cellular metabolism of both myelin and oligodendrocytes and neurons (Table II, Figs. 2, 3, and 4). These include CRMP-2, γ enolase, cofilin, αβ-crystallin, cyclophilin A, creatine kinase B, and Ubiquitin carboxy-terminal hydrolase L1 (UCH-L1/PGP 9.5). CRMP-2, a molecule that regulates neuronal polarity by way of axonal microtubule assembly, is found associated with degenerating neurons in its phosphorylated form (41–44). Together with Rho-GTP, CRMP2 is an intracellular effector of neurite retraction, primary involving myelin-associated inhibitory factors (39, 41). It regulates actin and tubulin dynamics after injury (41). Like CRMP-2, Cofilin is known to affect actin cytoskeleton dynamics, growth cone mobility and neurite outgrowth. The molecule of neuronal origin, γ enolase, is generally found elevated in CSF of patients with neurodegenerative disorders (45) and is involved in actin filament disassembly. Notably, we detected αβ-crystallin, a molecule present at enhanced levels in oligodendrocytes and astrocytes in MS lesions. Interestingly, the concentration of anti-αβ-crystallin antibodies in sera and CSF of MS patients correlates with activity as well as severity of the disease (42). Among the other molecules known to be up-regulated in inflammatory conditions and autoimmune diseases are Peptidylprolyl cis-trans isomerase A (Cyclophilin A), an immunophilin with a variety of intracellular function, including protein trafficking, intracellular signaling, and apoptosis, (46); creatine kinase B, a molecule involved in cellular energy homeostasis and Ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) also known as PGP 9.5 (47, 48). UCH-L1/PGP 9.5 is a highly conserved protein localized in neurons and neuroendocrine cells and has been implicated in several neurodegenerative disorders such as Parkinson's disease and Alzheimer (47, 48).
MS-CSF Reactivity to Proteins of Ubiquitous Nature
In addition to the molecules that are specifically associated with energy metabolism, oligodendrocytes, and myelin or neurons, we found MS CSF reactivity to proteins that are generally found in eukaryotic cells. These include, actin cytoplasmic 1 (β-actin), actin cytoplasmic 2 (γ-actin), tubulin β-4 chain, tubulin α-ubiquitous chain, tubulin α-3 chain, heat shock protein 70, dihydropteridine reductase and guanine nucleotide-binding protein (Transducin). In this context it is interesting to note that antibody responses to tubulin were seen in Leber's hereditary optic neuropathy, a mitochondrially inherited degeneration of retinal ganglion cells and their axons that lead to an acute or subacute loss of central vision, a clinical feature often seen in MS patients (42).
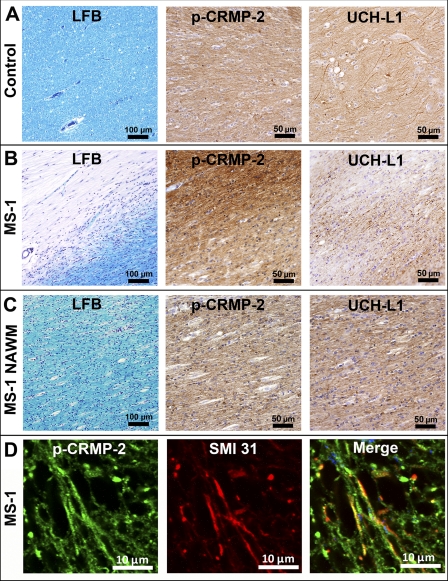
Expression of p-CRMP-2 and UCH-L1 in Chronic-active MS Lesions
The normal physiological role of CRMP-2 has been demonstrated to play a critical role in axon outgrowth and axon-dendrite specification (41, 43). Importantly, the phosphorylation of CRMP-2 at its C terminus is a major determinant in the neurodegenerative phases of several CNS diseases such as Alzheimer's disease (41, 44). Likewise, UCH-L1 has been linked to neurodegenerative disorders. This molecule is selectively and abundantly expressed in neurons and represents 1–2% of total soluble protein in the brain and immunoreactivity to UCH-L1 is a sensitive method to detect axonal dystrophy in pathological conditions of the CNS (47, 48). We therefore focused our attention on the phosphorylated form of CRMP-2 (p-CRMP-2) as well as the levels of UCH-L1/PGP 9.5 in MS plaques in order to gain some insight into the putative role of these two molecules during the active phase of MS. As shown in Fig. 5B (p-CRMP-2) and supplemental Figs. S3A and S3C, the cellular expression of p-CRMP-2 was increased in chronic active plaques, (as indicated by the absence of LFB staining, Fig. 5B; LFB) in the axons near the inflammatory lesions (supplemental Figs. S3A and S3C) As judged by the absence of such reactivity in normal brain (Fig. 5A; p-CRMP-2) and the normal appearing white matter (NAWM) of patient MS-1 (Fig. 5C) and total inhibition of the staining after the pre-incubation of the antibody with the phospho-peptide of CRMP-2 (data not shown), this reactivity was specific. p-CRMP-2 immuno-positive axons also stained strongly for phosphorylated neurofilaments (supplemental Figs. S3B and S3D). Double immunofluorescence staining with the anti-CRMP-2 and -SMI-31 antibodies clearly demonstrated the presence of degenerating axons within the chronic-active lesions (Fig. 5D). These results were further validated by the marked decreased of immunoreactivity for UCH-L1/PGP 9.5 within the demyelinating lesions (Fig. 5B; UCH-L1) as compared with normal brain and NAWM (Figs. 5A and 5C). Taken together, these results imply that the up-regulation of p-CRMP-2 and the down-regulation of UCH-L1/PGP 9.5 within chronic MS lesions may play an important role in the demyelinating process as well the degeneration of axons (41).
Fig. 5.
Levels of p-CRMP-2 and UCH-L1 in chronic-active MS lesions. Postmortem human brain paraffin sections (cut at 10 μm) were obtained from a 70-year-old control, who died of nonneurological causes (A) and a patient who had died with associated chronic-active MS lesions with 11 years duration of disease (B) (38 year old female). A, shows normal regions of white matter stained with LFB, the anti-p-CRMP-2 and the anti-UCH-L1 (PGP9.5) antibodies. B, a chronic-active MS lesion situated in the subcortical white matter of the frontal lobe stained as in panel A. Note the marked region of demyelination (LFB), increased levels of p-CRMP-2 immmunoreactivity and decreased levels of UCH-L1 staining, compared with that obtained from control brain and normal appearing white matter (NAWM) (C). C, shows that the reactivity obtained with the anti-p-CRMP-2 and UCH-L1 antibodies in the tissue adjacent to the MS lesion are similar to that of control tissue. D, shows a higher magnification of double immunofluorescence images of labeled axons with the anti-p-CRMP-2 antibody and SMI-3 from the chronic-active MS lesion shown in supplemental Figs. 3A and 3B.
