Abstract
HIV-associated neurological disorders (HAND) are estimated to affect almost 60% of HIV infected individuals. HIV-encephalitis (HIVE), the pathological correlate of the most severe form of HAND is often characterized by glial activation, cytokine/chemokine dysregulation, and neuronal damage and loss. However, the severity of HIVE correlates better with glial activation rather than viral load. Using the macaque model, it has been demonstrated that simian immunodeficiency virus encephalitis (SIVE) correlates with increased expression of the mitogen platelet-derived growth factor-B (PDGF-B) chain in the brain. The present study was aimed at exploring the role of PDGF-B chain in HIV-associated activation and proliferation of astrocytes. Specifically, the data herein demonstrate that exposure of rat and human astrocytes to the HIV-1 protein, Tat resulted in the induction of PDGF at both the mRNA and protein levels. Furthermore, PDGF-BB induction was regulated by activation of ERK1/2 and JNK signaling pathways and the downstream transcription factor, early growth response 1(Egr-1). Chromatin immunoprecipitation (ChIP) assays demonstrated binding of Egr-1 to the PDGF-B promoter. Exposure of astrocytes to PDGF-BB, in turn, led to both increased proliferation and release of pro-inflammatory cytokines MCP-1 and IL-1β. Since astrogliosis is linked to disease severity, understanding its regulation by PDGF-BB could aid in the development of therapeutic intervention strategies for HAND.
Introduction
HIV-1 is capable of penetrating the brain shortly after initial infection (1). However, while combined anti-retroviral therapy (cART) is able to control the virus in the periphery, the drugs have inferior penetration across the blood brain barrier (BBB) (2). So while HIV patients are living longer, they now have to deal with the long-term effects of having HIV in the brain. Despite the efficacy of cART, HIV-associated neurocognitive disorders (HAND), which includes HIV-associated dementia (HAD), Minor Cognitive Motor Disorders (MCMD), and other HIV related neuropsychiatric impairments, are estimated to now affect almost 60% of HIV patients (3, 4). These patients are diagnosed by changes in behavior and cognitive and motor abnormalities (4). HAD, the most severe form of HIV induced CNS impairment (5), is clinically characterized by motor and behavioral dysfunction that in the absence of therapy may lead to seizures, coma and death within six months of onset (6). Pathological correlates of HAD include astrogliosis, microglial activation, monocyte infiltration and neuronal damage. With the increasing prevalence of HAND it is thus essential to understand the cellular and molecular mechanisms by which HIV exerts its detrimental effects on the CNS.
Astrocytes, the most abundant cells in the CNS, are a type of glial cell capable of releasing toxic mediators following activation by either infection or injury. In addition to activation, these cells can also undergo active proliferation to replace apoptosing neurons (7). While astrocytes are not productively infected with HIV, the provirus in these cells is able to make the early HIV-1 genes Tat, Rev and Nef (8, 9). HIV-1 Tat is not only expressed in astrocytes and other productively infected cells like microglia, but it can also be released from these cells to activate other neighboring cells. Kutsch et. al. (2000) has reported that astrocytes activated by Tat have the ability to release toxic mediators (9).
One of the toxic mediators that has been shown to be up-regulated in the brains of macaques with SIVE, is the mitogen PDGF (10). PDGFs are a family of proteins comprising of four chains (A–D) encoded by four genes locates on different chromosomes, that are highly conserved throughout the animal kingdom (11). These proteins are usually expressed as dimers; PDGF-A and PDGF-B can form homodimers or heterodimers, while PDGF-C and D form homodimers. For the sake of clarity, PDGF (A–D) refers to the RNA expression whereas PDGF-AA, PDGF-BB, PDGF-CC and PDGF-DD refer to the protein expression of these genes. All four PDGF ligands exert their effects via tyrosine kinase receptors PDGF-Rα and PDGFR-β (12). Members of this family are disulfide-bonded polypeptides that have multi-functional roles ranging from embryonic development to wound healing (13–15). PDGF signaling has been implicated in a number of pathological disease states including liver, lung and cardiac fibrosis, atherosclerosis, restenosis, pulmonary hypertension, cancer, gliomas and stroke (11, 16–20), however, its role in HIVE/HAND, specifically as it pertains to astrogliosis has not been elucidated.
In the present work, we utilized rat and human astrocytes to study the effect of Tat on rat and human astrocytes. The data herein demonstrate that exposure of rat and human astrocytes to Tat resulted in the induction of PDGF at both the mRNA and protein levels. We further elucidated that signaling mechanisms involved in PDGF-BB induction was regulated by activation of ERK1/2 and JNK signaling pathways and the downstream transcription factor, early growth response gene 1 (Egr-1). Chromatin immunoprecipitation (ChIP) analysis revealed increased binding of Egr-1 to the PDGF-B promoter in rat glioma cells treated with Tat. Exposure of astrocytes to PDGF-BB, in turn, led to both increased proliferation and release of pro-inflammatory cytokines MCP-1 and IL-1β. Since PDGF is a known cerebrovascular permeant (21), the release of PDGF by astrocytes may have significant consequences including disruption of Blood Brain Barrier (BBB). This is particularly important since astrocyte endfeet processes are in close contact with the endothelial cells of the BBB. Furthermore, PDGF expression could also enhance astrocyte activation. An understanding of PDGF-BB regulation and its inhibition may thus aid in the development of therapeutic intervention strategies for those suffering from HAND.
Materials and Methods
Materials
Recombinant human PDGF-BB was purchased from R&D Systems (Minneapolis, MN). Since serum induces PDGF, all experiments involving the treatment of cells with exogenous PDGF-BB were conducted under serum-free conditions. STI-571, an inhibitor of tyrosine kinase receptors, was obtained from Novartis (Basel, Switzerland). The specific phosphatidinylinositol-3’ kinase (PI3K) inhibitor (LY294002), MEK inhibitor, (U0126) and p38 inhibitor, (SB203580) were purchased from Promega (Madison, WI). The JNK inhibitor (SP600125) was purchased from Assay Designs (Ann Arbor, MI). The concentrations of these inhibitors were based on the concentration-curve study and our previous reports (22, 23). Tat (1–72) (1–86) and 1–101 (supplied by Philip Ray, University of Kentucky) were used in these studies at a concentration of 200ng/ml. Details of Tat production and purification have been previously published (24–26). Control treatments included heat inactivated HIV-1 Tat and cells receiving no treatment. The concentration of Tat in the cerebral spinal fluid (CSF) has been reported at 16 ng/ml (27), however, the Tat concentration utilized in this in vitro study is generally accepted (28–32). HIV-1 LAI virus propagated in stimulated PBMC (33) supplied by Dr. Howard Gendelman (University of Nebraska Medical Center) was used in this study. The rationale for using CXCR4 (X4)-tropic virus was based upon previous studies demonstrating astrocyte activation in response to X4 viruses (34, 35). Dominant negative and constitutively active constructs of MEK and Egr-1 were provided by Dr. Young Han Lee (Konkuk University, Korea). Chromatin immunoprecipitation (ChIP) assay kit was purchased from Upstate (Billerica, MA).
