Abstract
The modest effects of neurotrophic factor (NTF) treatment on lifespan in both animal models and clinical studies of Amyotropic Lateral Sclerosis (ALS) may result from any one or combination of the four following explanations: 1.) NTFs block cell death in some physiological contexts but not in ALS; 2.) NTFs do not rescue motoneurons (MNs) from death in any physiological context; 3.) NTFs block cell death in ALS but to no avail; 4.) NTFs are physiologically effective but limited by pharmacokinetic constraints. The object of this review is to critically evaluate the role of both NTFs and the intracellular cell death pathway itself in regulating the survival of spinal and cranial (lower) MNs during development, after injury and in response to disease. Because the role of molecules mediating MN survival has been most clearly resolved by the in vivo analysis of genetically engineered mice, this review will focus on studies of such mice expressing reporter, null or other mutant alleles of NTFs, NTF-receptors, cell death or ALS-associated genes.
2. INTRODUCTION
The discovery of nerve growth factor (NGF) by Levi-Montalcini and Cohen 50 years ago strikingly supported the nascent concept, originated by Hamburger (1), that secreted molecules produced by the target of a developing neuron are required for it to survive programmed cell death (PCD), an embryonic period during which roughly half of developing postmitotic neurons die by the morphological process of apoptosis. This neurotrophic hypothesis, coined by Purves (2), provided an intellectual background for the identification of subsequently identified neurotrophic factors (NTFs): a candidate NTF would be added to or removed from a specific neuronal population during PCD and its effects on survival recorded by measuring the number of healthy or dying neurons. Simultaneous progress in the field of intracellular cell death machinery, largely performed by Horvitz and colleagues, led to the identification of many key molecules whose activity was required for the passage of a cell through its apoptotic, programmed death states (3). Thus, whereas early work focused on the nature of NTFs as extracellular signals regulating PCD, subsequent studies established that a.) neurons dying during developmental PCD activate a program of genes and b.) NTFs rescue neurons from PCD by inhibiting this program (4). The robust effect of NTFs and anti-PCD molecules on the survival of MNs during development soon led to the idea that they might ameliorate neurodegenerative disorders such as Amyotrophic Lateral Sclerosis (ALS) and thereby aid in the treatment for those suffering from these diseases (5).
The intent of this review is to critically evaluate the validity of the notion that NTFs are physiological survival-promoting factors for spinal MNs during development, after injury or in MN diseases such as ALS. The almost complete lack of success exhibited by NTFs in human clinical trials of ALS, coupled with recent data suggesting that several prominent NTFs are in fact dispensable for the survival of skeletomotor (α) MNs during PCD, have questioned the idea that cell death in some of these contexts reflects inadequate trophic support, and have therefore made such an evaluation timely. On the other hand, several observations indicate that NTF dysfunction may represent key clues to or intermediates within the pathogenesis of ALS: 1.) mutations in at least 3 NTFs cause MN disease 2.) expression of several target-derived NTFs is reduced in ALS, 3.) downstream signaling initiated by other target-derived NTFs is blocked in ALS and 4.) centrally, intrathecally and/or virally delivered NTFs often exhibit superior neuroprotective effects when compared to systemically administered NTF (the route used for NTF delivery in ALS clinical trials). These findings suggest a more complicated neuromuscular scenario underlying ALS, in which NTFs and their receptors are dynamically expressed by different subcellular regions of MNs as well as by many other interacting cell types such as glia, muscle and endothelial cells. Therefore, before dismissing the efficacy of NTFs based solely on poor performance in human clinical trials, we intend to put forth in their defense a summary of the hurdles NTFs face as therapeutics for MN disease. Because the regulation of MN survival by NTFs has been most well characterized during developmental PCD, we begin with the analysis of NTF expression and signaling during this period. After presenting a review of ALS pathogenesis with a detailed emphasis on the role of cell death, we return to the function of NTFs in ALS transgenic and NTF KO mice, focusing on sites of action (i.e, soma vs. axons vs. synaptic terminals), modes of action (i.e., anti-apoptotic vs. anti-excitotoxic) and overall effects on motor function and lifespan.
3. NEUROTROPHINS AND NEUROTROPHIC HETEROGENEITY
The presence of dying neurons in the developing nervous system was noted over a century ago (6) but not appreciated until nearly half a century later, when Hamburger and Levi-Montalcini noted that the number of dying neurons during development varied depending upon the size of the target field (7). Further evidence of a relationship between factors produced by the periphery and the number of dying neurons was provided by Hamburger (1), who observed a large increase in the number of dying sensory neurons and MNs several days after the surgical removal of their target limb. Because the increase in dying MNs occurred during the same period that dying MNs had normally been found, these experiments indicated that neurons undergoing death during normal development might also depend on target-derived signals (8). The presence of such natural-occurring cell death is observed in many different neuronal subpopulations of the central and peripheral nervous systems (CNS, PNS), such as the lateral motor column of the spinal cord, where approximately 50–60% of the MNs produced die this way (9). The extent of cell death is temporally and spatially reproducible across many neuronal populations and species (10) suggesting it occurs under the environmental control of a genetic program (4, 5,11). The idea that this process of programmed cell death (PCD) is not fixed but regulated by target-derived factors was further supported by experiments showing a decrease in normal MN death after transplantation of a supernumary limb (12). Evidence that skeletal muscle is the cell type mediating these effects on MN survival was provided by the finding that purified muscle extract reduces MN cell death in vitro (13) and in vivo (14,15). Together, these studies demonstrate that muscle-derived NTFs block the death of MNs normally fated to die during PCD. More recent work shows that elimination of muscle cells increases MN death (16–18), indicating that muscle-derived NTFs factors also block the death of MNs normally fated to live during this period. These studies also suggest that the concentration of target-derived NTFs is normally limiting and that an environment increasing the production of or access to NTFs would lead to a commensurately larger rescue of MNs from PCD (19). In this way, PCD may provide an element of plasticity in controlling neuronal number and therefore have evolved as a mechanism to match the number of neurons and their targets (20,21).
These studies suggested that extracellular, target-derived molecules profoundly regulate MN survival during PCD, but the discovery of NGF as such a factor for sympathetic and a subset of sensory neurons provided more specific evidence that the neurotrophic hypothesis was valid. Moreover, the demonstration that NGF is produced by the target of these neurons (22), is retrogradely transported from the target to their cell bodies (23, 24), and is necessary (25) and sufficient (26) for their survival implied that similar molecules might rescue other populations of developing neurons (27). The biochemical isolation and survival-promoting effects of a second trophic molecule structurally related to NGF, brain-derived neurotrophic factor (BDNF), supported this idea (28). The relatedness of these molecules combined with the technological advances of molecular cloning led to the identification of NT-3 and NT4/5, thus forming the neurotrophin family of molecules (29). Additional work demonstrated that each neurotrophin exerts its target-dependent, retrograde survival effect through binding and activation of high-affinity tyrosine kinase receptors: NGF with TrkA, BDNF and NT4/5 with TrkB and NT-3 with TrkC (30, 31). Studies of mice lacking any of the neurotrophins or their high-affinity receptors, however, reveal that whereas some populations of neurons act in accordance with the neurotrophic hypothesis, others don’t (32). For example, studies of NGF- and TrkA-deficient mice show a complete absence of sympathetic and nociceptive sensory neurons, as predicted from earlier reports, but no loss of TrkA-expressing basal forebrain cholinergic neurons (33, 34). Similarly, while TrkB-expressing sensory neurons in the vestibular ganglion depend on BDNF for their survival during PCD, TrkB-expressing hippocampal granule neurons require neither BDNF nor TrkB (35, 36). Finally, whereas TrkC-expressing proprioceptive sensory neurons require both NT-3 and TrkC for their survival (37, 38), TrkC-expressing cerebellar granule neurons survive in the absence of NT-3 or TrkC (39). In contrast, CNS neurons require multiple neurotrophins for survival, as combined genetic elimination of TrkB and TrkC, but not of either factor alone, increases the number of dying granule neurons in the dentate gyrus and cerebellum (40). These in vivo studies indicate that neurotrophins act in a redundant manner during developmental PCD of CNS but not PNS neurons (41).
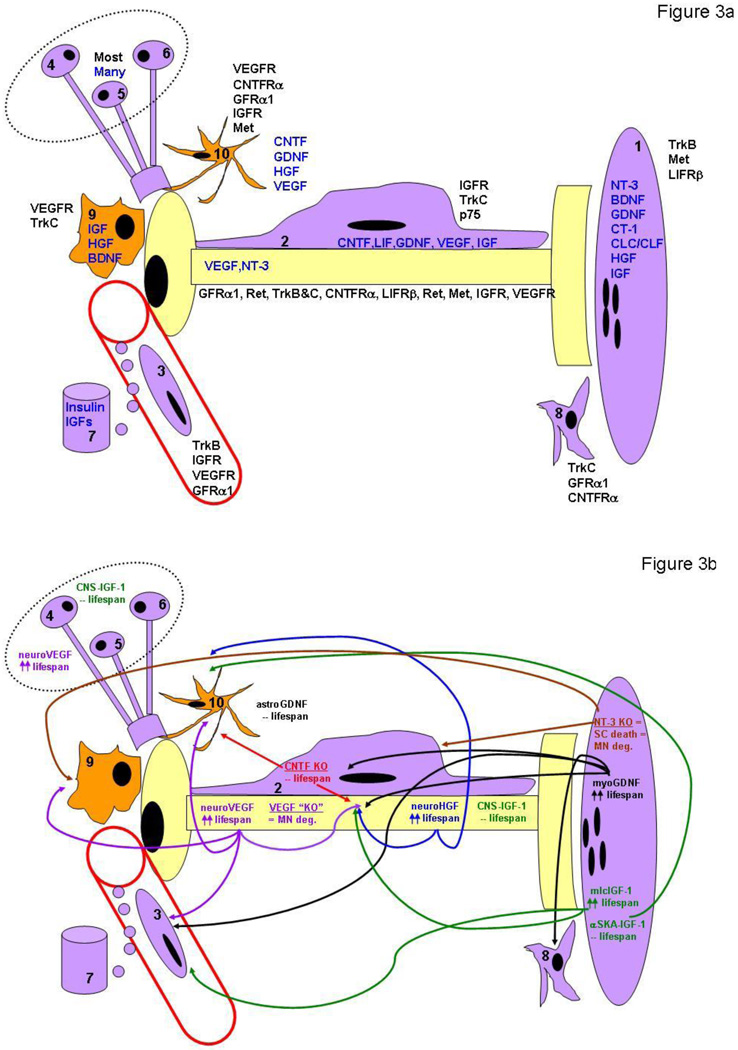
The discovery of multiple NTFs in addition to the neurotrophins allowed this idea to be further tested in MNs. In contrast to the results obtained with neurotrophins and CNS granule cells, the removal of single NTFs results in the loss of subpopulations of MNs (summarized in Table 1). Therefore, similar to PNS neurons, the loss of individual NTFs causes MN deficits, but in contrast to that of PNS neurons, the death of MNs appears less severe, with the greatest loss observed amounting to about 40%. The fact that the inactivation of different single NTFs each results in partial MN losses indicates that MNs may exhibit trophic heterogeneity during development; i.e., that different MN subtypes each respond to a distinct NTF or combination of NTFs (42–44). Analysis of mice deficient for some of these NTFs, which include glial cell line-derived neurotrophic factor (GDNF), ciliary neurotrophic factor (CNTF), insulin-like growth factors I and II (IGF-I, II), hepatocyte growth factor (HGF), and vascular endothelial-derived growth factor (VEGF), supports this idea (Table 1 and see the section below under GDNF). Such trophic heterogeneity may reflect a changing trophic environment encountered by MNs as they navigate toward and penetrate their targets, as has been demonstrated for cranial PNS neurons (45). Alternatively, the trophic dependence of each MN subpopulation on a different NTF may reflect the specific environments these subpopulations encounter during development, such as limb vs. trunk mesoderm, forelimb- vs. hindlimb-derived neural crest cells, cranial vs. spinal descending inputs. Evidence consistent with the idea that different cell types supply MNs with specific trophic support is provided by studies showing that selective elimination of supraspinal input, sensory afferent input, Schwann cells or astrocytes increase MN PCD (46–49). Moreover, pre-treatment with antibodies against GDNF or the cytokine cardiotrophin-1 (CT-1) abrogate the trophic effects of muscle and Schwann cells, respectively, on cultured MNs (50). Finally, MNs themselves express unique combinations of NTF receptors depending on position, target innervation and stage of maturation, suggesting that differential receptor and ligand distribution together contribute to MN neurotrophic heterogeneity (51). For example, exogenous BDNF only rescues lumbar MNs from the latter half of the PCD period because its receptor TrkB is only expressed at this time (52). Similarly restricted rescue effects are observed for HGF, whose receptor Met is expressed only by limb-innervating populations of spinal MNs (53, 54), and for cardiotrophin-like cytokine/cytokine-like factor for lumbar and facial MNs (CLC/CLF; 55). Recent evidence has demonstrated that smaller MN subpopulations known as motor pools also express discrete patterns of NTFRs, indicating that the trophic heterogeneity exhibited by MNs may be related to transcriptionally encoded patterns of target selection (56). A more detailed review of the rescue-promoting effects of individual NTFs is presented in Table 1 and below.
Table 1.
| NTF | NTFR(s) | Motoneuron Subtype Affected during Developmental PCD in KO mice |
|---|---|---|
| GDNF | GFRα1; Ret | Brachial: LD,CM - Ingrowth 171,172; Lumbar: Peroneal – Choicepoint173; sGM / TFL, iPsM - Ingrowth51 All spinal levels: 20–30% loss51, 113, 161–163; γ MNs (~90% loss)51; Facial: ~20% loss: Yes163; No161, 162, 174 |
| Neurturin | GFRα2, Ret | Spinal and cranial levels: No deficits noted in Neurturin KO or GFRα2 KO mice* |
| BDNF, NT-4/5 | TrkB; p75 | Spinal and cranial levels: No deficits noted in BDNF KO36, 187–189, NT-4/5 KO187–189, TrkB KO190; p75 KO149 |
| NT-3 | TrkC; p75 | NT-3 KOs: All spinal levels: γ MNs (complete loss)51, 192,195; Facial MNs: No deficits37,39 |
| CNTF | CNTFRα; LIFRβ; gp130 | CNTF or LIF KOs: Spinal and cranial levels: No deficits; CNTFRα223; LIFRβ224; gp130225 KOs: ~40% loss |
| LIF | LIFRβ; gp130 | Same as above |
| CT-1 | LIFRβ; gp130 | CT-1 KO: All spinal levels: 20–30% loss226; Facial: ~20% loss,226; Hypoglossal: ~20% loss226 |
| CLC/CLF | CNTFRα; LIFRβ; gp130 | CLC/CLF KO: Spinal levels: Lumbar (25% loss); Brachial55, Thoracic (No loss); Facial: - ~30% loss55; Hypoglossal: No loss55 |
| HGF | Met | Met KO: Brachial: LD,CM - Ingrowth239; Spinal and cranial levels: MN counts NE (embryonic lethality) |
| IGF-I, IGF-II | IGFR-1,IGFR-2 | IGF-I KO: Spinal levels: ~30 loss**; Facial: ~25% loss256; Trigeminal: ~30% loss256; IGR-1,2 KOs NEbut see 59 |
| VEGF | Flk-I;Flt-1;Flt-4 | VEGF KO: Facial: Migration267; Spinal and cranial levels: MN counts NE (embryonic lethality) |
APOPTOSIS DURING DEVELOPMENTAL AND NERVE INJURY-INDUCED PCD
Whereas the abundance of extracellular-derived, neurotrophic molecules illustrates the diversity of intercellular signaling mechanisms used to regulate neuronal PCD, the discovery of a relatively small group of evolutionarily conserved, intracellular death pathways activated by dying neurons indicates a common mien of cellular destruction during PCD (57). This interpretation is supported by the observation that NTF-deprived neurons during development typically die by a morphologically characteristic, physiologically efficient process known as apoptosis (58). Perhaps more importantly, the activation of this pathway and the presence of apoptosis in injured and degenerating neurons have fuelled the idea that the NTF regulation of apoptosis is operative or at least capable of exploitation in these pathological forms of cell death (5). While considerable support for this idea has been found in studies of experimental neuronal injury (59), the notion that either NTFs or apoptotic pathways of cell death underlie disease is less clear (60). In particular, recent observations suggest that caspase-deficient developing MNs, excitotoxically insulted MNs and degenerating MNs exhibit either necrosis or a third, autophagic form of PCD morphologically distinct from apoptosis and necrosis (61–64). In order to revisit this notion with respect to MN disease such as ALS, we will quickly review the basic biochemical pathways that mediate apoptotic and non-apoptotic death during mammalian developmental PCD or in response to injury. Invertebrate intracellular death pathways are not covered here but can be accessed in several excellent reviews (3, 57).
4.1 Development: intrinsic pathway of apoptosis
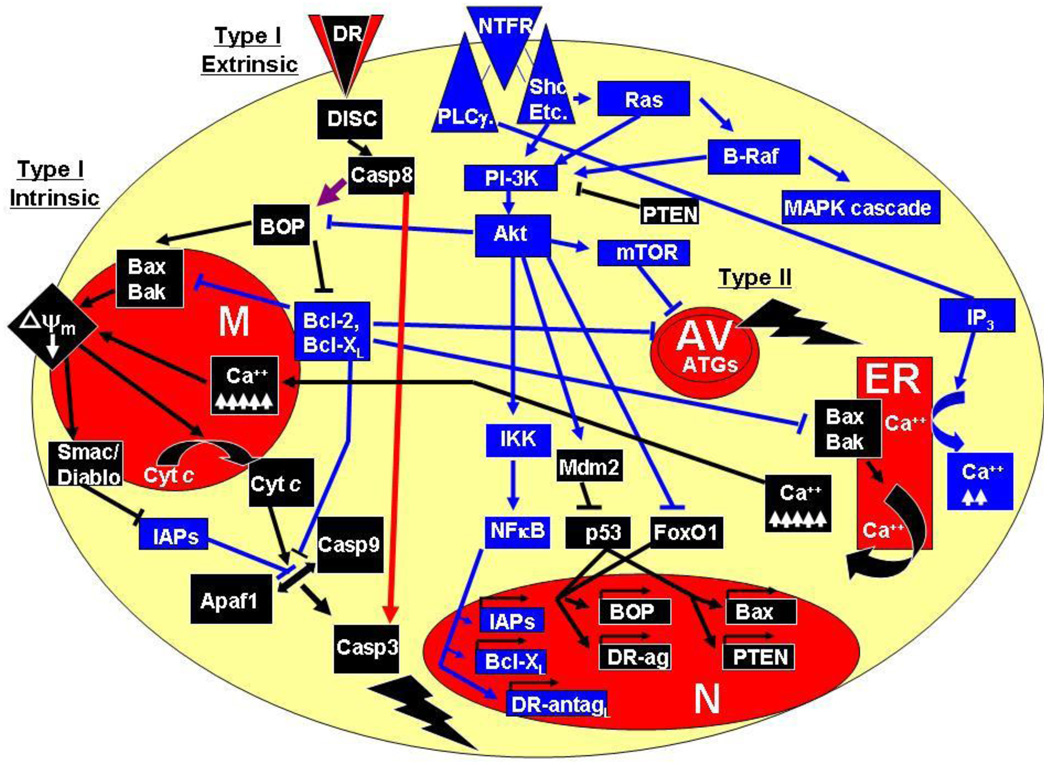
In the vertebrate system, developing neurons activate phosphoinositide-3 kinase (PI-3K) in response to NTF stimulation, which stimulates Akt and largely mediates the inhibition of the two major intracellular pathways of apoptosis, known as the intrinsic and extrinsic pathways (Figure 1; 65,66; but see also 67). The intrinsic pathway involves the mitochondria and endoplasmic reticulum and is composed of a variety of anti- and pro-apoptotic members containing one or several copies of the Bcl-2 Homology (BH) (68). This family of proteins can be further subdivided into proteins with several BH domains, the so-called multidomain proteins (MDPs; 69) and those with only 1 BH3 domain, the BH3-only proteins (BOPs; 70). Pro-apoptotic MDPs include Bax and Bak whereas at least 18 members of pro-apoptotic BOPs have been identified (71). Anti-apoptotic members include only MDPs such as Bcl-2, Bcl-XL, Bcl-w and MCP-1 (72). MDPs directly regulate apoptosis, whereas BOPs stimulate Bax/Bak or inhibit Bcl-2/Bcl-XL in response to NTF deprivation or other death signals (73, 74). BOPs are themselves either transcriptionally or post-translationally regulated by NTF signaling; for example, Akt inhibits the pro-apoptotic activity of Bad by direct phosphorylation (75, 76). The common result of NTF deprivation or many other forms of cell stress is the homo-oligomerization and translocation of Bax from cytosol to outer mitochondrial membrane (77, 78) as well as Bak activation within the mitochondrial membrane. Oligomerized, membrane-targeted Bax forms a pore and permits flow of cytochrome c (cyt c) from mitochondria to cytosol (79–81), while activated Bak causes the fragmentation of mitochondria (82). Cytosolic cyt c binds the apoptosis protease-activating factor-1 (Apaf-1), which facilitates the binding and activation of caspase-9 in a tertiary complex called the apoptosome (83). Caspase-9 is a member of the cysteine proteases that reside in the cytosol as inactive zymogens and initiate a proteolytic cascade when activated (84). Initiator caspases such as caspase-9 cause the activation of effector caspases such as caspase-3, whose activity dismantles the cell and results in the phenotypic expression of apoptosis (85, 86).
Figure 1.
Basic intracellular molecular pathways regulating MN survival during development. Cells die through apoptosis (Type I), autophagic cell death (Type II) or necrosis (not pictured). Two different pathways mediate apoptosis, called the intrinsic and extrinsic pathways. The intrinsic pathway involves activation of the pro-apoptotic multi-BH-domain proteins (MDP) Bak and Bax and inhibition of the anti-apoptotic MDPs Bcl-2 and Bcl-XL by the pro-apoptotic BH3-domain-only proteins (BOPs). Bak/Bax activation promote mitochondrial permeability transition (mPT), loss of mitochondrial membrane potential (ψm), release of mitochondrial cytochrome c (cyt c) and activation of caspases (casp3, casp9), which dismantle the cell (lightening strike). Bak/Bax activation also depletes (ψm) by causing the transfer of Ca++ from the endoplasmic reticulum (ER) to the mitochondrion (M). The extrinsic apoptotic pathway directly activates caspases (red arrow) or activates the intrinsic death pathway through the BOP Bid (purple arrow). Autophagic death (lightening strike) occurs independently of apoptosis and is characterized by the presence of abundant autophagic vacuoles (AV) and the activation of autophagic genes (ATGs) such as Beclin 1 (atg6). Cell death is regulated by extracellular anti-apoptotic and pro-apoptotic stimuli that operate through neurotrophic factor receptors (NTFRs) or death receptors (DRs). Activated DRs activate the extrinsic death pathway by assembling a death-inducing signaling complex (DISC). Activated NTFRs antagonize apoptotic, autophagic and necrotic death by activating cytosolic kinases such as phosphoinositide-3 kinase, Akt and B-Raf, inhibiting pro-apoptotic BOP activity, activating anti-apoptotic and inhibiting pro-apoptotic gene expression in the nucleus (N). Blue lines indicate anti-apoptotic function and black lines depict pro-apoptotic function; arrows indicate activation and flat lines indicate inhibition. For details see text.
In addition to cyt c, several other apoptogenic molecules are released from the mitochondria, including Smac/Diablo, which activates caspases by inhibiting a family of caspase-inhibiting molecules known as the inhibitors of apoptosis (87–88), apoptosis-inhibiting factor (AIF) and the endonuclease EndoG, both of which cause apoptosis in a caspase-independent fashion (89–91). However, AIF and EndoG may exit mitochondria significantly later than cyt c and thus occur only after caspase activation (92). While these molecules form the bulk of the core pathway mediating PCD, additional inputs stimulated by pathological cell stress often stimulate the intrinsic pathway (93). For example, genotoxic, osmotic or oxidative stress activates the c-Jun N-terminal kinases (JNKs), which activate a wide variety of targets including the transcription factors c-Jun and p53 and the pro-apoptotic BOP BIM (94–96). p53 subsequently triggers apoptosis by transcriptionally activating Bax and Bax-stimulating BOPs (97, 98). Similarly, protein misfolding activates an ER-dependent, Ca++-sensitive pathway which stimulates mitochondrial permeability transition (mPT), resulting in the dissipation of the mitochondrial electrochemical gradient, liberation of cyt c and activation of caspases (99, 100). The extent to which mPT also occurs in response to classic intrinsic pathway activation, i.e., Bax-dependent cyt c release and caspase activation, is still under debate (101).
While in vitro pharmacological studies of MNs have established a critical role for PI-3K- and Akt-mediated signaling in response to NTFs (102), analyses of neuronal PCD in mice genetically lacking these enzymes have not been performed on account of early embryonic lethality caused by constitutive inactivation (PI-3K; 103,104) or redundancy of multiple isoforms (Akt; 105). In contrast, genetic studies have shown that key regulators of post-mitotic neuronal PCD in vivo include MDPs such as Bax, Bcl-2 and Bcl-XL, but not BOPs (106). Although caspase activation is a feature of post-mitotic neurons undergoing PCD, caspase-3 is required only for the death of cycling neuronal progenitors (107). Accordingly, post-mitotic spinal MNs still die but in a caspase-independent manner in caspase-3, caspase-9 and Apaf-1 null mutant mice (61, 108). The morphological process by which caspase-deficient MNs die is similar to autophagic death, but this non-apoptotic form of death is unlikely to normally regulate MN survival because MN PCD is unaffected by deletion of ATG7, a gene required for autophagy (106). In marked contrast, overexpression of Bcl-2 and inactivation of Bax profoundly arrests neuronal PCD (109–111); MNs, for example, exhibit no developmental MN death whatsoever in Bax knockout (KO) mice (112). In fact, a recent analysis of total axons in the fourth lumbar ventral root (L4 VR) of Bax-deficient mice indicates that 2 of 3 rather than 1 of 2 MNs die during developmental PCD (113). MNs rescued from PCD by Bax-deficiency are smaller in diameter than wild-type MNs, and many are unmyelinated, suggesting that Bax deletion alone (i.e., in the absence of elevated NTF signaling), does not increase the number of MNs that are competent to innervate extrafusal muscle targets (113).
4.2 Nerve injury: intrinsic pathway of apoptosis
MNs maintain dependence on their targets even after the period of developmental PCD, because rapid apoptosis occurs if an axon is transected and prevented from re-innervating its muscle during early postnatal development, (114–118). In contrast, axotomy in the adult causes a much slower degeneration characterized by neuronal atrophy and loss of neurotransmitter expression (119, 120), as well as the increase in expression of factors involved in axonal regeneration (121). Apoptotic death of adult MNs is observed, however, in response to some types of axonal injury, such as proximal transection or avulsion of the sciatic nerve (122–124).
Viral-mediated expression of a constitutively active form of Akt prevents MNs from neonatal axotomy (125). Transgenic overexpression of Bcl-2 or Bcl-XL (126, 127) as well as deletion of Bax (110, 111) also inhibits this form of MN death. In contrast, inactivation of caspase-3 (128) or viral-mediated expression of caspase-inhibiting IAPs delays but does not block neonatal axotomy-induced MN cell death (129). The death of adult MNs in response to avulsion is also prevented by Bax deletion (130; but see 131) or viral-mediated Bcl-2 overexpression (132). Interestingly, whereas individual Bax-activating BOPs are dispensable for developmental PCD, their absence after nerve injury significantly attenuates MN death. For example, MNs lacking DR5/Harakiri or Noxa are protected from injury-induced death in adult mice (133, 134). Finally, apoptotic death caused by nerve injury involves the activation of several pathways that are dispensable for programmed cell death, such as the stress-activated transcription factors p53 and c-Jun (130, 135), suggesting that the intrinsic pathway of cell death, gated by Bcl-2, Bcl-XL and Bax, is activated by specific sets of stimuli in MNs undergoing developmental or injury-induced apoptotic death.
4.3 Development and nerve injury: extrinsic pathway of apoptosis
An additional pathway leading to apoptotic death can be initiated even in the presence of NTFs (136). This extrinsic pathway of apoptosis is initiated by cell surface receptors such as Fas, tumor necrosis factor alpha receptor (TNFR) and the low-affinity neurotrophin receptor p75NTR, which when activated by extracellular ligands exhibit a change in conformation that favors the assembly of a death-inducing signaling complex (DISC) on a region of their cytoplasmic tails known as the death domain (137). The association of activated “death receptors” and DISC causes the recruitment and activation of procaspase-8 (138), which leads to mitochondrial-independent activation caspase-3 and apoptotic death in so-called Type 1 cells or a mitochondrial-dependent, caspase-3 mediated apoptosis in Type II cells (139). This latter pathway is mediated by caspase-8-mediated proteolytic activation of BID, a BOP which activates the oligomerization and mitochondrial insertion of Bax, thus linking the extrinsic and intrinsic apoptotic pathways (140–143). Both Bcl-2 overexpression and Bax/Bak deficiency block this pathway (144,145).
Fas-mediated killing of MNs occurs in an in vitro model of PCD through this Type II pathway (136) as well as through another mitochondrial-dependent, caspase-8-independent pathway requiring the upregulation of neuronal nitric oxide synthase (nNOS; 146). However, Fas null mutant mice exhibit no difference in MN number at the end of the PCD (147). In contrast, MN survival after neonatal or adult nerve injury is robustly increased by Fas deletion (147, 148). Mitochondrial-dependent, apoptotic death of injured MNs is also blocked in nNOS-deficient mice, suggesting that Fas and NO underlie MN death caused by pathological disconnection of MNs from their targets (148). Similarly, MN survival in mice lacking p75NTR or both TNFR1 and TNFR2 is unaffected during developmental PCD, but increased after nerve injury (135, 149). Together, these studies show that injury-induced (but not developmental) apoptotic death of MNs occurs through an extrinsic, death receptor-mediated activation of the intrinsic pathway of apoptosis.
5. EFFECT OF NEUROTROPHIC FACTORS ON DEVELOPMENTAL AND NERVE INJURY-INDUCED PCD
5.1 GDNF
GDNF was first identified as a midbrain dopaminergic trophic factor produced by the supernatant of a clonal glial cell line (150). GDNF is expressed by Schwann cells and skeletal muscle during the period of MN PCD, and is upregulated after nerve injury (151, 152). GDNF is also expressed in astrocytes after excitotoxic injury to the spinal cord (153). GDNF is transported to MNs in both retrograde and anterograde directions, indicating a wide variety of potential cellular sources (154–156). Treatment with exogenous GDNF prevents the apoptotic death of neonatal MNs in response to axotomy (151, 157) and blocks MNs from undergoing PCD (158,159). GDNF also reduces the loss of ChAT expression and enhances the regeneration of adult MNs after axotomy (154, 160). Deletion of GDNF in mice causes a 20–30% additional loss of spinal MNs during the process of PCD (161–163). GDNF acts via a multicomponent receptor composed of the tyrosine kinase Ret and the glycosylphosphatidylinositol- (GPI-) linked GDNF family receptor α1 (GFRα1; 164). Although GDNF can induce the activation of the RAS/ERK, p38 MAPK, JNK, PI-3K, PKA and Rho kinase signaling pathways (165,166), GDNF-mediated survival signaling in MNs requires the phosphorylation of Ret on tyrosine (Y) 1062, recruitment and activation of PI-3K signaling (51, 167, 168). Ret and GFRα1 are expressed by spinal MNs during MN PCD and both GFRα1 and Ret KO mice exhibit a 20–30% loss of spinal MNs, similar to GDNF KOs (51, 113, 163, 169, 170). These results suggest that a specific subset of spinal MNs require GDNF for survival during development. Some MNs in these KOs fail to project to several muscles in the trunk and hindlimb (171–173), but these losses are insufficient to explain the full extent of MN loss in GDNF KOs (51). Recent reports suggest that the remaining component of MN loss in GDNF ligand or GDNF receptor KO mice is restricted to fusimotor γ MNs (51,174). More than 90% of lumbar fusimotor axons are missing in adult mice lacking the GDNF receptors Ret or GFRα1 exclusively in MNs, and muscle spindles receive severely diminished fusimotor innervation in MN-specific Ret KO mice (51). In contrast, in the 50% of GDNF KO mice that fail to exhibit an early pathfinding deficit (causing peroneal MNs to select the tibial nerve and subsequently die; 173), skeletomotor (α) MNs are completely unaffected (51). Moreover, MN number is unaffected in the facial nucleus, a cranial MN subpopulation which contains only α MNs (161, 162 but see 163).
