Abstract
Thioredoxin is an important reducing molecule in biological systems. Increasing CYP2E1 activity induces oxidative stress and cell toxicity. However, whether thioredoxin protects cells against CYP2E1 induced oxidative stress and toxicity is unknown. SiRNA were used to knockdown either cytosolic (TRX-1) or mitochondrial thioredoxin (TRX-2) in HepG2 cells expressing CYP2E1 (E47 cells) or without expressing CYP2E1 (C34 cells). Cell viability decreased 40–60% in E47 but not C34 cells with 80–90% knockdown of either TRX-1 or TRX-2. Depletion of either thioredoxin also potentiated the toxicity by either a glutathione synthesis inhibitor or TNFα in E47 cells. Generation of reactive oxygen species and 4-HNE protein adducts increased in E47 but not C34 cells with either thioredoxin knockdown. GSH was decreased and adding GSH completely blocked E47 cell death induced by either thioredoxin knockdown. Lowering TRX-1 or TRX-2 in E47 cells caused an early activation of ASK-1, followed by phosphorylation of JNK1 after 48 hrs of siRNA treatment. JNK inhibitor caused a partial recovery of E47 cell viability after thioredoxin knockdown. In conclusion, knockdown of TRX-1 or TRX-2 sensitizes cells to CYP2E1 induced oxidant stress partially via ASK-1 and JNK1 signaling pathways. Both TRX-1 and TRX-2 are important for defense against CYP2E1-induced oxidative stress.
Keywords: thioredoxin, CYP2E1, HepG2 cells, oxidative stress, cell toxicity
Introduction
The thioredoxin system plays a key role in modulating redox signaling pathways which regulate physiological as well as pathophysiological processes [1–2]. The thioredoxin system includes thioredoxin, thioredoxin reductase, and thioredoxin peroxidases. Thioredoxin has a conserved catalytic site (-Cys-Gly-Pro-Cys-Lys-) that undergoes reversible oxidation to the cystine disulfide. Oxidized thioredoxin is a major substrate for thioredoxin reductase, and reduced thioredoxin serves as an electron carrier to reduce peroxiredoxins. The oxidized thioredoxin is reduced back to the reduced form by thioredoxin reductase [3–4]. There are two main thioredoxins: thioredoxin-1 (TRX-1), a cytosolic form; and thioredoxin-2 (TRX-2), a mitochondrial form [3]. Activity has been found: outside the cell, where thioredoxin plays a role in regulating cell growth and chemotaxis [5]; in the cytoplasm, where it functions as an antioxidant and a reductant cofactor [6]; in the nucleus, regulating transcription factor activity [7]; and in the mitochondria, where it also functions as an antioxidant [8].
Thioredoxin is not only important because of its reducing power and antioxidant activity. Modification of thiols in thioredoxin interrupts signaling mechanisms involved in cell growth, proliferation, and apoptosis. The role of thioredoxin in the regulation of the activation of apoptosis signal-regulating kinase-1 (ASK-1) and downstream apoptosis pathways has been reported in multiple studies [9–11]. Thioredoxin can associate with the N-terminal portion of ASK-1 in vitro and in vivo. Expression of thioredoxin inhibited ASK-1 kinase activity and the subsequent ASK-1-dependent apoptosis [10]. In resting cells, endogenous ASK-1 constitutively forms a complex which includes thioredoxin. Upon ROS stimulation, the ASK-1 unbinds from thioredoxin and forms a fully activated higher-molecular-mass complex [12]. TNFα increases oxidative stress in mice with elevated CYP2E1, with subsequent activation of ASK-1 via a mechanism involving thioredoxin-ASK-1 dissociation, followed by activation of downstream MKK and MAPK [11]. A study with troglitazone also showed that increased intramitochondrial oxidant stress activates the TRX-2/ASK-1 pathway, leading to mitochondrial membrane permeabilization [13].
Both TRX-1 and TRX-2 are involved in the protection from oxidative stress. TRX-2 plays an important role in protecting the mitochondria against oxidative stress and in protecting cells from ROS-induced apoptosis. It is required for normal development of mice embryo and actively respiring cells. The absence of TRX-2 causes massive apoptosis and early embryonic lethality in homozygous mice [14]. Also, TRX-2 haploinsufficiency in mice results in impaired mitochondrial function and increased oxidative stress after diquat treatment [8]. Overexpression of human TRX-2 confers resistance to the oxidant tert-butylhydroperoxide-induced apoptosis in human osteosarcoma cells [15]. As for TRX-1, overexpression of human TRX-1 reduces oxidative stress in the placenta of transgenic mice and promotes fetal growth [16]. Supplementation of human recombinant TRX-1 to mice fed a Lieber DeCarli ethanol diet decreased several markers of oxidative stress, inflammatory cytokine expression and apoptosis in liver [17].
CYP2E1 is of interest in liver injury because of its ability to metabolize and activate many toxicological substrates, including ethanol, to more reactive toxic products. Levels of CYP2E1 are elevated under a variety of physiological and pathophysiological conditions, and after acute and chronic alcohol treatment. CYP2E1 is an effective generator of reactive oxygen species [18]. Since thioredoxin is a reducing molecule which can decrease oxidative stress, the goal of this study was to evaluate whether thioredoxin can inhibit the oxidative stress induced by CYP2E1, and whether there is any difference in the function of TRX-1 versus TRX-2 in blunting CYP2E1 oxidant stress. SiRNA for either TRX-1 or TRX-2 was added to HepG2 cells with CYP2E1 expression (E47 cells) or without CYP2E1 expression (C34 cells) to test: 1. whether thioredoxin decreases oxidative stress and injury induced by CYP2E1; 2. considering the compartmentation of thioredoxin, whether TRX-1 or TRX-2 has a stronger protective effect in preventing against this injury and oxidative stress? 3. what is the mechanism of the protection by thioredoxin from cell death in CYP2E1 expressing cells?
Materials and Methods
Reagents and chemicals
ON-TARGETplus Non-targeting pool siRNA, TRX (TRX-1) ON-TARGETplus SMARTpool siRNA, TRX-2 ON-TARGETplus SMARTpool siRNA, and Dharmafect I transfection reagent were from Dharmacon Research (Lafayette, CO, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), glutathione ethyl ester (GSSE), cycloheximide, and L-Buthionine-[R,S]-sulfoximine (BSO) were purchased from Sigma (St. Louis, MO, USA). Murine TNFα was from Fitzgerald (Concord, MA, USA). Antibody against TRX-1, p-ASK1 and PDI was from Cell Signaling Technology (Beverly, MA, USA). Antibody against TRX-2 was from Santa Cruz Biotechnology INC (Santa Cruz, CA, USA). Antibody against LC3 was purchased from Thermo Scientific (Rockford, IL, USA). Antibody against CYP2E1 was a generous gift from Dr. Jerry Lasker. Fluorescence-conjugated secondary antibodies used in the Odyssey infrared imaging system were from Li-Cor Biosciences (Lincoln, NE, USA). All other antibodies were from Santa Cruz Biotechnology INC (Santa Cruz, CA, USA). Total ROS detection kit was purchased from ENZO Life Sciences (Plymouth Meeting, PA, USA). Histostain Plus Broad Spectrum (ACE) kit was from Invitrogen (Camarillo, CA, USA). The JNK inhibitor, L-JNKI1 was purchased from EMD Chemicals (San Diego, CA, USA). Dihydroethidine (DHE) was purchased from Invitrogen (Camarillo, CA, USA). MitoSOX Red reagent was purchased from Molecular Probes (Eugene, OR, USA). ApoAlert Annexin V was from Clontech (Mountian View, CA, USA).
Cell culture and treatments
C34 and E47 HepG2 cells were cultured either on 96-well plates or 6-well plates or on glass cover slides according to the requirements of the experiments. E47 cells are HepG2 cells which were transfected with PCi plasmid containing human CYP2E1 cDNA. These cells constitutively express CYP2E1. C34 cells are HepG2 cells transfected with the empty plasmid. These cells express low or zero CYP2E1. E47 and C34 cells were maintained in DMEM containing 10% FBS plus 1% penicillin-streptomycin-glutamine mixture plus 0.1 mg/ml G418 [19]. Cells were plated at a density of 1×104 cells/ml in DMEM containing 10% FBS and 1% antibiotics (penicillin plus streptomycin). SiRNA of TRX-1, TRX-2, TRX-1 and TRX-2 together, or control were used at 10nM concentration in DMEM containing 2% FBS and were performed 24 hrs after cells were plated. The treatment was continued for 5, 24, 48, and 72 hrs. The respective siRNA was present during the entire culture period. The transfection reagent was Dharmafect I used at a concentration of 1µl/ml. For cells treated with either BSO or TNFα, 200µM BSO or 2ng/ml TNFα with 10µg/ml cycloheximide were added after 24 hrs of siRNA treatment. The cells continued to be incubated with the appropriate siRNA and either BSO or TNFα for another 48 hrs (total siRNA treatment for 72 hrs). To test the protection effect of glutathione ethyl ester, 5mM glutathione ethyl ester was added to the E47 cell culture medium after 24 hrs siRNA treatment and the cells were incubated with the respective siRNA and glutathione ethyl ester for another 48 hrs. To test the effect of a JNK inhibitor on cell viability, E47 cells were treated with 5µM L-JNKI1 for 3 hrs, then cells were treated with either control siRNA, or the respective thioredoxin siRNAs for another 72 hrs. Cells were collected and various analyses were carried out as described below.
Cell viability assay
Cells grown on 96-well plates and treated with siRNA for 72 hrs were incubated with MTT for 3 hrs. Cell culture medium was aspirated, and isopropanol was added and plates were shaken for 30 min. Absorbance at 590nm and 630nm was detected on a plate reader. Cell viability was calculated as the absorbance difference between 590nm and 630nm. Cell viability in the control siRNA group of both C34 and E47 cells was taken as 100%, and cell viability in the thioredoxin knockdown groups was expressed as the percentage of viability relative to that of the control siRNA group in corresponding C34 and E47 cells.
Propidium iodide and Annexin V staining
Cells on 6-well plates were treated with siRNA for 72 hrs. For PI staining, 5µg/ml PI was added to the culture medium and the cells incubated for 10 min at 37 °C. PI staining was analyzed using fluorescence microscopy. Images shown were the merged ones taken with both light and fluorescence microscopy. The ApoAlert Annexin V detection kit was used for Annexin V staining. After siRNA treatment for 72 hours, cells were digested with trypsin, and pelleted by centrifugation. Cells were washed twice with binding buffer supplied in the detection kit, then resuspended with 200µl binding buffer, treated with 5µl Annexin V stock solution (20µg/ml) and incubated for 15 min in the dark. Annexin V staining was analyzed by flow cytometry in the FITC channel.
ROS detection by microscopy
ROS was detected with the Total ROS Detection Kit from Enzo. Cytosolic ROS was detected by fluorescence of DHE, and mitochondrial ROS was detected by fluorescence of mitoSOX Red. Briefly, cells were allowed to attach to the glass cover slides for 24 hrs. Cells were treated with either TRX-1 siRNA, TRX-2 siRNA or both for 72 hrs. Cells were loaded with ROS detection solution or 40 µM DHE or 5 µM mitoSOX Red reagent at 37°C and incubated in the dark. Cover slides were washed with wash buffer twice, then overlaid on slides and observed immediately under a fluorescence microscope. Images were taken at 40× magnitude. Images for mitoSOX were merged images from light microscopy and fluorescence microscopy.
Flow cytometry and spectrofluorometry assay for ROS
Cells grown on 6-well plates were treated with siRNA as described above for ROS detection by microscopy. Cells were harvested by digestion with trypsin. The cells were washed twice with wash buffer. Cells were treated with ROS detection solution for 30 min at 37°C in the dark. Samples were directly analyzed by flow cytometry using the FITC channel or spectrofluorometry (Ex/Em: 490/525nm).