Cell culture and cell lines
The human astrocytic cell line A172 (ATCC #CRL-1620; American Type Culture Collection) were cultured as described previously (36). Rat glioma C6B2 cells were obtained from Dr. Myron Toews (University of Nebraska Medical Center). Human primary astrocytes were obtained from the Congenital Defects Lab (University of Washington). Rat primary astrocytes were isolated from embryonic day 18–19 Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA). Briefly, cortical tissues were dissected in hank’s balanced salt solution and trypsinized. Cells were pelleted, plated and sustained in CO2 incubator at 37°C for 7 days. The cells were then shaken to remove loosely attached cells. Attached cells that remain, primarily astroglia, were allowed to grow for 3–4 days then passaged and seeded. Seven days after plating, cultures consisted of 97% astroglia. C6B2 immortalized cell line and rat primary astrocytes were cultured in DMEM medium (Invitrogen Life Technologies, Carlsbad, CA) containing 10% heated-inactivated fetal bovine serum, 2 mM glutamine, penicillin (100 units/ml), streptomycin (100 µg/ml). Human primary astrocytes were cultured in DMEM medium (Invitrogen Life Technologies) containing 10% heated-inactivated fetal bovine serum, 2 mM glutamine, sodium bicarbonate, gentamycin, non-essential amino acids and vitamins.
Reverse transcription and Real-Time PCR
Total RNA was extracted using Trizol reagent according to the manufacturer’s protocol (Invitrogen Life Technologies). 1 µg of RNA was used for cDNA production according to manufacturer’s instructions (Thermo Scientific, Waltham, MA). Real Time RT2 qPCR primer sets for rat PDGF-A, PDGF-B, PDGF-C, PDGF-Rβ, Egr-1, 18s and human PDGF-B, PDGF-Rβ, Egr-1, MCP-1, IL-1β and 18s were obtained from SA Biosciences (Frederick, MD). Quantitative Analyses of mRNA were conducted using ABI 7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA). Data were normalized using Ct values for 18s in each sample. To calculate relative amounts mRNA, the average Ct values were subtracted from 18s values for each target gene to provide changes in Ct value. Fold change in expression was calculated as log2 relative units.
Western Blotting
Treated cells were lysed using the Mammalian Cell Lysis kit (Sigma, St. Louis, MO) and cell lysates were subjected to separation by 12% SDS-PAGE electrophoresis (30 µg protein per well) and transferred to polyvinylidene difluoride (PVDF) membranes. The blots were blocked with 5% nonfat dry milk in PBS. Western blots were then probed with antibodies recognizing Egr-1 (1:500), PDGF-BB (1:1000), β-actin (1:5000) (Santa Cruz Biotechnology, Santa Cruz, CA) and phosphorylated forms of ERK1/2, JNK, p38and Akt (1:200) (Cell Signaling, Danvers, MA). Cells were then incubated with goat-anti-rabbit secondary antibody (1:5000) (Invitrogen Life Technologies). Signals were detected by chemiluminescence (Pierce, Rockford, IL). All experiments were repeated four times individually and representative blots are presented in the figures.
Immunofluorescence labeling and image analyses
Immunocytochemical analysis of Egr-1 and PDGF-BB were performed in rat glioma cells grown on cover slips. Following Tat treatment, cells were fixed with 4% paraformaldehyde for 15 min at room temperature then permeabilized with PBS containing 0.3% Triton X-100. After blocking with PBS containing 10% normal goat serum, cells were incubated at 4° C overnight with anti-Egr-1 (1:200) or anti-PDGF-BB (1:200) rabbit polyclonal antibodies (Santa Cruz Biotechnology). After washing, cells were incubated with goat anti-rabbit Alexa Fluor® 488-conjugated secondary antibody (1:500). For negative controls, cells were treated as described above omitting the primary antibody. Cells were mounted with Prolong Gold containing 4’,6’-diamidino-2-phenylindole to stain nuclei. Images were caputured with a 40x objective. Slide specimens (5µm thick) of paraffin-embedded SIV infected rhesus macaque and HIV-1-infected human basal ganglia (National NeuroAIDS Tissue Consortium) were selected and treated with paired combinations of primary mouse and rabbit antibodies anti-PDGF-BB (1:50) (Santa Cruz Biotechnologies), PDGF-BB (1:1000) (PGF007, Mochida, Tokyo, Japan), anti-vWF (1:00) (Chemicon, Billerica, MA) and anti-GFAP (1:50) antibodies (Santa Cruz Biotechnologies). Primary antibodies were labeled with secondary anti-mouse and anti-rabbit antibodies conjugated to the fluorescent probes Alexa Fluor® 488 and Alexa Fluor® 594 and nuclei were labeled with 4', 6-diamidino-2-phenylindole (DAPI). Slides were coverslipped with ProLong Gold anti-fade reagent (Invitrogen, Carlsbad, CA), allowed to dry for 24 hr at room temp. Images were captured with a 20x objective.
Transfection with plasmid constructs
A172 cells were transfected with plasmid constructs containing either WT or DN forms of MEK and Egr-1. Knockdown efficiencies were determined by Western Blotting.
Short interfering (si)RNA transfection
siRNA target against Egr-1 were obtained from Dharmacon (Boulder, CO). Human A172 cells were plated in 24-well plates at a density of 4×104 cells per well one day prior to transfection. Cell culture medium was replaced with 250 µl pre-warmed culture medium. DharmaFECT 1 transfection reagent (Dharmacon) was then combined with serum-free DMEM medium (Invitrogen Life Technologies) for 5 min at room temperature. The Egr-1 siRNA was then added into the mixture described above to a final concentration of 5 µM. The siRNA and the reagent mixture were incubated for 20 min at room temperature following which the combined mixture was added to the cells. The cell culture plate was shaken gently for 5 seconds and incubated for 48 h at 37°C. Knockdown efficiencies were determined by Western Blotting.
Chromatin immunoprecipitation (ChIP) Assay
ChIP assay was performed according to the manufacturer’s instructions (Upstate, Billerica, MA) with slight modifications. Briefly, following treatment of the cells, 18.5% fresh formaldehyde was added directly into the medium at a final concentration of 1% formaldehyde and incubated for 10 min at room temperature, followed by quenching with 125 mM glycine. The cells were then scraped using 2 ml pre-chilled PBS containing 1×protease inhibitor cocktail. The cell pellet was harvested by spinning at 800×g at 4°C and lysis buffer was added (provided in the kit) to harvest nuclei. DNA was then sheared by sonication. Fifty µl of the sheared cross-linked chromatin was then mixed with 20µl of protein A magnetic beads and 5 µg of immunopreciptating antibodies against Egr-1, acetyl Histone H3 (as a positive control) and normal rabbit IgG (as a negative control) diluted in 450 µl dilution buffer overnight at 4°C. The magnetic beads binding antibody/chromatin complex was then washed with 0.5 ml each of a series of cold wash buffers in the order of low salt buffer, high salt buffer, LiCl buffer and finally with Tris-EDTA buffer. The cross-linking of protein/DNA complexes were reversed to free DNA by incubation at 62°C for 2 h and purified using DNA purification spin columns following the manufacturer’s instructions. Finally the purified DNA was amplified via PCR to identify the promoter region containing Egr-1 binding site “GCG GGG GCG“. The sequence of the primers used to identify the PDGF-B promoter bound to Egr-1 were as follows: sense, 5’-GCAGAGGCCTGAGCGCCTGATC-3’ anti–sense, 5’GCAGCGATTCATGCCGACTCCG-3’.
Assay for cell viability
Cell viability was measured by mitochondrial dehydrogenases [3(4,5-dimethylthiazol-2-yl)-2.5 diphenyltetrazolium bromide] (MTT) method. Human A172 and rat glioma cells were seeded in 96-well plates at a density of 105cells/cm2 and were exposed to PDGF-BB for 4days. After incubation, 20 µl MTT salt dissolved in Hank’s balanced salt solution at a final concentration of 5 mg/ml was added to each well for 4 h. The medium was aspirated from each well and 200 µl of DMSO was added to dissolve the formazan crystals. The absorbance of each well was obtained using a Synergy Mx (BioTek) plate counter at test and reference wavelengths of 570 and 630 nm, respectively.
Statistical Analysis
Statistical analysis was performed using one-way analysis of variance with a post hoc Student t test. Results were judged statistically significant if p < 0.05 by analysis of variance.