DISCUSSION
In an attempt to identify potential molecules of clinical and physiological significance in the development of MS, we first performed a two-dimensional immunoblotting of brain, myelin fraction and myelin-axolemmal proteins using biotinylated molecules from unprocessed CSF. This method overcomes the general limitation of two-dimensional proteome analysis of CSF, which often necessitates the pooling and concentrating of samples (19). Despite the fact that the amount of brain homogenate loaded in the western/far Western blot using biotinylated CSF (1 μg) was one third that of the silver stained gel (3 μg), the patterns of staining between silver-stained CSF gels and the biotinylated CSF blots probed with streptavidin-HRP were similar. This highlights the sensitivity and effectiveness of the biotinylation procedure used in this study and also indicates that in addition to immunoglobulins (Fig. 1C), other moieties are present in the MS CSF samples. Experiments attempting to decipher the nature of these additional molecules are currently in progress. However, given that the potential of CSF proteins interacting with denatured proteins on two-dimensional blots is small, the detected reactivity is most likely because of high affinity antigen-antibody mediated interactions. In order to reduce the possibility of false positives as well as enhancing the detection of putative protein candidates, CSF samples were not only probed on brain homogenate but also two different brain fractions. As illustrated in Fig. 2, supplemental Table S1 and supplemental Fig. S4, several independent CSF-reactive spots could be identified on blots from brain homogenate, myelin and myelin-axolemmal complex fractions. This differential reactivity is partly because of the enrichment of various proteins as a result of the fractionation of the brain tissue into myelin and myelin axolemmal complex as well as other factors, such as the avidity and affinity of the MS reactive moieties (e.g. antibodies) and possibly the stage of the disease.
The MALDI-TOF-TOF mass spectrometry analysis has permitted the identification of potential new candidate-molecules that are present in the MS-CSF. Based on the overall CSF analysis on brain proteins, we found that biotinylated MS-CSF molecules reacted predominantly to proteins related to mitochondrial metabolism pathways in general (33%) and axonal metabolism (29%) (Fig. 4). In particular, many cytoskeletal proteins and proteins regulating the cytoskeletal dynamics showed reactivity with MS-CSF (Table II). In line with previous reports, molecules such as αβ-crystallin, enolase, 14–3-3 protein and GAPDH were also identified (11, 15, 49) in the MS-CSF, thus validating our experimental approach. Interestingly, 17% of reactive proteins were myelin proteins and molecules from immunological origin (Fig. 4 and Table II). In this context, it is worth noting that several of the proteins identified by the MS CSFs were also reactive to the control diabetic demyelinating neuropathy CSF (Fig. 3, supplemental Table S1 and supplemental Fig. S4). These findings corroborate several studies showing that beside lymphocytic infiltrates in damaged ganglia and nerve bundles, cell-mediated immune responses and autoantibodies to autonomous nervous tissue structures typically affect such a condition (30–32). This further highlights the relevance of our approach in deciphering within a complex array of tissue antigens, potential pathogenic targets as well as providing insight into the pathogenesis of neurodegenerative as well as other disorders.
Significance of Glycolytic Pathway enzymes in MS
A significant proportion of the brain's energy needs are met from the metabolic breakdown of glucose (50). Therefore, it is not surprising that any events that may impair the function of molecules involved in glucose metabolism could lead to significant damage in the integrity and function of neurons or their supporting cells. In this context, it is noteworthy that antibodies to AE, TPI, and GAPDH have been shown to be present in the CSF from MS patients (15, 51, 52). Besides their glycolytic roles, these enzymes also have other functions. For example, AE can bind to c-myc binding protein and indirectly influence myelination as c-myc overexpression leads to hypomyelination (53). Similarly, GAPDH, TPI and PK are components of KATP channels and can be immunoprecipitated along with potassium channel subunit Kir 6.2, suggesting that these enzymes could influence the regulation of KATP channels function (37). KATP channels act as metabolic sensors and control the firing rate of neurons thereby protecting them from hypoxia. In disease states, activation of these channels can control the death of dopaminergic neurons (54). GAPDH, on the other hand is associated with γ-aminobutyric acid-type A receptors and functions as a kinase involved in maintaining γ-aminobutyric acid-type A receptors-mediated responses (55); inhibition of GAPDH activity leads to induction of apoptosis (56). Although antibodies are the most likely reactive candidates, the possibility that other reactive molecules could interact with intracellular proteins cannot be excluded. Nonetheless, Fc gamma receptor mediated uptake of antibodies has been shown in motor neurons (57) and their interaction with intracellular components has been implicated in a variety of diseases (58). As demonstrated here, reactivity to the intracellular component CRMP2 was not only detected in MS-CSF but also up-regulated in MS lesions, illustrating for the first time the role of CRMP-2 in MS axonal pathology. The generation of reactive molecules, particularly antibodies to GAPDH, TPI, or PK could influence the activity of these molecules and potentially be of pathological significance. However, the basis of the interactions with these MS protein targets as well as their relevance to the disease process, remains to be established. Experiments are currently underway to try and answer these critical questions. Indeed, it is pertinent to note that not all autoimmune responses are pathogenic. For example, whereas we have identified aldolase A, (another glycolytic enzyme) as a potential target, immunization of C57BL/6 and NOD mice or Lewis rats with this protein does not lead to the development of any diseases (59). Likewise, whereas antibodies to alpha-enolase are associated with autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus and mixed cryoglobulnemia, these are nonpathogenic (45).
Among the different glycolytic enzymes we detected in our study, is PGK, a molecule involved in regulating endocytosis, expressed in relatively high levels in cells (60). The presence of antibodies or other putative binding proteins could therefore inhibit the function of PGK and thus lead to impaired neural cell function. In addition to the glycolytic enzymes involved in the energy metabolism, creatine kinase B (CKB), a molecule involved in cellular energy homeostasis also reacted with MS-CSF (Table II, Figs. 3 and 4). CKB catalyzes the reversible transfer of phosphoryl group from phosphocreatine to ADP generating ATP thereby propelling cells with high-energy demands such as neurons. Moreover, CKB influences the cytoskeletal changes mediated by PAR-1 signaling (61). Recently, PAR-1 has been localized to the nodes of Ranvier and its activation leads to conduction block (62). Given the role of CKB in PAR-1 signaling, factors that influence CKB activity could lead to impairment in impulse conduction and cytoskeletal organizations as clearly illustrated by the collapse of oligodendrocyte membrane architecture after treatment with demyelinating antibodies (63, 64).