Immunostaining for TRX-1, TRX-2, CYP2E1, SODs, pASK-1, ASK-1, pJNK, JNK, pp38 MAPK, p38 MAPK, p-cJUN, and cJUN
C34 and E47 cells, either treated with control siRNA or thioredoxin siRNAs, were collected typically after 72 hrs siRNA treatment. For the MAPK experiments, cells were collected after 5, 24, 48, and 72 hrs siRNA treatment for staining of pASK-1, ASK-1, pJNK, JNK, pp38 MAPK, and p38 MAPK. Cells were lysed with RIPA lysis buffer containing protease inhibitors. Western blotting was performed with a Bio-Rad mini gel system. Membranes were blotted with corresponding antibody separately for overnight at 4°C. Fluorescence conjugated secondary antibody against mice or rabbit IgG were incubated with the membranes for 1 hr at room temperature in the dark. Fluorescence was detected by an Odyssey infrared imaging system from Li-Cor Biosciences. Quantitation of fluorescence intensity was performed with an Image J program (NIH).
Immunostaining for pASK-1
Cells grown on glass cover slides after 5, 24, 48, 72 hrs siRNA treatment were fixed in 10% buffered formalin for 15 min. Slides were rinsed with PBS twice, and treated with 0.1% Triton X-100 for 15 min. After rinsing twice in PBS, fixed cells were blotted with blotting buffer for 30 min at room temperature. Antibody against pASK-1 was added at 1:100 dilution and incubated at 4°C overnight. Slides were rinsed 3 times with PBS and incubated with Alexa 488 conjugated secondary antibody for 1 hr at room temperature. Fluorescence was detected under a fluorescence microscope at 40× magnitude.
Immunostaining for 4-HNE adducts
Cells grown on glass cover slides after 72 hrs siRNA treatment were fixed in 10% buffered formalin for 15 min. Slides were rinsed with PBS twice, and treated with 0.1% Triton X-100 for 15 min. After rinsing in PBS twice, fixed cells were blotted with blotting buffer for 30 min at room temperature. Antibody against 4-HNE was added at 1:200 dilution and incubated at 4°C overnight. Slides were rinsed 3 times with PBS and then were stained using a Histostain Plus Broad Spectrum (ACE) kit. 4-HNE staining was detected under a light microscope at 20× magnitude.
Total glutathione analysis
Cells were collected after 72 hrs siRNA treatment, and lysed. Cell lysates were mixed with 10% trichloroacetic acid (TCA) and incubated for 10 min to extract intracellular glutathione. The TCA supernatant was used to measure the glutathione content following the method of Tietze [20]. 0–100µM glutathione dissolved in 5% TCA solution was used for standard curve analysis. Results were expressed as nanograms of glutathione per milligram of protein. The relative GSH level was expressed with the control siRNA treatment group of E47 cells taken as 1.0. The relative GSH level in other groups was expressed as the ratio to that of control siRNA group of E47 cells.
Statistics
One-Way ANOVA was performed using the Statistical software SPSS (17.0) to compare the difference between control and treatment groups. Values reflect means ± standard error from 3–4 experiments. Statistical significance was expressed as p<0.05.
Results
Specific knock down of cytosolic and mitochondrial thioredoxin by TRX-1 and TRX-2 siRNA after 72h siRNA treatment
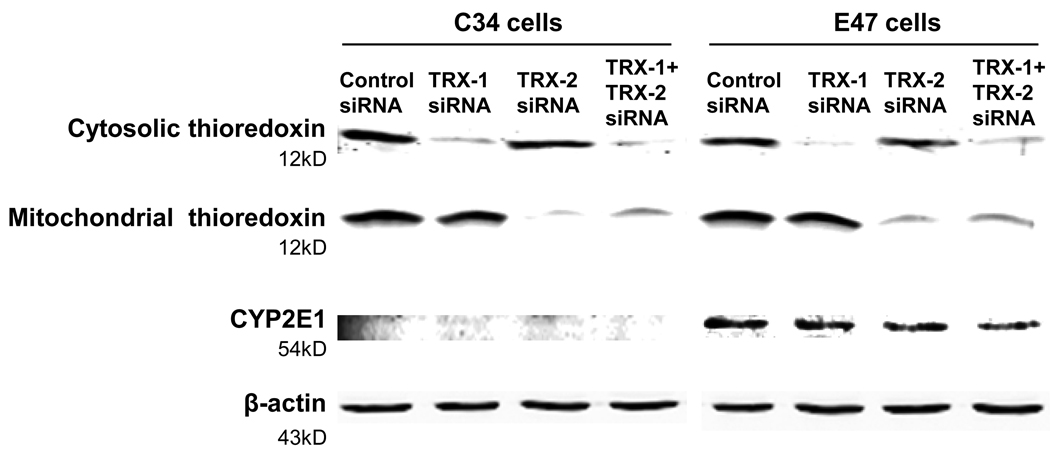
Both E47 and C34 cells were treated with either control siRNA, TRX-1 siRNA, or TRX-2 siRNA, or both TRX-1 and TRX-2 siRNA for 72 hrs. TRX-1 expression was decreased by 90% by either TRX-1 siRNA alone or TRX-1 and TRX-2 siRNA together in both cell lines. TRX-2 expression was decreased by 80–90% by TRX-2 siRNA alone or TRX-1 and TRX-2 siRNA together (Fig. 1) in both cell lines. TRX-1 siRNA is specific for cytosolic thioredoxin and had no effect on levels of mitochondrial thioredoxin, and TRX-2 siRNA is specific for decreasing mitochondrial thioredoxin and had no effect on levels of cytosolic thioredoxin. We tested the expression of thioredoxin after 24, 48, and 72 hrs siRNA treatment. Knock down of TRX-1 or TRX-2 was only about 10 and 50% after 24 and 48 hrs siRNA treatment (data not shown), so further experiments used 72 hrs siRNA treatment. As expected, there is no expression of CYP2E1 in C34 cells, and the expression of CYP2E1 in E47 cells was unaffected by either TRX-1 or TRX-2 siRNA (Fig. 1).
Fig. 1. Knockdown of cytosolic or mitochondrial thioredoxin by either TRX-1 or TRX-2 siRNA.
C34 and E47 cells were treated with TRX-1 siRNA, or TRX-2 siRNA, or both TRX-1 plus TRX-2 siRNA, or control siRNA for 72 hrs. Cells were harvested and lysed. SDS-PAGE was performed to detect the expression of TRX-1, TRX-2, and CYP2E1.
Thioredoxin knockdown induces cell death in a CYP2E1 dependent manner
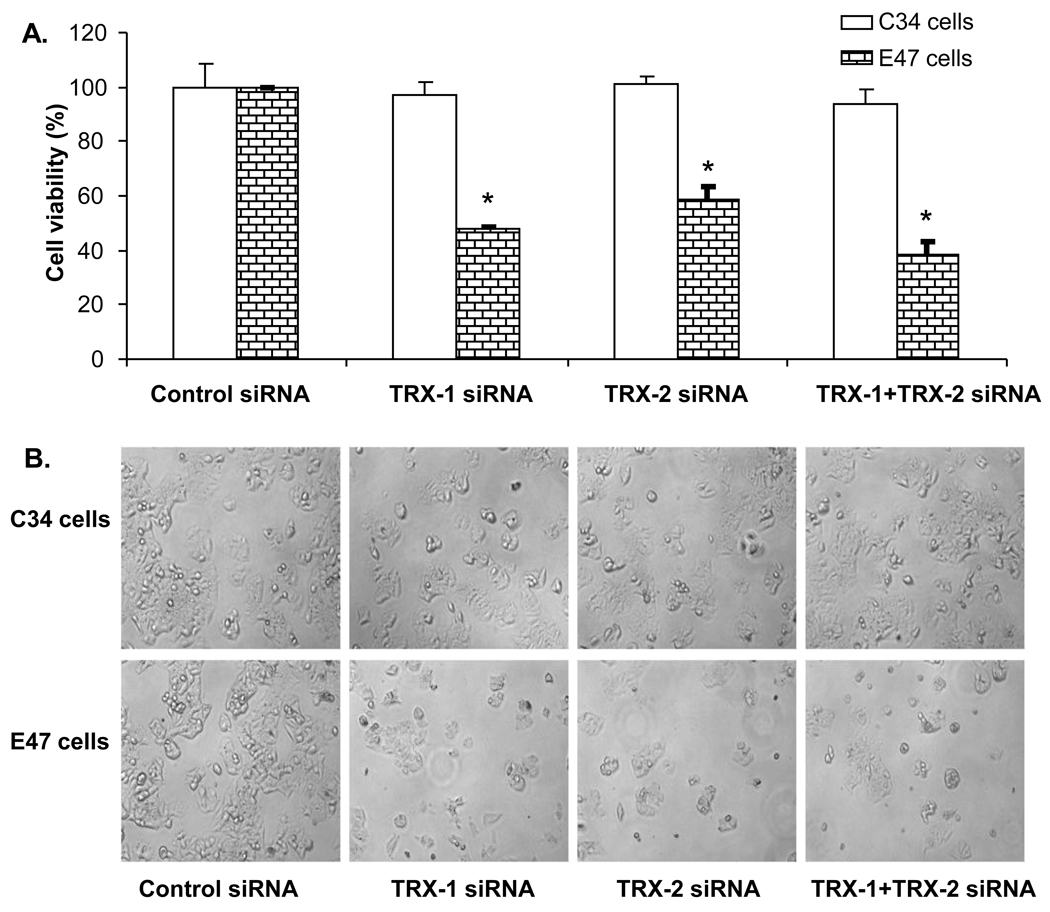
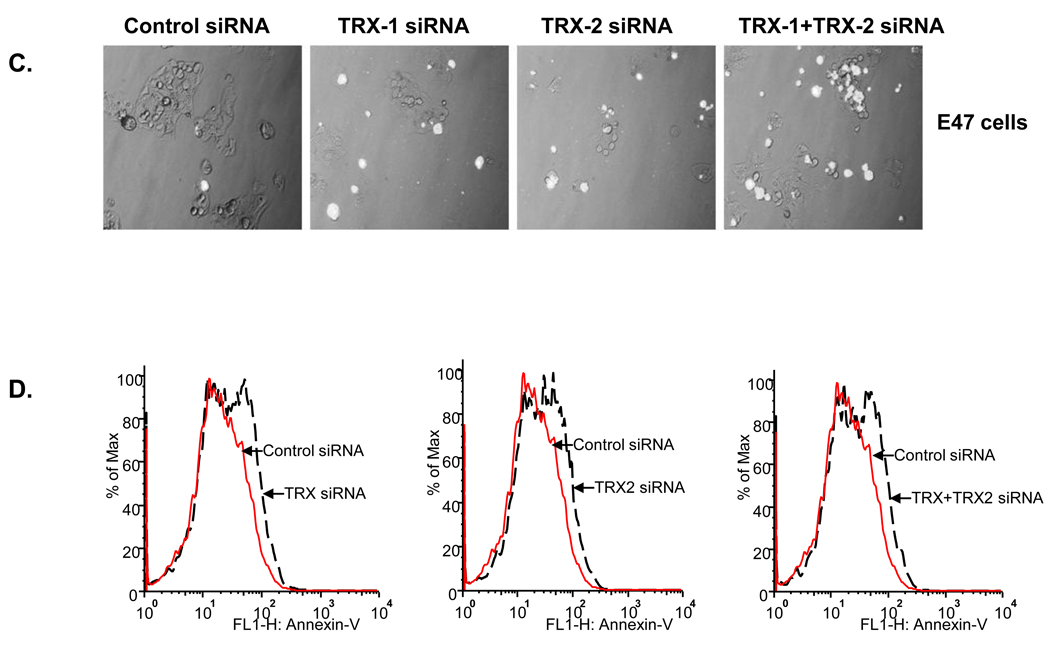
Cell viability was detected by MTT assay in both E47 and C34 cells after 72 hrs siRNA treatment. Knock down of TRX-1 or TRX-2 or both decreased cell viability of E47 cells by 40–60%, but cell viability of C34 cells was not affected with the knockdown of either TRX-1 or TRX-2 or both (Fig. 2A). Morphology evaluation showed a decreased cell density in E47 cells but not in C34 cells treated with either TRX-1, or TRX-2, or TRX-1 and TRX-2 siRNA together (Fig. 2B). These results indicate that cell death induced by thioredoxin knockdown under these conditions is CYP2E1 dependent and that decreasing either TRX-1 or TRX-2 promotes this toxicity. To assess the mode of cell death, experiments studying uptake of propidium iodide or annexin V staining were carried out. Uptake of propidium iodide into E47 cells was elevated upon knockdown of either TRX-1 or TRX-2 or both (Fig. 2C). Annexin V staining, taken as a reflection of apoptosis, was also elevated in the E47 cells upon knockdown of TRX-1 or TRX-2 or both (Fig. 2D). Thus, the cell death appears to be a mix of necrosis plus apoptosis, i.e. necroptosis.
