Abstract
Oxidative damage, neuroinflammation, and mitochondrial dysfunction contribute to the pathogenesis of ALS, and these pathologic processes are tightly regulated by the Nrf2/ARE (NF-E2 related factor 2/antioxidant response element) signaling program. Therefore, modulation of the Nrf2/ARE pathway is an attractive therapeutic target for neurodegenerative diseases such as ALS. We examined two triterpenoids, CDDO (2-cyano-3, 12-dioxooleana-1,9-dien-28-oic acid) ethylamide (CDDO-EA) and CDDO-trifluoroethylamide (CDDO-TFEA), that potently activate Nrf2/ARE in a cell culture model of ALS and in the G93A SOD1 mouse model of ALS. Treatment of NSC-34 cells stably expressing mutant G93A SOD1 with CDDO-TFEA upregulated Nrf2 expression, and resulted in translocation of Nrf2 into the nucleus. Western blot analysis showed an increase in the expression of Nrf2/ARE-regulated proteins. When treatment started at a “presymptomatic age”, of 30 days both of these compounds significantly attenuated weight loss, enhanced motor performance and extended the survival of G93A SOD1 mice. Treatment started at a “symptomatic age”, as assessed by impaired motor performance was neuroprotective and slowed disease progression. These findings provide further evidence that compounds which activate the Nrf2/ARE signaling pathway may be useful in the treatment of ALS.
Keywords: G93A, SOD1, Triterpenoids, Inflammation, Oxidative damage, Motor neuron degeneration
Introduction
ALS is a progressive, lethal neurodegenerative disorder in which the hallmark of the disease is the selective death of motor neurons in the brain and spinal cord, leading to paralysis of voluntary muscles. The degeneration of motor neurons is associated with activation and proliferation of astrocytes and microglia. The etiology of ALS is not fully known and the cause(s) of most cases of ALS are yet to be defined. Several mechanisms have been implicated including glutamate excitotoxicity [1, 2], aberrant protein aggregates containing mutant SOD1 [3], mislocalization and aggregation of neurofilaments [4], oxidative damage by enhanced free radical formation [5], neuroinflammation [6–10], proteasome dysfunction [11–13], mitochondrial dysfunction [14] and apoptosis [15, 16].
Over 150 clinical trials in human ALS patients have been conducted thus far, and the only drug which shows marginal efficacy is riluzole, which at best extends life for 3 months [17, 18]. Riluzole inhibits the release of glutamate from the synapses, hence reducing glutamate toxicity.
The Nrf2/ARE signaling program is an endogenous cytoprotective system which is ubiquitously expressed. The transcription factor Nrf2 [19] was identified based on its binding to Antioxidant Response Element (ARE), which is a cis-acting enhancer sequence that mediates transcriptional activation of genes in cells exposed to oxidative stress [20]. Its role involves the coordinated induction of a battery of over 250 cytoprotective genes, including those that encode antioxidant and anti-inflammatory proteins, collectively termed phase II detoxifying enzymes [21, 22]. The induction of Nrf2-regulated neuroprotective genes depends on the nuclear translocation of Nrf2 and its formation of a transcriptional complex with the Maf (musculo-aponeurotic fibrosarcoma) family proteins, which bind to the promoter region of genes with the ARE regulatory sequence. Binding of the Nrf2-Maf dimer to the ARE sequence results in coordinated transcriptional activation of antioxidant enzymes and detoxifying proteins.
In ALS postmortem brain and spinal cord tissues Nrf2 mRNA levels are reduced in the motor cortex and spinal cord while Keap1 mRNA levels were elevated [23]. This impairment of the Nrf2/ARE signaling system may increase the vulnerability of motor neurons to stressed-induced toxic insults.
In the present studies we found that triterpenoids activate the Nrf2/ARE pathway in NSC-34 G93A SOD1 cells, and they extended life-span in ALS transgenic mice with the G93A SOD1 mutation. Therefore, the Nrf2/ARE signaling pathway is a novel therapeutic target for treatment of ALS patients.
GENERAL METHODS
Measurement of reactive oxygen species in mouse embryonic fibroblasts
Mouse embryonic fibroblast cells were treated as previously described (Yang et al., 2009). Briefly, wild-type and Nrf2−/− mouse embryonic fibroblasts were pre-treated with CDDO-EA or CDDO-TFEA at various concentrations (1, 10 and 100 nM in DMSO) for 18 hours and incubated with 2’,7’-Dichlorodihydrofluorecein diacetate (H2DCFDA) (Invitrogen, CA) for 30 min. Cells were challenged with 250 µmol/L tBHP for 15–30 min and the mean fluorescence intensity for ~10,000 cells was analyzed by FACSan flow cytometry (Becton Dickinson, NJ) using a 480-nm excitation wavelength and a 525-nm emission wavelength.
NSC-34 Cell Culture
NSC-34 cells were cultured as described [24] with modifications and stably transfected with wild-type human SOD1 and G93A SOD1 as described [25]. Stably transfected NSC-34 cells were provided by Dr. Giovanni Manfredi (Weill Medical College of Cornell University). Briefly, NSC-34 wild-type SOD1 and NSC-34 G93A SOD1 were plated in 100 mm dishes with 10×106 cells or in 8-well Culture Slides (BD Biosciences, San Jose, California) using 0.5×103 cells per well in the presence of 500 mg/ml neomycin analog G418. NSC-34 G93A SOD1 or wild-type SOD1 were treated with CDDO-TFEA dissolved in DMSO (300 nM) for up to 48 hours. Total RNA and protein were extracted: the RNA was subjected to RT-PCR and the protein was analyzed by Western blotting. Cytoplasmic and nuclear fractions were prepared using Pierce kit (Thermo Fisher Scientific). Cells grown on 8-well Culture Slides were treated with CDDO-TFEA (300 nM) for 24 hours and fixed in a 4% para-formaldehyde solution, permeabilized with 0.1% Triton-X100, and immunostained with rabbit polyclonal antibody directed against Nrf2 (Abcam, Cambridge, UK) overnight at 4°C, followed the next day by incubation with biotinylated mouse anti-rabbit IgG (1:300 in PBS; DAKO), and developed with an Elite ABC kit purchased from Vector Laboratories.
Primary motor neuron culture
Purification of rat embryonic motor neurons was performed as previously described [26]. For harvesting of cells, a minimal number of rats was housed and sacrificed according to German law on animal care. Lumbar ventral spinal cords were dissected from Sprague-Dawley rat embryos (gestational age: E14/15). After tissue dissociation, the motor neurons were enriched by gradient density centrifugation using OptiPrep (Sigma-Aldrich, Steinheim, Germany). Culture medium (Neurobasal medium, Gibco Invitrogen, Germany; with 2% (v/v) horse serum; 2% (v/v) B27-supplement, Gibco Invitrogen, Germany; 0.5mM l-glutamax; 5 ng/ml rHuBDNF Peprotech, Germany and 5 ng/ml rHuCNTF Peprotech, Germany) was added to the resulting pellet. Highly enriched motor neurons were seeded on glass coverslips pre-incubated first with polyornithin (diluted 1:1000, Sigma), and then with laminin1 (conc. 2, 5µg/ml, Invitrogen). Cultures were fed every second day with culture medium for motor neurons.
Treatment with CDDO-TFEA (300nM)
Motor neuron-rich cell fractions were prepared as described above and seeded on laminin at an average density of 3×104 cells/cm2. At day in vitro (DIV) 10, cells were incubated for 24 h with either CDDO-TFEA (300 nM) or DMSO as a vehicle. Following incubation, cells were fixed with 4% para-formaldehyde (PFA) at DIV 11, and Nrf-2 expression was assessed by immunocytochemical analysis:
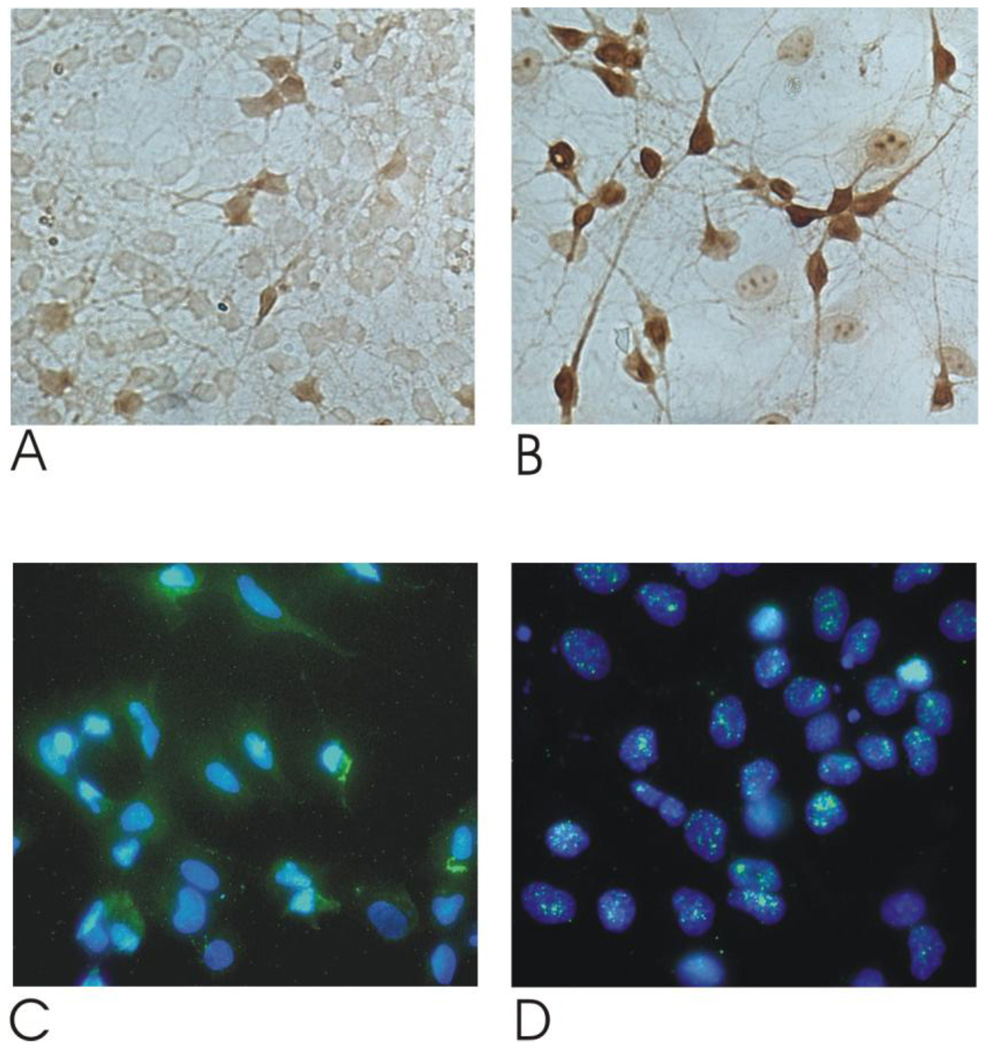
Endogenous peroxidase was blocked with 3% hydrogen peroxide and 10% methanol in 1×PBS. After pre-incubation with 1% bovine serum albumin (BSA) in 1×PBS/0.3% Triton X-100 for 30 minutes at room temperature, sections were incubated with a rabbit polyclonal antibody directed against Nrf2 (Abcam, Cambridge, UK) overnight at 4°C, followed the next day by incubation with biotinylated mouse anti-rabbit IgG (1:300 in PBS; DAKO, Glostrup, Denmark) and a streptavidin-biotin-horseradish peroxidase complex (Vector) (Figure 4A, B).
