Abstract
Maternal smoking during pregnancy greatly enhances perinatal morbidity/mortality and is the major risk factor for Sudden Infant Death Syndrome (SIDS). Studies in developing rodents indicate that nicotine is a neuroteratogen that targets monoamine pathways involved in the responses to hypoxia that are in turn, hypothesized to contribute to these adverse events. We administered nicotine to pregnant Rhesus monkeys from gestational day 30 through 160 by continuous infusion, achieving maternal plasma levels comparable to those in smokers; we examined neurochemical parameters immediately after Caesarean delivery at the end of the exposure period. Nicotine evoked elevations in brainstem serotonin levels and serotonin turnover, indicating hyperactivity of these pathways. The same treatment evoked a deficit in cardiac norepinephrine levels. Both effects were offset by coadministration of the antioxidant, Vitamin C. Brainstem serotonin hyperinnervation is a hallmark of SIDS, and the hyperactivity seen here can also account for the downregulation of serotonin receptors noted in this disorder. Deficient cardiac sympathetic innervation is also consistent with increased vulnerability to hypoxia during delivery or in the agonal event in SIDS. Our results thus indicate that nicotine exposure in a primate model produces brainstem and autonomic abnormalities of the key monoamine systems that govern the response to hypoxia, indicate an important role of oxidative stress in the adverse effects, and point to potential amelioration strategies that could offset these particular effects of nicotine.
Keywords: Brainstem, Nicotine, Norepinephrine, Oxidative Stress, Serotonin, Sudden Infant Death Syndrome, Vitamin C
Introduction
In developed countries, maternal smoking during pregnancy is the largest, preventable cause of perinatal morbidity and mortality and the majority of women who smoke continue to do so through pregnancy [31]. Indeed, with the success of the Back-To-Sleep campaign, tobacco exposure now accounts for the majority of the remaining incidence of Sudden Infant Death Syndrome (SIDS), the leading cause of death in the first 18 months of life [8,12,16,21,29]. These adverse outcomes are directly related to each other, since vaginal delivery and SIDS both involve the ability to survive acute hypoxia, involving patterns of cardiorespiratory responses that are unique to the neonate [18,19]. Although tobacco smoke contains thousands of biologically active compounds, nicotine itself is a major contributor to neurodevelopmental and autonomic abnormalities [33,34]. Accordingly, there are serious concerns as to whether the use of nicotine replacement products to achieve smoking cessation in pregnant women actually lessens perinatal risk or the subsequent appearance of neurodevelopmental deficits [4,5,20,26,34].
Although animal models have provided the key evidence for nicotine as a neuroteratogen, most of these studies have been carried out in rodents; because rats and mice are born much more immature than humans, this complicates the ability to examine vulnerability of the developing organism to nicotine over a span comparable to that in human fetal development. In the current study, we used a primate model to examine the effects of prenatal nicotine exposure on two of the neural pathways known to be targeted in SIDS, serotonergic pathways in the brainstem and cardiac sympathetic innervation from noradrenergic inputs [9,11,13,14,18,40]. Defects in both these targets are also characteristics of prenatal nicotine exposure in rodent models [9,22,35]. We evaluated the concentrations of serotonin (5HT) and its metabolite, 5-hydroxyindoleacetic acid (5HIAA) and calculated 5HT turnover (5HIAA/5HT) so as to evaluate the net impulse activity of this pathway. In the heart, we measured the norepinephrine concentration to determine the degree of sympathetic innervation. Finally, to assess the potential contribution of oxidative stress, we determined whether coadministration of Vitamin C could offset the effects of nicotine. Our specific aims were to characterize whether prenatal nicotine exposure in a primate model affects these particular monoaminergic pathways in a manner compatible with observations from SIDS victims, and whether these could be prevented with a strategy aimed at reducing oxidative stress from nicotine exposure.