Fig. 2. Effect of thioredoxin knockdown on E47 and C34 cell viability.
C34 and E47 cells were treated with TRX-1 siRNA, or TRX-2 siRNA, or both TRX-1 and TRX-2 siRNA, or control siRNA for 72 hours. Cell viability was detected by MTT assay and by cell morphology. A. Cell viability was expressed as the percentage of viable cells for the specific siRNA treatment to the control siRNA group in C34 and E47 cells respectively. * p<0.05 comparing specific siRNA treatment groups to control siRNA group in C34 and E47 cells respectively, n=3. B. Images were taken with the light microscope at 10× magnitude. C. PI staining of E47 cells under fluorescence and light microscopy. Images were merged from light and fluorescence microscopy. PI staining positive cells were shown as white dots. D. Annexin V staining of E47 cells by flow cytometry. E47 cells were treated with specific siRNA for 72 hrs, trypsinized from the plate and stained with Annexin V. Fluorescence was analyzed by flow cytometry under FITC channel.
Thioredoxin knockdown potentiated the toxic effect of the glutathione synthesis inhibitor BSO and of TNFα
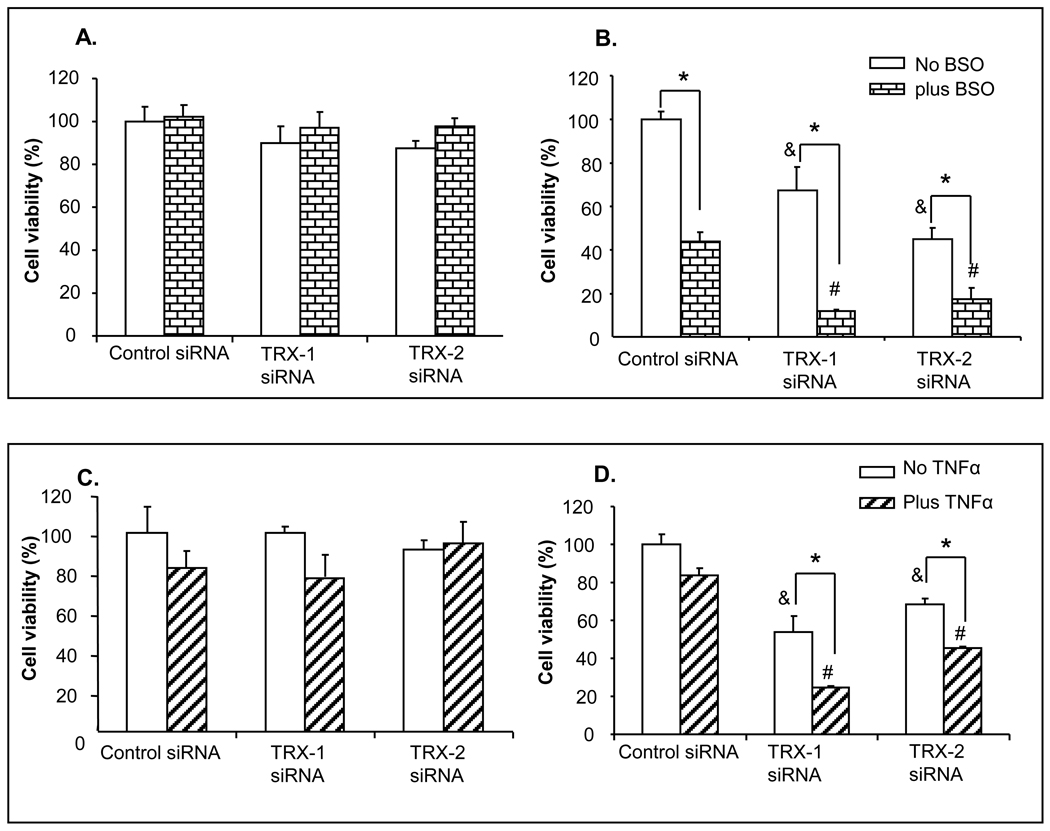
The cell toxicity described above is due to the effect of thioredoxin knockdown per se, as no other toxins or treatments besides the knockdown were carried out. Experiments were carried out to see whether thioredoxin knockdown potentiates the toxic effect of other cytotoxic chemicals, such as the glutathione synthesis inhibitor BSO, and TNFα. Lowering of cellular and mitochondrial GSH levels and elevating levels of cytokines such as TNFα have been implicated in alcohol-induced liver injury. C34 and E47 cells were treated with either TRX-1 or TRX-2 siRNA for 24 hours. Either BSO or TNFα plus cycloheximide was then added into the medium and cells were cultured for another 48 hours with the respective siRNA and either BSO or TNFα. Cell viability was analyzed by MTT assay. In C34 cells, there was no decrease of cell viability with either TRX-1 or TRX-2 siRNA alone, with or without BSO treatment (Fig 3A). However, in E47 cells, inhibiting GSH synthesis by BSO induced 50% cell death in the absence of TRX-1 and TRX-2 siRNA treatment (Fig. 3B). GSH was previously shown to be protective against CYP2E1 toxicity and decreases in GSH caused E47 cell toxicity [21]. In E47 cells treated with either TRX-1 or TRX-2 siRNA, there was a decrease of cell viability of about 40–60% in the absence of BSO. After BSO treatment, cell viability decreased 90 and 80% respectively in E47 cells after either TRX-1 or TRX-2 knockdown (Fig. 3B). TNFα per se did not have toxic effect on E47 cells. Toxicity by TNFα was amplified in E47 cells treated either with TRX-1 or TRX-2 siRNA (Fig. 3D). TNFα, with or without TRX-1 or TRX-2 knockdown had no significant effect on viability of the C34 cells (Fig. 3C). TNFα was used at relatively low concentrations in these experiments to allow effects by thioredoxin knockdown and evaluate possible differences in TNFα toxicity between E47 and C34 cells to be observed. Higher concentrations of TNFα and more prolonged incubation do produce toxicity to both the C34 and E47 cells. Thus, either TRX-1 or TRX-2 protect against the toxicity produced in CYP2E1 expressing HepG2 cells by relatively low concentration of TNFα.
Fig. 3. Thioredoxin knockdown potentiated E47 cell toxicity by BSO and TNFα.
After 24 hrs of TRX-1 siRNA, or TRX-2 siRNA, or control siRNA treatment, C34 (A and C) and E47 (B and D) cells were treated with either 200µM of BSO (A and B) or 2ng/ml TNFα plus 10ng/ml cycloheximide (C and D) along with the appropriate siRNA for another 48 hrs. Cell viability was expressed as the percentage of viable cells for the specific siRNA treatment to the control siRNA group in C34 and E47 cells respectively. * p<0.05 comparing either plus BSO or plus TNFα treatment group to either minus BSO or minus TNFα group with corresponding siRNA treatment, n=3. & p<0.05 represents TRX-1 or TRX-2 siRNA treatment compared to control siRNA treatment in minus BSO or TNFα treatment group in corresponding cell type, n=3. # p<0.05 represents TRX-1 or TRX-2 siRNA treatment compared to control siRNA treatment in plus BSO or TNFα treatment group in corresponding cell type, n=3.
Role of glutathione in thioredoxin knockdown induced E47 cell toxicity
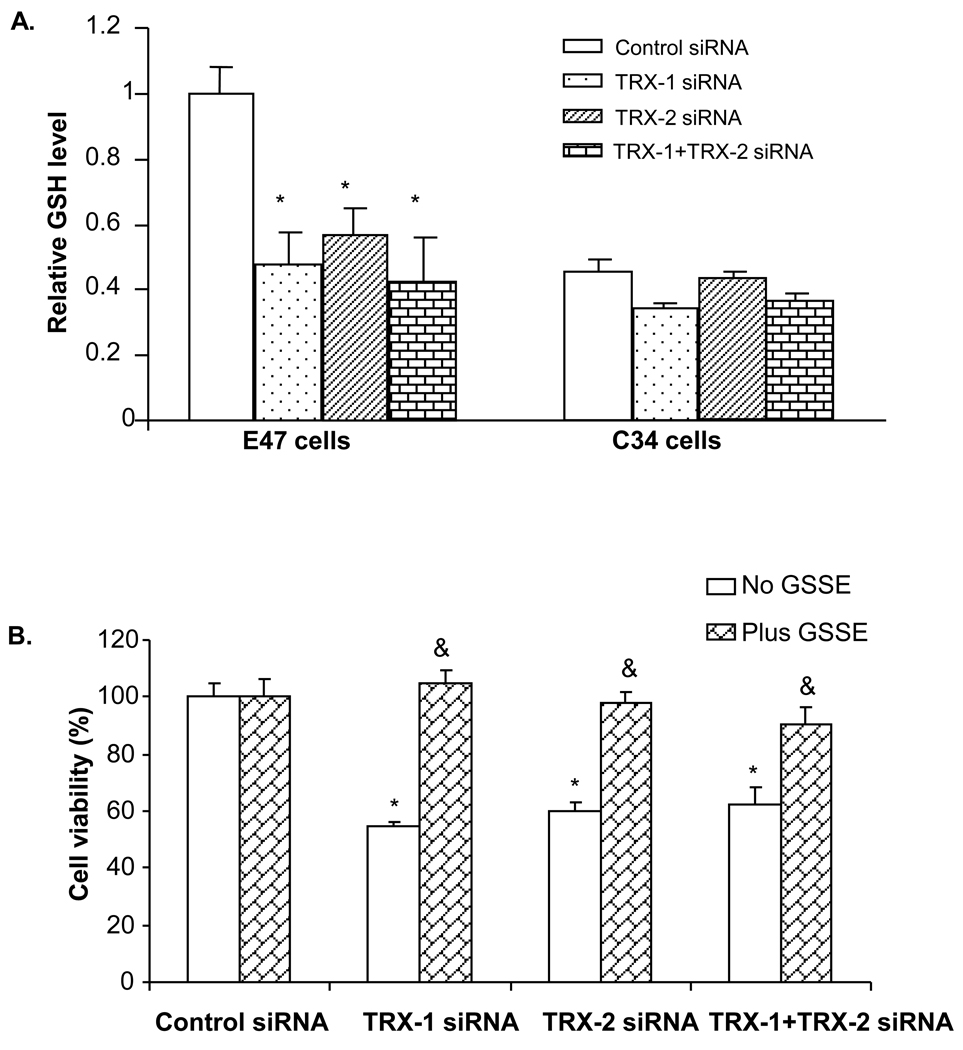
Total glutathione levels were assayed in both C34 and E47 cells after 72 hrs siRNA treatment. Total glutathione was 2 fold higher at baseline control siRNA treatment level in E47 cells compared to C34 cells (Fig. 4A). This suggests an adaptive response to ROS produced by CYP2E1 as described previously [22]. Treatment with either TRX-1 or TRX-2 siRNA or both, did not cause a significant change of total glutathione level in C34 cells, while a 50% decrease was found in E47 cells (Fig. 4A). This suggests that with knockdown of thioredoxin, glutathione was consumed as a major reducing molecule and antioxidant. Addition to the culture medium of glutathione ethyl ester prevented E47 cell death caused by either TRX-1 or TRX-2 siRNA or both together (Fig. 4B). The lowering of, and the protection by, glutathione suggests that the knock down of thioredoxin induced cell death is related to oxidative stress. This was further tested by studies on the production of ROS and other oxidative stress markers.