Figure 4. Nrf2 upregulation and translocation in rat embryonic motor neurons in response to CDDO-TFEA.
Rat embryonic motor neurons (Rat eMNs) were isolated as described in the Methods section. Rat eMNs were plated and treated with 300 nM CDDO-TFEA (Figure 4B, D) or DMSO (Figure 4A, C) for 24 hours. These cells were washed, fixed and then stained for Nrf2 using anti-Nrf2 (Figure 4A,B). Double immunofluorescence was performed to study nuclear translocation of Nrf2. CDDO-TFEA incubation led to a reduction of cytoplasmatic Nrf2 staining (green fluorescence) and increased nuclear colocalization (Figure 4 C,D).
Double immunofluorescence was performed to study nuclear translocation of Nrf-2. After incubation with the Nrf-2 antibody, cells were rinsed with PBS and incubated for 1 hour in the secondary antibody goat anti-rabbit Alexa Fluor 488 (Molecular Probes, Leiden, Denmark) at 1:250 in 0.5% BSA/PBS, washed with PBS and coverslipped in Mowiol 4–88 reagent (Merck, Darmstadt). The nuclei of the cells were stained with 4’,6-diamidino-2-phenylindole (DAPI, Sigma, Germany) (Figure 4 C,D).
Mouse Models of ALS
G93A SOD1 transgenic familial ALS mice (high copy number) B6SJL background strain (G93A SOD1, B6SJL-TgGur1) [27] were obtained from The Jackson Laboratory (Bar Harbor, ME). We maintained the transgenic G93A hemizygotes by mating transgenic males with B6SJLF1/J hybrid females. Transgenic offspring were genotyped by PCR assay of DNA obtained from tail tissue. Mice were housed at regulated room temperature in a 12h light/dark cycle with free access to food and water.
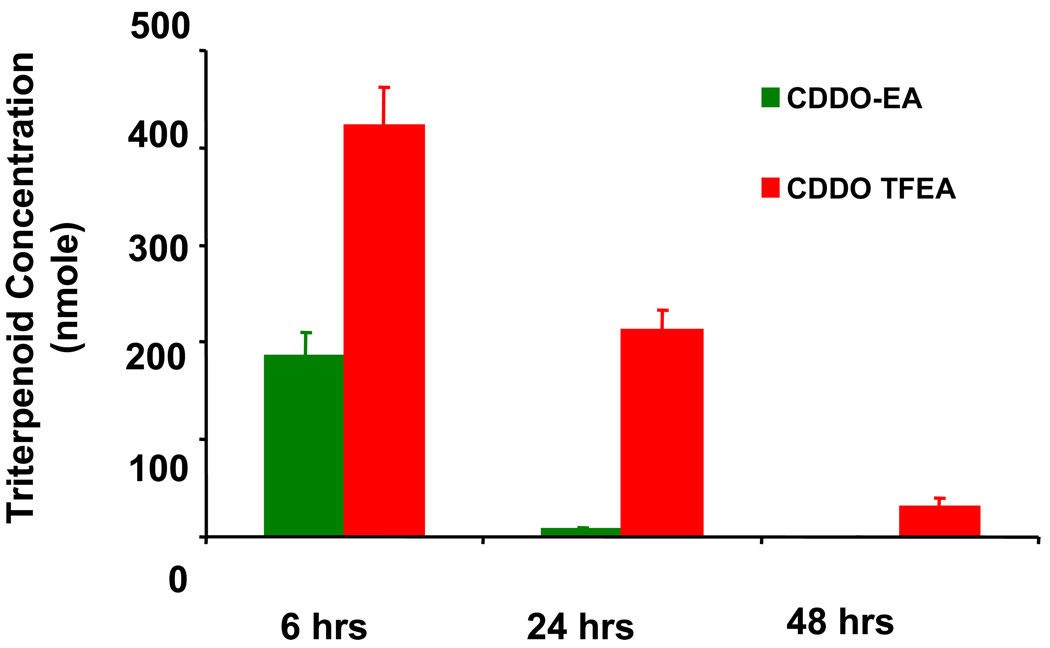
CDDO-EA or CDDO-TFEA were dissolved in Neobee oil, and administered to wild-type C57Bl6 mice by gavage (100 microliters) and about 2–3 nM of CDDO-EA or CDDO-TFEA accumulated in brain tissue (Figure 6).
Figure 6. Brain levels of triterpenoids measured by LC-MS.
Twelve week old male C57Bl6 mice were fed by gavage once with 100 microlitters of 2 micromolar CDDO-EA or CDDO TFEA dissolved in Neobee oil. The CDDOs were measured in the brain after 6, 24 and 48 hours of gavage. Data represent Mean ± SEM, (n=5 mice in each group for three time points). Animals fed with vehicle showed no triterpenoids in the brain (data not shown).
G93A transgenic mice were assigned randomly to the control (vehicle, mouse chaw only) and to mouse chaw containing either CDDO-EA or CDDO-TFEA (400 mg/kg of food, n=30 in both groups). This dose corresponds to about 80 mg/kg body weight/day, assuming each mouse consumes 5 grams of food per day. We found mice can tolerate this dose. Treatments started at two different time regimens: 1) “Early” at 30 days of age, about two months prior to symptom onset; 2) “At Onset” from the onset of the phenotype (80–90 days of age). A diet consisting of either 400 mg of CDDO-TFEA per kg of food or 400 mg of CDDO-EA per kg of food, and a control lab diet (Lab Diet #5002), were prepared by Purina (Purina-Mills, Richmond, IN, USA). CDDO-EA and CDDO-TFEA were provided by Reata Pharmaceuticals (Dallas, TX, USA). CDDO-EA and CDDO-TFEA were synthesized by the condensation of either ethyl amine or 2,2,2-trifluoroethyl amine, respectively, with CDDO acid chloride as described [28],
We have analyzed the data to determine whether there are any preferential effects due to the gender of the G93A mice. Our data shows there was no significant difference between the ages of death between females and males treated with triterpenoids. It is well established that female G93A mice have a longer life-span than that of males by an average of 5 days. This difference was also observed in this study (data not shown).
Six G93A mice on control food as the control group and six G93A mice from the CDDO-EA and CDDO-TFEA treated groups were killed at 110 days of age for histological evaluation. Six G93A mice were used from each group at 110 days of age for biochemical analysis. All animal handling and experiments are conducted in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines of Weill Medical College of Cornell University, New York.
Motor performance was assessed using a rotarod apparatus (Columbus Instruments, Columbus, OH, USA). The rotarod test measures the capability of mice to remain upright on a rod rotating at 12 rpm (rotations per minute). The time elapsed before a mouse falls off the rotarod measures its competency at the task, and provides a reliable indication of motor performance and progression of motor dysfunction. Mice were trained for one week on the rotarod, and subsequently tested twice per week starting at 60 days of age. Each mouse underwent three trials per session: the best result of three trials was recorded, with the maximum result set at 5 minutes. Mice were observed each morning and were weighed twice per week. Mice were deemed to have reached the end stage of the disorder when they were no longer able to initiate movement after being gently agitated for 2 minutes or could not right themselves within 30 seconds when placed on their backs. When they reached this stage, animals were euthanized by sodium pentobarbital overdose.
Mice were deeply anesthetized with sodium pentobarbital and transcardially perfused with 4% para-formaldehyde at pre-determined time-points. Spinal cords were removed, post-fixed with the perfusant for 2 hours, cryoprotected in a graded series of 10% and 20% glycerol/2% DMSO, and cut into frozen serial 50 or 35 µm-thick sections. Spinal cord tissue sections were stained with various antibodies, including Beta-actin (a house keeping protein), NQO1 protein, and Hemeoxygenase-1 antibodies (Santa Cruz Biotechnolgoy, CA) and Nrf2 (Abcam, Cambridge, UK). Secondary antibodies and the Elite ABC kit were purchased from Vector Laboratories.
Expression levels of SOD1, antioxidant and inflammatory genes modulated by Nrf2/ARE pathways were assessed by Western blot analysis of tissue extracts from the brain cortex and spinal cord using standard procedures that are routinely performed in our laboratory. Anti-SOD1 antibody purchased from Calbiochem (Calbiochem/Milipore, MA).