Mitochondrial Proteins in MS
It is now recognized that axonal injury occurs early in acute MS lesions (2, 3). In order to compensate for the impulse conduction at demyelinated regions along the axon, mitochondria migrate into the sites of axonal damage to provide ATP (65). However, with time, mitochondrial function is affected because of mitochondrial DNA damage mediated by free radicals generated from activated immune cells, a common feature of chronic-MS plaques (66). Thus, mitochondria play a central role in the function and survival of the neuron. The intricate mechanism by which mitochondrial function is impaired in MS remains to be established. One of the reactive molecules detected by our proteomic screening with biotinylated MS-CSF was the ATP synthase subunit alpha of the mitochondrial oxidative phosphorylation pathway from complex-V (Table II, Fig. 3 & 4). Interestingly, the cytosolic accumulation of ATP synthase alpha subunit is associated with early degenerating neurons in Alzheimer's disease (67). VDAC1, another molecule associated with mitochondrial membrane component also showed reactivity with MS-CSF. VDAC1 is critical for the release of cytochrome c, a molecule that induces apoptosis (68) and in addition plays an important role in the transport of ATP, calcium and other metabolites across the mitochondrial membrane (69). VDAC1, in conjunction with the pro-apoptotic Bcl-2 family of proteins Bax, Bak and chemical agents such as ruthenium red and fluoxetine (70) regulates the release of cytochrome c. Notably, VDAC1 is involved in the pathophysiology of neurodegenerative diseases such as Alzheimer's and Huntington's diseases (71, 72). Thus, regulators of VDAC1 activity present in MS-CSF could play a role in the neurodegenerative phase of MS.
Detection of MS-CSF reactivity to aspartate aminotransferase (AAT) is interesting given the mitochondrial involvement in neurodegeneration. Indeed, AAT utilizes oxaloacetate and glutamate to produce aspartate. Furthermore, αketo-glutarate and aspartate are indicators of axonal degeneration, commonly demonstrated by magnetic resonance spectroscopic imaging, to detect the extent of neurodegeneration (73). Notably, the substrate for AAT, N-acetyl aspartate (NAA), which is a key molecule used by oligodendrocytes for myelin lipid synthesis and is generated by neurons, has to be transported from the neuron to the myelinating oligodendrocytes (39, 40, 74). The compartmentalization of NAA in neurons and the exclusive requirement of NAA by oligodendrocytes for myelin synthesis suggest a complex relationship that is important for the survival of both neuron and oligodendrocytes. Thus, impairment in the regulation of enzymes and transporters that take part in the NAA metabolism could have an important role to play not only in the neuropathology of MS but also in other neurodegenerative diseases. Another interesting molecule we have identified in this study is cyclophilin A (CypA). Apart from its role in T-cell activation and protein chaperoning, CypA participates in the caspase-independent route of programmed cell death, where the apoptosis inducing factor recruits the endonuclease CypA for its DNA degrading capability and perhaps may play a role in the neurodegenerative process (75). Indeed, CypA deficiency affords neuroprotection in vivo (46). Given that the inactivation of cyclophilin D also protects axons after experimental autoimmune encephalomyelitis induction (76), the possibility that CypA may also play a role in the neurodegenerative aspects of MS cannot be excluded.
Role of Stress Proteins and Oligodendrocyte and Myelin Proteins in Neurodegeneration
The identification of MS-CSF reactivity to quaking protein is potentially an important finding, given that the 37 kDa isoform has been shown to be essential for the development of oligodendrocyte progenitor cells by regulating their gene expression toward a promyelinating phenotype (77). Similarly, HSP-70 is required for the optimal expression of MBP (78), with HSP-70 also found to be associated with MBP in MS lesions, in an ATP-dependent fashion (79). Complexes of MBP and HSP-70 can stimulate MBP-T cell lines and that HSP-70 null mice have a low clinical score of MOG-experimental autoimmune encephalomyelitis and a decreased proliferation of MOG-CD4+ T cells (79, 80). It is therefore tempting to speculate that in MS, HSP-70 may have the ability to modulate the immune response mediated by encephalitogenic T cells. In addition, when oligodendrocytes are treated with H2O2 and subjected to heat shock, both HSP-70 and αβ crystalline are expressed, suggesting that the detection of these two molecules with MS-CSF is indicative of an oxidative stress on oligodendrocytes (81). In this context, it is noteworthy that the apoptosis-inducing factor that interacts with cyclophilin A also interacts with HSP-70 (82). In line with the findings of Lovato and colleagues (15), we found that CNPase, one of the third most abundant myelin proteins of the CNS, reacted with MS-CSF. Although CNPase has been proposed as a putative autoantigen in MS, its encephalitogenic role has not yet been established and thus further investigations on its role in MS are warranted.