Results
HIV-1/Tat-mediated upregulation of PDGF in astrocytes
Since astrocytes in the CNS are exposed to HIV-1, we first sought to examine the modulation of PDGF-BB by HIV-1 exposure in astrocytes. Briefly, serum-starved human astrocyte cell line A172 was infected with HIV LAI at an MOI of 0.1 for 12 h followed by assessment of cell lysate for PDGF-BB expression by Western blot. As shown in Fig. 1A, there was HIV-1-mediated upregulation of PDGF-BB in cells exposed to the virus and as expected, heat inactivated virus failed to mediate induction of PDGF-BB.
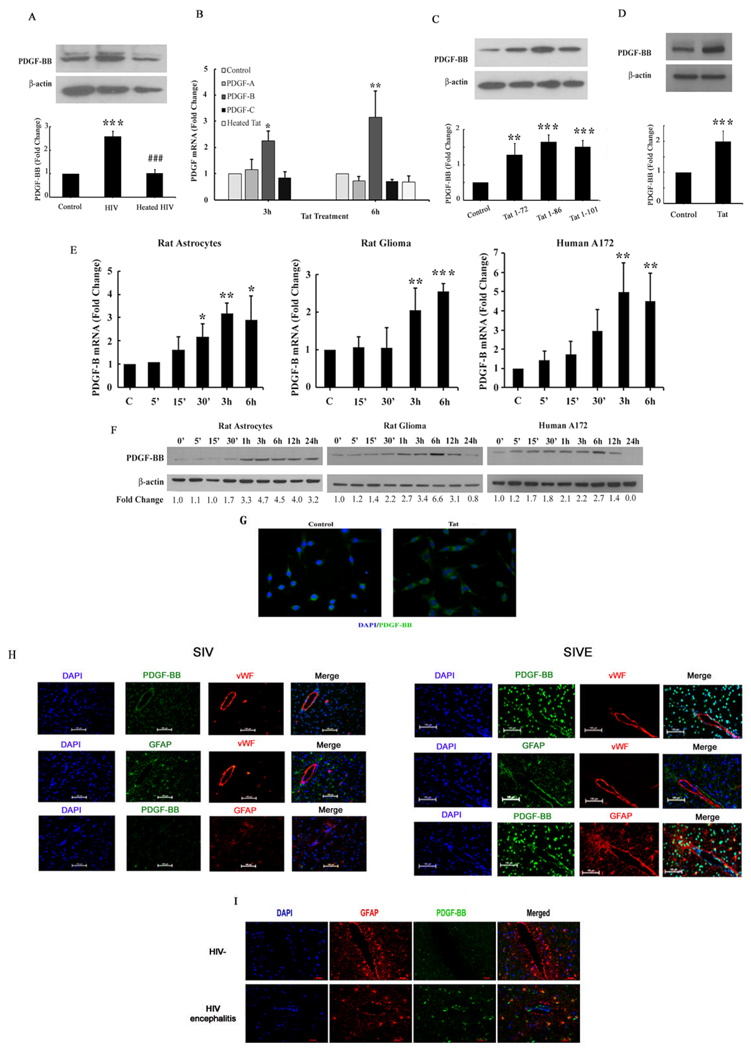
Figure 1. HIV-1/Tat-mediated up-regulation of PDGF-BB in astrocytes.
(A) HIV-mediated induction of PDGF-BB in human A172 astrocytes. Whole cell lysates from human A172 cells were subjected to Western blot analysis using antibodies specific for PDGF-BB. (B) Total RNA isolated from primary rat astrocytes were subjected to Real-Time PCR analysis using primer sets for PDGF-A, PDGF-B and PDGF-C primers. Tat mediated induction of PDGF-B mRNA expression, but no changes in PDGF-A and PDGF-C were evident. Heated Tat abolished Tat-mediated induction of PDGF-B. (C) All Tat preparations upregulated PDGF-BB protein expression equally. Human A172 were treated with Tat lengths 1–72, 1–86 and 1–101 and PDGF-BB levels were assessed by Western blot analysis. (D) Tat-mediated upregulation of PDGF-BB in human primary astrocytes. Whole cell lysates from human primary astrocytes treated with Tat for 12h were subjected to Western blot analysis. (E) Time-dependence of Tat-mediated induction of PDGF-B mRNA expression in rat primary astrocytes, rat gliomas and human A172 cells. (F) Whole cell lysates from rat primary astrocytes, rat gliomas and human A172 cells were subjected to Western blot analysis using antibodies specific for PDGF-BB. Time-dependence of Tat-mediated induction of PDGF-BB protein expression in rat primary astrocytes, rat gliomas and human A172 cells. All data are presented as mean±SD of three individual experiments. *p<0.05, **p<0.01, ***p<0.001 vs control group; ###p<0.001vs Tat treated group. (G) Representative image of PDGF-BB staining in rat glioma cells (Magnification X400). Images were acquired using a 40× oil-immersion lens and flourescent microscopy. (H) Representative image of GFAP + astrocytes expressing PDGF-BB surrounding the blood vessels (vWF positive) in the basal ganglia of SIV-infected rhesus macaques with (SIVE) or without SIVE (Magnification X200). Images were acquired using a 20x lens and flourescent microscopy. (I) Representative image of GFAP + astrocytes expressing PDGF-BB in the basal ganglia sections from humans with HIV encephalitis (HIVE) compared to un-infected (HIV-) controls (Magnification X200). Images were acquired using a 20x lens and flourescent microscopy. GFAP immunoreactivity was stained in red; PDGF-BB immunoreactivity was stained in green while DAPI staining was performed to visualize the nuclei (blue). n=3 per group.
Having determined the effect of whole virus on inducing PDGF-BB expression, the next step was to assess whether viral transactivator protein Tat, that is produced by both infected astrocytes as well as released from neighboring infected CNS cells, could also mediate this effect. Rat primary astrocytes were serum starved overnight followed by treatment with recombinant Tat (200 ng/ml) for 3 or 6 h. As an initial screening to identify which PDGF chains were expressed in response to Tat, mRNA levels of PDGF-A, PDGF-B and PDGF-C chains were determined by Real-Time-PCR. Following exposure of rat astrocytes to Tat for 3 and 6 h, there was up-regulation of PDGF-B mRNA (2.15 and 3.2 fold respectively), compared with the untreated cells and as expected, heated Tat had no effect on the induction of PDGF-B mRNA. In contrast no significant changes in the mRNA levels of PDGF-A and PDGF-C were evident (Fig. 1B). Since only PDGF-B chain was up-regulated in response to Tat, further studies were focused only on PDGF-B chain. Similar to Tat 1–72, both Tat 1–86 and the full length Tat 1–101 also mediated induction of PDGF-BB in human A172 cells (Fig 1C). Validation of our findings in human astrocyte cell line was also confirmed in human primary astrocytes (Fig.1D).
To assess the time course of Tat-mediated induction of PDGF-B, rat and human astrocyte cell lines (C6B2 and A172) as well as rat primary astrocytes were treated with Tat (200 ng/ml) for varying times (5 min to 6 h), followed by RNA extraction and assessment of PDGF-B chain mRNA levels by Real-Time PCR. As shown in Fig. 1E, PDGF-B mRNA was upregulated in a time-dependent manner in all the cell types examined, with a peak expression at 3–6 h. To examine whether the mRNA upregulation translated into increased protein expression, all the cell types were treated as described above, followed by cell lysis and Western blotting. Since PDGF-BB is an early response protein we also chose earlier time points for detection. As shown in Fig. 1F Tat up-regulated PDGF-BB protein expression in a time-dependent manner with a peak expression at 6 h and a decline thereafter. PDGF-BB expression in rat astrocytes appeared to peak earlier (3 h) compared with its expression in the cell lines (6 h). Confirmation of these findings by immunostaining also revealed increased PDGF-BB expression in Tat treated rat glioma cells at 6 h post-treatment (Fig. 1G). Images were captured with a 40x objective lens and fluorescence microscopy. Cumulatively, these data clearly demonstrate HIV-1/Tat-mediated induction of PDGF-BB protein in rat and human astrocytes.