In addition to the potent, restricted neurotrophic effect of GDNF on fusimotor neurons, overexpression of GDNF under either a muscle-specific (Myo-GDNF) or astrocyte-specific (GFAP-GDNF) promoter results in the hyper-innervation of both intra- and extrafusal motor fibers and a delay in the period of synapse elimination (175, 176). Myo-GDNF mice also exhibit a doubling of motor axons in the ventral root, nearly all of which represents an increase in the number of small-diameter axons in the lumbar ventral roots (113, 174). Therefore, the current data suggests that the trophic effects of GDNF during the developmental PCD period are restricted to muscle spindle-innervating γ MNs, despite the fact that all MNs express GDNF receptors and respond to exogenous GDNF. Moreover, these studies support the idea that specific populations of MNs require distinct, individual NTFs during this period.
5.2 BDNF
BDNF was originally isolated as a trophic factor for neurons from brain extract (177). Expression of BDNF (178) and the high-affinity BDNF receptor TrkB (179) is observed in skeletal muscle and MNs, respectively during MN PCD. BDNF and TrkB expression are increased in the peripheral nerve and MNs, respectively, after nerve injury (180, 181). MNs retrogradely transport BDNF and exogenous BDNF rescues MNs from embryonic PCD, neonatal axotomy-induced death, and embryonic deafferentation-induced death (182–185). Although MN death is increased in TrkB KO mice in response to neonatal axotomy (186), neither BDNF nor TrkB null mutant mice exhibit a loss of MNs after the period of MN PCD (36, 187–190). Therefore, the trophic effects of BDNF during development, in which a subpopulation of MNs is rescued from the second half of the cell death period (52), are restricted to MNs that would normally die rather than those that would normally live through the PCD period.
5.3 NT-3
The neurotrophin NT-3 and its high-affinity receptor TrkC are required for the survival during PCD of several populations of sensory neurons (37–39). In particular, proprioceptive sensory neurons of the dorsal root ganglia (DRG) depend on muscle-derived NT-3, which is expressed robustly by intrafusal muscle fibers (191–193). The loss of spinal proprioceptive afferent neurons prevents the induction of intrafusal muscle fibers, and the resulting absence of muscle spindles is believed to cause the death of fusimotor MNs, which are completely missing in NT-3 KO mice (51, 194, 195). In contrast, NT-3 is not required for the survival of skeletomotor MNs during PCD, because numbers of facial MNs, a population containing no fusimotor MNs, are unaffected in NT-3 KO mice (37, 39). Additionally, the number of large-diameter α MNs appears normal in the lumbar spinal cord of NT-3 null mutants, although they exhibit a 15% reduction in somal diameter (195). It is worth noting that while single deficits in BDNF, NT-4/5 or NT-3 cause no loss of facial MNs during MN PCD, mice deficient in all three of these neurotrophins contain fewer facial MNs at birth (196). Interestingly, muscles of NT-3 KO mice lose neuromuscular innervation postnatally (197). This deficit likely reflects the requirement of NT-3 for early postnatal Schwann cell survival (198), as Schwann cells themselves are required for MN survival (48) and maintenance of neuromuscular innervation (199). Future experiments aimed at deleting the TrkC receptor in selective cell types (e.g., MNs, Schwann cells, perineurial and muscle fibroblasts) should help confirm that NT-3 exerts these neuropathic effects on MNs indirectly through its effects on Schwann cells. Although exogenous NT-3 is required for fusimotor MNs to survive during MN PCD, it is insufficient to rescue MNs that would normally die during this period (15). Exogenous NT-3, similar to BDNF, also promotes the survival of MNs after neonatal axotomy and embryonic deafferentation (183; 200, 201). Together, these studies show that NT-3 is indirectly required for the survival of fusimotor MNs during PCD and for the maintenance of neuromuscular innervation in the adult.
5.4 CNTF, LIF, CT-1, CLC/CLF
The interleukin-6 (IL-6) family of neuropoietic cytokines includes leukemia inhibitory factor (LIF), CNTF, CT-1 and cardiotrophin-like cytokine/cytokine-like factor 1 (CLC/CLF), and each of these neurotrophic factors act through a signal-activating heterodimeric receptor composed of the tyrosine kinases LIF receptor-β (LIFR-β) and glycoprotein130 (gp130; 202–205). Additionally, CNTF and CLC/CLF require a GPI-linked co-receptor, CNTF receptor-α (CNTFRα), and CT-1 requires a similar, as-yet unidentified co-receptor. CNTF binding to LIFR-β/gp130 in MNs activates the janus kinase / signal transducer and activator of transcription-3 (JAK/STAT) and PI-3K signalling pathways, thus leading to the inhibition of the intrinsic apoptosis pathway (206, 207). CNTF was isolated as a neurotrophic factor for parasympathetic neurons of the ciliary ganglion (208, 209), and exogenous CNTF exerts trophic effects on dissociated embryonic MNs (210), neonatal MNs after axotomy (211) and MNs undergoing PCD (212). LIF also rescues embryonic MNs from PCD in vivo (213) or death in vitro (214). In contrast to other NTFs, CNTF is expressed only after MN PCD and in the nerve rather than muscle, and contains no leader sequence and hence is not classically secreted (215, 216). However, CNTF is found in the medium of cultured Schwann cells and is retrogradely transported by spinal MNs (217, 218), suggesting that CNTF is released in a non-classic manner. Inactivation of CNTF in mice has no effect on MN number during MN PCD, but leads to the atrophy and then loss of a subset of MNs postnatally, resulting in muscle weakness (219). Similarly, LIF null mutants fail to exhibit changes in the numbers of MNs after PCD (220, 221), although combined deletion of LIF and CNTF exacerbates the postnatal loss of MNs observed in CNTF KO mice (222).
In contrast to CNTF or LIF null mutants, mice lacking the neuropoietic cytokine receptors CNTFRα (223), LIFR-β (224) or gp130 (225) all show a profound (~40%) loss of spinal and cranial MNs during PCD. These results suggest that cytokines other than CNTF and LIF must account for these effects. Mice deficient for one such cytokines, CT-1, accordingly exhibit a 20–30% loss of MNs after the period of MN PCD (226). CT-1, which was expression cloned as a factor inducing cardiac myocyte hypertrophy, also lacks a classical leader sequence but is found in embryonic skeletal muscle during MN PCD (227, 228). CT-1 deletion is not required for the maintenance of MNs postnatally (226), and its absence does not potentiate the loss of MNs observed in CNTF-or LIF-deficient KO mice (229). Because the actions of CT-1 require gp130/LIFR-β but not CNTFRα (230) the loss of MNs observed in CNTFR KO mice cannot be accounted for by CT-1 deficiency. Accordingly, inactivation of the second CNTFRα ligand, CLC/CLF, also results in a 20–30% reduction of MNs during the PCD period (55). Interestingly, although CNTFRα, LIFR-β and gp130 are expressed in most murine MNs, the loss of spinal MNs in CLC/CLF KO mice is only observed in lumbar and not thoracic or brachial regions (55), suggesting that the subtype of MNs which is lost in these mice is different from fusimotor MNs. In the developing chicken spinal cord, lumbar MNs expressing CNTFRα are very well circumscribed and restricted to several motor pools, including those innervating the adductor muscles (56). However, due to the inability of CNTFRα and other cytokine KO mice to suckle, they fail to survive postnatally and thus a more detailed analysis on subtypes of murine MNs lost is still forthcoming.
5.5 HGF
Hepatocyte growth factor (HGF) / scatter factor (SF) was originally isolated as a mitogen for regenerating liver cells and a motogen for developing muscle cells, and HGF signals through the tyrosine kinase receptor Met (231–234). Mice deficient in either HGF or Met exhibit liver, muscle and nerve pathfinding deficits and die during embryogenesis before the completion of MN PCD (235). Nevertheless, the trophic effects of exogenous HGF on cultured MNs (53, 236, 237). MNs undergoing PCD in vivo (54) and neonatal MNs undergoing axotomy-induced death (238) indicate an important role for HGF in MN survival during development. Interestingly, the expression of Met is restricted to limb-innervating subtypes of MNs (53), and the expression of HGF in the limb mesenchyme is required for a subpopulation of MNs to innervate the LD and CM muscles of the forelimb, the same muscles which lack innervation in GDNF ligand/receptor KO mice (236, 239, 240). Recently, it was observed that these two NTFs collaborate to establish the transcriptional identity and thus target selection of MNs innervating these muscles (241). HGF-dependent limb innervation requires the presence of PI-3K-binding tyrosine residues on Met, emphasizing the central importance of this pathway in mediating both tropic and trophic effects of NTFs on developing MNs (240). A more detailed analysis of the effects of HGF / Met deletion on MN number has yet to be reported, but the recent generation of conditional Met mutants makes such a study possible (242).
5.6 IGFs
The family of insulin-like growth factor (IGF) includes IGF-I and IGF-II, two secreted proteins structurally similar to pro-insulin, the tetrameric tyrosine kinase receptors IGFR Type 1 and 2 (IGFR-1 and IGFR-2), and at least 6 IGF binding proteins (IGFBP); IGF-1 binds IGFR-1 as well as insulin receptor (IR), and IGF-II binds IGFR-1 and IGFR-2 (243, 244). The expression of IGFs is elevated in skeletal muscle and Schwann cells during synaptogenesis and after peripheral denervation and exogenous IGFs induce motor axon sprouting and reinnervation (245–247). Furthermore, the increase in neuromuscular branching observed after paralysis is blocked by treatment with IGFBP (248). Consistent with these studies, exogenous IGFs enhance regeneration after nerve injury and blockade of endogenous IGFs reduces this effect (249), and exogenous insulin also enhances motor reinnervation after sciatic nerve injury (250). IGFs are also necessary and sufficient for MN survival after neonatal axotomy (200, 251). During the process of developmental MN PCD, IGFs are expressed by muscle but not Schwann cells (245, 252), and IGFRs are expressed by embryonic MNs (246). Accordingly, exogenous treatment with IGF-I and IGF-II reduces MN PCD (253), and treatment with exogenous IGFBP blocks the survival-promoting effects of activity blockade (254). Conversely, administration of function-blocking antibodies to IGF-1 and targeted deletion of IGF-1 both result in an enhanced loss of spinal MNs during PCD (254; RW Oppenheim, personal communication). Although a wide variety of reports have indicated that the neurotrophic effects of IGFs are mediated through PI-3K activation, it has not been established whether signaling through this pathway is required for MN survival during PCD. IGFs also activate the JAK/STAT pathway (255), suggesting a potential link netween IGF and cytokine signaling, a hypothesis supported by the finding that LIF deficiency potentiates the loss of MNs in IGF-1 KOs despite exerting no such effect alone (256). In contrast, because the number of facial MNs is reduced in IGF-1-deficient mice, the trophic effects and signaling pathways of IGF-1 in MNs are likely different than those of either NT-3 or GDNF (256). IGFR-1 null mutant mice have been generated as well, but a detailed analysis of MN defects has not been reported (257). A recent study reported the generation of conditional IGFR-1 mutant mice (258), which when crossed with HB9-Cre mice will begin to address this issue.
5.7 VEGF
In contrast to each of the previous NTFs discussed, whose role in ALS has been extrapolated based on its developmental trophic effects, the discovery of VEGF as a candidate gene for sporadic ALS (see below) has reversed the equation and stimulated studies to elucidate its role in MN development. VEGF regulates the growth of blood vessels during development and after hypoxic injury (259), but has recently been shown to exert direct trophic effects on neurons (260). VEGF is a homodimeric glycoprotein that activates a variety of homodimeric tyrosine kinase receptors including VEGFR1 (FMS-like tyrosine kinase-1; Flt-1), VEGFR2 (Fetal liver kinase-1; Flk-1) VEGFR3 (FMS-like tyrosine kinase-4; Flt-4), as well as the neuropilin coreceptors Nrp1 and Nrp2, although the majority of effects on neurons are believed to occur through VEGFR2 (261). VEGF exerts trophic effects on MNs in culture and in spinal cord explants by activating the PI-3K signaling pathway and Bcl-2 (262–263). VEGF also enhances the regeneration of adult MNs after axotomy, but whether these effects are direct or indirect through an increase in angiogenesis are unclear (264). Although VEGF-KO and VEGFR-KO mice have been generated, the effect of VEGF on MN PCD has not been examined, because even haplo-insufficient VEGF mice die early during embryogenesis due to defective blood vessel formation (265, 266). Similarly, the expression of VEGF and VEGFR has not yet been thoroughly analyzed in the developing neuromuscular system, although a recent report demonstrated that VEGF is essential for the developmental migration of facial MNs (267). Conditional mutant mice lacking VEGF and VEGFR have been generated and will hopefully soon be crossed to mice expressing Cre recombinase in specific neuromuscular tissues in order to explore the role of this growth factor during development.
5.8 Summary of NTFs during MN developmental PCD
Although the first NTF/NTFR KO mice were generated nearly 15 years ago, the precise MN subtypes lost in each of them remains largely unknown. This is partially because constitutive NTF/NTFR null mutants often exhibit embryonic or neonatal lethality, thus allowing for gross but not precise analysis of missing MNs. Nevertheless, these studies demonstrate that the absence of an individual NTF nearly always enhances MN PCD, suggesting that like PNS neurons, MNs are sensitive to the removal of single NTFs. In the most thoroughly characterized example, such as conditional GDNF receptor KO mice, the near complete loss of γ MNs may indicate that NTFs are required for the complete survival of MN subtypes rather than for partial survival across many subtypes. Although this finding is consistent with the hypothesis that MNs which fail to gain access to individual NTFs are eliminated during PCD (268), it also suggests that different MN populations exhibit complete dependence on single and non-overlapping NTFs. In contrast, nearly all NTFs are effective in preventing the death of axotomized MNs. Finally, the inactivation of CNTF, NT-3 or VEGF causes a loss of MNs postnatally (see below), indicating that trophic deprivation may at least partially contribute to MN disease. However, the notion that all MNs exhibit a universal response to NTF deprivation caused by developmental, injury-induced or degenerative stimuli is not supported by these studies. Indeed, the idea that MN death during diseases such as ALS is similar to that during developmental PCD is itself questionable and is the subject of the following section of this review.
6. PATHOPHYSIOLOGY OF ALS
The death of MNs occurs and may play a pathogenetic role in MN diseases such as Amyotrophic Lateral Sclerosis (ALS). ALS causes death of both lower MNs (i.e., cranial and spinal MNs that innervate skeletal muscle) upper MNs (e.g., corticospinal neurons projecting to the spinal cord; 269), spinal interneurons (270, 271) and sensory neurons (272), and results in muscle weakness, paralysis and death by respiratory failure within 3–5 years of symptom onset. 90–95% of ALS occurs with unknown etiology but increasing subsets of sporadic ALS (sALS) cases are associated with mutations in a diverse set of genes, including dynactin, Neurofilament-H, Peripherin, Angiogenin and VEGF (273). The remaining 5–10% of ALS cases are inherited and approximately one-fifth of these familial ALS (fALS) mutations are found within the gene encoding superoxide dismutase 1 (SOD1; 273). SOD1, also known as Cu/ZnSOD in humans, encodes a dimeric metalloenzyme which catalyzes the dismutation of superoxide anion to oxygen and hydrogen peroxide (275). Transgenic mice containing various human SOD1 mutations develop progressive neurodegeneration and MN death, providing an animal model that has greatly contributed to the understanding of fALS pathogenesis (276). SOD1 mutations cause fALS by exerting a dominant gain of function, because the loss of SOD1 does not cause disease (277) and because disease-causing SOD1 mutations often fail to affect the antioxidant activity of SOD1 (278).
Wild-type (wt) mammalian SOD1 is robustly expressed by MNs (279). The 32-kD SOD1 homodimer is composed of two 16-kD subunits, each of which bind one zinc and one copper ion and contain one intrasubunit disulfide bond. After receiving zinc, SOD1 monomer forms a heterodimeric complex with the copper chaperone for SOD1 (CCS), which in the presence of oxygen results in the loading of copper onto SOD1, the formation of the disulfide, the disassociation of SOD1 from CCS, and the subsequent formation of dimeric SOD1 (280). The quaternary structure of dimeric SOD1 is very stable and the disulfide linkage is unusual for a protein present within the reducing environment of the cytosol (281, 282). Demetallated forms of SOD1 (apo-SOD1) are destabilized (283, 284) and demetallated, sulfide-reduced forms of SOD1 prevent the formation of dimeric SOD1 (281, 285). Interestingly, only this reduced, apo-form of SOD1 can be transported into mitochondria (286). The biological activity of dimeric SOD1 occurs in two half-reactions requiring the copper ion cofactor, which oxidizes one superoxide anion to form oxygen and then is re-oxidized by a second superoxide anion, creating hydrogen peroxide (275).
6.1 Molecular mechanisms of ALS
Two molecular mechanisms have been postulated to explain how mutant SOD1 causes ALS. The oxidative hypothesis proposes that mutant SOD1 generates the formation of toxic reactive oxygen species (ROS) through altered redox activity of the copper ion. These alterations in oxidative activity are believed to be caused by the reduced affinity for zinc exhibited by mutant SOD1 (287). One of these species, peroxynitrite, which also depends on the presence of the intercellular messenger nitric oxide (NO), causes the nitration of tyrosine residues on proteins (288). Elevated 3-nitrotyrosine products are detected within the spinal cord of both human ALS and mice models of fALS (289, 290). Nitrated proteins may cause degeneration of MNs by a variety of mechanisms including interference with cell signaling, protein degradation, axonal transport or activation of cell death pathways (291). The role of zinc in SOD1 oxidative dysfunction is also supported by the finding that WT SOD1 lacking zinc causes the death of MNs in vitro through a NO-dependent mechanism (292). Further evidence supporting the role of NO in MN degeneration is supported by the finding that inactivation of the inducible form of nitric oxide synthase (iNOS) significantly extends lifespan of mutant SOD1 mice (271). However, mice expressing mutant SOD1 in which the four copper-binding residues are also mutated nevertheless develop MN disease, suggesting that copper-mediated redox activity at the active site is not pathogenic (293). Nevertheless, the potential for mutant SOD1 to bind copper through non-native interactions and thereby cause aberrant oxidative activity remains unexplored.
The second possible molecular mechanism is based on the observation of SOD1-immunoreactive aggregates in neurons and glia in patients with fALS and in transgenic mouse models (294–297). SOD1 immunoreactive aggregates are composed of high molecular weight, detergent-insoluble macromolecules of SOD1 present only in CNS tissue at about the time of symptom onset in fALS mice (298). These insoluble aggregates are composed of intersubunit disulfide-linked multimers of SOD1, suggesting that immature disulfide-reduced forms of SOD1 monomer are the precursor for SOD1 aggregates (299–301). Together with reports that intrasubunit disulfides are more likely to be reduced in mutant SOD1 (302) and that protecting these bonds from reduction reduces the formation of aggregates (303), these studies indicate that both nascent SOD1 monomers and SOD1 monomers formed from the dissociation of unstable dimers may contribute to the disulfide cross-linked SOD1 multimers found within aggregates (304). However, it was recently demonstrated that fALS mutant SOD1 species lacking all four cysteine residues, and thus unable to form disulfide linkages, nevertheless retain the ability to form aggregates in vitro (305). Conversely, mice in which mutant SOD1-mediated disease is accelerated by overexpression of the copper chaperone CCS (306) exhibit increased disulfide reduction without the formation of aggregates (307). Aberrantly reduced and/or cross-linked, aggregated SOD1 may cause MN degeneration by a variety of mechanisms. Because only the demetallated, reduced form of SOD1 enters the mitochondria, mutant SOD1 expression within this organelle may increase to pathological levels. Alternatively, aggregates may represent the binding of mutant SOD1 to non-native substrates. Finally, the presence of aggregates in vesicles of the ER-Golgi system indicates that a percentage of mutant SOD1 is secreted and thus capable of exerting toxicity non-cell autonomously (308, 309). Together, these studies suggest that oxidative dysfunction and aggregation represent the major modes of molecular pathogenesis.
6.2 Cellular mechanisms of ALS
Several cellular mechanisms have been proposed to mediate the neuronal dysfunction caused by mutant SOD1 oxidative or aggregative properties (Figure 2). The first and perhaps most prevailing is that mutant SOD alters mitochondrial structure and/or function (310). Morphologically altered mitochondria are found in the spinal cord of human sALS and fALS patients (311, 312) and of mutant SOD1 transgenic mice (313–315). SOD1 immunoreactivity is found in a rimlike pattern around these vacuoles in histological sections of the spinal cord (316). Mitochondrial abnormalities are observed as early as P14 in the spinal cord of SOD1 G93A mutant mice (317), which exhibit symptom onset near P90 (318). Electron transport chain activity and ATP production are also reduced in mutant SOD1 mice, but not until symptom onset (319, 320). Although small amounts of cytosolic WT SOD1 are detected within the intramembrane space of mitochondria (321), a significantly larger percentage of mutant SOD1 is found within various regions of this organelle (322–324). Importantly, whereas fALS mutants can exhibit normal or severely deficient metal content and thus biophysical activity (325), both of these classes share a pathologically enhanced association with spinal cord mitochondria (326). Enhancing the levels of mitochondrial-associated mutant SOD1 by overexpressing the CCS chaperone accelerates the death of mutant SOD1 transgenic mice, further implicating mitochondria as an important locus of toxicity (306). Furthermore, overexpressing WT SOD1 in a mutant SOD1 background accelerates death and increases the percentage of mitochondrial WT SOD1, suggesting that the toxic effects of SOD1 mutations are mediated by enhanced distribution of SOD1 to this pool (327). Because demetallated, disulfide-reduced forms of SOD1 monomer are exclusively imported into mitochondria (328) and because mutant SOD1 exhibits altered antigenic accessibility (326) these data suggest that destabilized SOD1 species are tracked to mitochondria, where they misfold to form cytotoxic, non-native intersubunit-linked multimers (327, 329, 330). Interestingly, a recent study found that a novel, disulfide-independent non-native dimer of mutant SOD1 occurs in both familial and sporadic ALS, suggesting a common mechanism through which mutant and WT SOD1 cause ALS (331). Although the precise pathway by which mitochondrial dysfunction causes MN disease is unclear, several important possibilities include dysregulation of mitochondrial motility or transport (332), reduced energy production (333), altered ionic homeostasis (334) and/or activation of apoptosis (335). Interestingly, mutations preventing the movement of mitochondria to axons and presynaptic terminals are sufficient to cause defective neuromuscular transmission and death (336). Reduced numbers of mitochondria in MN presynaptic terminals was also recently demonstrated in transgenic mice expressing human TAR DNA-binding protein-43 (TDP-43), a recently generated ALS animal model, suggesting that mitochondrial distribution rather than function is affected in MN disease (337).
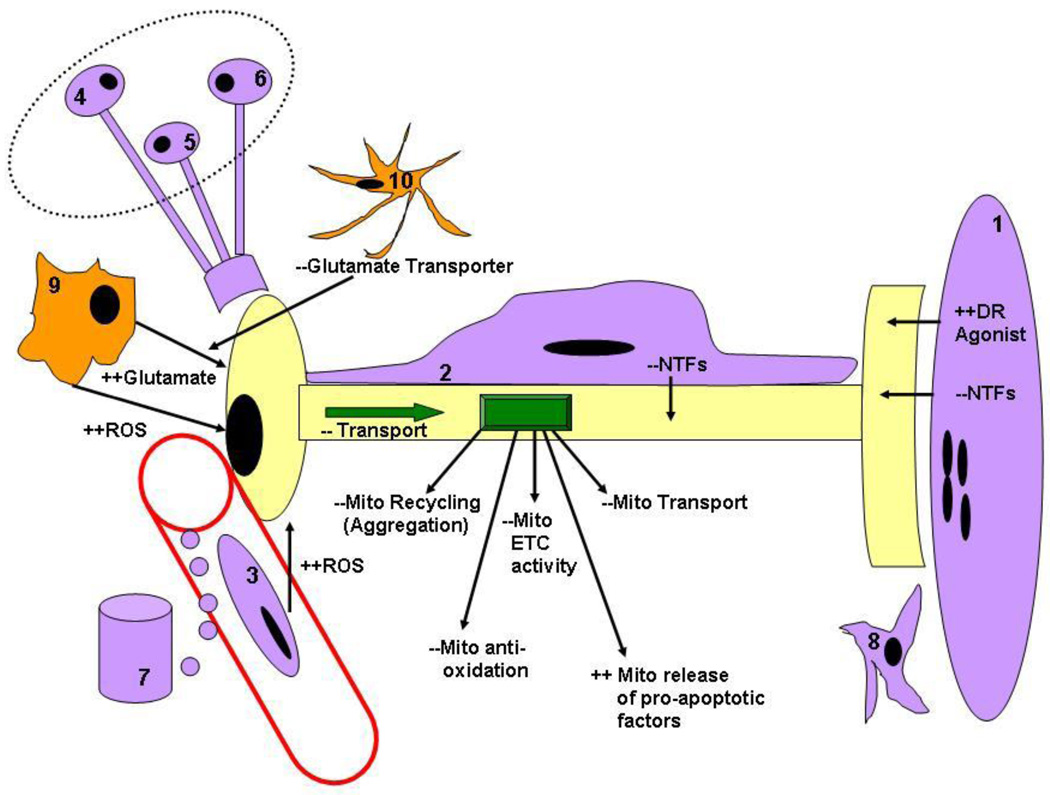
Figure 2.
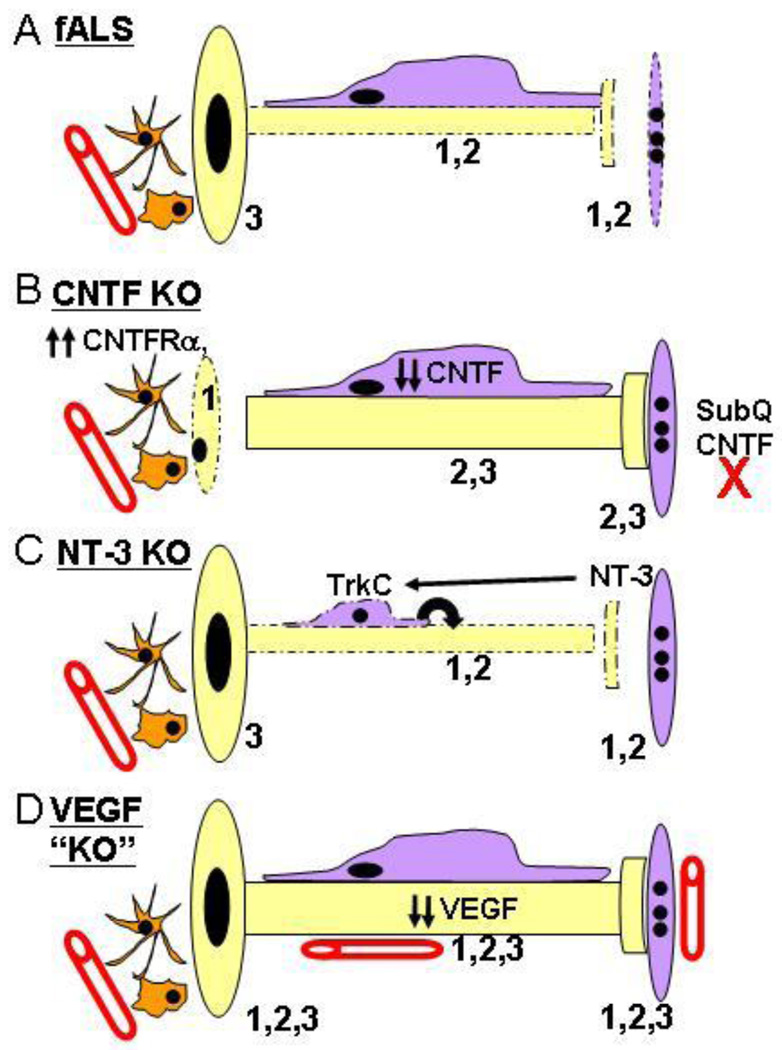
Cellular mechanisms of neurodegeneration in fALS mutant SOD1 mice. MNs may succumb by intrinsic (cell autonomous) or extrinsic (cell non-autonomous) signaling changes in ALS. Intrinsic mechanisms include defects in transport and/or mitochondrial function, and extrinsic signaling changes include altered trophic, excitotoxic (reactive oxygen species (ROS) or glutamate) or death receptor (DR) signaling from other cell types. A MN is depicted in yellow, and cell types known to positively and negatively regulate MN survival are shown in purple and orange, respectively, including muscle cells (1), Schwann cells (2) endothelial cells in spinal, nerve or muscle tissue (3), descending supraspinal neurons (4), local interneurons (5), spinal sensory ganglia (6), endocrine glands (7), nerve- or muscle-derived fibroblasts (8), microglia (9) or astrocytes. See text for further details.
The second potential cellular mechanism by which mutant SOD1 causes MN degeneration involves axonal transport, which as just mentioned may also overlap with perturbations in mitochondrial function and motility. A possible role for impaired axonal transport in ALS pathogenesis was suggested by the presence of neurofilamentous inclusions in the MN perikarya of human sALS patients and transgenic fALS mice (311, 338). Additionally, mutations in the heavy neurofilament gene (NF-H) are associated with a small number of sALS cases (339) and cause MN disease in mice (340). Slow anterograde axonal transport is impaired early in fALS mice (340, 341), as is retrograde transport (342) and fast anterograde transport (332). Additionally, mutations within members of the retrograde motor system, including dynein, dynactin and dynamitin produce are associated with sALS (343–345) and produce MN disease in mice (63, 344, 347, 348). Impaired axonal transport may result in MN degeneration by a variety of mechanisms, including focal damage to axons (349), alterations of SOD1 secretion (308, 350), interruption of vesicle trafficking and/or increases in protein aggregation (351, 352) impairment of retrograde neurotrophic signaling (353), activation of autophagic death (63) and impaired delivery of mitochondria to distal axons and synaptic terminals (332, 354). Together, these studies suggest mutant SOD1 is toxic to neurons by impairing several mutually non-exclusive cellular processes, including mitochondrial distriubution and axonal transport.
6.3 Tissue mechanisms of ALS
Because both the pathophysiology (e.g., motor weakness and paralysis) and pathology (e.g., degeneration of ventral root axons and ventral horn somata) of ALS indicate a disease whose primary locus is MNs, the role of other cells in mediating ALS has (until recently) been ignored, with the exception of defective astrocytic glutamate handling observed in human sALS patients (355). The findings that neuronal-specific overexpression of fALS-associated SOD1 mutants is insufficient to cause disease in mice (356, 357; but see 358), and that expression of mutant SOD1 in non-neuronal cells is sufficient to cause MN disease (359) has suddenly cast light on other cell types as candidates mediating ALS to MNs in a non cell-autonomous manner (360). These include, in principle, any endocrine cell in the body, surpaspinal, propriospinal or sensory ganglia neurons sending afferent input to MNs, multiple classes of interneurons mediating spinal reflexes, astrocytes in the ventral horns, oligodendrocytes in the ventrolateal funiculus, Schwann cells in the ventral root, peripheral nerve and neuromuscular junction, microglia in the spinal cord or nerve, skeletal muscle at the periphery, vascular smooth muscle in the arterioles irrigating the spinal cord, and vascular endothelial cells lining the walls of spinal, nerve and muscle capillaries (Figure 2). For the heroic efforts required to identify the offending cell type(s), conditional or excisable alleles of mutant SOD1 are proving indispensable. Conditional or floxed mutant SOD1 mice, crossed to mice expressing Cre-recombinase behind a promoter robustly expressed by microglia, delays the onset and progression of MN disease (361), suggesting that mutant SOD1 toxicity to microglia contributes to MN disease. Similarly, the removal of mutant SOD1-expressing microglia and replacement with wild-type (WT) SOD1-expressing microglia delays progression (but not onset) of fALS mice (362). Eliminating mutant SOD1 from astrocytes also delays progression but not onset of disease (363), consistent with a report showing that astrocytes expressing mutant SOD1 secrete toxic substances to MNs (354, 365). However, overexpression of mutant SOD1 in astrocytes alone is not sufficient to cause MN disease in mice (366). Removal of mutant SOD1 from skeletal muscle or endothelial cells fails to affect either disease onset or progression (367, 368). Interestingly, a recent study showed that expression mutant SOD1 in muscle cells is sufficient to cause MN disease (369). This study is in accord with the finding that muscle-restricted mitochondrial uncoupling causes mild, late-onset MN degeneration and pathology (370). However, because WT SOD1 also causes disease in this paradigm (369), the physiological significance of this finding remains unclear.