Fig. 4. Effect of thioredoxin knockdown on total glutathione levels. A.
C34 and E47 cells were treated with TRX-1 siRNA, or TRX-2 siRNA, or both, or control siRNA for 72 hours. Cells were trypsinized and sonicated and assayed for total glutathione. Glutathione level in each group was expressed as the value relative to that of the control siRNA treatment group in E47 cells. * p<0.05 comparing specific siRNA group to control siRNA in E47 cells, n=3. B. E47 cells grown on 96-well plates were treated with either TRX-1, or TRX-2 siRNA, or both, or control siRNA for 72 hours. At 24 hrs, 5mM glutathione ethyl ester was added into the culture medium and the cells were incubated along with the appropriate siRNAs for 48 hrs before MTT assay was performed. Cell viability was expressed as viability in each thioredoxin siRNA treatment group relative to that in the control siRNA group. * p<0.05 comparing specific siRNA group to control siRNA group, n=3. & p<0.05 comparing the plus GSSE group to the minus GSSE group in the corresponding siRNA treatment, n=3.
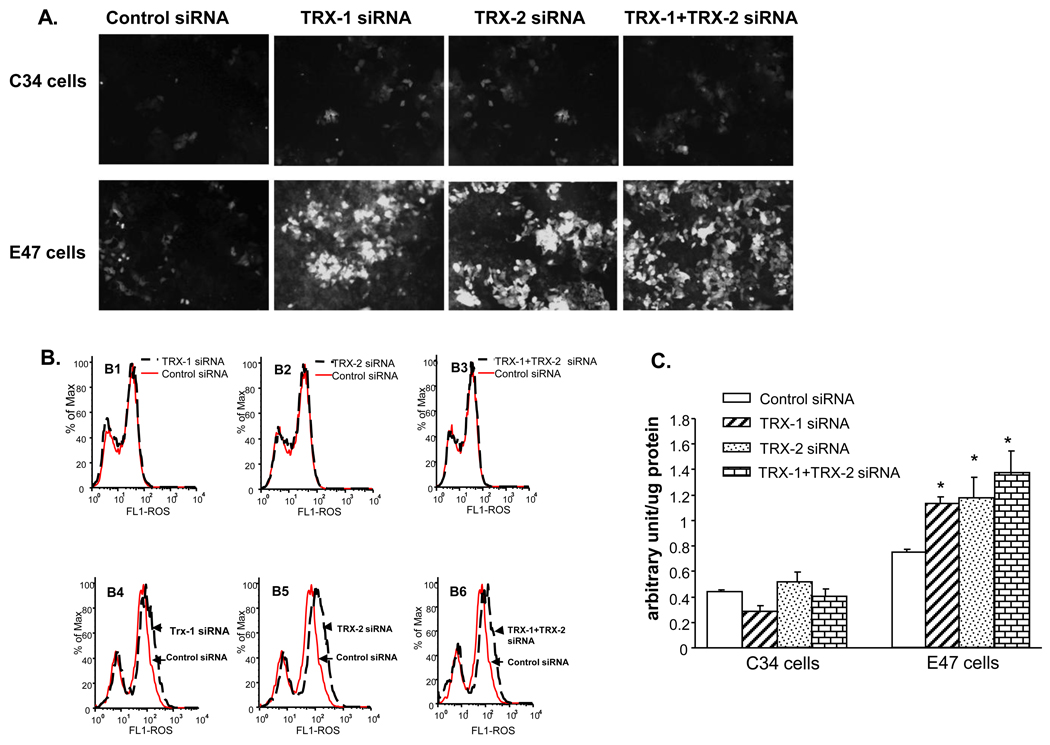
Thioredoxin knockdown increased ROS production and 4-HNE protein adduct formation in E47 cells but not in C34 cells
Administration of thioredoxin or induction of intracellular thioredoxin protects mice or cells from lipid peroxidation and ROS generation [17, 23]. We evaluated whether knocking down of thioredoxin intracellularly by siRNA induces ROS production and lipid peroxidation. Total ROS was detected both by fluorescence microscopy (Fig. 5A), flow cytometry assay (Fig. 5B), and spectrofluorimetry assay (Fig. 5C). An increase of ROS production was detected in E47 cells but not in C34 cells after 72 hrs treatment with either TRX-1 or TRX-2 siRNA or both (Fig. 5 A and B). Quantification of ROS production by spectrofluorimetry indicated that total ROS production was elevated 50–100% by thioredoxin knockdown in E47 cells (Fig 5C). There were no increases in ROS production in C34 cells upon thioredoxin knockdown. Although knocking down of TRX-1 did not affect the expression of TRX-2, and vice versa, there were significant increases of ROS production when either TRX-1 or TRX-2 was lowered. This suggests that TRX-1 or TRX-2 is not sufficient to protect the E47 cells from oxidative stress. It would appear that both TRX-1 or TRX-2 are essential for the protection of E47 cells from oxidative stress.
Fig. 5. Effect of thioredoxin knockdown on ROS production in E47 cells and C34 cells.
C34 and E47 cells were treated with TRX-1 siRNA, or TRX-2 siRNA, or both, or control siRNA for 72 hours. Cells were grown on glass cover slides for fluorescence microscopy, and on 6-well plates for flow cytometry or for spectrofluorometry assay. Total ROS was detected with a Total ROS Detection Kit as described in ‘Methods’. A. Total ROS detection by fluorescence microscopy. B. total ROS detected by flow cytometry. B1–B3: C34 cells; B4–B6: E47 cells. C. Spectrofluorimetry of ROS. Arbitrary units of fluorescence produced by E47 and C34 cells in the presence of control, TRX-1, TRX-2, and TRX-1 plus TRX-2 siRNA. P<0.05 compared to control siRNA. D. Dihydroethidium and E. mitoSOX fluorescence as an assay for cytosolic and mitochondrial production of superoxide, respectively. C34 and E47 cells were treated with control siRNA or TRX-1 siRNA or TRX-2 siRNA or TRX-1 plus TRX-2 siRNA for 72 hrs followed by the addition of either 40µM DHE or 5µM mitoSOX. Images of mitoSOX were the merged ones of light microscopy and fluorescence microscopy. Examples of mitoSOX red positive cells were pointed with white arrow.
As mentioned above, TNFα did not produce toxicity to the C34 cells even after TRX-1 and TRX-2 knockdown after a 48 hrs incubation with 2 ng/ml of TNFα. This lack of toxicity was associated with a corresponding lack of increase in ROS production by TNFα under these conditons (data not shown).
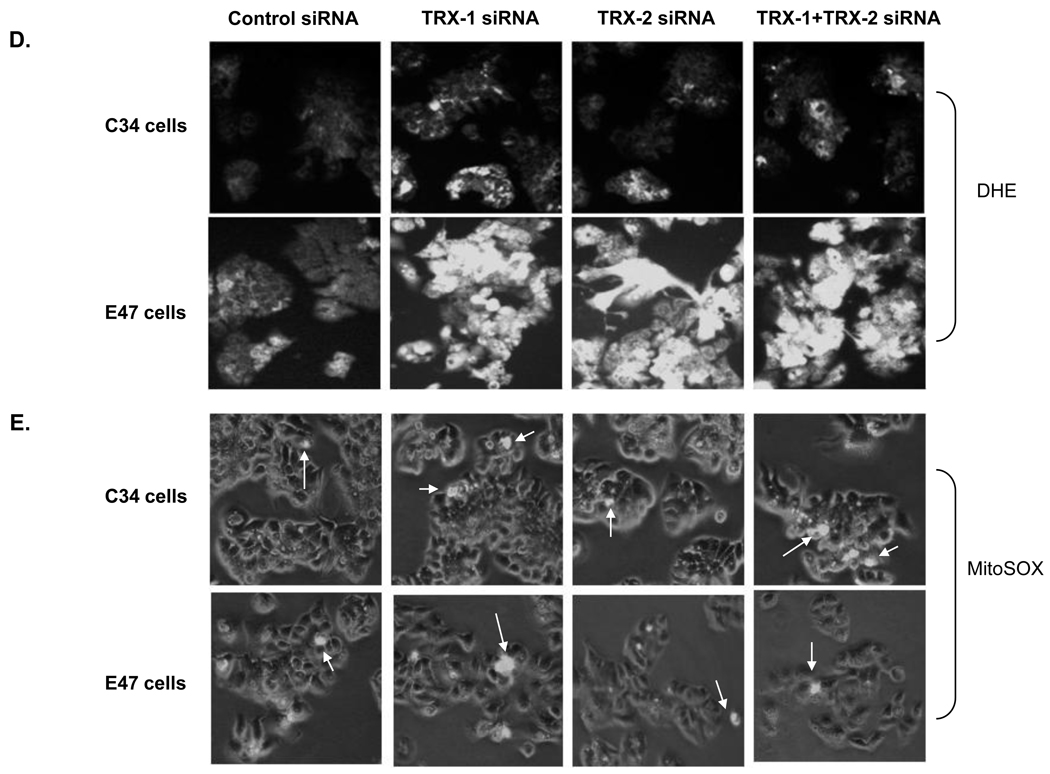
To gain more insight into where in the cell the increase of ROS production was occurring, the production of superoxide in the cytosol compartment was assayed using DHE as the probe while production of superoxide in the mitochondrial compartment was determined using mitoSOX Red as the probe. Knockdown of TRX-1 or TRX-2 or both increased DHE fluorescence in E47 cells, but not C34 cells (Fig. 5D). However, knockdown of thioredoxin did not increase mitSOX fluorescence in the E47 (or C34) cells (Fig 5E). These results suggest that most of the increase in superoxide production occurs in the cytosol compartment, where the CYP2E1 of the E47 cells is primarily located.
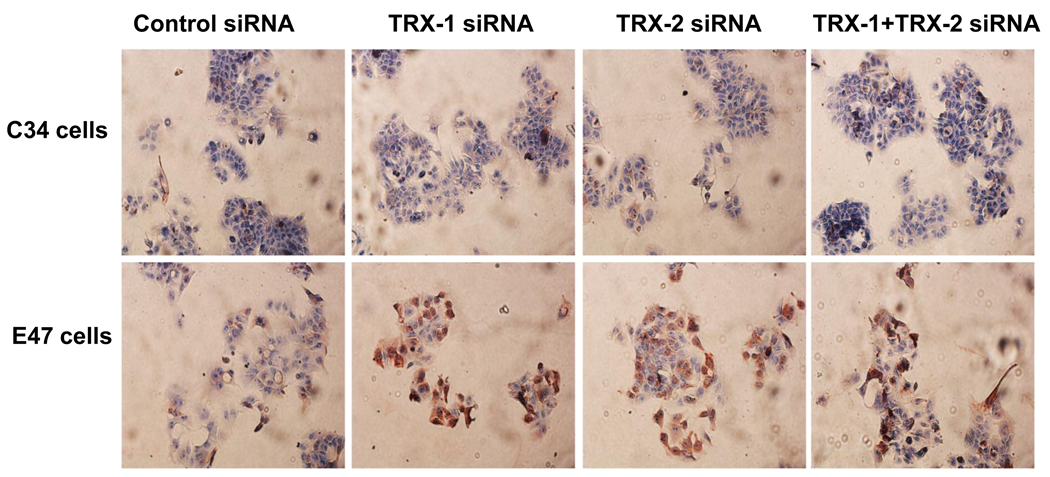
4-HNE adduct formation was analyzed by immunocytochemistry with fixed E47 and C34 cells. At baseline, 4-HNE adduct expression is higher in E47 cells than C34 cells when control siRNA was applied (Fig. 6), similar to the increase in fluorescence of E47 compared to C34 cells (Fig. 5A). There was no increase of 4-HNE adducts in C34 cells, but a significant increase of 4-HNE adducts was observed in E47 cells when comparing either TRX-1 or TRX-2 or both siRNA treatment to control siRNA treatment (Fig. 6). There was no increase of 3-nitrotyrosine protein adducts expression in either E47 or C34 cells with the treatment of TRX-1 or TRX-2 siRNA compared to control siRNA (data not shown).