To validate PDGF-BB expression in SIV/HIV infection, paraffin-embedded sections of basal ganglia from SIV-infected rhesus macaques with (SIV-E) and without encephalitis were stained for PDGF-BB, an endothelial cell marker, von Willebrand Factor (vWF) and GFAP. As shown in Figure 1H, there was upregulated expression of PDGF-BB in astrocytes surrounding the blood vessels in brains of macaques with SIVE (right panels) compared with the infected animals without encephalitis (left panels). Similar studies were performed on basal ganglia sections from human subjects with HIV encephalitis (HIVE) and uninfected controls and, as shown in Fig. 1I, there was increased expression of astrocytic PDGF-BB in the sections from HIVE versus uninfected controls. Images were captured with a 20x objective lens and fluorescence microscopy.
ERK1/2 and JNK MAPK but not p38 and PI3K/Akt pathways are involved in Tat-mediated PDGF-BB expression in astrocytes
Having determined Tat-mediated induction of PDGF-BB, we next sought to elucidate the signaling pathways involved in this process. Since Tat signaling involves mitogen- activated protein kinase (MAPK) pathways, we examined the involvement of ERK1/2, JNK and p38 kinases in Tat-mediated induction of PDGF-BB. Treatment of rat glioma cells with HIV-1 Tat resulted in a time-dependent increase in phosphorylation of ERK1/2, JNK, p38 and Akt, with maximal activation at 30 min following treatment (Fig. 2A). Specificity of these signaling pathways was subsequently assessed using a pharmacological approach. Pretreatment of cells with MAP kinase (MEK) inhibitor U0126, resulted in abrogation of Tat-induced phosphorylation of ERK as expected since MEK lies upstream of ERK1/2. However, pretreatment of cells with PI3K inhibitor, LY294002 failed to inhibit ERK phosphorylation. Conversely, treatment of cells with PI3K inhibitor resulted in the inhibition of Tat-induced activation of Akt, but not ERK1/2 (Fig. 2B).
Figure 2. ERK1/2 and JNK MAPK but not p38 and PI3K/Akt pathways are involved in Tat-mediated PDGF-BB expression in astrocytes.
(A) Western blot analysis of time-dependent activation ERK1/2, JNK, p38 and Akt by Tat. (B) ERK1/2 and Akt pathways are activated independently of each other. Pretreatment of cells with MAP kinase kinase (MEK) inhibitor (U0126), resulted in abrogation of Tat-induced phosphorylation of ERK1/2. However, pretreatment of cells with PI3K inhibitor (LY294002) failed to inhibit ERK1/2 phosphorylation. Conversely, treatment of cells with PI3K inhibitor resulted in the inhibition of Tat-induced activation of Akt, but not ERK1/2. (C) Pretreatment of cells with MEK inhibitor (U1026) and JNK inhibitor (SB600125) resulted in amelioration of Tat-mediated PDGF-BB expression. Conversely, pretreatment with PI3K inhibitor (LY294002) failed to inhibit Tat-mediated PDGF-BB expression. (D) Pretreatment of cells with p38 inhibitor (SB203580) did not ameliorate Tat-mediated induction of PDGF-BB. (E) Transfection with DN-MEK and not WT-MEK resulted in abrogation of Tat-mediated induction of PDGF-BB. All data are presented as mean±SD of three individual experiments.**p<0.01, ***p<0.001 vs control group; #p<0.05, ##p<0.01, ###p<0.001vs Tat treated group.
We next wanted to address the functional role of MAPK and PI3K/Akt in the PDGF-BB expression induced by Tat. Human A172 cells were pretreated with inhibitors specific for the respective signaling pathways prior to stimulation with Tat and assessed for expression of PDGF-BB. As shown in Fig. 2C, pretreatment of cells with MEK (U0126, 20 µM), JNK (SP600125, 20 µM), but not PI3K (LY294002, 10 µM) inhibitor, resulted in amelioration of Tat-mediated induction of PDGF-BB. Pretreatment of A172 astrocytes with p38 inhibitor (SB203580, 10 µM) however, did not result in amelioration of PDGF-BB expression in response to Tat (Fig. 2D). Further validation of the involvement of ERK pathway in this process was confirmed by transfecting cells with either the WT or DN constructs of MEK followed by treatment with Tat. Tat mediated induction of PDGF-BB was attenuated by DN-MEK, but not by WT-MEK construct (Fig. 2E). Taken together, these findings confirm the involvement of MEK MAPKs but not p38 and PI3K/Akt cascade in Tat-mediated induction of PDGF-BB in astrocytes.
Egr-1 expression is upregulated in astrocytes exposed to Tat
Having determined the involvement of ERK1/2 and JNK MAPKs in Tat-mediated PDGF-BB expression and from published reports suggesting the binding of Egr-1 to PDGF-B promoter, we rationalized the involvement of Egr-1 in Tat-mediated induction of PDGF-BB (37). Exposure of astrocytes to Tat resulted in a time-dependent increase of Egr-1 expression both at the mRNA and protein levels in rat and human astrocyte cell lines (C6B2 and A172) as well as rat primary astrocytes. As shown in Fig. 3A, Egr-1 mRNA was up-regulated in a time-dependent manner in all the cell types examined, with a peak at 15–30 min. To examine whether the mRNA upregulation translated into increased protein expression, all the cell types were treated as described above, followed by Western blotting. As shown in Fig. 3B, Tat upregulated Egr-1 expression in a time-dependent manner with a peak expression between 1–3 h and a decline thereafter. Confirmation of these findings by immunostaining also revealed increased Egr-1 expression in Tat treated human A172 cells at 1 h post-treatment (Fig. 3C). Cumulatively, these data clearly demonstrate that Tat mediated the induction of Egr-1 expression in rat and human astrocytes.
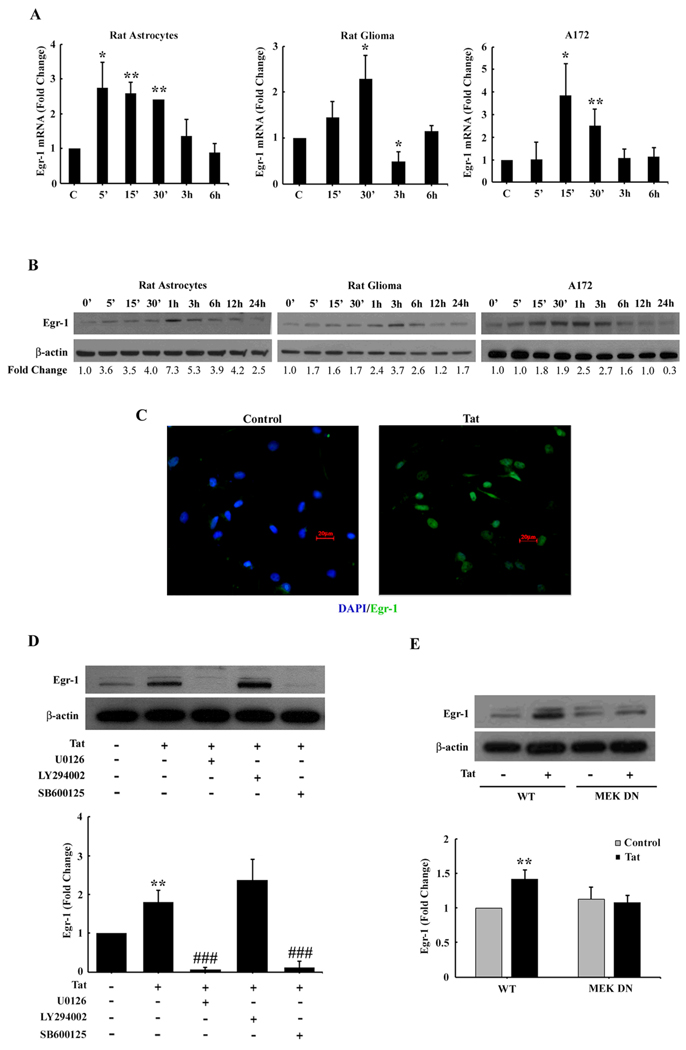
Figure 3. Egr-1 expression is up-regulated in astrocytes exposed to Tat.