Proteins of Cytoskeletal Organization and Degradation
Treatment of oligodendrocytes with demyelinating antibodies to galactocerebroside (GalC) and to myelin oligodendrocyte glycoprotein (MOG) has been shown to potentiate cytoskeletal collapse (63, 64), indicating that cytoskeletal components play an essential role in maintaining the structural architecture of oligodendrocytes. CRMP-2 is one such protein that regulates cytoskeletal dynamics in neurons (43) and in a subset of mature oligodendrocytes that exhibit Semaphorin 3A sensitivity (83). CRMP-2 interacts with tubulin heterodimers and takes part in the tubulin dynamics during axonal growth (84). Over-expression of CRMP-2 results in the induction of multiple axons (85). Phosphorylation of CRMP-2 on Thr555 leads to growth cone collapse and prevents the association of CRMP-2 with tubulin dimers (41, 43). This may in turn have important implications in regulating the axonal cytoskeletal dynamics. Notably, semaphorin 3A-sensitive oligodendrocytes show an increased expression of CRMP-2 in association with oligodendrocyte process retraction (83). Our finding that there is an increase in the expression levels of p-CRMP-2 (ROCK II substrate) in chronic-active MS lesion, particularly in SMI-31-positive axons, suggest that the collapse of cytoskeletal dynamics in axons may play a role in axonal degeneration. This contention is further supported by the demonstration that UCH-L1 is down-regulated in MS plaques, a finding also observed in the brains of Alzheimer's as well as Parkinson's disease patients (47, 48). In addition to it putative role in neurodegeneration, UCH-L1/PGP 9.5 is thought to be involved in the regulation of ATP receptors in neurons and the morphology of neuronal precursors (47). Thus, it is possible that in MS, the altered activity of this molecule leads to additional deleterious effects on axon. In this context, it is noteworthy that cofilin, another molecule involved in the actin filament disassembly reacts with MS-CSF (Figs. 3 and 4). Cofilin has also been shown to have a role in neurodegeneration associated with Alzheimer's disease, whereby dephosphorylation of cofilin is stimulated by beta-amyloid peptide (86). This leads to the activation of cofilin and the formation of rod shaped actin filaments that inhibit axonal transport (86). The finding that both CRMP-2 and cofilin were reactive to MS-CSF is important in view of some similarity existing between Leber hereditary optic neuropathy and MS (87). Increased anti-tubulin antibody levels with inflammatory demyelination similar to MS were also seen in Leber hereditary optic neuropathy patients, suggesting a role for these antibodies in inducing demyelination (42, 87). Besides the regulation of actin and tubulin dynamics by cofilin and CRMP-2, we also found as reported by others (88, 89), that the 14–3-3 protein reacted to MS-CSF. 14–3-3 interacts with cofilin (90) and controls the phosphorylation status of cofilin and LIM kinase via slingshot phosphatase 1L, along with F actin (91, 92) leading to the regulation of cytoskeletal dynamics. Thus, detection of MS-CSF reactivity to 14–3-3 and CRMP-2 is important in the context of the ability of demyelinating anti-MOG antibody in inducing cytoskeletal damage to oligodendrocytes in cultures (63, 64).
In summary, using an unbiased approach aimed at identifying putative biomarkers for MS, we have identified several novel molecules that are either involved in myelin metabolism, mitochondrial survival and/or apoptosis. As discussed above, these may play a significant role in the pathogenesis of MS. The group of MS patients and controls studied here is relatively small, thus precluding any definite conclusion on the potential pathogenic role of these molecules in MS. Longitudinal CSF analysis using a larger cohort of patients presenting with different forms of MS, across geographic and ethnic boundaries is required to validate these results and identify novel targets for research as well as the development of new treatments.
Acknowledgments
We thank Ms Naomi Campanale for expert technical assistance and Ms Sally Caine for her invaluable help in formatting the figures and manuscript. Martin Short is the recipient of a Biogen-Idec Clinical Fellowship.
Footnotes
* This work was supported by grants from the National Health and Medical Research Council of Australia, the Baker Foundation, The National Multiple Sclerosis Society of New York, The Diana Asmar Fund and the Bellberry Ltd Fund.
 This article contains supplemental Figs. S1 to S4 and Tables S1 and S2.
This article contains supplemental Figs. S1 to S4 and Tables S1 and S2.
1 The abbreviations used are:
- CNS
- central nervous system
- CSF
- cerebrospinal fluid
- MS
- Multiple Sclerosis
- VDAC-1
- voltage dependent anion-selective channel protein 1
- CRMP-2
- collapsin response mediator protein-2
- p-CRMP-2
- phosphorylated collapsin response mediator protein-2
- IgG
- immunoglobulins
- MS/MS
- tandem mass spectrometry
- TPI
- triose phosphate isomerase
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- AE
- alpha-enolase
- PK
- pyruvate kinase
- NAA
- N-acetyl-aspartate
- CNPase
- 2′,3′-cyclicnucleotide 3′-phosphodiesterase
- CaV2.2
- N-type calcium channel
- UCH-L1
- Ubiquitin carboxy-terminal hydrolase L1
- β-actin
- Actin cytoplasmic 1
- γ-actin
- Actin cytoplasmic 2
- CKB
- creatine kinase B
- CypA
- cyclophilin A
- HSP-70
- 70 kilodalton heat shock protein
- MBP
- myelin basic protein
- MOG
- myelin oligodendrocyte glycoprotein
- TBS-TW20
- TBS containing Tween 20.
REFERENCES
- 1. Imitola J., Chitnis T., Khoury S. J. (2006) Insights into the molecular pathogenesis of progression in multiple sclerosis potential implications for future therapies. Arch. Neurol. 63, 25–33 [DOI] [PubMed] [Google Scholar]