7. MN DEATH IN ALS: PASSERBY, PARTICIPATORY OR PARAMOUNT
Although degeneration of MNs is a prominent feature of ALS, whether activation of the cell death machinery represents a pathogenetic mechanism or a downstream effector of MN degeneration remains unclear. Four types of studies have helped clarify this issue. (1) Because certain forms of death have been historically more associated with active vs. passive killing of neurons (e.g., apoptosis vs. necrosis), the structural and molecular heterogeneity underlying these forms of cell death have been characterized in human cases and animal models of ALS. (2) The temporal correlation between cell death activation and other aspects of MN dysfunction such as neuromuscular denervation has been established in fALS mutant mice. (3) The extent to which cell death inhibition delays onset vs. progression of disease has been examined in transgenic fALS mice. (4) The correlation between MN death and disease has been investigated in which one or the other has been experimentally changed. A summary of the effects of these studies is presented in Table 2.
Table 2.
| Gene | Neurons - Knockout (KO) | Effect on MN death during PCD, after axotomy, or in response to ALS | ||
|---|---|---|---|---|
| PCD In vivo | PCD In vivo | Injury In vivo | ALS In vivo | |
| p75-NGFR | Sensory, symp neurons ↑↑540 | KO --149 | KO ↓↓149 | KO --395 |
| kRas | Central neurons ↑↑541 | KO ↑↑541 | OE ↓↓542 | ND |
| B-Raf | Sensory neurons --543 | ND* | ND | ND |
| PI3-K | ND* | ND | ND | ND |
| PTEN | ND* | ND | ND | ND |
| Akt | Reduced brian & body size544,545 | ND | DN ↑↑125 CA ↓↓125 | ND |
| Bcl-2, Bcl-XL | Postnatal neurons ↑↑546 | KO ↑↑547 OE ↓↓109 | KO--548; OE ↓↓126 | OE ↓↓399 |
| BH3-Only | Mild, no obvious defects106 | KO -- 106 | KO ↓↓133, 134 | KO ↓↓433 |
| Bax/Bak | Postmitotic neurons ↓↓110,549 | KO ↓↓112 OE ↑↑550 | KO ↓↓130,551 | KO ↓↓375 |
| IAPs | No Effect552,553 | ND | ND | ND |
| Apaf-1 | Neuronal precursors ↓↓554,555 | KO ↓↓108 | ND | ND |
| Caspase3 | Neuronal precursors ↓↓107 | KO ↓↓61 | ND | ND |
| Caspase9 | Neuronal precursors ↓↓556.557 | KO ↓↓557 | ND | ND |
| AIF | No Effect558 | KO -- 108 | ND* | ND |
| p53 | Sympathetic neurons ↓↓559 | KO --130 | KO ↓↓130,134 | KO --395 |
| Fas, FasL | KO -- 147 | KO --147 | KO ↓↓147 | KO ↓↓432 |
| TNF/TNFR | Sensory neurons -- ↓↓560 | KO --135 | KO ↓↓135 | KO -- 561 |
| iNOS | ND | ND | KO ↓↓148 | KO ↓↓271 |
MN=motoneuron; PCD=programmed cell death; KO = knockout; OE = overexpression; DN,CA=overexpression of constitutively or dominant negative activated alleles
Conditional mutants generated but not determined (ND); Red, Blue = Pro-, Anti-apoptotic
MN death underlies the pathology of both human cases and mouse models of familial and sporadic ALS (63, 276, 311, 371). Post-mortem studies of human ALS patients and end-stage fALS mice estimate the loss of spinal MNs to be near 60%, and morphometric analyses of both ventral root axons and ventral horn MN somata indicate that this loss is restricted to large-diameter, skeletomotor α MNs (271, 315, 318, 372–374). However, because MNs undergo atrophy in ALS (375), and muscle spindle innervation has only been systematically investigated in neonatal and not adult or fALS mice (51), it remains possible that a subset of dying MNs are spindle-innervating, fusimotor γ MNs and that a subset of remaining MNs are atrophied α MNs. Indeed, specific subsets of α MNs are retained even at advanced stages of disease in fALS mice (376).
In human ALS post-mortem tissue, dying MNs exhibit some but not all of the morphological features of apoptosis, such as cell shrinking and nuclear condensation, but not formation of apoptotic bodies or DNA fragmentation (371). Additionally, remaining MNs in human ALS spinal tissue exhibit prominent intracellular inclusions (377), damage to organelles such as Golgi (378) and mitochondria (379) and cytoskeletal alterations (311), none of which are observed in classical apoptosis. Similarly, Guégan and Przedborski (2003; 380) observed apoptosis in some but not all dying MNs within the spinal cord of symptomatic fALS mice. Other studies have completely failed to detect evidence for apoptosis in either human cases or mouse models of ALS (381, 382). One recent report demonstrated MN swelling and a reduction of membrane-associated Na+, K+ ATPase immunoreactivity in high-expressing G93A SOD1 mutant mice, reminiscent of necrosis (271). Another recent report described evidence for Type II autophagic cell death in dynactin sALS mutant mice (63) and autophagic dysfunction in SOD1 fALS mutant mice (64). Similarly, in a chick model of MN disease displaying tubulovesicular inclusions and neuromuscular dysfunction, autophagic but not necrotic or apoptotic cell death was observed (62, 383, 384). Together, these morphological studies do not support a role for widespread apoptosis in ALS.
Similarly, biochemical studies have supported a limited role for apoptosis in ALS. TNFα and Fas are extracellular activators of the extrinsic pathway of Type I cell death or apoptosis. Although TNFα is elevated in multiple cell types within the spinal cord of fALS mice (385, 386), genetic deletion of TNFα exerts no effect on their lifespan or MN number (387). Fas and FasL appear to exhibit enhanced MN-associated immunoreactivity in presymptomatic fALS mice (388), and the reduction of FasL expression modestly extends lifespan of SOD1 mutant mice. In contrast, intrathecal injection of Fas siRNA significantly lengthens the lifespan of fALS mice (389), but the more convincing study of crossing Fas KO to fALS mice has yet to be reported, despite the availability of both mice for over 15 years. Fas-mediated death of mutant SOD1-expressing embryonic MNs in vitro proceeds through a pathway including Daxx, ASK1 and p38 and NO (146), and the elevation of p38 MAPK and iNOS is increased in MNs of presymptomatic fALS mice (271, 390). Pharmacological inhibition of p38 MAPK increases lifespan of DO1 mutant mice by only 6 days, however (391). Inactivation of iNOS extends the lifespan of high- but not low-expressing G93A SOD1 mutant mice (271, 392), but because NO is provided by multiple sources and cytotoxic by multiple mechanisms its participation cannot by itself be taken to indicate a specific death pathway in ALS (393; see below). Finally, although the expression of p53 is upregulated in fALS mice, its import into the nucleus is blocked (271), a finding which may explain the failure for inactivation of p53 to extend the lifespan of fALS mice (394, 395). Together, these studies argue that the extrinsic pathway of apoptosis plays at most a minor role in ALS.
Similar studies have shown that upregulation of Bax, and Caspases-1 and -3 are observed in presymptomatic fALS mice, suggesting that the intrinsic apoptotic machinery operates during ALS (396–398). However, inhibition of upstream and downstream members of this pathway has yielded contradictory results: whereas Bcl-2 overexpression and Bax deletion extends the lifespan of fALS mice (375, 399) inactivation of Caspase-1 or Caspase-11, whose own activity is required for Caspase-3 activation, fails to increase lifespan (271, 400, 401 but see 402). Together with the finding that activated Caspase-3 immunoreactivity is predominantly associated with astrocytes (381), these findings suggest that although Bax translocation to mitochondria occurs, caspases are not activated in MNs of fALS mice. Bcl-2 overexpression may inhibit MN death by other mechanisms independent of apoptosis: (1) dilution of the toxic effects of direct physical association of mutant SOD1 with Bcl-2 (403); (2) inhibition of necrotic cell death (404); (3) prevention of autophagic death (405); (4) enhancement of axonal regeneration (406). Finally, the contribution of caspase-independent apoptosis to MN degeneration in ALS has recently been investigated. While genetic inactivation studies of either AIF or EndoG have yet to be performed, the expression of AIF is increased in MNs of SOD1 G93A mutant mice, but this elevation appears restricted to mitochondrial-associated vacuoles and not the nucleus (271), where AIF causes DNA fragmentation and apoptotic death (407). Together with the finding that Bax deletion completely prevents MN death in fALS mice (375), these results suggest that MNs die by a Bax-dependent, Bcl-2-sensitive pathway that differs from caspase-dependent apoptosis.
The occurrence of autophagic death has recently been shown to occur in a variety of neurodegenerative disorders (408), and the inhibition of autophagy is sufficient to cause neurodegeneration in mice (409, 410). Conversely, induction of autophagy by deletion of a gene required for the unfolded protein response pathway (UPR) enhances the survival of fALS mice (411). Autophagic clearance of both protein aggregates and damaged organelles depends on the activity of the retrograde motor complex (351), and mutations in dynactin that cause ALS in humans and mice lead to a pathological accumulation of membrane vesicles and autophagic death (63). Similarly, embryonic chick MNs subjected in vivo to sublethal excitotoxicity exhibit elevated protein retention in the ER and autophagic degeneration rather than apoptosis or necrosis (384, 412). A similar deficit in autophagy could either cause or result from mutant SOD1-containing protein aggregates in the ER (309) and in the dysfunctional, hypertrophic mitochondria observed in fALS. Together, these studies warrant further exploration of the autophagic death pathway in ALS.
Necrosis but not apoptosis or autophagic death triggers local inflammation, and the observation of increased levels of activated microglia in the spinal cord of fALS mice near the onset of MN death lends additional support to the notion that such cell death is necrotic (413). Activated microglia release pro-inflammatory cytokines, ROS, NO and glutamate (414, 415), and treatment of fALS mice with minocycline, a potent inhibitor of microglial activation, prolongs their lifespan (416, 417). Similarly, inhibition of NADPH oxidase diminishes the production of microglial-derived ROS and accordingly extends the lifespan of SOD1 mutant mice (418). Microglia may represent the source of elevated CNS glutamate in human ALS cases and fALS mice (176, 355). Alternatively, excessive extracellular glutamate in ALS may reflect dysfunction of the astroglial glutamate transporter EAAT2 (419–420). Glutamate-triggered depolarization allows Ca++ to enter MNs through voltage-sensitive and ligand-dependent, voltage-sensitive Ca++ channels, and excessive activation causes excitotoxic elevations of intracellular Ca++, which may perturb cellular viability by increasing neuronal NOS activity, stimulating the ER stress response, promoting mitochondrial permeability transition (mPT) and activating Ca++-sensitive enzymes such as calcineurin and calpain (393). Interestingly, sustained administration of NMDA to chick embryos causes a sublethal excitotoxicity that exhibits considerable morphological similarity to ALS, suggesting that elevated glutamate receptor-mediated signaling is sufficient to trigger these pathological alterations (383). Together, these studies provide evidence that the MN death observed in ALS contains features of necrosis.
Although these studies indicate the importance of microglia as important sources of excitotoxic signals to MNs in ALS, they fail to indicate by themselves which death pathway MNs adopt in the end. For example, NO activates the DNA repair enzyme PARP1, the excessive activity of which during stroke leads to an energydpeleting form of death most similar to necrosis (421). In contrast, NO mediates apoptosis after cerebral ischemia by catalyzing the S-nitrosylation of matrix metalloproteinase 9 (422). Similarly, excessive intracellular Ca++ can cause the death of neurons through apoptosis or autophagy triggered by ER stress (423–425), or necrosis triggered by mPT (426, 427). Interestingly, PUMA, a BOP activator of Bax/Bak, is required for apoptosis caused by ER stress in cultured MNs and is elevated in the spinal cord of presymptomatic fALS mice (428). Furthermore, elevations of misfolded proteins in the ER, which stimulate ER stress-mediated apoptosis through the UPR (429), have recently been reported in SOD1 mutant mice (309). However, inactivation of PUMA fails to extend the lifespan of fALS mice (428), suggesting that the mechanism by which high intracellular Ca++ kills cells is not ER stress-mediated apoptosis. Together, these studies suggest that dying MNs use upstream biochemical pathways such as Bax which are common to apoptosis, necrosis and autophagy, fail to either activate downstream caspases or to die by apoptosis, and instead exhibit a hybrid morphological character of necrotic and autophagic death. Most importantly, they suggest that cell death in ALS can be effectively blocked and that, in conjunction with therapies aimed at reversing more proximal molecular disease mechanisms such as protein oxidation or aggregation, therapies aimed at inhibiting Bax may protect and rejuvenate diseased MNs.
While it is reasonably straightforward to characterize the cell death pathway selected by MNs dying in ALS, providing data to argue for or against the cell death pathways as a principal pathogenetic mechanism in ALS or merely downstream executor is more complicated. One obvious strategy is to compare the temporal activation of cell death components in fALS mice with other measurements of disease progression. In high-expressing G93A SOD1 mutant fALS mice, cell death is initially observed at P65–P90, close to the onset of symptom onset, which occurs near P100 (271, 276, 318). Cell death pathway activation, measured by upregulation of Bax protein (375, 396), occurs significantly later than deficits in mitochondrial morphology (317), axonal transport (343) and neuromuscular innervation (430, 375), suggesting it acts as a downstream effector of MN degeneration. The coincident upregulation of cell death proteins and frank MN degeneration, between P60–P75, generally holds true for the measurement of most cell death proteins believed to mediate cell death. The implication of these results is that the core components of the cell death pathway are not the preponderant site of molecular damage underlying ALS, and that a key feature mediating MN dysfunction, namely progressive neuromuscular withdrawal, occurs long before this pathway is activated. Whether regressive events at the neuromuscular junction (NMJ) represent local, presynaptic toxicity or represent a response by MNs to toxicity initiated elsewhere is unclear (431). However, a recent study has challenged the interpretation that cell death in fALS is downstream of denervation by demonstrating that Bcl-2 represents an early, direct pathological target of mutant SOD1 (335). Future studies aimed at corroborating these data and elucidating its significance should prove interesting.
Another method to distinguish whether MN death is a disease target or a terminal process comes from studies in which particular experimental manipulations result in the extension of fALS lifespan. If cell death plays a principal role in mediating MN degeneration, then factors extending lifespan by blocking the cell death pathway might be expected to delay disease onset rather than progression, because inhibition of cell death would precede inhibition of denervation and motor weakness. In contrast, treatments resulting in the extension of disease progression imply that the cell death inhibition is downstream of denervation and weakness and therefore a downstream process of MN disease. With respect to members of the extrinsic apoptotic pathway, deletion of FasL and p38 MAPK both delayed progression but not onset of disease (391, 432), whereas this distinction was not reported after iNOS deletion (271). In contrast, overexpression of Bcl-2 or deletion of Bax, BIM or PUMA all extend disease onset, suggesting that the intrinsic pathway may be involved in disease pathogenesis (375, 399, 428, 433). However, the extent to which proteins in this pathway cause disease by promoting death or some other aspect of degeneration is not clear; for example, the ability of Bcl-2 to promote re-innervation after injury suggests a regenerative as well as anti-death role for this protein (406 but see 434).
In fact, the complete inhibition of MN death in SOD1 mutant, Bax-deficient mice supports the idea that these “pro-apoptotic” proteins delay disease onset through cell death-independent mechanisms: if the delay in disease onset by Bax deletion reflects the inhibition of Bax-mediated death, then indeed Bax-deficient SOD1 mutant mice should never die, as Bax-mediated MN death is completely inhibited. Instead, SOD1 mutant, Bax KO mice die despite retaining all MNs (375), suggesting that MN death itself is not required for MN dysfunction. This profound dissociation between MN survival and organism death has been reported in other models of MN disease (435). Moreover, recent studies show a similar discrepancy between MN survival and lifespan extension in fALS mice (391, 428). This likely represents a differential capacity for the specific regional compartments of the MN such as the soma, axon and synaptic terminals to withstand cellular stresses (431). Supporting this idea, transplantation of GDNF-overexpressing neural progenitors into the spinal cord of fALS rats robustly enhances the survival of MN cell bodies but fails to impede neuromuscular denervation and thus degeneration (436).
8. NTFs, MN DEATH AND ALS
Because MN death in ALS occurs late in disease, proceeds through a hybrid of necrotic-autophagic morphologies and is dispensable for disease, therapies aimed exclusively at inhibiting developmental or axotomy-induced apoptotic cell death pathways may not succeed in ameliorating symptoms. Nevertheless, because NTFs regulate maintenance and regeneration as well as developmental apoptotic death of MNs, their use as therapies for ALS has been extensively studied in animal models of disease and in human clinical trials. Additionally, a.) the degree to which NTF expression or signaling is affected in MN disease and b.) the ability of NTF treatment methodologies to restore some aspects of MN function and c.) the knowledge that specific NTFs and NTFRs are expressed in discrete cell types, has provided some insight into the functional status of different inter- and intracellular pathways (Figure 3). For example, the loss of GDNF expression in human ALS muscle tissue, together with the lifespan-extending effects of muscle- but not astrocyte-derived overexpression of GDNF, suggest that MN presynaptic terminals but not cell bodies are deprived of and maintain responsivity to this NTF (see below). The following section aims to summarize these studies, with an emphasis again being placed on transgenic animal studies employing fALS mutants.
Figure 3.
Neurotrophic signaling between MNs and other neuromuscular cell types in the context of ALS. (A) Expression of NTFs and NTFRs in cell types which may regulate MN survival during disease. MNs (in yellow) express nearly all NTFRs as well as several NTFs such as VEGF and NT-3. Expression of NTFs (blue type) and NTFRs (black type) by cell types known to positively and negatively regulate MN survival (purple and orange, respectively). Other NTF or NTFR sources not shown include oligodendrocytes in the ventrolateral funiculus and and smooth muscle cells of spinal, neural and muscular arterioles. Exogenously administered NTFs may bind NTFRs expressed by any of these cell types (B) Results of studies with transgenic or null mutant mice expressing elevated or reduced amounts of NTFs in specific cell types. Arrows indicate likely direction of signaling based on NTFR expression analysis. VEGF “KO” refers to HRE-VEGF mutant (see text). SC refers to Schwann cells. Two small arrows indicate a positive effect of NTF overexpression on lifespan of FALS mice; two dashes signify no such effect.
8.1 GDNF
Exogenous GDNF and the autophagy inhibitor 3-methyladenine prevent the death of excitotoxically injured MNs in spinal cord explants and promote motor axon outgrowth, suggesting that GDNF may be protective against oxidative stress (437, 438). Interestingly, the growth but not neuroprotective effects of GDNF are abolished by inhibitors of PI-3K, suggesting that GDNF inhibits developmental apoptotic death and excitotoxic necrotic-autophagic death by different mechanisms (437). Because GDNF also protects against the death-inducing effects of proteasomal inhibition in cultured dopaminergic (DAergic) neurons (439), the ability of GDNF to ameliorate autophagic dysfunction may result from its ability to attenuate aggregation. Together with the finding that GDNF blocks DAergic neuronal neurodegeneration caused by the mitochondrial toxin 3-nitropropionic acid (440), these studies suggest that exogenous GDNF protects neurons from cell death induced by a variety of oxidative/aggregative stimuli that involve the mitochondria. In contrast, endogenous GDNF appears dispensable for these neurotoxic effects, because deletion of Ret fails to increase the death of DAergic neurons in mice treated with the neurotoxin MPTP (441). In addition to disturbing mitochondrial morphology and function, ALS causes an early disturbance in axonal transport (see above). Although GDNF regulates axonal growth and synaptic maturation of MNs during development (175, 442), GDNF does not appear to enhance the retrograde transport of molecules within degenerating MNs (443). Finally, because ALS may result from disease to MN neighboring cells such as afferent neurons, astrocytes, Schwann cells or microglia, it is noteworthy that the expression of both GDNF and GFRα1 is increased in injured peripheral nerves (444). Exogenous GDNF induces the proliferation of Schwann cells and stimulates the myelination of unmyelinated axons in adult rats, indicating a non-cell-autonomous manner by which GDNF may influence motor function during disease (445).
The effects of GDNF on innervation, cell death and motor function have been studied in a variety of neurodegenerative conditions (446). In contrast to various animal models of Parkinson’s disease, where treatment with GDNF rescues DAergic neurons, prevents presynaptic terminal withdrawal and delays locomotor decline (447–449), studies of mice with MN disease reveal that GDNF preferentially protects the cell body rather than presynaptic terminals of MNs, with modest or no beneficial effects on disease onset and variable effects on disease progression (436, 450–453; see Table 3). Interestingly, when GDNF overexpression is targeted to the muscle rather than to the CNS, muscle innervation is maintained longer and disease progression is extended (454–456). In human ALS patients, GDNF is upregulated in muscle (457), Ret phosphorylation of Y1062 is detected (458), suggesting that MNs maintain responsivity to GDNF. These studies indicate that treatment with GDNF, by attenuating necrotic-autophagic MN death, represents a useful component of a multi-factor therapeutic approach to treat ALS, but that by itself is insufficient to arrest the motor axon withdrawal that precedes and likely drives this MN death.
Table 3.
| NTF | Familial ALS Manipulation | Onset | Duration | Treatment InitiationRef |
|---|---|---|---|---|
| GDNF |
OE Myoblasts in SOD1 Mouse Muscle Tg OE in SOD1 Mouse CNS Tg OE in SOD1 Mouse Muscle OE Neural Progenitors in SOD1 Rat CNS OE Mesenchymal SC in SOD1 Rat Muscle AdV-GDNF in SOD1 Mouse: Muscle to CNS AAV-GDNF in SOD1 Mouse: Muscle to CNS AAV-GDNF in SOD1 Mouse: Muscle to CNS GDNF Phase 1 in human ALS – ICV |
87 vs. 102 days No change 74 vs. 84 days No change No change 106 vs. 116 days 101 vs. 114 days 91 vs. 107 days NA |
Not examined No change 43 vs. 50 days No change 10 vs. 28 days 10 vs. 18 days 21 vs. 24 days 32 vs. 27 days No change |
Presymptomatic Injection 454 Embryonic OE 455 Embryonic OE 455 Presymptomatic Injection 436 Presymptomatic Injection456 Young postnatal OE 451 Presymptomatic OE452 Presymptomatic OE453 Symptom Onset562 |
| IGF-1 |
AAV-IGF1 in SOD1 Mouse: Muscle to CNS AAV-IGF1 in SOD1 Mouse: Muscle to CNS Tg OE in SOD1 Mouse CNS Tg OE in SOD1 Mouse Muscle Tg OE in SOD1 Mouse Muscle AAV-IGF1 in SOD1 Mouse: Intraparenchymal IGF1 Phase III in human ALS - Subcutaneous |
91 vs. 122 days NA No change No change 111 vs. 121 days 103 vs. 115 days* NA |
32 vs. 38 days 56 vs. 34 days No change No change 12 vs. 32 days No change No change |
Presymptomatic OE453 Symptomatic OE453 Presymptomatic OE524 Presymptomatic OE524 Embryonic OE525 Presymptomatic OE531 Symptom Onset528 |
| VEGF |
EIAV-VEGF in SOD1 Mouse CNS Recombinant VEGF in SOD1 Mouse - IP Recombinant VEGF in SOD1 Mouse – ICV Tg OE in SOD1 Mouse CNS |
95 vs. 123 days 127 vs. 139 days 107 vs. 120 days 95 vs. 115 days |
30 vs. 40 days 5 vs. 4 days 8 vs. 19 days No change |
Presymptomatic536 Presymptomatic537 Presymptomatic538 Young postnatal538 |
| BDNF |
OE Neural Progenitors in SOD1 Mouse CNS BDNF Phase III in human ALS - Subcutaneous |
No change NA |
No change No change |
Presymptomatic563 Symptom Onset485 |
| NT-3 | OE Neural Progenitors in SOD1 Mouse CNS | No change | No change | Presymptomatic563 |
| HGF | Tg OE in SOD1 Mouse CNS | 138 vs. 162 days | No change | Young postnatal509 |
| CNTF | CNTF Phase III in human ALS - Subcutaneous | NA | No change | Symptom Onset505 |
Male only
OE – Overexpressing
Tg – Transgenic
SC – Stem cells
NA – Not applicable
AdV – Adenovirus
AAV-Adeno-associated Virus
EIAV – Equine Infectious Anaemia Virus
IP – Intraperitoneal injection
ICV – Intracerebroventricular injection
The prospect of GDNF as a therapeutic factor for ALS is not only limited by these concerns of biological efficacy, but also those of pharmacological delivery. Like all peptide neurotrophic factors, GDNF exhibits pharmacokinetic constraints, including the inability to cross the blood-brain barrier (BBB) and the regulation by molecules in the plasma, CSF or local extracellular matrix such as heparan sulfate (459). Because GDNF receptors are expressed throughout the body and brain, delivery of this NTF outside of its immediate target region may also produce unwanted side effects (460). Additionally, prolonged GDNF treatment may reduce endogenous GDNF signaling by causing receptor downregulation or stimulating the production of GDNF antibodies (461). Some of these issues are encountered even when NTF delivery is optimized by surgical implantation of a catheter within the CNS proximal to the target. For example, despite the successful results of intrastriatal infusions of recombinant GDNF in both animal studies and Phase 1 clinical trials of Parkinson’s disease, a larger, double-blind Phase 2 trial failed as a result of poor penetration distal to the delivery site (462, 463). Moreover, GDNF activation of Purkinje cells in the cerebellum was observed to result from the contamination of CSF with GDNF from the catheter (463). Therefore, a variety of techniques to maximize the specificity and control of GDNF delivery are currently being evaluated, including surgical infusion of recombinant GDNF, transplantation of encapsulated, GDNF-overexpressing cells (464), infection of various types of viral vectors expressing GDNF (465, 466), administration of small lipophilic compounds capable of upregulating GDNF expression (467), chemical modification of GDNF to enhance its passage through the BBB (468) or increase its internalization by neurons (469, 470). Because one recent study demonstrated that GDNF overexpression in muscle cells but not astrocytes extends the survival of fALS mice (454), systemic or intramuscular delivery of viral or modified GDNF, rather than CNS-targeted delivery, may be a preferred therapeutic approach to achieve MN neuroprotection in ALS (Figure 3).
8.2 BDNF and NT-3
In contrast to their ability to protect striatal neurons from excitotoxicity (471–473), BDNF and NT-3 signaling through the high-affinity receptors TrkB or TrkC exerts no effect on MN survival in response of excessive glutamate receptor stimulation (474, 438). In some studies, BDNF actually increases the vulnerability of MNs to excitotoxic stress (475), consistent with findings that BDNF and TrkB increase the production of ROS and cause necrotic death (476, 477). Although the effects of BDNF and NT-3 on protein degradation or aggregation have not been examined, BDNF increases the oxidative efficiency of brain-derived mitochondria, suggesting a potential role for this NTF in ameliorating mitochondrial dysfunction in ALS (478), and NT-3 reduces diabetes-induced deficits in the mitochondrial membrane potential of sensory neurons (479). In contrast to GDNF, both BDNF and NT-3 increase retrograde transport within degenerating MNs (443). Interestingly, NT-3 regulates the survival of developing Schwann cells (480), and NT-3-deficient mice exhibit a postnatal loss of Schwann cells and motor neuropathy (197). These results suggest that alterations in NT-3 signaling may play a cell non-autonomous role in the degeneration of MNs.
Exogenous BDNF and NT-3 slow MN degeneration and increase lifespan in two models of inherited MN disease, the wobbler and progressive motor neuropathy (pmn) mice, respectively (481, 482). Similar to GDNF, the expression of neurotrophins and phosphorylated TrkB are detected in the muscle and spinal cord, respectively, of human ALS patients, suggesting that MNs can access and respond to these factors during disease (483). However, in both fALS mice and Phase 3 human clinical ALS trials, treatment with subcutaneous recombinant BDNF fails to affect motor decline or lifespan (484, 485). Crossing fALS mice to mice lacking the low-affinity neurotrophin receptor p75 also fails to significantly extend lifespan (483). Although BDNF is able to cross the endothelial cells of the BBB by a saturable transport mechanism, significant penetration into the CNS is limited by the parenchyma, to which most systemically BDNF is bound (486). Animal and human studies of intrathecally administered BDNF also show the trapping of BDNF by spinal parenchyma, but a significant portion of BDNF also binds to motor axons coursing through the subarachnoid space and is retrogradely transported to MN perikarya (487, 488). Nevertheless, this method of delivery still fails to increase motor performance or lifespan in clinical trials (489). Finally, because tyrosine kinase receptors are transactivated by G-protein-coupled receptors (GPCRs) such as the 2A adenosine receptor (Ad2A-R), therapies with small-molecule agonists of this receptor are being explored (490). The sum of these studies indicate that although neurotrophins appear unable to ameliorate ALS, they may retain use as therapies for MN diseases such as spinal motor atrophy (SMA), whose early onset and quick progression more closely resemble that of the pmn and wobbler mutant mice.
8.3 CNTF
Although CNTF reduces excitotoxicity in animal models of Huntington’s Disease (491, 492), exogenous CNTF, LIF or CT-1 fails to promote the long-term survival of MNs in spinal explants after exposure to excitotoxic stress (438, 474). Because oxidative stress inhibits signaling induced by CNTF in a MN cell line (493), ROS produced during ALS may prevent exogenous CNTF from rescuing excitotoxically injured MNs. Furthermore, oxidative stress may cause ALS in part by impairing endogenous CNTF signaling, because deletion of CNTF in mice causes the loss of MNs and a modest motor weakness (219). This idea is also supported by the findings that CNTF expression is reduced (494) and CNTFR 1 expression is increased (495) in the spinal cord of human ALS patients (but see 496). Similarly, after sciatic nerve axotomy, CNTF expression in nerve-derived Schwann cells is dramatically reduced (497) and CNTFR 1 expression in MNs is increased (498). CNTFRα1 is expressed by astrocytes and activation of these cells by CNTF mediates the neuroprotective effects observed in the striatum (491). CNTFRα is also expressed by Schwann cells (499) and CNTF is required for the proper maintenance of axon-Schwann cell networks (ASNs; 500). Together, these studies suggest that disruption of cytokine signaling in spinal or nerve-derived glia may contribute to non-cell autonomous MN disease.
Although systemic administration of CNTF to both pmn and wobbler mutant mice increases MN survival, improves motor performance and lengthens lifespan (183, 501), subcutaneously injected CNTF causes severe side effects (502–504) and exerted no effect on motor function or lifespan in human Phase 3 clinical trials of ALS (505). Whereas systemically administered CNTF induces MN sprouting in muscles of wild-type mice (506), it has yet to be shown whether diseased MNs, Schwann cells or astrocytes respond or gain access to systemic CNTF. Intrathecal implantation of encapsulated cells engineered to express CNTF results in long-term expression and the absence of major side effects in Phase 1 studies, providing hope that this route of CNTF may be better tolerated and more likely to activate CNTFRα expressed by MNs and Schwann cells (487, 507).
8.4 HGF
Although the trophic effects of HGF on developing MNs are well established, few studies have examined the role of this factor in ALS. Exogenous HGF prevents the decline in ChAT expression observed after adult hypoglossal axotomy (508), indicating that MNs retain responsivity to this NTF despite downregulating its receptor Met shortly after developmental PCD (53). HGF also reduces MN death in response to excitotoxic stess (508). Consistent with these studies, crossing fALS mice to transgenic mice overexpressing HGF extends the lifespan of FALS mice exclusively by delaying disease onset (509). In this study, HGF overexpression delayed the induction of iNOS but exerted no effect on mutant SOD1-mediated accumulation, suggesting that HGF interferes with oxidative but not aggregative processes associated with ALS (509). Astrocytes in fALS mice upregulate the expression of Met and HGF overexpression retards reactive gliosis in the spinal cord, suggesting that HGF, like CNTF, may also signal through astrocytes to ameliorate disease (509, 510). Intrathecally injected HGF also extends lifespan of fALS rats when given at symptom onset (511). Met is also expressed by endothelial cells, whose response to VEGF during hypoxia may contribute to ALS (see below). Similar to expression of receptors for GDNF and BDNF, Met is expressed in spinal cord MNs in human ALS (512). Levels of phosphorylated Met are strongly reduced in fALS rats (511), indicating that HGF levels decline during disease. HGF has not been developed as a therapy for human ALS, in part because of its association with tumor invasion and metastasis (513). However, HGF-expressing plasmid DNA injected intramuscularly promotes angiogenesis in patients with limb ischemia and neither leaks into blood nor influences the growth of an innoculated cancerous cell line, suggesting that this methodology of HGF expression may be viable for ALS (514). Because viral vectors encoding HGF retrogradely transduce neurons and promote recovery after nerve injury (512), intramuscular or intrathecal injection of such vectors might provide a safe way to target MNs in ALS.
