Abstract
All of the members of the Microsporidia possess a unique, highly specialised structure, the polar tube. This article reviews the available data on the organisation, structure and function of this invasion organelle. It was over 100 years ago that Thelohan accurately described the microsporidian polar tube and the triggering of its discharge. In the spore, the polar tube is connected at the anterior end, and then coils around the sporoplasm. Upon appropriate environmental stimulation the polar tube rapidly discharges out of the spore pierces a cell membrane and serves as a conduit for sporoplasm passage into the new host cell. The mechanism of germination of spores, however, remains to be definitively determined. In addition, further studies on the characterisation of the early events in the rupture of the anterior attachment complex, eversion of the polar tube as well as the mechanism of host cell attachment and penetration are needed in order to clarify the function and assembly of this structure. The application of immunological and molecular techniques has resulted in the identification of three polar tube proteins referred to as PTP1, PTP2 and PTP3. The interactions of these identified proteins in the formation and function of the polar tube remain to be determined. Data suggest that PTP1 is an O-mannosylated glycoprotein, a post-translational modification that may be important for its function. With the availability of the Encephalitozoon cuniculi genome it is now possible to apply proteomic techniques to the characterisation of the components of the microsporidian spore and invasion organelle.
Keywords: Microsporidia, Polar tube protein, Germination, Polaroplast, Encephalitozoon, Brachiola, Nosema
1. Overview
Microsporidia are eukaryotic, obligate intracellular, spore-forming parasites in the phylum Microsporidia (Sprague, 1977; Sprague and Becnel, 1998). They are ubiquitous in the animal kingdom with over a 1000 species parasitising a wide range of invertebrate and vertebrate hosts, including humans (Wittner and Weiss, 1999). While it had been suggested that the Microsporidia are primitive eukaryotes lacking mitochondria, recent data suggests they are related to the Fungi (Weiss et al., 1999; Keeling, 2003; Thomarat et al., 2004), have homologues for mitochondrial hsp70 (Hirt et al., 1997; Arisue et al., 2002) and a mitochondrial relic organelle (the mitosome) (Williams et al., 2002). Encephalitozoon species have very small genomes (2.9, 2.6, and 2.3 Mb in Encephalitozoon cuniculi, Encephalitozoon hellem and Encephalitozoon intestinalis, respectively) (Biderre et al., 1999; Wittner and Weiss, 1999). With the completion of the E. cuniculi genome, the phylogenetic relationship between microsporidia and fungi has been further solidified by the presence of numerous genes that on phylogenetic analysis cluster the Microsporidia with the Fungi (Katinka et al., 2001; Thomarat et al., 2004). The fungal origin of microsporidia has a significant impact on how we interpret their unusual characteristics. They no longer represent ancestral features, but instead are indicative of the highly derived nature of these intracellular parasites.
In 1857, microsporidia were first recognised as pathogens in silkworms, and long before they were described as human pathogens they were recognised as a cause of disease in many nonhuman hosts (Franzen and Muller, 2001). The first suggestion that microsporidia are associated with human infections was made in 1924, and up to 1985, there were less than a dozen reports of human microsporidiosis (Wittner and Weiss, 1999). Since 1985 with the recognition that Enterocytozoon bieneusi causes diarrhoea in patients with AIDS (Desportes et al., 1985), many infections with different species of microsporidia have been reported from all over the world and microsporidia are now frequently recognised as etiologic agents of opportunistic infections in persons with AIDS, and more recently, in organ transplant recipients, patients being treated with immunosuppressive drugs and immunocompetent patients (Weber and Bryan, 1994; Sax et al., 1995; Wanke et al., 1996; Bryan et al., 1997; Raynaud et al., 1998; Gumbo et al., 1999; Wittner and Weiss, 1999; Metge et al., 2000; Lores et al., 2002; Lewis et al., 2003).
Although the phylum Microsporidia consists of nearly 150 genera only seven genera Enterocytozoon, Encephalitozoon (including Septata), Pleistophora, Trachipleistophora, Vittaforma, Brachiola and Nosema as well as a few unclassified microsporidia (e.g. Microsporidium) have been described as pathogens in humans (Sprague et al., 1992; Wittner and Weiss, 1999; Franzen and Muller, 2001). Reported prevalence rates in the 25 studies conducted on patients with HIV infection before the widespread use of highly active antiretroviral therapy demonstrated rates that varied between 2 and 70% depending on the symptoms of the population studied and the diagnostic technique employed (Weber and Bryan, 1994; Drobniewski et al., 1995; van Gool et al., 1995; Weitz et al., 1995; Coyle et al., 1996; Deplazes et al., 2000). In immunocompromised patients, Encephalitozoon has been associated with hepatitis, peritonitis, keratoconjunctivitis, sinusitis and disseminated infections involving the lungs and/or kidneys (Zender et al., 1989; Moss et al., 1997; Weber et al., 1997, 1999; Wittner and Weiss, 1999; Franzen and Muller, 2001). Three cases of Pleistophora-like microsporidian infection involving skeletal muscles and myositis have been described in two HIV-infected patients and in a non-HIV-infected patient (Chupp et al., 1993; Cali and Takvorian, 2003). The genus Trachipleistophora contains two species Trachipleistophora hominis (Hollister et al., 1996), which has caused myositis and Trachipleistophora anthropophthera (Vavra et al., 1998), which has been associated with encephalitis and disseminated infection in immune compromised patients. The genus Brachiola has three species that have been reported in human infections Brachiola vesicularum, Brachiola conorii and Brachiola algerae (Coyle et al., 2004). Vittaforma corneae infection has presented as corneal disease as well as a disseminated infection (Deplazes et al., 1998).
Microsporidian spores are commonly found in surface water and human pathogenic Microsporidia have been found in municipal water supplies, tertiary sewage effluent and ground water (Avery and Undeen, 1987; Sparfel et al., 1997; Dowd et al., 1998; Cotte et al., 1999). It is possible that many of the Microsporidia are zoonotic human infections. Microsporidia of the genus Encephalitozoon are widely distributed parasites of mammals and birds and the onset of microsporidiosis has been associated with exposure to livestock, fowl and pets (Deplazes et al., 2000). Most microsporidian infections are transmitted by oral ingestion of spores with the site of initial infection being the gastrointestinal tract. Viable infective spores of Microsporidia are present in multiple body fluids (stool, urine, respiratory secretions, etc.) during infection suggesting that person to person transmission can occur and that ocular infection may be transmitted by external autoinoculation due to contaminated fingers (Schwartz et al., 1993b,c).
2. Structure of the microsporidian spore and polar tube
The microsporidian life cycle consists of a proliferative phase, the spore production phase (sporogony) and the mature spore or infective phase. The unicellular spore has a resistant spore wall, with a uninucleate or binucleate sporoplasm, and an extrusion apparatus consisting of a single polar tube with an anterior attachment complex, which is characteristic for the phylum (Wittner and Weiss, 1999; Fig. 1). Spores range in size from 1 to 12 μm. The spore coat consists of an electron dense, proteinaceous exospore, and electron lucent endospore composed of chitin and protein and an inner membrane or plasmalemma (Kudo, 1921; Vavra, 1976; Canning and Lom, 1986; Wittner and Weiss, 1999).
Fig. 1.
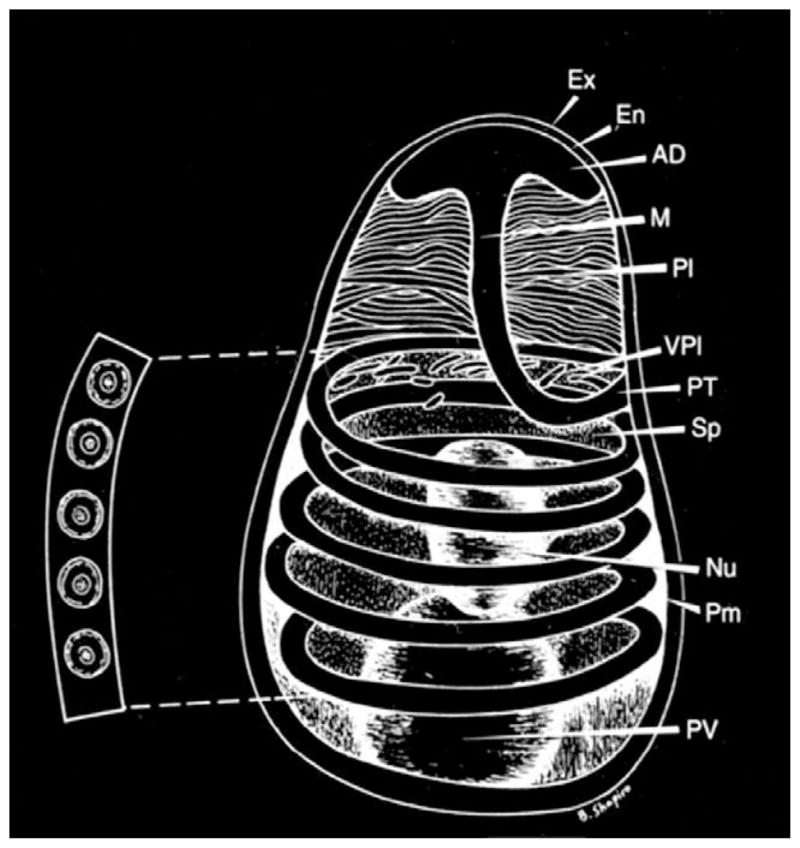
Diagram of a microsporidian spore. Spores range in size from 1 to 10 μm. The spore coat consists of an electron dense exospore (Ex), an electron lucent endospore (En) and plasma membrane (Pm). It is thinner at the anterior end of the spore. The sporoplasm (Sp) contains a single nucleus (Nu), the posterior vacuole (PV) and ribosomes. The polar filament is attached to the anterior end of the spore by an anchoring disc (AD), and is divided into two regions: the manubrium or straight portion (M), and the posterior region forming five coils (PT) around the sporoplasm. The manubrium is surrounded by the lamellar polaroplast (Pl) and vesicular polaroplast (VPl). The insert depicts a cross-section of the polar tube coils (five coils in this spore), demonstrating the various concentric layers of different electron density and electron dense core present in such cross-sections. [Reprinted with permission from Wittner, M., Weiss, L.M. (1999). The Microsporidia and Microsporidiosis. ASM Press, Washington, DC].
The invasion apparatus consists of a long polar tube which is divided into two regions: the anterior straight portion surrounded by a lamellar polaroplast and attached to the inside of the anterior end of the spore by an anchoring disc; and the posterior coiled region that forms from four to approximately 30 coils around the sporoplasm in the spore, depending on the species (Wittner and Weiss, 1999). While inside the spore, the core of the polar tube is sometimes referred to as a polar filament prior to discharge (Lom and Vavra, 1963; Weidner, 1972, 1976, 1982; Takvorian and Cali, 1986). Evagination of this filament during discharge forms a hollow tube that remains attached to the spore and permits the passage of the sporoplasm from the spore through this tube into its host cell (Ohshima, 1937; Walters, 1958; Lom and Vavra, 1963). The distal end of the polar filament has not been demonstrated and it remains unclear if it is blunt (i.e. closed) or open-ended in the spore (Erickson et al., 1968; Lom, 1972; Vavra, 1976; Chioralia et al., 1998). Depending on the genus, the sporoplasm may have one nucleus or may have two abutted nuclei called a diplokaryon. A posterior vacuole and numerous ribosomes, arranged in helical coils or in sheets, are also present in the sporoplasm (Vavra, 1976).