- 2. Ferguson B., Matyszak M. K., Esiri M. M., Perry V. H. (1997) Axonal damage in acute multiple sclerosis lesions. Brain 120, 393–399 [DOI] [PubMed] [Google Scholar]
- 3. Trapp B. D., Peterson J., Ransohoff R. M., Rudick R., Mörk S., Bö L. (1998) Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 338, 278–285 [DOI] [PubMed] [Google Scholar]
- 4. McFarland H. F., Martin R. (2007) Multiple Sclerosis: a complicated picture of autoimmunity. Nat. Immunol. 8, 913–919 [DOI] [PubMed] [Google Scholar]
- 5. Oksenberg J. R., Panzara M. A., Begovich A. B., Mitchell D., Erlich H. A., Murray R. S., Shimonkevitz R., Sherritt M., Rothbard J., Bernard C. C., Steinman L. (1993) Selection for T-cell receptor V beta-D beta-J beta gene rearrangements with specificity for a myelin basic protein peptide in brain lesions of multiple sclerosis. Nature 362, 68–70 [DOI] [PubMed] [Google Scholar]
- 6. Genain C. P., Cannella B., Hauser S. L., Raine C. S. (1999) Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat. Med. 5, 170–175 [DOI] [PubMed] [Google Scholar]
- 7. Baranzini S. E., Jeong M. C., Butunoi C., Murray R. S., Bernard C. C., Oksenberg J. R. (1999) B Cell repertoire diversity and clonal expansion in multiple sclerosis brain lesions. J. Immunol. 163, 5133–5144 [PubMed] [Google Scholar]
- 8. Bernard C. C., Randell V. B., Horvath L. B., Carnegie P. R., Mackay I. R. (1981) Antibody to myelin basic protein in extracts of multiple sclerosis brain. Immunology 43, 447–457 [PMC free article] [PubMed] [Google Scholar]
- 9. Salvetti M., Giovannoni G., Aloisi F. (2009) Epstein-Barr virus and multiple sclerosis. Curr. Opin. Neurol. 22, 201–206 [DOI] [PubMed] [Google Scholar]
- 10. Brankin B., Allen I. V., Hawkins S. A., Wisdom G. B. (1988) Screening of multiple sclerosis cerebrospinal fluid for autoantibodies. J. Neurol. Sci. 84, 29–40 [DOI] [PubMed] [Google Scholar]
- 11. Dumont D., Noben J. P., Raus J., Stinissen P., Robben J. (2004) Proteomic analysis of cerebrospinal fluid from multiple sclerosis patients. Proteomics 4, 2117–2124 [DOI] [PubMed] [Google Scholar]
- 12. Pasinetti G. M., Ungar L. H., Lange D. J., Yemul S., Deng H., Yuan X., Brown R. H., Cudkowicz M. E., Newhall K., Peskind E., Marcus S., Ho L. (2006) Identification of potential CSF biomarkers in ALS. Neurology 66, 1218–1222 [DOI] [PubMed] [Google Scholar]
- 13. Finehout E. J., Franck Z., Choe L. H., Relkin N., Lee K. H. (2007) Cerebrospinal Fluid Proteomic Biomarkers for Alzheimer's Disease. Ann. Neurol. 61, 120–129 [DOI] [PubMed] [Google Scholar]
- 14. Han M. H., Hwang S. I., Roy D. B., Lundgren D. H., Price J. V., Ousman S. S., Fernald G. H., Gerlitz B., Robinson W. H., Baranzini S. E., Grinnell B. W., Raine C. S., Sobel R. A., Han D. K., Steinman L. (2008) Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature 451, 1076–1081 [DOI] [PubMed] [Google Scholar]
- 15. Lovato L., Cianti R., Gini B., Marconi S., Bianchi L., Armini A., Anghileri E., Locatelli F., Paoletti F., Franciotta D., Bini L., Bonetti B. (2008) Transketolase and 2′,3′-Cyclic-nucleotide 3′-Phosphodiesterase Type I Isoforms Are Specifically Recognized by IgG Autoantibodies in Multiple Sclerosis Patients. Mol. Cell. Proteomics 7, 2337–2349 [DOI] [PubMed] [Google Scholar]
- 16. Berger T., Rubner P., Schautzer F., Egg R., Ulmer H., Mayringer I., Dilitz E., Deisenhammer F., Reindl M. (2003) Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N. Engl. J. Med. 349, 139–145 [DOI] [PubMed] [Google Scholar]
- 17. Robinson W. H., Fontoura P., Lee B. J., de Vegvar H. E., Tom J., Pedotti R., DiGennaro C. D., Mitchell D. J., Fong D., Ho P. P., Ruiz P. J., Maverakis E., Stevens D. B., Bernard C. C., Martin R., Kuchroo V. K., van Noort J. M., Genain C. P., Amor S., Olsson T., Utz P. J., Garren H., Steinman L. (2003) Protein microarrays guide tolerizing DNA vaccine treatment of autoimmune encephalomyelitis. Nat. Biotechnol. 21, 1033–1039 [DOI] [PubMed] [Google Scholar]
- 18. Quintana F. J., Farez M. F., Viglietta V., Iglesias A. H., Merbl Y., Izquierdo G., Lucas M., Basso A. S., Khoury S. J., Lucchinetti C. F., Cohen I. R., Weiner H. L. (2008) Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc. Natl. Acad. Sci. U.S.A. 105, 18889–18894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tumani H., Pfeifle M., Lehmensiek V., Rau D., Mogel H., Ludolph A. C., Brettschneider J. (2009) Candidate biomarkers of chronic inflammatory demyelination polyneuropathy (CIDP): proteome analysis of cerebrospinal fluid. J. Neuroimmunol. 214, 109–112 [DOI] [PubMed] [Google Scholar]
- 20. Ranganathan S., Williams E., Ganchev P., Gopalakrishnan V., Lacomis D., Urbinelli L., Newhall K., Cudkowicz M. E., Brown R. H., Jr., Bowser R. (2005) Proteomic profiling of cerebrospinal fluid identifies biomarkers for amyotrophic lateral sclerosis. J Neurochem. 95, 1461–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Winges K. M., Gilden D. H., Bennett J. L., Yu X., Ritchie A. M., Owens G. P. (2007) Analysis of multiple sclerosis cerebrospinal fluid reveals a continuum of clonally related antibody-secreting cells that are predominantly plasma blasts. J. Neuroimmunol. 192, 226–234 [DOI] [PubMed] [Google Scholar]
- 22. von Büdingen H. C., Harrer M. D., Kuenzle,. S., Meier M., Goebels N. (2008) Clonally expanded plasma cells in the cerebrospinal fluid of MS patients produce myelin-specific antibodies. Eur. J. Immunol. 38, 2014–2023 [DOI] [PubMed] [Google Scholar]
- 23. Karnezis T., Mandemakers W., McQualter J. L., Zheng B., Ho P. P., Jordan K. A., Murray B. M., Barres B., Tessier-Lavigne M., Bernard C. C. (2004) The neurite outgrowth inhibitor Nogo A is involved in autoimmune-mediated demyelination. Nat. Neurosci. 7, 736–744 [DOI] [PubMed] [Google Scholar]