8.5 IGF-1
Excess IGF-1 modestly protects MNs from excitotoxicity (473; 492). Oxidative stress directly carbonylates IGF-1 receptors (418) and antagonizes IGF-1-induced activation of Akt, suggesting that intracellular trophic and oxidative pathways intersect to govern neuronal survival (515–517). IGF-1 also induces sprouting in uninjured muscle and accelerates MN regeneration after axotomy (246, 518), indicating an important role for IGF-1 in the response to ALS-mediated denervation. IGF-1 receptors are expressed not only by MNs but also by oligodendrocytes, Schwann cells, astrocytes, smooth and skeletal muscle cells and endothelial cells, indicating a plethora of potential cellular non-autonomous mechanisms by which IGF-1 could regulate MN disease. Evidence for such signaling is provided by the finding that although the serum or spinal cord levels of IGF-1 are normal in ALS patients (519, 520), increased astrocytic IGF-1 receptor expression is observed in the spinal cord of human ALS patients and fALS mice (520–522).
When administered at symptom onset, exogenous IGF-1 attenuates the loss of muscle weight and decline of behavioural performance in wobbler mutant mice (523). Intramuscular injection of adeno-associated viral IGF-1 (AAV-IGF-1) also delays disease onset and progression of fALS mice (453). Similar treatment with lentiviral-IGF-1 exerts significant but less pronounced effects in fALS mice, demonstrating that the transduction of both muscle cells and MNs by AAV is more protective than the transduction of muscle cells alone by lentiviral-IGF-1 (453). Indeed, postnatally restricted, muscle-specific overexpression of IGF-1 fails to prolong the survival of fALS mice (524). Interestingly, however, embryonic and postnatal overexpression of IGF-1 in muscle delays denervation, disease onset and progression of fALS mice (525). These results may result from the myotrophic and neurotrophic effects of IGF-1 during embryonic development, and are consistent with embryonic deficits of retrograde transport reported in fALS MNs (343).
Although IGF-1, HGF and VEGF (below) all prolong survival of fALS animals when administered at symptom onset, clinical trials with subcutaneously delivered IGF-1 have produced mixed effects, with a large-scale Phase III study recently failing to show benefit (526–528). Although IGF-1 readily crosses the BBB (529), the disparate results may reflect diminished responsivity of MN terminals to subcutaneously delivered IGF-1 in MN disease or to the presence of IGF binding proteins or other factors that affect the bio-availability of this NTF when injected systemically. On the other hand, IGF-1 serum half-life is significantly increased when administered as a complex with IGF binding protein 3 (IGFBP-3), and approval was recently granted by the FDA for an early-phase clinical study of this IGF-1/IGFBP-3 complex, called IPLEX, in human ALS patients (530). Other strategies to elude such pharmacokinetic hurdles include direct intrathecal or intraparenchymal delivery of either recombinant IGF-1 or AAV-IGF-1 (531). However, human clinical studies with spinal-targeted AAV-IGF-1 have recently been suspended on account of AAV serotype complications as well as pre-existing axonal and neuromuscular degeneration that is observed in symptomatic patients (RT Bartus, personal communication).
8.6 VEGF
MN disease rather than MN PCD established the connection between VEGF and MNs; mice deficient for the hypoxia response element (HRE) in the VEGF promoter develop late-onset, paralytic MN disease (371). VEGF has subsequently been demonstrated to strongly protect MNs from excitotoxicity (263, 438). The protective effect of exogenous VEGF after axotomy is mediated by enhanced angiogenesis (264), and MNs are sensitive to ischemic hypoxia (532), suggesting that endothelial- or smooth muscle-derived VEGF receptors contribute non-cell autonomously to MN disease. On the other hand, VEGF receptors are expressed by MNs and VEGF directly rescues MNs in vitro, suggesting a more direct effect (262).
Consistent with the reduction of VEGF expression observed in the spinal cord of HRE-VEGF-deficient mice, VEGF promoter haplotypes resulting in diminished VEGF levels are correlated with human sporadic ALS (533). Other types of MN disease, including spinal and bulbar spinal muscular atrophy (SBMA), are also associated with a decrease in VEGF levels (534). Deletion of the HRE-VEGF in fALS (SOD1 mutant) mice accelerates disease, suggesting an overlap in pathogenesis between these two forms of ALS (534). The induction of VEGF after hypoxia is impaired early in fALS mice, further indicating such an interaction (535). Intramuscular injection of a retrogradely transportable VEGF delays disease onset when injected early postnatally into fALS mice and extends lifespan when injected at disease onset (536). Systemic and intraventricular injection of VEGF into fALS mice or rats, respectively, also delays disease onset and progression (537, 538). Overexpression of VEGF in neurons also delays disease onset and prolongs survival of fALS mice (539), suggesting that impaired production rather than reception of VEGF by MNs is associated with disease in fALS mice. Clinical studies studying the effects of VEGF are still forthcoming.
9. SUMMARY AND PERSPECTIVE
In an effort to discern which of 4 explanations might most likely explain the meager effects of NTFs in ALS clinical trials, we have reviewed studies which demonstrate that NTFs are bona fide regulators of MN survival during development and after injury (1, 2), and in some cases block or delay MN death in ALS despite exerting only modest effects on motor function and lifespan (3). Because NTF receptors are expressed by a diverse number of cell types known to interact with MNs, it also appears that systemically administered recombinant NTFs are severely limited by pharmacokinetic constraints (4). Together with the evidence suggesting that cell death in ALS is quite distinct from that in development or after injury, these data suggest that NTFs are unlikely to significantly affect MN degeneration in most forms of ALS. One exception to this conclusion is the spontaneous wobbler mutant, in which the disease onset and progression are significantly ameliorated by treatment with a variety of NTFs. Because this mutant predominantly affects the central soma rather than peripheral nerve terminal of affected cervical MNs, it may serve as a less valuable model of human ALS than the SOD1 transgenic mouse or even the pmn spontaneous mutant, in which dysfunction appears first in the distal nerve terminal and then later in the axon and soma.
Nevertheless, these studies provide astonishingly useful information on the inter- and intra-cellular mechanisms of MN degeneration in ALS. Moreover, their results when taken together are more insightful than when considered individually, and help identify common and selective NTF-resistant pathways of degeneration. For example, VEGF and GDNF provide strong protection against glutamate excitotoxicity in spinal explants, but CNTF has no effect (438). Furthermore, the location and propagation of MN disease subtypes can be hypothesized based on the interpretation of the a.) effects of different types of NTF delivery, b.) subcellular timecourse of degeneration observed in different ALS mutants missing or supplemented with NTFs, and c.) expression of NTFs and NTF receptors on different cell types in proximity to MNs (Figure 4). For example, the lack of effect of CNTF in human clinical trials of sALS, the proximal-distal gradient in MN dysfunction observed in CNTF-deficient mice (219), and the expression of CNTFRα in astrocytes and proximal MNs suggest that this form of MN disease is initiated centrally rather than peripherally. In contrast, MN degeneration in fALS mice exhibits a distal-proximal gradient (374) and treatment with GDNF peripherally but not centrally (via transgenic overexpression) increases lifespan (454).
Figure 4.
Subcellular degeneration of MNs in fALS and NTF KO mice. Cell types and colors are the same as those in figures 2 and 3. fALS mice exhibit degenerative changes (indicated by broken lines) at the neuromuscular junction (NMJ) and axon (1,2) before the soma (3). CNTF KO mice, similar to fALS mice, display reduced CNTF in the nerve and increased CNTFRα in the spinal cord, and a loss of countable MN soma (1) before neuromuscular changes at the axon and NMJ (2,3). Consistent with this scenario, systemic (subq=subcutaneous) CNTF treatment fails to ameliorate human ALS. NT-3 KO mice display a neuropathy that likely results from Schwann cell death and subsequent loss of axons and NMJs (1,2) before loss of MNs. Finally, HRE-VEGF mice (VEGF “KO” mice) exhibit a reduction in VEGF upon ischemic stress, which could arise from impaired vascular support of muscle, nerve or spinal tissue. Although the sequence of MN degeneration has not been elucidated, treatment with VEGF reduces both astrogliosis centrally and loss of NMJs peripherally.
In conclusion, although the evidence cited herein suggests that subcutaneously delivered NTFs are ineffective therapies for most forms of human ALS, several opportunities for specialized applications remain. First, as a general rule, it appears that NTF treatment via gene- or cell-based approaches are more likely to avoid the pharmacokinetic limitations that often reduce bio-availability and increase side effects. Second, it appears that NTFs are more effective in MN disease subtypes that exhibit central before peripheral dysfunction, such as the wobbler mouse mutant. However, even when NTFs fail to affect disease onset or lifespan, such as in SOD1 mutants, their potent effects on MN somal size may justify their inclusion in a cocktail of anti-ALS pharmacotherapies that also include axon-targeted and NMJ-targeted molecules. Therefore, while there is reason to question the validity of launching another clinical trial featuring the subcutaneous delivery of an NTF that has been identified on its ability to arrest developmental cell death, we still feel cautiously optimistic that when applied to the appropriate MN disease subtype with the appropriate delivery system, NTFs will “”live up” to their therapeutic expectations in ALS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hamburger V. Regression versus peripheral control of differentiation in motor hypoplasia. Am J Anat. 1958;102(3):365–409. doi: 10.1002/aja.1001020303. [DOI] [PubMed] [Google Scholar]
- 2.Purves D. Body and brain: a trophic theory of neural connections. Cambridge, UK: Harvard UP; 1988. [DOI] [PubMed] [Google Scholar]
- 3.Ellis RE, Yuan JY, Horvitz HR. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- 4.Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 5.Appel SH. A unifying hypothesis for the cause of amyotrophic lateral sclerosis, parkinsonism, and Alzheimer disease. Ann Neurol. 1981;10(6):499–505. doi: 10.1002/ana.410100602. [DOI] [PubMed] [Google Scholar]
- 6.Collin R. Recherches cytologiques sur le developpement de la cellule nerveus. Nevraxe. 1906;8:181–303. [Google Scholar]
- 7.Hamburger V, Levi-Montalcini R. Proliferation, differentiation and degeneration in the spinal ganglia of the chick embryo under normal and experimental conditions. J Exp Zool. 1949;111:457–502. doi: 10.1002/jez.1401110308. [DOI] [PubMed] [Google Scholar]
- 8.Oppenheim RW. Neuronal cell death and some related regressive phenomena during neurogenesis: a selective historical review and progress report. In: Cowan WM, editor. Studies in Developmental Neurobiology. Oxford, UK: Oxford University Press; 1981. pp. 74–133. [Google Scholar]
- 9.Hamburger V. Cell death in the development of the lateral motor column of the chick embryo. J Comp Neurol. 1975;160(4):535–546. doi: 10.1002/cne.901600408. [DOI] [PubMed] [Google Scholar]
- 10.Buss RR, Sun W, Oppenheim RW. Adaptive roles of programmed cell death during nervous system development. Annu Rev Neurosci. 2006;29:1–35. doi: 10.1146/annurev.neuro.29.051605.112800. [DOI] [PubMed] [Google Scholar]
- 11.Oppenheim RW, W R, von Bartheld C. Programmed cell death and neurotrophic factors. In: Squire L, Berg D, Bloom F, Du Lac S, Ghosh A, Spitzer N, editors. Fundamental Neuroscience. 3rd ed. New York: Elsevier; 2008. pp. 437–468. [Google Scholar]
- 12.Hollyday M, Hamburger V. Reduction of the naturally occurring motor neuron loss by enlargement of the periphery. J Comp Neurol. 1976;170(3):311–320. doi: 10.1002/cne.901700304. [DOI] [PubMed] [Google Scholar]
- 13.Calof AL, Reichardt LF. Motoneurons purified by cell sorting respond to two distinct activities in myotube-conditioned medium. Dev Biol. 1984;106(1):194–210. doi: 10.1016/0012-1606(84)90075-7. [DOI] [PubMed] [Google Scholar]
- 14.Oppenheim RW, Haverkamp LJ, Prevette D, McManaman JL, Appel SH. Reduction of naturally occurring motoneuron death in vivo by a target-derived neurotrophic factor. Science. 1988;240(4854):919–922. doi: 10.1126/science.3363373. [DOI] [PubMed] [Google Scholar]
- 15.Oppenheim RW, Prevette D, Haverkamp LJ, Houenou L, Yin QW, McManaman JL. Biological studies of a putative avian muscle-derived neurotrophic factor that prevents naturally occurring motoneuron death in vivo. J Neurobiol. 1993;4(8):1065–1079. doi: 10.1002/neu.480240806. [DOI] [PubMed] [Google Scholar]
- 16.Phelan KA, Hollyday M. Embryonic development and survival of brachial motoneurons projecting to muscleless chick wings. J Comp Neurol. 1991;311(3):313–320. doi: 10.1002/cne.903110302. [DOI] [PubMed] [Google Scholar]
- 17.Grieshammer U, Lewandoski MD, Prevette D, Oppenheim RW, Martin GR. Muscle-specific cell ablation conditional upon Cre-mediated DNA recombination in transgenic mice leads to massive spinal and cranial motoneuron loss. Dev Biol. 1998;197(2):234–247. doi: 10.1006/dbio.1997.8859. [DOI] [PubMed] [Google Scholar]
- 18.Kablar B, Rudnicki MA. Development in the absence of skeletal muscle results in the sequential ablation of motor neurons from the spinal cord to the brain. Dev Biol. 1999;208(1):93–109. doi: 10.1006/dbio.1998.9184. [DOI] [PubMed] [Google Scholar]
- 19.Oppenheim RW. The neurotrophic theory and naturally occurring motoneuron death. Trends Neurosci. 1989;12(7):252–255. doi: 10.1016/0166-2236(89)90021-0. [DOI] [PubMed] [Google Scholar]
- 20.McLennan IS. Size of motoneuron pool may be related to number of myotubes in developing muscle. Dev Biol. 92(1):263–265. doi: 10.1016/0012-1606(82)90171-3. [DOI] [PubMed] [Google Scholar]
- 21.Lamb AH. Target dependency of developing motoneurons in Xenopus laevis. J Comp Neurol. 1981;203(2):157–171. doi: 10.1002/cne.902030202. [DOI] [PubMed] [Google Scholar]
- 22.Cohen S. Purification of a nerve growth-promoting protein from the mouse salivary gland and its neuro-cytotoxic antiserum. Proc Natl Acad Sci U S A. 1960;46(3):302–311. doi: 10.1073/pnas.46.3.302. 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hendry IA, Stöckel K, Thoenen H, Iversen LL. The retrograde axonal transport of nerve growth factor. Brain Res. 1974;68(1):103–121. doi: 10.1016/0006-8993(74)90536-8. [DOI] [PubMed] [Google Scholar]
- 24.Korsching S, Thoenen H. Quantitative demonstration of the retrograde axonal transport of endogenous nerve growth factor. Neurosci Lett. 1983;39(1):1–4. doi: 10.1016/0304-3940(83)90155-6. [DOI] [PubMed] [Google Scholar]
- 25.Johnson EM, Jr, Gorin PD, Brandesi LD, Pearson J. Dorsal root ganglion neurons are destroyed by exposure in utero to maternal antibody to nerve growth factor. Science. 1980;210(4472):916–918. doi: 10.1126/science.7192014. [DOI] [PubMed] [Google Scholar]
- 26.Oppenheim RW, Maderdrut JL, Wells DJ. Cell death of motoneurons in the chick embryo spinal cord. VI. Reduction of naturally occurring cell death in the thoracolumbar column of Terni by nerve growth factor. J Comp Neurol. 1982;210(2):174–189. doi: 10.1002/cne.902100208. [DOI] [PubMed] [Google Scholar]
- 27.Korsching S. The neurotrophic factor concept: a re-examination. J Neurosci. 1983;13(7):2739–2748. doi: 10.1523/JNEUROSCI.13-07-02739.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barde Y-A. Trophic factors and neuronal survival. Neuron. 1989;2(6):1525–1534. doi: 10.1016/0896-6273(89)90040-8. [DOI] [PubMed] [Google Scholar]
- 29.Thoenen H. The changing scene of neurotrophic factors. Trends Neurosci. 1995;14(5):165–170. doi: 10.1016/0166-2236(91)90097-e. [DOI] [PubMed] [Google Scholar]
- 30.Barbacid M. Structural and functional properties of the TRK family of neurotrophin receptors. Ann N Y Acad Sci. 1995;766:442–458. doi: 10.1111/j.1749-6632.1995.tb26693.x. [DOI] [PubMed] [Google Scholar]
- 31.Klein R. Role of neurotrophins in mouse neuronal development. FASEB J. 1995;8(10):738–744. doi: 10.1096/fasebj.8.10.8050673. [DOI] [PubMed] [Google Scholar]
- 32.Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77(5):627–633. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 33.Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, Lira SA, Barbacid M. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994;368(6468):246–249. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- 34.Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, McMahon SB, Shelton DL, Levinson AD, et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76(6):1001–1011. doi: 10.1016/0092-8674(94)90378-6. 1994. [DOI] [PubMed] [Google Scholar]
- 35.Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL, Barbacid M. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell. 1993;75(1):113–122. 19931993. [PubMed] [Google Scholar]
- 36.Jones KR, Fariñas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76(6):989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ernfors P, Lee KF, Kucera J, Jaenisch R. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell. 1994a;77(4):503–512. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- 38.Klein R, Silos-Santiago I, Smeyne RJ, Lira SA, Brambilla R, Bryant S, Zhang L, Snider WD, Barbacid M. Disruption of the neurotrophin-3 receptor gene trkC eliminates la muscle afferents and results in abnormal movements. Nature. 1994;368(6468):249–251. doi: 10.1038/368249a0. [DOI] [PubMed] [Google Scholar]
- 39.Fariñas I, Jones KR, Backus C, Wang XY, Reichardt LR. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369(6482):658–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- 40.Minichiello L, Klein R. TrkB and TrkC neurotrophin receptors cooperate in promoting survival of hippocampal and cerebellar granule neurons. Genes Dev. 1996;10(22):2849–2858. doi: 10.1101/gad.10.22.2849. [DOI] [PubMed] [Google Scholar]
- 41.Goldberg JL, Barres BA. The relationship between neuronal survival and regeneration. Annu Rev Neurosci. 2000;23:579–612. doi: 10.1146/annurev.neuro.23.1.579. [DOI] [PubMed] [Google Scholar]
- 42.Kanning KC, Kaplan A, Henderson CE. Motor neuron diversity in development and disease. Annu Rev Neurosci. 2010;33:409–440. doi: 10.1146/annurev.neuro.051508.135722. [DOI] [PubMed] [Google Scholar]
- 43.Oppenheim RW. Neurotrophic survival molecules for motoneurons: an embarrassment of riches. Neuron. 1996;17(2):195–197. doi: 10.1016/s0896-6273(00)80151-8. [DOI] [PubMed] [Google Scholar]
- 44.Sendtner M, Pei G, Beck GM, Schweizer U, Wiese S. Developmental motoneuron cell death and neurotrophic factors. Cell Tissue Res. 2000;301(1):71–84. doi: 10.1007/s004410000217. [DOI] [PubMed] [Google Scholar]
- 45.Davies AM. The neurotrophic hypothesis: where does it stand? Philos Trans R Soc Lond B Biol Sci. 1996;351(1338):389–394. doi: 10.1098/rstb.1996.0033. [DOI] [PubMed] [Google Scholar]
- 46.Hamburger V, Balaban M, Oppenheim R, Wenger E. Periodic motility of normal and spinal chick embryos between 8 and 17 days of incubation. J Exp Zool. 1965;159(1):1–13. doi: 10.1002/jez.1401590102. [DOI] [PubMed] [Google Scholar]
- 47.Okado N, Oppenheim RW. Cell death of motoneurons in the chick embryo spinal cord. IX. The loss of motoneurons following removal of afferent inputs. J Neurosci. 1984;4(6):1639–1652. doi: 10.1523/JNEUROSCI.04-06-01639.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riethmacher D, Sonnenberg-Riethmacher E, Brinkmann V, Yamaai T, Lewin GR, Birchmeier C. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389(6652):725–730. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- 49.Taylor AR, Gifondorwa DJ, Newbern JM, Robinson MB, Strupe JL, prevette D, Oppenheim RW, Milligan CE. Astrocyte and muscle-derived secreted factors differentially regulate motoneuron survival. J Neurosci. 2007;27(3):634–644. doi: 10.1523/JNEUROSCI.4947-06.2007. 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arce V, Pollock RA, Philippe JM, Pennica D, Henderson CE, deLapeyrière O. Synergistic effects of schwann- and muscle-derived factors on motoneuron survival involve GDNF and cardiotrophin-1 (CT-1) J Neurosci. 1998;18(4):1440–1448. doi: 10.1523/JNEUROSCI.18-04-01440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gould TW, Yonemura S, Oppenheim RW, Ohmori S, Enomoto H. The neurotrophic effects of glial cell line-derived neurotrophic factor on spinal motoneurons are restricted to fusimotor subtypes. J Neurosci. 2008;28(9):2131–2146. doi: 10.1523/JNEUROSCI.5185-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKay SE, Garner A, Calderó AJ, Tucker RP, Large T, Oppenheim RW. The expression of trkB and p75 and the role of BDNF in the developing neuromuscular system of the chick embryo. Development. 1996;122(2):715–724. doi: 10.1242/dev.122.2.715. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto Y, Livet J, Pollock RA, Garces A, Arce V, deLapeyrière O, Henderson CE. Hepatocyte growth factor (HGF/SF) is a muscle-derived survival factor for a subpopulation of embryonic motoneurons. Development. 1997;124(15):2903–2913. doi: 10.1242/dev.124.15.2903. [DOI] [PubMed] [Google Scholar]
- 54.Novak KD, Prevette D, Wang S, Gould TW, Oppenheim RW. Hepatocyte growth factor/scatter factor is a neurotrophic survival factor for lumbar but not for other somatic motoneurons in the chick embryo. J Neurosci. 2000;20(1):326–337. doi: 10.1523/JNEUROSCI.20-01-00326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Forger NG, Prevette D, deLapeyrière O, de Bovis B, Wang S, Bartlett P, Oppenheim RW. Cardiotrophin-like cytokine/cytokine-like factor 1 is an essential trophic factor for lumbar and facial motoneurons in vivo. J Neurosci. 2003;23(26):8854–8858. doi: 10.1523/JNEUROSCI.23-26-08854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gould TW, Oppenheim RW. The function of neurotrophic factor receptors expressed by the developing adductor motor pool in vivo. J Neurosci. 2004;24(19):4668–4682. doi: 10.1523/JNEUROSCI.0580-04.2004. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan J, Shaham S, LeDoux S, Ellis HM, Horvitz HR. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993;75(4):641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- 58.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elliott JL, Snider WD. Motor neuron growth factors. Neurology. 1996;47(4 Suppl 2):S47–S53. doi: 10.1212/wnl.47.4_suppl_2.47s. [DOI] [PubMed] [Google Scholar]
- 60.Gould TW, Oppenheim RW. Synaptic dysfunction in disease and following injury in the developing and adult nervous system: caveats in the choice of therapeutic intervention. Neurosci Biobehav Rev. 2007;31(8):1073–1087. doi: 10.1016/j.neubiorev.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 61.Oppenheim RW, Flavell RA, Vinsant S, Prevette D, Kuan CY, Rakic P. Programmed cell death of developing mammalian neurons after genetic deletion of caspases. J Neurosci. 2001;21(13):4752–4760. doi: 10.1523/JNEUROSCI.21-13-04752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Calderó J, Prevette D, Mei X, Oakley RA, Li L, Milligan C, Houenou L, Burek M, Oppenheim RW. Peripheral target regulation of the development and survival of spinal sensory and motor neurons in the chick embryo. J Neurosci. 2007;18(1):356–370. doi: 10.1523/JNEUROSCI.18-01-00356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laird FM, Farah MH, Ackerley S, Hoke A, Maragakis N, Rothstein JD, Griffin J, Pric DL, Martin LJ, Wong PC. Motor neuron disease occurring in a mutant dynactin mouse model is characterized by defects in vesicular trafficking. J Neurosci. 2008;28(9):1997–2005. doi: 10.1523/JNEUROSCI.4231-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fornai F, Longone P, Cafaro L, Kastsiuchenka O, Ferrucci M, Manca ML, Lazzeri G, Spalloni A, Bellio N, Lenzi P, Modugno N, Siciliano G, Isidoro C, Murri L, Ruggieri S, Paparelli A. Lithium delays progression of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2008;105(6):2052–2057. doi: 10.1073/pnas.0708022105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91(2):231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 66.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wiese S, Pei G, Karch C, Troppmair J, Holtmann B, Rapp UR, Sendtner M. Specific function of B-Raf in mediating survival of embryonic motoneurons and sensory neurons. Nat Neurosci. 2001;(2):137–142. doi: 10.1038/83960. [DOI] [PubMed] [Google Scholar]
- 68.Yin XM, Oltvai ZN, Korsmeyer SJ. BH1 and BH2 domains of Bcl-2 are required for inhibition of apoptosis and heterodimerization with Bax. Nature. 1994;369(6478):321–323. doi: 10.1038/369321a0. [DOI] [PubMed] [Google Scholar]
- 69.Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PJ. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;(12):1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 70.Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. ID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10(22):2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 71.Reed JC. Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ. 2006;13(8):1378–1386. doi: 10.1038/sj.cdd.4401975. [DOI] [PubMed] [Google Scholar]
- 72.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116(2):205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 73.Willlis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, Strasser A, Kluck RM, Adams JM, Huang DC. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science. 2007;315(5813):856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- 74.Youle RJ. Cell biology. Cellular demolition and the rules of engagement. Science. 2007;315(5813):776–777. doi: 10.1126/science.1138870. [DOI] [PubMed] [Google Scholar]
- 75.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80(2):285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 76.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87(4):619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 77.Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139(5):1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nechushtan A, Smith CL, Hsu YT, Youle RJ. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18(9):2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, Maundrell K, Gambale F, Sadoul FR, Martinou JC. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277(5324):370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 80.Marzo I, Brenner C, Zamzami N, Jürgensmeier JM, Susin SA, Vieira HL, Prévost MC, Xie Z, Matsuyama S, Reed JC, Kroemer G. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science. 1998;281(5385):2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]
- 81.Shimizu S, Tsujimoto Y. Proapoptotic BH3-only Bcl-2 family members induce cytochrome c release, but not mitochondrial membrane potential loss, and do not directly modulate voltage-dependent anion channel activity. Proc Natl Acad Sci U S A. 2000;97(2):577–582. doi: 10.1073/pnas.97.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brooks C, Wei Q, Feng L, Dong G, Tao Y, Mei L, Xie ZJ, Dong Z. Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc Natl Acad Sci U S A. 2007;104(28):11649–11654. doi: 10.1073/pnas.0703976104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91(4):479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 84.Martin SJ, Green DJ. Protease activation during apoptosis: death by a thousand cuts? Cell. 1995;82(3):349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- 85.Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326(Pt 1):1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281(5381):1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 87.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102(1):33–42. doi: 10.1016/s0092-8674(00)00008-8. 7. [DOI] [PubMed] [Google Scholar]
- 88.Verhagen AM, Vaux DL. Cell death regulation by the mammalian IAP antagonist Diablo/Smac. Apoptosis. 2000;7(2):163–166. doi: 10.1023/a:1014318615955. [DOI] [PubMed] [Google Scholar]
- 89.Susin SA, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med. 1996;184(4):1331–1341. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412(6842):95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- 91.Parrish J, Li L, Klotz K, ledwich D, Wang X, Xue D. Mitochondrial endonuclease G is important for apoptosis in C. elegans. Nature. 2001;412(6842):90–94. doi: 10.1038/35083608. [DOI] [PubMed] [Google Scholar]
- 92.Arnoult D, Gaume B, Karbowski M, Sharpe JC, Cecconi F, Youle RJ. Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax/Bak-mediated permeabilization. EMBO J. 2003;22(17):4385–4399. doi: 10.1093/emboj/cdg423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Golstein P, Kroemer G. A multiplicity of cell death pathways. Symposium on apoptotic and non-apoptotic cell death pathways. EMBO Rep. 2007;8(9):829–833. doi: 10.1038/sj.embor.7401042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270(28):16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 95.Fuchs SY, Adler V, Pincus MR, Ronai Z. MEKK1/JNK signaling stabilizes and activates p53. Proc Natl Acad Sci U S A. 1998;95(18):10541–10546. doi: 10.1073/pnas.95.18.10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Putcha GV, Le S, Frank S, Besirli CG, Clark K, Chu B, Alix S, Youle RJ, LaMarche A, Maroney AC, Johnson EM., Jr JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron. 2003;38(6):899–914. doi: 10.1016/s0896-6273(03)00355-6. [DOI] [PubMed] [Google Scholar]
- 97.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80(2):293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 98.Villunger A, Michalak EM, Coultas L, Müllauer F, Böck G, Ausserlechner MJ, Adams JM, Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302(5647):1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 99.Crompton M, Virji S, Doyle V, Johnson N, Ward JM. The mitochondrial permeability transition pore. Biochem Soc Symp. 1999;66:167–179. doi: 10.1042/bss0660167. [DOI] [PubMed] [Google Scholar]