In cross-section, the polar filament inside the spore is composed of electron dense and electron lucent concentric layers that can range from as few as three to as many as 20 different layers in cross-section (Lom, 1972; Sinden and Canning, 1974; Vavra, 1976; Chioralia et al., 1998; Cali et al., 2002; Fig. 2). It appears that the thickness of the layers vary along the polar filament while number of layers vary with spore maturity (Vavra, 1976). Moreover, a different pattern of layers has been observed before, during and after extrusion (Lom, 1972; Vavra, 1976; Chioralia et al., 1998; Cali et al., 2002). An electron dense particulate material fills the centre of the filament (Kudo and Daniels, 1963; Lom and Vavra, 1963; Vavra, 1976) and it undergoes changes during the eversion process (Lom and Corliss, 1967; Cali et al., 2002). Weidner proposed that this material is unpolymerised polar tube protein (Weidner, 1972, 1976). Discharged polar tubes have a sheath which is sensitive to trypsin, is silver methenamine negative and is able to bind ferritin-conjugated concanavalin A (Weidner, 1972). Incompletely discharged tubes appear as a cylinder within a cylinder at their distal ends (Weidner, 1982). A homogenous pattern of subunits has been observed in completely and incompletely discharged tubes, which appears identical to the material inside the tube (Weidner, 1976, 1982).
Fig. 2.
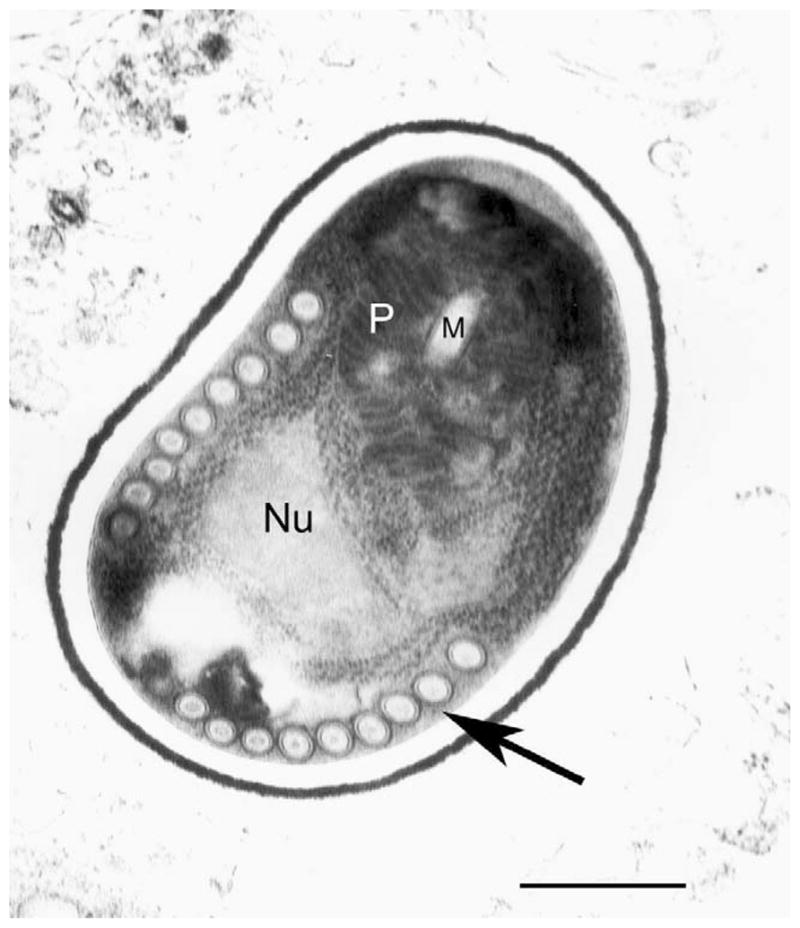
Ultrastructure of a microsporidian spore. Transmission electron micrograph of a spore from Brachiola algerae demonstrating the polar tube in cross-section (arrow). Nu, nucleus; M, manubrium; P, polaroplast. Bar=1 μm (courtesy of Ann Cali and Peter Takvorian, Rutgers University).
When triggered by appropriate stimuli, the polar tube rapidly discharges from the anterior pole of the spore forming a hollow tube that remains attached to the anterior end of the spore (Ohshima, 1937; Lom and Vavra, 1963; Weidner, 1972; Frixione et al., 1992) and the sporoplasm flows through the tube appearing as a droplet at its distal end (Ohshima, 1937; Lom and Vavra, 1963; Weidner, 1972; Frixione et al., 1992; Fig. 3). Lom (1972) and Weidner (1976) using electron microscopy demonstrated elongated sporoplasm in sections of extruded polar tube and the piercing of host cell membranes by the polar tube (Lom, 1972; Weidner, 1976). This process serves as a unique mechanism of infection resulting in sporoplasm transfer directly into the host cell cytoplasm (Ohshima, 1937; Lom and Vavra, 1963; Frixione et al., 1992; Weidner, 1972). In B. algerae, polar tube discharge is associated with the appearance of membrane infoldings surrounding the polar tube (Cali et al., 2002). These ultrastructural observations are suggestive that the polar tube is actually extracytoplasmic in the spore and explains how the sporoplasm can remain intact during the explosive germination reaction.
Fig. 3.
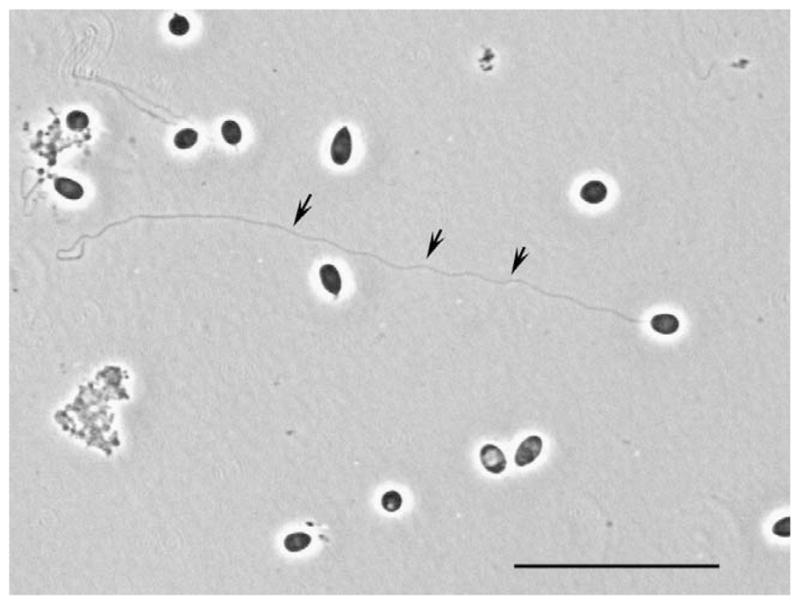
Germination of Brachiola algerae spores. Phase light microscopy demonstrating germination of a B. algerae spore in aqueous acidic media. The arrows point to an extruded polar tube. Bar = 25 μm.
Polar tubes range from 50 to 100 μm in length and 0.1 to 0.15 μm in diameter (Frixione et al., 1992). The polar tube has some flexibility in that it shows variation in diameter from 0.1 to 0.25 μm during discharge, its diameter can increase to 0.4 μm during sporoplasm passage, and its length shortens by 5–10% after sporoplasm passage (Ohshima, 1937; Lom and Vavra, 1963; Weidner, 1972; Frixione et al., 1992). The hollow discharged tubes appear to be 2–3 times as long as the dense, coiled tube inside the spore and it has been suggested that the internal contents of the tube are incorporated at its growing tip during discharge (Weidner, 1972, 1976, 1982; Frixione et al., 1992). The evagination of the polar filament has been likened to ‘reversing a finger of a glove’ (Oshshima 1937; Ishihara, 1968; Lom and Vavra, 1963; Weidner, 1972; Weidner and Byrd, 1982; Weidner et al., 1995). This theory is supported by observations using pulse labelling with latex particles and by video enhanced contrast microscopy (Weidner, 1982; Frixione et al., 1992). It has also been observed that the portion of the tube already everted remains unchanged while the tube elongates and even changes direction at the tip (Weidner, 1982; Frixione et al., 1992). It should be noted that spores can be broken by mechanical pressure or by glass bead disruption, thus releasing polar tubes from the sides of the spores (Kudo, 1921; Keohane et al., 1994, 1996a). It has been reported that polar tubes had a similar ultrastructural appearance regardless of whether they were triggered to activate and evert from the apical pole, or if they passively burst through the lateral walls of the spores (Weidner, 1982). Although it is now accepted that the sporoplasm flows through the discharged polar tube and into the host cell, the mechanisms of activation and tube formation during discharge still remain unclear.
3. The microsporidian spore wall
The spore wall is a major feature of microsporidian spores. Its mechanical properties provide resistance to environmental influences and allow the increase in hydrostatic pressure that causes spore discharge (Frixione et al., 1997). The anterior end of the spore, in front of the anchoring disk, has an area where the endospore is less thick and more electron-dense. Ultrastructural studies of the genus Encephalitozoon using TEM, freeze-fracture and deep-etching, demonstrated that the exospore is very complex and consists of three layers: an outer spiny layer, an intermediate electron-lucent lamina and an inner fibrous layer (Bigliardi et al., 1996). The endospore is observed as a space crossed by bridges connecting the exospore to the plasma membrane. Chitin, a major component of the endospore, has been suggested to be a component of the fibrils forming the bridges across the endospore and to be part of the fibrillar system of the exospore (Erickson and Blanquet, 1969; Vavra, 1976; Bigliardi et al., 1996). This hypothesis was confirmed by an immunohistochemical study in E. intestinalis (Prigneau et al., 2000).
There are hints that the spore surface, besides providing mechanical protection, is involved in the initiation of polar tube extrusion and that modifications of the spore wall architecture occur during this activation (Weidner, 1992; Weidner and Halonen, 1993). Electron microscopy revealed that the outer spore envelope of Spraguea lophii and Thelohania sp. completely disassembles at the time of spore activation (Weidner, 1992; Weidner and Halonen, 1993). Using antikeratin antibodies it was demonstrated that the outer spore wall of Thelohania sp. consists in part of keratin-like proteins that form 10-nm intermediate filaments which become phosphorylated and disassemble during spore activation (Weidner and Halonen, 1993).