- 24. Wang D., Ayers M. M., Catmull D. V., Hazelwood L. J., Bernard C. C., Orian J. M. (2005) Astrocyte-associated axonal damage in pre-onset stages of experimental autoimmune encephalomyelitis. Glia 51, 235–240 [DOI] [PubMed] [Google Scholar]
- 25. Sherman D. L., Brophy P. J. (2005) Mechanisms of axon ensheathment and myelin growth. Nat. Rev. Neurosci. 6, 683–690 [DOI] [PubMed] [Google Scholar]
- 26. Susuki K., Rasband M. N. (2008) Molecular mechanisms of node of Ranvier formation. Curr. Opin. Cell Biol. 20, 616–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goto K., Kurihara T., Takahashi Y., Kondo H. (1990) Expression of genes for the myelin-specific proteins in Oligodendrocytes in vivo demands the presences of axons. Neurosci. Lett. 117, 269–274 [DOI] [PubMed] [Google Scholar]
- 28. Peles E., Salzer J. L. (2000) Molecular domains of myelinated axons. Curr. Opin. Neurobiol. 10, 558–565 [DOI] [PubMed] [Google Scholar]
- 29. Menon K., Rasband M. N., Taylor C. M., Brophy P., Bansal R., Pfeiffer S. E. (2003) The myelin-axolemmal complex: biochemical dissection and the role of galactosphingolipids. J. Neurochem. 87, 995–1009 [DOI] [PubMed] [Google Scholar]
- 30. Segal P., Teitelbaum D., Ohry A. (1983) Cell-mediated immunity to nervous system antigens in diabetic patients with neuropathy. Isr. J. Med. Sci. 19, 7–10 [PubMed] [Google Scholar]
- 31. Ejskjaer N. T., Zanone M. M., Peakman M. (1998) Autoimmunity in diabetic autonomic neuropathy: does the immune system get on your nerves? Diabet. Med. 15, 723–729 [DOI] [PubMed] [Google Scholar]
- 32. Vinik A. I., Anandacoomaraswamy D., Ullal J. (2005) Antibodies to neuronal structures: innocent bystanders or neurotoxins? Diabetes Care 28, 2067–2072 [DOI] [PubMed] [Google Scholar]
- 33. Wu Y., Li Q., Chen X. Z. (2007) Detecting protein-protein interactions by far western blotting. Nat Protoc 2, 3278–3284 [DOI] [PubMed] [Google Scholar]
- 34. Azari M., Ozturk E., Profyris C., Wang S., Small D., Bernard C. C .A., Petratos S. (2008) LIF treatment reduces Nogo-A deposits in spinal cord injury, modulating Rho GTPase activity and CRMP-2 phosphorilation. J. Neurodegeneration Regeneration 1, 23–29 [Google Scholar]
- 35. Petratos S., Gonzales M. F., Azari M. F., Marriott M., Minichiello R. A., Shipham K. A., Profyris C., Nicolaou A., Boyle K., Cheema S. S., Kilpatrick T. J. (2004) Expression of the low-affinity neurotrophin receptor, p75(NTR), is upregulated by oligodendroglial progenitors adjacent to the Subventricular zone in response to demyelination. Glia 48, 64–75 [DOI] [PubMed] [Google Scholar]
- 36. Senatorov V. V., Charles V., Reddy P. H., Tagle D. A., Chuang D. M. (2003) Overexpression and nuclear accumulation of glyceraldehyde-3-phosphate dehydrogenase in a transgenic mouse model of Huntington's disease. Mol. Cell. Neurosci. 22, 285–297 [DOI] [PubMed] [Google Scholar]
- 37. Dhar-Chowdhury P., Harrell M. D., Han S. Y., Jankowska D., Parachuru L., Morrissey A., Srivastava S., Liu W., Malester B., Yoshida H., Coetzee W. A. (2005) The glycolytic enzymes, glyceraldehyde-3-phosphate dehydrogenase, triose-phosphate isomerase, and pyruvate kinase are components of the K(ATP) channel macromolecular complex and regulate its function. J. Biol. Chem. 280, 38464–38470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oláh J., Orosz F., Puskás L. G., Hackler L., Jr., Horányi M., Polgár L., Hollán S., Ovádi J. (2005) Triosephosphate isomerase deficiency: consequences of an inherited mutation at mRNA, protein and metabolic levels. Biochem. J. 392, 675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lu Z. H., Chakraborty G., Ledeen R. W., Yahya D., Wu G. (2004) N-Acetylaspartate synthase is bimodally expressed in microsomes and mitochondria of brain. Brain Res. Mol. Brain Res. 122, 71–78 [DOI] [PubMed] [Google Scholar]
- 40. Moffett J. R., Ross B., Arun P., Madhavarao C. N., Namboodiri A. M. (2007) N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog. Neurobiol. 81, 89–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Petratos S., Azari M. F., Ozturk E., Papadopoulos R., Bernard C. C. A. (2010). Novel Therapeutic targets for axonal degeneration in multiple sclerosis. J. Neuropathol. Exp. Neurol. 69, 323–334 [DOI] [PubMed] [Google Scholar]
- 42. Vyshkina T., Kalman B. (2008) Autoantibodies and neurodegeneration in multiple sclerosis. Lab. Invest. 88, 796–807 [DOI] [PubMed] [Google Scholar]
- 43. Arimura N., Kaibuchi K. (2007) Neuronal polarity: from extracellular signals t o intracellular mechanisms. Nat. Rev. Neurosci. 8, 194–205 [DOI] [PubMed] [Google Scholar]
- 44. Petratos S., Li Q. X., George A. J., Hou X., Kerr M. L., Unabia S. E., Hatzinisiriou I., Maskel D., Aguilar M. I., Small D. H. (2008) The beta-amyloid protein of Alzheimer's disease increases neuronal CRMP-2 phosphorylation by a Rho-GTP mechanism. Brain 131, 90–108 [DOI] [PubMed] [Google Scholar]
- 45. Forooghian F., Adamus G., Sproule M., Westall C., O'Connor P. (2007) Enolase autoantibodies and retinal function in multiple sclerosis patients. Graefes Arch. Clin. Exp. Ophthalmol. 245, 1077–1084 [DOI] [PubMed] [Google Scholar]
- 46. Zhu C., Wang X., Deinum J., Huang Z., Gao J., Modjtahedi N., Neagu M. R., Nilsson M., Eriksson P. S., Hagberg H., Luban J., Kroemer G., Blomgren K. (2007) Cyclophilin A participates in the nuclear translocation of apoptosis-inducing factor in neurons after cerebral hypoxia-ischemia. J. Exp. Med. 204, 1741–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Setsuie R., Wada K. (2007) The functions of UCH-L1 and its relation to neurodegenerative diseases. Neurochem. Int. 51, 105–111 [DOI] [PubMed] [Google Scholar]