(A) Time-dependence of Tat-mediated induction of Egr-1 mRNA expression in rat gliomas and human A172 cell lines and rat primary astrocytes. (B) Whole cell lysates from rat gliomas and human A172 cell lines and rat primary astrocytes were subjected to Western blot analysis using antibodies specific for Egr-1. Time-dependence of Tat-mediated induction of Egr-1 protein expression in rat gliomas and human A172 cell lines and rat primary astrocytes. (C) Representative picture of Egr-1 staining in rat gliomas. (D) Inhibition of the ERK1/2 and Akt pathways by MEK (U0126) and PI3K inhibitor (LY294002) resulted in amelioration of Tat-mediated Egr-1 expression. (E) Transfection with DN-MEK and not WT-MEK resulted in abrogation of Tat-mediated induction of Egr-1. All data are presented as mean±SD of three individual experiments. *p<0.05, **p<0.01 vs control group; ###p<0.001vs Tat treated group.
Next logical step was to examine whether there existed a link that could tie together the activation of ERK1/2, JNK MAPKs and PI3K/Akt with Egr-1. Similar to our studies on signaling molecules described above, astrocytes were pretreated with MEK, JNK or PI3K inhibitors followed by Tat treatment. As shown in Fig. 3D, MEK and JNK inhibitors but not PI3K inhibitor ameliorated Tat-mediated activation of Egr-1. These findings thus linked Tat-mediated activation of ERK1/2 and JNK to the downstream activation of Egr-1.
Further validation of the involvement of ERK1/2 pathway in this process was confirmed by transfecting cells with either the WT or DN constructs of MEK followed by treatment with Tat. Tat-mediated induction of Egr-1 was attenuated by DN-MEK, but not by the WT-MEK construct (Fig. 3E). Taken together, these findings underpin the involvement of MEK MAPKs but not PI3K/Akt cascade in Tat-mediated induction of Egr-1 in astrocytes.
Involvement of Egr-1 in Tat-induced expression of PDGF-BB in astrocytes
Since Egr-1 is a transcription factor implicated in the induction of PDGF-BB, we next wanted to examine the expression kinetics of Egr-1 and PDGF-B mRNAs. As shown in Fig. 4A, in both the cell lines and primary astrocytes, Egr-1 expression (peaking 15–30 min) preceded that of the PDGF-B chain expression (peaking 3–6 h).
Figure 4. Involvement of Egr-1 in Tat-induced expression of PDGF-BB in astrocytes.
(A) Kinetic profiles of Egr-1 and PDGF-B in rat primary astrocytes, rat gliomas and human A172 cells. (B) Whole cell lysates from A172 cells transfected with either Egr-1 or Nonsense (Non) siRNAs were subject to Western blot analysis using antibodies specific Egr-1. (C) Egr-1 siRNA, but not non-specific siRNA, inhibited HIV-1 Tat-mediated induction of PDGF-BB expression. (D) Whole cell lysates from A172 cells transfected with either wildtype (WT) or dominant negative (DN) forms of MEK were subject to Western blot analysis using antibodies specific Egr-1. DN-MEK, but not WT-MEK, inhibited Tat-mediated induction of PDGF-BB expression. (E) Schematic illustration of Egr-1 binding consensus sequence on the PDGF-B promoter region (F) ChIP assay demonstrating Tat-mediated binding of Egr-1 to the PDGF-B promoter. All data are presented as mean±SD of three individual experiments. **p<0.01, *** p<0.001 vs control group.
To confirm the role of Egr-1 in Tat-mediated induction of PDGF-BB, knocking down the expression of Egr-1 using the short interfering RNA (siRNA) approach was used. As shown in Fig. 4B, transfection of human A172 cells with Egr-1 siRNA resulted in efficient knockdown of Egr-1 protein using Western Blot assay. Furthermore, Egr-1 siRNA also significantly abrogated Tat-mediated up-regulation of PDGF-BB expression (Fig. 4C). To further validate the involvement of the Egr-1 in Tat-induced regulation of PDGF-BB, cells were transfected with either WT or DN constructs of Egr-1, followed by treatment with Tat. Tat-mediated induction of PDGF-BB was attenuated by the DN-Egr-1 construct, but not by the WT-Egr-1 construct (Fig. 4D). Collectively, these findings thus underscore the role of Egr-1 in Tat-mediated induction of PDGF-BB.
To further confirm the binding of Egr-1 with PDGF-B promoter in its natural chromatin context, we resorted to chromatin immunoprecipitation to reveal active sites accessible to Egr-1. Rat glioma cells were treated with Tat for 3 h followed by DNA extraction and processed using a ChIP Analysis kit. These experiments revealed increased binding of Egr-1 to the PDGF-B promoter in rat glioma cells treated with Tat (Fig. 4E–F).
PDGF-BB induces cell proliferation and pro-inflammatory cytokine expression in astrocytes
Having determined the induction of PDGF-BB by Tat, the next step was to explore the functional relevance of this upregulation. Since PDGF-BB is a known mitogen for various cell types, we hypothesized that Tat-induced PDGF-BB released from the astrocytes could, in fact, act on the astrocytes themselves via an autocrine loop. We thus set out to examine the effect of PDGF-BB on both proliferation and expression of pro-inflammatory cytokines in these cells. However, before proceeding with this, it was important to first examine whether astrocytes indeed expressed the PDGF-β receptor (PDGF-Rβ). As shown in Fig. 5A, rat glioma and human astrocyte cell lines (C6B2 and A172) as well as rat primary astrocytes cells expressed PDGF-Rβ mRNA as demonstrated by RT-PCR.
Figure 5. PDGF-BB induces cell proliferation and pro-inflammatory cytokines expression in astrocytes (A).
A representative RT-PCR gel of PDGF-Rβ mRNA expression in human A172, rat primary astrocytes and gliomas. (B) Rat gliomas and human A172 cell lines incubated with PDGF-BB showed increased proliferation by MTT assay (C) mRNA isolated from human A172 cells was subjected to Real-Time PCR analysis using MCP-1 and IL-1β primers. PDGF-BB-induction of MCP-1 and IL-1β mRNA expression in human A172 cell line. All data are presented as mean±SD of at least three individual experiments. *p<0.05, *** p<0.001 vs control group.
The effect of PDGF-BB on astrocyte proliferation demonstrated a significant increased in cell proliferation as evidenced by MTT assay in both rat glioma and human A172 cells (Fig. 5B). In addition to proliferation, astrocytes also respond to activation by releasing a plethora of cytokines/chemokines. To further elucidate the role of PDGF-BB in this praocess, since it is a known inducer of the chemokine, MCP-1 (38), we next examined the effect of PDGF-BB on the induction of both MCP-1 and the pro-inflammatory cytokine IL-1β in A172 cells. As shown in Fig. 5C, treatment of human A172 cells with PDGF-BB resulted in a dramatic upregulation of MCP-1 and a significant induction of IL-1β as measured by Real-Time PCR.