It is possible to distinguish subcompartments within the spore wall using polyclonal antisera against partially purified microsporidial proteins. A 30-kDa antigen was found to be located on the outer spore wall, while a 33-kDa protein was found in a region close to the plasma membrane (Delbac et al., 1998a). In addition, several monoclonal antibodies were reported to recognise spore wall antigens (Visvesvara et al., 1994; Beckers et al., 1996; Lujan et al., 1998). A glycine- and serine-rich 51-kDa protein named SWP1 is localised to the exospore in E. cuniculi (Bohne et al., 2000) and E. intestinalis (Hayman et al., 2001). The corresponding gene, swp1, has been identified in E. cuniculi (Bohne et al., 2000), E. hellem (Bohne et al., 2000) and E. intestinalis (Hayman et al., 2001). SWP1 is absent in meronts and first seen in early sporonts at a time when organisms translocate from the periphery to the centre of the parasitophorous vacuole (Bohne et al., 2000). A 150-kDa glycoprotein in the spore wall named SWP2 was identified in E. intestinalis (Hayman et al., 2001). SWP2 is found on mature spores. Cysteine residues and the N terminal signal sequences are conserved among these SWPs. E. cuniculi SWP1 has 11 cysteine residues and E. intestinalis SWP1 and SWP2 have 10 cystein residues with a conserved spacing suggesting that these proteins may have similar secondary structures and functions (Bohne et al., 2000; Hayman et al., 2001).
Techniques have been described that permit the purification of spore coat proteins as well as polar tube proteins (Keohane et al., 1994, 1996a, 1998, 1999). With the publication of the genome of E. cuniculi this has permitted investigators to initial proteomic studies of the composition of this structure (Texier et al., 2003; Weiss L.M., unpublished data). This has resulted in the identification of new components of the spore wall, such as an enzyme involved in the synthesis of chitin and a protein Ec-6 that is expressed during formation of the spore wall (Xu Y. and Weiss L.M., unpublished data). Some of the components of the spore wall appear to be modified by post-translational glycosylation involving mannosylation. These modifications may be important in adherence of the spore wall to mucin or to host cells during passage of the spores in the gastrointestinal tract, facilitating invasion. It has, for example, been demonstrated that exogenous glycosaminoglycans can decrease the adherence of spores to a host cell monolayer (Hayman et al., 2005).
4. Germination
Spore discharge is generally believed to occur in several phases: (1) activation, (2) increase in intrasporal osmotic pressure, (3) eversion of the polar tube, and (4) passage of sporoplasm through the polar tube. The exact mechanism(s) of this process is not well understood. Conditions that activate spores vary widely among species, presumably reflecting the organism’s adaptation to their host and external environment (Undeen and Epsky, 1990) [for a review see Keohane and Weiss (1999)]. Since microsporidia are found in a wide range of terrestrial and aquatic hosts, different species may require unique activation conditions for spore discharge. These specific conditions are also probably important to prevent accidental discharge in the environment (Undeen and Epsky, 1990).
Conditions that have been shown to promote spore discharge include incubation at an alkaline pH, acidic pH, or a pH shift from acid to alkaline or from alkaline to neutral (Undeen and Avery, 1984; Undeen and Epsky, 1990). Other species have demonstrated spore discharge at both acidic and alkaline conditions (Hashimoto et al., 1976). Dehydration by drying or hyperiosmotic solutions followed by rehydration has been effective in promoting spore discharge in some species, while dehydration followed by rehydration at an alkaline pH was effective in others (Olson, 1976; Undeen and Epsky, 1990). Various cations including potassium, lithium, sodium, cesium and anions such as bromide, chloride, iodide and fluoride have been used to promote discharge (Undeen and Avery, 1988; Undeen and Epsky, 1990; Frixione et al., 1994). Mucin or polyanions, hydrogen peroxide, low dose ultraviolet radiation and calcium ionophore A 23187 have also been used to trigger discharge (Lom and Vavra, 1963; Weidner and Byrd, 1982; Undeen and Vandermeer, 1990; Leitch et al., 1993).
Inhibitors of spore discharge include 0.01–0.1 M magnesium chloride, ammonium chloride, low salt concentrations (10–50 mM), sodium fluoride, silver ions, gamma radiation, ultraviolet light, temperatures greater than 40 °C, calcium channel antagonists, calmodulin inhibitors (chlorpromazine, trifluroperazine), a microfilament disrupter (cytochalasin D), a microtubule disrupter (demecolcine) and itraconazole (Undeen and Avery, 1988; Undeen and Vandermeer, 1990; Leitch et al., 1993). Calcium chloride (0.001–0.1 M) has been found to inhibit spore discharge in some studies, while 0.2 M CaCl2 at pH 9.0 and 1 mM CaCl2 promoted discharge in other studies (Weidner and Byrd, 1982; Pleshinger and Weidner, 1985; Leitch et al., 1993). EGTA in the presence of calcium also promoted spore discharge in one study and inhibited discharge in another (Pleshinger and Weidner, 1985; Malone, 1990). Removal of clathrin and calmodulin from the intermediate filament cage assembly, which envelopes the spores of Glugea americanus (formerly S. lophii), results in irreversible inactivation of spore discharge (Weidner, 1992).
It has been theorised that, regardless of the mode of activation, microsporidia exhibit the same response to the stimuli, that is, increased intrasporal osmotic pressure (Ohshima, 1937; Lom and Vavra, 1963; Weidner and Byrd, 1982; Undeen, 1990; Undeen and Frixione, 1990). This increase in osmotic pressure results in an influx of water into the spore accompanied by swelling of the polaroplast and posterior vacuole prior to spore discharge (Lom and Vavra, 1963; Undeen, 1990; Undeen and Frixione, 1990). It is this pressure that forces the eversion of the polar tube and expulsion of sporoplasm (Undeen, 1990; Undeen and Frixione, 1990). In hyperosmotic solutions, polar tube discharge is inhibited or slowed down, and sporoplasm passage does not occur, thus providing indirect evidence for the osmotic pressure theory (Lom and Vavra, 1963; Weidner and Byrd, 1982; Undeen, 1990; Undeen and Frixione, 1990; Frixione et al., 1992).
Several theories have been proposed for the mechanism by which osmotic pressure is increased in the spore. One of the earliest explanations was that the activation simply increases the permeability of the spore coat to water (Lom and Vavra, 1963). Another theory involved the creation of a proton gradient by the alkaline environment surrounding the spore (Dall, 1983). The proton gradient drives a protoncation exchange mechanism consisting of a carboxylic acid ionophore. As protons in the sporoplasm are depleted, the increase in alkalinity triggers the same mechanisms in the membrane of organelles, particularly the polaroplast and posterior vacuole. Water flows into the spore, due to the generalised osmotic imbalance, increasing the intrasporal pressure (Dall, 1983). It should be noted, however, that not all microsporidia require an alkaline pH for spore discharge. Another theory was based on the finding of decreased trehalose levels in discharged spores as compared with undischarged spores of B. algerae, an aquatic microsporidium (Undeen and Vandermeer, 1994). In this mechanism, activation causes changes in the spore that brings the trehalose in contact with the enzyme trehalase, perhaps by a disruption of compartments within the spore (Undeen, 1990). The trehalose is degraded into a larger number of small molecules, causing an increase in osmotic pressure. The subsequent flow of water into the spore results in increase in intrasporal pressure and spore discharge (Undeen, 1990). Recently it has been suggested that the posterior vacuole may function as a peroxisome containing both catalase and Acyl CoA oxidase (Findley et al., 2005). The oxidation of fatty acids in the posterior vacuole may therefore provide the force needed for germination (Findley et al., 2005).
Calcium has been proposed to play a major role in spore discharge, in which the displacement of calcium from the polaroplast membrane would either activate a contractile mechanism or combine with the polaroplast matrix causing polaroplast swelling (Weidner and Byrd, 1982). Calcium ionophore A23187, sodium citrate and phosphate were found to trigger polaroplast swelling and polar tube discharge, while calcium chloride inhibited the reaction (Weidner and Byrd, 1982).
The first sign of spore discharge is a visible protrusion at the anterior end of the spore at the polar cap, which is followed by the rapid emergence of the polar tube in a helicoidal fashion along nearly a straight line (Lom and Vavra, 1963; Frixione et al., 1992). In B. algerae, activation is associated with several other morphologic changes in the spore including the appearance of membrane infoldings that surround the polar tube (Cali et al., 2002). This ultra-structural data is suggestive that the polar tube is actually extracytoplasmic in the spore and explains how the sporoplasm can remain intact during the explosive germination reaction.
After complete discharge of the polar tube, the sporoplasm flows through the polar tube and appears as a droplet at its distal end (Ohshima, 1937; Weidner, 1972; Frixione et al., 1992). Using video enhanced contrast microscopy, there is a time delay between completion of discharge and appearance of the droplet of about 15–500 ms (Frixione et al., 1992). It has been suggested that the delay might be due to the eversion of a blind ended tube that needs to be opened by some mechanism prior to sporoplasm release (Frixione et al., 1992). Sporoplasm passage has not been observed in partially discharged tubes (Weidner, 1972; Frixione et al., 1992). While in contact with the tip of the polar tube, the sporoplasm droplet enlarges to a volume in excess of what might be expected from the size of the spore (Frixione et al., 1992). This might be due to the movement of water into the sporoplasm due to the osmotic gradient (Frixione et al., 1992). If the polar tube is discharged next to a cell it pierces the cell and transfers the sporoplasm into it (Ohshima, 1937; Weidner, 1972; Frixione et al., 1992). If there are no adjacent cells, the droplet of sporoplasm remains attached to the polar tube for a period of time. It has been suggested that a new membrane for discharged sporoplasms may be provided by the polaroplast (Weidner et al., 1984).
The polar tube provides a bridge to deliver the sporoplasm to the host cell. It serves to protect the sporoplasm from the harsh external environment during its passage into its host cell. Ultrastructural data have demonstrated that the polar tube can invaginate the host cell membrane creating a microenvironment for the interaction of the sporoplasm and the host cell membrane. Traditionally, the polar tube has been believed to pierce the host cell membrane delivering the sporoplasm directly into the host cell’s cytoplasm. The mechanism, however, by which the polar tube or sporoplasm interacts with the host cell’s membrane is not known. Some data suggests that the final penetration of the sporoplasm into the host cell may require the participation of host cell proteins such as actin (Foucault and Drancourt, 2000). In addition to its role in delivering the sporoplasm to the host cell, the polar tube has also been demonstrated to be a mechanism of escape from phagosomes for ingested spores (Couzinet et al., 2000; Franzen, 2004). In fact, spore germination in a phagosome has been demonstrated to allow the sporoplasm to escape the phagosome and penetrate out of one host cell and into an adjacent cell (Franzen, 2004). This may be an important mechanism for dissemination of infection through macrophages transporting spores in their phagosomes to other tissues.