Total RNA was isolated from respective tissues of control and G93A SOD1 mice according to the manufacturer’s protocol using Trizol reagent (Invitrogen, CA). The resultant RNA samples were treated with RNase free DNase (Ambion, TX) in order to remove genomic DNA contamination. The purity of total RNA and its integrity was confirmed by measuring absorbance ratios at 260/280 nm using ND-1000 NanoDrop (NanoDrop Technologies DE) and 2100 Bioanalyzer (Agilent Technologies CA), respectively. Only those RNA samples that showed an absorbance ratio ranging from 1.9 to 2.1 and an average RNA Integrity Number (RIN) ranging from 5.0 to 7.5 were used to synthesize cDNA. Real-time PCR was performed using an ABI prism 7900 HT sequence detection system (Applied Biosystems, Foster City, CA) based on the 5'-nuclease assay (Holland et al., 1991). Relative expression was calculated using the ΔΔ Ct method (Livak, KJ and Schmittgen TD, 2001). TaqMan assays were used for Nrf2/ARE-regulated antioxidant genes like NQO-1, HO-1, GSTa3, GCL and neuroinflammatory genes like iNOS and COX-2. Beta actin and GAPDH TaqMan assays were used as housekeeping genes (Applied Biosystems, Foster City, CA).
Unless otherwise noted, data analysis was done by analysis of variance (ANOVA) and/or t-tests to compare sample groups, with Bonferroni correction used to correct for multiple comparisons if appropriate using GraphPad (San Diego, CA) and InStat software. Survival statistics were calculated from Kaplan-Meyer survival curves using Mantel-Cox Log-rank analysis. Statistical significance is at the p < 0.05 level.
RESULTS
Synthetic triterpenoids activate the neuroprotective Nrf2/ARE signaling program in ALS
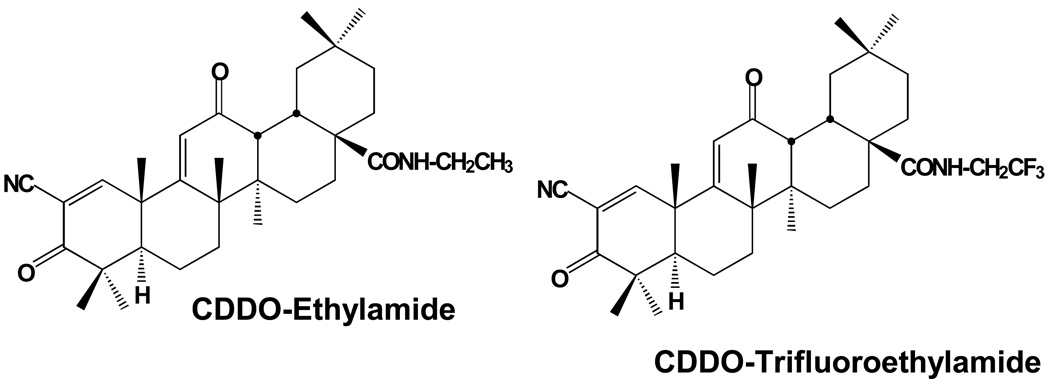
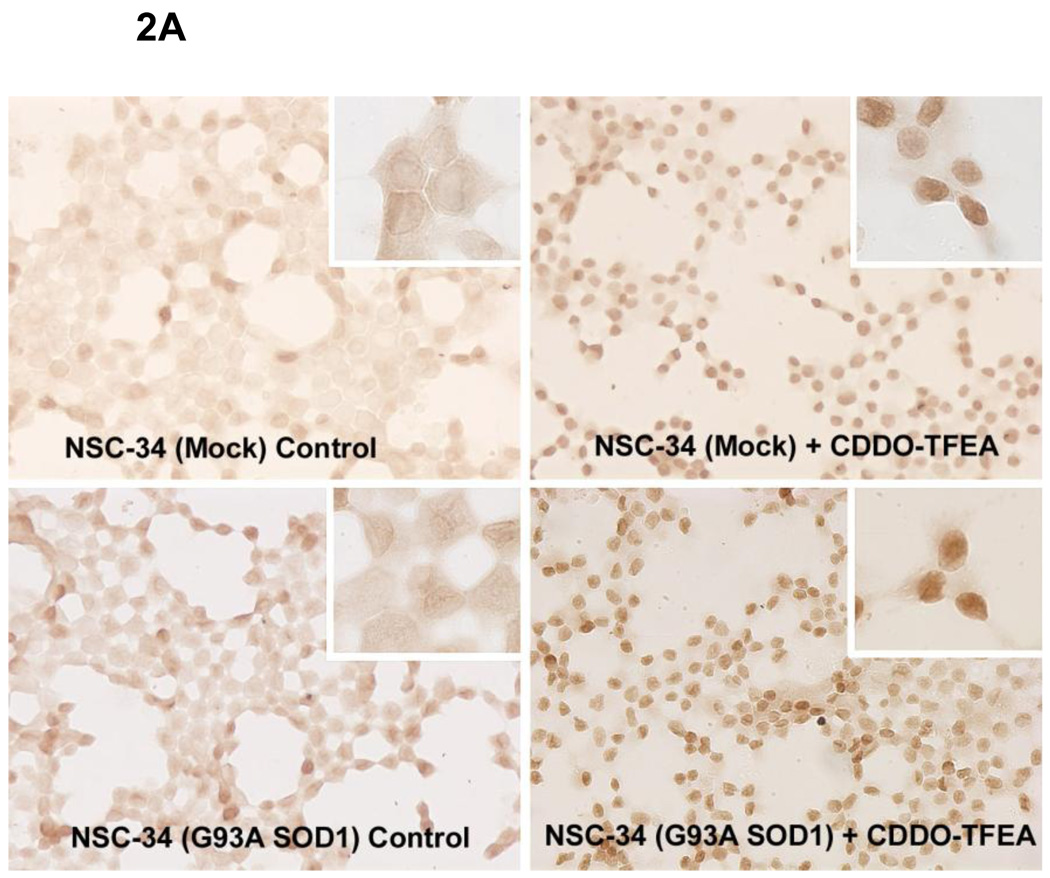
CDDO-EA and CDDO-TFEA (Figure 1) are synthetic triterpenoid analogs derived from oleanolic acid. We used NSC-34 cells [24] stably transfected with mutant G93A SOD1 in our laboratory which have been widely used as a cell culture model of ALS. We treated NSC-34 G93A SOD1 cells with CDDO-TFEA (300 nmolar) and found an increase in expression of Nrf2 as assessed by immunoreactivity to Nrf2 and analyzed by Western blotting. Vehicle treated NSC-34 G93A SOD1 cells showed modest Nrf2 expression as evidenced by diffuse staining in the cytoplasm and weak nuclear staining in some cells. It is expected that oxidative stress due to mutant SOD1 would trigger the activation of Nrf2; however, we found that in mutant SOD1 transfected NSC-34 cells, Nrf2 activation was only slightly upregulated, and Nrf2 was not translocated to the nucleus (Figure 2A). This suggests that Nrf2 upregulation and translocation into the nucleus may have been blocked. Interestingly, when CDDO-TFEA was added to NSC-34 cells, Nrf2 was found in the nucleus (Figure 2A). Next, we asked whether Nrf2 translocation to the nucleus results in upregulation of ARE-dependant genes. We isolated total RNA from NSC-34 G93A SOD1 cells treated with DMSO or CDDO-TFEA and analyzed the expression of NQO1, HO-1 and Glutathione S-transferase-a3. Total protein extracted from CDDO-TFEA or vehicle-treated NSC-34 cells was analyzed by Western blotting. We found known Nrf2-regulated genes products were upregulated in CDDO-TFEA-treated cells (Figure 3A). We show Nrf2 found in the nuclear fractions in CDDOTFEA treated NSC-34 cells where as in DMSO treated cell Nrf2 was only found in cytoplasm fractions (Figure 3B). Consistent with immunohistochemical and RT-PCR data, we found that Nrf2 and downstream proteins (NQO-1, HO-1 and Glutathione reductase) were upregulated in response to CDDO-TFEA (Figure 4).
Figure 1. Chemical structures of amide derivatives of CDDO.
Figure 2.
A) CDDO-TFEA treatment increased Nrf2 expression in NSC-34 cells. NSC-34 cells with and without the G93A SOD1 mutation were seeded on glass-bottom chamber slides and treated with CDDO-TFEA (300 nmolar) for 24 hours. Cells were fixed and stained for Nrf2 using anti-Nrf2 (Abcam). This data is representative of three experiments.