Discussion
Anti-retroviral therapies have proven highly effective in controlling systemic viral infection, thus leading to increased longevity in patients with AIDS. The inability of some of these drugs to cross the BBB results in slow and smoldering infection in the CNS. Subsequently, the brain has become a sanctuary of virus-induced toxicity leading to increased prevalence of HAND in HIV-infected individuals. One of the hallmark features of HAND is increased astrogliosis comprising of increased numbers of activated astrocytes, culminating ultimately into increased neuronal degeneration. It is well recognized that activation of astrocytes leads to the release of a plethora of inflammatory cytokines and chemokines as well as factors such as PDGF-BB. PDGF-BB has been implicated in a variety of pathological conditions, however, its role in HIV pathogenesis remains poorly defined.
PDGF-BB has been shown to be upregulated in the brains of macaques with SIV encephalitis (10). It belongs to a family of five dimeric ligands (PDGF-AA, -AB, -BB, -CC and –DD) assembled from four gene products (PDGF A–D) that act through two classical receptor-tyrosine kinases, PDGF-Rα and PDGF-Rβ (10, 11, 18, 39).
HIV Tat protein that is released from HIV infected cells is often taken up by the neighboring cells in the CNS. It has been previously reported that Tat-expressing astrocytes caused astrocytosis, astrocyte dysfunction, and subsequent neuronal death (40), suggesting thereby that both astrocyte dysfunction and certain factors induced by HIV-1/HIV-Tat may contribute to neurotoxicity. Since astrocytes, the most abundant cells within the CNS play a key role in the pathogenesis of HAND via the release of pro-inflammatory cytokines, chemokines and other toxic mediators, the present study was undertaken to explore the role of yet another mediator PDGF-BB that is released by astrocytes in response to HIV-1/HIV-1 Tat. We demonstrate that exposure of HIV-1/Tat to rat and human astrocyte cell lines as well as rat primary astrocytes resulted in the induction of PDGF at both the transcriptional and translational levels.
In our efforts to dissect Tat-mediated downstream signaling events, we demonstrated the activation of ERK1/2, JNK, p38 MAPK and PI3K/Akt pathways by Tat. Further dissection of the signaling pathways involved in Tat-mediated induction of PDGF-BB using both the pharmacologic and genetic approaches revealed activation of ERK1/2, JNK, p38 MAPKs, and PI3K/Akt pathways. Despite the activation of all these pathways, Tat-mediated induction of PDGF-BB involved only the ERK1/2 and JNK, but not p38 and PI3K/Akt signaling.
The transcription factor, Egr-1, has emerged as a major regulatory transcription factor for a number of genes including growth factors such as PDGF (41–43). Our findings demonstrated a time-dependent upregulation of Egr-1 again at both the transcriptional and translational levels in rat and human astrocytes. Further dissection of Egr-1 regulation using both the pharmacological and genetic approaches revealed the activation of upstream ERK1/2 and JNK MAPK pathways in the activation of Egr-1. In agreement with our findings, requirement of ERK1/2 and JNK activation for Egr-1 expression has been reported in FGF2 treated astrocytes (44).
Taking into account that Egr-1 is an early response gene that regulates a number of other target genes, it was of interest to examine its expression profile compared with that of PDGF-B expression. Interestingly, Egr-1 expression preceded that of PDGF-B leading us to speculate their interaction. In an effort to determine a link between Egr-1 and PDGF-B expression, we demonstrated increase Egr-1 binding to the PDGF-B promoter in astrocytes treated with Tat, lending credence to the role of Egr-1 in PDGF-BB expression. Further support of Egr-1 involvement in Tat-mediated PDGF-BB induction was also demonstrated using both the siRNA and genetic approaches. Our findings are in agreement with the report by Khachigan et al 1996, who demonstrated that Egr-1 interacts with the PDGF-B promoter in arterial endothelial cells (37).
Since Tat induced PDGF-BB, it was critical to understand what role released PDGF-BB played in these cells. Based on the mitogenic function of PDGF-BB, we rationalized that PDGF-BB may be involved in increasing astrocyte proliferation. As expected, exogenous PDGF-BB did indeed increase astrocyte proliferation and in addition, also led to the release of the chemokine MCP-1. These findings are consistent with the reports on PDGF mediated induction of MCP-1 in fibroblast cell line and in smooth muscle cells (45, 46). MCP-1 is a known biomarker of HIV neuropathogenesis (47, 48), PDGF-mediated up-regulation of this chemokine can thus have ramifications for accelerated CNS disease in the context of HIV-1 infection. MCP-1 is a potent chemokine whose elevated levels are closely associated with the progression of HAND (49, 50) and whose function includes the recruitment of monocytes from the blood to the brain (51). Interestingly, in addition to MCP-1, PDGF-BB also induced IL-1β expression in astrocytes, which can have further implications in the amplification of toxic responses in the CNS.
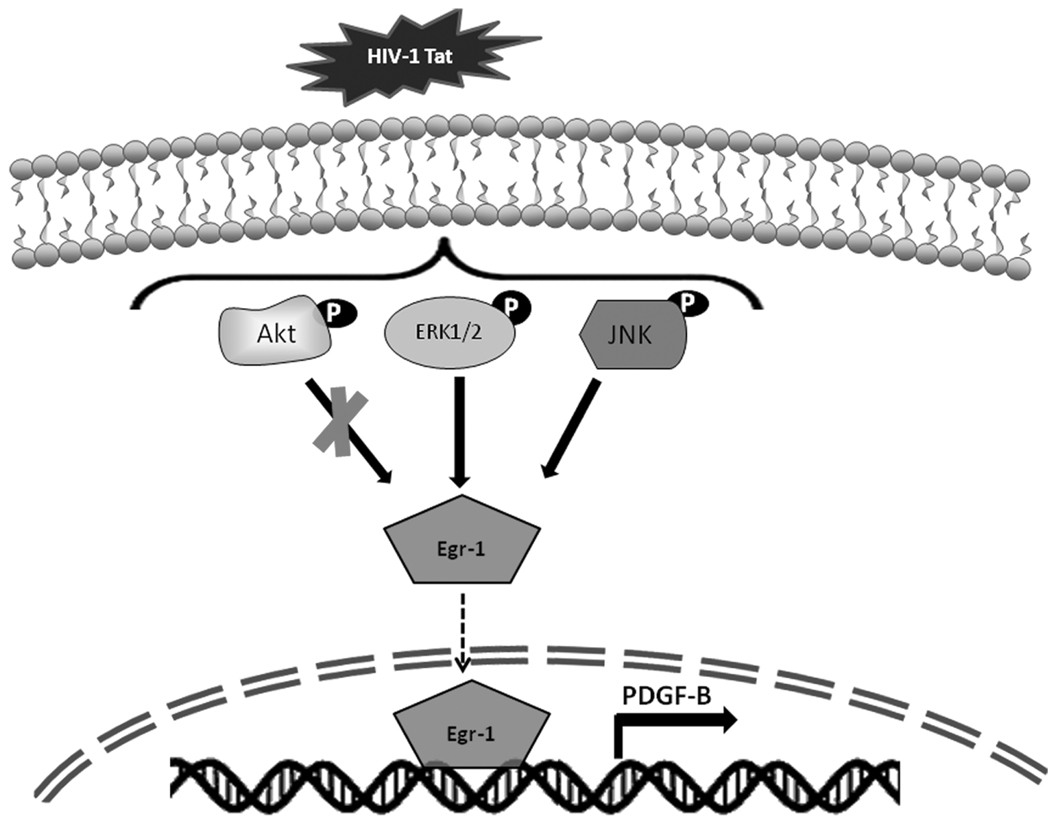
In summary, our findings have mapped out a detailed molecular pathway of Tat-mediated PDGF-BB expression represented in Figure 6. Tat induction of PDGF-BB in astrocytes involves ERK1/2 and JNK MAPK activation, with the subsequent activation of Egr-1 resulting in increased PDGF-BB expression, ultimately leading to increased astrogliosis and pro-inflammatory responses in the brains of individuals infected with HIV.