Fig. 6. Effect of thioredoxin knockdown on 4-HNE expression in E47 cells and C34 cells.
Cells grown on cover slides were treated with TRX-1 siRNA, or TRX-2 siRNA, or both, or control siRNA for 72 hrs. Cells were fixed with 10% formalin and stained for 4-HNE as described in ‘Methods’. Images were taken with the light microscope at 10× magnitude.
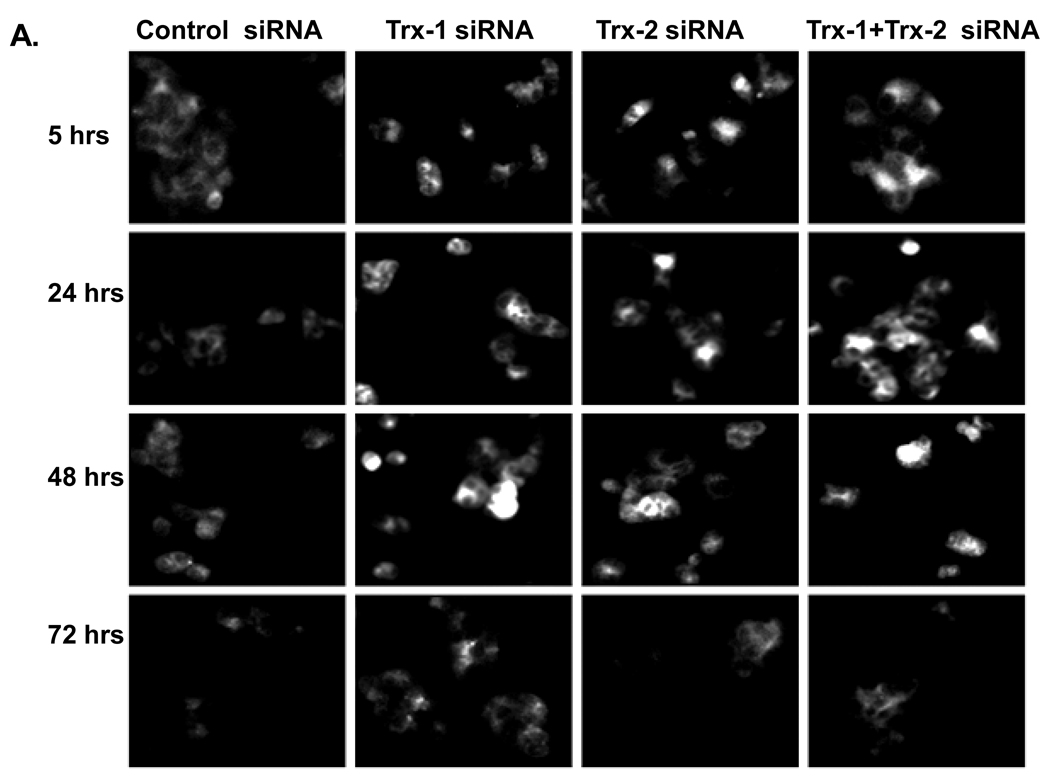
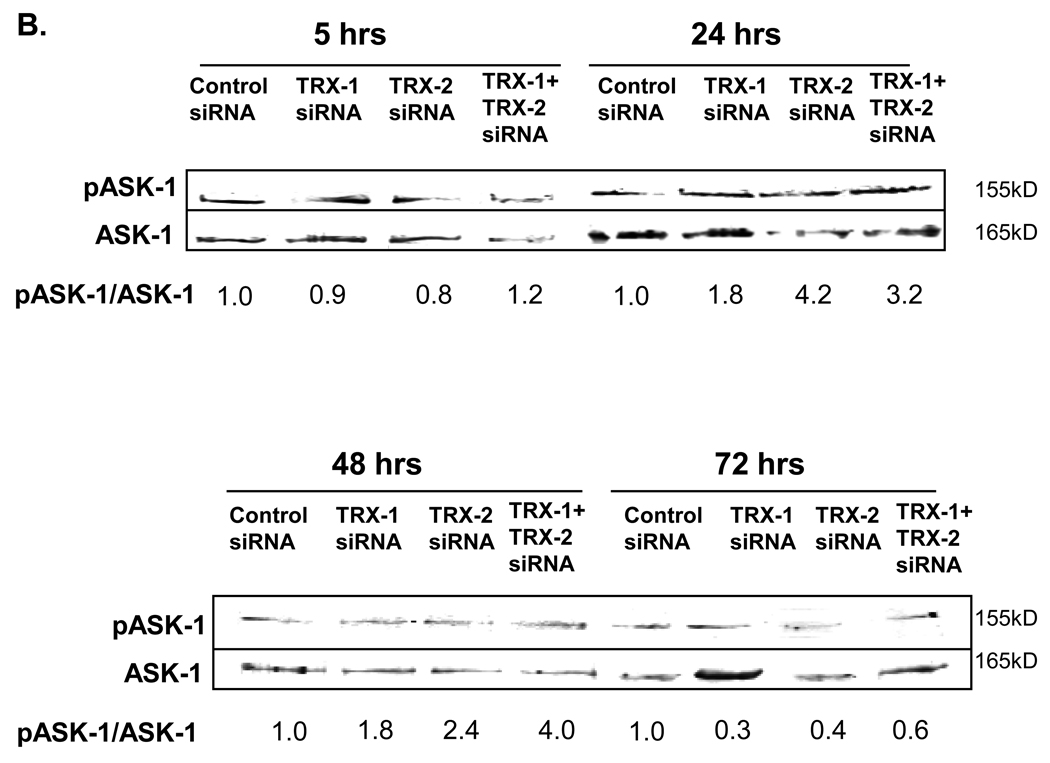
Thioredoxin knockdown promotes phosphorylation of ASK-1 and JNK1 in E47 cells
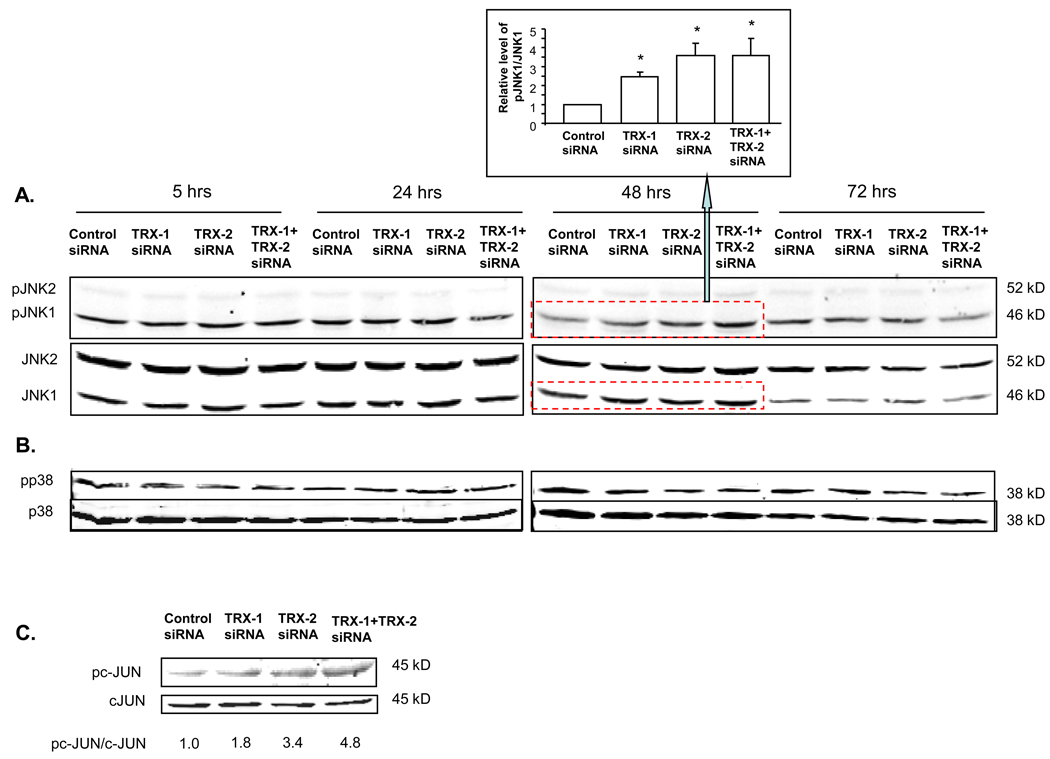
Since thioredoxin is bound to ASK-1 and inhibits the activation of ASK-1 [9–11, 24], experiments were carried out to evaluate whether thioredoxin knockdown activates ASK-1 and downstream MAPK signaling pathways in the E47 cells. Increased ASK-1 phosphorylation was seen by immunohistochemistry in E47 cells upon treatment with TRX-1, or TRX-2 siRNA or both at 5, 24, and 48 hrs, but not after 72 hrs of siRNA treatment (Fig. 7A). Western blot analysis revealed a 2–4 fold increase in the pASK-1/ASK-1 ratio 24 hrs and 48 hrs but not after 72 hrs of thioredoxin knockdown (Fig. 7B), confirming the immunohistochemistry data. ASK-1 activates downstream MAPK such as JNK and p38 MAPK, ultimately by promoting their phosphorylation to pJNK or pp38 MAPK [11]. Increased JNK1 but not JNK2 phosphorylation was seen in E47 cells treated with either TRX-1 or TRX-2 siRNA for 48 hrs (Fig. 8A). No such activation persisted at 72 hrs after treatment. Thus, activation of JNK1 occurs after the earlier activation of ASK-1 (5–48 hrs) and declines when activation of ASK-1 terminates (72 hrs). p38 MAPK was not activated under these conditions as there was no increase in pp38 MAPK levels (Fig. 8B). One downstream target of JNK1 is cJUN phosphorylation. There was an increase in the pc-JUN/c-JUN ratio (Fig. 8C) 72 hrs after treatment with siRNA for TRX-1, TRX-2 or both, a time point after the activation of JNK1 (48 hrs).
Fig. 7. Effect of thioredoxin knockdown on ASK-1 phosphorylation in E47 cells.
E47 cells grown on cover slides or on 6-well plates were treated with TRX-1 siRNA, or TRX-2 siRNA, or both, or control siRNA for 5, 24, 48 and 72 hrs. A. Cells were fixed with 10% formalin and stained for p-ASK-1 as described in ‘Methods’. Images were taken using a fluorescence microscope at 20× magnitude. B. Cell lysates were prepared and western blots were carried out to detect p-ASK-1 and ASK-1 levels. Numbers under the blots refer to the ratio of p-ASK-1/ASK-1. Images were quantified with the Image J program.
Fig. 8. Effect of thioredoxin knockdown on the activation of JNK in E47 cells.