Materials and Methods
All studies were carried out with the approval of the Oregon National Primate Research Center s Institutional Animal Care and Use Committee, in accordance with the declaration of Helsinki and with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. Sixty-seven pregnant Rhesus macaque monkeys were divided into eight treatment groups that received treatments beginning on day 30 of pregnancy (the earliest point at which pregnancy can be reliably detected), continuing through day 160. Four groups received nicotine by continuous infusion using subcutaneously-implanted osmotic minipumps with a four-week capacity (Alzet type 2ML4; Durect Corp., Cupertino CA) containing nicotine bitartrate (Sigma Chemical Co., St. Louis, MO) dissolved in bacteriostatic water, set to deliver 2 mg/kg/day (equivalent to 0.7 mg/kg/day of nicotine free base), whereas the four groups not receiving nicotine had pumps containing only bacteriostatic water. Pumps were implanted under ketamine anesthesia and were changed every three weeks to maintain a steady-state infusion throughout pregnancy; minipump changes were carried out under the direction of a veterinary surgeon following strict sterile protocols. This regimen produces maternal plasma levels of about 30 ng/ml for nicotine and 120 for cotinine [39], both well within the range of values typical of maternal smoking in pregnant smokers consuming 10-20 cigarettes per day [23][39]; importantly, total nicotine delivery and average daily nicotine plasma levels are similar in pregnant smokers and in users of transdermal nicotine patches [24]. Concurrently with the nicotine or vehicle infusion, animals received daily oral supplementation with Vitamin C in the form of a chewable vitamin pill; there were four different Vitamin C dose levels (0, 50, 100, 250 mg per day). These Vitamin C regimens have been shown previously to offset the effects of prenatal nicotine on pulmonary function [27] and deliver approximately 5-10 times the amount of Vitamin C per kg body weight as would be taken by a pregnant woman in a standard dietary supplement although it is important to note that Vitamin C is substantially reduced by maternal smoking [30].
On the 160th day of pregnancy (full term is 165 days), animals were anesthetized with ketamine and isoflurane and the fetuses delivered by Caesarean section as described previously [32], so as to ensure that all fetuses were sampled at the same gestational age. The sex was noted and tissue samples were dissected just as in our earlier study [39], taking the right half of the brainstem and a cardiac section across the left and right ventricle, being careful to use exactly the same location in every animal All tissue samples were shipped on dry ice by overnight express to Duke University and were stored at -80° C until analyzed.
For determinations of 5HT and 5HIAA, brainstem samples were thawed and homogenized in ice-cold 0.1 M perchloric acid and sedimented for 20 min at 40,000 × g. The supernatant solution was collected and aliquots were analyzed by high-performance liquid chromatography with electrochemical detection [38,41]. Cardiac samples were subjected to trace enrichment by alumina adsorption prior to chromatography, with dihydroxybenzylamine included as an internal standard to correct for recovery. Concurrently-run standards, containing each of the neurotransmitters and metabolites (Sigma), were used to calculate the concentration of each neurochemical.
The treatment groups resulted in 9 controls (4 males, 5 females), 9 nicotine alone (6 males, 3 females), 8 Vitamin C 50 mg/day (5 males, 3 females), 7 Vitamin C 100 mg/day (5 males, 2 females), 8 Vitamin C 250 mg/day (2 males, 6 females, 9 nicotine + Vitamin C 50 mg/day (5 males, 3 females), 9 nicotine + Vitamin C 100 mg/day (7 males, 2 females), and 8 nicotine + Vitamin C 250 mg/day (3 males, 5 females). Data were compiled as mean ± SE. Statistical evaluations were carried out first by multivariate ANOVA, with factors of drug treatment (control, nicotine), Vitamin C dose (0, 50, 100, 250 mg/day) and sex. In this initial test, there were no significant differences among the various Vitamin C treatments, so those groups were collapsed into two divisions (with or without Vitamin C). Similarly, we did not observe any interactions between treatment and sex, so values are presented combined for males and females. The results are thus presented as two-factor comparisons (drug treatment, with/without Vitamin C). Where the ANOVA indicated a significant interaction between Vitamin C and nicotine, we performed lower-order pairwise tests (Fisher s Protected Least Significant Difference) to establish which groups differed from each other; where there was no interaction, only main effects of each treatment are reported. Significance was assumed at p < 0.05.