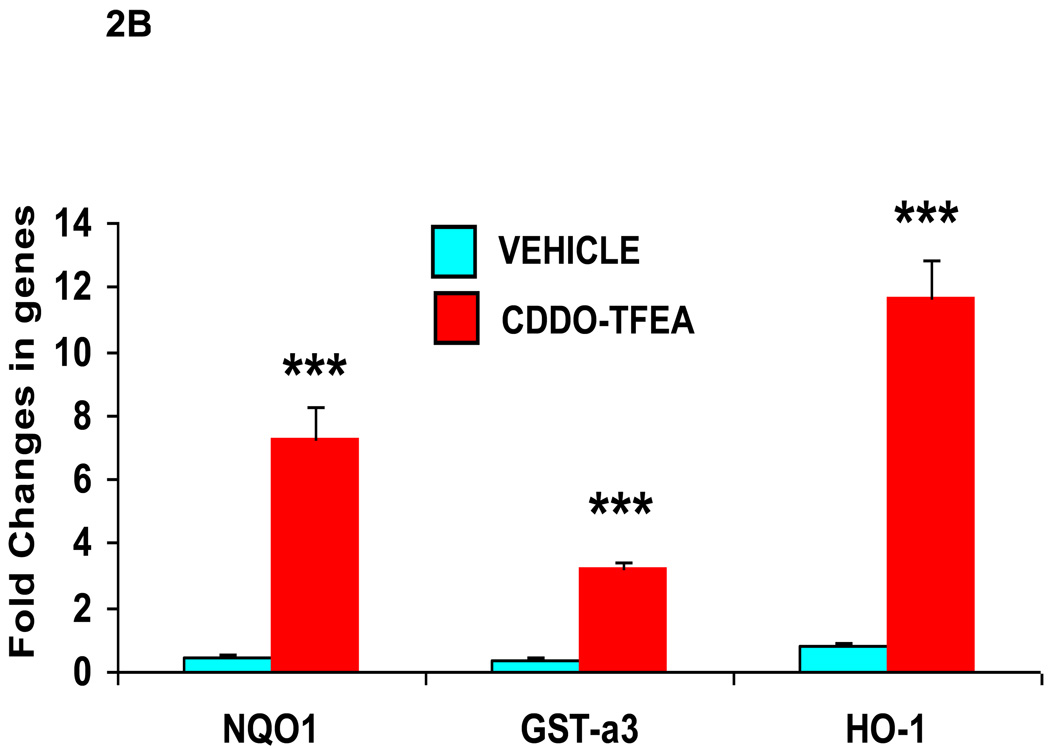
B) Nrf2/ARE gene upregulation in NSC-34 G93A SOD1 cells treated with CDDO-TFEA. NSC-34 cells were grown on plastic tissue culture plates and allowed to grow to confluency. These cells were treated with CDDO-TFEA or DMSO (vehicle) for 24 hours. Cells were washed and total RNA was extracted using TRIZOL. RNAs were analyzed by real time quantitative RT-PCR using TaqMan assays and Applied Biosystem PCR systems. Nrf2-regulated genes like NQO1, GST-a3 and HO-1 were significantly upregulated (p<0.0001, student t-test). Beta actin TaqMan assay was used as house-keeping gene to compare and calculate the fold difference in control and treated cells. All experiments were done in triplicates.
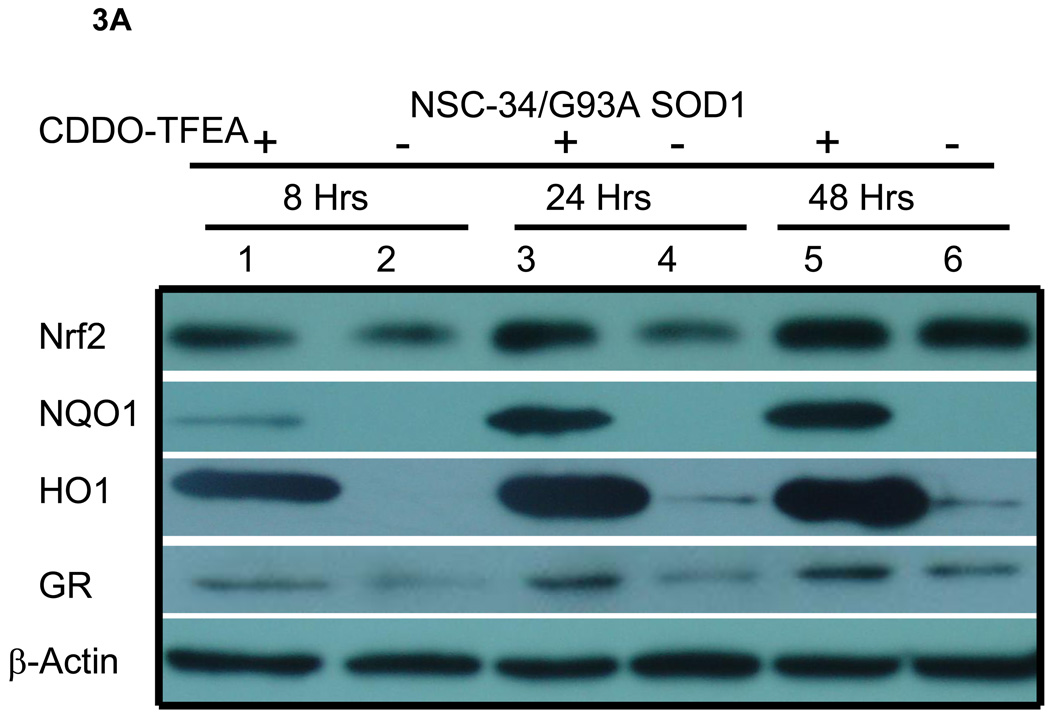
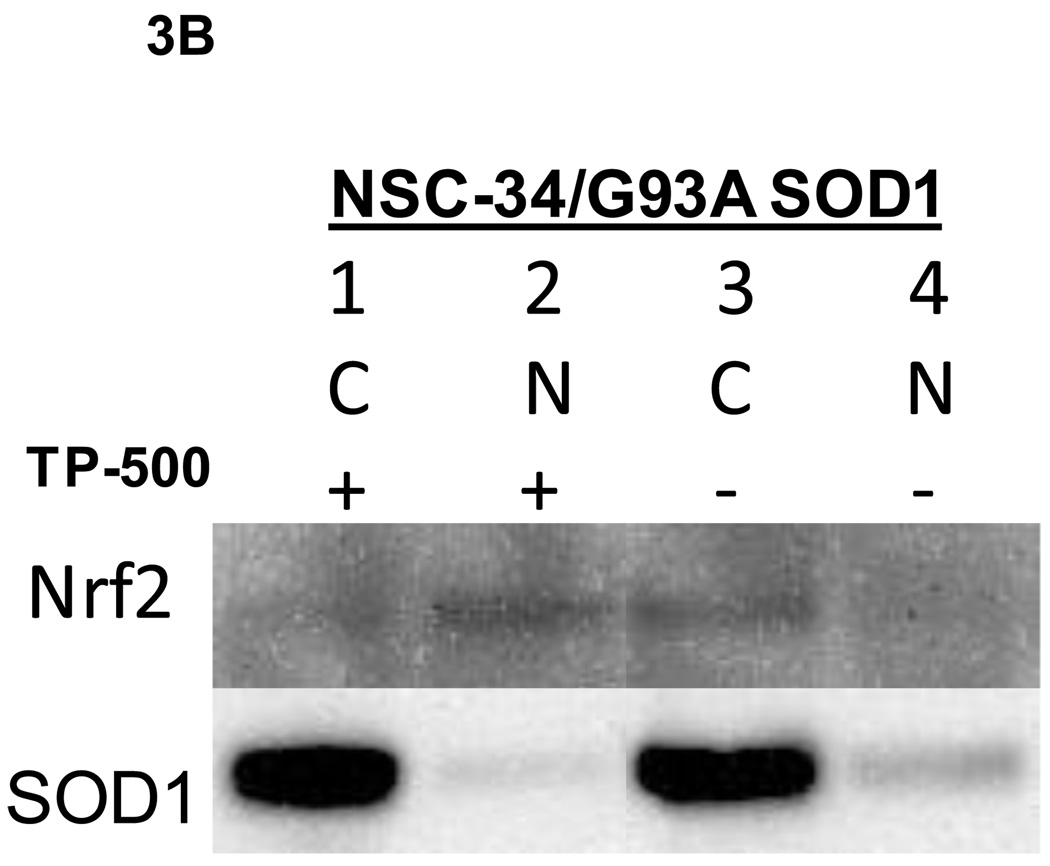
Figure 3. Western blot analysis of NSC-34/G93A A) Cell lysates for Nrf2/ARE proteins. B) Cytoplasmic and nuclear fractions for Nrf2 protein.
NSC-34/G93A cells were plated and treated with CDDO-TFEA or DMSO as described in Figure 2. Cell lysates were prepared at the indicated time points. A) Lanes 1, 3, and 5 each contain 30 µg of total lysates from CDDO-TFEA-treated cells and lanes 2, 4, and 6 each contain 30 µg of total lysates from DMSO-treated cells. Nrf2 is upregulated as early as 8 hours. NQO1, HO1 and GR were upregulated and reached peak levels at 48 hours. Beta-actin, a house-keeping protein is shown for a loading control. B) Lanes 1 and 3 are cytoplasmic (C) and lanes 2 and 4 are nuclear (N) fractions, 20 µg per lane loaded. . SOD1 used as cytoplasmic protein.
We also tested the effect of CDDO-TFEA in rat primary motor neurons. Following incubation with CDDO-TFEA, Nrf2 was upregulated as shown by immunocytochemistry (Figure 4A, B). By double immunofluorescence labeling with the nuclear marker 4’,6-diamidino-2-phenylindole (DAPI), nuclear translocation of Nrf2 following CDDO-TFEA treatment was observed (Figure 4 C, D).
Recently, we demonstrated that CDDO-MA activates the Nrf2/ARE pathway in an Nrf2-dependent manner (Yang et al., 2009). CDDO-EA and CDDO-TFEA pre-treated wild-type and Nrf2-deficient mouse (Yoh et al., 2001) embryonic fibroblasts were pre-loaded with H2DCFDA for 30 minutes and then challenged with tert-butylhydroperoxide (tBHP) for 30 minutes. ROS generation was assayed in these cells: ROS production was observed to be blocked dose-dependently by CDDO-EA or CDDO-TFEA in wild-type fibroblasts, while ROS generation showed an 8-fold increase in Nrf2-deficient mouse fibroblasts treated identically (Fig. 5). These data demonstrate that the Nrf2/ARE signaling pathway is activated by triterpenoids in vitro.