E47 cells grown on 6-well plates were treated with TRX-1 siRNA or TRX-2 siRNA separately or both together, or control siRNA for 5, 24, 48 and 72 hrs. Cells were lysed and western blot was performed for the analysis of A: pJNK, JNK; B: pP38, and p38 MAPK as described in ‘Methods’. Images were quantified with the Image J program. The ratio of the phosphorylated JNK1 to the total JNK1 was expressed relative to the value of the control siRNA group at the 48 hrs time point as shown in the bar graph. * p<0.05 comparing the ratio of pJNK1/JNK1 in specific siRNA to that in control siRNA group after 48 hrs siRNA treatment, n=4. C: Western blot for p-cJUN and cJUN was carried out at 72 hrs after siRNA treatment. Numbers under the blots refer to the p-cJUN/cJUN ratio.
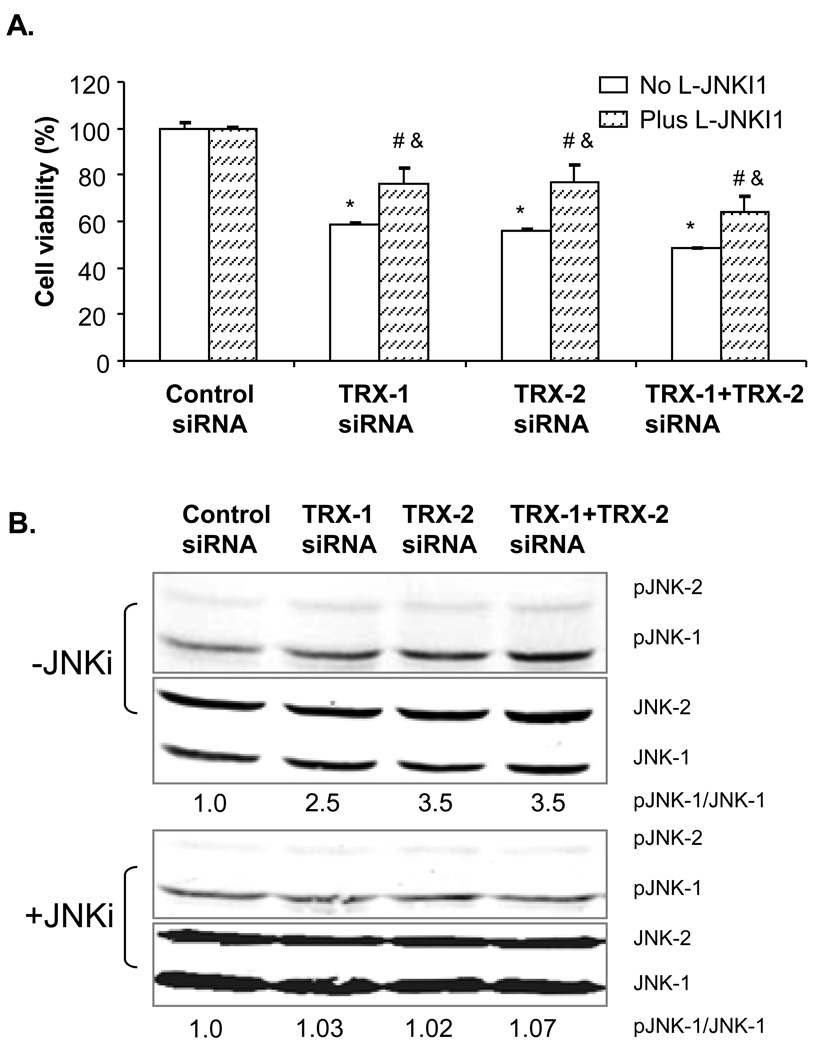
Could the CYP2E1 plus thioredoxin knockdown induced cell death be mediated through ASK-1 and JNK1 signaling pathways? The JNK inhibitor, L-JNKI1 which specifically inhibits the phosphorylation of JNK, lowered the decline in E47 cell viability from 45–50% in the absence of L-JNKI1 to about 20–30% in the presence of L-JNKI1 plus TRX-1, or TRX-2 siRNA, or both TRX-1 and TRX-2 siRNA treatment (Fig 9A). Under these conditions, L-JNKI1 strongly blunted the activation of JNK which occurs 48 hrs after thioredoxin knockdown; the pJNK1/JNK1 ratio was elevated 2.5 to 3.5 fold by siRNA for TRX-1 or TRX-2 or both in the absence of JNKI1 whereas no increase in pJNK1/JNK1 was observed in the presence of the inhibitor (Fig 9B). The partial protection by L-JNKI1 suggests that the cell death induced by thioredoxin knockdown was partly via JNK signaling pathways, although non JNK-dependent pathways are likely also involved.
Fig. 9. A JNK inhibitor partially protects E47 cells from loss of viability from thioredoxin knockdown.
E47 cells were treated with 5 µM of the JNK inhibitor- L-JNKI1 for 3 hours, then cells were treated with TRX-1 siRNA, TRX-2 siRNA or both or control siRNA for another 72 hours. E47 cells were also treated in parallel in the absence of L-JNKI1. A: Cell viability was performed with the MTT assay. Cell viability was expressed as the percentage of each siRNA in the absence or presence of the JNK inhibitor with the control siRNA taken as 100% viability. * p<0.05 comparing the specific siRNA treatment group to the control siRNA group without L-JNKI1 treatment, n=3. & p<0.05 comparing the plus L-JNKI1 group to the minus L-JNKI1 group with corresponding siRNA treatment, n=3. # p<0.05 comparing the specific siRNA treatment group to the control siRNA group with L-JNKI1 treatment, n=3. B: Inhibition of JNK activiation by L-JNKI1. Immunoblots for p-JNK and JNK in the absence or presence of L-JNKI1 were carried out 48 hrs after thioredoxin knockdown. Numbers under the blots refer to the p-JNK/JNK ratio. The minus JNKi results are repeated from Fig. 8 48 hrs data in order to show the effectiveness of the JNK inhibitor in blunting JNK activation.
Effect of thioredoxin knockdown on levels of antioxidant enzymes
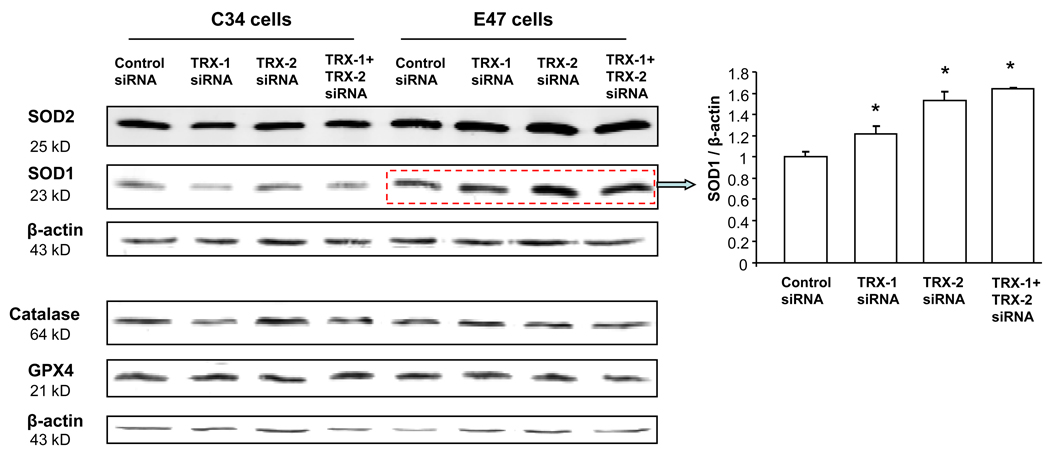
With either TRX-1 or TRX-2 knockdown, there was about a 20–60% increase of SOD-1 expression in E47 cells, but not in C34 cells (Fig 10). There was no significant increase in SOD-2 in either E47 cells or in C34 cells (Fig. 10). This suggests that in E47 cells, with thioredoxin knockdown, the antioxidant enzyme SOD-1 may have increased as a compensation reaction, to protect E47 cells from oxidative stress induced by CYP2E1. However, this increase of SOD-1 was unable to prevent the E47 cell death upon TRX-1 or TRX-2 knockdown. Other antioxidant enzymes, such as catalase and GPX-4 were not changed at the protein level in both E47 and C34 cells upon thioredoxin knockdown (Fig. 10). Failure to elevate these H2O2 detoxification enzymes may contribute to the loss of viability of E47 cells upon thioredoxin knockdown even when SOD-1 was slightly elevated.
Fig. 10. Effect of thioredoxin knockdown on the expression of anti-oxidant enzymes.
C34 and E47 cells were treated with TRX-1 siRNA, or TRX-2 siRNA, or both, or control siRNA for 72 hrs. Cells were lysed and Western Blots to detect SOD-1, SOD-2, GPX4, and catalase were performed as described in ‘Methods’. Images were quantified with the Image J program. Only the SOD-1/β-actin ratios showed some difference between the thioredoxin knockdown versus the control siRNA (bar graph). * p<0.05 comparing specific siRNA treatment to control siRNA group, n=4.
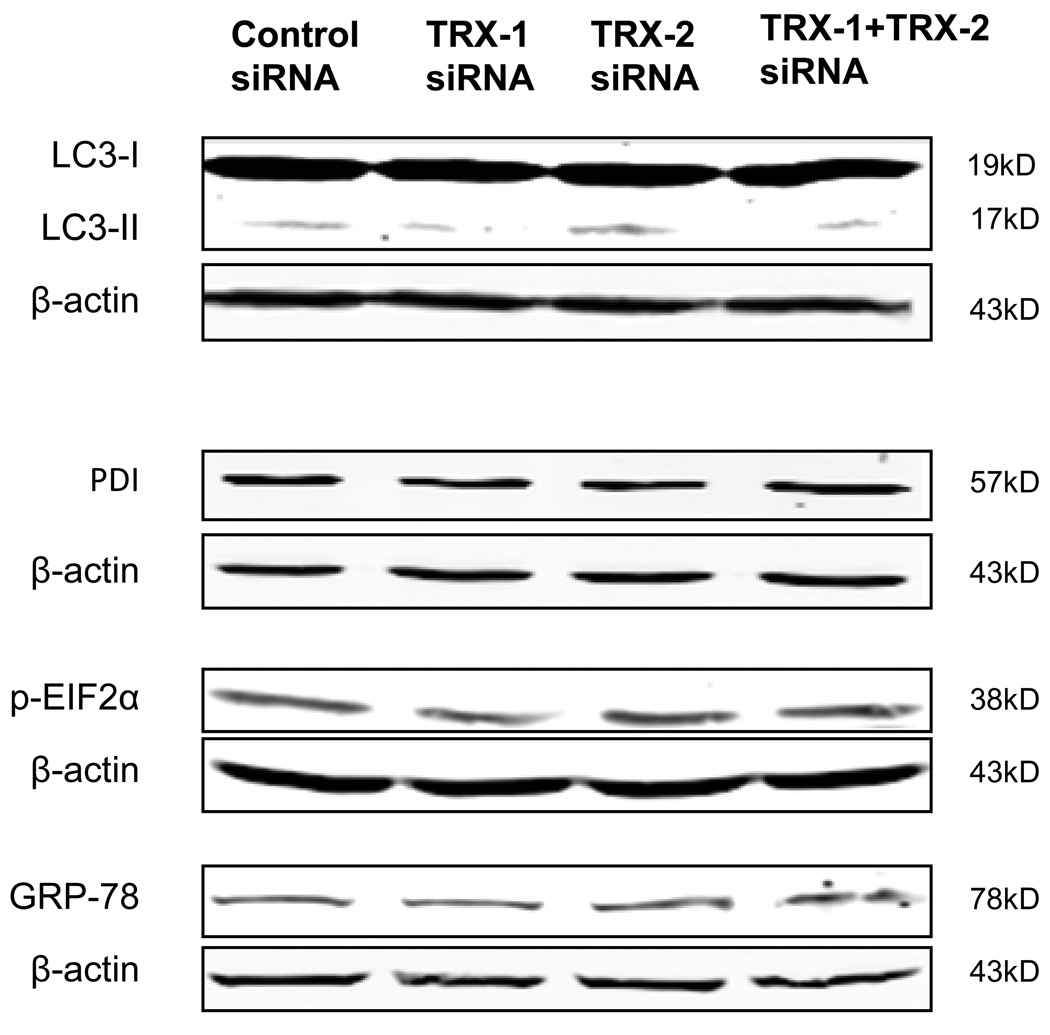
Since ER stress and autophagy can be related to thioredoxin status [25–27], we studied whether ER stress and autophagy occurred or played a role in the cell death induced by thioredoxin knockdown. However, no significant changes in the expression of ER stress markers such as PDI, GRP 78, or pEIF2α, and no increase in autophagy markers such as the LC3-II/LC3-I ratio were observed in E47 cells after 72 hrs thioredoxin knockdown (Fig. 11).