5. Composition of the microsporidian polar tube
A single coiled polar tube is present, in a similar structural and functional form, in all microsporidia regardless of species, host or geographical location. It is likely that this structure evolved prior to divergence of microsporidia into various genera, and is not the result of convergence of independently-evolved polar tube structure in different microsporidia. Therefore, the proteins comprising the polar tube are likely to be members of a protein family that evolved from the same ancestral genes. Several observers have suggested that the polar tube originates from the coalescence of vacuoles of the Golgi, forming a tube with a limiting membrane (Vavra, 1976). Weidner (1970) proposed that the central core of the filament arose from the Golgi-like saccules and the outer envelope from the endoplasmic reticulum (Weidner, 1970). In Glugea stephani, thiamine pyrophosphatase was present on membranes and dense material that formed the polar filament suggesting a trans-Golgi association (Takvorian and Cali, 1994). However, staining of the polar filament core, its outer sheath and its originating vacuoles was also seen with nucleoside disphosphatase (NDPase), which is a marker for endoplasmic reticulum as well as cis-Golgi membrane (Takvorian and Cali, 1996). The polaroplast has also been reported to be derived from the Golgi (Sprague and Vernick, 1968; Jensen and Wellings, 1972) and the endoplasmic reticulum (Lom and Corliss, 1967; Weidner, 1970).
Early studies on the properties of polar tubes found them to be insoluble in water and saliva but completely digested by trypsin in 24 h (Kudo, 1921). Polar tubes have been observed to be rapidly digested after extrusion in digestive fluid or in the midgut of insects (Ohshima, 1937; Undeen and Epsky, 1990). The polar tube resists dissociation in 1–3% sodium dodecyl sulfate (SDS), 5–8 N H2SO4, 1–2 N HCl, chloroform, 1% guanidine HCl, 0.1 M proteinase K and 8–10 M Urea, but is soluble in 50% 2-mercaptoethanol (2-ME) or 1% dithiothreitol (DTT) (Weidner, 1972, 1976; Keohane et al., 1994, 1996a, 1998a, 1999; Keohane and Weiss, 1998). The polar tube inside the spore has been found to react in the same manner to reducing agents and detergents as everted polar tubes (Weidner, 1976, 1982).
Polar tube proteins (PTPs) appear to be highly immunogenic in both experimental and natural infections. In a large serosurvey, antibodies reacting to E. intestinalis by immunofluorescence techniques were present in 5% of pregnant French women and 8% of Dutch blood donors (van Gool et al., 1997). Studies have demonstrated that polyclonal antibodies raised to whole spore lysates in experimental animals usually react with the polar tube (Schwartz et al., 1993a; Zierdt et al., 1993; Weiss L.M., unpublished data). In a study of the immunologic response to spore antigens of Glugea atherinae and E. cuniculi, several candidate antibodies to PTPs were identified (Delbac et al., 1996, 1997) which demonstrated cross reactivity to the polar tubes of G. atherinae and E. cuniculi by immunogold electron microscopy. In addition, E. cuniculi antibodies reacted with the polar tube of S. lophii (aka G. americanus) by immunofluorescence (Delbac et al., 1996). These findings suggest that shared epitopes must exist. In the process of eversion of the polar tube, unique immunologic epitopes may be exposed. For example, monoclonal antibody Si91 is specific for extruded polar tubes of E. intestinalis and does not react with polar tubes within the spore nor does it react with the polar tube of other Encephalitozoonidae (Beckers et al., 1996).
The solubility properties of polar tube proteins have facilitated their separation from other proteins in the spore permitting the development of a method for the purification of the major polar tube protein (PTP1) from microsporidian spores (Keohane et al., 1994, 1996a, 1998, 1999). Soluble polar tube preparations of G. americanus, E. hellem, E. cuniculi, E. intestinalis and B. algerae were prepared by sequentially extracting glass bead disrupted spores with 1% SDS and 9 M urea, followed by solubilisation of the residual polar tubes in 2% DTT (Keohane et al., 1994, 1996a, 1998, 1999). PTP1 in the DTT-solubilised material was then purified to homogeneity using reverse phase high performance liquid chromatography (HPLC) (Keohane et al., 1994, 1996a, 1998, 1999). By SDS-PAGE and silver staining this purified fraction migrated at 43 kDa for G. americanus, 45 kDa for E. cuniculi and E. intestinalis, 55 kDa for E. hellem (Keohane et al., 1994, 1996a, 1998, 1999). Monoclonal and or polyclonal antibodies raised to the purified PTP1 demonstrated reactivity with polar tubes by immunofluorescence (IF) and immunogold electron microscopy (EM), and demonstrated cross reactivity among the species by immunoblotting and immmunogold EM (Keohane et al., 1994, 1996a, 1998, 1999; Fig. 4). Weidner (1976) purified a potential polar tube protein of 23 kDa from Ameson michaelis by utilising the unusual solubility properties of polar tubes (Weidner, 1976). Amino acid analysis of this protein demonstrated the presence of multiple cysteine residues consistent with the hypothesis that disulfide bridging is important in polar tube proteins (Weidner, 1976).
Fig. 4.
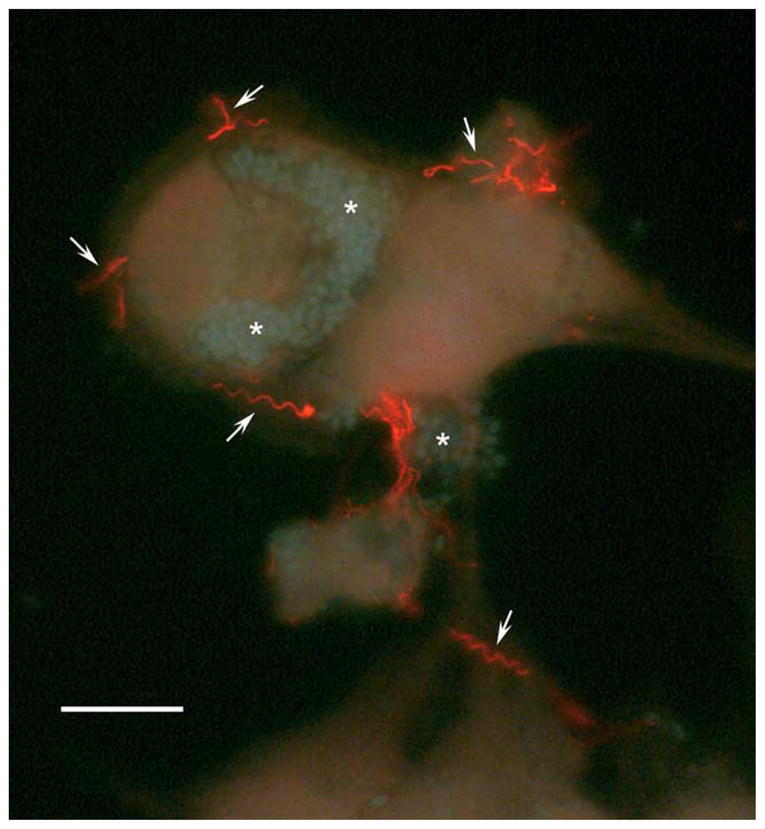
Staining of the polar tubes with antibody to PTP1. Encephalitozoon hellem cultured in RK13 (rabbit kidney) cells was stained with antibody to EhPTP1 (secondary antibody Cy3 anti-mouse IgG) demonstrating the presence of PTP1 in the polar tube (arrows) and the occurrence of germination in cell culture in vitro. Spores of E. hellem stained with 0.5% Calcoflour white M3R (Xu et al., 2004) are indicated by an ‘*’. Bar=25 μm (courtesy of Peter Takvorian, Rutgers University).
All of the major polar tube proteins (PTP1s) purified to date display similarities in hydrophobicity, high proline content, and immunologic epitopes. Amino acid analysis of the Encephalitozoonidae, G. americanus and B. algerae PTP1s demonstrated that proline was a significant component of these proteins (Keohane et al., 1994, 1996a, 1998, 1999). Proline is a hydrophobic imino acid and due to its ring structure it forms a fixed kink in a polypeptide resulting in chain rigidity. High proline content is a feature of several structural proteins such as collagen and elastin that are known for their high tensile strength, elasticity and ability to recoil. Such properties are important for function of PTPs during the discharge and passage of sporoplasm through the polar tube.
The E. cuniculi, E. hellem and E. intestinalis ptp1 have been cloned and the corresponding protein (PTP1) expressed in vitro for E. cuniculi and E. hellem (Delbac et al., 1998b, 2001; Keohane et al., 1998). Clones have also been obtained for the ptp1 of several different isolates of E. hellem as well as G. americanus and B. algerae (Peuvel et al., 2000; Weiss, 2001; Xiao et al., 2001; Haro et al., 2003; Weiss L.M., unpublished data). The E. hellem ptp1 is 1362 bp and encodes a protein of 453 amino acids with a predicted molecular mass of 43 kDa while the ptp1 of E. cuniculi is 1188 bp and encodes a protein of 395 amino acids with a predicted molecular mass of 37 kDa (Delbac et al., 1998b; Keohane et al., 1998). These two microsporidia are in the same genus (Encephalitozoon) and cannot be distinguished ultrastructurally, their native PTP1s have similarities in overall amino acid composition, hydrophobicity, mass and immunologic epitopes, and their polar tubes have functional identity (Weiss, 2001). It was therefore somewhat surprising that the translated proteins have only limited identity in amino acid sequences. Further comparison does, however, reveal striking similarities. Both translated proteins have a high number of proline and glycine residues, a similar percentage of cysteine, and lack arginine, tryptophane and phenylalanine. The spatial distribution of both the cysteine and proline residues is conserved in both proteins. This suggests that conservation of function of these proteins may be provided by conservation of secondary structural motifs (Weiss, 2001). From previous studies on DTT solubilisation of polar tubes it is probable that disulfide bonds are important for polar tube stability. The preservation of cysteine residues in these two PTP1s suggests that these amino acids may be critical in the formation and function of the polar tube.
All of the cloned PTP1s have central amino acid repeat regions that are predominantly hydrophilic. However, the repeats are different in composition and number. It is possible that this region is not important for the assembly of the polar tube and may function as an immunologic mask. In the process of evolution, a similar duplication of internal sequences has been noted in malaria and other protozoan genes and this mechanism may be operative in the microsporidia ptp1 (Rich and Ayala, 2000). Analysis of ptp1 from several isolates of E. hellem supports this view as the number of repeats in the central region of their ptp1 is variable (Peuvel et al., 2000; Weiss, 2001; Xiao et al., 2001; Haro et al., 2003).
The Encephalitozoonidae PTP1s contain almost identical N-terminal signal sequences that are cleaved to form the mature protein, as predicted by SignalP V1.1 and confirmed by N-terminal sequencing in E. hellem (Keohane et al., 1998), E. cuniculi and E. intestinalis (Keohane and Weiss, unpublished data). There are probably similar intracellular targeting and processing pathways in these organisms for PTP1. The signal peptide is predicted by PSORT to target PTP1 for processing through the endoplasmic reticulum and golgi complex, which is consistent with morphologic observations of polar tube development (Wittner and Weiss, 1999). The N- and C-terminus of these proteins display conservation suggesting that these areas may have important structural or functional domains. In the Encephalitozoonidae PTP1s, the C-terminus is high in cysteine residues and the last amino acid is a cysteine, which may be important for interaction between proteins.