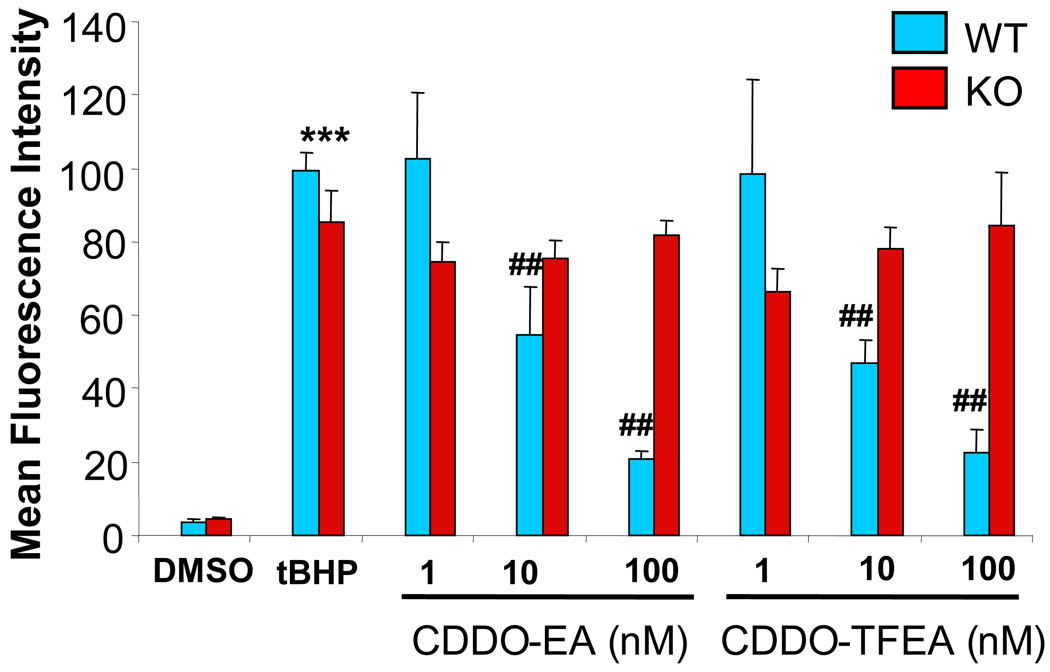
Figure 5. CDDOs specifically activate the Nrf2/ARE signaling program.
Wild-type and Nrf2 knockout mouse embryonic fibroblasts were pre-treated with either CDDO-EA or CDDO-TFEA (1, 10, 100 and 300 nM) or DMSO (as control) overnight. Then all of these cells were treated with 250 µM of tert-butylhydroperoxide (tBHP). Generation of reactive oxygen species (ROS) was measured 15 minutes later by flow cytometry. CDDO-EA or CDDO-TFEA treatments resulted in a significant dose-dependent reduction of tert-butylhydroperoxide-induced ROS generation in wild-type fibroblasts. Nrf2 knockout fibroblasts failed to show a reduction in tBHP-induced ROS generation. This data is representative of three separate experiments. *** indicates a significant increase in ROS production caused by tBHP treatment compared to DMSO treatment (p<0.0001). ## indicates significant reduction in ROS in CDDO-EA or CDDO TFEA pre-treated as wild-type cells compared to Nrf2 KO cells (p<.001, ANOVA).
Synthetic triterpenoids activate Nrf2 in the mouse model of ALS
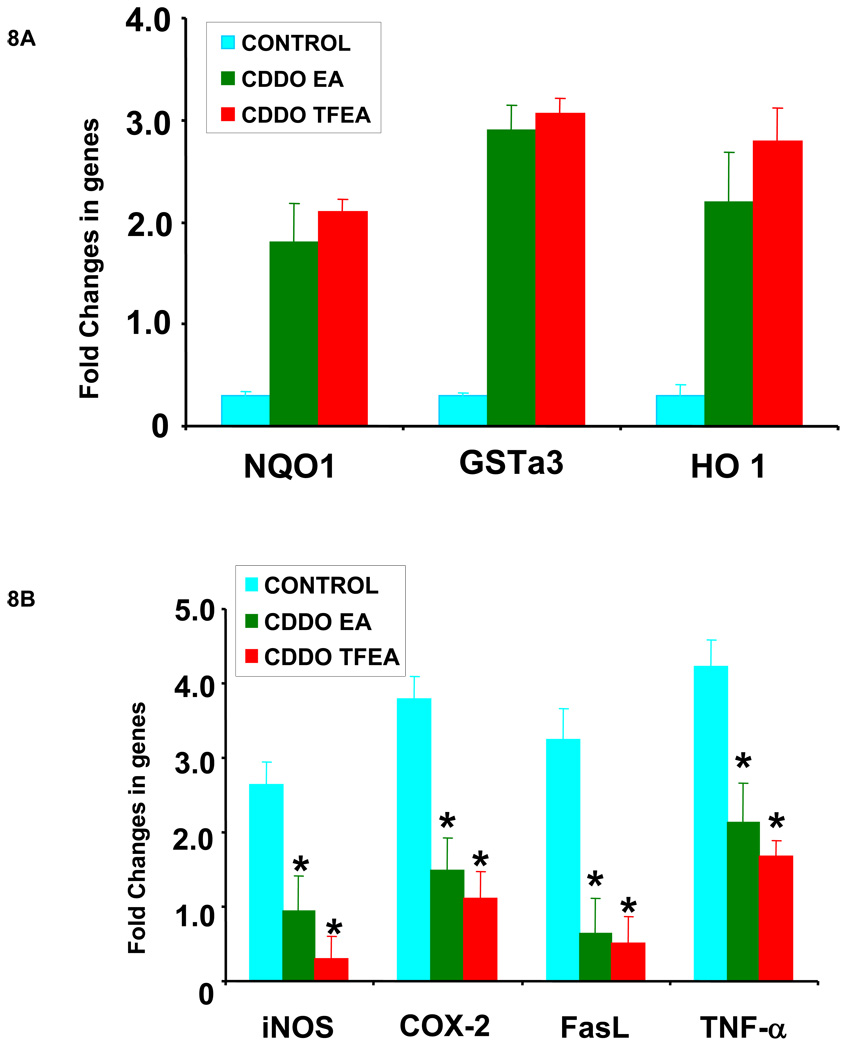
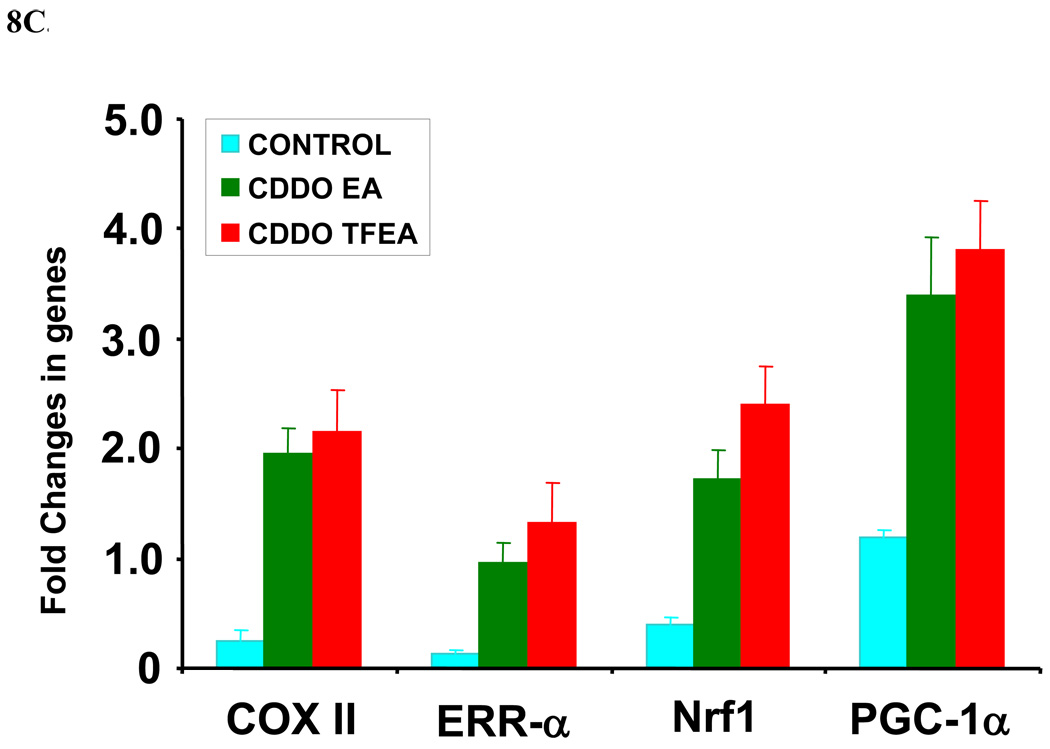
CDDO-EA and CDDO-TFEA are bio-available, potent and penetrate the blood-brain-barrier in mice (Figure 6). Chronic administration of triterpenoids (400 mg/kg food) resulted up to 500 nM of triterpenoids in the brains HD mice [29]. We studied the effects of CDDO-EA and CDDO-TFEA (400 mg/kg food) in a mouse model of ALS. We treated G93A SOD1 transgenic mice with CDDO-TFEA and CDDO-EA for 10 days and analyzed coronal sections of spinal cord from CDDO-treated and vehicle-treated G93A mice for Nrf2 expression and localization. We found that both CDDO-EA and CDDO-TFEA upregulated the expression and translocation of Nrf2 from the cytoplasm to the nucleus of neurons in the spinal cord. The spinal cord sections from vehicle-treated G93A mice were examined and we noticed a diffuse although faint staining of Nrf2 in the cytoplasm of larger neurons (Figure 7). To determine whether this activation of Nrf2 and nuclear translocation modulate the Nrf2/ARE-regulated genes, we isolated total RNA from mouse spinal cord and tested a sample of genes that are known to be regulated by the Nrf2/ARE system using real time RT-PCR. Expression of 7 genes (NQO-1, HO-1, GST-a3, iNOS, COX-2, TNF-alpha and FasL) was examined. We found the antioxidant genes were upregulated and that neuroinflammatory genes were down-regulated. Both CDDO-EA and CDDO-TFEA caused upregulation of NQO-1, HO-1 and GST-a3 cDNA (Figure 8A) and down-regulation of iNOS, COX-2, FasL and TNF-alpha (Figure 8B). Furthermore, we found that these triterpenoids upregulated a key mitochondria biogenesis promoting gene, PGC1-alpha, and the downstream genes, COX II, NRF-1, and ERRalpha-1 (Figure 8C).