Figure 6. Schematic of the signaling pathways involved in the increased induction of PDGF-BB in astrocytes stimulated with Tat.
Tat-mediated activation of ERK1/2, JNK MAPKs and PI3K/Akt signaling pathways. ERK1/2 and JNK MAPKs but not and PI3K/Akt signaling resulted in the subsequent activation of the downstream transcription factor, Egr-1. Activation of Egr-1, in turn, leads to enhanced PDGF-BB expression.
Acknowledgements
We thank Dr. Randall Davis (Oklahoma State University, OK) for providing the A172 cell line. We thank Dr. Myron Toews (University of Nebraska Medical Center, NE) for providing C6B2 rat glioma cell lines. We thank Dr. Young Han Lee (Konkuk University, Korea) for providing the dominant negative and constitutively active constructs of MEK and Egr-1. We thank Dr. Sasahara, Mochida Company, Japan for providing us with the PDGF007 antibody.
This work was supported by grants MH-068212, DA020392, DA023397, DA024442, DA027729 (SB).
This publication was also made possible from NIH funding through the NIMH and NINDS Institutes by the following grants: Manhattan HIV Brain Bank: U01MH083501, R24MH59724; Texas NeuroAIDS Research Center U0MH083507, R24NS45491; National Neurological AIDS Bank 5U01MH083500, NS38841; California NeuroAIDS Tissue Network U0MH083506, R24MH59745; Statistics and Data Coordinating Center U01MH083545, N01MH32002. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NNTC or NIH.
Abbreviations used in this paper
- HAND
HIV-associate neurocognitive disorder
- HIVE
HIV Encephalitis
- SIV
Simian Immunodeficiency Virus
- SIVE
SIV Encephalitis
- cART
combinatorial anti-retroviral therapy
- Egr-1
early growth response gene 1
- PDGF-BB
platelet-derived growth factor-BB
- GFAP
Glial fibrillary acidic protein
- vWF
von Willebrand Factor
- ChIP
chromatin immunoprecipitation
Footnotes
The Journal of Immunology - "This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org."
References
- 1.Kaul M, Lipton SA. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res. 2006;4:307–318. doi: 10.2174/157016206777709384. [DOI] [PubMed] [Google Scholar]
- 2.Gilgun-Sherki Y, Melamed E, Offen D. Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001;40:959–975. doi: 10.1016/s0028-3908(01)00019-3. [DOI] [PubMed] [Google Scholar]
- 3.Ozdener H. Molecular mechanisms of HIV-1 associated neurodegeneration. J Biosci. 2005;30:391–405. doi: 10.1007/BF02703676. [DOI] [PubMed] [Google Scholar]
- 4.Giunta B, Obregon D, Hou H, Zeng J, Sun N, Nikolic V, Ehrhart J, Shytle D, Fernandez F, Tan J. EGCG mitigates neurotoxicity mediated by HIV-1 proteins gp120 and Tat in the presence of IFN-gamma: role of JAK/STAT1 signaling and implications for HIV-associated dementia. Brain Res. 2006;1123:216–225. doi: 10.1016/j.brainres.2006.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albright AV, Soldan SS, Gonzalez-Scarano F. Pathogenesis of human immunodeficiency virus-induced neurological disease. J Neurovirol. 2003;9:222–227. doi: 10.1080/13550280390194073. [DOI] [PubMed] [Google Scholar]
- 6.Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- 7.Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 8.van Marle G, Henry S, Todoruk T, Sullivan A, Silva C, Rourke SB, Holden J, McArthur JC, Gill MJ, Power C. Human immunodeficiency virus type 1 Nef protein mediates neural cell death: a neurotoxic role for IP-10. Virology. 2004;329:302–318. doi: 10.1016/j.virol.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Kutsch O, Oh J, Nath A, Benveniste EN. Induction of the chemokines interleukin-8 and IP-10 by human immunodeficiency virus type 1 tat in astrocytes. J Virol. 2000;74:9214–9221. doi: 10.1128/jvi.74.19.9214-9221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potula R, Dhillion N, Sui Y, Zien CA, Funa K, Pinson D, Mayo MS, Singh DK, Narayan O, Buch S. Association of platelet-derived growth factor-B chain with simian human immunodeficiency virus encephalitis. Am J Pathol. 2004;165:815–824. doi: 10.1016/S0002-9440(10)63344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seifert RA, Hart CE, Phillips PE, Forstrom JW, Ross R, Murray MJ, Bowen-Pope DF. Two different subunits associate to create isoform-specific platelet-derived growth factor receptors. J Biol Chem. 1989;264:8771–8778. [PubMed] [Google Scholar]
- 13.Brown RL, Breeden MP, Greenhalgh DG. PDGF and TGF-alpha act synergistically to improve wound healing in the genetically diabetic mouse. J Surg Res. 1994;56:562–570. doi: 10.1006/jsre.1994.1090. [DOI] [PubMed] [Google Scholar]
- 14.Lepisto J, Kujari H, Niinikoski J, Laato M. Effects of heterodimeric isoform of platelet-derived growth factor PDGF-AB on wound healing in the rat. Eur Surg Res. 1994;26:267–272. doi: 10.1159/000129345. [DOI] [PubMed] [Google Scholar]
- 15.Graham S, Leonidou A, Lester M, Heliotis M, Mantalaris A, Tsiridis E. Investigating the role of PDGF as a potential drug therapy in bone formation and fracture healing. Expert Opin Investig Drugs. 2009;18:1633–1654. doi: 10.1517/13543780903241607. [DOI] [PubMed] [Google Scholar]
- 16.Sturzl M, Roth WK, Brockmeyer NH, Zietz C, Speiser B, Hofschneider PH. Expression of platelet-derived growth factor and its receptor in AIDS-related Kaposi sarcoma in vivo suggests paracrine and autocrine mechanisms of tumor maintenance. Proc Natl Acad Sci U S A. 1992;89:7046–7050. doi: 10.1073/pnas.89.15.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao Y, Cao R, Hedlund EM. R Regulation of tumor angiogenesis and metastasis by FGF and PDGF signaling pathways. J Mol Med. 2008;86:785–789. doi: 10.1007/s00109-008-0337-z. [DOI] [PubMed] [Google Scholar]
- 18.Claesson-Welsh L. Mechanism of action of platelet-derived growth factor. Int J Biochem Cell Biol. 1996;28:373–385. doi: 10.1016/1357-2725(95)00156-5. [DOI] [PubMed] [Google Scholar]
- 19.Hede SM, Hansson I, Afink GB, Eriksson A, Nazarenko I, Andrae J, Genove G, Westermark B, Nister M. GFAP promoter driven transgenic expression of PDGFB in the mouse brain leads to glioblastoma in a Trp53 null background. Glia. 2009;57:1143–1153. doi: 10.1002/glia.20837. [DOI] [PubMed] [Google Scholar]