A study of the assembly properties of isolated PTP from A. michaelis demonstrated that upon acidification this PTP reassembled into sheets or shells, appearing more fluid than PTP of discharged spores (Weidner, 1976). Reassembly was not observed to occur if PTP was alkylated after 2-ME treatment, nor after reduction by 1% DTT and subsequent removal of the DTT (Weidner, 1976). We found that DTT solubilised E. hellem PTP1 would aggregate when DTT was removed by dialysis, but reductive alkylation of cysteine residues using 4-vinylpyridine prevented such aggregation (Keohane et al., 1996b; Weiss L.M., unpublished data). Polar tubes have been reported to show branches and coalesce into networks when suspended in 0.05–0.1 M CaCl2 (Weidner and Byrd, 1982).
Encephalitozoon hellem PTP1 has nine N-linked glycosylation sites, six of which are in the central core and 93 O-linked glycosylation sites, 19 of which are in the central core. Many of these are conserved among the Encephalitozoonidae PTP1 proteins. Glycosylation is likely to have functional significance for the polar tube structure. This concept is supported by studies demonstrating carbohydrate residues on intact polar tubes (Vavra, 1972; Wittner and Weiss, 1999; Delbac et al., 2001; Xu et al., 2004). For example, concanavalin A bound to PTP1 and to the polar tube of several different microsporidia species (Xu et al., 2004). Analysis of the glycosylation of E. hellem PTP1 suggests it is modified by O-linked mannosylation and that ConA binds to these O-linked mannose residues (Xu et al., 2004). The E. cuniculi genome contains all of the genes required for O-mannosylation, however, the genes for N-glycosylation appear to be absent (Katinka et al., 2001). Mannose pretreatment of host cells decreased their infection by E. hellem consistent with an interaction between the mannosylation of PTP1 and some unknown host cell mannose-binding molecule (Xu et al., 2004).
While PTP1 is the major component of the polar tube, other polar tube proteins (PTPs) are clearly present in the DTT solubilised polar tube fraction. For example, several putative PTPs of 23, 27 and 34 kDa have been identified in G. americanus using mAbs produced to the DTT solubilised polar tube (Keohane et al., 1994). Using 2D electrophoresis additional proteins can be seen in DTT solubilised E. hellem polar tube preparations (Weiss, 2001). In addition, polyclonal and monoclonal antibodies that localised to the polar tube by IFA and immunogold EM and recognised proteins of 34, 75, and 170 kDa in G. atherinae and 35, 52/55, 150 kDa in E. cuniculi, 60 and 120 kDa in E. intestinalis and 46, 34, 21 and 15 kDa in Nosema grylli have been reported (Beckers et al., 1996; Delbac et al., 1998a; Dolgikh and Semenov, 2003).
The gene encoding a 35-kDa protein band (i.e. PTP2) seen on SDS-PAGE of E. cuniculi polar tube proteins has been cloned (Delbac et al., 2001). Encephalitozoon cuniculi PTP2 is a 277 amino-acid polypeptide with a predicted molecular mass of 30 kDa with no significant homology to any other GeneBank protein (Delbac et al., 2001). The N-terminal sequence of PTP2 has a characteristic signal peptide (for Golgi-ER processing similar to PTP1) and the central region contains a lysine-rich octapeptide motif (KPKKKKSK) (Delbac et al., 2001). The C-terminal region of 27 residues is devoid of any basic residues and possesses four aspartate and five glutamate residues forming an acidic tail (Delbac et al., 2001). One putative N-glycosylation site and one RGD motif, possibly involved in some protein-protein interactions, are present (Delbac et al., 2001). Similar to ptp1, E. cuniculi ptp2 exists as a single copy per haploid genome. Encephalitozoon cuniculi ptp1 and ptp2 mRNAs are polyadenylated and have reduced 5′ and 3′ UTRs (Delbac et al., 2001). Both ptp1 and ptp2 are located on chromosome VI in E. cuniculi as a gene cluster, which has a conserved orientation and spacing in E. intestinalis and E. hellem (Delbac et al., 2001). Preservation of gene order has recently been described in studies of microsporidian genomes and may be due to constraints of evolution on these small eukaryotic genomes (Slamovits et al., 2004). All of the Encephalitozoonidae PTP2 s are basic proteins of about 30 kDa, with a maximal size difference of five residues (E. cuniculi PTP2 versus E. hellem PTP2) (Delbac et al., 2001). Three potential O-glycosylation sites are present in PTP2.
By immunoscreening of a cDNA library of E. cuniculi, a third polar tube protein, PTP3, has been identified and cloned (Peuvel et al., 2002). This protein is predicted to be synthesized as a 1256-amino acid precursor (136 kDa) with a cleavable signal peptide and is encoded by a single transcription unit (3990 bp) located on the chromosome XI of E. cuniculi (Peuvel et al., 2002). Unlike PTP1 and PTP2, PTP3 is solubilised in the presence of SDS alone without the need for a reducing agent such as DTT (Peuvel et al., 2002). Only one cysteine residue is present, which is located in the potential N-terminal signal peptide. Charged residues (171 acidic and 144 basic residues) are highly dispersed along the protein (Peuvel et al., 2002). Highly basic N- and C-terminal domains can be distinguished in the sequence of the predicted mature protein (Peuvel et al., 2002). Aspartate and glutamate (16%) are the major amino acids of the protein core (Peuvel et al., 2002). Immunolocalisation data indicated that PTP3 is involved in the sporoblast-to-spore polar tube biogenesis (Peuvel et al., 2002). A transcriptional up-regulation during sporogony is supported by a strong increase in the relative amount of E. cuniculi PTP mRNAs within host cells sampled at late p.i. times (Peuvel et al., 2002).
The regular multi-layered organisation of the microsporidian polar tube must be dependent on specific interactions between its protein components. Previous studies suggested that disulfide bridges play an essential role. Both PTP1 and PTP2 are cysteine-rich and the positions of most cysteine residues are conserved in the three Encephalitozoon species (Delbac et al., 2001), however, PTP3 protein lacks significant cysteine residues (Peuvel et al., 2002). To explore polar tube-associated protein interactions, spore proteins were extracted in the presence of SDS and dithiothreitol then incubated with a chemical cross-linker (DSP or sulfo-EGS) (Peuvel et al., 2002). A large multimeric complex was formed and shown to contain PTP1–PTP3 with a few other proteins (Peuvel et al., 2002). Considering that PTP3 is extractable from E. cuniculi spores in the absence of thiol-reducing agent, and lacks cysteine but is rich in charged residues, it has been suggested that PTP3 interacts with PTP1 and/or PTP2 via ionic bonds and may play a role in the control of the conformational state of the PTP1–PTP2 polymers (Peuvel et al., 2002). For example, when the polar tube exists as a coiled structure inside the spore interactions with PTP3 may permit the maintenance of PTP1–PTP2 polymers in a condensed form (Peuvel et al., 2002). Recently we have utilised DTT solubilised polar preparations for proteomic studies of the polar tube (Weiss L.M., unpublished data). These studies suggest that in addition to PTP1–PTP3 other proteins may be present in the polar tube and these proteins may also contribute to polar tube function and stability (Weiss L.M., unpublished data).
6. Summary
The invasion organelle of the Microsporidia has successfully served this diverse phylum, resulting in a group of obligate intracellular organisms capable of infecting almost any cell type. Investigations have resulted in the identification of three polar tube proteins (PTP1–PTP3) in this structure. Mass spectrometry approaches may yield additional components of the polar tube. Despite the fact that this structure was described over 100 years ago (Thelohan, 1894), fundamental information on the mechanism of germination and the interaction of the polar tube with the host cell still need to be obtained. Further study of this structure may lead to novel strategies for control of these important parasitic protists.
Acknowledgments
This work was supported by NIH Grants AI31788, GM60067, & NCRR 1S10—RR13959. We would like to thank Jiri Vavra, Elaine Keohane, Ann Cali, Peter Takvorian, George Orr and Herbert Tanowitz for advice and discussion about polar tube function and structure.