Fig. 11. Effect of thioredoxin knockdown on autophagy and ER stress.
E47 cells were treated with control siRNA, or TRX-1 siRNA, or TRX-2 siRNA or TRX-1 plus TRX-2 siRNA for 72 hrs. Cell lysates were prepared and immunoblots for detection of autophagy markers LC3-I and LC3-II or ER stress markers PDI, p-EIF2α, and GRP-78 were carried out. There was no effect of thioredoxin knockdown on the LC3-II/LC3-I ratio or on level of PDI, p-EIF2α, or GRP-78.
Discussion
Thioredoxin is expressed widely in biological systems. It increases life span and protects different organs or cell types from oxidative stress [28]. It protects against oxidative stress in ischemia reperfusion injury in kidney [29], protects from severe acute pancreatitis [30], from acute hepatitis and fibrosis [31–32], and focal ischemic brain damage [33]. It also prevents alcohol induced oxidative stress and inhibits apoptosis [17]. Thioredoxin has also been validated as a cancer drug target, since cancer cells rely on thioredoxin for protection against stress-disregulated redox signaling [34]. There is much interest in that thioredoxin can be a therapeutic target for treatment of oxidative stress-induced inflammation in different organs and as a target for cancer therapy.
The goal of the current study was to evaluate whether cytosolic and /or mitochondrial thioredoxin could protect against CYP2E1-dependent toxicity using a HepG2 cell model. Thioredoxin siRNAs were used to knock down either TRX-1 or TRX-2 or both. About 40–60% of E47 cells lost their viability after knockdown of either TRX-1 or TRX-2 individually or in combination. The fact that knockdown of both TRX-1 and TRX-2 did not promote greater toxicity than the knockdown of either alone suggests that other cellular protective systems besides thioredoxin contribute to the cell defense against ROS, e.g. the glutathione system. There was no cell death with thioredoxin knock down in HepG2 cells not expressing CYP2E1, indicating that cell death induced by CYP2E1 is enhanced when thioredoxin is decreased. TRX-1 and TRX-2 are both important in protecting cells from cytotoxicity and both are effective and necessary in protecting against CYP2E1 toxicity since loss of either one causes a decline in E47 cell viability. Knockdown of TRX-1 did not affect levels of TRX-2 and knockdown of TRX-2 did not affect levels of TRX-1, therefore, TRX-1 or TRX-2 alone is not sufficient to protect against the CYP2E1 toxicity. Since CYP2E1 is largely expressed in the endoplasmic reticulum (ER), TRX-1 may be necessary for removal of ROS generated in the cytosol. Indeed experiments with DHE suggest elevation of cytosolic superoxide upon thioredoxin knockdown. Previous studies showed that damage to mitochondria plays a key role in CYP2E1-dependent toxicity [35–36]. TRX-2 may be necessary for protection against CYP2E1-ROS mitochondrial dysfunction, which may explain why knockdown of TRX-2 protects against cell toxicity in the absence of an increase in mitochondrial superoxide production. Hence, both TRX-1 and TRX-2 would generate maximal protection against CYP2E1 toxicity and therefore loss of either thioredoxin would promote CYP2E1 toxicity. This is analogous to previous studies showing that both SOD-1 and SOD-2 were necessary for maximal protection against CYP2E1 toxicity [37].
The fact that adding glutathione ethyl ester prevented the cell death caused by thioredoxin knockdown suggests that oxidative stress played a key role in the E47 cell death. There was increased ROS production and 4-HNE protein adducts formation with thioredoxin knockdown in E47 cells but not in C34 cells. The levels of GSH in E47 cells were lowered by thioredoxin knockdown, most likely a reflection of the elevated oxidant stress. Treatment of cells with BSO to lower GSH levels was previously shown to cause loss of E47 cell, but not C34 cell viability [38]. A low concentration of BSO induced the death of 50% of E47 cells by itself. With thioredoxin knockdown, BSO treatment killed 80–90% of E47 cells, showing that thioredoxin knockdown potentiated the toxicity of BSO. In C34 cells, no cell death from either BSO alone or combined with thioredoxin knockdown occurred. Thus, both thioredoxin and GSH protect cells from the cytotoxicity from CYP2E1 induction. The fact that there was only 40–60% cell death from 80–90% thioredoxin knockdown in E47 cells suggests other protection factors in cells may increase as an adaption for the protection from cell death. There was a two fold increase of total glutathione in E47 cells compared to C34 cells. There was no change of total glutathione in C34 cells with thioredoxin knockdown. The reduction of glutathione level after thioredoxin knockdown in E47 cells suggests GSH was consumed in protecting against CYP2E1 induced cell death when thioredoxin was lowered.
Since thioredoxin is an important inhibitor of ASK-1 [9–10], we tested whether thioredoxin knockdown activated ASK-1. Both TRX-1 and TRX-2 knockdown increased phosphorylation of ASK-1, followed by an increased phosphorylation of downstream JNK1 and then cJUN in E47 cells. The phosphorylation of JNK2 and p38 MAPK were not changed with either thioredoxin knockdown. It is not clear why these MAP kinses were not activated as was JNK1 and this will require further study. There are many reports in the literature that e.g. activation of ASK-1 results in activation of either JNK1 or of JNK2 [11, 39–41] or of JNK isoforms but not p38 MAP kinase [42–45], but it is not clear why this occurs. This activation of ASK-1 and JNK1 may be involved in the E47 cell death pathway induced by thioredoxin knockdown. Addition of a JNK inhibitor caused a partial recovery of E47 cell viability produced from the thioredoxin knockdown. Enhancement of metabolic oxidative stress induced cell death by the thioredoxin inhibitor 1-methylpropyl 2-imidazolyl disulfide which was mediated through an ASK-1-JNK1 pathway [46]. While the mechanism is complicated and might be cell specific, knocking down of TRX-1 in Hela cells did not increase phosphorylation of JNK and activate the MAPK pathway [47]. Further studies are needed to evaluate whether activation of JNK and MAPK signaling by thioredoxin knockdown is cell-specific. While it is generally thought that activation of the transcription factor cJUN plays a role in JNK1 toxicity, exact mechanism are not clear [48–50] and further studies will be needed to evaluate the role of cJUN activation, if any, in the E47 cell-induced toxicity upon thioredoxin knockdown.
Other mechanisms may be also involved in thioredoxin knockdown induced cell death, such as changes of expression of antioxidant enzymes. SOD1 expression increased in E47 cells but not C34 cells with thioredoxin knockdown, perhaps as a compensation reaction to protect the E47 cells against the increased oxidative stress. However, the increase of SOD1 was not accompanied by a corresponding increase in removing the elevated H2O2 produced by CYP2E1, as no elevation of other antioxidant enzymes, e.g. catalase or GPX-4 occurred. There was also an increase of the baseline glutathione level in E47 cells compared to C34 cells as discussed above.
In conclusion, both cytosolic and mitochondrial thioredoxin are important in protecting HepG2 cells from cell death by oxidative stress induced by CYP2E1. Thioredoxin knockdown increased cellular production of ROS and increased lipid peroxidation in HepG2 cells expressing CYP2E1. The signaling pathway which induced cell death by thioredoxin knockdown may involve, at least in part, the activation of ASK-1 and JNK1. This protection by both TRX-1 and TRX-2 against CYP2E1-dependent toxicity may play a role in the ability of thioredoxin to protect against ethanol-induced hepatotoxicity [17] and suggests that antioxidative protection in both the cytosol and mitochondria is necessary for effective protection against liver injury potentiated by CYP2E1.
Acknowledgements
These studies were supported by USPHS Grants 1 R01 AA017423 and 018790 from The National Institute on Alcohol Abuse and Alcoholism.