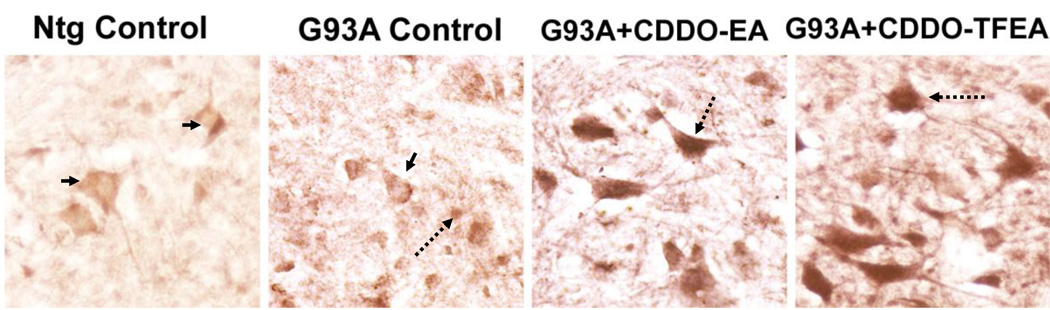
Figure 7. CDDO-EA and CDDO-TFEA treatment cause translocation of Nrf2 from the cytoplasm to the nucleus in motor neurons in the spinal cords of ALS mice.
G93A SOD1 mice were fed food containing CDDO-EA or CDDO-TFEA (400 mg/kg) for 10 days. Non-transgenic controls and G93A controls were fed control food. Spinal cord sections were prepared and stained with anti-Nrf2. Non-transgenic control spinal cord sections were weakly stained with anti-Nrf2 in the cytoplasm. G93A control spinal cord sections show mild Nrf2 staining. Larger motor neurons show Nrf2 only in the cytoplasm, while some smaller neurons/glial cells show nuclear staining. G93A mice treated with CDDO-EA or CDDO-TFEA produced strong nuclear staining for Nrf2 (n=5 per group). Arrows point to the nucleus (lightly stained in controls and darkly stained in CDDO-EA or CDDO-TFEA panels).
Figure 8.
A: Nrf2/ARE activation is upregulated by antioxidant genes in G93A spinal cord following triterpenoid treatment. Seventy-five day old G93A SOD1 transgenic mice were fed with either vehicle or CDDO-EA or CDDO-TFEA (400 mg/kg in food) daily for ten days. Spinal cords were analyzed for genes involved in antioxidant response by quantitative real time RT-PCR. Data represent Mean ± SEM (n=4, *p<0.001). Beta actin or GAPDH TaqMan assays were used as control genes in real time RT-PCR to compare the difference in expression level of antioxidant genes in samples from treated and untreated G93A SOD1 mice. Student’s t-test compared to control.
B: Nrf2/ARE activation upregulated inflammatory genes in G93A spinal cord following triterpenoid treatment. Seventy-five day old G93A SOD1 transgenic mice were fed with either vehicle or CDDO-EA or CDDO-TFEA (400 mg/kg in food) daily for ten days. Spinal cords were analyzed for genes involved in inflammatory response by quantitative real time RT-PCR. Data represent Mean ± SEM (n=4, *p<0.001). Beta actin or GAPDH TaqMan assays were used as control genes in real time RT-PCR to compare the difference in expression level in inflammatory genes in samples from treated and untreated G93A SOD1 mice. Student’s t-test compared to control.
C: Nrf2/ARE activation upregulated mitochondrial biogenesis genes in G93A spinal cord following triterpenoid treatment. Seventy-five day old G93A SOD1 transgenic mice were fed with either vehicle or CDDO-EA or CDDO-TFEA (400 mg/kg in food) daily for ten days. Spinal cords were analyzed for genes involved in mitochondrial biogenesis by quantitative real time RT-PCR. Data represent Mean ± SEM (n=4, *p<0.001). Beta actin or GAPDH TaqMan assays were used as control genes in real time RT-PCR to compare the difference in expression level in mitochondrial enhancing genes in samples from treated and untreated G93A SOD1 mice. Students t-test compared to control.
Nrf2/ARE activation by triterpenoids increased survival of G93A SOD1 mice
a) Treatment with triterpenoids at a “presymptomatic Age”
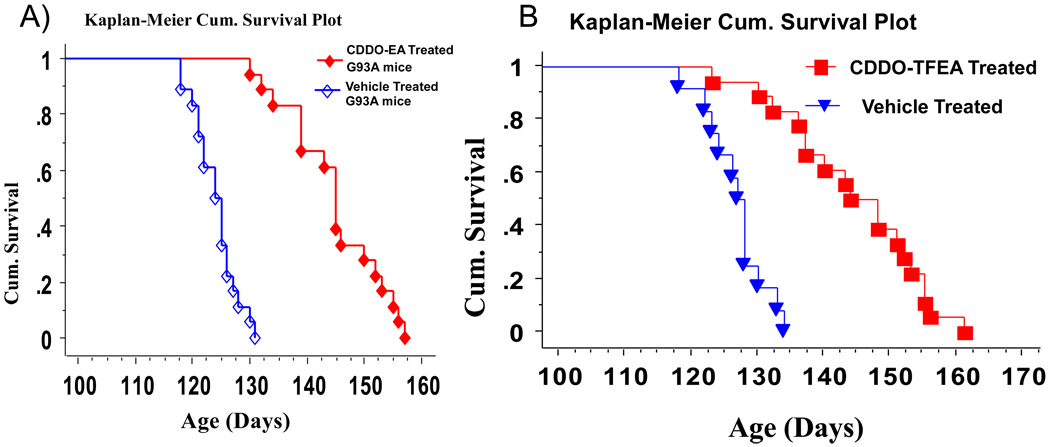
We treated G93A SOD1 mice with CDDO-EA and CDDO-TFEA starting at 30 days of age in two separate cohorts. In these experiments, we found that triterpenoid-treated mice retained higher weights and enhanced motor performance compared to G93A SOD1 mice treated with vehicle (control food) (data not shown). The survival analysis showed that G93A mice treated with CDDO-EA or CDDO-TFEA, compared to G93A littermate controls, lived significantly longer. CDDO-EA treatment increased the life-span by 20.6 days from 124.05 ± 3.7 days to 144.72 ± 8.1 days (16.6%) (Figure 8A) (p<0.001). CDDO-TFEA treatment increased the life-span by 17.6 days from 126.4 ± 3.9 days to 144 ± 10.4 days (14.3%) (Figure 8B) (p<0.001).
b) Treatment with Triterpenoids at a “symptomatic age”
We treated G93A SOD1 mice with the triterpenoid Nrf2/ARE activators; CDDO-EA and CDDO-TFEA, from the onset of symptoms assessed by impairment of rotarod performance, and monitored their weights, motor performance and survival. We found that triterpenoid treatment attenuated weight loss, and motor performance was preserved as compared to G93A SOD1 mice treated with control food (data non shown).
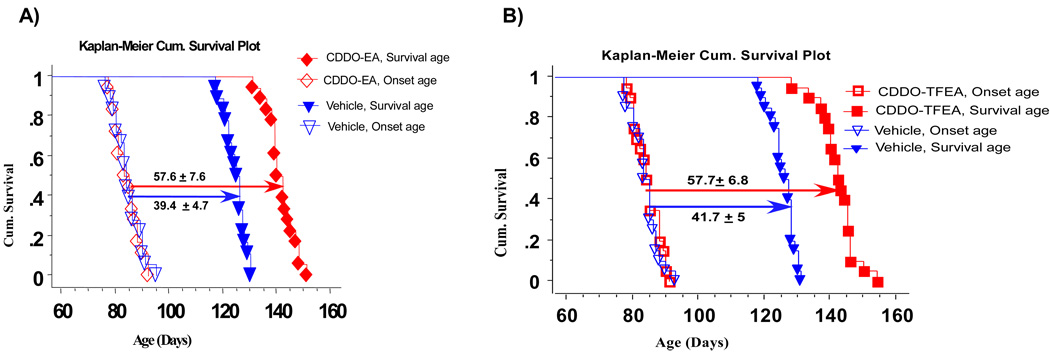
We measured the age of onset and the age of death in both groups. The average age of onset was 84.4 ± 5 days, and this was the age when treatment began. The age of death in the control group was 124.5 ± 3.9 days and the duration from the age of onset to the age of death was 40.1 ± 4.7 days. In CDDO-EA-treated G93A mice, the age of death was 141.4 ± 5.2 days and the duration from the age of onset to the age of death was 57.6 ± 7.6 days, which means that the age of death from onset was prolonged by 17.5 days (43%) (Figure 9A). In the CDDO-TFEA treatment cohort, the duration from onset to age of death was extended by 16 days (38%) (Figure 9B) (p<;0.001).
Figure 9.
A: CDDO-EA treatment extends survival in G93A SOD1 mice. G93A SOD1 mice (n=18) were treated with CDDO-EA (starting at 30 days of age). The control group (n=18) was treated with control food (Chaw diet # 5002). The data were analyzed and compared using the Kaplan-Meier plot. Survival is significantly longer in CDDO-EA treated G93A SOD1 mice vs controls (p=0.001, Logrank, Mental-Cox).
B: CDDO-TFEA treatment extends survival in G93A SOD1 mice. G93A SOD1 mice (n=18) were treated with CDDO-TFEA (starting at 30 days of age). The control group (n=18) was treated with control food (Chaw diet # 5002). The data were analyzed and compared using the Kaplan-Meier plot. Survival is significantly longer in CDDO-TFEA-treated G93A SOD1 mice vs controls (P=0.001, Logrank, Mental-Cox).