References
- Arisue N, Sanchez LB, Weiss LM, Muller M, Hashimoto T. Mitochondrial-type hsp70 genes of the amitochondriate protistsGiardia intestinalis, Entamoeba histolytica and two microsporidians. Parasitol Int. 2002;51:9–16. doi: 10.1016/s1383-5769(01)00093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SW, Undeen AH. The isolation of microsporidia and other pathogens from concentrated ditch water. J Am Mosq Control Assoc. 1987;3:54–58. [PubMed] [Google Scholar]
- Beckers PJ, Derks GJ, Gool T, Rietveld FJ, Sauerwein RW. Encephalitozoon intestinalis-specific monoclonal antibodies for laboratory diagnosis of microsporidiosis. J Clin Microbiol. 1996;34:282–285. doi: 10.1128/jcm.34.2.282-285.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biderre C, Mathis A, Deplazes P, Weber R, Metenier G, Vivares CP. Molecular karyotype diversity in the microsporidian Encephalitozoon cuniculi. Parasitology. 1999;118:439–445. doi: 10.1017/s0031182099004023. [DOI] [PubMed] [Google Scholar]
- Bigliardi E, Selmi MG, Lupetti P, Corona S, Gatti S, Scaglia M, Sacchi L. Microsporidian spore wall: ultrastructural findings on Encephalitozoon hellem exospore. J Eukaryot Microbiol. 1996;43:181–186. doi: 10.1111/j.1550-7408.1996.tb01388.x. [DOI] [PubMed] [Google Scholar]
- Bohne W, Ferguson DJ, Kohler K, Gross U. Developmental expression of a tandemly repeated, glycine- and serine-rich spore wall protein in the microsporidian pathogen Encephalitozoon cuniculi. Infect Immun. 2000;68:2268–2275. doi: 10.1128/iai.68.4.2268-2275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan RT, Weber R, Schwartz DA. Microsporidiosis in patients who are not infected with human immunodeficiency virus. Clin Infect Dis. 1997;24:534–535. doi: 10.1093/clinids/24.3.534. [DOI] [PubMed] [Google Scholar]
- Cali A, Takvorian PM. Ultrastructure and development of Pleistophora ronneafiei n. sp., a microsporidium (Protista) in the skeletal muscle of an immune-compromised individual. J Eukaryot Microbiol. 2003;50:77–85. doi: 10.1111/j.1550-7408.2003.tb00237.x. [DOI] [PubMed] [Google Scholar]
- Cali A, Weiss LM, Takvorian PM. Brachiola algerae spore membrane systems, their activity during extrusion, and a new structural entity, the multilayered interlaced network, associated with the polar tube and the sporoplasm. J Eukaryot Microbiol. 2002;49:164–174. doi: 10.1111/j.1550-7408.2002.tb00361.x. [DOI] [PubMed] [Google Scholar]
- Canning EU, Lom J. The Microsporidia of Vertebrates. Academic press; London: 1986. [Google Scholar]
- Chioralia G, Trammer T, Maier WA, Seitz HM. Morphologic changes in Nosema algerae (Microspora) during extrusion. Parasitol Res. 1998;84:123–131. doi: 10.1007/s004360050368. [DOI] [PubMed] [Google Scholar]
- Chupp GL, Alroy J, Adelman LS, Breen JC, Skolnik PR. Myositis due to Pleistophora (Microsporidia) in a patient with AIDS. Clin Infect Dis. 1993;16:15–21. doi: 10.1093/clinids/16.1.15. [DOI] [PubMed] [Google Scholar]
- Cotte L, Rabodonirina M, Chapuis F, Bailly F, Bissuel F, Raynal C, Gelas P, Persat F, Piens MA, Trepo C. Waterborne outbreak of intestinal microsporidiosis in persons with and without human immunodeficiency virus infection. J Infect Dis. 1999;180:2003–2008. doi: 10.1086/315112. [DOI] [PubMed] [Google Scholar]
- Couzinet S, Cejas E, Schittny J, Deplazes P, Weber R, Zimmerli S. Phagocytic uptake of Encephalitozoon cuniculi by nonprofessional phagocytes. Infect Immun. 2000;68:6939–6945. doi: 10.1128/iai.68.12.6939-6945.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle CM, Wittner M, Kotler DP, Noyer C, Orenstein JM, Tanowitz HB, Weiss LM. Prevalence of microsporidiosis due to Enterocytozoon bieneusi and Encephalitozoon (Septata) intestinalis among patients with AIDS-related diarrhea: determination by polymerase chain reaction to the microsporidian small-subunit rRNA gene. Clin Infect Dis. 1996;23:1002–1006. doi: 10.1093/clinids/23.5.1002. [DOI] [PubMed] [Google Scholar]
- Coyle CM, Weiss LM, Rhodes LV, 3rd, Cali A, Takvorian PM, Brown DF, Visvesvara GS, Xiao L, Naktin J, Young E, Gareca M, Colasante G, Wittner M. Fatal myositis due to the microsporidian Brachiola algerae, a mosquito pathogen. N Engl J Med. 2004;351:42–47. doi: 10.1056/NEJMoa032655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall DJ. A theory for the mechanism of polar filament extrusion in the Microspora. J Theor Biol. 1983;105:647–659. doi: 10.1016/0022-5193(83)90225-4. [DOI] [PubMed] [Google Scholar]
- Delbac F, Duffieux F, Peyret P, David D, Metenier G, Vivares C. Identification of sporal proteins in two microsporidian species: an immunoblotting and immunocytochemical study. J Eukaryot Microbiol. 1996;43:101S. doi: 10.1111/j.1550-7408.1996.tb05024.x. [DOI] [PubMed] [Google Scholar]
- Delbac F, David D, Metenier G, Vivares C. First complete amino acid sequence of a polar tube protein in a microsporidian species, Encephalitozoon cuniculi. J Eukaryot Microbiol. 1997;44:77S. doi: 10.1111/j.1550-7408.1997.tb05791.x. [DOI] [PubMed] [Google Scholar]
- Delbac F, Duffieux F, David D, Metenier G, Vivares CP. Immunocytochemical identification of spore proteins in two microsporidia, with emphasis on extrusion apparatus. J Eukaryot Microbiol. 1998a;45:224–231. doi: 10.1111/j.1550-7408.1998.tb04529.x. [DOI] [PubMed] [Google Scholar]
- Delbac F, Peyret P, Metenier G, David D, Danchin A, Vivares CP. On proteins of the microsporidian invasive apparatus: complete sequence of a polar tube protein of Encephalitozoon cuniculi. Mol Microbiol. 1998b;29:825–834. doi: 10.1046/j.1365-2958.1998.00975.x. [DOI] [PubMed] [Google Scholar]
- Delbac F, Peuvel I, Metenier G, Peyretaillade E, Vivares CP. Microsporidian invasion apparatus: identification of a novel polar tube protein and evidence for clustering of ptp1 and ptp2 genes in three Encephalitozoon species. Infect Immun. 2001;69:1016–1024. doi: 10.1128/IAI.69.2.1016-1024.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplazes P, Mathis A, van Saanen M, Iten A, Keller R, Tanner I, Glauser MP, Weber R, Canning EU. Dual microsporidial infection due to Vittaforma corneae and Encephalitozoon hellem in a patient with AIDS. Clin Infect Dis. 1998;27:1521–1524. doi: 10.1086/515023. [DOI] [PubMed] [Google Scholar]
- Deplazes P, Mathis A, Weber R. Epidemiology and zoonotic aspects of microsporidia of mammals and birds. Contrib Microbiol. 2000;6:236–260. doi: 10.1159/000060363. [DOI] [PubMed] [Google Scholar]
- Desportes I, Le Charpentier Y, Galian A, Bernard FB, Cochand-Priollet, Lavergne A, Ravisse F, Modigliani R. Occurrence of a new microsporidian: Enterocytozoon bieneusi n. g., n sp., in the enterocytes of a human patient with AIDS. J Protozool. 1985;32:250–245. doi: 10.1111/j.1550-7408.1985.tb03046.x. [DOI] [PubMed] [Google Scholar]
- Dolgikh VV, Semenov PB. The spore wall and polar tube proteins of the microsporidian Nosema grylli: the major spore wall protein is released before spore extrusion. Tsitologiia. 2003;45:324–329. [PubMed] [Google Scholar]
- Dowd SE, Gerba CP, Pepper IL. Confirmation of the human-pathogenic microsporidia Enterocytozoon bieneusi, Encephalitozoon intestinalis, and Vittaforma corneae in water. Appl Environ Microbiol. 1998;64:3332–3335. doi: 10.1128/aem.64.9.3332-3335.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobniewski F, Kelly P, Carew A, Ngwenya B, Luo N, Pankhurst C, Farthing M. Human microsporidiosis in African AIDS patients with chronic diarrhea. J Infect Dis. 1995;171:515–516. doi: 10.1093/infdis/171.2.515. [DOI] [PubMed] [Google Scholar]
- Erickson B, Blanquet R. The occurrence of chitin in the spore wall of Glugea weissenbergi. J Invertebr Pathol. 1969;14:358–364. doi: 10.1016/0022-2011(69)90162-1. [DOI] [PubMed] [Google Scholar]
- Erickson BWJ, Vernick SH, Sprague V. Electron study of the everted polar filament of Glugea weissenbergi (Microsporida Nosematidae) J Protozool. 1968;15:758–761. doi: 10.1111/j.1550-7408.1968.tb02207.x. [DOI] [PubMed] [Google Scholar]
- Findley AM, Weidner EH, Carman KR, Xu Z, Godbar JS. Role of the posterior vacuole in Spraguea lophii (Microsporidia) spore hatching. Folia Parasitol. 2005;52:111–117. doi: 10.14411/fp.2005.014. [DOI] [PubMed] [Google Scholar]
- Foucault C, Drancourt M. Actin mediates Encephalitozoon intestinalis entry into the human enterocyte-like cell line, Caco-2. Microb Pathog. 2000;28:51–58. doi: 10.1006/mpat.1999.0329. [DOI] [PubMed] [Google Scholar]
- Franzen C. Microsporidia: how can they invade other cells? Trends Parasitol. 2004;20:275–279. doi: 10.1016/j.pt.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Franzen C, Muller A. Microsporidiosis: human diseases and diagnosis. Microbes Infect. 2001;3:389–400. doi: 10.1016/s1286-4579(01)01395-8. [DOI] [PubMed] [Google Scholar]
- Frixione E, Ruiz L, Santillan M, de Vargas LV, Tejero JM, Undeen AH. Dynamics of polar filament discharge and sporoplasm expusion by microsporidian spores. Cell Motil Cytoskel. 1992;22:38–50. [Google Scholar]
- Frixione E, Ruiz L, Undeen AH. Monovalent cations induce microsporidian spore germination in vitro. J Eukaryot Microbiol. 1994;41:464–468. [Google Scholar]
- Frixione E, Ruiz L, Cerbon J, Undeen AH. Germination of Nosema algerae (Microspora) spores: conditional inhibition by D2O, ethanol and Hg-2+ suggests dependence of water influx upon membrane hydration and specific transmembrane pathways. J Eukaryot Microbiol. 1997;44:109–116. doi: 10.1111/j.1550-7408.1997.tb05946.x. [DOI] [PubMed] [Google Scholar]
- Gumbo T, Hobbs RE, Carlyn C, Hall G, Isada CM. Microsporidia infection in transplant patients. Transplantation. 1999;67:482–484. doi: 10.1097/00007890-199902150-00024. [DOI] [PubMed] [Google Scholar]
- Haro M, Del Aguila C, Fenoy S, Henriques-Gil N. Intraspecies genotype variability of the microsporidian parasite Encephalitozoon hellem. J Clin Microbiol. 2003;41:4166–4171. doi: 10.1128/JCM.41.9.4166-4171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Sasaki Y, Takinami K. Conditions for extrustion of the polar filament of the spore of Plistophora anguillarum, a microsporidian parasite in Anguilla japonica. Bull Jpn Soc Sci Fish. 1976;42:837–845. [Google Scholar]