- 100.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300(5616):135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 101.Chipuk JE, Bouchier-Hayes L, Green DR. Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ. 2006;13(8):1396–1402. doi: 10.1038/sj.cdd.4401963. [DOI] [PubMed] [Google Scholar]
- 102.Newbern JM, Taylor AR, Robinson M, Lively MO, Milligan CE. c-Jun N-terminal kinase signaling regulates events associated with both health and degeneration in motoneurons. Neuroscience. 2005;147(3):680–692. doi: 10.1016/j.neuroscience.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 103.Bi L, Okabe I, Bernard DJ, Wynshaw-Boris A, Nussbaum RL. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110alpha subunit of phosphoinositide 3-kinase. J Biol Chem. 1999;274(16):10963–10968. doi: 10.1074/jbc.274.16.10963. [DOI] [PubMed] [Google Scholar]
- 104.Bi L, Okabe I, Bernard DJ, Nussbaum RL. Early embryonic lethality in mice deficient in the p110beta catalytic subunit of PI 3-kinase. Mamm Genome. 2002;13(3):169–172. doi: 10.1007/BF02684023. [DOI] [PubMed] [Google Scholar]
- 105.Dummler B, Tschopp O, Hynx D, Yang ZZ, Dirnhofer S, Hemmings BA. Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol Cell Biol. 2006;26(21):8042–8051. doi: 10.1128/MCB.00722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 107.Kuida K, Zheng TS, Na S, Kuan C, Yang D, Karasuyama H, Rakic P, Flavell RA. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384(6607):368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- 108.Oppenheim RW, Blomgren K, Ethell DW, Koike M, Komatsu M, Prevette D, Roth KA, Uchiyama Y, Vinsant S, Zhu C. Developing postmitotic mammalian neurons in vivo lacking Apaf-1 undergo programmed cell death by a caspase-independent, nonapoptotic pathway involving autophagy. J Neurosci. 2008;28(6):1490–1497. doi: 10.1523/JNEUROSCI.4575-07.2008. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martinou JC, Dubois-Dauphin M, Staple JK JK, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C, et al. Overexpression of BCL-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron. 1994;13(4):1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 110.Deckwerth TL, Elliott JL, Knudson CM, Johnson EM, Jr, Snider WD, Korsmeyer SJ. BAX is required for neuronal death after trophic factor deprivation and during development. Neuron. 1996;17(3):401–411. doi: 10.1016/s0896-6273(00)80173-7. [DOI] [PubMed] [Google Scholar]
- 111.Sun W, Oppenheim RW. Response of motoneurons to neonatal sciatic nerve axotomy in Bax-knockout mice. Mol. Cell. Neurosci. 2003;24:875–886. doi: 10.1016/s1044-7431(03)00219-7. [DOI] [PubMed] [Google Scholar]
- 112.Sun W, Gould TW, Vinsant S, Prevette D, Oppenheim RW. Neuromuscular development after the prevention of naturally occurring neuronal death by Bax deletion. J Neurosci. 2003;23(19):7298–7310. doi: 10.1523/JNEUROSCI.23-19-07298.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Buss RR, Gould TW, Ma J, Vinsant S, Prevette D, Winseck A, Toops KA, Hammarback JA, Smith TL, Oppenheim RW. Neuromuscular development in the absence of programmed cell death: phenotypic alteration of motoneurons and muscle. J Neurosci. 2006;26(52):13413–13427. doi: 10.1523/JNEUROSCI.3528-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Romanes GJ. Motor localization and the effects of nerve injury on the ventral horn cells of the spinal cord. J Anat. 1946;80:117–131. [PubMed] [Google Scholar]
- 115.Schmalbruch H. Motoneuron death after sciatic nerve section in newborn rats. J Comp Neurol. 1984;224:252–258. doi: 10.1002/cne.902240206. [DOI] [PubMed] [Google Scholar]
- 116.Kashihara Y, Kuno M, Miyata Y. Cell death of axotomized motoneurones in neonatal rats, and its prevention by peripheral reinnervation. J Physiol. 1987;386:135–148. doi: 10.1113/jphysiol.1987.sp016526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lowrie MB, Vrbová G. Dependence of postnatal motoneurones on their targets: review and hypothesis. Trends Neurosci. 1992;15(3):80–84. doi: 10.1016/0166-2236(92)90014-y. [DOI] [PubMed] [Google Scholar]
- 118.de Bilbao F, Giannakopoulos P, Srinivasan A, Dubois-Dauphin M. In vivo study of motoneuron death induced by nerve injury in mice deficient in the caspase 1/ interleukin-1 beta-converting enzyme. Neuroscience. 2000;98(3):573–583. doi: 10.1016/s0306-4522(00)00100-7. [DOI] [PubMed] [Google Scholar]
- 119.Lams BE, Isacson O, Sofroniew MV. Loss of transmitter-associated enzyme staining following axotomy does not indicate death of brainstem cholinergic neurons. Brain Res. 1988;475(2):401–406. doi: 10.1016/0006-8993(88)90635-x. [DOI] [PubMed] [Google Scholar]
- 120.Snider WD, Elliott JL, Yan Q. Axotomy-induced neuronal death during development. J Neurobiol. 1992;23(9):1231–1246. doi: 10.1002/neu.480230913. [DOI] [PubMed] [Google Scholar]
- 121.Wu W. Potential roles of gene expression change in adult rat spinal motoneurons following axonal injury: a comparison among c-jun, off-affinity nerve growth factor receptor (LNGFR), and nitric oxide synthase (NOS) Exp Neurol. 1996;141(2):190–200. doi: 10.1006/exnr.1996.0153. [DOI] [PubMed] [Google Scholar]
- 122.Wu W, Li L. Inhibition of nitric oxide synthase reduces motoneuron death due to spinal root avulsion. Neurosci Lett. 1993;153(2):121–124. doi: 10.1016/0304-3940(93)90303-3. [DOI] [PubMed] [Google Scholar]
- 123.Li L, Houenou LJ, Wu W, Lei M, Prevette DM, Oppenheim RW. Characterization of spinal motoneuron degeneration following different types of peripheral nerve injury in neonatal and adult mice. J Comp Neurol. 1998;396(2):158–168. [PubMed] [Google Scholar]
- 124.Martin LJ, Kaiser A, Price AC. Motor neuron degeneration after sciatic nerve avulsion in adult rat evolves with oxidative stress and is apoptosis. J Neurobiol. 1999;40(2):185–201. [PubMed] [Google Scholar]
- 125.Namikawa K, Honma M, Abe K, Takeda M, Mansur K, Obata T, Miwa A, Okado H, Kiyama H. Akt/protein kinase B prevents injury-induced motoneuron death and accelerates axonal regeneration. J Neurosci. 2000;20(8):2875–2886. doi: 10.1523/JNEUROSCI.20-08-02875.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dubois-Dauphin M, Frankowski H, Tsujimoto Y, Huarte J, Martinou JC. Neonatal motoneurons overexpressing the bcl-2 protooncogene in transgenic mice are protected from axotomy-induced cell death. Proc Natl Acad Sci U S A. 1994;91(8):3309–3301. doi: 10.1073/pnas.91.8.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Parsadanian AS, Cheng Y, Keller-Peck CR, Holtzman DM, Snider WD. Bcl-xL is an antiapoptotic regulator for postnatal CNS neurons. J Neurosci. 1998;18(3):1009–1019. doi: 10.1523/JNEUROSCI.18-03-01009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vanderluit JL, McPhail LT, Fernandes KJ, McBride CB, Huguenot C, Roy S, Robertson GS, Nicholson DW, Tetzlaff W. Caspase-3 is activated following axotomy of neonatal facial motoneurons and caspase-3 gene deletion delays axotomy-induced cell death in rodents. Eur J Neurosci. 2000;12(10):3469–3480. doi: 10.1046/j.1460-9568.2000.00241.x. [DOI] [PubMed] [Google Scholar]
- 129.Perrelet D, Ferri A, MacKenzie AE, Smith GM, Korneluk RG, Liston P, Sagot Y, Terrado J, Monnier D, Kato AC. IAP family proteins delay motoneuron cell death in vivo. Eur J Neurosci. 2000;12(6):2059–2067. doi: 10.1046/j.1460-9568.2000.00098.x. [DOI] [PubMed] [Google Scholar]
- 130.Martin LJ, Liu Z. Injury-induced spinal motor neuron apoptosis is preceded by DNA single-strand breaks and is p53- and Bax-dependent. J Neurobiol. 2002;50(3):181–197. doi: 10.1002/neu.10026. [DOI] [PubMed] [Google Scholar]
- 131.Park OH, Lee KJ, Rhyu IJ, Geum D, Kim H, Buss RR, Oppenheim RW, Sun W. Bax-dependent and - independent death of motoneurons after facial nerve injury in adult mice. Eur J Neurosci. 2007;26(6):1421–1432. doi: 10.1111/j.1460-9568.2007.05787.x. [DOI] [PubMed] [Google Scholar]
- 132.Yamada M, Natsume A, Mata M, Oligino T, Goss J, Glorioso J, Fink DJ. Herpes simplex virus vector-mediated expression of Bcl-2 protects spinal motor neurons from degeneration following root avulsion. Exp Neurol. 2001;168(2):225–230. doi: 10.1006/exnr.2000.7597. [DOI] [PubMed] [Google Scholar]
- 133.Imaizumi K, Benito A, Kiryu-Seo S, Gonzalez V, Inohara N, Lieberman AP, Kiyama H, Nuñez G. Critical role for DP5/Harakiri, a Bcl-2 homology domain 3-only Bcl-2 family member, in axotomy-induced neuronal cell death. J Neurosci. 2004;24(15):3721–3725. doi: 10.1523/JNEUROSCI.5101-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kiryu-Seo S, Hirayama T, Kato R, Kiyama H. Noxa is a critical mediator of p53-dependent motor neuron death after nerve injury in adult mouse. J Neurosci. 2005;25(6):1442–1447. doi: 10.1523/JNEUROSCI.4041-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Raivich G, Liu ZQ, Kloss CU, Labow M, Bluethmann H, Bohatschek M. Cytotoxic potential of proinflammatory cytokines: combined deletion of TNF receptors TNFR1 and TNFR2 prevents motoneuron cell death after facial axotomy in adult mouse. Exp Neurol. 2002;178(2):186–193. doi: 10.1006/exnr.2002.8024. [DOI] [PubMed] [Google Scholar]
- 136.Raoul C, Henderson CE, Pettmann B. Programmed cell death of embryonic motoneurons triggered through the Fas death receptor. J Cell Biol. 1999;147(5):1049–1062. doi: 10.1083/jcb.147.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nagata S. Apoptosis by death factor. Cell. 1997;88(3):355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 138.Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14(22):5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17(6):1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94(4):491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 141.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94(4):481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 142.Eskes R, Desagher S, Antonsson B, Martinou JC. JC: Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20(3):929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, Thompson CB, Korsmeyer SJ. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14(16):2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 144.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8(3):705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 145.Wei MC, Zong XW, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Raoul C, Estévez AG, Nishimune H, Cleveland DW, deLapeyrière O, Henderson CE, Haase G, Pettmann B. Motoneuron death triggered by a specific pathway downstream of Fas potentiation by ALS-linked SOD1 mutations. Neuron. 2002;35(6):1067–1083. doi: 10.1016/s0896-6273(02)00905-4. [DOI] [PubMed] [Google Scholar]
- 147.Ugolini G, Raoul C, Ferri A, Haenggeli C, Yamamoto Y, Salaün D, Henderson CE, Kato AC, Pettmann B, Hueber AO. Fas/tumor necrosis factor receptor death signaling is required for axotomy-induced death of motoneurons in vivo. J Neurosci. 2003;23(24):8526–8531. doi: 10.1523/JNEUROSCI.23-24-08526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Martin LJ, Chen K, Liu Z. Adult motor neuron apoptosis is mediated by nitric oxide and Fas death receptor linked by DNA damage and p53 activation. J Neurosci. 2005;25(27):6449–6459. doi: 10.1523/JNEUROSCI.0911-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ferri CC, Moore FA, Bisby MA. Effects of facial nerve injury on mouse motoneurons lacking the p75 low-affinity neurotrophin receptor. J Neurobiol. 1998;34(1):1–9. [PubMed] [Google Scholar]
- 150.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 151.Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, et al. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266(5187):1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- 152.Hammarberg H, Piehl F, Cullheim S, Fjell J, Hökfelt T, Fried K. GDNF mRNA in Schwann cells and DRG satellite cells after chronic sciatic nerve injury. Neuroreport. 1996;7(4):857–860. doi: 10.1097/00001756-199603220-00004. [DOI] [PubMed] [Google Scholar]
- 153.Tokumine J, Sugahara K, Kakinohana O, Marsala M. The spinal GDNF level is increased after transient spinal cord ischemia in the rat. Acta Neurochir Suppl. 2003;86:231–234. doi: 10.1007/978-3-7091-0651-8_50. [DOI] [PubMed] [Google Scholar]
- 154.Yan Q, Matheson C, Lopez OT. In vivo neurotrophic effects of GDNF on neonatal and adult facial motor neurons. Nature. 1995;373(6512):341–344. doi: 10.1038/373341a0. [DOI] [PubMed] [Google Scholar]
- 155.Leitner ML, Molliver DC, Osborne PA, Vejsada R, Golden JP, Lampe PA, Kato AC, Milbrandt J, Johnson EN., Jr Analysis of the retrograde transport of glial cell line-derived neurotrophic factor (GDNF), neurturin, and persephin suggests that in vivo signaling for the GDNF family is GFRalpha coreceptor-specific. J Neurosci. 1999;19(21):9322–9331. doi: 10.1523/JNEUROSCI.19-21-09322.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rind HB, Butowt R, von Bartheld C. Synaptic targeting of retrogradely transported trophic factors in motoneurons: comparison of glial cell line-derived neurotrophic factor, brain-derived neurotrophic factor, and cardiotrophin-1 with tetanus toxin. J Neurosci. 2002;25(3):539–549. doi: 10.1523/JNEUROSCI.4322-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Perrelet D, Ferri A, Liston P, Muzzin P, Korneluk RG, Kato AC. IAPs are essential for GDNF-mediated neuroprotective effects in injured motor neurons in vivo. Nat Cell Biol. 2002;4(2):175–179. doi: 10.1038/ncb751. [DOI] [PubMed] [Google Scholar]
- 158.Oppenheim RW, Houenou LJ, Johnson JE, Lin LF, Li L, Lo AC, Newsome AL, Prevette DM, Wang S. Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature. 1995;373(6512):344–346. doi: 10.1038/373344a0. [DOI] [PubMed] [Google Scholar]
- 159.Houenou LJ, Oppenheim RW, Li L, Lo AC, Prevette D. Regulation of spinal motoneuron survival by GDNF during development and following injury. Cell Tissue Res. 1996;286(2):219–223. doi: 10.1007/s004410050690. [DOI] [PubMed] [Google Scholar]
- 160.Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003;27(3):277–324. doi: 10.1385/MN:27:3:277. [DOI] [PubMed] [Google Scholar]
- 161.Moore MW, Klein RD, Fariñas I, Sauer H, Armanini M, Phillips H, et al. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1995;382(6586):76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- 162.Sánchez MP, Silos-Santiago I, Frisén J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1995;382(6586):70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 163.Oppenheim RW, Houenou L, Parsadanian AS, Prevette D, Snider WD, Shen L. Glial cell line-derived neurotrophic factor and developing mammalian motoneurons: regulation of programmed cell death among motoneuron subtypes. J Neurosci. 2000;20(13):5001–5011. doi: 10.1523/JNEUROSCI.20-13-05001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Airaksinen MS, Titievsky A, Saarma M. GDNF family neurotrophic factor signaling: four masters, one servant? Mol Cell Neurosci. 1999;13(5):313–325. doi: 10.1006/mcne.1999.0754. [DOI] [PubMed] [Google Scholar]
- 165.Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3(5):383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 166.Takahashi M. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev. 2001;12(4):361–373. doi: 10.1016/s1359-6101(01)00012-0. [DOI] [PubMed] [Google Scholar]
- 167.Soler RM, Dolcet X, Encinas M, Egea J, Bayascas JR, Comella JX. Receptors of the glial cell line-derived neurotrophic factor family of neurotrophic factors signal cell survival through the phosphatidylinositol 3-kinase pathway in spinal cord motoneurons. J Neurosci. 1998;19(21):9160–9169. doi: 10.1523/JNEUROSCI.19-21-09160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Coulpier M, Anders J, Ibáñez CF. Coordinated activation of autophosphorylation sites in the RET receptor tyrosine kinase: importance of tyrosine 1062 for GDNF mediated neuronal differentiation and survival. J Biol Chem. 2002;277(3):1991–1999. doi: 10.1074/jbc.M107992200. [DOI] [PubMed] [Google Scholar]
- 169.Cacalano G, Fariñas I, Wang LC, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF, Hynes M, Davies AM, Rosenthal A. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21(1):53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Garcès A, Haase G, Airaksinen MS, Livet J, Filippi P, deLapeyrière O. GFRalpha 1 is required for development of distinct subpopulations of motoneuron. J Neurosci. 2000;(13):4992–5000. doi: 10.1523/JNEUROSCI.20-13-04992.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Haase G, Dessaud E, Garcès A, de Bovis B, Birling M, Filippi P, Schmalbruch H, Arber S, deLapeyrière O. GDNF acts through PEA3 to regulate cell body positioning and muscle innervation of specific motor neuron pools. Neuron. 2002;35(5):893–905. doi: 10.1016/s0896-6273(02)00864-4. [DOI] [PubMed] [Google Scholar]
- 172.Livet J, Sigrist M, Stroebel S, De Paola V, Price SR, Henderson CE, Jessell TM, Arber S. ETS gene Pea3 controls the central position and terminal arborization of specific motor neuron pools. Neuron. 2002;35(5):877–892. doi: 10.1016/s0896-6273(02)00863-2. [DOI] [PubMed] [Google Scholar]
- 173.Kramer ER, Knott L, Su F, Dessaud E, Krull CE, Helmbacher F, Klein R. Cooperation between GDNF/Ret and ephrinA/EphA4 signals for motor-axon pathway selection in the limb. Neuron. 2006;50(1):35–47. doi: 10.1016/j.neuron.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 174.Whitehead J, Keller-Peck C, Kucera J, Tourtellotte WG. Glial cell-line derived neurotrophic factor-dependent fusimotor neuron survival during development. Mech Dev. 2005;122(1):27–41. doi: 10.1016/j.mod.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 175.Nguyen QT, Parsadanian AS, Snider WD, Lichtman JD. Hyperinnervation of neuromuscular junctions caused by GDNF overexpression in muscle. Science. 1998;279(5357):1725–1729. doi: 10.1126/science.279.5357.1725. [DOI] [PubMed] [Google Scholar]
- 176.Zhao Z, Alam S, Oppenheim RW, Prevette DM, Evenson A, Parsadanian A. Overexpression of glial cell line-derived neurotrophic factor in the CNS rescues motoneurons from programmed cell death and promotes their long-term survival following axotomy. Exp Neurol. 2004;190(2):356–372. doi: 10.1016/j.expneurol.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 177.Barde Y-A, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Henderson CE, Camu W, Mettling C, Gouin A, Poulsen K, Karihaloo M, Rullamas J, Evans T, McMahon SB, Armanini MP. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature. 1993;363(6426):266–270. doi: 10.1038/363266a0. [DOI] [PubMed] [Google Scholar]
- 179.Yan Q, Elliot J, Snider WD. Brain-derived neurotrophic factor rescues spinal motor neurons from axotomy-induced cell death. Nature. 1993;360(6406):753–755. doi: 10.1038/360753a0. [DOI] [PubMed] [Google Scholar]
- 180.Meyer M, Matsuoka I, Wetmore C, Olson L, Thoenen H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J Cell Biol. 1992;119(1):45–54. doi: 10.1083/jcb.119.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Piehl F, Frisén J, Risling M, Hökfelt T, Cullheim S. Increased trkB mRNA expression by axotomized motoneurones. Neuroreport. 1994;5(6):697–700. doi: 10.1097/00001756-199402000-00009. [DOI] [PubMed] [Google Scholar]
- 182.Oppenheim RW, Yin QW, Prevette D, Yan Q. Brain-derived neurotrophic factor rescues developing avian motoneurons from cell death. Nature. 1992;360(6406):755–757. doi: 10.1038/360755a0. [DOI] [PubMed] [Google Scholar]
- 183.Sendtner M, Holtmannm B, Kolbeck R, Thoenen H, Barde YA. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature. 1992;360(6406):757–759. doi: 10.1038/360757a0. [DOI] [PubMed] [Google Scholar]
- 184.Yan Q, Elliott J, Snider WD. Brain-derived neurotrophic factor rescues spinal motor neurons from axotomy-induced cell death. Nature. 1992;360(6406):753–755. doi: 10.1038/360753a0. [DOI] [PubMed] [Google Scholar]
- 185.Koliatsos VE, Clatterbuck RE, Winslow JW, Cayouette MH, Price DL. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron. 1993;10(3):359–367. doi: 10.1016/0896-6273(93)90326-m. [DOI] [PubMed] [Google Scholar]
- 186.Alcántara S, Frisén J, del Río JA, Soriano E, Barbacid M, Silos-Santiago I. TrkB signaling is required for postnatal survival of CNS neurons and protects hippocampal and motor neurons from axotomy-induced cell death. J Neurosci. 1997;17(10):3623–3633. doi: 10.1523/JNEUROSCI.17-10-03623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994b;368(6467):147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- 188.Conover JC, Erickson JT, Katz DM, Bianchi LM, Poueymirou WT, McClain J, Pan L, Helgren M, Ip NY, Boland P, et al. Neuronal deficits, not involving motor neurons, in mice lacking BDNF and/or NT4. Nature. 1995;375(6528):235–238. doi: 10.1038/375235a0. [DOI] [PubMed] [Google Scholar]
- 189.Liu X, Ernfors P, Wu H, Jaenisch R. Sensory but not motor neuron deficits in mice lacking NT4 and BDNF. Nature. 1995;375(6528):238–241. doi: 10.1038/375238a0. [DOI] [PubMed] [Google Scholar]
- 190.Silos-Santiago I, Fagan AM, Garber M, Fritzsch B, Barbacid M. Severe sensory deficits but normal CNS development in newborn mice lacking TrkB and TrkC tyrosine protein kinase receptors. Eur J Neurosci. 1997;9(10):2045–2056. doi: 10.1111/j.1460-9568.1997.tb01372.x. [DOI] [PubMed] [Google Scholar]
- 191.Copray JC, Brouwer N. Selective expression of neurotrophin-3 messenger RNA in muscle spindles of the rat. Neuroscience. 1994;63(4):1125–1135. doi: 10.1016/0306-4522(94)90578-9. [DOI] [PubMed] [Google Scholar]
- 192.Kucera J, Ernfors P, Walro J, Jaenisch R. Reduction in the number of spinal motor neurons in neurotrophin-3-deficient mice. Neuroscience. 1995a;69(1):321–330. doi: 10.1016/0306-4522(95)00221-4. [DOI] [PubMed] [Google Scholar]
- 193.Wright DE, Zhou L, Kucera J, Snider WD. Introduction of a neurotrophin-3 transgene into muscle selectively rescues proprioceptive neurons in mice lacking endogenous neurotrophin-3. Neuron. 1997;19(3):503–517. doi: 10.1016/s0896-6273(00)80367-0. [DOI] [PubMed] [Google Scholar]
- 194.Kucera J, Fan G, Jaenisch R, Linnarsson S, Ernfors P. Dependence of developing group Ia afferents on neurotrophin-3. J Comp Neurol. 1995b;363(2):307–320. doi: 10.1002/cne.903630211. [DOI] [PubMed] [Google Scholar]
- 195.Woolley AG, Sheard PW, Dodds K, Duxson MJ. Alpha motoneurons are present in normal numbers but with reduced soma size in neurotrophin-3 knockout mice. Neurosci Lett. 1999;272(2):107–110. doi: 10.1016/s0304-3940(99)00587-x. [DOI] [PubMed] [Google Scholar]
- 196.Liu X, Jaenisch R. Severe peripheral sensory neuron loss and modest motor neuron reduction in mice with combined deficiency of brain-derived neurotrophic factor, neurotrophin 3 and neurotrophin 4/5. Dev Dyn. 2000;218(1):94–101. doi: 10.1002/(SICI)1097-0177(200005)218:1<94::AID-DVDY8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 197.Woolley AG, Sheard PW, Duxson MJ. Neurotrophin-3 null mutant mice display a postnatal motor neuropathy. Eur J Neurosci. 2005;21(8):2100–2110. doi: 10.1111/j.1460-9568.2005.04052.x. [DOI] [PubMed] [Google Scholar]
- 198.Woolley AG, Tait KJ, Hurren BJ, Fisher L, Sheard PW, Duxson MJ. Developmental loss of NT-3 in vivo results in reduced levels of myelin-specific proteins, a reduced extent of myelination and increased apoptosis of Schwann cells. Glia. 2008;56(3):306–317. doi: 10.1002/glia.20614. [DOI] [PubMed] [Google Scholar]
- 199.Reddy LV, Koirala S, Sugiura Y, Herrera AA, Ko CP. Glial cells maintain synaptic structure and function and promote development of the neuromuscular junction in vivo. Neuron. 2006;40(3):563–580. doi: 10.1016/s0896-6273(03)00682-2. [DOI] [PubMed] [Google Scholar]
- 200.Li L, Oppenheim RW, Lei M, Houenou LJ. Neurotrophic agents prevent motoneuron death following sciatic nerve section in the neonatal mouse. J Neurobiol. 1994;25(7):759–766. doi: 10.1002/neu.480250702. [DOI] [PubMed] [Google Scholar]
- 201.Yin QQ, Johnson J, Prevette D, Oppenheim RW. Cell death of spinal motoneurons in the chick embryo following deafferentation: rescue effects of tissue extracts, soluble proteins, and neurotrophic agents. J Neurosci. 1995;14(12):7629–7640. doi: 10.1523/JNEUROSCI.14-12-07629.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ip NY, Yancopoulos GD. The neurotrophins and CNTF: two families of collaborative neurotrophic factors. Annu Rev Neurosci. 1996;19:491–515. doi: 10.1146/annurev.ne.19.030196.002423. [DOI] [PubMed] [Google Scholar]
- 203.Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 204.Senaldi G, Varnum BC, Sarmiento U, Starnes C, Lile J, Scully S, Guo J, Elliott G, McNinch J, Shaklee CL, Freeman D, Manu F, Simonet WS, Boone T, Chang MS. Novel neurotrophin-1/B cell-stimulating factor-3: a cytokine of the IL-6 family. Proc Natl Acad Sci U S A. 1999;96(20):11458–11463. doi: 10.1073/pnas.96.20.11458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Shi Y, Wang W, Yourey PA, Gohari S, Zukauskas D, Zhang J, Ruben S, Alderson RF. Computational EST database analysis identifies a novel member of the neuropoietic cytokine family. Biochem Biophys Res Commun. 1999;262(1):132–138. doi: 10.1006/bbrc.1999.1181. [DOI] [PubMed] [Google Scholar]
- 206.Haas CA, Hofmann HD, Kirsch M. Expression of CNTF/LIF-receptor components and activation of STAT3 signaling in axotomized facial motoneurons: evidence for a sequential postlesional function of the cytokines. J Neurobiol. 1999;41(4):559–571. doi: 10.1002/(sici)1097-4695(199912)41:4<559::aid-neu11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 207.Dolcet X, Soler RM, Gould TW, Egea J, Oppenheim RW, Comella JX. Cytokines promote motoneuron survival through the Janus kinase-dependent activation of the phosphatidylinositol 3-kinase pathway. Mol Cell Neurosci. 2001;18(6):619–631. doi: 10.1006/mcne.2001.1058. [DOI] [PubMed] [Google Scholar]
- 208.Adler R, Landa KB, Manthorpe M, Varon S. Cholinergic neuronotrophic factors: intraocular distribution of trophic activity for ciliary neurons. Science. 1979;204(4400):1434–1436. doi: 10.1126/science.451576. [DOI] [PubMed] [Google Scholar]
- 209.Barbin G, Manthorpe M, Varon S. Purification of the chick eye ciliary neuronotrophic factor. J Neurochem. 1984;43(5):1468–1478. doi: 10.1111/j.1471-4159.1984.tb05410.x. [DOI] [PubMed] [Google Scholar]
- 210.Arakawa Y, Sendtner M, Thoenen H. Survival effect of ciliary neurotrophic factor (CNTF) on chick embryonic motoneurons in culture: comparison with other neurotrophic factors and cytokines. J Neurosci. 1990;10(11):3507–3515. doi: 10.1523/JNEUROSCI.10-11-03507.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sendtner M, Kreutzberg GW, Thoenen H. Ciliary neurotrophic factor prevents the degeneration of motor neurons after axotomy. Nature. 1990;345(6274):440–441. doi: 10.1038/345440a0. [DOI] [PubMed] [Google Scholar]
- 212.Oppenheim RW, Prevette D, Yin QW, Collins F, MacDonald J. Control of embryonic motoneuron survival in vivo by ciliary neurotrophic factor. Science. 1991;251(5001):1616–1618. doi: 10.1126/science.2011743. [DOI] [PubMed] [Google Scholar]
- 213.McManaman JL, Oppenheim RW, Prevette D, Marchetti D. Rescue of motoneurons from cell death by a purified skeletal muscle polypeptide: effects of the ChAT development factor, CDF. Neuron. 1990;4(6):891–898. doi: 10.1016/0896-6273(90)90142-3. [DOI] [PubMed] [Google Scholar]
- 214.Martinou JC, Martinou I, Kato AC. Cholinergic differentiation factor (CDF/LIF) promotes survival of isolated rat embryonic motoneurons in vitro. Neuron. 1992;8(4):737–744. doi: 10.1016/0896-6273(92)90094-t. [DOI] [PubMed] [Google Scholar]
- 215.Stöckli KA, Lottspeich F, Sendtner M, Masiakowski P, Carroll P, Götz R, Lindholm D, Thoenen H. Molecular cloning, expression and regional distribution of rat ciliary neurotrophic factor. Nature. 1989;342(6252):920–923. doi: 10.1038/342920a0. [DOI] [PubMed] [Google Scholar]
- 216.Stöckli KA, Lillien LE, Näher-Noé M, Breitfeld G, Hughes RA, Raff MC, Thoenen H, Sendtner M. Regional distribution, developmental changes, and cellular localization of CNTF-mRNA and protein in the rat brain. J Cell Biol. 1991;115(2):447–459. doi: 10.1083/jcb.115.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Meyer M, Matsuoka I, Wetmore C, Olson L, Thoenen H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J Cell Biol. 1992;119(1):45–54. doi: 10.1083/jcb.119.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Curtis R, Adryan KM, Zhu Y, Harkness PJ, Lindsay RM, DiStefano PS. Retrograde axonal transport of ciliary neurotrophic factor is increased by peripheral nerve injury. Nature. 1993;365(6443):253–255. doi: 10.1038/365253a0. [DOI] [PubMed] [Google Scholar]
- 219.Masu Y, Wolf E, Holtmann B, Sendtner M, Brem G, Thoenen H. Disruption of the CNTF gene results in motor neuron degeneration. Nature 2. 1993;365(6441):27–32. doi: 10.1038/365027a0. [DOI] [PubMed] [Google Scholar]
- 220.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Köntgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359(6390):76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 221.Escary JL, Perreau J, Duménil D, Ezine S, Brûlet P. Leukaemia inhibitory factor is necessary for maintenance of haematopoietic stem cells and thymocyte stimulation. Nature. 1993;363(6427):361–364. doi: 10.1038/363361a0. [DOI] [PubMed] [Google Scholar]
- 222.Sendtner M, Götz R, Holtmann B, Escary JL, Masu Y, Carroll P, Wolf E, Brem G, Brület P, Thoenen H. Cryptic physiological trophic support of motoneurons by LIF revealed by double gene targeting of CNTF and LIF. Curr Biol. 1996;6(6):686–694. doi: 10.1016/s0960-9822(09)00450-3. [DOI] [PubMed] [Google Scholar]
- 223.DeChiara TM, Vejsada R, Poueymirou WT, Acheson A, Suri C, Conover JC, Friedman B, McClain J, Pan L, Stahl N, Ip NY, Yancopoulos GD. Mice lacking the CNTF receptor, unlike mice lacking CNTF, exhibit profound motor neuron deficits at birth. Cell. 1995;83(2):313–322. doi: 10.1016/0092-8674(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 224.Li M, Sendtner M, Smith A. Essential function of LIF receptor in motor neurons. Nature. 1995;378(6558):724–727. doi: 10.1038/378724a0. [DOI] [PubMed] [Google Scholar]
- 225.Nakashima K, Wiese S, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Yoshida K, Kishimoto T, Sendtner M, Taga T. Developmental requirement of gp130 signaling in neuronal survival and astrocyte differentiation. J Neurosci. 1999;19(13):5429–5434. doi: 10.1523/JNEUROSCI.19-13-05429.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Oppenheim RW, Wiese S, Prevette D, Armanini M, Wang S, Houenou LJ, Holtmann B, Gotz R, Pennica D, Sendtner M. Cardiotrophin-1, a muscle-derived cytokine, is required for the survival of subpopulations of developing motoneurons. J Neurosci. 2001;21(4):1283–1291. doi: 10.1523/JNEUROSCI.21-04-01283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Pennica D, Shaw KJ, Swanson TA, Moore MW, Shelton DL, Zioncheck KA, Rosenthal A, Taga T, Paoni NF, Wood WI. Cardiotrophin-1. Biological activities and binding to the leukemia inhibitory factor receptor/gp130 signaling complex. J Biol Chem. 1995;270(18):10915–10922. doi: 10.1074/jbc.270.18.10915. [DOI] [PubMed] [Google Scholar]