DISCUSSION
The Nrf2/ARE signaling system is a powerful defense system that exists in higher organisms and which regulates the expression of over 250 phase II detoxification genes and protects against xenobiotics and cellular insults [30]. Activation of the Nrf2/ARE system by triterpenoids has therapeutic potential for neurodegenerative diseases such as ALS. Triterpenoids (CDDO-EA and CDDO-TFEA) are potent activators of the Nrf2/ARE signaling program. We recently reported the neuroprotective effects of CDDO-methylamide in Parkinson’s disease (PD) mouse models [31] and in Alzheimer’s disease (AD) transgenic mouse models [32], and neuroprotective effects of CDDOethylamide and CDDO-ethylamide trifluoride in Huntington’s disease (HD) animal models [29].
Oxidative damage, neuroinflammation and mitochondrial dysfunction are postulated to play important roles in the pathogenesis of neurodegenerative diseases. The Nrf2/ARE signaling pathway controls the upregulation of an array of genes involved in antioxidant responses, heat shock chaperone proteins and mitochondria-protective genes, and the down-regulation of genes involved in neuroinflammation, as shown previously and in this study [29, 31, 32].
Our observation of reduced Nrf2 expression in untreated G93A SOD1 mice spinal cord suggests that neuronal cells may not be able to induce the Nrf2/ARE system on their own even under oxidative stress. The increase in Nrf2 expression [33] and its nuclear translocation by CDDOs in the cell culture and in the ALS mouse model, as well as the subsequent upregulation of classical Nrf2-regulated genes (Figures 3–6), provides evidence that the neuroprotective effects of CDDOs are mediated by the Nrf2/ARE signaling system. Earlier studies found that DJ-1 (Park 7) stabilizes Nrf2 [34] and is involved in pathway modulation for Nrf2 activation and nuclear translocation [35].
Although Nrf2-deficient mice are born and develop normally [36], they are highly sensitive to environmental stress [37]. Aged Nrf2-deficient mice develop a degenerative disorder characterized by destruction of myelin sheaths in the nervous system, which is accompanied by the widespread presence of activated glia [38]. Transgenic mice overexpressing Nrf2 in astrocytes under control of glial fiblilary acidic protein promoter crossed with G93A SOD1 transgenic mouse model of ALS significantly delayed onset and extended survival possibly due to increase in glutathione in the spinal cord [39]. Experiments using Nrf2 knockout mice, and primary astroglial and neuronal cells derived from these mice, showed that disruption of Nrf2 renders neuronal tissue more susceptible to oxidative stress, induced by either H2O2, or mitochondrial complex inhibitors [40, 41]. Conversely, Nrf2 overexpression in rat-mixed neuronal-glial cortical cultures enhances anti-oxidant capacity in both neuronal and astroglial cells, and protects cortical neurons from excitotoxicity [42]. These effects were associated with upregulation of glutathione synthesis. Pre-conditioning of tissue by transplantation of Nrf2 over-expressing astrocytes protects against the neurotoxic effects of the mitochondrial complex 2 inhibitor malonate [42]. Nrf2-deficient mice are also more susceptible to 3-NP toxicity, and it has been shown that increasing Nrf2 activity with an inducer attenuates 3-NP toxicity in wild type mice [41]. Transgenic mice overexpressing Nrf2 in astrocytes shown to be resistant to malonate lesioning [43].
The cytoprotective potency of the Nrf2/ARE-mediated response, led us to hypothesize that therapeutic strategies that lead to the stabilization and translocation of Nrf2 to the nucleus may be effective in combating oxidative stress and ongoing neurodegeneration in ALS. A number of recent studies demonstrate involvement of Nrf2 expression in astrocytes in ALS [44]. One of the critical enzymes induced by the Nrf2 signaling system is NADPH quinone oxidoreductase 1 (NQO-1). This enzyme is involved in detoxification of protein-bound quinones. It also functions to maintain both alpha-tocopherol and coenzyme Q10 in their reduced, antioxidant states.
The transcription factor, PGC-1α (peroxisome proliferator-activated receptor-γ coactivator 1α) plays a role in energy homeostasis, adaptive thermogenesis, β-oxidation of fatty acids and glucose metabolism (reviewed by Puigserver & Spiegelman [45]). Originally identified as a PPAR-γ-interacting protein in brown adipose tissue, PGC-1α and its close ortholog, PGC-1β, are highly expressed in tissues with increased energy demands and large numbers of mitochondria, including brown adipose tissue, heart muscle and slow-twitch skeletal muscle [46]. PGC-1α has the ability to activate different sets of metabolic programs in different tissues due to its ability to form heteromeric complexes with a variety of transcription factors, including NRF-1 and NRF-2 (nuclear respiratory factor) and the nuclear hormone receptors PPARα, PPARδ and ERRα [47]. Heterodimers with NRF-1, NRF-2 and ERRα regulate the expression of many nuclear-encoded mitochondrial genes, including cytochrome c, complexes I–V and mitochondrial transcription factor A (Tfam) [48]. Its role in the regulation of energy metabolism and antioxidant enzymes suggests that upregulation of PGC-1α could be beneficial to ALS patients. Another ARE-dependent protein is the stress-responsive and inducible enzyme hemoxygenase-1 (HO-1). HO-1 is involved in the metabolism of the pro-oxidant heme to the antioxidant pigment biliverdin, ferrous iron, and carbon monoxide. HO-1 also has profound anti-inflammatory properties. The Nrf2/ARE signaling pathway also regulates aldo-keto reductases (AKRCs). These enzymes function to eliminate chemically reactive carbonyl groups [49]. AKRCs are valuable in the study of activation of Nrf2 because of their enzymatic reductase activity [50].
An important proinflammatory cytokine receptor is Fas which plays a role in ALS pathogenesis [10, 51, 52]. Nrf2 directly inhibits Fas-mediated apoptosis [53]. Two genes that are down-regulated by Nrf2/ARE are iNOS and the prostanoid synthesizing enzyme cyclooxygenase-2 (COX-2); these two proinflammatory genes are implicated in the pathogenesis of ALS [6, 7, 51, 54–56]. There is therefore considerable evidence that Nrf2-induced, ARE-dependent gene transcription produces neuroprotective effects.
Sarlette et al. is the first study which examined Nrf2 in ALS patients and it showed a deficiency of Nrf2 in the ALS motor cortex and spinal cord [23]. We found that treating G93A SOD1 mice with Nrf2/ARE activators (triterpenoids) resulted in a significant increase in survival, which indicates that activation of Nrf2 has a neuroprotective effect.
Our previous studies have shown that compounds that diminish oxidative stress and inflammation, result in reduction of neuronal cell loss and inflammatory markers in G93A SOD1 mice. However, a critical barrier to progress in development of better therapeutic strategies is lack of an agent that acts on multiple pathways simultaneously to effectively block neuronal death. It may be necessary to block multiple toxic pathways to develop an effective therapy for ALS.
In summary, our study showed that CDDO-EA and CDDO-TFEA are able to activate the Nrf2/ARE system in cell cultures and in a mouse model of ALS. The compounds regulate three families of genes with neuroprotective effects: antioxidants, anti-inflammatory and mitochondria-enhancing genes. CDDOs therefore have great clinical promise for patients with cancer, metabolic and neurodegenerative diseases. They are currently being investigated in clinical trials sponsored by Reata Pharmaceuticals for cancer and diabetes type II. Therefore, targeting the Nrf2/ARE system to induce endogenous anti-oxidative and anti-inflammatory responses is of great interest for neurodegenerative cases.
Figure 10. A) Treatment with CDDO-EA and B) CDDO-TFEA from the onset of ALS extends survival in G93A SOD1 mice.
G93A SOD1 mice (n=18) were treated with CDDO-EA or CDDO-TFEA from the age of onset of ALS. The control group (n=18) was treated with control food (Chaw diet # 5002). The data were analyzed and compared using the Kaplan-Meier plot. Survival is significantly longer for CDDO-EA and CDDO-TFEA-treated G93A SOD1 mice vs controls (P=0.001, Logrank, Mental-Cox).