- Hayman JR, Hayes SF, Amon J, Nash TE. Developmental expression of two spore wall proteins during maturation of the microsporidian Encephalitozoon intestinalis. Infect Immun. 2001;69:7057–7066. doi: 10.1128/IAI.69.11.7057-7066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman JR, Southern TR, Nash TE. Role of sulfated glycans in adherence of the microsporidian Encephalitozoon intestinalis to host cells in vitro. Infect Immun. 2005;73:841–848. doi: 10.1128/IAI.73.2.841-848.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt RP, Healy B, Vossbrinck CR, Canning EU, Embley TM. A mitochondrial Hsp70 orthologue in Vairimorpha necatrix: molecular evidence that microsporidia once contained mitochondria. Curr Biol. 1997;7:995–998. doi: 10.1016/s0960-9822(06)00420-9. [DOI] [PubMed] [Google Scholar]
- Hollister WS, Canning EU, Weidner E, Field AS, Kench J, Marriott DJ. Development and ultrastructure of Trachipleistophora hominis n. g., n.sp after in vitro isolation from an AIDS patient and inoculation into athymic mice. Parasitology. 1996;112:143–154. doi: 10.1017/s0031182000065185. [DOI] [PubMed] [Google Scholar]
- Ishihara R. Some observations on the fine structure of sporoplasm discharged from spores of a microsporidian, Nosema bombycis. J Invertebr Pathol. 1968;12:245–258. [Google Scholar]
- Jensen HM, Wellings SR. Development of the polar filament–polaroplast complex in a microsporidian parasite. J Protozool. 1972;19:297–305. doi: 10.1111/j.1550-7408.1972.tb03463.x. [DOI] [PubMed] [Google Scholar]
- Katinka MD, Duprat S, Cornillot E, Metenier G, Thomarat F, Prensier G, Barbe V, Peyretaillade E, Brottier P, Wincker P, Delbac F, El Alaoui H, Peyret P, Saurin W, Gouy M, Weissenbach J, Vivares CP. Genome sequence and gene compaction of the eukaryote parasite Encephalitozoon cuniculi. Nature. 2001;414:450–453. doi: 10.1038/35106579. [DOI] [PubMed] [Google Scholar]
- Keeling PJ. Congruent evidence from alpha-tubulin and beta-tubulin gene phylogenies for a zygomycete origin of microsporidia. Fungal Genet Biol. 2003;38:298–309. doi: 10.1016/s1087-1845(02)00537-6. [DOI] [PubMed] [Google Scholar]
- Keohane EM, Weiss LM. Characterization and function of the microsporidian polar tube: a review. Folia Parasitol. 1998;45:117–127. [PubMed] [Google Scholar]
- Keohane E, Takvorian PM, Cali A, Tanowitz HB, Wittner M, Weiss LM. The identification and characterization of a polar tube reactive monoclonal antibody. J Eukaryot Microbiol. 1994;41:48S. [PubMed] [Google Scholar]
- Keohane EM, Orr GA, Takvorian PM, Cali A, Tanowitz HB, Witter M, Weiss LM. Purification and characterization of a microsporidian polar tube protein Mol. Biochem Parasitol. 1996a;79:255–259. doi: 10.1016/0166-6851(96)02666-7. [DOI] [PubMed] [Google Scholar]
- Keohane EM, Orr GA, Takvorian PM, Cali A, Tanowitz HB, Wittner M, Weiss LM. Purification and characterization of human microsporidian polar tube proteins. J Eukaryot Microbiol. 1996b;43:100S. doi: 10.1111/j.1550-7408.1996.tb05023.x. [DOI] [PubMed] [Google Scholar]
- Keohane EM, Orr GA, Zhang HS, Takvorian PM, Cali A, Tanowitz HB, Wittner M, Weiss LM. The molecular characterization of the major polar tube protein gene from Encephalitozoon hellem, a microsporidian parasite of humans. Mol Biochem Parasitol. 1998;94:227–236. doi: 10.1016/s0166-6851(98)00071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keohane EM, Orr GA, Takvorian PM, Cali A, Tanowitz HB, Wittner M, Weiss LM. Polar tube proteins of microsporidia of the family Encephalitozoonidae. J Eukaryot Microbiol. 1999;46:1–5. doi: 10.1111/j.1550-7408.1999.tb04569.x. [DOI] [PubMed] [Google Scholar]
- Kudo R. On the nature of structures characteristic of Cnidosporidian spores. Trans Am Microsc Soc. 1921;40:59–74. [Google Scholar]
- Kudo RR, Daniels EW. An electron microscope study of the spore of a microsporidian, Thelohania californica. J Protozool. 1963;10:112–120. doi: 10.1111/j.1550-7408.1963.tb01645.x. [DOI] [PubMed] [Google Scholar]
- Leitch GJ, He Q, Wallace S, Visvesvara GS. Inhibition of the spore polar filament extrusion of the microsporidium, Encephalitozoon hellem, isolated from an AIDS patient. J Eukaryot Microbiol. 1993;40:711–717. doi: 10.1111/j.1550-7408.1993.tb04463.x. [DOI] [PubMed] [Google Scholar]
- Lewis NL, Francis IC, Hawkins GS, Coroneo MT. Bilateral microsporidial keratoconjunctivitis in an immunocompetent non-contact lens wearer. Cornea. 2003;22:374–376. doi: 10.1097/00003226-200305000-00018. [DOI] [PubMed] [Google Scholar]
- Lom J. On the structure of the extruded microsporidian polar filament. Z Parasitenkd. 1972;38:200–213. [Google Scholar]
- Lom J, Corliss JO. Ultrastructural observations on the development of the microsporidian protozoon Plistophora hyphessobryconis schaperclaus. J Protozool. 1967;14:141–152. [Google Scholar]
- Lom J, Vavra J. The mode of sporoplasm extrusion in microsporidian spores. Acta Protozool. 1963;1:81–89. [Google Scholar]
- Lores B, Lopez-Miragaya I, Arias C, Fenoy S, Torres J, del Aguila C. Intestinal microsporidiosis due to Enterocytozoon bieneusi in elderly human immunodeficiency virus—negative patients from Vigo, Spain. Clin Infect Dis. 2002;34:918–921. doi: 10.1086/339205. [DOI] [PubMed] [Google Scholar]
- Lujan HD, Conrad JT, Clark CG, Touz MC, Delbac F, Vivares CP, Nash TE. Detection of microsporidia spore-specific antigens by monoclonal antibodies. Hybridoma. 1998;17:237–243. doi: 10.1089/hyb.1998.17.237. [published erratum appears in Hybridoma 1998 Dec; 17(6):581] [DOI] [PubMed] [Google Scholar]
- Malone LA. In vitro spore hatching of two microsporidia, Nosema costelytrae and Vavraia oncoperae, frrom New Zealand pasture insects. J Invertebr Pathol. 1990;55:441–443. [Google Scholar]
- Metge S, Van Nhieu JT, Dahmane D, Grimbert P, Foulet F, Sarfati C, Bretagne S. A case of Enterocytozoon bieneusi infection in an HIV-negative renal transplant recipient. Eur J Clin Microbiol Infect Dis. 2000;19:221–223. doi: 10.1007/s100960050463. [DOI] [PubMed] [Google Scholar]
- Moss RB, Beaudet LM, Wenig BM, Nelson AM, Firpo A, Punja U, Scott TS, Kaliner MA. Microsporidium-associated sinusitis. Ear Nose Throat J. 1997;76:95–101. [PubMed] [Google Scholar]
- Ohshima K. On the function of the polar filament of Nosema bombycis. Parasitology. 1937;29:220–224. [Google Scholar]
- Olson RE. Laboratory and field studies on Glugea stephani (Hagenmuller), a microsporidan parasite of pleuronectid flatfishes. J Protozool. 1976;23:158–164. doi: 10.1111/j.1550-7408.1976.tb05262.x. [DOI] [PubMed] [Google Scholar]
- Peuvel I, Delbac F, Metenier G, Peyret P, Vivares CP. Polymorphism of the gene encoding a major polar tube protein PTP1 in two microsporidia of the genus Encephalitozoon. Parasitology. 2000;121 (6):581–587. doi: 10.1017/s0031182000006910. [DOI] [PubMed] [Google Scholar]
- Peuvel I, Peyret P, Metenier G, Vivares CP, Delbac F. The microsporidian polar tube: evidence for a third polar tube protein (PTP3) in Encephalitozoon cuniculi. Mol Biochem Parasitol. 2002;122:69–80. doi: 10.1016/s0166-6851(02)00073-7. [DOI] [PubMed] [Google Scholar]
- Pleshinger J, Weidner E. The microsporidian spore invasion tube. IV Discharge activation begins with pH-triggered Ca2+ influx. J Cell Biol. 1985;100:1834–1838. doi: 10.1083/jcb.100.6.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigneau O, Achbarou A, Bouladoux N, Mazier D, Desportes-Livage I. Identification of proteins in Encephalitozoon intestinalis, a microsporidian pathogen of immunocompromised humans: an immunoblotting and immunocytochemical study. J Eukaryot Microbiol. 2000;47:48–56. doi: 10.1111/j.1550-7408.2000.tb00010.x. [DOI] [PubMed] [Google Scholar]
- Raynaud L, Delbac F, Broussolle V, Rabodonirina M, Girault V, Wallon M, Cozon G, Vivares CP, Peyron F. Identification of Encephalitozoon intestinalis in travelers with chronic diarrhea by specific PCR amplification. J Clin Microbiol. 1998;36:37–40. doi: 10.1128/jcm.36.1.37-40.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich SM, Ayala FJ. Population structure and recent evolution of Plasmodium falciparum. Proc Natl Acad Sci USA. 2000;97:6994–7001. doi: 10.1073/pnas.97.13.6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sax PE, Rich JD, Pieciak WS, Trnka YM. Intestinal microsporidiosis occurring in a liver transplant recipient. Transplantation. 1995;60:617–618. doi: 10.1097/00007890-199509270-00018. [DOI] [PubMed] [Google Scholar]
- Schwartz DA, Visvesvara GS, Diesenhouse MC, Weber R, Font RL, Wilson LA, Corrent G, Serdarevic ON, Rosberger DF, Keenen PC, et al. Pathologic features and immunofluorescent antibody demonstration of ocular microsporidiosis (Encephalitozoon hellem) in seven patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1993a;115:285–292. doi: 10.1016/s0002-9394(14)73577-9. [DOI] [PubMed] [Google Scholar]
- Schwartz DA, Visvesvara GS, Diesenhouse MC, Weber R, Font RL, Wilson LA, Corrent G, Serdarevic ON, Rosberger DF, Keenen PC, Grossniklaus HE, Hewanlowe K, Bryan RT. Pathologic features and immunofluorescent antibody demonstration of ocular microsporidiosis (Encephalitozoon hellem) in seven patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1993b;115:285–292. doi: 10.1016/s0002-9394(14)73577-9. [DOI] [PubMed] [Google Scholar]
- Schwartz DA, Visvesvara GS, Leitch GJ, Tashjian L, Pollack M, Holden J, Bryan RT. Pathology of symptomatic microsporidial (Encephalitozoon hellem) bronchiolitis in the acquired immunodeficiency syndrome: a new respiratory pathogen diagnosed from lung biopsy, bronchoalveolar lavage, sputum, and tissue culture. Hum Pathol. 1993c;24:937–943. doi: 10.1016/0046-8177(93)90106-q. [DOI] [PubMed] [Google Scholar]
- Sinden RE, Canning EU. The ultrastructure of the spore of Nosema algerae (Protozoa Microsporida), in relation to the hatching mechanism of microsporidian spores. J Gen Microbiol. 1974;85:350–357. [Google Scholar]
- Slamovits CH, Fast NM, Law JS, Keeling PJ. Genome compaction and stability in microsporidian intracellular parasites. Curr Biol. 2004;14:891–896. doi: 10.1016/j.cub.2004.04.041. [DOI] [PubMed] [Google Scholar]