- 228.Pennica D, Arce V, Swanson TA, Vejsada R, Pollock RA, Armanini M, Dudley K, Phillips HS, Rosenthal A, Kato AC, Henderson CE. Cardiotrophin-1, a cytokine present in embryonic muscle, supports long-term survival of spinal motoneurons. Neuron. 1996;17(1):63–74. doi: 10.1016/s0896-6273(00)80281-0. [DOI] [PubMed] [Google Scholar]
- 229.Holtmann B, Wiese S, Samsam M, Grohmann K, Pennica D, Martini R, Sendtner M. Triple knockout of CNTF, LIF, and CT-1 defines cooperative and distinct roles of these neurotrophic factors for motoneuron maintenance and function. J Neurosci. 2005;25(7):1778–1787. doi: 10.1523/JNEUROSCI.4249-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Arce V, Garces A, de Bovis B, Filippi P, Henderson CE, Pettmann B, deLapeyrière O. Cardiotrophin-1 requires LIFRbeta to promote survival of mouse motoneurons purified by a novel technique. J Neurosci Res. 1999;55(1):119–126. doi: 10.1002/(SICI)1097-4547(19990101)55:1<119::AID-JNR13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 231.Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984;122(3):1450–1459. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- 232.Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987;327(6119):239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- 233.Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, Aaronson SA. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251(4995):802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- 234.Naldini L, Weidner KM, Vigna E, Gaudino G, Bardelli A, Ponzetto C, Narsimhan RP, Hartmann G, Zarnegar R, Michalopoulos GK, et al. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J. 1991;10(10):2867–2878. doi: 10.1002/j.1460-2075.1991.tb07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Birchmeier C, Gherardi E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998;8(10):404–410. doi: 10.1016/s0962-8924(98)01359-2. [DOI] [PubMed] [Google Scholar]
- 236.Ebens A, Brose K, Leonardo ED, Hanson MG, Jr, Bladt F, Birchmeier C, Barres BA, Tessier-Lavigne M. Hepatocyte growth factor/scatter factor is an axonal chemoattractant and a neurotrophic factor for spinal motor neurons. Neuron. 1996;17(6):1157–1172. doi: 10.1016/s0896-6273(00)80247-0. [DOI] [PubMed] [Google Scholar]
- 237.Wong V, Glass DJ, Arriaga R, Yancopoulos GD, Lindsay RM, Conn G. Hepatocyte growth factor promotes motor neuron survival and synergizes with ciliary neurotrophic factor. J Biol Chem. 1997;272(8):5187–5191. doi: 10.1074/jbc.272.8.5187. [DOI] [PubMed] [Google Scholar]
- 238.Koyama J, Yokouchi K, Fukushima N, Kawagishi K, Higashiyama F, Moriizumi T. Neurotrophic effect of hepatocyte growth factor on neonatal facial motor neurons. Neurol Res. 2003;25(7):701–707. doi: 10.1179/016164103101202192. [DOI] [PubMed] [Google Scholar]
- 239.Maina F, Hilton MC, Ponzetto C, Davies AM, Klein R. Met receptor signaling is required for sensory nerve development and HGF promotes axonal growth and survival of sensory neurons. Genes Dev. 1997;11(24):3341–3350. doi: 10.1101/gad.11.24.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Maina F, Panté G, Helmbacher F, Andres R, Porthin A, Davies AM, Ponzetto C, Klein R. Coupling Met to specific pathways results in distinct developmental outcomes. Mol Cell. 2001;7(6):1293–1306. doi: 10.1016/s1097-2765(01)00261-1. [DOI] [PubMed] [Google Scholar]
- 241.Helmbacher F, Dessaud E, Arber S, deLapeyrière O, Henderson CE, Klein R, Maina F. Met signaling is required for recruitment of motor neurons to PEA3-positive motor pools. Neuron. 2003;39(5):767–777. doi: 10.1016/s0896-6273(03)00493-8. [DOI] [PubMed] [Google Scholar]
- 242.Roccisana J, Reddy V, Vasavada RC, Gonzalez-Pertusa JA, Magnuson MA, Garcia-Ocaña A. Targeted inactivation of hepatocyte growth factor receptor c-met in beta-cells leads to defective insulin secretion and GLUT-2 downregulation without alteration of beta-cell mass. Diabetes. 2005;54(7):2090–2102. doi: 10.2337/diabetes.54.7.2090. [DOI] [PubMed] [Google Scholar]
- 243.LeRoith D, McGuinness M, Shemer J, Stannard B, Lanau F, Faria TN, Kato H, Werner H, Adamo M, Roberts CT., Jr Insulin-like growth factors. Biol Signals. 1993;1(4):173–181. doi: 10.1159/000109323. [DOI] [PubMed] [Google Scholar]
- 244.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16(1):3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 245.Ishii DN. Relationship of insulin-like growth factor II gene expression in muscle to synaptogenesis. Proc Natl Acad Sci U S A. 1989;86(8):2898–2902. doi: 10.1073/pnas.86.8.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Caroni P, Grandes P. Nerve sprouting in innervated adult skeletal muscle induced by exposure to elevated levels of insulin-like growth factors. J Cell Biol. 1990;110(4):1307–1317. doi: 10.1083/jcb.110.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hansson HA. Insulin-like growth factors and nerve regeneration. Ann N Y Acad Sci. 1993;692:61–71. doi: 10.1111/j.1749-6632.1993.tb26214.x. [DOI] [PubMed] [Google Scholar]
- 248.Caroni P, Schneider C, Kiefer MC, Zapf J. Role of muscle insulin-like growth factors in nerve sprouting: suppression of terminal sprouting in paralyzed muscle by IGF-binding protein 4. J Cell Biol. 1994;125(4):893–902. doi: 10.1083/jcb.125.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Near SL, Whalen LR, Miller JA, Ishii DN. Insulin-like growth factor II stimulates motor nerve regeneration. Proc Natl Acad Sci USA. 1992;89(24):11716–11720. doi: 10.1073/pnas.89.24.11716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Xu QG, Li XQ, Kotecha SA, Cheng C, Sun HS, Zochodne DW. Insulin as an in vivo growth factor. Exp Neurol. 2004;188(1):43–51. doi: 10.1016/j.expneurol.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 251.Pu SF, Zhuang HX, Marsh DJ, Ishii DN. Insulin-like growth factor-II increases and IGF is required for postnatal rat spinal motoneuron survival following sciatic nerve axotomy. J Neurosci Res. 1999;55(1):9–16. doi: 10.1002/(SICI)1097-4547(19990101)55:1<9::AID-JNR2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 252.Ralphs JR, Wylie L, Hill DJ. Distribution of insulin-like growth factor peptides in the developing chick embryo. Development. 1990;109(1):51–58. doi: 10.1242/dev.109.1.51. [DOI] [PubMed] [Google Scholar]
- 253.Neff NT, Prevette D, Houenou LJ, Lewis ME, Glicksman MA, Yin QW, Oppenheim RW. Insulin-like growth factors: putative muscle-derived trophic agents that promote motoneuron survival. J Neurobiol. 1993;24(12):1578–1588. doi: 10.1002/neu.480241203. [DOI] [PubMed] [Google Scholar]
- 254.D'Costa AP, Prevette DM, Houenou LJ, Wang S, Zackenfels K, Rohrer H, Zapf J, Caroni P, Oppenheim RW. Mechanisms of insulin-like growth factor regulation of programmed cell death of developing avian motoneurons. J Neurobiol. 1998;36(3):379–394. [PubMed] [Google Scholar]
- 255.Yadav A, Kalita A, Dhillon S, Banerjee K. JAK/STAT3 pathway is involved in survival of neurons in response to insulin-like growth factor and negatively regulated by suppressor of cytokine signaling-3. J Biol Chem. 2005;280(36):31830–31840. doi: 10.1074/jbc.M501316200. [DOI] [PubMed] [Google Scholar]
- 256.Vicario-Abejón C, Fernández-Moreno C, Pichel JG, de Pablo F. Mice lacking IGF-I and LIF have motoneuron deficits in brain stem nuclei. Neuroreport. 2004;15(18):2769–2772. [PubMed] [Google Scholar]
- 257.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75(1):59–72. [PubMed] [Google Scholar]
- 258.Mason JL, Xuan S, Dragatsis I, Efstratiadis A, Goldman JE. Insulin-like growth factor (IGF) signaling through type 1 IGF receptor plays an important role in remyelination. J Neurosci. 2003;23(20):7710–7718. doi: 10.1523/JNEUROSCI.23-20-07710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 260.Sondell M, Sundler F, Kanje M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur J Neurosci. 2000;12(12):4243–4254. doi: 10.1046/j.0953-816x.2000.01326.x. [DOI] [PubMed] [Google Scholar]
- 261.Lambrechts D, Carmeliet P. VEGF at the neurovascular interface: therapeutic implications for motor neuron disease. Biochim Biophys Acta. 2006;1762(11–12):1109–1121. doi: 10.1016/j.bbadis.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 262.Van Den Bosch L, Storkebaum E, Vleminckx V, Moons L, Vanopdenbosch L, Scheveneels W, Carmeliet P, Robberecht W. Effects of vascular endothelial growth factor (VEGF) on motor neuron degeneration. Neurobiol Dis. 2004;17(1):21–28. doi: 10.1016/j.nbd.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 263.Tolosa L, Mir M, Asensio VJ, Olmos G, Lladó J. Vascular endothelial growth factor protects spinal cord motoneurons against glutamate-induced excitotoxicity via phosphatidylinositol 3-kinase. J Neurochem. 2008;105(4):1080–1090. doi: 10.1111/j.1471-4159.2007.05206.x. [DOI] [PubMed] [Google Scholar]
- 264.Hobson MI, Green CJ, Terenghi G. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J Anat. 2000;197(Pt 4):591–605. doi: 10.1046/j.1469-7580.2000.19740591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380(6573):435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 266.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380(6573):439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 267.Schwarz Q, Gu C, Fujisawa H, Sabelko K, Gertsenstein M, Nagy A, Taniguchi M, Kolodkin AL, Ginty DD, Shima DT, Ruhrberg C. Vascular endothelial growth factor controls neuronal migration and cooperates with Sema3A to pattern distinct compartments of the facial nerve. Genes Dev. 2004;18(22):2822–2834. doi: 10.1101/gad.322904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Oppenheim RW, Cole T, Prevette D. Early regional variations in motoneuron numbers arise by differential proliferation in the chick embryo spinal cord. Dev Biol. 1989;133(2):468–474. doi: 10.1016/0012-1606(89)90050-x. [DOI] [PubMed] [Google Scholar]
- 269.Ince PG, Evans J, Knopp M, Forster G, Hamdalla HH, Wharton SB, Shaw PJ. Corticospinal tract degeneration in the progressive muscular atrophy variant of ALS. Neurology. 2003;60(8):1252–1258. doi: 10.1212/01.wnl.0000058901.75728.4e. [DOI] [PubMed] [Google Scholar]
- 270.Stephens B, Guiloff RJ, Navarrete R, Newman P, Nikhar N, Lewis P. Widespread loss of neuronal populations in the spinal ventral horn in sporadic motor neuron disease. A morphometric study. J Neurol Sci. 2006;244(1–2):41–58. doi: 10.1016/j.jns.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 271.Martin LJ, Liu Z, Chen K, Price AC, Pan Y, Swaby JA, Golden WC. Motor neuron degeneration in amyotrophic lateral sclerosis mutant superoxide dismutase-1 transgenic mice: mechanisms of mitochondriopathy and cell death. J Comp Neurol. 2007;500(1):20–46. doi: 10.1002/cne.21160. [DOI] [PubMed] [Google Scholar]
- 272.Isaacs JD, Dean AF, Shaw CE, Al-Chalabi A, Mills KR, Leigh PN. Amyotrophic lateral sclerosis with sensory neuropathy: part of a multisystem disorder? J Neurol Neurosurg Psychiatry. 2007;78(7):750–753. doi: 10.1136/jnnp.2006.098798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Schymick JC, Talbot K, Traynor BJ. Genetics of sporadic amyotrophic lateral sclerosis. Hum Mol Genet. 2007;16(Spec No. 2):R233–R242. doi: 10.1093/hmg/ddm215. [DOI] [PubMed] [Google Scholar]
- 274.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 275.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244(22):6049–6055. [PubMed] [Google Scholar]
- 276.Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264(5166):1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 277.Reaume AG, Elliott JL, Hoffman EK, Kowall NJ, Ferrante RJ, Siwek DF, Wilcox HM, Flood DG, Beal MF, Brown RH, Jr, Scott RW, Snider WD. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nat Genet. 1996;13(1):43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- 278.Borchelt DR, Lee MK, Slunt HS, Guarnieri M, Xu ZS, Wong PC, Brown RH, Jr, Price DL, Sisodia SS, Cleveland DW. Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc Natl Acad Sci U S A. 1994;91(17):8292–8296. doi: 10.1073/pnas.91.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Pardo CA, Xu Z, Borchelt DR, Price DL, Sisodia SS, Cleveland DW. Superoxide dismutase is an abundant component in cell bodies, dendrites, and axons of motor neurons and in a subset of other neurons. Proc Natl Acad Sci U S A. 1995;92(4):954–958. doi: 10.1073/pnas.92.4.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Culotta VC, Yang M, O'Halloran TV. Activation of superoxide dismutases: putting the metal to the pedal. Biochim Biophys Acta. 2006;1763(7):747–758. doi: 10.1016/j.bbamcr.2006.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Arnesano F, Banci L, Bertini I, Martinelli M, Furukawa Y, O'Halloran TV. The unusually stable quaternary structure of human Cu,Zn-superoxide dismutase 1 is controlled by both metal occupancy and disulfide status. J Biol Chem. 2004;279(46):47998–48003. doi: 10.1074/jbc.M406021200. [DOI] [PubMed] [Google Scholar]
- 282.Furukawa Y, Torres AS, O'Halloran TV. Oxygen-induced maturation of SOD1: a key role for disulfide formation by the copper chaperone CCS. EMBO J. 2004;23(14):2872–2881. doi: 10.1038/sj.emboj.7600276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Lindberg MJ, Tibell L, Oliveberg M. Common denominator of Cu/Zn superoxide dismutase mutants associated with amyotrophic lateral sclerosis: decreased stability of the apo state. Proc Natl Acad Sci U S A. 2002;99(26):16607–16612. doi: 10.1073/pnas.262527099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Lindberg MJ, Byström R, Boknäs N, Andersen PM, Oliveberg M. Systematically perturbed folding patterns of amyotrophic lateral sclerosis (ALS)-associated SOD1 mutants. Proc Natl Acad Sci U S A. 2005;102(28):9754–9759. doi: 10.1073/pnas.0501957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Doucette PA, Whitson LJ, Cao X, Schirf V, Demeler B, Valentine JS, Hansen JC, Hart PJ. Dissociation of human copper-zinc superoxide dismutase dimers using chaotrope and reductant. Insights into the molecular basis for dimer stability. J Biol Chem. 2004;279(52):54558–54566. doi: 10.1074/jbc.M409744200. [DOI] [PubMed] [Google Scholar]
- 286.Field LS, Furukawa Y, O'Halloran TV, Culotta VC. Factors controlling the uptake of yeast copper/zinc superoxide dismutase into mitochondria. J Biol Chem. 2003;278(30):28052–28059. doi: 10.1074/jbc.M304296200. [DOI] [PubMed] [Google Scholar]
- 287.Lyons TJ, Liu H, Goto JJ, Nersissian A, Roe JA, Graden JA, Café C, Ellerby LM, Bredesen DE, Gralla EB, Valentine JS. Mutations in copper-zinc superoxide dismutase that cause amyotrophic lateral sclerosis alter the zinc binding site and the redox behavior of the protein. Proc Natl Acad Sci U S A. 1996;93(22):12240–12244. doi: 10.1073/pnas.93.22.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Crow JP, Sampson JB, Zhuang Y, Thompson JA, Beckman JS. Decreased zinc affinity of amyotrophic lateral sclerosis-associated superoxide dismutase mutants leads to enhanced catalysis of tyrosine nitration by peroxynitrite. J Neurochem. 1997;69(5):1936–1944. doi: 10.1046/j.1471-4159.1997.69051936.x. [DOI] [PubMed] [Google Scholar]
- 289.Beal MJ, Ferrante RJ, Browne SE, Matthews RT, Kowall NW, Brown RH., Jr Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann Neurol. 1997;42(4):644–654. doi: 10.1002/ana.410420416. [DOI] [PubMed] [Google Scholar]
- 290.Ferrante RJ, Shinobu LA, Schulz JB, Matthews RT, Thomas CE, Kowall NW, Gurney ME, Beal MF. Increased 3-nitrotyrosine and oxidative damage in mice with a human copper/zinc superoxide dismutase mutation. Ann Neurol. 1997;42(3):326–334. doi: 10.1002/ana.410420309. [DOI] [PubMed] [Google Scholar]
- 291.Casoni F, Basso M, Massignan T, Gianazza E, Cheroni C, Salmona M, Bendotti C, Bonetto V. Protein nitration in a mouse model of familial amyotrophic lateral sclerosis: possible multifunctional role in the pathogenesis. J Biol Chem. 2005;280(16):16295–16304. doi: 10.1074/jbc.M413111200. [DOI] [PubMed] [Google Scholar]
- 292.Estévez AG, Crow JP, Sampson JB, Reiter C, Zhuang Y, Richardson GJ, Tarpey MM, Barbeito L, Beckman JS. Induction of nitric oxide-dependent apoptosis in motor neurons by zinc-deficient superoxide dismutase. Science. 1999;286(5449):2498–2500. doi: 10.1126/science.286.5449.2498. [DOI] [PubMed] [Google Scholar]
- 293.Wang J, Slunt H, Gonzales V, Fromholt D, Coonfield M, Copeland NG, Jenkins NA, Borchelt DR. Copper-binding-site-null SOD1 causes ALS in transgenic mice: aggregates of non-native SOD1 delineate a common feature. Hum Mol Genet. 2003;12(21):2753–2764. doi: 10.1093/hmg/ddg312. [DOI] [PubMed] [Google Scholar]
- 294.Ince PG, Shaw PJ, Slade JY, Jones C, Hudgson P. Familial amyotrophic lateral sclerosis with a mutation in exon 4 of the Cu/Zn superoxide dismutase gene: pathological and immunocytochemical changes. Acta Neuropathol. 1996;92(4):395–403. doi: 10.1007/s004010050535. [DOI] [PubMed] [Google Scholar]
- 295.Kato S, Kawata A, Oda M, Arai N, Komori T, Tanabe H. Absence of SOD1 gene abnormalities in familial amyotrophic lateral sclerosis with posterior column involvement without Lewy-body-like hyaline inclusions. Acta Neuropathol. 1996;92(5):528–533. doi: 10.1007/s004010050557. [DOI] [PubMed] [Google Scholar]
- 296.Bruijn LI, Houseweart MK, Kato S, Anderson KL, Anderson SD, Ohama E, Reaume AG, Scott RW, Cleveland DW. Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science. 1998;281(5384):1851–1854. doi: 10.1126/science.281.5384.1851. [DOI] [PubMed] [Google Scholar]
- 297.Johnston JA, Dalton MJ, Gurney ME, Kopito RR. Formation of high molecular weight complexes of mutant Cu, Zn-superoxide dismutase in a mouse model for familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2000;97(23):12571–12576. doi: 10.1073/pnas.220417997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Wang J, Xu G, Borchelt DR. High molecular weight complexes of mutant superoxide dismutase 1: age-dependent and tissue-specific accumulation. Neurobiol Dis. 2002;9(2):139–148. doi: 10.1006/nbdi.2001.0471. [DOI] [PubMed] [Google Scholar]
- 299.Furukawa Y, Fu R, Deng HX, Siddique T, O'Halloran TV. Disulfide cross-linked protein represents a significant fraction of ALS-associated Cu, Zn-superoxide dismutase aggregates in spinal cords of model mice. Proc Natl Acad Sci U S A. 2006;103(18):7148–7153. doi: 10.1073/pnas.0602048103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Jonsson PA, Graffmo KS, Andersen PM, Brännström T, Lindberg M, Oliveberg M, Marklund SL. Disulphide-reduced superoxide dismutase-1 in CNS of transgenic amyotrophic lateral sclerosis models. Brain. 2006;129(Pt 2):451–464. doi: 10.1093/brain/awh704. [DOI] [PubMed] [Google Scholar]
- 301.Wang J, Xu G, Borchelt DR. Mapping superoxide dismutase 1 domains of non-native interaction: roles of intra- and intermolecular disulfide bonding in aggregation. J Neurochem. 2006;96(5):1277–1288. doi: 10.1111/j.1471-4159.2005.03642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Tiwari A, Hayward LJ. Familial amyotrophic lateral sclerosis mutants of copper/zinc superoxide dismutase are susceptible to disulfide reduction. J Biol Chem. 2003;278(8):5984–5992. doi: 10.1074/jbc.M210419200. [DOI] [PubMed] [Google Scholar]
- 303.Ray SS, Nowak RJ, Strokovich K, Brown RH, Jr, Walz T, Lansbury PT., Jr An intersubunit disulfide bond prevents in vitro aggregation of a superoxide dismutase-1 mutant linked to familial amytrophic lateral sclerosis. Biochemistry. 2004;43(17):4899–4905. doi: 10.1021/bi030246r. [DOI] [PubMed] [Google Scholar]
- 304.Rakhit R, Cunningham P, Furtos-Matei A, Dahan S, Qi QF, Crow JP, Cashman NR, Kondejewski LH, Chakrabartty A. Oxidation-induced misfolding and aggregation of superoxide dismutase and its implications for amyotrophic lateral sclerosis. J Biol Chem. 2002;277(49):47551–47556. doi: 10.1074/jbc.M207356200. [DOI] [PubMed] [Google Scholar]
- 305.Karch CM, Borchelt DR. A limited role for disulfide cross-linking in the aggregation of mutant SOD1 linked to familial amyotrophic lateral sclerosis. J Biol Chem. 2008;283(20):13528–13537. doi: 10.1074/jbc.M800564200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Son M, Puttaparthi K, Kawamata H, Rajendran B, Boyer PJ, Manfredi G, Elliott JL. Overexpression of CCS in G93A-SOD1 mice leads to accelerated neurological deficits with severe mitochondrial pathology. Proc Natl Acad Sci U S A. 2007;104(14):6072–6077. doi: 10.1073/pnas.0610923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Proescher JB, Son M, Elliott JL, Culotta VC. Biological effects of CCS in the absence of SOD1 enzyme activation: implications for disease in a mouse model for ALS. Hum Mol Genet. 2008;17(12):1728–1737. doi: 10.1093/hmg/ddn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Urushitani M, Sik A, Sakurai T, Nukina N, Takahashi R, Julien JP. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat Neurosci. 2006;9(1):108–118. doi: 10.1038/nn1603. [DOI] [PubMed] [Google Scholar]
- 309.Urushitani M, Ezzi SA, Matsuo A, Tooyama I, Julien JP. The endoplasmic reticulum-Golgi pathway is a target for translocation and aggregation of mutant superoxide dismutase linked to ALS. FASEB J. 2008;22(7):2476–2487. doi: 10.1096/fj.07-092783. [DOI] [PubMed] [Google Scholar]
- 310.Manfredi G, Xu Z. Mitochondrial dysfunction and its role in motor neuron degeneration in ALS. Mitochondrion. 2005;5(2):77–87. doi: 10.1016/j.mito.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 311.Hirano A, Nakano I, Kurland LT, Mulder DW, Holley PW, Saccomanno G. Fine structural study of neurofibrillary changes in a family with amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 1984;43(5):471–480. doi: 10.1097/00005072-198409000-00002. [DOI] [PubMed] [Google Scholar]
- 312.Sasaki S, Iwata M. Ultrastructural study of the synapses of central chromatolytic anterior horn cells in motor neuron disease. J Neuropathol Exp Neurol. 1996;55(8):932–939. doi: 10.1097/00005072-199608000-00009. [DOI] [PubMed] [Google Scholar]
- 313.Dal Canto MC, Gurney ME. Neuropathological changes in two lines of mice carrying a transgene for mutant human Cu,Zn SOD, and in mice overexpressing wild type human SOD: a model of familial amyotrophic lateral sclerosis (FALS) Brain Res. 1995;676(1):25–40. doi: 10.1016/0006-8993(95)00063-v. [DOI] [PubMed] [Google Scholar]
- 314.Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14(6):1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- 315.Kong J, Xu Z. Massive mitochondrial degeneration in motor neurons triggers the onset of amyotrophic lateral sclerosis in mice expressing a mutant SOD1. J Neurosci. 1998;18(9):3241–3250. doi: 10.1523/JNEUROSCI.18-09-03241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Jaarsma D, Rognoni F, van Duijn W, Verspaget HW, Haasdijk ED, Holstege JC. CuZn superoxide dismutase (SOD1) accumulates in vacuolated mitochondria in transgenic mice expressing amyotrophic lateral sclerosis-linked SOD1 mutations. Acta Neuropathol. 2001;102(4):293–305. doi: 10.1007/s004010100399. [DOI] [PubMed] [Google Scholar]
- 317.Bendotti C, Calvaresi N, Chiveri L, Prelle A, Moggio M, Braga M, Silani V, De Biasi S. Early vacuolization and mitochondrial damage in motor neurons of FALS mice are not associated with apoptosis or with changes in cytochrome oxidase histochemical reactivity. J Neurol Sci. 2001;191(1–2):25–33. doi: 10.1016/s0022-510x(01)00627-x. [DOI] [PubMed] [Google Scholar]
- 318.Chiu AY, Zhai P, Dal Canto MC, Peters TM, Kwon YW, Prattis SM, Gurney ME. Age-dependent penetrance of disease in a transgenic mouse model of familial amyotrophic lateral sclerosis. Mol Cell Neurosci. 1995;6(4):349–362. doi: 10.1006/mcne.1995.1027. [DOI] [PubMed] [Google Scholar]
- 319.Mattiazzi M, D'Aurelio M, Gajewski CD, Martushova K, Kiaei M, Beal MF, Manfredi G. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J Biol Chem. 2002;277(33):29626–29633. doi: 10.1074/jbc.M203065200. [DOI] [PubMed] [Google Scholar]
- 320.Kirkinezos IG, Bacman SR, Hernandez D, Oca-Cossio J, Arias LJ, Perez-Pinzon MA, Bradley WG, Moraes CT. Cytochrome c association with the inner mitochondrial membrane is impaired in the CNS of G93A-SOD1 mice. J Neurosci. 2005;25(1):164–172. doi: 10.1523/JNEUROSCI.3829-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Sturtz LA, Diekert K, Jensen LT, Lill R, Culotta VC. A fraction of yeast Cu,Zn-superoxide dismutase and its metallochaperone, CCS, localize to the intermembrane space of mitochondria. A physiological role for SOD1 in guarding against mitochondrial oxidative damage. J Biol Chem. 2001;276(41):38084–38089. doi: 10.1074/jbc.M105296200. [DOI] [PubMed] [Google Scholar]
- 322.Higgins CM, Jung C, Ding H, Xu Z. Mutant Cu, Zn superoxide dismutase that causes motoneuron degeneration is present in mitochondria in the CNS. J Neurosci. 2002;22(6):RC215. doi: 10.1523/JNEUROSCI.22-06-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Liu J, Lillo C, Jonsson PA, Vande Velde C, Ward CM, Miller TM, Subramaniam JR, Rothstein JD, Marklund S, Andersen PM, Brännström T, Gredal O, Wong PC, Williams DS, Cleveland DW. Toxicity of familial ALS-linked SOD1 mutants from selective recruitment to spinal mitochondria. Neuron. 2004;43(1):5–17. doi: 10.1016/j.neuron.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 324.Vijayvergiya C, Beal MF, Buck J, Manfredi G. Mutant superoxide dismutase 1 forms aggregates in the brain mitochondrial matrix of amyotrophic lateral sclerosis mice. J Neurosci. 2005;25(10):2463–2470. doi: 10.1523/JNEUROSCI.4385-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Hart PJ. Pathogenic superoxide dismutase structure, folding, aggregation and turnover. Curr Opin Chem Biol. 2006;10(2):131–138. doi: 10.1016/j.cbpa.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 326.Vande Velde C, Miller TM, Cashman NR, Cleveland DW. Selective association of misfolded ALS-linked mutant SOD1 with the cytoplasmic face of mitochondria. Proc Natl Acad Sci U S A. 2008;105(10):4022–4027. doi: 10.1073/pnas.0712209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Deng HX, Shi Y, Furukawa Y, Zhai H, Fu R, Liu E, Gorrie GH, Khan MS, Hung WY, Bigio EH, Lukas T, Dal Canto MC, O'Halloran TV, Siddique T. Conversion to the amyotrophic lateral sclerosis phenotype is associated with intermolecular linked insoluble aggregates of SOD1 in mitochondria. Proc Natl Acad Sci U S A. 2007;103(18):7142–7147. doi: 10.1073/pnas.0602046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Field LS, Furukawa Y, O'Halloran TV, Culotta VC. Factors controlling the uptake of yeast copper/zinc superoxide dismutase into mitochondria. J Biol Chem. 2003;278(30):28052–28059. doi: 10.1074/jbc.M304296200. [DOI] [PubMed] [Google Scholar]
- 329.Ferri A, Cozzolino M, Crosio C, Nencini M, Casciati A, Gralla EB, Rotilio G, Valentine JS, Carrì MT. Familial ALS-superoxide dismutases associate with mitochondria and shift their redox potentials. Proc Natl Acad Sci U S A. 2006;103(37):13860–13865. doi: 10.1073/pnas.0605814103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Niwa J, Yamada S, Ishigaki S, Sone J, Takahashi M, Katsuno M, Tanaka F, Doyu M, Sobue G. Disulfide bond mediates aggregation, toxicity, and ubiquitylation of familial amyotrophic lateral sclerosis-linked mutant SOD1. J Biol Chem. 2007;282(38):28087–28095. doi: 10.1074/jbc.M704465200. [DOI] [PubMed] [Google Scholar]
- 331.Gruzman A, Wood WL, Alpert E, Prasad MD, Miller RG, Rothstein JD, Bowser R, Hamilton R, Wood TD, Cleveland DW, Lingappa VR, Liu J. Common molecular signature in SOD1 for both sporadic and familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2007;104(30):12524–12529. doi: 10.1073/pnas.0705044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.De Vos KJ, Chapman AL, Tennant ME, Manser C, Tudor EL, Lau KF, Brownlees J, Ackerley S, Shaw PJ, McLoughlin DM, Shaw CE, Leigh PN, Miller CC, Grierson AJ. Familial amyotrophic lateral sclerosis-linked SOD1 mutants perturb fast axonal transport to reduce axonal mitochondria content. Hum Mol Genet. 2007;16(22):2720–2728. doi: 10.1093/hmg/ddm226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Gonzalez de Aguilar JL, Dupuis L, Oudart H, Loeffler JP. The metabolic hypothesis in amyotrophic lateral sclerosis: insights from mutant Cu/Zn-superoxide dismutase mice. Biomed Pharmacother. 2005;59(4):190–196. doi: 10.1016/j.biopha.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 334.Siklós L, Engelhardt JI, Alexianu ME, Gurney ME, Siddique T, Appel SH. Intracellular calcium parallels motoneuron degeneration in SOD-1 mutant mice. J Neuropathol Exp Neurol. 1998;57(6):571–587. doi: 10.1097/00005072-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 335.Pasinelli P, Belford ME, Lennon N, Bacskai BJ, Hyman BT, Trotti D, Brown RH., Jr Amyotrophic lateral sclerosis-associated SOD1 mutant proteins bind and aggregate with Bcl-2 in spinal cord mitochondria. Neuron. 2004;43(1):19–30. doi: 10.1016/j.neuron.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 336.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118(Pt 23):5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Shan X, Chiang PM PM, Price DL DL, Wong PC. Altered distributions of Gemini of coiled bodies and mitochondria in motor neurons of TDP-43 transgenic mice. Proc Natl Acad Sci U S A. 2010;107(37):16325–16330. doi: 10.1073/pnas.1003459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Rouleau GA, Clark AW, Rooke K, Pramatarova A, Krizus A, Suchowersky O, Julien JP, Figlewicz D. SOD1 mutation is associated with accumulation of neurofilaments in amyotrophic lateral sclerosis. Ann Neurol. 1996;39(1):128–131. doi: 10.1002/ana.410390119. [DOI] [PubMed] [Google Scholar]
- 339.Cleveland DW. From Charcot to SOD1: mechanisms of selective motor neuron death in ALS. Neuron. 1999;24(3):515–520. doi: 10.1016/s0896-6273(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 340.Côté F, Collard JF, Julien JP. Progressive neuronopathy in transgenic mice expressing the human neurofilament heavy gene: a mouse model of amyotrophic lateral sclerosis. Cell. 1993;73(1):35–46. doi: 10.1016/0092-8674(93)90158-m. [DOI] [PubMed] [Google Scholar]
- 341.Zhang B, Tu P, Abtahian F, Trojanowski JQ, Lee VM. Neurofilaments and orthograde transport are reduced in ventral root axons of transgenic mice that express human SOD1 with a G93A mutation. J Cell Biol. 1997;139(5):1307–1315. doi: 10.1083/jcb.139.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Williamson TL, Cleveland DW. Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nat Neurosci. 1999;2(1):50–56. doi: 10.1038/4553. [DOI] [PubMed] [Google Scholar]