- 20.Nunnari G, Smith JA, Daniel R. HIV-1 Tat and AIDS-associated cancer: targeting the cellular anti-cancer barrier? J Exp Clin Cancer Res. 2008;27:3. doi: 10.1186/1756-9966-27-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su EJ, Fredriksson L, Geyer M, Folestad E, Cale J, Andrae J, Gao Y, Pietras K, Mann K, Yepes M, Strickland DK, Betsholtz C, Eriksson U, Lawrence DA. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med. 2008;14:731–737. doi: 10.1038/nm1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao H, Allen JE, Zhu X, Callen S, Buch S. Cocaine and human immunodeficiency virus type 1 gp120 mediate neurotoxicity through overlapping signaling pathways. J Neurovirol. 2009;15:164–175. doi: 10.1080/13550280902755375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao H, Peng F, Dhillon N, Callen S, Bokhari S, Stehno-Bittel L, Ahmad SO, Wang JQ, Buch S. Involvement of TRPC channels in CCL2-mediated neuroprotection against tat toxicity. J Neurosci. 2009;29:1657–1669. doi: 10.1523/JNEUROSCI.2781-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turchan J, Anderson C, Hauser KF, Sun Q, Zhang J, Liu Y, Wise PM, Kruman I, Maragos W, Mattson MP, Booze R, Nath A. Estrogen protects against the synergistic toxicity by HIV proteins, methamphetamine and cocaine. BMC Neurosci. 2001;2:3. doi: 10.1186/1471-2202-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hollman AM, Christian DA, Ray PD, Galey D, Turchan J, Nath A, Bhattacharyya D. Selective isolation and purification of tat protein via affinity membrane separation. Biotechnol Prog. 2005;21:451–459. doi: 10.1021/bp049804z. [DOI] [PubMed] [Google Scholar]
- 26.Ma M, Nath A. Molecular determinants for cellular uptake of Tat protein of human immunodeficiency virus type 1 in brain cells. J Virol. 1997;71:2495–2499. doi: 10.1128/jvi.71.3.2495-2499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 28.Bonavia R, Bajetto A, Barbero S, Albini A, Noonan DM, Schettini G. HIV-1 Tat causes apoptotic death and calcium homeostasis alterations in rat neurons. Biochem Biophys Res Commun. 2001;288:301–308. doi: 10.1006/bbrc.2001.5743. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi K, Pu H, Andras IE, Eum SY, Yamauchi A, Hennig B, Toborek M. HIV-TAT protein upregulates expression of multidrug resistance protein 1 in the blood-brain barrier. J Cereb Blood Flow Metab. 2006;26:1052–1065. doi: 10.1038/sj.jcbfm.9600254. [DOI] [PubMed] [Google Scholar]
- 30.Oshima T, Flores SC, Vaitaitis G, Coe LL, Joh T, Park JH, Zhu Y, Alexander B, Alexander JS. HIV-1 Tat increases endothelial solute permeability through tyrosine kinase and mitogen-activated protein kinase-dependent pathways. Aids. 2000;14:475–482. doi: 10.1097/00002030-200003310-00002. [DOI] [PubMed] [Google Scholar]
- 31.Prendergast MA, Rogers DT, Mulholland PJ, Littleton JM, Wilkins LH, Jr, Self RL, Nath A. Neurotoxic effects of the human immunodeficiency virus type-1 transcription factor Tat require function of a polyamine sensitive-site on the N-methyl-D-aspartate receptor. Brain Res. 2002;954:300–307. doi: 10.1016/s0006-8993(02)03360-7. [DOI] [PubMed] [Google Scholar]
- 32.Speth C, Schabetsberger T, Mohsenipour I, Stockl G, Wurzner R, Stoiber H, Lass-Florl C, Dierich MP. Mechanism of human immunodeficiency virus-induced complement expression in astrocytes and neurons. J Virol. 2002;76:3179–3188. doi: 10.1128/JVI.76.7.3179-3188.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorantla S, Santos K, Meyer V, Dewhurst S, Bowers WJ, Federoff HJ, Gendelman HE, Poluektova L. Human dendritic cells transduced with herpes simplex virus amplicons encoding human immunodeficiency virus type 1 (HIV-1) gp120 elicit adaptive immune responses from human cells engrafted into NOD/SCID mice and confer partial protection against HIV-1 challenge. J Virol. 2005;79:2124–2132. doi: 10.1128/JVI.79.4.2124-2132.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 35.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 36.Davis RL, Dertien J, Syapin PJ. Ethanol-induced modulation of inducible nitric-oxide synthase activity in human A172 astrocytoma cells. Alcohol Clin Exp Res. 2002;26:1404–1411. doi: 10.1097/01.ALC.0000030841.92766.80. [DOI] [PubMed] [Google Scholar]
- 37.Khachigian LM, Lindner V, Williams AJ, Collins T. Egr-1-induced endothelial gene expression: a common theme in vascular injury. Science. 1996;271:1427–1431. doi: 10.1126/science.271.5254.1427. [DOI] [PubMed] [Google Scholar]
- 38.Dhillon NK, Peng F, Ransohoff RM, Buch S. PDGF synergistically enhances IFN-gamma-induced expression of CXCL10 in blood-derived macrophages: implications for HIV dementia. J Immunol. 2007;179:2722–2730. doi: 10.4049/jimmunol.179.5.2722. [DOI] [PubMed] [Google Scholar]
- 39.Luo J, Miller MW. Platelet-derived growth factor-mediated signal transduction underlying astrocyte proliferation: site of ethanol action. J Neurosci. 1999;19:10014–10025. doi: 10.1523/JNEUROSCI.19-22-10014.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou BY, Liu Y, Kim B, Xiao Y, He JJ. Astrocyte activation and dysfunction and neuron death by HIV-1 Tat expression in astrocytes. Mol Cell Neurosci. 2004;27:296–305. doi: 10.1016/j.mcn.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 41.James AB, Conway AM, Morris BJ. Genomic profiling of the neuronal target genes of the plasticity-related transcription factor -- Zif268. J Neurochem. 2005;95:796–810. doi: 10.1111/j.1471-4159.2005.03400.x. [DOI] [PubMed] [Google Scholar]
- 42.Silverman ES, Collins T. Pathways of Egr-1-mediated gene transcription in vascular biology. Am J Pathol. 1999;154:665–670. doi: 10.1016/S0002-9440(10)65312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng F, Yao H, Bai X, Zhu X, Reiner BC, Beazely M, Funa K, Xiong H, Buch S. Platelet-derived growth factor-mediated induction of the synaptic plasticity gene Arc/Arg3.1. J Biol Chem. doi: 10.1074/jbc.M110.107003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shin HJ, Lee JB, Park SH, Chang J, Lee CW. T-bet expression is regulated by EGR1-mediated signaling in activated T cells. Clin Immunol. 2009;131:385–394. doi: 10.1016/j.clim.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Freter RR, Alberta JA, Hwang GY, Wrentmore AL, Stiles CD. Platelet-derived growth factor induction of the immediate-early gene MCP-1 is mediated by NF-kappaB and a 90-kDa phosphoprotein coactivator. J Biol Chem. 1996;271:17417–17424. doi: 10.1074/jbc.271.29.17417. [DOI] [PubMed] [Google Scholar]
- 46.Poon M, Hsu WC, Bogdanov VY, Taubman MB. Secretion of monocyte chemotactic activity by cultured rat aortic smooth muscle cells in response to PDGF is due predominantly to the induction of JE/MCP-1. Am J Pathol. 1996;149:307–317. [PMC free article] [PubMed] [Google Scholar]
- 47.Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26:1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dhillon NK, Williams R, Callen S, Zien C, Narayan O, Buch S. Roles of MCP-1 in development of HIV-dementia. Front Biosci. 2008;13:3913–3918. doi: 10.2741/2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci U S A. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanders VJ, Pittman CA, White MG, Wang G, Wiley CA, Achim CL. Chemokines and receptors in HIV encephalitis. Aids. 1998;12:1021–1026. [PubMed] [Google Scholar]
- 51.Bell JE. The neuropathology of adult HIV infection. Rev Neurol (Paris) 1998;154:816–829. [PubMed] [Google Scholar]