- Sparfel JM, Sarfati C, Liguory O, Caroff B, Dumoutier N, Gueglio B, Billaud E, Raffi F, Molina JM, Miegeville M, Derouin F. Detection of microsporidia and identification of Enterocytozoon bieneusi in surface water by filtration followed by specific PCR. J Eukaryot Microbiol. 1997;44:78S. doi: 10.1111/j.1550-7408.1997.tb05792.x. [DOI] [PubMed] [Google Scholar]
- Sprague V. Systematics of the Microsporidia. Plenum Press; New York, NY: 1977. [Google Scholar]
- Sprague VV, Becnel JJ. Note on the name-author-date combination for the Taxon MICROSPORIDIES Balbiani, 1882, when ranked as a phylum. J Invertebr Pathol. 1998;71:91–94. doi: 10.1006/jipa.1997.4702. [DOI] [PubMed] [Google Scholar]
- Sprague V, Vernick SH. The golgi complex of microsporida and its role in spore morphogenesis. Am Zool. 1968;8:824. [Google Scholar]
- Sprague V, Becnel JJ, Hazard EI. Taxonomy of phylum Microspora. Crit Rev Microbiol. 1992;18:285–395. doi: 10.3109/10408419209113519. [DOI] [PubMed] [Google Scholar]
- Takvorian PM, Cali A. The ultrastructure of spores (Protozoa: Microspora) from Lophius americanus, the Angler fish. J Protozool. 1986;33:570–575. doi: 10.1111/j.1550-7408.1986.tb05664.x. [DOI] [PubMed] [Google Scholar]
- Takvorian PM, Cali A. Enzyme histochemical identification of the Golgi apparatus in the microsporidian, Glugea stephani. J Eukaryot Microbiol. 1994;41:63S–64S. [PubMed] [Google Scholar]
- Takvorian PM, Cali A. Polar tube formation and nucleoside diphosphatase activity in the microsporidian, Glugea stephani. J Eukaryot Microbiol. 1996;43:102S–103S. doi: 10.1111/j.1550-7408.1996.tb05025.x. [DOI] [PubMed] [Google Scholar]
- Texier C, Roux F, Brosson D, Delbac F, Garin J, Vivares CP. Proteomics analysis of Encephalitozoon cuniculi. Groupment des Protistologues de Langue Francasie Abstract 114. J Eukaryot Microbiol. 2003;50 (supplement):32A. [Google Scholar]
- Thelohan P. Sur la presence d’une capsule a filament dans les spores des microsporidies. CR Acad Sci. 1894;118:1425–1427. [Google Scholar]
- Thomarat F, Vivares CP, Gouy M. Phylogenetic analysis of the complete genome sequence of Encephalitozoon cuniculi supports the fungal origin of microsporidia and reveals a high frequency of fast-evolving genes. J Mol Evol. 2004;59:780–791. doi: 10.1007/s00239-004-2673-0. [DOI] [PubMed] [Google Scholar]
- Undeen AH. A proposed mechanism for the germination of microsporidian (Protozoa Microspora) spores. J Theor Biol. 1990;142:223–235. [Google Scholar]
- Undeen AH, Avery SE. Germination of experimentally nontransmissible microsporidia. J Invertebr Pathol. 1984;43:299–301. [Google Scholar]
- Undeen AH, Avery SW. Ammonium chloride inhibition of the germination of spores of Nosema algerae (Microspora: Nosematidae) J Invertebr Pathol. 1988;52:326–334. [Google Scholar]
- Undeen AH, Epsky ND. In vitro and vivo germination of Nosema locustae (Microspora: Nosematidae) spores. J Invertebr Pathol. 1990;56:371–379. [Google Scholar]
- Undeen AH, Frixione E. The role of osmotic pressure in the germination of Nosema algerae spores. J Protozool. 1990;37:561–567. doi: 10.1111/j.1550-7408.1990.tb01265.x. [DOI] [PubMed] [Google Scholar]
- Undeen AH, Vandermeer RK. The effect of ultraviolet radiation on the germination of Nosema algerae Vávra and Undeen (Microsporida: Nosematidae) spores. J Protozool. 1990;37:194–199. doi: 10.1111/j.1550-7408.1990.tb01127.x. [DOI] [PubMed] [Google Scholar]
- Undeen AH, Vandermeer RK. Conversion of intrasporal trehalose into reducing sugars during germination of Nosema algerae (Protista: Microspora) spores—a quantitative study. J Eukaryot Microbiol. 1994;41:129–132. [Google Scholar]
- van Gool T, Luderhoff E, Nathoo KJ, Kiire CF, Dankert J, Mason PR. High prevalence of Enterocytozoon bieneusi infections among HIV-positive individuals with persistent diarrhoea in Harare, Zimbabwe. Trans R Soc Trop Med Hyg. 1995;89:478–480. doi: 10.1016/0035-9203(95)90073-x. [DOI] [PubMed] [Google Scholar]
- van Gool T, Vetter JC, Weinmayr B, Van Dam A, Derouin F, Dankert J. High seroprevalence of Encephalitozoon species in immunocompetent subjects. J Infect Dis. 1997;175:1020–1024. doi: 10.1086/513963. [DOI] [PubMed] [Google Scholar]
- Vavra J. Detection of polysaccarides in microsporidian spores by means of the periodic acid-thiosemicarbazide-silver proteinate test. J Microsc. 1972;14:357–360. [Google Scholar]
- Vavra J. In: Structure of the microsporidia Comparative Pathobiology. Bulla LA, Cheng TC, editors. Vol. 1. Plenum Press; New York, NY: 1976. pp. 1–85. [Google Scholar]
- Vavra J, Yachnis AT, Shadduck JA, Orenstein JM. Microsporidia of the genus Trachipleistophora—causative agents of human microsporidiosis: description of Trachipleistophora anthropophthera n. sp (Protozoa: Microsporidia) J Eukaryot Microbiol. 1998;45:273–283. doi: 10.1111/j.1550-7408.1998.tb04536.x. [DOI] [PubMed] [Google Scholar]
- Visvesvara GS, Leitch GJ, da Silva AJ, Croppo GP, Moura H, Wallace S, Slemenda SB, Schwartz DA, Moss D, Bryan RT, et al. Polyclonal and monoclonal antibody and PCR-amplified small-subunit rRNA identification of a microsporidian, Encephalitozoon hellem, isolated from an AIDS patient with disseminated infection. J Clin Microbiol. 1994;32:2760–2768. doi: 10.1128/jcm.32.11.2760-2768.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters VA. Structure, hatching and size variation of the spores in a species of Nosema (Microsporidia) found in Hyalophora cecropia (Lepidoptera) Parasitology. 1958;48:113–120. doi: 10.1017/s0031182000021107. [DOI] [PubMed] [Google Scholar]
- Wanke CA, DeGirolami P, Federman M. Enterocytozoon bieneusi infection and diarrheal disease in patients who were not infected with human immunodeficiency virus: case report and review. Clin Infect Dis. 1996;23:816–818. doi: 10.1093/clinids/23.4.816. [DOI] [PubMed] [Google Scholar]
- Weber R, Bryan RT. Microsporidial infections in immunodeficient and immunocompetent patients. Clin Infect Dis. 1994;19:517–521. doi: 10.1093/clinids/19.3.517. [DOI] [PubMed] [Google Scholar]
- Weber R, Deplazes P, Flepp M, Mathis A, Baumann R, Sauer B, Kuster H, Luthy R. Cerebral microsporidiosis due to Encephalitozoon cuniculi in a patient with human immunodeficiency virus infection. N Engl J Med. 1997;336:474–478. doi: 10.1056/NEJM199702133360704. [DOI] [PubMed] [Google Scholar]
- Weber R, Deplazes P, Schwartz D. Diagnosis and clinical aspects of human microsporidiosis. Contrib Microbiol. 2000;6:166–192. doi: 10.1159/000060360. [DOI] [PubMed] [Google Scholar]
- Weidner E. Ultrastructural study of microsporidian development. J Parasitol. 1970;56:362. [PubMed] [Google Scholar]
- Weidner E. Ultrastructural study of microsporidian invasion into cells. Z Parasitenkd. 1972;40:227–242. doi: 10.1007/BF00329623. [DOI] [PubMed] [Google Scholar]
- Weidner E. The microsporidian spore invasion tube. The ultrastructure, isolation, and characterization of the protein comprising the tube. J Cell Biol. 1976;71:23–34. doi: 10.1083/jcb.71.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner E. The microsporidian spore invasion tube. III Tube extrusion and assembly. J Cell Biol. 1982;93:976–979. doi: 10.1083/jcb.93.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner E. Cytoskeletal proteins expressed by microsporidian parasites. Subcell Biochem. 1992;18:385–399. doi: 10.1007/978-1-4899-1651-8_12. [DOI] [PubMed] [Google Scholar]
- Weidner E, Byrd W. The microsporidian spore invasion tube. II Role of calcium in the activation of invasion tube discharge. J Cell Biol. 1982;93:970–975. doi: 10.1083/jcb.93.3.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner E, Halonen SK. Microsporidian spore envelope keratins phosphorylate and disassemble during spore activation. J Eukaryot Microbiol. 1993;40:783–788. [Google Scholar]
- Weidner E, Byrd W, Scarborough A, Pleshinger J, Sibley D. Microsporidian spore discharge and the transfer of polaroplast organelle membrane into plasma membrane. J Protozool. 1984;31:195–198. [Google Scholar]
- Weidner E, Manale SB, Halonen SK, Lynn JW. Protein–membrane interaction is essential to normal assembly of the microsporidian spore invasion tube. Biol Bull. 1995;188:128–135. doi: 10.2307/1542078. [DOI] [PubMed] [Google Scholar]
- Weiss LM. Microsporidia: emerging pathogenic protists. Acta Trop. 2001;78:89–102. doi: 10.1016/s0001-706x(00)00178-9. [DOI] [PubMed] [Google Scholar]
- Weiss LM, Edlind TD, Vossbrinck CR, Hashimoto T. Microsporidian molecular phylogeny: the fungal connection. J Eukaryot Microbiol. 1999;46:17S–18S. [PubMed] [Google Scholar]
- Weitz JC, Botehlo R, Bryan R. Microsporidiosis in patients with chronic diarrhea and AIDS, in HIV asymptomatic patients and in patients with acute diarrhea. Rev Med Chil. 1995;123:849–856. [PubMed] [Google Scholar]
- Williams BA, Hirt RP, Lucocq JM, Embley TM. A mitochondrial remnant in the microsporidian Trachipleistophora hominis. Nature. 2002;418:865–869. doi: 10.1038/nature00949. [DOI] [PubMed] [Google Scholar]
- Wittner M, Weiss LM. The Microsporidia and Microsporidiosis. ASM Press; Washington, DC: 1999. [Google Scholar]
- Xiao L, Li L, Visvesvara GS, Moura H, Didier ES, Lal AA. Genotyping Encephalitozoon cuniculi by multilocus analyses of genes with repetitive sequences. J Clin Microbiol. 2001;39:2248–2253. doi: 10.1128/JCM.39.6.2248-2253.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Takvorian PM, Cali A, Orr G, Weiss LM. Glycosylation of the major polar tube protein of Encephalitozoon hellem, a microsporidian parasite that infects humans. Infect Immun. 2004;72:6341–6350. doi: 10.1128/IAI.72.11.6341-6350.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zender HO, Arrigoni E, Eckert J, Kapanci Y. A case of Encephalitozoon cuniculi peritonitis in a patient with AIDS. Am J Clin Pathol. 1989;92:352–356. doi: 10.1093/ajcp/92.3.352. [DOI] [PubMed] [Google Scholar]
- Zierdt CH, Gill VJ, Zierdt WS. Detection of microsporidian spores in clinical samples by indirect fluorescent-antibody assay using whole-cell antisera to Encephalitozoon cuniculi and Encephalitozoon hellem. J Clin Microbiol. 1993;31:3071–3074. doi: 10.1128/jcm.31.11.3071-3074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