- 48. Wilkinson K. D., Lee K. M., Deshpande S., Duerksen-Hughes P., Boss J. M., Pohl J. (1989) The neuron-specific protein PGP 9.5 is a ubiuitin carboxyl-terminal hydrolase. Science 246, 670–673 [DOI] [PubMed] [Google Scholar]
- 49. Teunissen C. E., Dijkstra C., Polman C. (2005) Biological markers in CSF and blood for axonal degeneration in multiple sclerosis. Lancet Neurol. 4, 32–41 [DOI] [PubMed] [Google Scholar]
- 50. Sokoloff L. (1977) Relation between physiological function and energy metabolism in the central nervous system. J. Neurochem. 29, 13–26 [DOI] [PubMed] [Google Scholar]
- 51. Kolln J., Ren H. M., Da R. R., Zhang Y., Spillner E., Olek M., Hermanowicz N., Hilgenberg L. G., Smith M. A., van den Noort S., Qin Y. (2006) Triosephosphate isomerase- and glyceraldehyde-3-phosphate dehydrogenase-reactive autoantibodies in the cerebrospinal fluid of patients with multiple sclerosis. J. Immunol. 177, 5652–5658 [DOI] [PubMed] [Google Scholar]
- 52. Terrier B., Degand N., Guilpain P., Servettaz A., Guillevin L., Mouthon L. (2007) Alpha-enolase: a target of antibodies in infectious and autoimmune diseases. Autoimmun. Rev. 6, 176–182 [DOI] [PubMed] [Google Scholar]
- 53. Jensen N. A., Pedersen K. M., Celis J. E., West M. J. (1998) Failure of central nervous system myelination in MBP/c-myc transgenic mice: evidence for c-myc cytotoxicity. Oncogene. 16, 2123–2129 [DOI] [PubMed] [Google Scholar]
- 54. Liss B., Haeckel O., Wildmann J., Miki T., Seino S., Roeper J. (2005) K-ATP channels promote the differential degeneration of dopaminergic midbrain neurons. Nat. Neurosci. 8, 1742–1751 [DOI] [PubMed] [Google Scholar]
- 55. Laschet J. J., Minier F., Kurcewicz I., Bureau M. H., Trottier S., Jeanneteau F., Griffon N., Samyn B., Van Beeumen J., Louvel J., Sokoloff P., Pumain R. (2004) Glyceraldehyde-3-phosphate dehydrogenase is a GABAA receptor kinase linking glycolysis to neuronal inhibition. J. Neurosci. 24, 7614–7622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mazzola J. L., Sirover M. A. (2001) Reduction of glyceraldehyde-3-phosphate dehydrogenase activity in Alzheimer's disease and in Huntington's disease fibroblasts. J. Neurochem. 76, 442–449 [DOI] [PubMed] [Google Scholar]
- 57. Mohamed H. A., Mosier D. R., Zou L. L., Siklós L., Alexianu M. E., Engelhardt J. I., Beers D. R., Le W. D., Appel S. H. (2002) Immunoglobulin Fc gamma receptor promotes immunoglobulin uptake, immunoglobulin-mediated calcium increase, and neurotransmitter release in motor neurons. J. Neurosci. Res. 69, 110–116 [DOI] [PubMed] [Google Scholar]
- 58. Yamamoto T., Iwasaki Y., Konno H., Iizuka H., Zhao J. X. (1987) Retrograde transport and differential accumulation of serum proteins in motor neurons: implications for motor neuron diseases. Neurology 37, 843–846 [DOI] [PubMed] [Google Scholar]
- 59. Mor F., Izak M., Cohen I. R. (2005) Identification of aldolase as a target antigen in Alzheimer's disease. J. Immunol. 175, 3439–3445 [DOI] [PubMed] [Google Scholar]
- 60. Wang P., Saraswati S., Guan Z., Watkins C. J., Wurtman R. J., Littleton J. T. (2004) A Drosophila temperature-sensitive seizure mutant in phosphoglycerate kinase disrupts ATP generation and alters synaptic function. J. Neurosci. 24, 4518–4529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mahajan V. B., Pai K. S., Lau A., Cunningham D. D. (2000) Creatine kinase, an ATP-generating enzyme, is required for thrombin receptor signaling to the cytoskeleton. Proc. Natl. Acad. Sci. U.S.A. 97, 12062–12067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shavit E., Beilin O., Korczyn A. D., Sylantiev C., Aronovich R., Drory V. E., Gurwitz D., Horresh I., Bar-Shavit R., Peles E., Chapman J. (2008) Thrombin receptor PAR-1 on myelin at the node of Ranvier: a new anatomy and physiology of conduction block. Brain 131, 1113–1122 [DOI] [PubMed] [Google Scholar]
- 63. Dyer C. A., Matthieu J. M. (1994) Antibodies to myelin/oligodendrocyte-specific protein and myelin/oligodendrocyte glycoprotein signal distinct changes in the organization of cultured oligodendroglial membrane sheets. J. Neurochem. 62, 777–787 [DOI] [PubMed] [Google Scholar]
- 64. Marta C. B., Taylor C. M., Coetzee T., Kim T., Winkler S., Bansal R., Pfeiffer S. E. (2003) Antibody cross-linking of myelin oligodendrocyte glycoprotein leads to its rapid repartitioning into detergent-insoluble fractions, and altered protein phosphorylation and cell morphology. J. Neurosci. 23, 5461–5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mutsaers S. E., Carroll W. M. (1998) Focal accumulation of intraaxonal mitochondria in demyelination of the cat optic nerve. Acta Neuropathol. (Berl) 96, 139–143 [DOI] [PubMed] [Google Scholar]
- 66. Lu F., Selak M., O'Connor J., Croul S., Lorenzana C., Butunoi C., Kalman B. (2000) Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J. Neurol. Sci. 177, 95–103 [DOI] [PubMed] [Google Scholar]
- 67. Sergeant N., Wattez A., Galván-valencia M., Ghestem A., David J. P., Lemoine J., Sautiére P. E., Dachary J., Mazat J. P., Michalski J. C., Velours J., Mena-López R., Delacourte A. (2003) Association of ATP synthase alpha-chain with neurofibrillary degeneration in Alzheimer's disease. Neuroscience 117, 293–303 [DOI] [PubMed] [Google Scholar]
- 68. Yuan S., Fu Y., Wang X., Shi H., Huang Y., Song X., Li L., Song N., Luo Y. (2008) Voltage-dependent anion channel 1 is involved in endostatin-induced endothelial cell apoptosis. FASEB J. 22, 2809–2820 [DOI] [PubMed] [Google Scholar]
- 69. Shoshan-Barmatz V., Gincel D. (2003) The voltage dependent anion channel:characterization, modulation, and role in mitochondrial function in cell life and death. Cell. Biochem. Biophys. 39, 279–292 [DOI] [PubMed] [Google Scholar]