Results
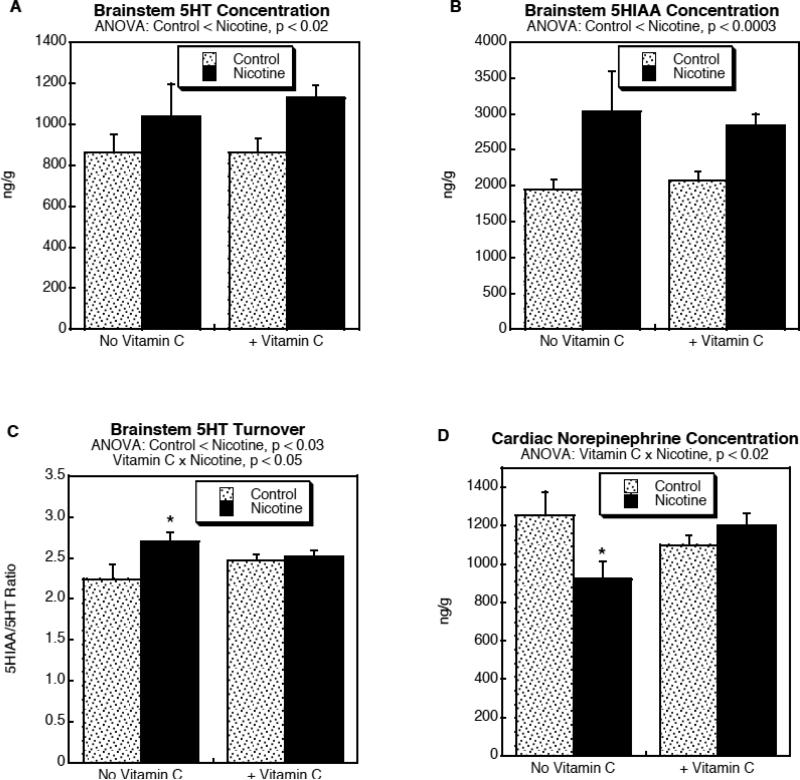
Prenatal nicotine exposure evoked significant increases in both brainstem 5HT (Fig. 1A; p < 0.02 for the main treatment effect of nicotine) and 5HIAA (Fig. 1B; p < 0.0003 for the main treatment effect of nicotine), regardless of whether the animals received cotreatment with Vitamin C. The nicotine effect on 5HIAA was twice as large as that on 5HT, so that nicotine exposure evoked a significant increase in 5HT turnover (Fig. 1C; p < 0.03 for the main treatment effect of nicotine). However, there was a differential effect on the two parameters when animals were cotreated with Vitamin C (p < 0.05 for Vitamin C × Nicotine interaction), so that 5HT turnover was unaffected by the combined treatment (Fig. 1C). In the heart, prenatal nicotine exposure evoked a significant reduction in norepinephrine levels (p < 0.02 for the main treatment effect of nicotine) and again, this effect was reversed by Vitamin C cotreatment (Fig. 1D; p < 0.02 for Vitamin C × Nicotine interaction).
Figure 1.
Effects of prenatal nicotine exposure, with and without Vitamin C treatment, on brainstem 5HT (A), 5HIAA (B) and 5HT turnover, (C) and on cardiac norepinephrine levels (D). Data represent mean ± SE. ANOVA for each variable appears at the top of the panels, showing the main treatment effects and interactions; where there was a significant interaction between Vitamin C and nicotine (C,D), asterisks denote the values where the nicotine group differs from the corresponding control.