- 343.Kieran D, Hafezparast M, Bohnert S, Dick JR, Martin J, Schiavo G, Fisher EM, Greensmith L. A mutation in dynein rescues axonal transport defects and extends the life span of ALS mice. J Cell Biol. 2005;169(4):561–567. doi: 10.1083/jcb.200501085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Hafezparast M, Klocke R, Ruhrberg C, Marquardt A, Ahmad-Annuar A, Bowen S, Lalli G, Witherden AS, Hummerich H, Nicholson S, Morgan PJ, Oozageer R, Priestley JV, Averill S, King VR, Ball S, Peters J, Toda T, Yamamoto A, Hiraoka Y, Augustin M, Korthaus D, Wattler S, Wabnitz P, Dickneite C, Lampel S, Boehme F, Peraus G, Popp A, Rudelius M, Schlegel J, Fuchs H, Hrabe de Angelis M, Schiavo G, Shima DT, Russ AP, Stumm G, Martin JE, Fisher EM. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science. 2003;300(5620):808–812. doi: 10.1126/science.1083129. [DOI] [PubMed] [Google Scholar]
- 345.Puls I, Jonnakuty C, LaMonte BH, Holzbaur EL, Tokito M, Mann E, Floeter MK, Bidus K, Drayna D, Oh SJ, Brown RH, Jr, Ludlow CL, Fischbeck KH. Mutant dynactin in motor neuron disease. Nat Genet. 2003;33(4):455–456. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- 346.Münch C, Sedlmeier R, Meyer T, Homberg V, Sperfeld AD, Kurt A, Prudlo J, Peraus G, Hanemann CO, Stumm G, Ludolph AC. Point mutations of the p150 subunit of dynactin (DCTN1) gene in ALS. Neurology. 2004;63(4):724–726. doi: 10.1212/01.wnl.0000134608.83927.b1. [DOI] [PubMed] [Google Scholar]
- 347.LaMonte BH, Wallace KE, Holloway BA, Shelly SS, Ascaño J, Tokito M, Van Winkle T, Howland DS, Holzbaur EL. Disruption of dynein/dynactin inhibits axonal transport in motor neurons causing late-onset progressive degeneration. Neuron. 2002;34(5):715–727. doi: 10.1016/s0896-6273(02)00696-7. [DOI] [PubMed] [Google Scholar]
- 348.Chevalier-Larsen E, Holzbaur EL. Axonal transport and neurodegenerative disease. Biochim Biophys Acta. 2006;1762(11–12):1094–1108. doi: 10.1016/j.bbadis.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 349.Borchelt DR, Wong PC, Becher MW, Pardo CA, Lee MK, Xu ZS, Thinakaran G, Jenkins NA, Copeland NJ, Sisodia SS, Cleveland DW, Price DL, Hoffman PN. Axonal transport of mutant superoxide dismutase 1 and focal axonal abnormalities in the proximal axons of transgenic mice. Neurobiol Dis. 1998;5(1):27–35. doi: 10.1006/nbdi.1998.0178. [DOI] [PubMed] [Google Scholar]
- 350.Turner BJ, Atkin JD, Farg MA, Zang DW, Rembach A, Lopes EC, Patch JD, Hill AF, Cheema SS. Impaired extracellular secretion of mutant superoxide dismutase 1 associates with neurotoxicity in familial amyotrophic lateral sclerosis. J Neurosci. 2005;25(1):108–117. doi: 10.1523/JNEUROSCI.4253-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Ravikumar B, Acevedo-Arozena A, Imarisio S, Berger Z, Vacher C, O'Kane CJ, Brown SD, Rubinsztein DC. Dynein mutations impair autophagic clearance of aggregate-prone proteins. Nat Genet. 2005;37(7):771–776. doi: 10.1038/ng1591. [DOI] [PubMed] [Google Scholar]
- 352.Levy JR, Sumner CJ, Caviston JP, Tokito MK, Ranganathan S, Ligon LA, Wallace KE, LaMonte BH, Harmison GG, Puls I, Fischbeck KH, Holzbaur EL. A motor neuron disease-associated mutation in p150Glued perturbs dynactin function and induces protein aggregation. J Cell Biol. 2006;172(5):733–745. doi: 10.1083/jcb.200511068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.DiStefano PS, Friedman B, Radziejewski C, Alexander C, Boland P, Schick CM, Lindsay RM, Wiegand SJ. The neurotrophins BDNF, NT-3, and NGF display distinct patterns of retrograde axonal transport in peripheral and central neurons. Neuron. 1992;8(5):983–993. doi: 10.1016/0896-6273(92)90213-w. [DOI] [PubMed] [Google Scholar]
- 354.Tong JJ. Mitochondrial delivery is essential for synaptic potentiation. Biol Bull. 2007;212(2):169–175. doi: 10.2307/25066594. [DOI] [PubMed] [Google Scholar]
- 355.Rothstein JD, Martin LJ, Kuncl RW. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med. 1992;326(22):1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- 356.Lino MM, Schneider C, Caroni P. Accumulation of SOD1 mutants in postnatal motoneurons does not cause motoneuron pathology or motoneuron disease. J Neurosci. 2002;22(12):4825–4832. doi: 10.1523/JNEUROSCI.22-12-04825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Pramatarova A, Laganière J, Roussel J, Brisebois K, Rouleau GA. Neuron-specific expression of mutant superoxide dismutase 1 in transgenic mice does not lead to motor impairment. J Neurosci. 2001;21(10):3369–3374. doi: 10.1523/JNEUROSCI.21-10-03369.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Jaarsma JaarsmaD, Teuling E, Haasdijk ED, De Zeeuw CI, Hoogenraad CC. Neuron-specific expression of mutant superoxide dismutase is sufficient to induce amyotrophic lateral sclerosis in transgenic mice. J Neurosci. 2008;28(9):2075–2088. doi: 10.1523/JNEUROSCI.5258-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillée S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, Brown RH, Jr, Julien JP, Goldstein LS, Cleveland DW. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302(5642):113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- 360.Boillée S, Cleveland DW. Gene therapy for ALS delivers. Trends Neurosci. 2006a;27(5):235–238. doi: 10.1016/j.tins.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 361.Boillée S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006b;312(5778):1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 362.Beers DR, Henkel JS, Xiao Q, Zhao W, Wang J, Yen AA, Siklos L, McKercher SR, Appel SH. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2006;103(43):16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Yamanaka K, Chun SJ, Boillée S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11(3):251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10(5):608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10(5):615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Gong YH, Parsadanian AS, Andreeva A, Snider WD, Elliott JL. Restricted expression of G86R Cu/Zn superoxide dismutase in astrocytes results in astrocytosis but does not cause motoneuron degeneration. J Neurosci. 2002;20(2):660–665. doi: 10.1523/JNEUROSCI.20-02-00660.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Miller TM, Kim SH, Yamanaka K, Hester M, Umapathi P, Arnson H, Rizo L, Mendell JR, Gage FH, Cleveland DW, Kaspar BK. Gene transfer demonstrates that muscle is not a primary target for non-cell-autonomous toxicity in familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2006;103(51):19546–19551. doi: 10.1073/pnas.0609411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Zhong Z, Ilieva H, Hallagan L, Bell R, Singh I, Paquette N, Thiyagarajan M, Deane R, Fernandez JA, Lane S, Zlokovic AB, Liu T, Griffin JH, Chow N, Castellino FJ, Stojanovic K, Cleveland DW, Zlokovic BV. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J Clin Invest. 2009;119(11):3437–3449. doi: 10.1172/JCI38476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Wong M, Martin LJ. Skeletal muscle-restricted expression of human SOD1 causes motor neuron degeneration in transgenic mice. Hum Mol Genet. 2010;19(11):2284–2302. doi: 10.1093/hmg/ddq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Dupuis L, Gonzalez de Aguilar JL, Echaniz-Laguna A, Eschbach J, Rene F, Oudart H, Halter B, Huze C, Schaeffer L, Bouillaud F, Loeffler JP JP. Muscle mitochondrial uncoupling dismantles neuromuscular junction and triggers distal degeneration of motor neurons. PLoS One. 2009;4(4):5390. doi: 10.1371/journal.pone.0005390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Oosthuyse B, Moons L, Storkebaum E, Beck H, Nuyens D, Brusselmans K, Dorpe VanJ, Hellings P, Gorselink M, Heymans S, Theilmeier G, Dewerchin M, Laudenbach V, Vermylen P, Raat H, Acker T, Vleminckx V, Van Den Bosch L, Cashman N, Fujisawa H, Drost MR, Sciot R, Bruyninckx F, Hicklin DJ, Ince C, Gressens P, Lupu F, Plate KH, Robberecht W, Herbert JM, Collen D, Carmeliet P. Deletion of the hypoxia-response element in the vascular endothelial growth factor promoter causes motor neuron degeneration. Nat Genet. 2001;28(2):131–138. doi: 10.1038/88842. [DOI] [PubMed] [Google Scholar]
- 372.Kawamura Y, Dyck PJ, Shimono M, Okazaki H, Tateishi J, Doi H. Morphometric comparison of the vulnerability of peripheral motor and sensory neurons in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 1981;40(6):667–675. doi: 10.1097/00005072-198111000-00008. [DOI] [PubMed] [Google Scholar]
- 373.Mohajeri MH, Figlewicz DA, Bohn MC. Selective loss of alpha motoneurons innervating the medial gastrocnemius muscle in a mouse model of amyotrophic lateral sclerosis. Exp Neurol. 1998;150(2):329–336. doi: 10.1006/exnr.1998.6758. [DOI] [PubMed] [Google Scholar]
- 374.Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185(2):232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 375.Gould TW, Buss RR, Vinsant S, Prevette D, Sun W, Knudson CM, Milligan CE, Oppenheim RW. Complete dissociation of motor neuron death from motor dysfunction by Bax deletion in a mouse model of ALS. J Neurosci. 2006;26(34):8774–8786. doi: 10.1523/JNEUROSCI.2315-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Schaefer AM, Sanes JR, Lichtman JW. A compensatory subpopulation of motor neurons in a mouse model of amyotrophic lateral sclerosis. J Comp Neurol. 2005;490(3):209–219. doi: 10.1002/cne.20620. [DOI] [PubMed] [Google Scholar]
- 377.Hirano A. Neuropathology of ALS: an overview. Neurology. 1996;47(4 Suppl 2):S63–S66. doi: 10.1212/wnl.47.4_suppl_2.63s. [DOI] [PubMed] [Google Scholar]
- 378.Gonatas NK, Stieber A, Gonatas JO. Fragmentation of the Golgi apparatus in neurodegenerative diseases and cell death. J Neurol Sci. 2006;246(1–2):21–30. doi: 10.1016/j.jns.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 379.Sasaki S, Iwata M. Mitochondrial alterations in the spinal cord of patients with sporadic amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2007;66(1):10–16. doi: 10.1097/nen.0b013e31802c396b. [DOI] [PubMed] [Google Scholar]
- 380.Guégan C, Przedborski S. Programmed cell death in amyotrophic lateral sclerosis. J Clin Invest. 2003;111(2):153–161. doi: 10.1172/JCI17610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Migheli A, Atzori C, Piva R, Tortarolo M, Girelli M, Schiffer D, Bendotti C. Lack of apoptosis in mice with ALS. Nat Med. 1999;5(9):966–967. doi: 10.1038/12381. [DOI] [PubMed] [Google Scholar]
- 382.He BP, Strong MJ. Motor neuronal death in sporadic amyotrophic lateral sclerosis (ALS) is not apoptotic. A comparative study of ALS and chronic aluminium chloride neurotoxicity in New Zealand white rabbits. Neuropathol Appl Neurobiol. 2000;26(2):150–160. doi: 10.1046/j.1365-2990.2000.026002150.x. [DOI] [PubMed] [Google Scholar]
- 383.Tarabal O, Calderó J, Lladó J, Oppenheim RW, Esquerda JE. Long-lasting aberrant tubulovesicular membrane inclusions accumulate in developing motoneurons after a sublethal excitotoxic insult: a possible model for neuronal pathology in neurodegenerative disease. J Neurosci. 2001;21(20):8072–8081. doi: 10.1523/JNEUROSCI.21-20-08072.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Calderó J, Tarabal O, Casanovas A, Ciutat D, Casas C, Lladó J, Esquerda JE. Excitotoxic motoneuron disease in chick embryo evolves with autophagic neurodegeneration and deregulation of neuromuscular innervation. J Neurosci Res. 1997;85(12):2726–2740. doi: 10.1002/jnr.21174. [DOI] [PubMed] [Google Scholar]
- 385.Elliott JL. Cytokine upregulation in a murine model of familial amyotrophic lateral sclerosis. Brain Res Mol Brain Res. 2001;95(1–2):172–178. doi: 10.1016/s0169-328x(01)00242-x. [DOI] [PubMed] [Google Scholar]
- 386.Hensley K, Fedynyshyn J, Ferrell S, Floyd RA, Gordon B, Grammas P, Hamdheydari L, Mhatre M, Mou S, Pye QN, Stewart C, West M, West S, Williamson KS. Message and protein-level elevation of tumor necrosis factor alpha (TNF alpha) and TNF alpha-modulating cytokines in spinal cords of the G93A-SOD1 mouse model for amyotrophic lateral sclerosis. Neurobiol Dis. 2003;14(1):74–80. doi: 10.1016/s0969-9961(03)00087-1. [DOI] [PubMed] [Google Scholar]
- 387.Gowing G, Dequen F, Soucy G, Julien JP. Absence of tumor necrosis factor-alpha does not affect motor neuron disease caused by superoxide dismutase 1 mutations. J Neurosci. 2006;26(44):11397–11402. doi: 10.1523/JNEUROSCI.0602-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Raoul C, Buhler E, Sadeghi C, Jacquier A, Aebischer P, Pettmann B, Henderson CE, Haase G. Chronic activation in presymptomatic amyotrophic lateral sclerosis (ALS) mice of a feedback loop involving Fas, Daxx, and FasL. Proc Natl Acad Sci U S A. 2006;103(15):6007–6012. doi: 10.1073/pnas.0508774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Locatelli F, Corti S, Papadimitriou D, Fortunato F, Del Bo R, Donadoni C, Nizzardo M, Nardini M, Salani S, Ghezzi S, Strazzer S, Bresolin N, Comi GP. Fas small interfering RNA reduces motoneuron death in amyotrophic lateral sclerosis mice. Ann Neurol. 2007;62(1):81–92. doi: 10.1002/ana.21152. [DOI] [PubMed] [Google Scholar]
- 390.Tortarolo M, Veglianese P, Calvaresi N, Botturi A, Rossi C, Giorgini A, Migheli A, Bendotti C. Persistent activation of p38 mitogen-activated protein kinase in a mouse model of familial amyotrophic lateral sclerosis correlates with disease progression. Mol Cell Neurosci. 2003;23(2):180–192. doi: 10.1016/s1044-7431(03)00022-8. [DOI] [PubMed] [Google Scholar]
- 391.Dewil M, dela Cruz VF, Van Den Bosch L, Robberecht W. Inhibition of p38 mitogen activated protein kinase activation and mutant SOD1(G93A)-induced motor neuron death. Neurobiol Dis. 2007;26(2):332–341. doi: 10.1016/j.nbd.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 392.Son M, Fathallah-Shaykh HM, Elliott JL. Survival in a transgenic model of FALS is independent of iNOS expression. Ann Neurol. 2001;50(2):273. doi: 10.1002/ana.1104. [DOI] [PubMed] [Google Scholar]
- 393.Hara MR, Snyder SH. Cell signaling and neuronal death. Annu Rev Pharmacol Toxicol. 2007;47:117–141. doi: 10.1146/annurev.pharmtox.47.120505.105311. [DOI] [PubMed] [Google Scholar]
- 394.Prudlo J, Koenig J, Gräser J, Burckhardt E, Mestres P, Menger M, Roemer K. Motor neuron cell death in a mouse model of FALS is not mediated by the p53 cell survival regulator. Brain Res. 2000;879(1–2):183–187. doi: 10.1016/s0006-8993(00)02745-1. [DOI] [PubMed] [Google Scholar]
- 395.Kuntz C, 4th, Kinoshita Y, Beal MF, Donehower LA, Morrison RS. Absence of p53: no effect in a transgenic mouse model of familial amyotrophic lateral sclerosis. Exp Neurol. 2000;165(1):184–190. doi: 10.1006/exnr.2000.7464. [DOI] [PubMed] [Google Scholar]
- 396.Vukosavic S, Dubois-Dauphin M, Romero N, Przedborski S. Bax and Bcl-2 interaction in a transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem. 1999;73(6):2460–2468. doi: 10.1046/j.1471-4159.1999.0732460.x. [DOI] [PubMed] [Google Scholar]
- 397.Guégan C, Vila M, Rosoklija G, Hays AP, Przedborski S. Recruitment of the mitochondrial-dependent apoptotic pathway in amyotrophic lateral sclerosis. J Neurosci. 2001;21(17):6569–6576. doi: 10.1523/JNEUROSCI.21-17-06569.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Pasinelli P, Houseweart MK, Brown RH, Jr, Cleveland DW. Caspase-1 and -3 are sequentially activated in motor neuron death in Cu, Zn superoxide dismutase-mediated familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2000;97(25):13901–13906. doi: 10.1073/pnas.240305897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Kostic V, Jackson-Lewis V, de Bilbao F, Dubois-Dauphin M, Przedborski S. Bcl-2: prolonging life in a transgenic mouse model of familial amyotrophic lateral sclerosis. Science. 1997;277(5325):559–562. doi: 10.1126/science.277.5325.559. [DOI] [PubMed] [Google Scholar]
- 400.Nguyen MD, Julien JP, Rivest S. Induction of proinflammatory molecules in mice with amyotrophic lateral sclerosis: no requirement for proapoptotic interleukin-1beta in neurodegeneration. Ann Neurol. 2001;50(5):630–639. doi: 10.1002/ana.1256. [DOI] [PubMed] [Google Scholar]
- 401.Kang SJ, Sanchez I, Jing N, Yuan J. Dissociation between neurodegeneration and caspase-11-mediated activation of caspase-1 and caspase-3 in a mouse model of amyotrophic lateral sclerosis. J Neurosci. 2003;23(13):5455–5460. doi: 10.1523/JNEUROSCI.23-13-05455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Li M, Ona VO, Guégan C, Chen M, Jackson-Lewis V, Andrews LJ, Olszewski AJ, Stieg PE, Lee JP, Przedborski S, Friedlander RM. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science. 1999;288(5464):335–339. doi: 10.1126/science.288.5464.335. [DOI] [PubMed] [Google Scholar]
- 403.Pasinelli P, Belford ME, Lennon N, Bacskai BJ, Hyman BT, Trotti D, Brown RH., Jr Amyotrophic lateral sclerosis-associated SOD1 mutant proteins bind and aggregate with Bcl-2 in spinal cord mitochondria. Neuron. 2004;43(1):19–30. doi: 10.1016/j.neuron.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 404.Shimizu S, Eguchi Y, Kosaka H, Kamiike W, Matsuda H, Tsujimoto Y. Prevention of hypoxia-induced cell death by Bcl-2 and Bcl-xL. Nature. 1995;374(6525):811–813. doi: 10.1038/374811a0. [DOI] [PubMed] [Google Scholar]
- 405.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122(6):927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 406.Chen DF, Schneider GE, Martinou JC, Tonegawa S. Bcl-2 promotes regeneration of severed axons in mammalian CNS. Nature. 1997;385(6615):434–439. doi: 10.1038/385434a0. [DOI] [PubMed] [Google Scholar]
- 407.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397(6718):441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- 408.Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443(7113):780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- 409.Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima M. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441(7095):885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 410.Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441(7095):880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 411.Hetz C, Thielen P, Matus S, Nassif M, Court F, Kiffin R, Martinez G, Cuervo AM, Brown RH, Glimcher LH. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 2009;23(19):2294–2306. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Tarabal O, Calderó J, Casas C, Oppenheim RW, Esquerda JE. Protein retention in the endoplasmic reticulum, blockade of programmed cell death and autophagy selectively occur in spinal cord motoneurons after glutamate receptor-mediated injury. Mol Cell Neurosci. 2005;29(2):283–298. doi: 10.1016/j.mcn.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 413.Hall ED, Oostveen JA, Gurney ME. Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia. 1998;23(3):249–256. doi: 10.1002/(sici)1098-1136(199807)23:3<249::aid-glia7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 414.Le W, Rowe D, Xie W, Ortiz I, He Y, Appel SH. Microglial activation and dopaminergic cell injury: an in vitro model relevant to Parkinson's disease. J Neurosci. 2001;21(21):8447–8455. doi: 10.1523/JNEUROSCI.21-21-08447.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Barger SW, Basile AS. Activation of microglia by secreted amyloid precursor protein evokes release of glutamate by cystine exchange and attenuates synaptic function. J Neurochem. 2001;76(3):846–854. doi: 10.1046/j.1471-4159.2001.00075.x. [DOI] [PubMed] [Google Scholar]
- 416.Kriz J, Nguyen MD, Julien JP. Minocycline slows disease progression in a mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2002;10(3):268–278. doi: 10.1006/nbdi.2002.0487. [DOI] [PubMed] [Google Scholar]
- 417.Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu DC, Gullans S, Ferrante RJ, Przedborski S, Kristal BS BS, Friedlander RM. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417(6884):74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- 418.Wu DC, Ré DB, Nagai M, Ischiropoulos H, Przedborski S. The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice. Proc Natl Acad Sci U S A. 2006;103(32):12132–12137. doi: 10.1073/pnas.0603670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Rothstein JD, Van Kammen M, Levey AI, Martin LJ, Kuncl RW. Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann Neurol. 1995;38(1):73–84. doi: 10.1002/ana.410380114. [DOI] [PubMed] [Google Scholar]
- 420.Trotti D, Rolfs A, Danbolt NC, Brown RH, Jr, Hediger MA. SOD1 mutants linked to amyotrophic lateral sclerosis selectively inactivate a glial glutamate transporter. Nat Neurosci. 1999;2(5):427–433. doi: 10.1038/8091. [DOI] [PubMed] [Google Scholar]
- 421.Zhang J, Dawson VL, Dawson TM, Snyder SH. Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science. 1994;263(5147):687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]
- 422.Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297(5584):1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 423.Lam M, Dubyak G, Chen L, Nuñez G, Miesfeld RL, Distelhorst CW. Evidence that BCL-2 represses apoptosis by regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc Natl Acad Sci U S A. 1994;91(14):6569–6573. doi: 10.1073/pnas.91.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Sarkar S, Floto RA, Berger Z, Imarisio S, Cordenier A, Pasco M, Cook LJ, Rubinsztein DC. Lithium induces autophagy by inhibiting inositol monophosphatase. J Cell Biol. 2005;170(7):1101–1111. doi: 10.1083/jcb.200504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Criollo A, Maiuri MC, Tasdemir E, Vitale I, Fiebig AA, Andrews D, Molgó J, Díaz J, Lavandero S, Harper F, Pierron G, di Stefano D, Rizzuto R, Szabadkai G, Kroemer G. Regulation of autophagy by the inositol trisphosphate receptor. Cell Death Differ. 2007;14(5):1029–1039. doi: 10.1038/sj.cdd.4402099. [DOI] [PubMed] [Google Scholar]
- 426.Decaudin D, Geley S, Hirsch T, Castedo M, Marchetti P, Macho A, Kofler R, Kroemer G. Bcl-2 and Bcl-XL antagonize the mitochondrial dysfunction preceding nuclear apoptosis induced by chemotherapeutic agents. Cancer Res. 1997;57(1):62–67. [PubMed] [Google Scholar]
- 427.Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3(11):E255–E263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- 428.Kieran D, Woods I, Villunger A, Strasser A, Prehn JH. Deletion of the BH3-only protein puma protects motoneurons from ER stress-induced apoptosis and delays motoneuron loss in ALS mice. Proc Natl Acad Sci U S A. 2007;104(51):20606–20611. doi: 10.1073/pnas.0707906105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Patil C, Walter P. Intracellular signaling from the endoplasmic reticulum to the nucleus: the unfolded protein response in yeast and mammals. Curr Opin Cell Biol. 2001;13(3):49–55. doi: 10.1016/s0955-0674(00)00219-2. [DOI] [PubMed] [Google Scholar]
- 430.Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci. 2000;20(7):2534–2542. doi: 10.1523/JNEUROSCI.20-07-02534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Gould TW, Oppenheim RW. Synaptic dysfunction in disease and following injury in the developing and adult nervous system: caveats in the choice of therapeutic intervention. Neurosci Biobehav Rev. 2007;31(8):1073–1087. doi: 10.1016/j.neubiorev.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 432.Petri S, Kiaei M, Wille E, Calingasan NY, Beal MF. Loss of Fas ligand-function improves survival in G93A-transgenic ALS mice. J Neurol Sci. 2006;251(1–2):44–49. doi: 10.1016/j.jns.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 433.Hetz C, Thielen P, Fisher J, Pasinelli P, Brown RH, Korsmeyer S, Glimcher L. The proapoptotic BCL-2 family member BIM mediates motoneuron loss in a model of amyotrophic lateral sclerosis. Cell Death Differ. 2007;14(7):1386–1389. doi: 10.1038/sj.cdd.4402166. [DOI] [PubMed] [Google Scholar]
- 434.Inoue T, Hosokawa M, Morigiwa K, Ohashi Y, Fukuda Y. Bcl-2 overexpression does not enhance in vivo axonal regeneration of retinal ganglion cells after peripheral nerve transplantation in adult mice. J Neurosci. 2002;22(11):4468–4477. doi: 10.1523/JNEUROSCI.22-11-04468.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Sagot Y, Dubois-Dauphin M, Tan SA, de Bilbao F, Aebischer P, Martinou JC, Kato AC. Bcl-2 overexpression prevents motoneuron cell body loss but not axonal degeneration in a mouse model of a neurodegenerative disease. J Neurosci. 1995;15(11):7727–7733. doi: 10.1523/JNEUROSCI.15-11-07727.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Suzuki M, McHugh J, Tork C, Shelley B, Klein SM, Aebischer P, Svendsen CN. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS ONE. 2007;2(1):e689. doi: 10.1371/journal.pone.0000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Ho TW, Bristol LA, Coccia C, Li Y, Milbrandt J, Johnson E, Jin L, Bar-Peled O, Griffin JW, Rothstein JD. TGFbeta trophic factors differentially modulate motor axon outgrowth and protection from excitotoxicity. Exp Neurol. 2000;161(2):664–675. doi: 10.1006/exnr.1999.7290. [DOI] [PubMed] [Google Scholar]
- 438.Brunet N, Tarabal O, Portero-Otín M, Oppenheim RW, Esquerda JE, Calderó J. Survival and death of mature avian motoneurons in organotypic slice culture: trophic requirements for survival and different types of degeneration. J Comp Neurol. 2007;501(5):669–690. doi: 10.1002/cne.21157. [DOI] [PubMed] [Google Scholar]
- 439.Li X, Peng C, Li L, Ming M, Yang D, Le W. Glial cell-derived neurotrophic factor protects against proteasome inhibition-induced dopamine neuron degeneration by suppression of endoplasmic reticulum stress and caspase-3 activation. J Gerontol A Biol Sci Med Sci. 2007;62(9):943–950. doi: 10.1093/gerona/62.9.943. [DOI] [PubMed] [Google Scholar]
- 440.Araujo DM, Hilt DC. Glial cell line-derived neurotrophic factor attenuates the locomotor hypofunction and striatonigral neurochemical deficits induced by chronic systemic administration of the mitochondrial toxin 3-nitropropionic acid. Neuroscience. 1998;82(1):117–127. doi: 10.1016/s0306-4522(97)00266-2. [DOI] [PubMed] [Google Scholar]
- 441.Kowsky S, Pöppelmeyer C, Kramer ER, Falkenburger BH, Kruse A, Klein R, Schulz JB. RET signaling does not modulate MPTP toxicity but is required for regeneration of dopaminergic axon terminals. Proc Natl Acad Sci U S A. 2007;104(50):20049–20054. doi: 10.1073/pnas.0706177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Baudet C, Pozas E, Adameyko I, Andersson E, Ericson J, Ernfors P. Retrograde signaling onto Ret during motor nerve terminal maturation. J Neurosci. 2008;28(4):963–975. doi: 10.1523/JNEUROSCI.4489-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Sagot Y, Rossé T, Vejsada R, Perrelet D, Kato AC. Differential effects of neurotrophic factors on motoneuron retrograde labeling in a murine model of motoneuron disease. J Neurosci. 1998;18(3):1132–1141. doi: 10.1523/JNEUROSCI.18-03-01132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Naveilhan P, ElShamy WM, Ernfors P. Differential regulation of mRNAs for GDNF and its receptors Ret and GDNFR alpha after sciatic nerve lesion in the mouse. Eur J Neurosci. 1997;9(7):1450–1460. doi: 10.1111/j.1460-9568.1997.tb01499.x. [DOI] [PubMed] [Google Scholar]
- 445.Höke A, Ho T, Crawford TO, LeBel C, Hilt D, Griffin JW. Glial cell line-derived neurotrophic factor alters axon schwann cell units and promotes myelination in unmyelinated nerve fibers. J Neurosci. 2003;23(2):561–567. doi: 10.1523/JNEUROSCI.23-02-00561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Bohn MC. Motoneurons crave glial cell line-derived neurotrophic factor. Exp Neurol. 2005;190(2):263–267. doi: 10.1016/j.expneurol.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 447.Choi-Lundberg DL, Lin Q, Chang YN, Chiang YL, Hay CM, Mohajeri H, Davidson BL, Bohn MC. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science. 1997;275(5301):838–841. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- 448.Connor B, Kozlowski DA, Unnerstall JD, Elsworth JD, Tillerson JL, Schallert T, Bohn MC. Glial cell line-derived neurotrophic factor (GDNF) gene delivery protects dopaminergic terminals from degeneration. Exp Neurol. 2001;169(1):83–95. doi: 10.1006/exnr.2001.7638. [DOI] [PubMed] [Google Scholar]
- 449.Fink DJ, Glorioso J, Mata M. Therapeutic gene transfer with herpes-based vectors: studies in Parkinson's disease and motor nerve regeneration. Exp Neurol. 2004;184 Suppl 1:S19–S24. doi: 10.1016/s0014-4886(03)00358-3. [DOI] [PubMed] [Google Scholar]
- 450.Sagot Y, Tan SA, Hammang JP, Aebischer P, Kato AC. GDNF slows loss of motoneurons but not axonal degeneration or premature death of pmn/pmn mice. J Neurosci. 1996;16(7):2335–2344. doi: 10.1523/JNEUROSCI.16-07-02335.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 451.Acsadi G, Anguelov RA, Yang H, Toth G, Thomas R, Jani A, Wang Y, Ianakova E, Mohammad S, Lewis RA, Shy ME. Increased survival and function of SOD1 mice after glial cell-derived neurotrophic factor gene therapy. Hum Gene Ther. 2002;13(9):1047–1059. doi: 10.1089/104303402753812458. [DOI] [PubMed] [Google Scholar]
- 452.Wang LJ, Lu YY, Muramatsu S, Ikeguchi K, Fujimoto KK, Okada T, Mizukami TH, Matsushita T, Hanazono Y, Kume A, Nagatsu T, Ozawa K, Nakano I. I: Neuroprotective effects of glial cell line-derived neurotrophic factor mediated by an adeno-associated virus vector in a transgenic animal model of amyotrophic lateral sclerosis. J Neurosci. 2002;22(16):6920–6928. doi: 10.1523/JNEUROSCI.22-16-06920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Kaspar BK, Lladó J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2005;301(5634):839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- 454.Mohajeri MH, Figlewicz DA, Bohn MC. Intramuscular grafts of myoblasts genetically modified to secrete glial cell line-derived neurotrophic factor prevent motoneuron loss and disease progression in a mouse model of familial amyotrophic lateral sclerosis. Hum Gene Ther. 1999;10(11):1853–1866. doi: 10.1089/10430349950017536. [DOI] [PubMed] [Google Scholar]
- 455.Li W, Brakefield D, Pan Y, Hunter D, Myckatyn TM, Parsadanian A. Muscle-derived but not centrally derived transgene GDNF is neuroprotective in G93A-SOD1 mouse model of ALS. Exp Neurol. 2007;203(2):457–471. doi: 10.1016/j.expneurol.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 456.Suzuki M, McHugh J, Tork C, Shelley B, Hayes A, Bellantuono I, Aebischer P, Svendsen CN. Direct muscle delivery of GDNF with human mesenchymal stem cells improves motor neuron survival and function in a rat model of familial ALS. Mol Ther. 2008;16(12):2002–2010. doi: 10.1038/mt.2008.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Grundström E, Askmark H, Lindeberg J, Nygren I, Ebendal T, Aquilonius SM. Increased expression of glial cell line-derived neurotrophic factor mRNA in muscle biopsies from patients with amyotrophic lateral sclerosis. J Neurol Sci. 1999;162(2):169–173. doi: 10.1016/s0022-510x(98)00333-5. [DOI] [PubMed] [Google Scholar]