List of abbreviations
- CYP2E1
cytochrome P4502E1
- ASK-1
apoptosis signal-regulating kinase-1
- JNK
c-Jun NH2-terminal kinase
- MAPK
Mitogen-activated protein kinase
- ER
endoplasmic reticulum
- TNFα
tumor necrosis factor α
- 4-HNE
4-Hydroxynonenal
- GSSE
Glutathione ethyl ester
- BSO
l-Buthionine-[R,S]-sulfoximine
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- ROS
reactive oxygen species
- TRX-1
cytosolic thioredoxin
- TRX-2
mitochondrial thioredoxin
- PDI
prolyl disulfide isomerase
- DHE
dihydroethidine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- 2.Ebrahimian T, Touyz RM. Thioredoxin in vascular biology: role in hypertension. Antioxid Redox Signal. 2008;10:1127–1136. doi: 10.1089/ars.2007.1985. [DOI] [PubMed] [Google Scholar]
- 3.Powis G, Montfort WR. Properties and biological activities of thioredoxins. Annu Rev Pharmacol Toxicol. 2001;41:261–295. doi: 10.1146/annurev.pharmtox.41.1.261. [DOI] [PubMed] [Google Scholar]
- 4.Powis G, Montfort WR. Properties and biological activities of thioredoxins. Annu Rev Biophys Biomol Struct. 2001;30:421–455. doi: 10.1146/annurev.biophys.30.1.421. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura H, Masutani H, Tagaya Y, Yamauchi A, Inamoto T, Nanbu Y, Fujii S, Ozawa K, Yodoi J. Expression and growth-promoting effect of adult T-cell leukemia-derived factor. A human thioredoxin homologue in hepatocellular carcinoma. Cancer. 1992;69:2091–2097. doi: 10.1002/1097-0142(19920415)69:8<2091::aid-cncr2820690814>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 6.Bjornstedt M, Xue J, Huang W, Akesson B, Holmgren A. The thioredoxin and glutaredoxin systems are efficient electron donors to human plasma glutathione peroxidase. J Biol Chem. 1994;269:29382–29384. [PubMed] [Google Scholar]
- 7.White DW, Gilmore TD. Transcription factors, oncogenes, and apoptosis. Science. 1997;276:185. doi: 10.1126/science.276.5310.181f. [DOI] [PubMed] [Google Scholar]
- 8.Perez VI, Lew CM, Cortez LA, Webb CR, Rodriguez M, Liu Y, Qi W, Li Y, Chaudhuri A, Van Remmen H, Richardson A, Ikeno Y. Thioredoxin 2 haploinsufficiency in mice results in impaired mitochondrial function and increased oxidative stress. Free Radic. Biol. & Med. 2008;44:882–892. doi: 10.1016/j.freeradbiomed.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Nishitoh H, Ichijo H, Kyriakis JM. Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol Cell Biol. 2000;20:2198–2208. doi: 10.1128/mcb.20.6.2198-2208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu D, Cederbaum AI. Activation of ASK-1 and downstream MAP kinases in cytochrome P4502E1 potentiated tumor necrosis factor alpha liver injury. Free Radic. Biol. & Med. 2010;49:348–360. doi: 10.1016/j.freeradbiomed.2010.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujino G, Noguchi T, Matsuzawa A, Yamauchi S, Saitoh M, Takeda K, Ichijo H. Thioredoxin and TRAF family proteins regulate reactive oxygen species-dependent activation of ASK1 through reciprocal modulation of the N-terminal homophilic interaction of ASK1. Mol Cell Biol. 2007;27:8152–8163. doi: 10.1128/MCB.00227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim PL, Liu J, Go ML, Boelsterli UA. The mitochondrial superoxide/thioredoxin-2/Ask1 signaling pathway is critically involved in troglitazone-induced cell injury to human hepatocytes. Toxicol Sci. 2008;101:341–349. doi: 10.1093/toxsci/kfm273. [DOI] [PubMed] [Google Scholar]
- 14.Nonn L, Williams RR, Erickson RP, Powis G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol Cell Biol. 2003;23:916–922. doi: 10.1128/MCB.23.3.916-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Cai J, Murphy TJ, Jones DP. Overexpressed human mitochondrial thioredoxin confers resistance to oxidant-induced apoptosis in human osteosarcoma cells. J Biol Chem. 2002;277:33242–33248. doi: 10.1074/jbc.M202026200. [DOI] [PubMed] [Google Scholar]
- 16.Umekawa T, Sugiyama T, Kihira T, Murabayashi N, Zhang L, Nagao K, Kamimoto Y, Ma N, Yodoi J, Sagawa N. Overexpression of thioredoxin-1 reduces oxidative stress in the placenta of transgenic mice and promotes fetal growth via glucose metabolism. Endocrinology. 2008;149:3980–3988. doi: 10.1210/en.2007-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen JI, Roychowdhury S, DiBello PM, Jacobsen DW, Nagy LE. Exogenous thioredoxin prevents ethanol-induced oxidative damage and apoptosis in mouse liver. Hepatology (Baltimore, Md) 2009;49:1709–1717. doi: 10.1002/hep.22837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cederbaum AI. CYP2E1--biochemical and toxicological aspects and role in alcohol-induced liver injury. Mt Sinai J Med. 2006;73:657–672. [PubMed] [Google Scholar]
- 19.Wu D, Cederbaum AI. Development and properties of HepG2 cells that constitutively express CYP2E1. Methods Mol Biol. 2008;447:137–150. doi: 10.1007/978-1-59745-242-7_11. [DOI] [PubMed] [Google Scholar]
- 20.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 21.Wu D, Cederbaum AI. Glutathione depletion in CYP2E1-expressing liver cells induces toxicity due to the activation of p38 mitogen-activated protein kinase and reduction of nuclear factor-kappaB DNA binding activity. Mol Pharmacol. 2004;66:749–760. doi: 10.1124/mol.104.002048. [DOI] [PubMed] [Google Scholar]
- 22.Mari M, Cederbaum AI. CYP2E1 overexpression in HepG2 cells induces glutathione synthesis by transcriptional activation of gamma-glutamylcysteine synthetase. J Biol Chem. 2000;275:15563–15571. doi: 10.1074/jbc.M907022199. [DOI] [PubMed] [Google Scholar]
- 23.Didier C, Kerblat I, Drouet C, Favier A, Beani JC, Richard MJ. Induction of thioredoxin by ultraviolet-A radiation prevents oxidative-mediated cell death in human skin fibroblasts. Free Radic. Biol. & Med. 2001;31:585–598. doi: 10.1016/s0891-5849(01)00617-7. [DOI] [PubMed] [Google Scholar]
- 24.Zhang R, Al-Lamki R, Bai L, Streb JW, Miano JM, Bradley J, Min W. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circ Res. 2004;94:1483–1491. doi: 10.1161/01.RES.0000130525.37646.a7. [DOI] [PubMed] [Google Scholar]
- 25.Chen CL, Lin CF, Chang WT, Huang WC, Teng CF, Lin YS. Ceramide induces p38 MAPK and JNK activation through a mechanism involving a thioredoxin-interacting protein-mediated pathway. Blood. 2008;111:4365–4374. doi: 10.1182/blood-2007-08-106336. [DOI] [PubMed] [Google Scholar]
- 26.Honeggar M, Beck R, Moos PJ. Thioredoxin reductase 1 ablation sensitizes colon cancer cells to methylseleninate-mediated cytotoxicity. Toxicol Appl Pharmacol. 2009;241:348–355. doi: 10.1016/j.taap.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yacoub A, Hamed HA, Allegood J, Mitchell C, Spiegel S, Lesniak MS, Ogretmen B, Dash R, Sarkar D, Broaddus WC, Grant S, Curiel DT, Fisher PB, Dent P. PERK-dependent regulation of ceramide synthase 6 and thioredoxin play a key role in mda-7/IL-24-induced killing of primary human glioblastoma multiforme cells. Cancer Res. 2010;70:1120–1129. doi: 10.1158/0008-5472.CAN-09-4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitsui A, Hamuro J, Nakamura H, Kondo N, Hirabayashi Y, Ishizaki-Koizumi S, Hirakawa T, Inoue T, Yodoi J. Overexpression of human thioredoxin in transgenic mice controls oxidative stress and life span. Antioxid Redox Signal. 2002;4:693–696. doi: 10.1089/15230860260220201. [DOI] [PubMed] [Google Scholar]
- 29.Kasuno K, Nakamura H, Ono T, Muso E, Yodoi J. Protective roles of thioredoxin, a redox-regulating protein, in renal ischemia/reperfusion injury. Kidney Int. 2003;64:1273–1282. doi: 10.1046/j.1523-1755.2003.00224.x. [DOI] [PubMed] [Google Scholar]
- 30.Ohashi S, Nishio A, Nakamura H, Kido M, Ueno S, Uza N, Inoue S, Kitamura H, Kiriya K, Asada M, Tamaki H, Matsuura M, Kawasaki K, Fukui T, Watanabe N, Nakase H, Yodoi J, Okazaki K, Chiba T. Protective roles of redox-active protein thioredoxin-1 for severe acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G772–G781. doi: 10.1152/ajpgi.00425.2005. [DOI] [PubMed] [Google Scholar]
- 31.Okuyama H, Nakamura H, Shimahara Y, Uyama N, Kwon YW, Kawada N, Yamaoka Y, Yodoi J. Overexpression of thioredoxin prevents thioacetamide-induced hepatic fibrosis in mice. J Hepatol. 2005;42:117–123. doi: 10.1016/j.jhep.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 32.Okuyama H, Nakamura H, Shimahara Y, Araya S, Kawada N, Yamaoka Y, Yodoi J. Overexpression of thioredoxin prevents acute hepatitis caused by thioacetamide or lipopolysaccharide in mice. Hepatology (Baltimore, Md) 2003;37:1015–1025. doi: 10.1053/jhep.2003.50203. [DOI] [PubMed] [Google Scholar]
- 33.Takagi Y, Mitsui A, Nishiyama A, Nozaki K, Sono H, Gon Y, Hashimoto N, Yodoi J. Overexpression of thioredoxin in transgenic mice attenuates focal ischemic brain damage. Proc Natl Acad Sci USA. 1999;96(7):4131–4136. doi: 10.1073/pnas.96.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powis G, Kirkpatrick DL. Thioredoxin signaling as a target for cancer therapy. Curr Opin Pharmacol. 2007;7:392–397. doi: 10.1016/j.coph.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Bai J, Cederbaum AI. Overexpression of CYP2E1 in mitochondria sensitizes HepG2 cells to the toxicity caused by depletion of glutathione. J Biol Chem. 2006;281:5128–5136. doi: 10.1074/jbc.M510484200. [DOI] [PubMed] [Google Scholar]
- 36.Zhuge J, Cederbaum AI. Inhibition of the mitochondrial permeability transition by cyclosporin A prevents pyrazole plus lipopolysaccharide-induced liver injury in mice. Free Radic. Biol. & Med. 2009;46:406–413. doi: 10.1016/j.freeradbiomed.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perez MJ, Cederbaum AI. Adenovirus-mediated expression of Cu/Zn- or Mn-superoxide dismutase protects against CYP2E1-dependent toxicity. Hepatology (Baltimore, Md) 2003;38:1146–1158. doi: 10.1053/jhep.2003.50479. [DOI] [PubMed] [Google Scholar]
- 38.Wu D, Cederbaum AI. Removal of glutathione produces apoptosis and necrosis in HepG2 cells overexpressing CYP2E1. Alcohol Clin Exp Re. 2001;25:619–628. [PubMed] [Google Scholar]
- 39.Liu J, Minemoto Y, Lin A. c-Jun N-terminal protein kinase 1 (JNK1), but not JNK2, is essential for tumor necrosis factor alpha-induced c-Jun kinase activation and apoptosis. Mol Cell Biol. 2004;24:10844–10856. doi: 10.1128/MCB.24.24.10844-10856.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Singh R, Lefkowitch JH, Rigoli RM, Scherer PE, Czaja MJ. Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J Biol Chem. 2006;281:15258–15267. doi: 10.1074/jbc.M512953200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh R, Wang Y, Xiang Y, Tanaka KE, Gaarde WA, Czaja MJ. Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology (Baltimore, Md) 2009;49:87–96. doi: 10.1002/hep.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Magne L, Blanc E, Marchand A, Fafournoux P, Barouk R, Rouach H, Garlatli M. Stabilization of IGFBP-1 mRNA by ethanol in hepatoma cells involves the JNK pathway. J Hepatol. 2007;47:691–698. doi: 10.1016/j.jhep.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 43.Blatt NB, Boitano AE, Lyssiotic CA, Opipari AW, Glick GD. Bz-423 superoxide signals apoptosis via selective activation of JNK, Bak, and Bax. Free radical biology & medicine. 2008;45:1232–1242. doi: 10.1016/j.freeradbiomed.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh R, Wang Y, Schattenerg JM, Xiang Y, Czaja MJ. Chronic oxidative stress sensitizes hepatocytes to death from 4-hydroxynonenal by JNK/c-Jun overactivation. Am J Physiol Gastrointest Liver Physiol. 2009;297:G907–G917. doi: 10.1152/ajpgi.00151.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chung J, Chavez RG, Russell RM, Wang XD. Retinoic acid inhibits hepatic Jun N-terminal kinase-dependent signaling pathway in ethanol-fed rats. Oncogene. 2002;21:1539–1547. doi: 10.1038/sj.onc.1205023. [DOI] [PubMed] [Google Scholar]
- 46.Lee YJ, Kim JH, Chen J, Song JJ. Enhancement of metabolic oxidative stress-induced cytotoxicity by the thioredoxin inhibitor 1-methylpropyl 2-imidazolyl disulfide is mediated through the ASK1-SEK1-JNK1 pathway. Mol Pharmacol. 2002;62:1409–1417. doi: 10.1124/mol.62.6.1409. [DOI] [PubMed] [Google Scholar]
- 47.Jeong W, Chang TS, Boja ES, Fales HM, Rhee SG. Roles of TRP14, a thioredoxin-related protein in tumor necrosis factor-alpha signaling pathways. J Biol Chem. 2004;279:3151–3159. doi: 10.1074/jbc.M307959200. [DOI] [PubMed] [Google Scholar]
- 48.Shen HM, Pervaiz S. TNF receptor superfamily-induced cell death: redox-dependent execution. FASEB J. 2006;20:1589–1598. doi: 10.1096/fj.05-5603rev. [DOI] [PubMed] [Google Scholar]
- 49.Schwabe RF, Brenner DA. Mechanisms of Liver Injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583–G589. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- 50.Ventura JJ, Cogswll P, Flavell RA, Baldwin AS, Davis RJ. JNK potentiates TNF-stimulated necrosis by increasing the production of cytotoxic reactive oxygen species. Genes Dev. 2004;18:2905–2915. doi: 10.1101/gad.1223004. [DOI] [PMC free article] [PubMed] [Google Scholar]