Discussion
Our studies thus point to two major abnormalities in monoamine pathways known to play a role in the ability of the neonate to survive of hypoxia, and also known to be affected in SIDS: brainstem serotonergic pathways and cardiac sympathetic innervation. Examination of postmortem tissues from infants that died of SIDS show an increased density of 5HT neurons in the brainstem, along with decreased expression of 5HT receptors [18]; these sites are involved in autonomic function, arousal and cardiorespiratory responses to challenges such as hypoxia. Similarly, as seen here, nicotine administration to fetal monkeys produced elevations in both the 5HT concentration and 5HT turnover, indicative of generalized hyperactivity of these pathways. In turn, presynaptic hyperactivity would be expected to evoke 5HT receptor downregulation, which implies that the receptor deficit seen in SIDS is not the primary deficit, but rather an adaptive reaction to alterations arising from the abnormality in presynaptic innervation.
The other limb of hypoxia/SIDS vulnerability is peripheral autonomic imbalance, with loss of sympathetic response relative to parasympathetic [9,11,13,14,40]. Studies in rodents show that prenatal nicotine exposure can recapitulate these outcomes [9,22,35]. Here, we similarly found a reduction in cardiac norepinephrine levels, consistent with a reduction in sympathetic input. By itself, reduced cardiac sympathetic function is known to increase the mortality associated with hypoxic challenge [22,36,37]. This clearly could provide a mechanistic connection to adverse outcomes during the hypoxia associated with delivery or the triggering event in SIDS, especially in the subset of individuals predisposed to cardiac conduction defects [3].
Perhaps our interesting finding, though, was that coadministration of Vitamin C offset some of the effects. Although these animals still displayed elevated brainstem 5HT, the antioxidant prevented the increase in 5HT turnover, implying that, although innervation was still augmented, the nerve terminals did not become hyperactive. Similarly, Vitamin C completely prevented the decrement in cardiac norepinephrine levels. The implications are clear both in terms of the underlying mechanism for the effects of nicotine and for potential therapeutic interventions that might reduce the risks associated with maternal smoking during pregnancy. Prevention by Vitamin C implicates oxidative stress as a significant contributor to the adverse effects of nicotine on these systems. Nicotine is known to cause oxidative stress in the fetus [6,15,17] and the offspring of women who smoke during pregnancy similarly show hallmarks of oxidative damage [2,10,25,28]. Nevertheless, prevention by Vitamin C is not uniformly advantageous. In earlier work, we showed that the same cotreatment worsened fetal brain damage associated with nicotine s other major target, activation of nicotinic acetylcholine receptors [39], likely because of pharmacokinetic interactions that increased the concentration of nicotine and cotinine in the fetal compartment [39]. This points out the importance of recognizing that nicotine acts through multiple toxicant mechanisms, with major contributions from targeting of receptor-driven pathways as well as more generalized effects involving oxidative stress. Nevertheless, the current findings begin to point to interventions that may offset some of the major liabilities of maternal smoking during pregnancy. Because we did not examine lower nicotine exposures in our primate model, it is difficult to assess whether our conclusions also extend to lower nicotine exposures such as those involving second-hand smoke or nicotine replacement products that deliver less total nicotine than transdermal patches. Nevertheless, second-hand smoke exposure is also a risk factor for SIDS [1,7], so it would be worthwhile to pursue whether lower doses produce similar neurotransmitter abnormalities and whether these, too, are prevented by Vitamin C.
The present results thus provide some of the first evidence in a primate model that prenatal exposure to nicotine targets the same neural pathways that operate in SIDS and show how identification of an underlying mechanism can lead to therapeutic strategies to prevent or offset an eventual, adverse outcome. Equally important, the fact that these changes are seen with nicotine alone supports the need for caution in recommending nicotine replacement therapy in pregnant smokers as a strategy for reduced fetal harm.
Acknowledgments
Acknowledgments/disclaimers Research was supported by NIH ES10356, RR00163 and HL087710. TAS has provided expert witness testimony in the past three years at the behest of the following law firms: The Calwell Practice (Charleston WV), Weltchek Mallahan & Weltchek (Lutherville MD), Finnegan Henderson Farabow Garrett & Dunner (Washington DC), Carter Law (Peoria IL), Gutglass Erickson Bonville & Larson (Madison WI), The Killino Firm (Philadelphia PA), Alexander Hawes (San Jose, CA) and the Shanahan Law Group (Raleigh, NC).