- 458.Yamamoto M, Li M, Mitsuma N, Ito S, Kato M, Takahashi M, Sobue G. Preserved phosphorylation of RET receptor protein in spinal motor neurons of patients with amyotrophic lateral sclerosis: an immunohistochemical study by a phosphorylation-specific antibody at tyrosine 1062. Brain Res. 2001;912(1):89–94. doi: 10.1016/s0006-8993(01)02542-2. [DOI] [PubMed] [Google Scholar]
- 459.Barnett MW, Fisher CE, Perona-Wright G, J.A Davies. Signalling by glial cell line-derived neurotrophic factor (GDNF) requires heparan sulphate glycosaminoglycan. J Cell Sci. 2002;115(Pt 23):4495–4503. doi: 10.1242/jcs.00114. [DOI] [PubMed] [Google Scholar]
- 460.Nabeshima T, Yamada K. Neurotrophic factor strategies for the treatment of Alzheimer disease. Alzheimer Dis Assoc Disord. 2000;14 Suppl 1:S39–S46. doi: 10.1097/00002093-200000001-00007. [DOI] [PubMed] [Google Scholar]
- 461.Sherer TB, Fiske BK, Svendsen CN, Lang AE, Langston JW. Crossroads in GDNF therapy for Parkinson's disease. Mov Disord. 2006;21(2):136–141. doi: 10.1002/mds.20861. [DOI] [PubMed] [Google Scholar]
- 462.Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, Brodsky MA, Burchiel K, Kelly P, Dalvi A, Scott B, Stacy M, Turner D, Wooten VG, Elias WJ, Laws ER, Dhawan V, Stoessl AJ, Matcham J, Coffey RJ, Traub M. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2006;59(3):459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- 463.Salvatore MF, Ai Y, Fischer B, Zhang AM, Grondin RC, Zhang Z Z, Gerhardt GA, Gash DM. Point source concentration of GDNF may explain failure of phase II clinical trial. Exp Neurol. 2006;202(2):497–505. doi: 10.1016/j.expneurol.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 464.Lindvall O, Wahlberg LU. Encapsulated cell biodelivery of GDNF: a novel clinical strategy for neuroprotection and neuroregeneration in Parkinson's disease? Exp Neurol. 2008;209:82–88. doi: 10.1016/j.expneurol.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 465.Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181(2):115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- 466.Guillot S, Azzouz M, Déglon N, Zurn A, Aebischer P. Local GDNF expression mediated by lentiviral vector protects facial nerve motoneurons but not spinal motoneurons in SOD1(G93A) transgenic mice. Neurobiol Dis. 2004;16(1):139–149. doi: 10.1016/j.nbd.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 467.Visanji NP, Orsi A, Johnston TH, Howson PA, Dixon K, Callizot N, Brotchie JM, Rees DD. PYM50028, a novel, orally active, nonpeptide neurotrophic factor inducer, prevents and reverses neuronal damage induced by MPP+ in mesencephalic neurons and by MPTP in a mouse model of Parkinson's disease. FASEB J. 2008;22(7):2488–2489. doi: 10.1096/fj.07-095398. [DOI] [PubMed] [Google Scholar]
- 468.Boado RJ, Zhang Y, Wang Y, Pardridge WM. GDNF fusion protein for targeted-drug delivery across the human blood-brain barrier. Biotechnol Bioeng. 2007;100(2):387–396. doi: 10.1002/bit.21764. [DOI] [PubMed] [Google Scholar]
- 469.Kilic U, Kilic E, Dietz GP, Bähr M. The TAT protein transduction domain enhances the neuroprotective effect of glial-cell-line-derived neurotrophic factor after optic nerve transection. Neurodegener Dis. 2004;1(1):44–49. doi: 10.1159/000076669. [DOI] [PubMed] [Google Scholar]
- 470.Larsen KE, Benn SC, Ay I, Chian RJ, Celia SA, Remington MP, Bejarano M, Liu M, Ross J, Carmillo P, Sah D, Phillips KA, Sulzer D, Pepinsky RB, Fishman PS, Brown RH, Jr, Francis JW. A glial cell line-derived neurotrophic factor (GDNF):tetanus toxin fragment C protein conjugate improves delivery of GDNF to spinal cord motor neurons in mice. Brain Res. 2006;1120(1):1–12. doi: 10.1016/j.brainres.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 471.Alberch J, Pérez-Navarro E, Canals JM. Neuroprotection by neurotrophins and GDNF family members in the excitotoxic model of Huntington's disease. Brain Res Bull. 2002;57(6):817–822. doi: 10.1016/s0361-9230(01)00775-4. [DOI] [PubMed] [Google Scholar]
- 472.Torres-Peraza J, Pezzi S, Canals JM, Gavaldà N, García-Martínez JM, Pérez-Navarro E, Alberch J. Mice heterozygous for neurotrophin-3 display enhanced vulnerability to excitotoxicity in the striatum through increased expression of N-methyl-D-aspartate receptors. Neuroscience. 2007;144(2):462–471. doi: 10.1016/j.neuroscience.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 473.Kells AP, Henry RA, Connor B. AAV-BDNF mediated attenuation of quinolinic acid-induced neuropathology and motor function impairment. Gene Ther. 15(13):966–977. doi: 10.1038/gt.2008.23. [DOI] [PubMed] [Google Scholar]
- 474.Corse AM, Bilak MM, Bilak SR, Lehar M, Rothstein JD, Kuncl RW. Preclinical testing of neuroprotective neurotrophic factors in a model of chronic motor neuron degeneration. Neurobiol Dis. 1999;6(5):335–346. doi: 10.1006/nbdi.1999.0253. [DOI] [PubMed] [Google Scholar]
- 475.Fryer HJ, Wolf DH, Knox RJ, Strittmatter SM, Pennica D, O'Leary RM, Russell DS, Kalb RG. Brain-derived neurotrophic factor induces excitotoxic sensitivity in cultured embryonic rat spinal motor neurons through activation of the phosphatidylinositol 3-kinase pathway. J Neurochem. 2000;74(2):582–595. doi: 10.1046/j.1471-4159.2000.740582.x. [DOI] [PubMed] [Google Scholar]
- 476.Kim SH, Won SJ, Sohn S, Kwon HJ, Lee JY, Park JH, Gwag BJ. Brain-derived neurotrophic factor can act as a pronecrotic factor through transcriptional and translational activation of NADPH oxidase. J Cell Biol. 2002;159(5):821–831. doi: 10.1083/jcb.200112131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Kim HJ, Hwang JJ, Behrens MM, Snider BJ, Choi DW, Koh JY. TrkB mediates BDNF-induced potentiation of neuronal necrosis in cortical culture. Neurobiol Dis. 2003;14(1):110–119. doi: 10.1016/s0969-9961(03)00103-7. [DOI] [PubMed] [Google Scholar]
- 478.Markham A, Cameron I, Franklin P, Spedding M. BDNF increases rat brain mitochondrial respiratory coupling at complex I, but not complex II. Eur J Neurosci. 2004;20(5):1189–1196. doi: 10.1111/j.1460-9568.2004.03578.x. [DOI] [PubMed] [Google Scholar]
- 479.Huang TJ, Sayers NM, Verkhratsky A, Fernyhough P. Neurotrophin-3 prevents mitochondrial dysfunction in sensory neurons of streptozotocin-diabetic rats. Exp Neurol. 2005;194(1):279–283. doi: 10.1016/j.expneurol.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 480.Meier C, Parmantier E, Brennan A, Mirsky R, Jessen KR. Developing Schwann cells acquire the ability to survive without axons by establishing an autocrine circuit involving insulin-like growth factor, neurotrophin-3, and platelet-derived growth factor-BB. J Neurosci. 1999;19(10):3847–3859. doi: 10.1523/JNEUROSCI.19-10-03847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Ikeda K, Klinkosz B, Greene T, Cedarbaum JM, Wong V, Lindsay RM, Mitsumoto H. Effects of brain-derived neurotrophic factor on motor dysfunction in wobbler mouse motor neuron disease. Ann Neurol. 1995;37(4):505–511. doi: 10.1002/ana.410370413. [DOI] [PubMed] [Google Scholar]
- 482.Haase G, Kennel P, Pettmann B, Vigne E, Akli S, Revah F, Schmalbruch H, Kahn A. Gene therapy of murine motor neuron disease using adenoviral vectors for neurotrophic factors. Nat Med. 1997;3(4):429–436. doi: 10.1038/nm0497-429. [DOI] [PubMed] [Google Scholar]
- 483.Küst BM, Brouwer N, Mantingh IJ, Boddeke HW, Copray JC. Reduced p75NTR expression delays disease onset only in female mice of a transgenic model of familial amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4(2):100–105. [PubMed] [Google Scholar]
- 484.Gurney ME. The use of transgenic mouse models of amyotrophic lateral sclerosis in preclinical drug studies. J Neurol Sci. 1997;152 Suppl 1:S67–S73. doi: 10.1016/s0022-510x(97)00247-5. [DOI] [PubMed] [Google Scholar]
- 485.BDNF Study Group. A controlled trial of recombinant methionyl human BDNF in ALS: The BDNF Study Group (Phase III) Neurology. 1999;52(7):1427–1433. doi: 10.1212/wnl.52.7.1427. [DOI] [PubMed] [Google Scholar]
- 486.Pan Y, Anthony M, Clarkson TB. Evidence for up-regulation of brain-derived neurotrophic factor mRNA by soy phytoestrogens in the frontal cortex of retired breeder female rats. Neurosci Lett. 1999;261(1–2):17–20. doi: 10.1016/s0304-3940(98)00994-x. [DOI] [PubMed] [Google Scholar]
- 487.Dittrich F, Ochs G, Grosse-Wilde A, Berweiler U, Yan Q, Miller JA, Toyka KV, Sendtner M. Pharmacokinetics of intrathecally applied BDNF and effects on spinal motoneurons. Exp Neurol. 1996;141(2):225–239. doi: 10.1006/exnr.1996.0157. [DOI] [PubMed] [Google Scholar]
- 488.Ochs G, Penn RD, York M, Giess R, Beck M, Tonn J, Haigh J, Malta E, Traub M, Sendtner M, Toyka KV. A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(3):201–206. doi: 10.1080/14660820050515197. [DOI] [PubMed] [Google Scholar]
- 489.Beck M, Flachenecker P, Magnus T, Giess R, Reiners K, Toyka KV, Naumann M. Autonomic dysfunction in ALS: a preliminary study on the effects of intrathecal BDNF. Amyotroph Lateral Scler Other Motor Neuron Disord. 2005;6(2):100–103. doi: 10.1080/14660820510028412. [DOI] [PubMed] [Google Scholar]
- 490.Wiese S, Jablonka S, Holtmann B, Orel N, Rajagopal R, Chao MV, Sendtner M. Adenosine receptor A2A-R contributes to motoneuron survival by transactivating the tyrosine kinase receptor TrkB. Proc Natl Acad Sci U S A. 2007;104(43):17210–17215. doi: 10.1073/pnas.0705267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Escartin C, Brouillet E, Gubellini P, Trioulier Y, Jacquard C, Smadja C, Knott GW, Kerkerian-Le Goff L, Déglon N, Hantraye P, Bonvento G. Ciliary neurotrophic factor activates astrocytes, redistributes their glutamate transporters GLAST and GLT-1 to raft microdomains, and improves glutamate handling in vivo. J Neurosci. 2006;26(22):5978–5989. doi: 10.1523/JNEUROSCI.0302-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Escartin C, Pierre K, Colin A, Brouillet E, Delzescaux T, Guillermier M, Dhenain M, Déglon N, Hantraye P, Pellerin L, Bonvento G. Activation of astrocytes by CNTF induces metabolic plasticity and increases resistance to metabolic insults. J Neurosci. 2007;27(27):7094–7104. doi: 10.1523/JNEUROSCI.0174-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.Kaur N, Lu B, Monroe RK, Ward SM, Halvorsen SW. Inducers of oxidative stress block ciliary neurotrophic factor activation of Jak/STAT signaling in neurons. J Neurochem. 2005;92(6):1521–1530. doi: 10.1111/j.1471-4159.2004.02990.x. [DOI] [PubMed] [Google Scholar]
- 494.Anand P, Parrett A, Martin J, Zeman S, Foley P, Swash M, Leigh PN, Cedarbaum JM, Lindsay RM, Williams-Chestnut RE, et al. Regional changes of ciliary neurotrophic factor and nerve growth factor levels in post mortem spinal cord and cerebral cortex from patients with motor disease. Nat Med. 1995;1(2):168–172. doi: 10.1038/nm0295-168. [DOI] [PubMed] [Google Scholar]
- 495.Duberley RM, Johnson IP, Anand P, Swash M, Martin J, Leigh PN, Zeman S. Ciliary neurotrophic factor receptor expression in spinal cord and motor cortex in amyotrophic lateral sclerosis. J Neurol Sci. 1995;129 Suppl:109–113. doi: 10.1016/0022-510x(95)00079-h. [DOI] [PubMed] [Google Scholar]
- 496.Laaksovirta H, Soinila S, Hukkanen V, Röyttä M, Soilu-Hänninen M. Serum level of CNTF is elevated in patients with amyotrophic lateral sclerosis and correlates with site of disease onset. Eur J Neurol. 15(4):355–359. doi: 10.1111/j.1468-1331.2008.02080.x. [DOI] [PubMed] [Google Scholar]
- 497.Friedman B, Scherer SS, Rudge JS, Helgren M, Morrisey D, McClain J, Wang DY, Wiegand SJ, Furth ME, Lindsay RM, et al. Regulation of ciliary neurotrophic factor expression in myelin-related Schwann cells in vivo. Neuron. 1992;9(2):295–305. doi: 10.1016/0896-6273(92)90168-d. [DOI] [PubMed] [Google Scholar]
- 498.Mata M, Jin CF, Fink DJ. Axotomy increases CNTF receptor mRNA in rat spinal cord. Brain Res. 1993;610(1):162–165. doi: 10.1016/0006-8993(93)91231-g. [DOI] [PubMed] [Google Scholar]
- 499.Ip NY, McClain J, Barrezueta NX, Aldrich TH, Pan L, Li Y, Wiegand SJ, Friedman B, Davis S, Yancopoulos GD. The alpha component of the CNTF receptor is required for signaling and defines potential CNTF targets in the adult and during development. Neuron. 1993;10(1):89–102. doi: 10.1016/0896-6273(93)90245-m. [DOI] [PubMed] [Google Scholar]
- 500.Gatzinsky KP, Holtmann B, Daraie B, Berthold CH, Sendtner M. Early onset of degenerative changes at nodes of Ranvier in alpha-motor axons of cntf null (−/−) mutant mice. Glia. 2003;42(4):340–349. doi: 10.1002/glia.10221. [DOI] [PubMed] [Google Scholar]
- 501.Mitsumoto H, Ikeda K, Klinkosz B, Cedarbaum JM, Wong V, Lindsay RM. Arrest of motor neuron disease in wobbler mice cotreated with CNTF and BDNF. Science. 1994;265(5175):1107–1110. doi: 10.1126/science.8066451. [DOI] [PubMed] [Google Scholar]
- 502.ALS CNTF Treatment Study (ACTS) Phase I-II Study Group. The pharmacokinetics of subcutaneously administered recombinant human ciliary neurotrophic factor (rHCNTF) in patients with amyotrophic lateral sclerosis: relation to parameters of the acute-phase response. Clin Neuropharmacol. 1995;18(6):500–514. doi: 10.1097/00002826-199512000-00003. [DOI] [PubMed] [Google Scholar]
- 503.Cedarbaum JM, DeStefano PS, Lakings DB, Lindsay RM. Pharmacokinetics and pharmacodynamics of rHCNTF in rodents. Ann Neurol. 1996;39(4):552–554. doi: 10.1002/ana.410390420. [DOI] [PubMed] [Google Scholar]
- 504.Miller RG, Bryan WW, Dietz MA, Munsat TL, Petajan JH, Smith SA, Goodpasture JC. Toxicity and tolerability of recombinant human ciliary neurotrophic factor in patients with amyotrophic lateral sclerosis. Neurology. 1996;47(5):1329–1331. doi: 10.1212/wnl.47.5.1329. [DOI] [PubMed] [Google Scholar]
- 505.ALS CNTF Treatment Study Group. A double-blind placebo-controlled clinical trial of subcutaneous recombinant human ciliary neurotrophic factor (rHCNTF) in amyotrophic lateral sclerosis. Neurology. 1996;46(5):1244–1249. doi: 10.1212/wnl.46.5.1244. [DOI] [PubMed] [Google Scholar]
- 506.Kwon YW, Gurney ME. Systemic injections of ciliary neurotrophic factor induce sprouting by adult motor neurons. Neuroreport. 1994;5(7):789–792. doi: 10.1097/00001756-199403000-00013. [DOI] [PubMed] [Google Scholar]
- 507.Aebischer P, Schluep M, Déglon N, Joseph JM, Hirt L, Heyd B, Goddard M, Hammang JP, Zurn AD, Kato AC, Regli F, Baetge EE. Intrathecal delivery of CNTF using encapsulated genetically modified xenogeneic cells in amyotrophic lateral sclerosis patients. Nat Med. 1996;2(6):696–699. doi: 10.1038/nm0696-696. [DOI] [PubMed] [Google Scholar]
- 508.Okura Y, Arimoto H, Tanuma N, Matsumoto K, Nakamura T, Yamashima T, Miyazawa T, Matsumoto Y. Analysis of neurotrophic effects of hepatocyte growth factor in the adult hypoglossal nerve axotomy model. Eur J Neurosci. 1999;(11):4139–4144. doi: 10.1046/j.1460-9568.1999.00832.x. [DOI] [PubMed] [Google Scholar]
- 509.Sun W, Funakoshi H, Nakamura T. Overexpression of HGF retards disease progression and prolongs life span in a transgenic mouse model of ALS. J Neurosci. 2002;22(15):6537–6548. doi: 10.1523/JNEUROSCI.22-15-06537.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Kadoyama K, Funakoshi H, Ohya W, Nakamura T. Hepatocyte growth factor (HGF) attenuates gliosis and motoneuronal degeneration in the brainstem motor nuclei of a transgenic mouse model of ALS. Neurosci Res. 2007;59(4):446–456. doi: 10.1016/j.neures.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 511.Ishigaki A, Aoki M, Nagai M, Warita H, Kato S, Kato M, Nakamura T, Funakoshi H, Itoyama Y. Intrathecal delivery of hepatocyte growth factor from amyotrophic lateral sclerosis onset suppresses disease progression in rat amyotrophic lateral sclerosis model. J Neuropathol Exp Neurol. 2007;66(11):1037–1044. doi: 10.1097/nen.0b013e318159886b. [DOI] [PubMed] [Google Scholar]
- 512.Kato S, Funakoshi H, Nakamura T, Kato M, Nakano I, Hirano A, Ohama E. Expression of hepatocyte growth factor and c-Met in the anterior horn cells of the spinal cord in the patients with amyotrophic lateral sclerosis (ALS): immunohistochemical studies on sporadic ALS and familial ALS with superoxide dismutase 1 gene mutation. Acta Neuropathol. 2003;106(2):112–120. doi: 10.1007/s00401-003-0708-z. [DOI] [PubMed] [Google Scholar]
- 513.Matsumoto K, Nakamura T. NK4 gene therapy targeting HGF-Met and angiogenesis. Front Biosci. 2008;13:1943–1951. doi: 10.2741/2813. [DOI] [PubMed] [Google Scholar]
- 514.Matsuki A, Yamamoto S, Nakagami H, Aoki M, Tamai K, Matsumoto K, Nakamura T, Ogihara T, Kaneda Y, Morishita R. No influence of tumor growth by intramuscular injection of hepatocyte growth factor plasmid DNA: safety evaluation of therapeutic angiogenesis gene therapy in mice. Biochem Biophys Res Commun. 2004;315(1):59–65. doi: 10.1016/j.bbrc.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 515.Heck S, Lezoualc'h F, Engert S, Behl C C. Insulin-like growth factor-1-mediated neuroprotection against oxidative stress is associated with activation of nuclear factor kappaB. J Biol Chem. 1999;274(14):9828–9835. doi: 10.1074/jbc.274.14.9828. [DOI] [PubMed] [Google Scholar]
- 516.Zhong J, Lee WH. Hydrogen peroxide attenuates insulin-like growth factor-1 neuroprotective effect, prevented by minocycline. Neurochem Int. 51(6–7):398–404. doi: 10.1016/j.neuint.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 517.Dávila D, Torres-Aleman I. Neuronal death by oxidative stress involves activation of FOXO3 through a two-arm pathway that activates stress kinases and attenuates insulin-like growth factor I signaling. Mol Biol Cell. 2008;19(5):2014–2025. doi: 10.1091/mbc.E07-08-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Rabinovsky ED, Gelir E, Gelir S, Lui H, Kattash M, DeMayo FJ, Shenaq SM, Schwartz RJ. Targeted expression of IGF-1 transgene to skeletal muscle accelerates muscle and motor neuron regeneration. FASEB J. 2003;17(1):53–55. doi: 10.1096/fj.02-0183fje. [DOI] [PubMed] [Google Scholar]
- 519.Braunstein GD, Reviczky AL. Serum insulin-like growth factor-I levels in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 1987;50(6):792–794. doi: 10.1136/jnnp.50.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Adem A, Ekblom J, Gillberg PG, Jossan SS, Höög A, Winblad B, Aquilonius AM, Wang LH, Sara V. Insulin-like growth factor-1 receptors in human spinal cord: changes in amyotrophic lateral sclerosis. J Neural Transm Gen Sect. 1994;97(1):73–84. doi: 10.1007/BF01277964. [DOI] [PubMed] [Google Scholar]
- 521.Chung YH, Joo KM, Shin CM, Lee YJ, Shin DH, Lee KH, Cha CI. Immunohistochemical study on the distribution of insulin-like growth factor I (IGF-I) receptor in the central nervous system of SOD1(G93A) mutant transgenic mice. Brain Res. 2003;994(2):253–259. doi: 10.1016/j.brainres.2003.09.047. [DOI] [PubMed] [Google Scholar]
- 522.Dagvajantsan B, Aoki M, Warita H, Suzuki N, Itoyama Y. Up-regulation of insulin-like growth factor-II receptor in reactive astrocytes in the spinal cord of amyotrophic lateral sclerosis transgenic rats. Tohoku J Exp Med. 2008;214(4):303–310. doi: 10.1620/tjem.214.303. [DOI] [PubMed] [Google Scholar]
- 523.Hantaï D, Akaaboune M, Lagord C, Murawsky M, Houenou LJ, Festoff BW, Vaught JL, Rieger F, Blondet B B. Beneficial effects of insulin-like growth factor-I on wobbler mouse motoneuron disease. J Neurol Sci. 1995;129 Suppl:122–126. doi: 10.1016/0022-510x(95)00081-c. [DOI] [PubMed] [Google Scholar]
- 524.Messi ML, Clark HM, Prevette DM, Oppenheim RW, Delbono O. The lack of effect of specific overexpression of IGF-1 in the central nervous system or skeletal muscle on pathophysiology in the G93A SOD-1 mouse model of ALS. Exp Neurol. 2007;207(1):52–63. doi: 10.1016/j.expneurol.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Dobrowolny G, Giacinti C, Pelosi L, Nicoletti C, Winn N, Barberi L, Molinaro M, Rosenthal N, Musarò A. Muscle expression of a local Igf-1 isoform protects motor neurons in an ALS mouse model. J Cell Biol. 2005;168(2):193–199. doi: 10.1083/jcb.200407021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Lai EC, Felice KJ, Festoff BW, Gawel MJ, Gelinas DF, Kratz R, Murphy MF, Natter HM, Norris FH, Rudnicki SA. Effect of recombinant human insulin-like growth factor-I on progression of ALS. A placebo-controlled study. The North America ALS/IGF-I Study Group. Neurology. 1997;49(6):1621–1630. doi: 10.1212/wnl.49.6.1621. [DOI] [PubMed] [Google Scholar]
- 527.Borasio GD, Robberecht W, Leigh PN, Emile J, Guiloff RJ, Jerusalem F, Silani V, Vos PE, Wokke JH, Dobbins T. A placebo-controlled trial of insulin-like growth factor-I in amyotrophic lateral sclerosis. European ALS/IGF-I Study Group. Neurology. 1998;51(2):583–586. doi: 10.1212/wnl.51.2.583. [DOI] [PubMed] [Google Scholar]
- 528.Sorenson EJ, et al. Subcutaneous IGF-1 is not beneficial in 2-year ALS trial. Neurology. 2008;71(22):1770–1775. doi: 10.1212/01.wnl.0000335970.78664.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 529.Pan W, Kastin AJ. Interactions of IGF-1 with the blood-brain barrier in vivo and in situ. Neuroendocrinology. 2000;72(3):171–178. doi: 10.1159/000054584. [DOI] [PubMed] [Google Scholar]
- 530.Bedlack RS, Silani V, Cudkowicz ME. IPLEX and the telephone game: the difficulty in separating myth from reality on the internet. Amyotroph Lateral Scler. 2009;10(3):182–184. doi: 10.1080/17482960802673059. [DOI] [PubMed] [Google Scholar]
- 531.Lepore AC, Haenggeli C, Gasmi M, Bishop KM, Bartus RT, Maragakis NJ, Rothstein JD. Intraparenchymal spinal cord delivery of adeno-associated virus IGF-1 is protective in the SOD1G93A model of ALS. Brain Res. 2007;1185:256–265. doi: 10.1016/j.brainres.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 532.Sakurai M, Hayashi T, Abe K, Yaginuma G, Meguro T, Itoyama Y, Tabayashi K. Induction of glial cell line-derived neurotrophic factor and c-ret porto-oncogene-like immunoreactivity in rabbit spinal cord after transient ischemia. Neurosci Lett. 2000;276(2):123–126. doi: 10.1016/s0304-3940(99)00804-6. [DOI] [PubMed] [Google Scholar]
- 533.Lambrechts D, Storkebaum E, Morimoto M, Del-Favero J, Desmet F, Marklund SL, Wyns S, Thijs V, Andersson J, van Marion I, Al-Chalabi A, Bornes S, Musson R, Hansen V, Beckman L, Adolfsson R, Pall HS, Prats H, Vermeire S, Rutgeerts P, Katayama S, Awata T, Leigh N, Lang-Lazdunski L, Dewerchin M, Shaw C, Moons L, Vlietinck R, Morrison KE, Robberecht W, Van Broeckhoven C, Collen D, Andersen PM, Carmeliet P. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nat Genet. 2003;34(4):383–394. doi: 10.1038/ng1211. [DOI] [PubMed] [Google Scholar]
- 534.Sopher BL, Thomas PS, Jr, LaFevre-Bernt MA, Holm IE, Wilke SA, Ware CB, Jin LW, Libby RT, Ellerby LM, La Spada AR AR. Androgen receptor YAC transgenic mice recapitulate SBMA motor neuronopathy and implicate VEGF164 in the motor neuron degeneration. Neuron. 2004;41(5):687–699. doi: 10.1016/s0896-6273(04)00082-0. [DOI] [PubMed] [Google Scholar]
- 535.Murakami T, Ilieva H, Shiote M, Nagata T, Nagano I, Shoji M, Abe K. Hypoxic induction of vascular endothelial growth factor is selectively impaired in mice carrying the mutant SOD1 gene. Brain Res. 2003;989(2):231–237. doi: 10.1016/s0006-8993(03)03374-2. [DOI] [PubMed] [Google Scholar]
- 536.Azzouz M, Ralph GS, Storkebaum E, Walmsley LE, Mitrophanous KA, Kingsman SM, Carmeliet P, Mazarakis ND. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429(6990):413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- 537.Zheng C, Nennesmo I, Fadeel B, Henter JI. Vascular endothelial growth factor prolongs survival in a transgenic mouse model of ALS. Ann Neurol. 2004;56(4):564–567. doi: 10.1002/ana.20223. [DOI] [PubMed] [Google Scholar]
- 538.Storkebaum E, Lambrechts D, Dewerchin M, Moreno-Murciano MP, Appelmans S, Oh H, Van Damme P, Rutten B, Man WY, De Mol M, Wyns S, Manka D, Vermeulen K, Van Den Bosch L, Mertens N, Schmitz C, Robberecht W, Conway EM, Collen D, Moons L, Carmeliet P. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. 2005;8(1):85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- 539.Wang Y, Mao XO, Xie L, Banwait S, Marti HH, Greenberg DA, Jin K. Vascular endothelial growth factor overexpression delays neurodegeneration and prolongs survival in amyotrophic lateral sclerosis mice. J Neurosci. 2007;27(2):304–307. doi: 10.1523/JNEUROSCI.4433-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540.Lee KF, Li E, Huber LJ, Landis SC, Sharpe AH, Chao MV, Jaenisch R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69(5):737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- 541.Koera K, Nakamura K, Nakao K, Miyoshi J, Toyoshima K, Hatta T, Otani H H, Aiba A, Katsuki M. K-ras is essential for the development of the mouse embryo. Oncogene. 1997;15(10):1151–1159. doi: 10.1038/sj.onc.1201284. [DOI] [PubMed] [Google Scholar]
- 542.Heumann R, Goemans C, Bartsch D, Lingenhöhl K, Waldmeier PC, Hengerer B, Allegrini PR, Schellander K, Wagner EF, Arendt T, Kamdem RH, Obst-Pernberg K, Narz F, Wahle P, Berns H. Transgenic activation of Ras in neurons promotes hypertrophy and protects from lesion-induced degeneration. J Cell Biol. 2000;151(7):1537–1548. doi: 10.1083/jcb.151.7.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Zhong J, Li X, McNamee C, Chen AP, Baccarini M, Snider WD. Raf kinase signaling functions in sensory neuron differentiation and axon growth in vivo. Nat Neurosci. 2007;10(5):598–607. doi: 10.1038/nn1898. [DOI] [PubMed] [Google Scholar]
- 544.Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VM, Szabolcs M, de Jong R, Oltersdorf T, Ludwig T, Efstratiadis A, Birnbaum MJ. Role for Akt3/protein kinase Bgamma in attainment of normal brain size. Mol Cell Biol. 2005;25(5):1869–1878. doi: 10.1128/MCB.25.5.1869-1878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman KG, Hay N. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17(11):1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Michaelidis TM, Sendtner M, Cooper JD, Airaksinen MS, Holtmann B, Meyer BM, Thoenen H. Inactivation of bcl-2 results in progressive degeneration of motoneurons, sympathetic and sensory neurons during early postnatal development. Neuron. 1996;17(1):75–89. doi: 10.1016/s0896-6273(00)80282-2. [DOI] [PubMed] [Google Scholar]
- 547.Hui K, Kucera J, Henderson JT. Differential sensitivity of skeletal and fusimotor neurons to Bcl-2-mediated apoptosis during neuromuscular development. Cell Death Differ. 2007;15(4):691–699. doi: 10.1038/sj.cdd.4402294. [DOI] [PubMed] [Google Scholar]
- 548.Dietz GP, Kilic E, Bähr M, Isenmann S. Bcl-2 is not required in retinal ganglion cells surviving optic nerve axotomy. Neuroreport. 2001;12(15):3353–3356. doi: 10.1097/00001756-200110290-00041. [DOI] [PubMed] [Google Scholar]
- 549.White FA, Keller-Peck CR, Knudson CM, Korsmeyer SJ, Snider WD. Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J Neurosci. 1998;18(4):1428–1439. doi: 10.1523/JNEUROSCI.18-04-01428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Sato N, Sakuma C, Sato Y, Gould TW, Oppenheim RW, Yaginuma H. Distinct susceptibility of developing neurons to death following Bax overexpression in the chicken embryo. Cell Death Differ. 2006;13(3):435–445. doi: 10.1038/sj.cdd.4401760. [DOI] [PubMed] [Google Scholar]
- 551.Kinugasa T, Ozaki S, Hamanaka S, Kudo N. The effects of sciatic nerve axotomy on spinal motoneurons in neonatal Bax-deficient mice. Neurosci Res. 2002;44(4):439–446. doi: 10.1016/s0168-0102(02)00171-2. [DOI] [PubMed] [Google Scholar]
- 552.Harlin H, Reffey SB, Duckett CS, Lindsten T, Thompson CB. Characterization of XIAP-deficient mice. Mol Cell Biol. 2001;21(10):3604–3608. doi: 10.1128/MCB.21.10.3604-3608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Conze DB, Albert L, Ferrick DA, Goeddel DV, Yeh WC, Mak T, Ashwell JD. Posttranscriptional downregulation of c-IAP2 by the ubiquitin protein ligase c-IAP1 in vivo. Mol Cell Biol. 2005;25(8):3348–3356. doi: 10.1128/MCB.25.8.3348-3356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Yoshida H, Kong YY, Yoshida R, Elia AJ, Hakem A, Hakem R, Penninger JM, Mak TW. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell. 1998;94(6):739–750. doi: 10.1016/s0092-8674(00)81733-x. [DOI] [PubMed] [Google Scholar]
- 555.Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell. 1998;94(6):727–737. doi: 10.1016/s0092-8674(00)81732-8. [DOI] [PubMed] [Google Scholar]
- 556.Hakem R, Hakem A, Duncan GS, Henderson JT, Woo M, Soengas MS, Elia A, de la Pompa JL, Kagi D, Khoo W, Potter J, Yoshida R, Kaufman SA, Lowe SW, Penninger JM, Mak TW. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1996;94(3):339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- 557.Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, Su MS, Rakic P, Flavell RA. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94(3):325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 558.Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, Ferri KF, Zamzami N, Wakeham A, Hakem R, Yoshida H, Kong YY, Mak TW, Zúñiga-Pflücker JC, Kroemer G, Penninger JM. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001;410(6828):549–554. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]
- 559.Aloyz RS, Bamji SX, Pozniak CD, Toma JG, Atwal J, Kaplan DR, Miller FD. p53 is essential for developmental neuron death as regulated by the TrkA and p75 neurotrophin receptors. J Cell Biol. 1998;143(6):1691–1703. doi: 10.1083/jcb.143.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 560.Barker V, Middleton G, Davey F, Davies AM. TNFalpha contributes to the death of NGF-dependent neurons during development. Nat Neurosci. 2001;4(12):1194–1198. doi: 10.1038/nn755. [DOI] [PubMed] [Google Scholar]
- 561.Gowing G, Dequen F, Soucy G, Julien JP. Absence of tumor necrosis factor-alpha does not affect motor neuron disease caused by superoxide dismutase 1 mutations. J Neurosci. 2006;26(44):11397–11402. doi: 10.1523/JNEUROSCI.0602-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Amgen unpublished results. [Google Scholar]
- 563.Park S, Kim HT, Yun S, Kim IS, Lee J, Lee IS, Park KI. Growth factor-expressing human neural progenitor cell grafts protect motor neurons but do not ameliorate motor performance and survival in ALS mice. Exp Mol Med. 2009;41(7):487–500. doi: 10.3858/emm.2009.41.7.054. [DOI] [PMC free article] [PubMed] [Google Scholar]