Acknowledgements
This work was supported by Research Sponsored Grant from NIH grant (NS062302) to MK. We thank Reata Pharmaceuticals (Dallas, TX, USA) for providing Triterpenoids for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maragakis NJ, Rothstein JD. Glutamate transporters in neurologic disease. Arch Neurol. 2001;58(3):365–370. doi: 10.1001/archneur.58.3.365. [DOI] [PubMed] [Google Scholar]
- 2.Rothstein JD. Excitotoxic mechanisms in the pathogenesis of amyotrophic lateral sclerosis. Adv Neurol. 1995;68:7–20. discussion 21–7. [PubMed] [Google Scholar]
- 3.Wood JD, Beaujeux TP, Shaw PJ. Protein aggregation in motor neurone disorders. Neuropathol Appl Neurobiol. 2003;29(6):529–545. doi: 10.1046/j.0305-1846.2003.00518.x. [DOI] [PubMed] [Google Scholar]
- 4.Lariviere RC, Julien JP. Functions of intermediate filaments in neuronal development and disease. J Neurobiol. 2004;58(1):131–148. doi: 10.1002/neu.10270. [DOI] [PubMed] [Google Scholar]
- 5.Agar J, Durham H. Relevance of oxidative injury in the pathogenesis of motor neuron diseases. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4(4):232–242. doi: 10.1080/14660820310011278. [DOI] [PubMed] [Google Scholar]
- 6.Kiaei M, et al. Integrative role of cPLA with COX-2 and the effect of non-steriodal anti-inflammatory drugs in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurochem. 2005;93(2):403–411. doi: 10.1111/j.1471-4159.2005.03024.x. [DOI] [PubMed] [Google Scholar]
- 7.Almer G, et al. Increased expression of the pro-inflammatory enzyme cyclooxygenase-2 in amyotrophic lateral sclerosis. Ann Neurol. 2001;49(2):176–185. [PubMed] [Google Scholar]
- 8.Kiaei M, et al. Thalidomide and lenalidomide extend survival in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci. 2006;26(9):2467–2473. doi: 10.1523/JNEUROSCI.5253-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hensley K, et al. Message and protein-level elevation of tumor necrosis factor alpha (TNF alpha) and TNF alpha-modulating cytokines in spinal cords of the G93A-SOD1 mouse model for amyotrophic lateral sclerosis. Neurobiol Dis. 2003;14(1):74–80. doi: 10.1016/s0969-9961(03)00087-1. [DOI] [PubMed] [Google Scholar]
- 10.Raoul C, et al. Motoneuron death triggered by a specific pathway downstream of Fas. potentiation by ALS-linked SOD1 mutations. Neuron. 2002;35(6):1067–1083. doi: 10.1016/s0896-6273(02)00905-4. [DOI] [PubMed] [Google Scholar]
- 11.Kabashi E, et al. Focal dysfunction of the proteasome: a pathogenic factor in a mouse model of amyotrophic lateral sclerosis. J Neurochem. 2004;89(6):1325–1335. doi: 10.1111/j.1471-4159.2004.02453.x. [DOI] [PubMed] [Google Scholar]
- 12.Urushitani M, et al. Proteasomal inhibition by misfolded mutant superoxide dismutase 1 induces selective motor neuron death in familial amyotrophic lateral sclerosis. J Neurochem. 2002;83(5):1030–1042. doi: 10.1046/j.1471-4159.2002.01211.x. [DOI] [PubMed] [Google Scholar]
- 13.Urushitani M, et al. CHIP promotes proteasomal degradation of familial ALS-linked mutant SOD1 by ubiquitinating Hsp/Hsc70. J Neurochem. 2004;90(1):231–244. doi: 10.1111/j.1471-4159.2004.02486.x. [DOI] [PubMed] [Google Scholar]
- 14.Xu Z, et al. Mitochondrial degeneration in amyotrophic lateral sclerosis. J Bioenerg Biomembr. 2004;36(4):395–399. doi: 10.1023/B:JOBB.0000041774.12654.e1. [DOI] [PubMed] [Google Scholar]
- 15.Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7(9):710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- 16.Przedborski S. Programmed cell death in amyotrophic lateral sclerosis: a mechanism of pathogenic and therapeutic importance. Neurologist. 2004;10(1):1–7. doi: 10.1097/01.nrl.0000106920.84668.37. [DOI] [PubMed] [Google Scholar]
- 17.DiBernardo AB, Cudkowicz ME. Translating preclinical insights into effective human trials in ALS. Biochim Biophys Acta. 2006;1762(11–12):1139–1149. doi: 10.1016/j.bbadis.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Bellingham MC. A Review of the Neural Mechanisms of Action and Clinical Efficiency of Riluzole in Treating Amyotrophic Lateral Sclerosis: What have we Learned in the Last Decade? CNS Neurosci Ther. 2010 doi: 10.1111/j.1755-5949.2009.00116.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moi P, et al. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci U S A. 1994;91(21):9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rushmore TH, Morton MR, Pickett CB. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991;266(18):11632–11639. [PubMed] [Google Scholar]
- 21.Itoh K, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236(2):313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 22.Kwak MK, et al. Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol Cell Biol. 2003;23(23):8786–8794. doi: 10.1128/MCB.23.23.8786-8794.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarlette A, et al. Nuclear erythroid 2-related factor 2-antioxidative response element signaling pathway in motor cortex and spinal cord in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2008;67(11):1055–1062. doi: 10.1097/NEN.0b013e31818b4906. [DOI] [PubMed] [Google Scholar]
- 24.Cashman NR, et al. Neuroblastoma x spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev Dyn. 1992;194(3):209–221. doi: 10.1002/aja.1001940306. [DOI] [PubMed] [Google Scholar]
- 25.Magrane J, et al. Mutant SOD1 in neuronal mitochondria causes toxicity and mitochondrial dynamics abnormalities. Hum Mol Genet. 2009;18(23):4552–4564. doi: 10.1093/hmg/ddp421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haastert K, et al. Rat embryonic motoneurons in long-term co-culture with Schwann cells--a system to investigate motoneuron diseases on a cellular level in vitro. J Neurosci Methods. 2005;142(2):275–284. doi: 10.1016/j.jneumeth.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Gurney ME, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264(5166):1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 28.Honda T, et al. A novel dicyanotriterpenoid, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-onitrile, active at picomolar concentrations for inhibition of nitric oxide production. Bioorg Med Chem Lett. 2002;12(7):1027–1030. doi: 10.1016/s0960-894x(02)00105-1. [DOI] [PubMed] [Google Scholar]
- 29.Stack C, et al. Triterpenoids CDDO-ethyl amide and CDDO-trifluoroethyl amide improve the behavioral phenotype and brain pathology in a transgenic mouse model of Huntington's disease. Free Radic Biol Med. 2010 doi: 10.1016/j.freeradbiomed.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sykiotis GP, Bohmann D. Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell. 2008;14(1):76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang L, et al. Neuroprotective effects of the triterpenoid, CDDO methyl amide, a potent inducer of Nrf2-mediated transcription. PLoS One. 2009;4(6):e5757. doi: 10.1371/journal.pone.0005757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dumont M, et al. Triterpenoid CDDO-methylamide improves memory and decreases amyloid plaques in a transgenic mouse model of Alzheimer's disease. J Neurochem. 2009;109(2):502–512. doi: 10.1111/j.1471-4159.2009.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lewerenz J, et al. Induction of Nrf2 and xCT are involved in the action of the neuroprotective antibiotic ceftriaxone in vitro. J Neurochem. 2009;111(2):332–343. doi: 10.1111/j.1471-4159.2009.06347.x. [DOI] [PubMed] [Google Scholar]
- 34.Clements CM, et al. DJ-1, a cancer- and Parkinson's disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci U S A. 2006;103(41):5091–5096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sykiotis GP, Bohmann D. Stress-activated cap'n'collar transcription factors in aging and human disease. Sci Signal. 2010;3(112):re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan K, et al. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc Natl Acad Sci U S A. 1996;93(24):13943–13948. doi: 10.1073/pnas.93.24.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Derjuga A, et al. Complexity of CNC transcription factors as revealed by gene targeting of the Nrf3 locus. Mol Cell Biol. 2004;24(8):3286–3294. doi: 10.1128/MCB.24.8.3286-3294.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hubbs AF, et al. Vacuolar leukoencephalopathy with widespread astrogliosis in mice lacking transcription factor Nrf2. Am J Pathol. 2007;170(6):2068–2076. doi: 10.2353/ajpath.2007.060898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vargas MR, et al. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci. 2008;28(50):13574–13581. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JM, et al. NF-E2-related factor-2 mediates neuroprotection against mitochondrial complex I inhibitors and increased concentrations of intracellular calcium in primary cortical neurons. J Biol Chem. 2003;278(39):37948–37956. doi: 10.1074/jbc.M305204200. [DOI] [PubMed] [Google Scholar]
- 41.Calkins MJ, et al. Protection from mitochondrial complex II inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc Natl Acad Sci U S A. 2005;102(1):244–249. doi: 10.1073/pnas.0408487101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shih AY, et al. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23(8):3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calkins MJ, et al. Astrocyte-specific overexpression of Nrf2 protects striatal neurons from mitochondrial complex II inhibition. Toxicol Sci. 2010;115(2):557–568. doi: 10.1093/toxsci/kfq072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vargas MR, et al. Increased glutathione biosynthesis by Nrf2 activation in astrocytes prevents p75NTR-dependent motor neuron apoptosis. J Neurochem. 2006;97(3):687–696. doi: 10.1111/j.1471-4159.2006.03742.x. [DOI] [PubMed] [Google Scholar]
- 45.Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24(1):78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 46.Puigserver P, et al. Involvement of the retinoblastoma protein in brown and white adipocyte cell differentiation: functional and physical association with the adipogenic transcription factor C/EBPalpha. Eur J Cell Biol. 1998;77(2):117–123. doi: 10.1016/s0171-9335(98)80079-4. [DOI] [PubMed] [Google Scholar]
- 47.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1(6):361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18(4):357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- 49.Bachur NR. Cytoplasmic aldo-keto reductases: a class of drug metabolizing enzymes. Science. 1976;193(4253):595–597. doi: 10.1126/science.959821. [DOI] [PubMed] [Google Scholar]
- 50.Felsted RL, Richter DR, Bachur NR. Rat liver aldehyde reductase. Biochem Pharmacol. 1977;26(12):1117–1124. doi: 10.1016/0006-2952(77)90054-5. [DOI] [PubMed] [Google Scholar]
- 51.Kiaei M, et al. Matrix metalloproteinase-9 regulates TNF-alpha and FasL expression in neuronal, glial cells and its absence extends life in a transgenic mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2007;205(1):74–81. doi: 10.1016/j.expneurol.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 52.Petri S, et al. Loss of Fas ligand-function improves survival in G93A-transgenic ALS mice. J Neurol Sci. 2006;251(1–2):44–49. doi: 10.1016/j.jns.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 53.van Muiswinkel FL, Kuiperij HB. The Nrf2-ARE Signalling pathway: promising drug target to combat oxidative stress in neurodegenerative disorders. Curr Drug Targets CNS Neurol Disord. 2005;4(3):267–281. doi: 10.2174/1568007054038238. [DOI] [PubMed] [Google Scholar]
- 54.Klivenyi P, et al. Additive neuroprotective effects of creatine and cyclooxygenase 2 inhibitors in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurochem. 2004;88(3):576–582. doi: 10.1046/j.1471-4159.2003.02160.x. [DOI] [PubMed] [Google Scholar]
- 55.Almer G, et al. Increased levels of the pro-inflammatory prostaglandin PGE2 in CSF from ALS patients. Neurology. 2002;58(8):1277–1279. doi: 10.1212/wnl.58.8.1277. [DOI] [PubMed] [Google Scholar]
- 56.Drachman DB, et al. Cyclooxygenase 2 inhibition protects motor neurons and prolongs survival in a transgenic mouse model of ALS. Ann Neurol. 2002;52(6):771–778. doi: 10.1002/ana.10374. [DOI] [PubMed] [Google Scholar]