Abbreviations
- 5HT
serotonin (5-hydroxytryptamine)
- 5HIAA
5-hydroxyindoleacetic acid
- ANOVA
analysis of variance
- SIDS
Sudden Infant Death Syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Adgent MA. Environmental tobacco smoke and sudden infant death syndrome: a review. Birth Defects Res B. 2006;77:69–85. doi: 10.1002/bdrb.20068. [DOI] [PubMed] [Google Scholar]
- 2.Aycicek A, Ipek A. Maternal active or passive smoking causes oxidative stress in cord blood. Eur J Pediatr. 2008;167:81–85. doi: 10.1007/s00431-007-0433-z. [DOI] [PubMed] [Google Scholar]
- 3.Baruteau AE, Baruteau J, Joomye R, Martins R, Treguer F, Baruteau R, Daubert JC, Mabo P, Roussey M. Role of congenital long-QT syndrome in unexplained sudden infant death: proposal for an electrocardiographic screening in relatives. Eur J Pediatr. 2009;168:771–777. doi: 10.1007/s00431-009-0951-y. [DOI] [PubMed] [Google Scholar]
- 4.Blood-Siegfried J, Rende EK. The long-term effects of prenatal nicotine exposure on neurologic development. J Midwifery Women's Health. 2010;55:143–152. doi: 10.1016/j.jmwh.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruin JE, Gerstein HC, Holloway AC. Long-term consequences of fetal and neonatal nicotine exposure: a critical review. Toxicol Sci. 2010;116:364–374. doi: 10.1093/toxsci/kfq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruin JE, Petre MA, Lehman MA, Raha S, Gerstein HC, Morrison KM, Holloway AC. Maternal nicotine exposure increases oxidative stress in the offspring. Free Radical Biol Med. 2008;44:1919–1925. doi: 10.1016/j.freeradbiomed.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 7.DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics. 2004;113:1007–1015. [PubMed] [Google Scholar]
- 8.DiFranza JR, Lew RA. Effect of maternal cigarette smoking on pregnancy complications and Sudden Infant Death Syndrome. J Family Pract. 1995;40:385–394. [PubMed] [Google Scholar]
- 9.Duncan JR, Garland M, Myers MM, Fifer WP, Yang M, Kinney HC, Stark RI. Prenatal nicotine-exposure alters fetal autonomic activity and medullary neurotransmitter receptors: implications for sudden infant death syndrome. J Appl Physiol. 2009;107:1579–1590. doi: 10.1152/japplphysiol.91629.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fayol L, Gulian JM, Dalmasso C, Calaf R, Simeoni U, Millet V. Antioxidant status of neonates exposed in utero to tobacco smoke. Biol Neonate. 2005;87:121–126. doi: 10.1159/000082128. [DOI] [PubMed] [Google Scholar]
- 11.Fifer WP, Fingers ST, Youngman M, Gomez-Gribben E, Myers MM. Effects of alcohol and smoking during pregnancy on infant autonomic control. Dev Psychobiol. 2009;51:234–242. doi: 10.1002/dev.20366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleming P, Blair PS. Sudden Infant Death Syndrome and parental smoking. Early Human Dev. 2007;83:721–725. doi: 10.1016/j.earlhumdev.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 13.Franco P, Szliwowski H, Dramaix M, Kahn A. Decreased autonomic responses to obstructive sleep events in future victims of sudden infant death syndrome. Pediatr Res. 1999;46:33–39. doi: 10.1203/00006450-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Gootman PM, Gootman N, Sica AL. A neuro-cardiac theory for Sudden Infant Death Syndrome: role of the autonomic nervous system. J Sudden Infant Death Infant Mort. 1996;1:169–182. [Google Scholar]