- 70. Zaid H., Abu-Hamad S., Israelson A., Nathan I., Shoshan-Barmatz V. (2005) The voltage dependent anion channel-1 modulates apoptotic cell death. Cell. Death. Differ. 12, 751–760 [DOI] [PubMed] [Google Scholar]
- 71. Butterfield D. A., Perluigi M., Sultana R. (2006) Oxidative stress in Alzheimer's disease brain: new insights from redox proteomics. Eur. J. Pharmacol. 545, 39–50 [DOI] [PubMed] [Google Scholar]
- 72. Ghosh T., Pandey N., Maitra A., Brahmachari S. K., Pillai B. (2007) A role for voltage-dependent anion channel Vdac1 in polyglutamine-mediated neuronal cell death. PLoS One. 2, e1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Siger-Zajdel M., Selmaj K. W. (2005) Proton magnetic resonance spectroscopy of normal appearing white matter in familial and sporadic multiple sclerosis. J. Neurol. 252, 830–832 [DOI] [PubMed] [Google Scholar]
- 74. Madhavarao C. N., Chinopoulos C., Chandrasekaran K., Namboodiri M. A. (2003) Characterization of the N-acetylaspartate biosynthetic enzyme from rat brain. J. Neurochem. 86, 824–835 [DOI] [PubMed] [Google Scholar]
- 75. Krantic S., Mechawar N., Reix S., Quirion R. (2005) Molecular basis of programmed cell death involved in neurodegeneration. Trends Neurosci. 28, 670–676 [DOI] [PubMed] [Google Scholar]
- 76. Forte M., Gold B. G., Marracci G., Chaudhary P., Basso E., Johnsen D., Yu X., Fowlkes J., Rahder M., Stem K., Bernardi P., Bourdette D. (2007) Cyclophilin D inactivation protects axons in experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Proc. Natl. Acad. Sci. U.S.A. 104, 7558–7563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen Y., Tian D., Ku L., Osterhout D. J., Feng Y. (2007) The selective RNA-binding protein quaking I (QKI) is necessary and sufficient for promoting oligodendroglia differentiation. J. Biol. Chem. 282, 23553–23560 [DOI] [PubMed] [Google Scholar]
- 78. Aquino D. A., Peng D., Lopez C., Farooq M. (1998) The constitutive heat shock protein-70 is required for optimal expression of myelin basic protein during differentiation of oligodendrocytes. Neurochem. Res. 23, 413–420 [DOI] [PubMed] [Google Scholar]
- 79. Lund B. T., Chakryan Y., Ashikian N., Mnatsakanyan L., Bevan C. J., Aguilera R., Gallaher T., Jakowec M. W. (2006) Association of MBP peptides with Hsp70 in normal appearing human white matter. J. Neurol. Sci. 249, 122–134 [DOI] [PubMed] [Google Scholar]
- 80. Mycko M. P., Cwiklinska H., Walczak A., Libert C., Raine C. S., Selmaj K. W. (2008) A heat shock protein gene (Hsp70.1) is critically involved in the generation of the immune response to myelin antigen. Eur. J. Immunol. 38, 1999–2013 [DOI] [PubMed] [Google Scholar]
- 81. Goldbaum O., Richter-Landsberg C. (2001) Stress proteins in oligodendrocytes: differential effects of heat shock and oxidative stress. J. Neurochem. 78, 1233–1242 [DOI] [PubMed] [Google Scholar]
- 82. Gurbuxani S., Schmitt E., Cande C., Parcellier A., Hammann A., Daugas E., Kouranti I., Spahr C., Pance A., Kroemer G., Garrido C. (2003) Heat shock protein 70 binding inhibits the nuclear import of apoptosis-inducing factor. Oncogene. 22, 6669–6678 [DOI] [PubMed] [Google Scholar]
- 83. Ricard D., Rogemond V., Charrier E., Aguera M., Bagnard D., Belin M. F., Thomasset N., Honnorat J. (2001) Isolation and expression pattern of human Unc-33-like phosphoprotein 6/collapsing response mediator protein 5 (Ulip6/CRMP5): coexistence with Ulip2/ CRMP2 in Sema3a- sensitive oligodendrocytes. J. Neurosci. 21, 7203–7214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fukata Y., Itoh T. J., Kimura T., Ménager C., Nishimura T., Shiromizu T., Watanabe H., Inagaki N., Iwamatsu A., Hotani H., Kaibuchi K. (2002) CRMP-2 binds to tubulin Mechanisms of Neuronal Polarization 45 heterodimers to promote microtubule assembly. Nat. Cell Biol. 4, 583–591 [DOI] [PubMed] [Google Scholar]
- 85. Inagaki N., Chihara K., Arimura N., Ménager C., Kawano Y., Matsuo N., Nishimura T., Amano M., Kaibuchi K. (2001) CRMP-2 induces axons in cultured hippocampal neurons. Nat. Neurosci. 4, 781–782 [DOI] [PubMed] [Google Scholar]
- 86. Maloney M. T., Bamburg J. R. (2007) Cofilin-mediated neurodegeneration in Alzheimer's disease and other amyloidopathies. Mol. Neurobiol. 35, 21–44 [DOI] [PubMed] [Google Scholar]
- 87. Lees F., MacDonald A. M., Turner J. W. (1964) Leber's disease with symptoms resembling disseminated sclerosis. J. Neurol. Neurosurg. Psychiatry 27, 415–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Smith P. R., Cooper J. M., Govan G. G., Riordan-Eva P., Harding A. E., Schapira A. H. (1995) Antibodies to human optic nerve in Leber's hereditary optic neuropathy. J. Neurol. Sci. 130, 134–138 [DOI] [PubMed] [Google Scholar]
- 89. Martínez-Yélamos A., Rovira A., Sánchez-Valle R., Martínez-Yélamos S., Tintoré M., Blanco Y., Graus F., Montalban X., Arbizu T., Saiz A. (2004) CSF 14–3-3 protein assay and MRI as prognostic markers in patients with a clinically isolated syndrome suggestive of MS. J. Neurol. 251, 1278–1279 [DOI] [PubMed] [Google Scholar]
- 90. Bartosik-Psujek H., Archelos J. J. (2004) Tau protein and 14–3-3 are elevated in the cerebrospinal fluid of patients with multiple sclerosis and correlate with intrathecal synthesis of IgG. J. Neurol. 251, 414–420 [DOI] [PubMed] [Google Scholar]
- 91. Birkenfeld J., Betz H., Roth D. (2003) Identification of cofilin and LIM domain-containing protein kinase 1 as novel interaction partners of 14–3-3z. Biochem. J. 369, 45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Soosairajah J., Maiti S., Wiggan O., Sarmiere P., Moussi N., Sarcevic B., Sampath R., Bamburg J. R., Bernard O. (2005) Interplay between components of a novel LIM kinase-slingshot phosphatase complex regulates cofilin. EMBO J. 24, 473–486 [DOI] [PMC free article] [PubMed] [Google Scholar]