- 15.Gunes T, Koklu E, Gunes I, Narin F, Koklu S, Koklu S. Influence of maternal nicotine exposure on neonatal rat oxidant-antioxidant system and effect of ascorbic acid supplementation. Human Exp Toxicol. 2008;27:781–786. doi: 10.1177/0960327107082229. [DOI] [PubMed] [Google Scholar]
- 16.Habek D, Habek JC, Ivanisevic M, Djelmis J. Fetal tobacco syndrome and perinatal outcome. Fetal Diag Ther. 2002;17:367–371. doi: 10.1159/000065387. [DOI] [PubMed] [Google Scholar]
- 17.Halima BA, Sarra K, Kais R, Salwa E, Najoua G. Indicators of oxidative stress in weanling and pubertal rats following exposure to nicotine via milk. Hum Exp Toxicol. 2010;29:489–496. doi: 10.1177/0960327109354440. [DOI] [PubMed] [Google Scholar]
- 18.Kinney HC. Brainstem mechanisms underlying the sudden infant death syndrome: evidence from human pathologic studies. Dev Psychobiol. 2009;51:223–233. doi: 10.1002/dev.20367. [DOI] [PubMed] [Google Scholar]
- 19.Lagercrantz H, Slotkin TA. The “stress” of being born. Sci Am. 1986;254(April):100–107. doi: 10.1038/scientificamerican0486-100. [DOI] [PubMed] [Google Scholar]
- 20.Maritz GS. Are nicotine replacement therapy, varenicline or bupropion options for pregnant mothers to quit smoking? Effects on the respiratory system of the offspring. Ther Adv Respir Dis. 2009;3:193–210. doi: 10.1177/1753465809343712. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell EA, Milerad J. Smoking and the sudden infant death syndrome. Rev Environ Health. 2006;21:81–103. doi: 10.1515/reveh.2006.21.2.81. [DOI] [PubMed] [Google Scholar]
- 22.Navarro HA, Mills E, Seidler FJ, Baker FE, Lappi SE, Tayyeb MI, Spencer JR, Slotkin TA. Prenatal nicotine exposure impairs β-adrenergic function: persistent chronotropic subsensitivity despite recovery from deficits in receptor binding. Brain Res Bull. 1990;25:233–237. doi: 10.1016/0361-9230(90)90066-9. [DOI] [PubMed] [Google Scholar]
- 23.Ogburn PL, Hurt RD, Croghan IT, Schroeder DR, Ramin KD, Offord KP, Moyer TP. Nicotine patch use in pregnant smokers: nicotine and cotinine levels and fetal effects. Am J Obstet Gynecol. 1999;181:736–743. doi: 10.1016/s0002-9378(99)70521-1. [DOI] [PubMed] [Google Scholar]
- 24.Oncken CA, Hardardottir H, Hatsukami DK, Lupo VR, Rodis JF, Smeltzer JS. Effects of transdermal nicotine or smoking on nicotine concentrations and maternal-fetal hemodynamics. Obstet Gynecol. 1997;90:569–574. doi: 10.1016/s0029-7844(97)00309-8. [DOI] [PubMed] [Google Scholar]
- 25.Orhon FS, Ulukol B, Kahya D, Cengiz B, Baskan S, Tezcan S. The influence of maternal smoking on maternal and newborn oxidant and antioxidant status. Eur J Pediatr. 2009;168:975–981. doi: 10.1007/s00431-008-0873-0. [DOI] [PubMed] [Google Scholar]
- 26.Pauly JR, Slotkin TA. Maternal tobacco smoking, nicotine replacement and neurobehavioural development. Acta Pædiatr. 2008;97:1331–1337. doi: 10.1111/j.1651-2227.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- 27.Proskocil BJ, Sekhon HS, Clark JA, Lupo SL, Jia Y, Hull WM, Whitsett JA, Starcher BC, Spindel ER. Vitamin C prevents the effects of prenatal nicotine on pulmonary function in newborn monkeys. Am J Respir Crit Care Med. 2005;171:1032–1039. doi: 10.1164/rccm.200408-1029OC. [DOI] [PubMed] [Google Scholar]
- 28.Rossner P, Jr., Milcova A, Libalova H, Novakova Z, Topinka J, Balascak I, Sram RJ. Biomarkers of exposure to tobacco smoke and environmental pollutants in mothers and their transplacental transfer to the foetus. Part II. Oxidative damage. Mutation Res. 2009;669:20–26. doi: 10.1016/j.mrfmmm.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Salihu HM, Wilson RE. Epidemiology of prenatal smoking and perinatal outcomes. Early Human Dev. 2007;83:713–720. doi: 10.1016/j.earlhumdev.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES) Am J Clin Nutr. 2009;90:1252–1263. doi: 10.3945/ajcn.2008.27016. [DOI] [PubMed] [Google Scholar]
- 31.Schneider S, Huy C, Schutz J, Diehl K. Smoking cessation during pregnancy: a systematic literature review. Drug Alcohol Rev. 2010:81–90. doi: 10.1111/j.1465-3362.2009.00098.x. [DOI] [PubMed] [Google Scholar]
- 32.Sekhon HS, Keller JA, Benowitz NL, Spindel ER. Prenatal nicotine exposure alters pulmonary function in newborn rhesus monkeys. Am J Respir Crit Care Med. 2001;164:989–994. doi: 10.1164/ajrccm.164.6.2011097. [DOI] [PubMed] [Google Scholar]
- 33.Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Slotkin TA. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol Teratol. 2008;30:1–19. doi: 10.1016/j.ntt.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Slotkin TA, Cho H, Whitmore WL. Effects of prenatal nicotine exposure on neuronal development: selective actions on central and peripheral catecholaminergic pathways. Brain Res Bull. 1987;18:601–611. doi: 10.1016/0361-9230(87)90130-4. [DOI] [PubMed] [Google Scholar]
- 36.Slotkin TA, Lappi SE, McCook EC, Lorber BA, Seidler FJ. Loss of neonatal hypoxia tolerance after prenatal nicotine exposure: implications for Sudden Infant Death Syndrome. Brain Res Bull. 1995;38:69–75. doi: 10.1016/0361-9230(95)00073-n. [DOI] [PubMed] [Google Scholar]
- 37.Slotkin TA, Saleh JL, McCook EC, Seidler FJ. Impaired cardiac function during postnatal hypoxia in rats exposed to nicotine prenatally: implications for perinatal morbidity and mortality, and for Sudden Infant Death Syndrome. Teratology. 1997;55:177–184. doi: 10.1002/(SICI)1096-9926(199703)55:3<177::AID-TERA2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 38.Slotkin TA, Seidler FJ, Ali SF. Cellular determinants of reduced adaptability of the aging brain: neurotransmitter utilization and cell signaling responses after MDMA lesions. Brain Res. 2000;879:163–173. doi: 10.1016/s0006-8993(00)02767-0. [DOI] [PubMed] [Google Scholar]
- 39.Slotkin TA, Seidler FJ, Qiao D, Aldridge JE, Tate CA, Cousins MM, Proskocil BJ, Sekhon HS, Clark JA, Lupo SL. Effects of prenatal nicotine exposure on primate brain development and attempted amelioration with supplemental choline or vitamin C: neurotransmitter receptors, cell signaling and cell development biomarkers in fetal brain regions of Rhesus monkeys. Neuropsychopharmacology. 2005;30:129–144. doi: 10.1038/sj.npp.1300544. others. [DOI] [PubMed] [Google Scholar]
- 40.Tong S, Ingenito S, Anderson JE, Gootman N, Sica AL, Gootman PM. Development of a swine animal model for the study of sudden infant death syndrome. Lab Animal Sci. 1995;45:398–403. [PubMed] [Google Scholar]
- 41.Xu Z, Seidler FJ, Ali SF, Slikker W, Slotkin TA. Fetal and adolescent nicotine administration: effects on CNS serotonergic systems. Brain Res. 2001;914:166–178. doi: 10.1016/s0006-8993(01)02797-4. [DOI] [PubMed] [Google Scholar]